Abstract

Ultrathin WS2 films are promising functional materials for electronic and optoelectronic devices. Therefore, their synthesis over a large area, allowing control over their thickness and structure, is an essential task. In this work, we investigated the influence of atomic layer deposition (ALD)-grown WO3 seed-film thickness on the structural and electrical properties of WS2 nanosheets obtained via a sulfurization technique. Transmission electron microscopy indicated that the thinnest (1.9 nm) film contains rather big (up to 50 nm) WS2 grains in the amorphous matrix. The signs of incomplete sulfurization, namely, oxysulfide phase presence, were found by X-ray photoemission spectroscopy analysis. The increase in the seed-film thickness of up to 4.7 nm resulted in a visible grain size decrease down to 15–20 nm, which was accompanied by defect suppression. The observed structural evolution affected the film resistivity, which was found to decrease from ∼106 to 103 (μΩ·cm) within the investigated thickness range. These results show that the thickness of the ALD-grown seed layer may strongly affect the resultant WS2 structure and properties. Most valuably, it was shown that the growth of the thinnest WS2 film (3–4 monolayers) is most challenging due to the amorphous intergrain phase formation, and further investigations focused on preventing the intergrain phase formation should be conducted.
Introduction
Tungsten disulfide is a representative of the class of two-dimensional (2D) transition-metal dichalcogenides (TMDs), which are characterized by a layered structure with adjacent layers bonded with van der Waals forces.1−3 2H-WS2 is a semiconductor with the band gap varying from 1.3 to 2.1 eV, depending on the number of layers.4 In addition to this, an indirect-to-direct band gap transition occurs on decreasing the thickness down to a single monolayer. Theoretical calculations predict that WS2 should possess the highest carrier mobility among other semiconducting 2D TMDs due to the reduced effective carrier mass.5,6 Relatively high mobility and tunable band gap in the visible optical range make this material promising for use in electronic and optoelectronic devices.7,8 Moreover, it also demonstrated good catalytic,9,10 gas-sensing,11,12 and chemical-sensitive properties.13 Each of these applications requires a specific film structure. While the large-area films with a terrace-terminated structure and a given number of monolayers are most relevant for optoelectronic applications, an edge-terminated film structure with a large number of dangling bonds is desirable for catalysis applications.14,15 In this regard, the controllable WS2 synthesis with a given structure over a large area is an essential task for practical applications. Bulk crystal exfoliation cannot satisfy this requirement since it does not provide precise control over the thickness and material properties16 as well as not scalable to industrial standards. In contrast, chemical vapor deposition (CVD)17 and direct atomic layer deposition (ALD)18,19 easily provide wafer-scale coverage with a precise thickness control. Currently, the superior WS2 layers containing the big crystalline domains up to several hundreds of μm were obtained by CVD.20 However, the CVD technique is quite challenging and is strongly dependent on the precursors’ chemistry and process parameters. The direct ALD synthesis of WS2 allows only obtaining small crystalline domains of several nm due to the limited process temperature;18 however, the interesting ability to control the film structure has been demonstrated.19 Another indirect WS2 synthesis technique, namely, seed-film sulfurization, was suggested.21 The size of crystal domains in films produced by this method is significantly lower than that, for example, in films produced by the CVD method.22 However, due to its relative simplicity and a high degree of controllability, this method remains relevant.23−28
Since the thickness significantly influences both the optical and electrical properties of the WS2 films, a lot of attention is usually paid to the precise control of the number of its monolayers.21 The purpose of earlier studies was to show the possibility of obtaining a given number of WS2 monolayers by changing the thickness of seed films. In subsequent studies, considering tungsten seed films as an example, it was shown that the thickness of the initial film not only determines the number of monolayers but affects the structure of WS2 films.27 In particular, sulfurization at 800 °C of sputtered tungsten films with a different thicknesses (1–28 nm) was used to obtain WS2.27 A thinner W seed layer resulted in a discontinuous WS2 formation. Instead, agglomeration of nanosize grains in a combination of triangles and flakes was visible. Thicker films had a lamellar structure and contained plenty of nanorods embedded. At the same time, the data on the effect of the thickness of ALD-grown WO3 seed films on the structure and properties of WS2 is scarce.
One of the most suitable methods to obtain the WO3 seed-film is ALD since it is governed by the chemical saturation concept, which results in perfect reproducibility, wafer-scale uniformity, and conformality over three-dimensional (3D) structures. The question of the effect of the thickness of ALD seed WO3 films requires separate consideration since these films differ in their microstructure and impurity content from films obtained by other methods. This work investigates the influence of the ALD-grown WO3 seed-film thickness on the structure and electrical properties of WS2 films and thus partially fills this gap in knowledge. The sulfurization technique with the preliminary WO3 hydrogen reduction was chosen as the method for the fabrication of WS2 films. The ability to fabricate a continuous WS2 film with a thickness down to three monolayers was demonstrated previously, while the thickness was limited by the chosen ALD process.22 In the current work, the films with the thickness in the range between ∼1.9 and ∼4.7 nm, which corresponds to 2–8 WS2 monolayers, were formed and systematically investigated. According to the literature data, such films can be used in field-effect transistors21,26 and possess a high photoelectric response,29,30 which reflects the interest in the WS2 within this thickness range.
Experimental Section
WO3 seed films were grown in a commercially available hot-wall PICOSUN R200adv ALD reactor. Bis(cyclopentadienyl)tungsten dihydride (WH2(Cp)2) and O3 were used as the precursor and reactant (oxygen source), respectively. The precursor feed and active oxygen exposure times were fixed at 2.0 and 12 s, respectively, which corresponds to the saturated ALD growth. The deposition temperature (Tdep) was fixed at 300 °C. The other details of the utilized ALD process can be found elsewhere.31 Sapphire pieces of 20 × 10 mm2 in size were used as substrates. They were cleaned (piranha solution and deionized water) and subsequently annealed in a tube furnace in air for 1 h at 1000 °C prior to WO3 growth. Several identical series of samples were fabricated. Each one of them contained seed films grown during 20, 30, 40, and 50 cycles of ALD processes.
The oxide film reduction, as well as its sulfurization, was carried out in a three-zone tube furnace HZS-1200 (Carbolite Gero) equipped with a 32 mm outer diameter quartz tube. After loading the samples, the tube was purged for 1 h with a 5% H2/Ar mixture (99.9999%) to obtain a controllable gas atmosphere (total gas flow was fixed at 150 sccm). The reduction temperature was 600 °C, while the process dwell-time was fixed at 60 min (H2/Ar flow was set to be 10 sccm during the whole process). After the process termination, the H2/Ar flow rate was increased to 50 sccm, and the tube was cooled down to room temperature before subsequent sulfurization (no atmosphere break took place). For the sulfurization process, a crucible containing 500 mg of sulfur flakes (99.98% purity, Sigma-Aldrich) was located upstream from the sample in a tube. Sulfur flakes were loaded in a boat placed at the end of the heated section of the split-tube three-zone furnace (all zones were maintained at the same temperature). The H2/Ar flow rate was reduced down to 10 sccm when the temperature reached 350 °C. This flow rate was maintained until the end of the process. The heating rate was set to be 10 °C/min during the entire processes time. The sample remained for 30 min at the target temperature (900 °C). Then, the heating system was switched off, allowing the furnace to cool down.
The films’ morphology was examined by atomic force microscopy (AFM, NT-MDT Ntegra) and Solver tools in a semicontact mode using a silicon tip with a radius < 10 nm (HA-NC, SCANSENS). Images were taken over a 5 × 5 μm2 area. The details of the film crystal structure were studied by transmission electron microscopy (TEM) (JEOL JEM-2100) at an accelerating voltage of 200 kV. Raman spectroscopy was used to obtain insights into the detailed film structure. A LabRAM Evolution (HORIBA Scientific) instrument with a 532 nm laser source with 1 cm–1 spectral resolution was used to perform the spectral measurements. A diffraction grating of 1800 lines/mm and a x100 objective lens (numerical aperture = 0.90) were utilized in these experiments. The laser spot diameter was 0.45 μm. In addition to this, WS2 films were characterized by Raman spectroscopy with 473 nm laser excitation acquired with a Ntegra Spectra II confocal Raman and SNOM microscope (NT-MDT) with a spectral resolution better than 1 cm–1. The laser intensity was kept under 0.5 mW to ensure that laser-induced heating did not introduce artifacts. Measurements were performed in a temperature-controlled room at standard conditions. The 520 cm–1 phonon mode from the silicon wafer was used for calibration. All peaks were fitted with Lorentzian functions. The films’ chemical state and composition were analyzed by X-ray photoelectron spectroscopy (XPS) in the Theta Probe tool (Thermo Scientific) under high-vacuum conditions with a monochromatic Al Kα X-ray source (1486,6 eV).
Electrical measurements were carried out by the transfer length method (TLM) on the prepared line-type structures. Ti (5 nm)/Au (50 nm) bilayers formed on WS2 by electron-beam evaporation served as metal contacts. Contact pads were defined by the lift-off procedure. They were separated by various distances to provide the WS2 channels with different lengths from 4 to 37 μm. WS2 channel widths were also different: 50, 75, and 100 μm. Rectangular WS2 regions were previously formed using etching in O2 + CHF3 plasma and standard lithography methods. After the formation of contact pads, the samples were annealed for 30 min at 350 °C in vacuum (10–6 mbar) to reduce the resistance. Before and after annealing, the elemental composition of the films was investigated by XPS. The ratio of atomic concentrations of sulfur and tungsten decreased by about 5% after annealing, but in both cases, it remained greater than the stoichiometric value. No WS2 film transfer was carried out, i.e., the experiment was carried out on the primary sapphire substrates.
Results and Discussion
As Figure S1 demonstrates, the WO3 seed-film thickness influences the morphology of the resulting WS2 films. In the thinnest film, corresponding to 20 ALD cycles (Figure S1a), the grain structure is clearly distinguishable. The estimated grain size is several tens of nanometers. The dark spots in some places are probably holes in the film. Increasing the number of ALD cycles to 30 (Figure S1b) results in the disappearance of the grain structure and higher surface uniformity. Further increase in the number of cycles leads to the reoccurrence of a grained structure (Figure S1c,d), but with a significantly smaller lateral grain size than that in the case of the thinnest film. Such morphology shift manifests itself in nonmonotonous dependence of the root-mean-square (RMS) values from thickness. Calculated RMS values are given in Table 1. Overall, it should be noted that the films under investigation have smooth surfaces, and RMS values are significantly lower than the ones previously reported for WS2 films (about 5 nm), fabricated using the same method.22 A RMS value of ≈0.2 nm is typical for monolayer WS2 films.32 For comparison, Figure S1e shows an AFM image of a sapphire substrate. Terraces are visible on the surface of the substrate after annealing. Consequently, the morphology observed in Figure S1a–d is not related to the substrate relief.
Table 1. Thickness and RMS of WS2 Films.
| number of ALD cycles | 20 | 30 | 40 | 50 |
| WS2 film thickness (nm) | 1.9 | 2.8 | 3.7 | 4.7 |
| RMS (nm) | 0.28 | 0.23 | 0.49 | 0.35 |
To measure the thickness, films’ fragments were transferred onto SiO2/Si substrates after soaking in a 5% KOH solution. Figure S2 provides the AFM profiles recorded across the edges of these fragments. These measurements show that film thickness increases linearly with the increasing number of WO3 ALD cycles. Measured thickness values are given in Table 1.
Taking into account the fact that separate grains in a film might comprise a different number of monolayers and that the interlayer spacing in bulk WS2 is 0.625 nm, the number of monolayers should be considered to increase from 2–3 to 6–8, with the WS2 film thickness increasing from 1.9 to 4.7 nm.
For Raman spectroscopy measurements, two different excitation wavelengths were used: 473 and 532 nm. The former is close to the resonance peak, corresponding to the interband transitions in WS2, and the latter corresponds to the B exciton peak resonance.33 When the 473 nm irradiation is used, the peaks corresponding to the first-order vibrational modes E2′(Γ) and A1′(Γ) prevail in Raman spectra (Figure 1a). It is well known that the difference in these peak positions determines the number of monolayers in thin TMD films.34 Recorded spectra make it possible to precisely determine the peak positions and, therefore, the number of monolayers without peak decomposition. This is especially important for WS2 films since the position of each separate peak in this material depends weakly on the number of monolayers.21 The red shift of the E2′(Γ) peak and the blue shift of the A1′(Γ) peak are observed with increasing thickness of films. Thus, the difference in their positions (Δf) increases, as shown in Figure 1b. According to the previous research,33 a Δf value slightly above 63 cm–1 corresponds to three monolayers, and a value slightly above 64 cm–1 corresponds to four monolayers of WS2. Since the samples studied in previous work33 were obtained by mechanical exfoliation, it is not correct to directly compare the Δf values from previous work with those of the samples investigated in this work. However, the observed trend of the changing value of Δf coincides. Noteworthily, the full width at half-maximum (FWHM) of the A1′(Γ) peak was 3.9–4.0 (cm–1), and it remained almost constant with a change in the film thickness change from 1.9 to 3.7 nm and increased to 4.5 cm–1 for a 5 nm-thick film.
Figure 1.
Raman spectra of WS2 films obtained with 473 nm excitation (a). Difference in the positions of the A1′(Γ) and E2′(Γ) peaks and the ratio of the intensities of these peaks as a function of the thickness of WS2 films (b). Lines serve as a guide to the eye.
Excitation with 532 nm wavelength revealed overtones and combination peaks in all Raman spectra (Figure 2a). Moreover, a peak corresponding to the vibrational mode LA(M), a first-order Raman-active mode representing the longitudinal acoustic (LA) phonons at the edge of the Brillouin zone (the M point), is clearly seen at 173 cm–1. According to the momentum conservation rule, LA(M) is a zone-edge mode and cannot be observed by conventional Raman measurements in a perfect WS2 sample. However, in a WS2 sample with defects, the momentum conservation condition can be satisfied by the phonon scattering from a defect, enabling the observation of zone-edge modes by conventional Raman measurement.35 It was shown previously that radiation-induced defects affect this peak intensity in MoS2.36 LA(M) peak intensity evolution was observed by varying the synthesis conditions of monolayer WS2 films,35 as well as by changing the MoS2 sulfurization temperature.37 Therefore, it is possible to estimate the extent of defects in films by the relative magnitude of this peak.
Figure 2.
Raman spectra of WS2 films obtained with 532 nm excitation (a). Ratio of the intensities of peaks LA(M) and A1′(Γ) as a function of the thickness of WS2 films (b).
The ratios of peak intensities I(LA(M))/I(A1′(Γ)) were calculated, and the dependence of this ratio on the thickness was plotted, assuming that the shape of the lines in the spectra of films of different thicknesses does not change. Within the margin of error, this dependence is linear. As Figure 2b illustrates, in thinner WS2 films, the peak intensity relation I(LA(M))/I(A1′(Γ)) increases. This leads to the conclusion that thinner WS2 films have higher defect densities.
The structure of WS2 films was investigated in more detail by TEM. Plan-view TEM images and selected area electron diffraction (SAED) patterns for the films with different thicknesses are provided in Figure 3a,c,e,g. Figure 3b,d,f,h illustrates the high-resolution TEM images from the same films. According to Figure 3a, the thinnest 1.9 nm-thick film consists of 10–50 nm grains. It can be seen in Figure 3b that the material in the intergrain regions is amorphous. The distance between grains is several nanometers. According to Figure 3c, the 2.8 nm-thick film consists of approximately 10 nm grains. As in the thinner film, crystal grains are separated by the amorphous phase, and the distance between them is also several nanometers. Increasing the film thickness to 3.7 nm results in an increase in the size of crystal grains to 10–20 nm (Figure 3e). The boundaries of grains become sharp, and the amorphous phase is only sparsely observed in triangular intergrain joints (Figure 4f). Further increase in the film thickness to 4.7 nm does not change the situation qualitatively (Figure 3g,h), and the grain size continues to increase. The size of the observed grains varies between 15 and 25 nm approximately.
Figure 3.
Plan-view TEM images of WS2 films with thicknesses of 1.9 nm (a, b), 2.8 nm (c, d), 3.7 nm (e, f), and 4.7 nm (g, h). Insets show SAED patterns.
Figure 4.
XPS W 4f and S 2p spectra of WS2 films with thicknesses of 1.9 nm (a, b) and 3.7 nm (c, d).
Observed grain size can be compared to the ones reported earlier. In previous research, the grain size in WS2 films fabricated using the same method was about 20 nm.22 In another work, 2.8 nm-thick WS2 films, also fabricated using sulfurization with preliminary reduction, consisted of the grains with 5 nm size approximately.26
SAED patterns for all samples introduce bright circles, corresponding to the (101) and (110) WS2 plane reflections. The basal (001) plane reflection is absent, which confirms that WS2 planes are mostly oriented parallel to the film surface. The blurry SAED pattern of the 1.9 nm-thick film is due to the relative lack of crystal grains in the analysis area and their slight relative disorientation in plane. Overall, TEM measurements showed a nonmonotonous change in the average crystallite size in films with varying thicknesses within the investigated range. The largest crystallites are found in thinnest films and the smallest in 2.8 nm-thick films. Apparently, the smallest crystallite size is the reason why 2.8 nm-thick films are the smoothest according to AFM data. Noteworthily, TEM results correspond perfectly with the surface morphology observed by AFM in the whole range of the WS2 thickness.
XPS elemental composition and chemical state analysis also revealed notable differences for the films of different thicknesses. For each film, core level spectra W 4f, W 4d, S 2p, and S 2s were recorded. In elem ental composition XPS analysis, Scofield’s factors for bulk material are employed in calculations, which might lead to distorted results in thin films if electron kinetic energies for elements under investigation differ significantly. Hence, relative atomic concentrations [S]/[W] were calculated from W 4d and S 2s spectra, as their binding energies (BEs) are very close. Below are the spectra of WS2 samples obtained after vacuum annealing. Therefore, they can be correlated with the measured electrical properties.
In Figure 4, W 4f and S 2p spectra for 1.9 and 3.7 nm-thick films are presented. The absence of the spectra from 2.8- and 4.7 nm-thick films spectra is explained by their similarity to those recorded for the 3.7 nm-thick film. For all films, the W 4f spectrum was decomposed into two doublets. A more intense one with a BE of 32.4 eV typical for the WS2 material corresponds to the W4+ state.13,26 Second, a much weaker W6+ state doublet with a BE of 35.8 eV corresponds to the WO3. In addition, a W5p3/2 line with a BE of 37.8 eV is found in W 4f spectra. In W 4f spectra of the 1.9 nm-thick film, W4+ doublet lines are slightly broader and the intensity of the W6+ doublet is more than two times higher than that of the 3.7 nm-thick film. S 2p spectra were fitted with a single doublet, and the BE value for S 2p3/2 was about 162 eV. The spectrum corresponding to the 1.9 nm-thick film is shifted toward a lower BE by 0.3 eV and is slightly broader in comparison to that of the 3.7 nm-thick film doublet.
In addition, it should be noted that according to the data obtained by Raman spectroscopy, XPS, and TEM, there were no signs of the presence of other phases in addition to amorphous and 2H-WS2, in particular, 1T-WS2.
In Figure 5, W 4d and S 2s spectra for 1.9 and 3.7 nm-thick films are also presented. W 4d spectra were fitted with a single doublet with a BE of 244.2 eV for the W 4d5/2 line and a spin–orbit split value of 12.4 eV in both cases. In the case of the 1.9 nm-thick film, this doublet is slightly broadened in comparison to the 3.7 nm-thick film. The S 2s spectrum was fitted with a single line for the 3.7 nm-thick film with a BE value of ∼ 226 eV. In the case of a 1.9 nm-thick film, a second peak with a BE of 228.6 appears. In addition, a higher intensity peak is found to be shifted toward a lower BE by 0.2 eV and to be slightly broadened in comparison to this peak for the 3.7 nm-thick film. A similar peak centered at 228.5 eV was earlier observed in the S 2s spectra of MoS2 films.38,39 It is attributed to oxysulfides or S–H states.38 In the case of this study, the appearance of the 228.6 eV peak is accompanied by an increased W 4f doublet intensity, corresponding to the W6+ state. This is also most possibly due to oxysulfide formation in the film. It should be noted that the presence of oxysulfide seems to be distinctive for the films with the highest amount of amorphous phase observed in TEM, and that leads to the conclusion that this amorphous phase contains an oxysulfide component.
Figure 5.
XPS W 4d and S 2s spectra of WS2 films with thicknesses of 1.9 nm (a, b) and 3.7 nm (c, d).
In the bulk material, the oxysulfide phase is metastable. When heated, a WS2 compound should be formed in sulfur vapor. However, the oxysulfide phase can probably be stabilized with a small film thickness. In the previous work,40 the stability of oxysulfide domains during sulfurization in the temperature range of 800–1000 °C is noted.
The calculated relative atomic concentrations x = [S]/[W] for 1.9, 2.8, 3.7, and 4.7 nm-thick films are 2.0, 2.1, 2.2, and 2.2, respectively. The XPS results show that in films with a thickness of 2.8 nm or more, the sulfur concentration exceeds the stoichiometric one. The reason for this may lie in the features of the sulfurization process used. The decrease in the x value for thinner films is probably due to the presence of an oxysulfide phase. In this phase, the ratio of the atomic concentrations of sulfur and tungsten can be less than 2. These results show that utilization of WO3 films grown with a different number of ALD cycles and further sulfurized at the same conditions lead to the formation of significantly different WS2 films in terms of the crystalline structure, morphology, and chemical composition. These differences should also manifest themselves in film’s electrical properties.
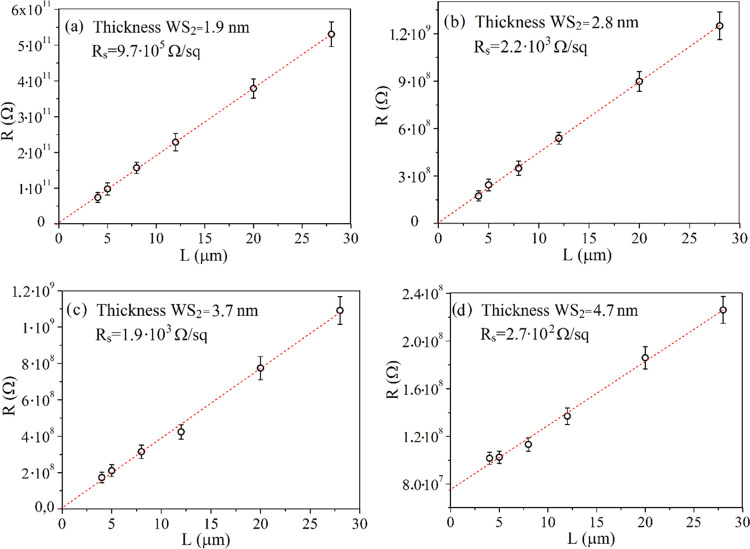
Electrical characteristic measurement results for WS2 films employing the TLM method are provided in Figure 6, as well as in Table 2. Figure S3 shows the DC current–voltage (IV) characteristics for 1.9 and 3.7 nm-thick films. In Figure 6, the measured resistance value relation dependencies on the channel length are presented for WS2 with different thicknesses. In Table 2, the calculated values of contact resistance and contact resistivity are listed. Calculated sheet resistance values are shown in each of Figure 6. From these values, the film resistivity (ρ) was calculated. In Figure 7, the plot of ρ dependency on WS2 film thickness is presented (the solid red line serves as a guide to the eye). From the plot, it is evident that the ρ value changes weakly for 3.7 and 4.7 nm-thick films. However, it increases abruptly with a decrease in thickness to 1.9 nm.
Figure 6.
Electrical resistance as a function of channel length for WS2 films with thicknesses of 1.9 nm (a), 2.8 nm (b), 3.7 nm (c), and 4.7 nm (d).
Table 2. Contact Resistance and Contact Resistivity of the Investigated Structures.
| WS2 film thickness (nm) | 1.9 | 2.8 | 3.7 | 4.7 |
| Rc (Ω) | 1 × 109 | 2.3 × 106 | 3.0 × 106 | 3.8 × 107 |
| ρc (Ω·cm2) | 1.7 × 102 | 4.2 | 6.1 | 8.7 |
Figure 7.

Resistivity as a function of the WS2 film thickness.
A lot of work is dedicated to the research on the influence of TMD film structure on its electrical properties.18,41 In particular, a strong influence of the intercrystallite interface was put forward.40−42 The decreasing crystallite size should lead to increased charge carrier scattering and, consequently, increased resistance. However, among the samples under investigation, the film with the largest crystallites has the highest specific resistance. This might be attributed to the stronger influence of the amorphous phase, containing oxysulfides, at the intercrystallite interface. Analysis of the literature data shows that the conductivity of molybdenum and tungsten oxysulfides can vary widely. Thus, MoSxOy compounds are characterized by high conductivity at a relatively low oxygen concentration, due to the presence of Mo5+ states.43 Due to this, they are successfully used in electrocatalysis.44 On the other hand, at a high oxygen concentration, when the metal is mainly in an oxidation state of 6+, the conductivity values are 10–6 Ω–1·cm–1.43 Therefore, the presence of an oxysulfide phase with a high concentration of oxygen should increase the value of the electrical resistance.
Conclusions
In this study, the structure and electrical properties of WS2 films obtained via sulfurization technique were investigated as a function of the thickness of the ALD-grown WO3 seed film. According to Raman spectroscopy data, defect concentration in films decreased monotonously with increasing thickness of the WO3 seed layer and, accordingly, the thickness of WS2 in the range of 1.9–4.7 nm. TEM and AFM measurements showed that the largest crystalline grains could be found in the thinnest 1.9 nm-thick film corresponding to 2–3 monolayers of WS2 and the smallest in the 2.8 nm-thick film. However, a decrease in the grain size, contrary to the expectation, does not lead to poorer electrical properties. Instead, the thinnest films demonstrated enhanced film resistivity. The origin of this phenomenon was found by a combination of XPS and TEM analysis. In relatively thin 1.9 and 2.8 nm-thick films, an amorphous phase was observed in the intergrain space. XPS investigation revealed that this amorphous phase contains tungsten oxysulfide. The presence of the amorphous phase between crystalline grains increases film resistivity significantly, while its disappearance leads to a significant improvement of the resistivity despite the smaller grains. Moreover, it was found that the further increase in the WO3 seed layer thickness provides a monotonic increase in the crystal grain size and, eventually, resistivity improvement. These results show that the thickness of the ALD-grown seed layer may strongly affect the resultant WS2 structure and properties. Moreover, because WS2 films thinner than four monolayers with a quality crystal structure may be useful for electronic applications, further detailed investigation focused on preventing the formation of the amorphous intergrain phase is required. In addition, the larger grain size in these films, revealed by TEM, might endow them with even superior properties.
Acknowledgments
The main part of the work was supported by the Russian Science Foundation (Project. No 19-19-00504). The Raman studies were carried by the authors S.M.N and V.S.V. with financial support from the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-606). The authors also acknowledge the MIPT Shared Facilities Center for access to the equipment.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04532.
AFM images of WS2 films obtained during 20, 30, 40, and 50 ALD cycles and WO3 film sulfurization and a sapphire substrate; AFM images and height profiles of the WS2 corresponding to 20 and 30 ALD cycles on WO3 seed films; and current–voltage curves of WS2 films measured at channel widths of 75 μm (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Choi W.; Choudhary N.; Han G. H.; Park J.; Akinwande D.; Lee Y. H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. 10.1016/j.mattod.2016.10.002. [DOI] [Google Scholar]
- Choi K.; Lee Y. T.; Im S. Two-dimensional van der Waals nanosheet devices for futureelectronics and photonics. Nano Today 2016, 11, 626–643. 10.1016/j.nantod.2016.08.009. [DOI] [Google Scholar]
- McDonnell S. J.; Wallace R. M. Atomically-thin layered films for device applications based upon 2D TMDC materials. Thin Solid Films 2016, 616, 482–501. 10.1016/j.tsf.2016.08.068. [DOI] [Google Scholar]
- Gutiérrez H. R.; Perea-Lopez N.; Elias A. L.; Berkdemir A.; Wang B.; Lv R.; Lopez-Urias F.; Crespi V. H.; Terrones H.; Terrones M. Extraordinary room-temperature photoluminescence in triangular WS2 monolayers. Nano Lett. 2013, 13, 3447–3454. 10.1021/nl3026357. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Huang Z.; Zhang W.; Li Y. Two-dimensional semiconductors with possible high room temperature mobility. Nano Res. 2014, 7, 1731–1737. 10.1007/s12274-014-0532-x. [DOI] [Google Scholar]
- Ovchinnikov D.; Allain A.; Huang Y.-S.; Dumcenco D.; Kis A. Electrical transport properties of single-layer WS2. ACS Nano 2014, 8, 8174–8181. 10.1021/nn502362b. [DOI] [PubMed] [Google Scholar]
- Lopez-Sanchez O.; Llado E. A.; Koman V.; Morral A. F.; Radenovic A.; Kis A. Light generation and harvesting in a van der Waals heterostructures. ACS Nano 2014, 8, 3042–3048. 10.1021/nn500480u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Sheng Y.; Zhou Y.; Chang R.; Wang X.; Huang H.; et al. High Photoresponsivity in Ultrathin 2D Lateral Graphene:WS2:Graphene Photodetectors Using Direct CVD Growth. ACS Appl. Mater. Interfaces 2019, 11, 6421–6430. 10.1021/acsami.8b20321. [DOI] [PubMed] [Google Scholar]
- Voiry D.; Yang J.; Chhowala M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv. Mater. 2016, 28, 6197–6206. 10.1002/adma.201505597. [DOI] [PubMed] [Google Scholar]
- Noh S. H.; Hwang J.; Kang J.; Seo M. H.; Choi D.; Han B. Tuning the catalytic activity of heterogeneous two-dimensional transition metal dichalcogenides for hydrogen evolution. J. Mater. Chem. A 2018, 6, 20005–20014. 10.1039/C8TA07141A. [DOI] [Google Scholar]
- Sun J.; Lin N.; Ren H.; Tang C.; Yang L.; Zhao X. Gas adsorption on MoS2/WS2 in-plane heterojunctions and the I-V response: a first principles study. RSC Adv. 2016, 6, 17494–17503. 10.1039/C5RA24592C. [DOI] [Google Scholar]
- Chen X.; Liu C.; Mao S. Environmental analysis with 2D transition-metal-dichalcogenide-based field-effect transistors. Nano-Micro Lett. 2020, 12, 95 10.1007/s40820-020-00438-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K. Y.; Song J.-G.; Kim Y.; Choi T.; Shin S.; Lee C. W.; Lee K.; Koo J.; Lee H.; Kim J.; Lee T.; Park J.; Kim H. Improvement of gas-sensing performance of large-area tungsten disulfide nanosheets by surface functionalization. ACS Nano 2016, 10, 9287–9296. 10.1021/acsnano.6b03631. [DOI] [PubMed] [Google Scholar]
- Voiry D.; Yamaguchi H.; Li J.; Silva R.; Alves D. C. B.; Fujita T.; Chen M.; Asefa T.; Shenoy V. B.; Eda G.; Chhowalla M. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 2013, 12, 850–855. 10.1038/nmat3700. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam S.; Shirazi M.; Bloodgood M. A.; Wu L.; Verheijen M. A.; Vandalon V.; Kessels W. M. M.; Hofmann J. P.; Bol A. A. Edge-site nanoengineering of WS2 by low-temperature plasma-enhanced atomic layer deposition for electrocatalytic hydrogen evolution. Chem. Mater. 2019, 31, 5104–5115. 10.1021/acs.chemmater.9b01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addou R.; McDonnell S.; Barrer D.; Guo Z.; Azcat A.; Wang J.; Zhu H.; Hinkle C. L.; Quevedo-Lopez M.; Alshareef H. N.; Colombo L.; Hsu J. W. P.; Wallace R. M. Impurities and Electronic Property Variations of Natural MoS2 Crystal Surfaces. ACS Nano 2015, 9, 9124–9133. 10.1021/acsnano.5b03309. [DOI] [PubMed] [Google Scholar]
- Chubarov M.; Choudhury T. H.; Hickey D. R.; Bachu S.; Zhang T.; Sebastian A.; Bansal A.; Zhu H.; Trainor N.; Das S.; Terrones M.; Alem N.; Redwing J. M. Wafer-Scale Epitaxial Growth of Unidirectional WS2 Monolayers on Sapphire. ACS Nano 2021, 15, 2532–2541. 10.1021/acsnano.0c06750. [DOI] [PubMed] [Google Scholar]
- Groven B.; Heyne M.; Mehta A. N.; Bender H.; Nuytten T.; Meersschaut J.; Conard T.; Verdonck P.; Van Elshocht S.; Vandervorst W.; De Gendt S.; Heyns M.; Radu I.; Caymax M.; Delabie A. Plasma-Enhanced Atomic Layer Deposition of Two-Dimensional WS2 from WF6, H2 Plasma, and H2S. Chem. Mater. 2017, 29, 2927–2938. 10.1021/acs.chemmater.6b05214. [DOI] [Google Scholar]
- Balasubramanyam S.; Shirazi M.; Bloodgood M. A.; Wu L.; Verheijen M. A.; Vandalon V.; Kessels W. M. M.; Hofmann J. P.; Bol A. A. Edge-site nanoengineering of WS2 by low-temperature plasma-enhanced atomic layer deposition for electrocatalytic hydrogen evolution. Chem. Mater. 2019, 31, 5104–5115. 10.1021/acs.chemmater.9b01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.; Luo T.; Xing J.; Hao H.; Liu H.; Dong J.; et al. Large-Area WS2 Film with Big Single Domains Grown by Chemical Vapor Deposition. Nanoscale Res. Lett. 2017, 12, 558 10.1186/s11671-017-2329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-G.; Park J.; Lee W.; Choi T.; Jung H.; Lee C. W.; Hwang S.-H.; Myoung J. M.; Jung J.-H.; Kim S.-H.; Lansalot-Matras C.; H Kim C. Layer-Controlled, Wafer-Scale, and Conformal Synthesis of Tungsten Disulfide Nanosheets Using Atomic Layer Deposition. ACS Nano 2013, 7, 11333–11340. 10.1021/nn405194e. [DOI] [PubMed] [Google Scholar]
- Markeev A. M.; Kozodaev M. G.; Slavich A. S.; Romanov R. I.; Zarubin S. S. Influence of reducing agent on properties of thin WS2 nanosheets prepared by sulfurization of atomic layer-deposited WO3. J. Phys. Chem. C 2020, 124, 28169–28177. 10.1021/acs.jpcc.0c09769. [DOI] [Google Scholar]
- Kim S. J.; Luo D.; Park K.; Choe M.; Kim D. W.; Wang M.; Jung W.-B.; Lee Z.; Ruoff R. S.; Jung H.-T. Mapping Graphene Grain Orientation by the Growth of WS2 Films with Oriented Cracks. Chem. Mater. 2020, 32, 7484–7491. 10.1021/acs.chemmater.0c02551. [DOI] [Google Scholar]
- Seok H.; Megra Y. T.; Kanade C. K.; Cho J.; Kanade V. K.; Kim M.; Lee I.; Yoo P. J.; Kim H.-U.; Suk J. W.; Kim T. Low-Temperature Synthesis of Wafer-Scale MoS2–WS2 Vertical Heterostructures by Single-Step Penetrative Plasma Sulfurization. ACS Nano 2021, 15, 707–718. 10.1021/acsnano.0c06989. [DOI] [PubMed] [Google Scholar]
- Chang X.; Xu S.; Liu S.; Wang N.; Sun S.; Zhu X.; Li J.; Ola O.; Zhu Y. Highly sensitive acetone sensor based on WO3 nanosheets derived from WS2 nanoparticles with inorganic fullerene-like structures. Sens. Actuators, B 2021, 343, 130135 10.1016/j.snb.2021.130135. [DOI] [Google Scholar]
- Zeng W.; Feng L.-P.; Su J.; Pan H.-X.; Liu Z.-T. Layer-controlled and atomically thin WS2 films prepared by sulfurization of atomic-layer deposited WO3 films. J. Alloys Compd. 2018, 745, 834–839. 10.1016/j.jallcom.2018.02.046. [DOI] [Google Scholar]
- Hotovy I.; Spiess L.; Mikolasek M.; Kostic I.; Sojkova M.; Romanus H.; Hulman M.; Buc D.; Rehacek V. Layered WS2 thin films prepared by sulfurization of sputtered W films. Appl. Surf. Sci. 2021, 544, 148719 10.1016/j.apsusc.2020.148719. [DOI] [Google Scholar]
- Hotovy I.; Spiess L.; Sojkova M.; Kostic I.; Mikolasek M.; Predanocy M.; Romanus H.; Hulman M.; Rehacek V. Structural and optical properties of WS2 prepared using sulfurization of different thick sputtered tungsten films. Appl. Surf. Sci. 2018, 461, 133–138. 10.1016/j.apsusc.2018.05.209. [DOI] [Google Scholar]
- Perea-López N.; Elías A. L.; Berkdemir A.; Castro-Beltran A.; Gutiérrez H. R.; Feng S.; Lv R.; Hayashi T.; López-Urías F.; Ghosh S.; Muchharla B.; Talapatra S.; Terrones H.; Terrones M. Photosensor Device Based on Few-Layered WS2 Films. Adv. Funct. Mater. 2013, 23, 5511–5517. 10.1002/adfm.201300760. [DOI] [Google Scholar]
- Xue Y.; Zhang Y.; Liu Y.; Liu H.; Song J.; Sophia J.; Liu J.; Xu Z.; Xu Q.; Wang Z.; Zheng J.; Liu Y.; Li S.; Bao Q. Scalable Production of a Few-Layer MoS2/WS2 Vertical Heterojunction Array and Its Application for Photodetectors. ACS Nano 2016, 10, 573–580. 10.1021/acsnano.5b05596. [DOI] [PubMed] [Google Scholar]
- Kozodaev M. G.; Romanov R. I.; Chernikova A. G.; Markeev A. M. Atomic Layer Deposition of Ultrathin Tungsten Oxide Films from WH2(Cp)2 and Ozone. J. Phys. Chem. A 2020, 125, 21663–21669. 10.1021/acs.jpcc.1c06149. [DOI] [Google Scholar]
- Tang B.; Yu Z. G.; Huang L.; Chai J.; Wong S. L.; Deng J.; Yang W.; Gong H.; Wang S.; Ang K.-W.; Zhang Y.-W.; Chi D. Direct n- to p-Type Channel Conversion in Monolayer/Few-Layer WS2 Field-Effect Transistors by Atomic Nitrogen Treatment. ACS Nano 2018, 12, 2506–2513. 10.1021/acsnano.7b08261. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Ghorannevis Z.; Amara K. K.; Pang J. R.; Toh M.; Zhang X.; Kloc C.; Tan P. H.; Eda G. Lattice dynamics in mono- and few-layer sheets of WS2 and WSe2. Nanoscale 2013, 5, 9677–9683. 10.1039/c3nr03052k. [DOI] [PubMed] [Google Scholar]
- Wang Q. H.; Kalantar-Zadeh K.; Kis A.; Coleman J. N.; Strano M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- Li J.; Su W.; Chen F.; Fu L.; Ding S.; Song K.; Huang X.; Zhang L. Atypical Defect-Mediated Photoluminescence and Resonance Raman Spectroscopy of Monolayer WS2. J. Phys. Chem. C 2019, 123, 3900–3907. 10.1021/acs.jpcc.8b11647. [DOI] [Google Scholar]
- Mignuzzi S.; Pollard A. J.; Bonini N.; Brennan B.; Gilmore I. S.; Pimenta M. A.; Richards D.; Roy D. Effect of disorder on Raman scattering of single-layer MoS2. Phys. Rev. B 2015, 91, 195411 10.1103/PhysRevB.91.195411. [DOI] [Google Scholar]
- Romanov R. I.; Kozodaev M. G.; Myakota D. I.; Chernikova A. G.; Novikov S. M.; Volkov V. S.; Slavich A. S.; Zarubin S. S.; Chizhov P. S.; Khakimov R. R.; Chouprik A. A.; Hwang C. S.; Markeev A. M. Synthesis of Large Area Two-Dimensional MoS2 Films by Sulfurization of Atomic Layer Deposited MoO3 Thin Film for Nanoelectronic Applications. ACS Appl. Nano Mater. 2019, 2, 7521–7531. 10.1021/acsanm.9b01539. [DOI] [Google Scholar]
- Liu J.; Goswami A.; Jiang K.; Khan F.; Kim S.; McGee R.; Li Z.; Hu Z.; Lee J.; Thundat T. Direct-current triboelectricity generation by sliding-Schottky nanocontact on MoS2 multilayers. Nat. Nanotechnol. 2018, 13, 112–116. 10.1038/s41565-017-0019-5. [DOI] [PubMed] [Google Scholar]
- Xie M. Z.; Zhou J. Y.; Ji H.; Ye Y.; Wang X.; Jiang K.; Shang L. Y.; Hu Z. G.; Chu J. H. Annealing effects on sulfur vacancies and electronic transport of MoS2 films grown by pulsed-laser deposition. Appl. Phys. Lett. 2019, 115, 121901 10.1063/1.5135375. [DOI] [Google Scholar]
- Najmaei S.; Liu Z.; Zhou W.; Zou X.; Shi G.; Lei S.; Yakobson B. I.; Idrobo J.-C.; Ajayan P. M.; Lou J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. 10.1038/nmat3673. [DOI] [PubMed] [Google Scholar]
- Van der Zande A. M.; Huang P. Y.; Chenet D. A.; Berkelbach T. C.; You Y.; Lee G.-H.; Heinz T. F.; Reichman D. R.; Muller D. A.; Hone J. C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. 10.1038/nmat3633. [DOI] [PubMed] [Google Scholar]
- Reifsnyder Hickey D. R.; Nayir N.; Chubarov M.; Choudhury T. H.; Bachu S.; Miao L.; Wang Y.; Qian C.; Crespi V. H.; Redwing J. M.; van Duin A. C. T.; Alem N. Illuminating Invisible Grain Boundaries in Coalesced Single-Orientation WS2 Monolayer Films. Nano Lett. 2021, 21, 6487–6495. 10.1021/acs.nanolett.1c01517. [DOI] [PubMed] [Google Scholar]
- Schmidt E.; Sourisseaub C.; Meunier G.; Levasseur A. Amorphous molybdenum oxysulfide thin films and their physical characterization. Thin Solid Films 1995, 260, 21–25. 10.1016/0040-6090(94)06463-6. [DOI] [Google Scholar]
- Shin S.; Jin Z.; Ham S.-Y.; Lee S.; Shin D.-S.; Min Y.-S. Effect of oxygen incorporation in amorphous molybdenum sulfide on electrochemical hydrogen evolution. Appl. Surf. Sci. 2019, 487, 981–989. 10.1016/j.apsusc.2019.05.188. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.