Abstract
The aim of this study was to investigate the effect of bexagliflozin on glycemic control in poorly regulated diabetic cats and to evaluate for adverse events associated with this medication.
Sodium-glucose cotransporter 2 inhibitors are a newer class of drugs used in the management of humans with type 2 diabetes mellitus. The objective of this study was to evaluate the effect of the orally administered drug, bexagliflozin in a group of poorly regulated diabetic cats over a 4-week study period. Five client-owned cats with poorly controlled diabetes mellitus receiving insulin therapy were enrolled. Bexagliflozin was administered once daily. Serum fructosamine, serum biochemistry profile, and 10-hour blood glucose curves were assessed at baseline (Day 0), Day 14, and Day 28. All cats had a significant reduction in insulin dose requirement (P = 0.015) and insulin was discontinued in 2 cats. There was a significant decrease in blood glucose concentration obtained from blood glucose concentration curves during the study period (P = 0.022). Serum fructosamine decreased in 4 of the 5 cats with a median decrease of 152 μmol/L (range: 103 to 241 μmol/L), which was not statistically significant (P = 0.117). No cats had any documented episodes of hypoglycemia. Adverse effects were mild. The addition of bexagliflozin significantly improved diabetic management in this group of cats.
Résumé
Le but de cette étude était d’étudier l’effet de la bexagliflozine sur la maitrise de la glycémie chez les chats diabétiques mal régulés et d’évaluer les événements indésirables associés à ce médicament.
Les inhibiteurs du cotransporteur sodium-glucose 2 sont une nouvelle classe de médicaments utilisés dans la prise en charge des personnes atteintes de diabète de type 2. L’objectif de cette étude était d’évaluer l’effet du médicament administré par voie orale, la bexagliflozine, dans un groupe de chats diabétiques mal régulés sur une période d’étude de 4 semaines. Cinq chats appartenant à des clients atteints de diabète sucré mal maitrisé et recevant une insulinothérapie ont été inclus. La bexagliflozine a été administrée une fois par jour. La fructosamine sérique, le profil biochimique sérique et les courbes de glycémie sur 10 heures ont été évalués au départ (jour 0), au jour 14 et au jour 28. Tous les chats ont présenté une réduction significative de la dose d’insuline requise (P = 0,015) et l’insuline a été interrompue chez deux chats. Il y avait une diminution significative de la concentration de glucose dans le sang obtenue à partir des courbes de concentration de glucose dans le sang au cours de la période d’étude (P = 0,022). La fructosamine sérique a diminué chez 4 des 5 chats avec une diminution médiane de 152 μmol/L (plage : 103 à 241 μmol/L), ce qui n’était pas statistiquement significatif (P = 0,117). Aucun chat n’a eu d’épisodes documentés d’hypoglycémie. Les effets indésirables étaient légers. L’ajout de bexagliflozine a considérablement amélioré la gestion du diabète dans ce groupe de chats.
(Traduit par Docteur Serge Messier)
Introduction
Diabetes mellitus is a common endocrine disorder that affects many animals, including cats (1). The mainstay of treatment for cats with diabetes is insulin administration coupled with a high-protein, low-carbohydrate diet (2). Treatment commitments associated with insulin administration, including daily needle injections, potential for hypoglycemic crises, and costs of therapy, have been shown to significantly impact the daily routine and life quality of pet owners with diabetic animals (3–5). The commitment that is required may result in poor compliance or limit an owner’s willingness to treat, which could result in the diabetic pet being relinquished or euthanized (5).
A drug that could be easily administered to cats to safely lower blood glucose with minimal adverse effects and low risk of hypoglycemia would have significant clinical application in treating cats with diabetes. The use of oral diabetic medications, including sulfonylureas, biguanides, alpha-glucosidase inhibitors, and incretin mimetics, has been described in cats with mostly unfavorable results (2,6–12).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a newer class of orally administered antidiabetic agents that inhibit glucose absorption from the kidney. SGLT2 is located within the early proximal tubule and is responsible for 90% of renal glucose reabsorption (13). Inhibition of renal glucose reabsorption has the highly desirable effect of lowering blood glucose with minimal risk of inducing hypoglycemia (13–15). The SGLT2 inhibitors dapagliflozin and velagliflozin have been evaluated in healthy cats and found to be well-tolerated and result in significant urinary glucose excretion without inducing hypoglycemia (16,17).
Bexagliflozin is a potent and highly selective SGLT2 inhibitor that has completed phase-3 clinical trials in adults with type-2 diabetes mellitus and is waiting for approval from the Food and Drug Administration (FDA) (18). Pharmacokinetic and pharmacodynamic studies conducted in healthy research cats showed that bexagliflozin yielded similar results to prior studies of SGLT2 inhibitor use in cats (19).
The aim of this study was to investigate the effect of bexagliflozin on glycemic control in poorly regulated diabetic cats and to evaluate for adverse events associated with this medication. Our hypothesis was that bexagliflozin would lower both blood glucose and serum fructosamine concentrations and would be well-tolerated in cats.
Materials and methods
Study cats
Five client-owned cats with poorly regulated diabetes mellitus were enrolled. Cats were considered poorly regulated if they had polyuria, polydipsia, polyphagia, and weight loss in addition to a nadir blood glucose concentration of > 250 mg/dL despite insulin dose adjustments over a minimum period of 8 wk before enrollment.
Exclusion criteria included age < 2 y or > 15 y, weight < 3.0 kg, chronic kidney disease IRIS stage ≥ 2, elevated serum bilirubin, alanine aminotransferase (ALT) elevation ≥ 2.5 times the upper limit of normal, active urinary tract infection, use of corticosteroids within the past 8 wk, congestive heart failure, uncontrolled hyperthyroidism, and a history of diabetic ketoacidosis within the preceding 2 mo. Cats with a history of lower urinary tract infections were included if the infection responded to a course of antibiotics and the urine culture was negative at the time of enrollment following discontinuation of antibiotics.
The study was approved by the Tufts University Cummings School of Veterinary Medicine Clinical Studies Review Committee (Protocol number 003-16 approved on April 11, 2016). Informed consent was obtained from the owner of all animals for the procedures undertaken during this prospective study. Informed consent was also obtained for any animals or humans individually identifiable for their use in this article.
Study design
Cats were enrolled for a 4-week study period with designated time points of measurement on Days 0, 14, and 28. On Day 0, each cat was thoroughly evaluated by obtaining a complete history, including a diet history, physical examination, and assessing body weight and body condition score (BCS) using the WSAVA 9-point scale and WSAVA muscle condition score (MCS) as described for use in cats (20).
A complete blood (cell) count (CBC), serum biochemical profile, urinalysis, aerobic bacterial urine culture, and total thyroxine was carried out on all cats. Urine samples were obtained by cystocentesis. An in-hospital serial blood glucose curve (BGC) was carried out and serum fructosamine concentration was measured. For serial BGC, the blood glucose concentration was measured every 2 h for 10 h using a portable glucometer (alphaTRAK 2; Zoetis, Parsippany, New Jersey, USA), validated for use in cats (21).
Measurement of the in-hospital BGC began in the morning after insulin was administered and the cat had eaten a full meal and was discontinued before the meal and insulin administration in the evening. The mean blood glucose concentration was calculated as the average of 5 blood glucose measurements that were obtained during the 10-hour BGC.
Capillary blood was sampled using a 22-gauge needle. Blood samples for CBC, serum biochemical analysis, and urinalyses were analyzed by standard laboratory methods. Serum was centrifuged immediately after sample collection at 1500 × g for 5 min. Urine and blood samples were stored at 4°C before analysis. Fructosamine concentrations were measured with a commercial analyzer and commercial reagent (IDEXX Laboratories, Westbrook, Maine, USA).
At discharge on Day 0, all owners were shown how to measure blood glucose concentration at home using the alphaTRAK 2 glucometer and asked to measure blood glucose concentrations twice daily and at times of suspected hypoglycemic events. All cats remained on their typical diet and type/brand of insulin. To minimize the risk of hypoglycemia, the insulin dose was reduced by 50% on Day 0 and further reductions were based on blood glucose concentrations obtained from at-home and in-hospital assessments.
Hypoglycemia was defined as the presence of supportive clinical signs and/or a blood glucose of ≤ 60 mg/dL assessed on an alpha-TRAK 2 portable glucometer. Owners were advised to check BG before administering insulin, to reduce insulin dose by at least 25% if the blood glucose was persistently 100 to 200 mg/dL, and to withhold insulin if blood glucose was < 100 mg/dL to circumvent a hypoglycemic crisis. Owners were also instructed to monitor for signs of hypoglycemia, including weakness, ataxia, twitching, abnormal behaviors, or seizure activity and advised to give 2 mL of Karo Syrup orally (ACH Food Companies, Oakbrook Terrace, Illinois, USA), encourage the cat to eat, and measure blood glucose if hypoglycemia was suspected.
Owners were required to record at-home blood glucose readings at each scheduled hospital visit on Days 0, 14, and 28 and complete a questionnaire about the insulin dose and times and dates of bexagliflozin administration. Any changes in thirst, urination, attitude, appetite, and any additional abnormalities were also reported. Before each scheduled hospital visit (Days 0, 14, and 28), the owners fed the cat and administered insulin and bexagliflozin before coming to hospital.
Bexagliflozin was dispensed to the owners, with instructions that it be started on Day 1. The first cat received a dose of 10 mg, PO, q24h. Subsequent cats received 15 mg, PO, q24h based on further research by the manufacturer that demonstrated maximal renal glucose excretion at the higher dose (19). Follow-up evaluations on Days 14 and 28 included a history, physical examination, body weight, BCS, MCS, BGC, serum biochemical profile, and serum fructosamine, in addition to a CBC on Day 28. Serum was collected on Days 0, 14, and 28 for measuring beta-hydroxybutyrate (β-OHB), which was done at the Animal Health Diagnostic Center at Cornell University College of Veterinary Medicine.
Statistical analysis
All variables were reported as medians and ranges. Parameters were analyzed with nonparametric tests for non-normally distributed data. Continuous variables included age, body weight, BCS, MCS, insulin dose, duration of clinical signs before diagnosis of diabetes mellitus, duration of diabetic management before starting bexagliflozin, and laboratory data, including the mean blood glucose (MBG) concentration of the BGCs conducted on Days 0, 14, and 28. Time points of measurements were Days 0, 14, and 28 of the study period.
Friedman repeated measures for paired continuous variables were used to compare MBG, β-OHB, insulin dose, fructosamine, MCS, BCS, weight, and biochemical parameters at Days 0, 14, and 28. Data were analyzed with StatView 5.0 software (SAS Institute, Cary, North Carolina, USA). Significance was set as P ≤ 0.05.
Results
Five cats with poorly regulated diabetes mellitus were enrolled in the study over a 9-month period. Breeds included domestic shorthair (n = 2), domestic longhair (n = 2), and Siamese (n = 1). Age at enrollment ranged from 5 to 10 y (median: 8 y). Four cats were castrated males and 1 was a spayed female. Median body weight of enrolled cats was 6.7 kg (range: 3.2 to 7.8 kg) with a median BCS of 7 (range: 3 to 8) based on a 9-point scale.
Comorbidities included dental disease in 2 cats, hypertrophic cardiomyopathy in 2 cats, and inflammatory bowel disease and asthma in 1 cat. One of the 2 cats with hypertrophic cardiomyopathy was receiving clopidogrel and lisinopril. This cat also had concurrent inflammatory bowel disease that was controlled with diet and probiotic therapy, as well as asthma that was managed with environmental modification.
Cats had been receiving insulin treatment for a median of 86 d (range: 76 to 318 d) before enrollment. Four cats were on prescription diets formulated for diabetic cats. One cat was on a commercially available feline maintenance diet (dry and canned) as previous attempts to transition to a diabetic diet resulted in hyporexia. All cats were receiving insulin glargine, SQ, q12h. At Day 0, the median insulin dose was 0.67 U/kg body weight (BW) (0.53 to 1.56 U/kg BW), q12h.
Baseline diagnostics
On Day 0, CBC results were within normal limits for all enrolled cats (data not shown). Serum biochemistry parameters are presented in Table I. All cats were hyperglycemic. One cat had an ALT 1.07 times the upper reference range that was unchanged from 2 mo earlier. Urinalyses revealed glucosuria in all cats, but results were otherwise unremarkable. No cats had ketonuria and urine cultures were negative for all cats. Serum total thyroxine concentrations were assessed in all cats before enrollment in the study and were within normal limits (median: 2.2 μg/L; range: 1.2 to 2.4 μg/L). The median serum β-OHB concentration was 0.14 mmol/L (range: 0.00 to 0.18 mmol/L) and the median fructosamine concentration was 507 μmol/L (range: 410 to 594 μmol/L). The median mean blood glucose (MBG) concentration during the in-hospital blood glucose curve (BGC) was 380 mg/dL (range: 312 to 479 mg/dL).
Table I.
Serum chemistry values at baseline (Day 0) and at end of study period (Day 28).
| Day 0 | Day 14 | Day 28 | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Variable | Median (range) | Median (range) | Median (range) | Reference interval | P-value |
| Glucose (mg/dL) | 337 (318 to 652) | 140 (87 to 285) | 164 (87 to 274) | 70 to 120 | 0.0193 |
| BUN (mg/dL) | 38 (22 to 39) | 40 (24 to 49) | 39 (27 to 48) | 15 to 33 | 0.2466 |
| Creatinine (mg/dL) | 0.9 (0.6 to 1.3) | 0.9 (0.8 to 1.4) | 0.9 (0.8 to 1.6) | 0.9 to 2.1 | 0.2122 |
| Phosphorus (mg/dL) | 4.3 (3.8 to 5.4) | 5.4 (4.3 to 6.0) | 5.2 (4.1 to 5.6) | 3.0 to 6.3 | 0.1653 |
| Calcium, total (mg/dL) | 10.3 (9.5 to 10.8) | 10.6 (10.3 to 11.9) | 10.8 (10.3 to 11.6) | 8.8 to 12.0 | 0.0429 |
| Total protein (g/dL) | 7.8 (6.8 to 8.8) | 8.5 (7.4 to 8.9) | 8.1 (7.0 to 9.4) | 6.0 to 8.4 | 0.0907 |
| Albumin (g/dL) | 3.8 (3.0 to 4.0) | 4.2 (3.5 to 4.9) | 3.9 (3.7 to 4.6) | 2.2 to 4.0 | 0.0743 |
| Globulin (g/dL) | 4.0 (2.8 to 5.8) | 3.6 (3.2 to 5.4) | 3.7 (3.2 to 5.6) | 2.5 to 5.8 | 0.7047 |
| Sodium (mEq/L) | 147 (141 to 151) | 151 (144 to 162) | 153 (146 to 153) | 146 to 158 | 0.2466 |
| Potassium (mEq/L) | 4.6 (4.4 to 5.0) | 4.3 (3.6 to 4.8) | 4.2 (4.1 to 5.3) | 3.4 to 5.2 | 0.2466 |
| Anion gap (mEql/L) | 20 (17 to 25) | 23 (21 to 32) | 23 (20 to 34) | 9 to 21 | 0.0474 |
| Total bilirubin (mg/dL) | 0 (0 to 0.1) | 0 (0 to 0.1) | 0 (0 to 0.1) | 0.1 to 0.3 | 0.8607 |
| ALP (U/L) | 53 (28 to 119) | 36 (17 to 83) | 28 (17 to 78) | 10 to 79.0 | 0.0193 |
| GGT (U/L) | 0 (0 to 1) | 0 (0 to 0) | 0 (0 to 1) | 0 to 5 | 0.6376 |
| ALT (U/L) | 40 (28 to 158) | 53 (35 to 127) | 47 (34 to 168) | 25 to 145 | 0.5488 |
| AST (U/L) | 18 (11 to 55) | 26 (10 to 35) | 28 (10 to 43) | 5.0 to 42 | 0.3867 |
| Creatinine kinase (U/L) | 110 (95 to 153) | 143 (82 to 384) | 92 (84 to 287) | 14 to 528 | 0.8187 |
| Cholesterol (mg/dL) | 221 (169 to 256) | 244 (230 to 394) | 281 (204 to 436) | 77 to 258 | 0.5488 |
| Triglycerides (mg/dL) | 195 (55 to 817) | 108 (40 to 4277) | 147 (125 to 7140) | 25 to 191 | 0.1738 |
BUN — blood urea nitrogen; ALP — alkaline phosphatase; GGT — γ-glutamyl transferase; ALT — alanine aminotransferase; AST — aspartate aminotransferase.
Response to treatment with bexagliflozin
The MBG concentration obtained from the in-hospital BGCs decreased significantly during the 4-week study period (P = 0.0224) (Figure 1). The median decrease of MBG from the blood glucose curves (BGCs) in this group of cats was 193 mg/dL (range: 81 to 313 mg/dL). The serum fructosamine concentration decreased in 4 of the 5 cats with a median decrease of 152 μmol/L (range: 103 to 241 μmol/L), although this did not reach statistical significance (P = 0.1165; Figure 2).
Figure 1.
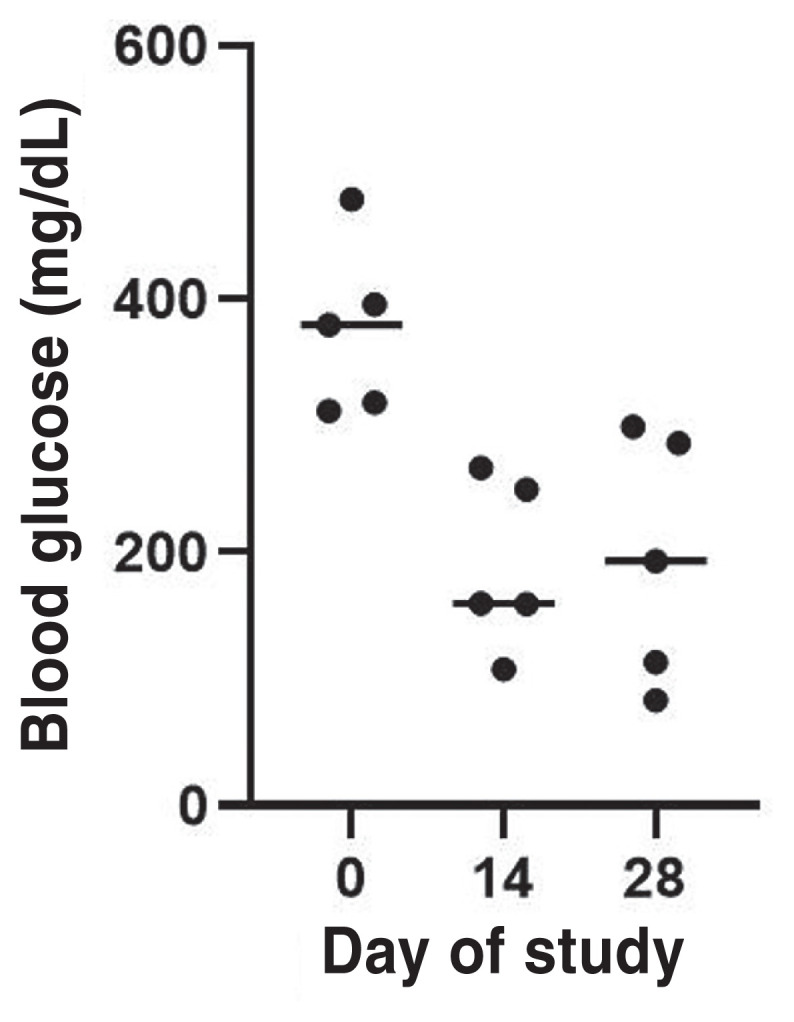
Serial blood glucose concentrations of the blood glucose curves (BGCs) of diabetic cats (N = 5) before (Day 0) and after receiving oral bexagliflozin daily for 2 wk (Day 14) and 4 wk (Day 28) during the 4-week study period. Dots represent the mean blood glucose (MBG) concentration obtained from the 10-hour BGCs of each cat on Days 0, 14, and 28. Horizontal line represents median values of the MBG concentration obtained during the 10-hour BGC of all cats on Days 0, 14, and 28.
Figure 2.
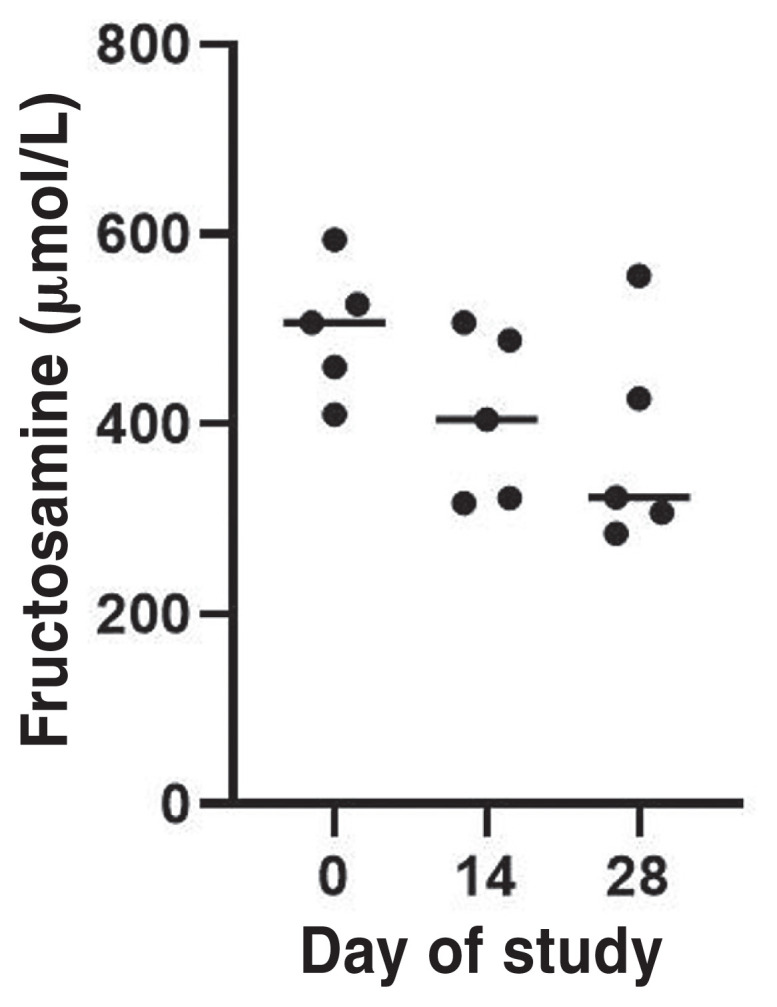
Fructosamine concentrations (median) of diabetic cats (N = 5) before (Day 0) and after receiving oral bexagliflozin daily for 2 wk (Day 14) and 4 wk (Day 28) during the 4-week study period. Dots represent the MBG concentration. Horizontal line represents median.
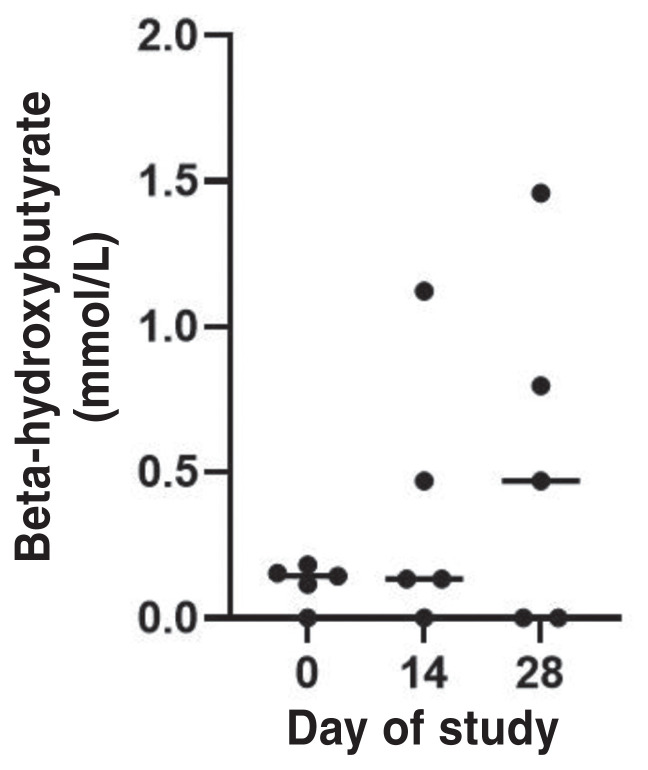
Insulin dose was decreased significantly in all cats during the study period (P = 0.015; Figure 3). The median insulin dose decreased 0.55 U/kg BW (range: 0.52 to 0.99 U/kg BW). Insulin was discontinued in 2 of the 5 cats on Day 14 (Figure 3). The median β-OHB concentration increased 0.33 mmol/L (range: 0.00 to 1.31 mmol/L), which was not statistically significant (P = 0.52; Figure 4).
Figure 3.
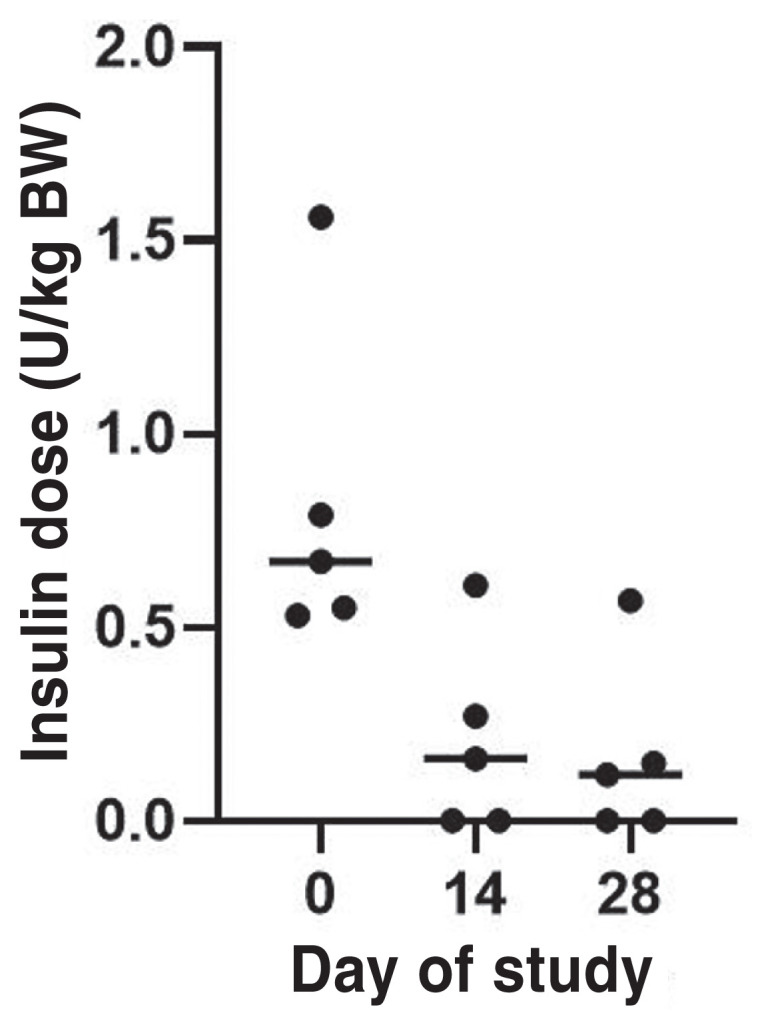
Insulin dose (median) administered every 12 h to diabetic cats (N = 5) before (Day 0) and after receiving oral bexagliflozin daily for 2 wk (Day 14) and 4 wk (Day 28) during the 4-week study period. Dots represent the MBG concentration. Horizontal line represents median.
Figure 4.
Beta-hydroxybutyrate (β-OHB) values (median) of diabetic cats (N = 5) before (Day 0) and after receiving oral bexagliflozin daily for 2 wk (Day 14) and 4 wk (Day 28) during the 4-week study period. Dots represent the MBG concentration. Horizontal line represents median.
Serum biochemistry parameters were compared on Days 0, 14, and 28 and are shown in Table I. The median serum glucose concentration decreased significantly during the study period (P = 0.0193). Total calcium concentration increased during the study period (P = 0.0429), but the total calcium concentration in all cats remained within the reference range. None of the cats was classified as hypercalcemic (total calcium ≤ 12.0 mg/dL). Alkaline phosphatase (ALP) decreased significantly during the study period (P = 0.0193), whereas the anion gap increased significantly (P = 0.0474). Anion gap increased in 4 of the 5 cats, with mild elevations (≤ 23.0 mEq/L) at Day 28 in all but 1 cat. This cat had a slightly elevated anion gap at Day 0 (25 mEq/L), which further increased during the study to 34 mEq/L on Day 28. No statistically significant changes occurred in any other laboratory parameters.
All cats completed the study. Compliance was excellent with no cat reported to miss any bexagliflozin administration and there were no leftover tablets at the end of the trial when the owners were asked to return the prescription provided for the 28-day study. No cat had any documented episodes of hypoglycemia assessed during at-home or in-hospital blood glucose monitoring or reported clinical signs consistent with hypoglycemia during the study period.
The owners of 3 cats reported that clinical signs were resolved at the end of the 28-day study period. Clinical signs reported in the remaining 2 cats included polyuria, polydipsia, and polyphagia, although these were reportedly improved based on subjective client assessment. Weight loss stopped in 3 cats during the study period, whereas the remaining 2 cats lost 0.09 kg and 0.25 kg, which was a percentage weight loss of 2.3% and 3.8%, respectively. These changes in body weight were not statistically significant (P = 0.6376). There was no change in body condition score (BCS) during the 28-day study period in any of the cats.
Adverse effects
Given our secondary aim, we evaluated patients to determine if any adverse effects were noted during this study. No significant adverse effects occurred in any cats during this study. No cat had to be discontinued early from the study due to adverse effects or difficulty administering bexagliflozin orally. All cats continued to maintain a good appetite during the study period. One cat with a prior history of severe pancreatitis developed mild hyporexia on the last day of the study (Day 28). This cat also experienced a marked, progressive increase in triglyceride serum concentration at Day 14 (4277 mg/dL) and Day 28 (7140 mg/dL), compared to Day 0 (208 mg/dL), as well as increases in β-OHB concentrations from Day 0 (0.15 mmol/L) to Day 28 (1.46 mmol/L).
Long-term follow-up
Insulin was discontinued in 2 cats during the study. One of these cats continued to receive bexagliflozin for over 2 y and was receiving this medication as sole therapy when this article was submitted. The other cat received bexaglifozin for an additional 5 wk until it was stopped and insulin was restarted due to the development of small bowel diarrhea that the owner reported as moderately severe. The diarrhea resolved after bexagliflozin was discontinued. This cat had a history of inflammatory bowel disease and prior episodes of diarrhea.
The cat receiving 10 mg of bexagliflozin once daily continued to require insulin therapy for the duration of the 4-week study period. On completion of the study, the dosage of bexagliflozin was increased to 15 mg/d and insulin was discontinued, with successful management of diabetes for a further 17 mo until an episode of clinically suspected pancreatitis resulted in the need to supplement with 1 U of insulin glargine twice daily to control hyperglycemia.
The fourth cat in this study continued on 1 U of insulin twice daily in addition to bexagliflozin until hyporexia developed at Day 28. Immediately after completion of the study, bexagliflozin was discontinued and insulin was increased to 2 U twice daily, which resolved the hyporexia. One month after completion of this study, bloodwork revealed marked hypertriglyceridemia (1643 mg/dL) and this cat was gradually increased to 4 U of glargine twice daily, which resulted in ongoing control of clinical signs of diabetes mellitus.
The fifth cat was continued on 2 U of glargine twice daily in addition to bexagliflozin after completion of the study due to ongoing clinical improvement of diabetes mellitus. Although bexagliflozin was ultimately discontinued due to concerns about diarrhea, the diarrhea did not improve. The insulin dosage was gradually increased to 4 U twice daily after bexagliflozin was discontinued.
Discussion
Our results showed that the oral administration of the SLGT2 inhibitor, bexagliflozin, significantly lowered blood glucose and insulin dose in poorly regulated diabetic cats over a 28-day period. Insulin was discontinued in 2 out of 5 cats. Bexagliflozin was well-tolerated by the cats and administration compliance was excellent during the study period. Our results therefore suggest that bexagliflozin may have potential promise in managing feline diabetes.
Although serum fructosamine concentration decreased in 4/5 cats during the study period, this decrease did not reach statistical significance. This could be due to the small number of cats in this study and lack of power to reach statistical significance. Additionally, fructosamine concentrations can vary among individual cats and may be influenced by non-diabetic factors, including body weight, sex, serum protein concentration, and hydration status (22,23).
Three cats in the study did experience asymptomatic ketosis as evidenced by elevations in β-OHB during the study. This is not unexpected as mild elevations of β-OHB would likely result from decreased exogenous insulin until beta cell recovery and improvement in endogenous insulin secretion occurs (24). Given the mechanism of action of SGLT2 inhibitors, the body must be able to produce some endogenous insulin to allow complete withdrawal of exogenous insulin or else ketosis will ensue.
In light of this potential risk, it is advised that ketones be monitored in cats receiving SGLT2 inhibitors, especially if there are signs of illness. Although the elevations in β-OHB in this group of cats were not high enough to be considered clinically significant, we cannot rule out the possibility that ketonemia may have contributed to the hyporexia observed in 1 cat at the end of the study. This cat also experienced marked hypertriglyceridemia at Day 28, however, which may have contributed to clinical signs of hyporexia. Hypertriglyceridemia in this cat may have been due to lack of adequate insulin production. Although we cannot rule out the possibility that hypertriglyceridemia is an adverse effect related to the use of SGLT2 inhibitor in cats, SGLT2 inhibitors have been shown to reduce serum triglyceride levels in humans and mice (25,26).
In clinical trials of SGLT2 inhibitors in humans with type-2 diabetes mellitus, the risk of hypoglycemia is rare due to the selectivity of these drugs. SGLT1 function is preserved as are counter-regulatory mechanisms that prevent hypoglycemia, including decreased insulin secretion and increased glucagon levels. However, the risk of hypoglycemia increases with co-administration of sulfonylureas or insulin (27,28). Since the cats in this study were receiving insulin in addition to bexagliflozin, the risk of hypoglycemia was deemed to be higher. The insulin dose was therefore reduced by 50% at the onset of enrollment before Day 0. The dosage of insulin was subsequently titrated further based on at-home blood glucose monitoring and in-hospital BGC.
As osmotic diuresis accompanies glycosuria, urine output often increases during treatment with SGLT2 inhibition in human patients (29). This side effect has also been reported in healthy cats (17). Unexpectedly, 3/5 cats in this study had client-reported resolution of polyuria and polydipsia and no cat had worsening of these signs. Two of the 3 cats with resolution of polyuria and polydipsia were taken off insulin and improvement in hyperglycemia may have helped control these signs.
Because of the presence of SGLT1 in the small intestine, diarrhea occurs with nonselective SGLT2 inhibitors but is not expected to occur with highly selective SGLT2 inhibitors such as bexagliflozin (27,30). One cat with a history of inflammatory bowel disease experienced diarrhea when bexagliflozin was continued beyond the study period. The diarrhea resolved in this cat when the drug was discontinued. One cat had a mildly elevated anion gap on Day 0 that increased moderately during the study. This cat was reportedly doing clinically well during the study, although continued signs of polyphagia, polyuria, and polydipsia were reported. This cat also had an increase in blood urea nitrogen during the study, potentially indicating pre-renal azotemia due to progressive dehydration that may have contributed to an elevated anion gap.
This study had several limitations. The sample size was small and therefore the study may have been underpowered to detect differences in some parameters. A second limitation was the fact that the first cat enrolled in the study received a lower dose (10 mg) than the other cats (15 mg). The dose was increased after industry-sponsored data from the drug manufacturer suggested that urine glucose excretion was maximal at 15 mg. Despite the possibility that 1 cat’s dose was not optimized, there was a statistically significant decrease in the median blood glucose concentration and in insulin dose over the course of the study. It is of note that the cat on this lower dose was later changed to 15 mg once daily and achieved adequate glycemic control after the study ended.
A third limitation was the lack of a control group in this study. A control group would have helped to support the fact that the findings were directly due to bexagliflozin treatment, rather than other explanatory factors. Given that our goal was to establish a proof of concept for the use of bexagliflozin in diabetic cats, we aimed to collect comprehensive baseline data in an effort to provide valuable information for use in future larger studies to evaluate clinical use of bexagliflozin and potentially other SGLT2 inhibitors in diabetic cats. Therefore, the addition of a control group was not sought after and would not be expected to alter our findings in this small population. Nevertheless, future studies should focus on a larger population with placebo controls and ideally be double-blinded.
A fourth limitation was that the drug was used in cats with poorly regulated diabetes mellitus. Although this was the group we selected for, monitoring glycemic control in newly diagnosed diabetic cats may have yielded different results. The fifth limitation was the short duration of this study. Further studies evaluating SGLT2 inhibitors over a longer period would help to identify when the maximal effects on glucose regulation occur and if adverse effects are noted with long-term use.
In conclusion, bexagliflozin appears to be effective in lowering blood glucose and the dose of insulin required in cats with poorly controlled diabetes mellitus receiving conventional therapy. Further studies are warranted.
Acknowledgments
Bexagliflozin was provided free of charge by IncreVet Inc. for use in this study. Diagnostics and monitoring costs associated with the study were provided by the Cummings School of Veterinary Medicine Companion Animal Health Fund.
The authors thank Cynthia R.L. Webster, DVM, DACVIM, for assistance with statistical evaluation and editing.
The figures were created using GraphPad Prism version 9.2.0 for windows (GraphPad Software, San Diego, California, USA) www.graphpad.com
References
- 1.O’Neill DG, Gostelow R, Orme C, et al. Epidemiology of diabetes mellitus among 193 435 cats attending primary-care veterinary practices in England. J Vet Int Med. 2016;30:964–972. doi: 10.1111/jvim.14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparkes AH, Cannon M, Church D, et al. ISFM consensus guidelines on the practical management of diabetes mellitus in cats. J Feline Med Surg. 2015;17:235–250. doi: 10.1177/1098612X15571880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aptekmann KP, Armstrong J, Coradini M, Rand J. Owner experiences in treating dogs and cats diagnosed with diabetes mellitus in the United States. J Am Anim Hosp Assoc. 2014;50:247–253. doi: 10.5326/JAAHA-MS-6101. [DOI] [PubMed] [Google Scholar]
- 4.Niessen SJM, Powney S, Guitian J, et al. Evaluation of a quality-of-life tool for cats with diabetes mellitus. J Vet Intern Med. 2010;24:1098–1105. doi: 10.1111/j.1939-1676.2010.0579.x. [DOI] [PubMed] [Google Scholar]
- 5.Niessen SJM, Hazuchova K, Powney SL, et al. The big pet diabetes survey: Perceived frequency and triggers for euthanasia. Vet Sci. 2017;4:27. doi: 10.3390/vetsci4020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson RW. Oral medications for treating diabetes mellitus in dogs and cats. J Small Anim Pract. 2000;41:486–490. doi: 10.1111/j.1748-5827.2000.tb03969.x. [DOI] [PubMed] [Google Scholar]
- 7.Miller AB, Nelson RW, Kirk CA, Neal L, Feldman EC. Effect of glipizide on serum insulin and glucose concentrations in healthy cats. Res Vet Sci. 1992;52:177–181. doi: 10.1016/0034-5288(92)90007-o. [DOI] [PubMed] [Google Scholar]
- 8.Ford SL. NIDDM in the cat: Treatment with the oral hypoglycemic medication, glipizide. Vet Clin North Am Small Anim Pract. 1995;25:599–615. doi: 10.1016/s0195-5616(95)50056-7. [DOI] [PubMed] [Google Scholar]
- 9.Nelson RW, Feldman EC, Ford SL. Effect of an orally administered sulfonylurea, glipizide, for treatment of diabetes mellitus in cats. J Am Vet Med Assoc. 1993;203:821–827. [PubMed] [Google Scholar]
- 10.Mazzaferro EM, Greco DS, Turner AS, Fettman MJ. Treatment of feline diabetes mellitus using an α-glucosidase inhibitor and a low-carbohydrate diet. J Feline Med Surg. 2003;5:183–189. doi: 10.1016/S1098-612X(03)00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson R, Spann D, Elliott D, Brondos A, Vulliet R. Evaluation of the oral antihyperglycemic drug metformin in normal and diabetic cats. J Vet Intern Med. 2004;18:18–24. doi: 10.1892/0891-6640(2004)18<18:eotoad>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Riederer A, Zini E, Salesov E, et al. Effect of the glucagon-like peptide-1 analogue exenatide extended release in cats with newly diagnosed diabetes mellitus. J Vet Intern Med. 2016;30:92–100. doi: 10.1111/jvim.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallon V, Sharma K. Sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Curr Opin Nephrol Hypertens. 2010;19:425–431. doi: 10.1097/MNH.0b013e32833bec06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idris I, Donnelly R. Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug. Diabetes Obes Metab. 2009;11:79–88. doi: 10.1111/j.1463-1326.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 15.Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: Preclinical and clinical data. Pharmacol Ther. 2013;139:51–59. doi: 10.1016/j.pharmthera.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Gal A, Burton SE, Weidgraaf K, et al. The effect of the sodium-glucose cotransporter type-2 inhibitor dapagliflozin on glomerular filtration rate in healthy cats. Domest Anim Endocrinol. 2020;70:106376. doi: 10.1016/j.domaniend.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Hoenig M, Clark M, Schaeffer DJ, Reiche D. Effects of the sodium-glucose cotransporter 2 (SGLT2) inhibitor velagliflozin, a new drug with therapeutic potential to treat diabetes in cats. J Vet Pharmacol Ther. 2018;41:266–273. doi: 10.1111/jvp.12467. [DOI] [PubMed] [Google Scholar]
- 18.McMurray JJV, Freeman MW, Massaro J, et al. 32-OR: The bexagliflozin efficacy and safety trial (BEST): A randomized, double-blind, placebo-controlled, phase III, clinical trial. Diabetes. 2020:69. doi: 10.2337/db20-32-OR. [DOI] [Google Scholar]
- 19.Hadd M, Collinson A, Seed B. A compound for the management of feline diabetes. International application number PCT/US2020/022567. [Last accessed October 31, 2021]. Filed 13 March 2020. Available from: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2020186142.
- 20.World Small Animal Veterinary Association (WSAVA) Global Nutrition Guidelines. 2020. [Last accessed October 31, 2021]. Available from: https://wsava.org/global-guidelines/global-nutrition-guidelines/
- 21.Zini E, Moretti S, Tschuor F, Reusch CE. Evaluation of a new portable glucose meter designed for the use in cats. Schweiz Arch Tierheilkd. 2009;151:448–451. doi: 10.1024/0036-7281.151.9.448. [DOI] [PubMed] [Google Scholar]
- 22.Reusch CE, Haberer B. Evaluation of fructosamine in dogs and cats with hypo- or hyperproteinaemia, azotaemia, hyperlipidaemia and hyperbilirubinaemia. Vet Rec. 2001;148:370–376. doi: 10.1136/vr.148.12.370. [DOI] [PubMed] [Google Scholar]
- 23.Gilor C, Graves TK, Lascelles B, et al. The effects of body weight, body condition score, sex, and age on serum fructosamine concentrations in clinically healthy cats. Vet Clin Pathol. 2010;39:322–328. doi: 10.1111/j.1939-165X.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- 24.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu D, Huggins LA, Scerbo D, et al. Mechanism of increased LDL (low-density lipoprotein) and decreased triglycerides with SGLT2 (sodium-glucose cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38:2207–2216. doi: 10.1161/ATVBAHA.118.311339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szekeres Z, Toth K, Szabados E. The effects of SGLT2 inhibitors on lipid metabolism. Metabolites. 2021;11:87. doi: 10.3390/metabo11020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheen AJ. SGLT2 inhibition: Efficacy and safety in type 2 diabetes treatment. Expert Opin Drug Saf. 2015;14:1879–1904. doi: 10.1517/14740338.2015.1100167. [DOI] [PubMed] [Google Scholar]
- 28.Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457–466. doi: 10.1111/dom.12244. [DOI] [PubMed] [Google Scholar]
- 29.Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 30.Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24:73–79. doi: 10.1097/MED.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]