Abstract

Sulfonamides and lipids are widely found in natural products, bioactive substances, and pharmaceuticals. Here, we report N-sulfonylation and esterification of carboxylic acids in an environment-friendly one-pot tandem protocol involving 1,2-dichloroethane (DCE). Moreover, 1,8-diazabicyclo (5.4.0) undec-7-ene was necessary for this reaction as a strong base, which drives the reaction to completion. Although DCE is a very low activity reagent, it acts not only as a solvent but also as a reactant in the reaction. The β-chloroester contained in the reaction product can be easily dissociated to react with N, S, and O atoms, increasing the possibility for subsequent synthesis.
Introduction
With the widespread use of sulfonamides and lipids,1−3 sulfonamides have become the most commonly used antibacterial drugs today, with a wide range of biological and pharmacological activities, including antibacterial, antitumor, and antiviral effects,4 while carboxylic acid esters also have important uses in the coatings and pharmaceutical industries. Over time, researchers have identified a wide range of indications, such as Alzheimer’s disease and other central nervous system disorders, diabetes mellitus, and various cancers.5 Recently, studies have found that N-substituted sulfonamides are effective against dengue fever and Ebola virus infection, and new carboxyamide derivatives of substituted benzenesulfonamides have antitrypanosomal and anti-inflammatory activities (Figure 1).6,7
Figure 1.
Selected Examples of Biologically Active Sulfonamides.
Over the past decades, great efforts have been made in the preparation of N-functionalized sulfonamides.8 Among them, one of the most common methods to synthesize sulfonamides is the palladium- or copper-catalyzed cross-coupling reaction of primary sulfonamides with aryl halides,9 pseudohalides,10 sodium arylsulfinates,11 or alkylated ketoesters.12 Iridium-catalyzed for transfer hydrogenation reduction of N-sulfonylimine has recently been developed.13 Traditionally, sulfonyl azides are rarely used as sulfonyl donors in sulfonation reactions by direct sulfonamylation (Scheme 1a).14 Subsequently, it was shown that Ugwu’s group promoted the reaction of l-proline and l-4-hydroxyproline with substituted benzenesulfonyl chloride via bases (Scheme 1b).6 In addition, Herrera’s group used nitrogen-centered radicals for intramolecular C–H amination of unactivated methyl groups and developed a chemoselective procedure to synthesize pyrrolidine under mild conditions (Scheme 1c).15 Finally, we observed that the pyrrole structure can be obtained directly from alkanes by halogen bond-sequential Csp3-H amination (Scheme 1d).16,17
Scheme 1. Previous Reports and Our Design.
Being one of the most basic and important central bridging bonds of liquid crystal compounds, the synthesis of ester groups is significant. For a long time now, the synthesis of esters has been mainly performed by using some traditional methods, such as acid-catalyzed, ester exchange, and chloride methods (Scheme 1e).18 Besides, with the study of various new catalysts and the exploration of organic reaction mechanisms, some novel synthesis methods have emerged. For example, Rigo’s group used HMDS to catalyze the synthesis of esters.19 Moreover, the Mitsunobu reaction was also applied to the synthesis of esters from carboxylic acids.20 Moreover, here, we use 1,8-diazabicyclo (5.4.0) undec-7-ene (DBU) as a one-pot reagent to complete N-sulfonylation and carboxylic acid esterification, which provides a new idea for their synthesis. As we all know that 1,2-dichloroethane (DCE) is an inert reagent and rarely participates in the reaction, herein encouraged by these works and our group’s previous application of DCE and continuous efforts on tandem reactions,21−25 we report a one-step tandem N-sulfonylation and esterification involving DCE as both the solvent and reactant, where DBU acts as a strong basic reagent to transfer the proton. This reaction atom is economical, raw materials are readily available, and no hazardous reagents are used, and it is well-tolerated for different functional groups and provides a novel idea of esterification. Furthermore, this is the first report on the synthesis of proline-derived benzenesulfonamides by three components such as sulfonyl azide.
Results and Discussion
To start with, we selected p-toluenesulfonyl azide (1a) and proline (2a) as model substrates to screen the reaction parameters. The raw materials 1a (0.2 mmol) and 2a (0.3 mmol) were treated with DBU (1.0 equiv) in 1,2-dichloroethane (DCE, 2 mL) at a temperature at 60 °C to produce the desired 2-chloroethyl tosylprolinate (4a), a kind of yellow liquid, in 88% yield after 6 h. The structure of 4a was determined by 1H NMR, 13C NMR, HPLC–MS, and NOESY (Table 1, entry 3) for identification. Other known bases including KOH, Cs2CO3, NaHCO3, triethylamine, and so forth were ineffective for the reaction (Table 1, entries 1–8), but the coupling reaction between 1a and 2a in the presence of organobasic pyridine also proceeded with poor conversion (Table 1, entry 9). When considering the effect of temperature on the reaction, we noticed a slight decrease in yield when the reaction temperature was lowered to 40 °C or raised to 80 °C (Table 1, entries 10 and 11). Next, the desired product was not obtained when the reaction time was shortened to 2 h (Table 1, entries 12). On the contrary, the yields of 4a decreased to 74 and 86% when the reaction time was 4 h and extended to 8 h (Table 1, entries 13 and 14).
Table 1. Reaction Optimizationa.
| entry | base | temp (°C) | time (h) | yield (%) |
|---|---|---|---|---|
| 1 | KOH | 60 | 6 | trace |
| 2 | Cs2CO3 | 60 | 6 | n.r |
| 3 | DBU | 60 | 6 | 88 |
| 4 | NaHCO3 | 60 | 6 | n.r |
| 5 | CH3COOK | 60 | 6 | n.r |
| 6 | Et3N | 60 | 6 | trace |
| 7 | KOtBu | 60 | 6 | n.r |
| 8 | K2CO3 | 60 | 6 | trace |
| 9 | pyridine | 60 | 6 | n.r |
| 10 | DBU | 40 | 6 | 46 |
| 11 | DBU | 80 | 6 | 80 |
| 12 | DBU | 60 | 2 | n.r |
| 13 | DBU | 60 | 4 | 74 |
| 14 | DBU | 60 | 8 | 86 |
Reaction conditions: 1a (0.2 mmol), 2 (0.3 mmol), base (1.0 equiv) and solvent (2.0 mL), in a sealed tube with air atmosphere stirred for 6 h. Isolated yield.
With the knowledge of the optimal conditions, the range of phenylsulfonyl azides was explored using 2a as a model reaction partner for the first time. As shown in Table 2, benzenesulfonyl azide derivatives bearing different electron-donating and electron-withdrawing substituents at the para-position of the benzene ring were suitable for this protocol, providing the corresponding 2-chloroethyl (phenylsulfonyl)prolinate in moderate to good yields. The reaction of the benzenesulfonyl azide containing electron-donating substituents such as methyl (4a), tert-butyl (4c), and methoxy (4d) with 2 smoothly provided the desired product in 84–88% yield. Phenylsulfonyl azides with electron-withdrawing groups such as fluorine (4e), chlorine (4f), bromine (4g), and CF3 (4i) at different positions of the benzene ring were tolerated, providing the corresponding products in moderate to good yields (78–83%). In addition, electron-donating groups were proved to be more favorable for the reaction than electron-withdrawing groups. Moreover, there is excellent tolerance for the neighboring substituents with electron-donating character (4o), halogen substituents (4p, 4q, 4r) and electron-withdrawing character (4s). Notably, 2-CH3 (4o) required extended time to reach 70% yield. When meta-substituted benzenesulfonyl azides, such as methyl (4j), halogen (4k, 4l, 4m), and trifluoromethyl (4n), were used in the reaction, they showed good compatibility, yielding the corresponding products in about 70–78% yield. Multi-substituted compounds 4t (2-CH3, 4-CH3), 4u (2-CH3, 4-CH3, 6-CH3) showed slightly lower yields than the compounds with other substituents. Besides, thiophene-2-sulfonyl azide gave 78% yield of 4v.
Table 2. Scope for the Synthesis of 4a–4va.
Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), and DCE (2.0 mL), air, sealed tube, 60 °C, 6 h, isolated yield.
24 h.
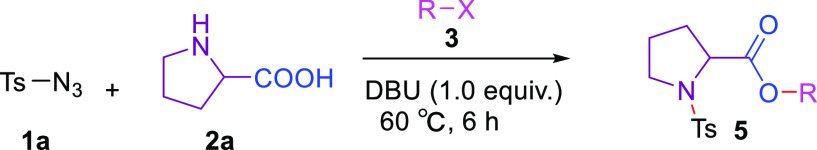
According to the above results, the range of substrates varied with the solvent. As shown in Table 3, replacing 1,2-dichloroethane with 1-chloro-2-bromoethane, 1,2-dibromoethane, 2-chloropropane, 1,3-dichloropropane, 1-chlorobutane, and 1,4-dichlorobutane, we could obtain long-chain halogenated tosylprolinate (5a–5f) in good yields. However, dichloromethane is not a good solvent for this reaction and the corresponding product was not obtained. To our delight, we used pyrrolidine-3-carboxylic acid and 2-methylpyrrolidine-2-carboxylic acid instead of proline under standard conditions and were able to obtain 5g and 5h of product in good yields. Unfortunately, proline derivatives (6a–6c), indoline-2-carboxylic acid (6d), piperidine-2-carboxylic acid (6e), and uncyclized N-alkyl α-carboxylic acids (6f–6h) had been studied under standard conditions, and no products were found.
Table 3. Scope for the Synthesis of 5a–5ta.
Reaction conditions: 1 (0.2 mmol), 2a (0.3 mmol), and DCE (2.0 mL), air, sealed tube, 60 °C, 6 h.
Isolated yield.
A gram-scale preparation of 4a was carried out to demonstrate the practicality of the method. Pleasantly, we were able to obtain the desired product smoothly in 68% yield when in the standard condition (Scheme 2).
Scheme 2. Scale-Up Experiment.
To investigate the mechanism of the process, some exploratory experiments were performed. When TEMPO (3.0 equiv) was added, the desired 4a product was obtained with a slight decrease in yield, indicating the absence of a free radical pathway (Scheme 3a). Furthermore, in order to verify the sequential nature of the reaction, no benzenesulfonyl azide was added in the standard state and it was found that the desired product 6 was not obtained (Scheme 3b), indicating that the presence of benzenesulfonyl azide is essential for the reaction. Finally, we replaced the solvent with acetonitrile, and no ideal product 7 was obtained (Scheme 3c), clearly indicating that DCE plays a key role in the reaction. Additionally, we observed product 4a when tosylproline 8 was reacted under standard reactions conditions (Scheme 3d). At the same time, about 5 min after the start of the reaction, we detected intermediate B and C (Scheme 4) from the reaction mixture by HPLC–MS (Supporting Information). In addition, we did a controlled experiment to substitute proline with pyrrolidine and finally succeeded in obtaining product 9 to confirm the correctness of the product (Scheme 3e). To elucidate the connection between the sulfonation of amines and the alkylation of carboxylic acids, we found that the carboxylic acids were not alkylated in the presence of cyclopentane carboxylic acids (Scheme 3f).
Scheme 3. Mechanistic Studies.
Scheme 4. Postulated Reaction Mechanism.
Based on the above experimental investigations and some well-documented reports,14,27 a plausible mechanism is proposed (Scheme 4). First, the base action of DBU, which dehydrogenates the proline ion, leading to the formation of prolinate (A), which subsequently forms a known intermediate of 4-methylbenzenesulfonic pyrrolidine-2-carboxylic anhydride (B) with benzenesulfonyl azide. Meanwhile, two molecules of intermediate B underwent intermolecular ester exchange to form tosylproline C. Finally, the target product 4a was obtained by the esterification reaction of DCE with deprotonated intermediate D.
Virtual screening of the products revealed that the products showed good antitumor activity against MCF-7 and SKOV3 cells. Compound 4d showed a relatively good antitumor activity against MCF-7 cells compared to the positive control drug, as shown in Table S1 with an IC50 value of 147.4 ± 0.8 μM comparing to 81.59 ± 9.2 μM of the positive control drug.26 More detailed experimental results are available in the Supporting Information. Furthermore, because the O-chloroethyl of the product has more room for structural modification, it can provide a structural basis for subsequent work, and this study can provide a good foundation for the next step of drug mechanism research and identification. Therefore, our laboratory is currently working on the structural modification and modification of the subsequent products.
Herein, we developed an efficient general strategy to provide proline-derived benzenesulfonamides under metal-free conditions. In contrast to benzenesulfonyl azide compounds providing sulfonyl groups, we can obtain the benzenesulfonamide derivatives by reacting with useful amino acids. In this paper, we present a simple, practical, green, and environmentally friendly method to prepare 2-chloroethyl tosylprolinate in moderate to good yields via a one-pot coupling reaction of benzenesulfonyl azides, avoiding the use of transition metal. In addition, the reaction is carried out under convenient handling and has a high tolerance of functional groups.
Experimental Section
General Experiment Information
1H NMR (400 MHz) and 13C NMR spectra (101 MHz) were recorded on the Bruker Ascend 400 spectrometer using CDCl3 and DMSO-d6 as the solvent. Chemical shifts are given in ppm and coupling constants in Hz. 1H spectra were calibrated in relation to the reference measurement of TMS (0.000 ppm). The following abbreviations were used for 1H NMR spectra to indicate the signal multiplicity: s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet) and combinations of them. Flash chromatography was performed on silica gel 200–300 mesh (purchased from Qingdao Haiyang Chemical, China). MS spectra were recorded on an Agilent 6546 LC/Q-TOF.
General Procedure for Synthesis of 2-Chloroethyl Tosylprolinate (4a)
A mixture of substrate 1a (39.4 mg, 0.2 mmol), 2 (34.5 mg, 0.3 mmol), in solvent (2.0 mL) was charged in a glass sealed-tube and stirred under air atmosphere at 60 °C for 6 h. When over, the reaction mixture was extracted with saturated aqueous NaHCO3/EA. Then the organic layer was dried with Na2SO4, and concentrated to dryness. The crude product was purified by silica gel chromatography (silica gel, PE) to afford the product 4a as a light-yellow oil with the yield of 88%. 1H NMR (400 MHz, DMSO-d6): δ 7.84–7.65 (m, 2H), 7.43 (d, J = 8.0 Hz, 2H), 4.42–4.32 (m, 2H), 4.25 (dd, J = 8.3, 4.3 Hz, 1H), 3.88–3.78 (m, 2H), 3.42–3.37 (m, 1H), 3.15 (dt, J = 9.6, 7.2 Hz, 1H), 2.40 (s, 3H), 2.02–1.88 (m, 2H), 1.87–1.76 (m, 1H), 1.63 (dtt, J = 9.9, 4.9, 2.6 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.9, 144.1, 134.9, 130.4, 127.7, 65.0, 60.7, 48.8, 42.9, 30.9, 24.6, 21.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18ClNO4S, 332.0718; found, 332.0726. The remaining reactions were performed following this typical procedure.
2-Chloroethyl (Phenylsulfonyl)prolinate (4b)
Yellow liquid, 53.9 mg, 85% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.89–7.81 (m, 2H), 7.76–7.68 (m, 1H), 7.64 (dd, J = 8.3, 6.7 Hz, 2H), 4.39–4.31 (m, 2H), 4.28 (dd, J = 8.4, 4.2 Hz, 1H), 3.82 (td, J = 4.6, 1.3 Hz, 2H), 3.45–3.40 (m, 1H), 3.18 (dt, J = 9.7, 7.2 Hz, 1H), 2.05–1.89 (m, 2H), 1.88–1.79 (m, 1H), 1.64 (dddd, J = 12.1, 7.2, 4.7, 2.3 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 137.8, 133.7, 129.9, 127.6, 65.0, 60.7, 48.8, 42.9, 30.9, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H16ClNO4S, 318.0561; found, 318.0564.
2-Chloroethyl ((4-(tert-Butyl)phenyl)sulfonyl)prolinate (4c)
Colorless solid, 64.1 mg, 86% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.81–7.72 (m, 2H), 7.67–7.60 (m, 2H), 4.32 (ddd, J = 6.1, 4.5, 1.9 Hz, 2H), 4.27 (dd, J = 8.6, 4.0 Hz, 1H), 3.85–3.75 (m, 2H), 3.45–3.38 (m, 1H), 3.19 (dt, J = 9.7, 7.2 Hz, 1H), 2.04–1.89 (m, 2H), 1.89–1.82 (m, 1H), 1.67 (dddd, J = 12.0, 7.3, 4.6, 2.5 Hz, 1H), 1.30 (s, 9H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 156.6, 135.1, 127.5, 126.7, 65.0, 60.7, 48.8, 42.8, 35.3, 31.2, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H24ClNO4S, 374.1187; found 374.1196.
2-Chloroethyl ((4-Methoxyphenyl)sulfonyl)prolinate (4d)
Colorless liquid, 58.3 mg, 84% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.87–7.68 (m, 2H), 7.21–7.04 (m, 2H), 4.44–4.30 (m, 2H), 4.22 (dd, J = 8.4, 4.3 Hz, 1H), 3.86 (s, 3H), 3.85–3.81 (m, 2H), 3.37–3.34 (m, 1H), 3.15 (dt, J = 9.7, 7.1 Hz, 1H), 1.95 (dddd, J = 20.8, 9.9, 5.8, 2.3 Hz, 2H), 1.87–1.77 (m, 1H), 1.70–1.55 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.9, 163.2, 129.9, 129.3, 115.0, 65.0, 60.7, 56.1, 48.8, 42.90, 30.9, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18ClNO5S, 348.0667; found 348.0663.
2-Chloroethyl ((4-Fluorophenyl)sulfonyl)prolinate (4e)
Yellow liquid, 54.4 mg, 81% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.99–7.88 (m, 2H), 7.50–7.40 (m, 2H), 4.41–4.33 (m, 2H), 4.31 (dd, J = 8.7, 4.0 Hz, 1H), 3.85–3.80 (m, 2H), 3.44–3.38 (m, 1H), 3.19 (dt, J = 9.5, 7.1 Hz, 1H), 2.07–1.90 (m, 2H), 1.89–1.78 (m, 1H), 1.73–1.62 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 166.4 (d, JCF = 253.5), 163.8, 134.3 (d, JCF = 3.0), 134.3, 130.8 (d, JCF = 9.7), 130.7, 117.2 (d, JCF = 22.6), 116.9, 65.1, 60.7, 48.8, 42.9, 31.0, 24.6. 19F NMR (377 MHz, DMSO): δ −105.83. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15ClFNO4S, 336.0467; found, 336.0470.
2-Chloroethyl ((4-Chlorophenyl)sulfonyl)prolinate (4f)
Colorless liquid, 58.4 mg, 83% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.91–7.82 (m, 2H), 7.73–7.64 (m, 2H), 4.37–4.33 (m, 2H), 4.33–4.30 (m, 1H), 3.88–3.77 (m, 2H), 3.44–3.38 (m, 1H), 3.19 (dt, J = 9.5, 7.2 Hz, 1H), 2.08–1.90 (m, 2H), 1.90–1.81 (m, 1H), 1.74–1.60 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 138.6, 136.8, 130.0, 129.6, 65.1, 60.7, 48.8, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15Cl2NO4S, 352.0172; found, 352.0174.
2-Chloroethyl ((4-Bromophenyl)sulfonyl)prolinate (4g)
Colorless liquid, 64.6 mg, 82% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.84 (d, J = 8.7 Hz, 2H), 7.78 (d, J = 8.7 Hz, 2H), 4.37–4.33 (m, 2H), 4.31 (dd, J = 8.7, 3.8 Hz, 1H), 3.88–3.79 (m, 2H), 3.45–3.38 (m, 1H), 3.19 (dt, J = 9.5, 7.1 Hz, 1H), 2.09–1.90 (m, 2H), 1.89–1.79 (m, 1H), 1.69 (dddd, J = 12.0, 7.3, 4.6, 2.5 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 137.2, 133.0, 129.6, 127.6, 65.1, 60.7, 48.8, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15BrClNO4S, 395.9667; found, 395.9667.
2-Chloroethyl ((4-(Trifluoromethyl)phenyl)sulfonyl)prolinate (4h)
Yellow liquid, 61.6 mg, 80% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.14–8.04 (m, 2H), 8.00 (d, J = 8.3 Hz, 2H), 4.39 (dd, J = 8.7, 3.7 Hz, 1H), 4.37–4.29 (m, 2H), 3.82 (td, J = 4.7, 1.4 Hz, 2H), 3.44 (ddd, J = 9.4, 7.4, 4.6 Hz, 1H), 3.24 (dt, J = 9.5, 7.2 Hz, 1H), 2.13–2.01 (m, 1H), 1.99–1.91 (m, 1H), 1.91–1.81 (m, 1H), 1.77–1.65 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.7, 142.0, 133.7 (q, JCF = 32.3), 133.4, 133.1, 132.8, 128.6, 128.0 (q, JCF = 273.9), 127.1 (q, JCF = 3.3), 127.0, 127.0, 127.0, 125.2, 122.5, 119.8, 65.1, 60.8, 48.8, 42.8, 31.0, 24.6. 19F NMR (377 MHz, DMSO): δ −61.80. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H15ClF3NO4S, 386.0435; found, 386.0438.
2-Chloroethyl ((4-Cyanophenyl)sulfonyl)prolinate (4i)
Colorless liquid, 53.5 mg, 78% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.15–8.07 (m, 2H), 8.07–7.98 (m, 2H), 4.40 (dd, J = 8.7, 3.8 Hz, 1H), 4.38–4.27 (m, 2H), 3.86–3.75 (m, 2H), 3.42 (ddd, J = 9.5, 7.4, 4.6 Hz, 1H), 3.23 (dt, J = 9.6, 7.2 Hz, 1H), 2.13–1.98 (m, 1H), 2.00–1.86 (m, 1H), 1.92–1.78 (m, 1H), 1.79–1.65 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.7, 142.1, 134.0, 128.4, 118.1, 116.0, 65.1, 60.8, 48.8, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C14H15ClN2O4S, 365.0333; found, 365.0339.
2-Chloroethyl (m-Tolylsulfonyl)prolinate (4j)
Colorless liquid, 53.6 mg, 81% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.69–7.65 (m, 1H), 7.65–7.61 (m, 1H), 7.55–7.48 (m, 2H), 4.38–4.31 (m, 2H), 4.28 (dd, J = 8.4, 4.2 Hz, 1H), 3.87–3.78 (m, 2H), 3.41–3.36 (m, 1H), 3.17 (dt, J = 9.7, 7.2 Hz, 1H), 2.41 (s, 3H), 2.02–1.86 (m, 2H), 1.88–1.77 (m, 1H), 1.69–1.58 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.9, 139.8, 137.7, 134.3, 129.7, 127.7, 124.8, 65.0, 60.7, 48.8, 42.9, 31.0, 24.6, 21.3. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18ClNO4S, 332.0718; found, 332.0726.
2-Chloroethyl ((3-Fluorophenyl)sulfonyl)prolinate (4k)
Colorless liquid, 51.6 mg, 77% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.72–7.70 (m, 2H), 7.68 (dd, J = 3.9, 2.2 Hz, 1H), 7.62–7.55 (m, 1H), 4.41–4.36 (m, 1H), 4.36–4.32 (m, 2H), 3.86–3.80 (m, 2H), 3.41 (ddd, J = 9.6, 7.4, 4.7 Hz, 1H), 3.23 (dt, J = 9.6, 7.2 Hz, 1H), 2.08–1.98 (m, 1H), 1.97–1.89 (m, 1H), 1.89–1.80 (m, 1H), 1.75–1.64 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 163.6 (d, JCF = 249.6), 161.1, 140.1 (d, JCF = 6.7), 140.0, 132.3(d, JCF = 8.1), 132.2, 123.9 (d, JCF = 3.2), 123.9, 121.0 (d, JCF = 21.3), 120.7, 114.8 (d, JCF = 24.3), 114.6, 65.1, 60.8, 48.8, 42.9, 31.0, 24.6. 19F NMR (377 MHz, DMSO): δ −110.13. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H16FN3O2S, 336.0467; found, 336.0468.
2-Chloroethyl ((3-Chlorophenyl)sulfonyl)prolinate (4l)
Colorless liquid, 53.3 mg, 76% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.87 (t, J = 1.9 Hz, 1H), 7.83 (ddd, J = 7.8, 1.8, 1.1 Hz, 1H), 7.79 (ddd, J = 8.1, 2.1, 1.0 Hz, 1H), 7.67 (t, J = 7.9 Hz, 1H), 4.39 (dd, J = 8.7, 3.8 Hz, 1H), 4.36–4.32 (m, 2H), 3.86–3.80 (m, 2H), 3.41 (ddd, J = 9.5, 7.3, 4.7 Hz, 1H), 3.23 (dt, J = 9.5, 7.2 Hz, 1H), 2.08–2.00 (m, 1H), 2.00–1.89 (m, 1H), 1.90–1.81 (m, 1H), 1.78–1.65 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.7, 139.9, 134.6, 133.6, 131.9, 127.1, 126.3, 65.1, 60.7, 48.8, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15Cl2NO4S, 352.0172; found, 352.0174.
2-Chloroethyl ((3-Bromophenyl)sulfonyl)prolinate (4m)
Yellow liquid, 61.6 mg, 78% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.98 (t, J = 1.9 Hz, 1H), 7.93 (ddd, J = 8.0, 2.0, 1.0 Hz, 1H), 7.87 (ddd, J = 7.9, 1.8, 1.0 Hz, 1H), 7.60 (t, J = 7.9 Hz, 1H), 4.39 (dd, J = 8.7, 3.8 Hz, 1H), 4.36–4.31 (m, 2H), 3.86–3.80 (m, 2H), 3.43–3.38 (m, 1H), 3.22 (dt, J = 9.5, 7.2 Hz, 1H), 2.10–2.01 (m, 1H), 1.97–1.91 (m, 1H), 1.89–1.80 (m, 1H), 1.76–1.68 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 140.1, 136.5, 132.2, 129.9, 126.7, 122.9, 65.1, 60.7, 48.8, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15BrClNO4S, 395.9667; found, 395.9666.
2-Chloroethyl ((3-(Trifluoromethyl)phenyl)sulfonyl)prolinate (4n)
Colorless liquid, 57.0 mg, 74% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.21–8.16 (m, 1H), 8.10 (d, J = 8.0 Hz, 2H), 7.92–7.87 (m, 1H), 4.45 (dd, J = 8.7, 3.7 Hz, 1H), 4.34 (td, J = 4.7, 1.4 Hz, 2H), 3.82 (td, J = 4.8, 1.4 Hz, 2H), 3.42 (ddd, J = 9.5, 7.4, 4.6 Hz, 1H), 3.25 (dt, J = 9.5, 7.3 Hz, 1H), 2.15–2.01 (m, 1H), 1.99–1.92 (m, 1H), 1.91–1.80 (m, 1H), 1.79–1.69 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.7, 139.5, 131.7, 131.6, 131.0 (q, JCF = 32.8), 130.7, 130.4 (q, JCF = 3.5), 130.4, 130.3, 130.3, 130.0, 127.9 (q, JCF = 273.8), 125.2, 124.1 (q, JCF = 3.7), 124.1, 124.1, 124.0, 122.5, 119.8, 65.1, 60.8, 48.8, 42.9, 31.0, 24.6. 19F NMR (377 MHz, DMSO): δ −61.38. 19F NMR (377 MHz, DMSO): δ −61.38. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H15ClF3NO4S, 386.0435; found, 386.0437.
2-Chloroethyl (o-Tolylsulfonyl)prolinate (4o)
Yellow liquid, 46.3 mg, 70% yield. 1H NMR (400 MHz, chloroform-d): δ 7.91 (dd, J = 8.2, 1.4 Hz, 1H), 7.38 (td, J = 7.5, 1.4 Hz, 1H), 7.26–7.21 (m, 2H), 4.41 (dd, J = 8.8, 3.2 Hz, 1H), 4.14 (dd, J = 6.3, 5.2 Hz, 2H), 3.50 (dd, J = 6.2, 5.2 Hz, 2H), 3.47–3.42 (m, 2H), 2.59 (s, 3H), 2.26–2.16 (m, 1H), 2.09–1.96 (m, 2H), 1.94–1.87 (m, 1H). 13C NMR (101 MHz, chloroform-d): δ 170.7, 137.4, 136.4, 131.9, 131.5, 128.7, 125.0, 63.4, 58.7, 47.4, 40.2, 30.3, 23.7, 19.4. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18ClNO4S, 332.0718; found, 332.0723.
2-Chloroethyl ((2-Fluorophenyl)sulfonyl)prolinate (4p)
Colorless liquid, 48.2 mg, 72% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.83 (td, J = 7.6, 1.8 Hz, 1H), 7.79–7.72 (m, 1H), 7.49 (ddd, J = 10.9, 8.4, 1.1 Hz, 1H), 7.42 (td, J = 7.7, 1.1 Hz, 1H), 4.38 (dd, J = 8.8, 3.4 Hz, 1H), 4.29 (td, J = 4.8, 1.8 Hz, 2H), 3.84–3.75 (m, 2H), 3.49 (ddd, J = 9.5, 7.5, 4.7 Hz, 1H), 3.29 (dt, J = 9.5, 7.2 Hz, 1H), 2.22–2.11 (m, 1H), 2.02–1.96 (m, 1H), 1.95–1.86 (m, 1H), 1.85–1.77 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.6, 159.9 (d, JCF = 253.9), 157.4, 136.4 (d, JCF = 8.8), 136.3, 131.1, 126.5 (d, JCF = 14.9), 126.4, 125.6 (d, JCF = 3.4), 125.5, 118.1 (d, JCF = 21.8), 117.9, 65.0, 60.6 (d, JCF = 2.9), 60.5, 48.6 (d, JCF = 2.0), 48.5, 42.8, 31.0, 24.6. 19F NMR (377 MHz, DMSO): δ −108.95. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15ClFNO4S, 336.0467; found, 336.0470.
2-Chloroethyl ((2-Chlorophenyl)sulfonyl)prolinate (4q)
Colorless liquid, 53.3 mg, 75% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.00 (dd, J = 7.9, 1.5 Hz, 1H), 7.72–7.65 (m, 2H), 7.56 (ddd, J = 7.9, 7.0, 1.7 Hz, 1H), 4.52 (dd, J = 8.8, 3.0 Hz, 1H), 4.30–4.24 (m, 2H), 3.82–3.76 (m, 2H), 3.52 (ddd, J = 9.2, 7.6, 4.6 Hz, 1H), 3.28 (dt, J = 9.2, 7.2 Hz, 1H), 2.31–2.21 (m, 1H), 2.06–2.00 (m, 1H), 1.98–1.80 (m, 2H).13C NMR (101 MHz, DMSO-d6): δ 171.8, 136.9, 134.9, 132.6, 131.9, 131.3, 128.3, 65.1, 61.0, 48.6, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15Cl2NO4S, 352.0172; found, 352.0174.
2-Chloroethyl ((2-Bromophenyl)sulfonyl)prolinate (4r)
Yellow liquid, 60.0 mg, 76% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.03 (dd, J = 7.6, 2.0 Hz, 1H), 7.88 (dd, J = 7.5, 1.6 Hz, 1H), 7.66–7.48 (m, 2H), 4.59 (dd, J = 8.8, 2.9 Hz, 1H), 4.39–4.17 (m, 2H), 3.94–3.70 (m, 2H), 3.52 (ddd, J = 9.2, 7.7, 4.5 Hz, 1H), 3.25 (dt, J = 9.2, 7.3 Hz, 1H), 2.39–2.20 (m, 1H), 2.09–2.00 (m, 1H), 1.99–1.84 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 138.7, 136.1, 134.8, 132.0, 128.7, 119.9, 65.1, 61.3, 48.5, 42.9, 30.9, 24.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C13H15BrClNO4S, 395.9667; found, 395.9667.
2-Chloroethyl ((2-(Trifluoromethyl)phenyl)sulfonyl)prolinate (4s)
Yellow liquid, 60.0 mg, 78% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.12 (dd, J = 7.7, 1.5 Hz, 1H), 8.01 (dd, J = 7.5, 1.7 Hz, 1H), 7.94–7.84 (m, 2H), 4.52 (dd, J = 8.8, 2.9 Hz, 1H), 4.33–4.22 (m, 2H), 3.83–3.71 (m, 2H), 3.51 (ddd, J = 9.4, 6.9, 5.1 Hz, 1H), 3.38–3.34 (m, 1H), 2.34 (dq, J = 12.3, 8.6 Hz, 1H), 2.10–2.02 (m, 1H), 2.02–1.92 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 138.5, 134.0, 133.7, 130.7, 129.1 (q, JCF = 6.4), 129.0, 129.0, 128.9, 127.3 (q, JCF = 274.9), 127.1 (q, JCF = 32.7), 126.8, 126.5, 126.1, 124.5, 121.8, 119.1, 65.1, 60.9, 48.9, 42.8, 31.1, 24.6. 19F NMR (377 MHz, DMSO): δ −56.15. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H16F3N3O2S, 386.0435; found, 386.0439.
2-Chloroethyl ((2,5-Dimethylphenyl)sulfonyl)prolinate (4t)
Colorless liquid, 54.4 mg, 79% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.66 (d, J = 1.8 Hz, 1H), 7.35 (dd, J = 7.8, 1.8 Hz, 1H), 7.30 (d, J = 7.8 Hz, 1H), 4.34 (dd, J = 8.8, 3.2 Hz, 1H), 4.23 (dd, J = 6.0, 4.4 Hz, 2H), 3.80–3.68 (m, 2H), 3.43–3.38 (m, 1H), 3.31 (dt, J = 9.4, 7.2 Hz, 1H), 2.52 (s, 3H), 2.34 (s, 3H), 2.23 (ddt, J = 10.3, 5.1, 2.8 Hz, 1H), 2.04–1.97 (m, 1H), 1.97–1.79 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 137.1, 136.4, 134.7, 134.1, 133.1, 129.6, 64.9, 60.1, 48.6, 42. 8, 31.2, 24.7, 20.8, 20.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H20ClNO4S, 346.0874; found, 346.0882.
2-Chloroethyl (Mesitylsulfonyl)prolinate (4u)
Yellow liquid, 53.8 mg, 75% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.04 (s, 2H), 4.26 (dd, J = 8.9, 3.1 Hz, 1H), 4.09 (td, J = 5.3, 1.7 Hz, 2H), 3.72–3.63 (m, 2H), 3.38–3.27 (m, 3H), 2.55 (s, 6H), 2.26 (s, 3H), 2.27–2.19 (m, 1H), 2.04–1.97 (m, 1H), 1.97–1.86 (m, 2H). 13C NMR (101 MHz, DMSO-d6): δ 171.6, 143.1, 140.2, 132.8, 132.2, 64.8, 59.4, 48.2, 42.6, 31.4, 24.6, 22.8, 20.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H22ClNO4S, 360.1031; found, 360.1039.
2-Chloroethyl (Thiophen-2-ylsulfonyl)prolinate (4v)
Colorless liquid, 50.4 mg, 78% yield. 1H NMR (400 MHz, DMSO-d6): δ 8.04 (dd, J = 5.0, 1.3 Hz, 1H), 7.75 (dd, J = 3.8, 1.4 Hz, 1H), 7.28 (dd, J = 5.0, 3.8 Hz, 1H), 4.37 (ddd, J = 5.2, 4.4, 1.5 Hz, 2H), 4.22 (dd, J = 7.7, 5.0 Hz, 1H), 3.84 (dd, J = 5.7, 4.8 Hz, 2H), 3.47 (ddd, J = 9.8, 7.0, 4.8 Hz, 1H), 3.21 (dt, J = 9.9, 7.1 Hz, 1H), 2.00–1.93 (m, 2H), 1.91–1.82 (m, 1H), 1.69–1.61 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.6, 137.1, 134.2, 133.3, 128.8, 65.1, 61.0, 49.3, 42.9, 31.0, 24.6. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C11H14ClNO4S2, 324.0126; found, 324.0124.
2-Chloroethyl Tosylprolinate (5a)
Yellow liquid, 54.3 mg, 82% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.76–7.69 (m, 2H), 7.44 (d, J = 8.0 Hz, 2H), 4.36–4.32 (m, 2H), 4.24 (dd, J = 8.4, 4.2 Hz, 1H), 3.83 (dd, J = 6.3, 4.3 Hz, 2H), 3.43–3.38 (m, 1H), 3.15 (dt, J = 9.7, 7.2 Hz, 1H), 2.40 (s, 3H), 2.02–1.89 (m, 2H), 1.86–1.78 (m, 1H), 1.68–1.57 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.9, 144.1, 134.9, 130.4, 127.7, 65.0, 60.7, 48.8, 42.9, 30.9, 24.6, 21.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18ClNO4S, 332.0718; found, 332.0723.
2-Bromoethyl Tosylprolinate (5b)
Yellow liquid, 52.5 mg, 70% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.80–7.63 (m, 2H), 7.44 (d, J = 8.0 Hz, 2H), 4.49–4.33 (m, 2H), 4.24 (dd, J = 8.1, 4.6 Hz, 1H), 3.68 (t, J = 5.5 Hz, 2H), 3.47–3.37 (m, 1H), 3.15 (dt, J = 9.7, 7.2 Hz, 1H), 2.40 (s, 3H), 2.01–1.89 (m, 2H), 1.88–1.77 (m, 1H), 1.64 (dddd, J = 12.0, 7.1, 4.8, 2.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 171.8, 144.1, 134.9, 130.4, 127.7, 64.8, 60.7, 48.8, 31.1, 31.0, 24.6, 21.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18BrNO4S, 376.0213; found, 376.0220.
Isopropyl Tosylprolinate (5c)
Yellow liquid, 54.3 mg, 75% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.75–7.68 (m, 2H), 7.47–7.39 (m, 2H), 4.99–4.79 (m, 1H), 4.12 (dd, J = 8.6, 3.9 Hz, 1H), 3.43–3.36 (m, 1H), 3.14 (dt, J = 9.8, 7.0 Hz, 1H), 2.41 (s, 3H), 2.02–1.88 (m, 1H), 1.87–1.74 (m, 2H), 1.60 (dtd, J = 11.6, 7.7, 6.8, 4.1 Hz, 1H), 1.22 (d, J = 6.3 Hz, 3H), 1.18 (d, J = 6.3 Hz, 3H). 13C NMR (101 MHz, DMSO-d6): δ 171.6, 144.0, 135.0, 130.4, 127.6, 68.7, 60.9, 48.8, 30.9, 24.6, 21.9, 21.8, 21.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H21NO4S, 312.1264; found, 312.1270.
3-Chloropropyl Tosylprolinate (5d)
Colorless liquid, 55.2 mg, 80% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.75–7.71 (m, 2H), 7.47–7.43 (m, 2H), 4.24–4.19 (m, 2H), 4.19–4.17 (m, 1H), 3.72 (t, J = 6.5 Hz, 2H), 3.48–3.38 (m, 1H), 3.15 (dt, J = 9.8, 7.1 Hz, 1H), 2.41 (s, 3H), 2.05 (p, J = 6.3 Hz, 2H), 1.97–1.74 (m, 3H), 1.60 (dddd, J = 12.1, 7.2, 5.0, 2.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 172.1, 144.1, 134.9, 130.4, 127.6, 62.2, 60.8, 48.9, 42.1, 31.5, 30.9, 24.7, 21.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H20ClNO4S, 346.0875; found, 346.0883.
Butyl Tosylprolinate (5e)
Colorless liquid, 50.0 mg, 77% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.76–7.64 (m, 2H), 7.49–7.38 (m, 2H), 4.17 (dd, J = 8.5, 3.9 Hz, 1H), 4.06 (ddt, J = 10.8, 6.6, 4.3 Hz, 2H), 3.38 (dd, J = 5.9, 3.7 Hz, 1H), 3.15 (dt, J = 9.7, 7.0 Hz, 1H), 2.40 (s, 3H), 2.01–1.89 (m, 1H), 1.88–1.75 (m, 2H), 1.67–1.58 (m, 1H), 1.58–1.48 (m, 2H), 1.39–1.26 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6): δ 172.2, 144.0, 135.0, 130.4, 127.6, 64.8, 60.8, 48.8, 30.9, 30.5, 24.7, 21.5, 19.0, 14.0. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H23NO4S, 326.1421; found, 326.1431.
4-Chlorobutyl Tosylprolinate (5f)
Colorless liquid, 56.0 mg, 78% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.73 (d, J = 8.3 Hz, 2H), 7.44 (d, J = 7.7 Hz, 2H), 4.19 (dd, J = 8.5, 4.1 Hz, 1H), 4.17–4.04 (m, 2H), 3.68 (t, J = 6.4 Hz, 2H), 3.43–3.36 (m, 0H), 3.16 (dt, J = 9.6, 7.0 Hz, 1H), 2.41 (s, 3H), 2.03–1.90 (m, 1H), 1.93–1.80 (m, 2H), 1.83–1.74 (m, 2H), 1.77–1.67 (m, 2H), 1.66–1.54 (m, 1H). 13C NMR (101 MHz, DMSO-d6): δ 172.1, 144.0, 134.9, 130.4, 127.6, 64.5, 60.8, 48.9, 45.4, 30.9, 29.1, 26.0, 24.7, 21.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H22ClNO4S, 360.1031; found, 360.1042.
2-Chloroethyl 1-Tosylpyrrolidine-3-carboxylate (5g)
Colorless liquid, 53.8 mg, 81% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.73–7.64 (m, 2H), 7.45 (dd, J = 7.3, 1.4 Hz, 2H), 4.29–4.08 (m, 2H), 3.76 (t, J = 5.3 Hz, 2H), 3.43–3.35 (m, 2H), 3.24–3.15 (m, 2H), 3.13–3.02 (m, 1H), 2.41 (s, 3H), 2.05–1.93 (m, 1H), 1.89 (ddd, J = 12.7, 6.3, 1.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 172.49, 144.02, 133.24, 130.32, 127.94, 64.78, 49.98, 47.76, 42.85, 42.33, 28.34, 21.46. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C14H18ClNO4S, 332.0718; found, 332.0716.
2-Chloroethyl 3-Methyl-1-tosylpyrrolidine-3-carboxylate (5h)
Colorless liquid, mg, 63% yield. 1H NMR (400 MHz, DMSO-d6): δ 7.87–7.56 (m, 2H), 7.40 (d, J = 8.1 Hz, 2H), 4.29 (dddd, J = 72.0, 12.2, 6.5, 4.0 Hz, 2H), 3.87–3.69 (m, 2H), 3.49–3.40 (m, 1H), 3.34–3.27 (m, 1H), 2.39 (s, 3H), 2.18 (dd, J = 11.0, 5.2 Hz, 1H), 1.94 (tdd, J = 12.7, 5.6, 2.9 Hz, 2H), 1.91–1.84 (m, 1H), 1.50 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 173.55, 143.45, 138.24, 130.12, 127.17, 67.88, 65.14, 49.22, 42.87, 40.67, 23.48, 23.35, 21.44. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C15H20ClNO4S, 346.0874; found, 346.0871.
1-Tosylpyrrolidine (9)
White solids, 121.8–123.6 °C, 40.5 mg, 90% yield. 1H NMR (400 MHz, chloroform-d): δ 7.78–7.62 (m, 2H), 7.33 (d, J = 8.0 Hz, 2H), 3.29–3.13 (m, 4H), 2.42 (s, 3H), 1.82–1.64 (m, 4H). 13C NMR (101 MHz, chloroform-d): δ 143.34, 133.76, 129.62, 127.48, 47.91, 25.14, 21.46.
Acknowledgments
The authors gratefully thank the Academic promotion programme of Shandong First Medical University (no. 2019LJ003) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05331.
Experimental procedures and spectral data (PDF)
Author Contributions
§ H.L., B.Z. and W.Z. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Huang Y.; Pi C.; Tang Z.; Wu Y.; Cui X. Cp*Co(III)-catalyzed C-H amidation of azines with dioxazolones. Chin. Chem. Lett. 2020, 31, 3237–3240. 10.1016/j.cclet.2020.08.046. [DOI] [Google Scholar]
- Feng Y.; Liu Y.; Fu Q.; Zou Z.; Shen J.; Cui X. Construction of diaminobenzoquinone imines via ferrocene-initiated radical reaction of benzoquinone with amines. Chin. Chem. Lett. 2020, 31, 733–735. 10.1016/j.cclet.2019.09.026. [DOI] [Google Scholar]
- Fu R.; Kou L.; Gao K.; Zhou S.; Bao X. Understanding mechanistic differences between 3-diazoindolin-2-imines and N-sulfonyl-1,2,3-triazoles in the Rh2(II)-catalyzed reactions with nitrosoarenes. Chin. J. Chem. 2021, 39, 1565–1572. 10.1002/cjoc.202100033. [DOI] [Google Scholar]
- a Scozzafava A.; Owa T.; Mastrolorenzo A.; Supuran C. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. 10.2174/0929867033457647. [DOI] [PubMed] [Google Scholar]; b Moeker J.; Peat T. S.; Bornaghi L. F.; Vullo D.; Supuran C. T.; Poulsen S.-A. Cyclic secondary sulfonamides: Unusually good inhibitors of cancer-related carbonic anhydrase enzymes. J. Med. Chem. 2014, 57, 3522–3531. 10.1021/jm500255y. [DOI] [PubMed] [Google Scholar]; c Bag S.; Tulsan R.; Sood A.; Cho H.; Redjeb H.; Zhou W.; LeVine H.; Török B.; Török M. Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015, 25, 626–630. 10.1016/j.bmcl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Apaydın S.; Török M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg. Med. Chem. Lett. 2019, 29, 2042–2050. 10.1016/j.bmcl.2019.06.041. [DOI] [PubMed] [Google Scholar]
- Ugwu D. I.; Okoro U. C.; Mishra N. K. Synthesis of proline derived benzenesulfonamides: A potent anti-Trypanosoma brucei gambiense agent. Eur. J. Med. Chem. 2018, 154, 110–116. 10.1016/j.ejmech.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Kulman D. G.; Lama R.; Li B.; Su B.. Evaluation of the selectivity of sulfonamide tubulin inhibitors against human African trypanosomiasis; Cleveland State University, 2017. Retrieved on 22nd February 22. www.csuohio.edu/sites/default/files/29-%202015.pdf.
- Mondal S.; Malakar S. Synthesis of sulfonamide and their synthetic and therapeutic applications: Recent advances. Tetrahedron 2020, 76, 131662. 10.1016/j.tet.2020.131662. [DOI] [Google Scholar]
- Rosen B. R.; Ruble J. C.; Beauchamp T. J.; Navarro A. Mild Pd-catalyzed N-arylation of methanesulfonamide and related nucleophiles: Avoiding potentially genotoxic reagents and byproducts. Org. Lett. 2011, 13, 2564–2567. 10.1021/ol200660s. [DOI] [PubMed] [Google Scholar]
- Shekhar S.; Dunn T. B.; Kotecki B. J.; Montavon D. K.; Cullen S. C. A general method for Palladium-catalyzed reactions of primary sulfonamides with aryl nonaflates. J. Org. Chem. 2011, 76, 4552–4563. 10.1021/jo200443u. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; You Y.; Dong W.; Peng Z.; Zhang Y.; An D. Copper-promoted desulfitative N-arylation of sulfonamides and sulfoximines with sodium arylsulfinates. J. Org. Chem. 2017, 82, 5810–5818. 10.1021/acs.joc.7b00633. [DOI] [PubMed] [Google Scholar]
- Liu T.-L.; Jhou M.-L.; Hsieh C.-E.; Lin C.-J.; Su H.-H.; Chou C.-M. Palladium-catalyzed intramolecular allylic amidation via decarboxylative aromatization: Synthesis of N-allyl-N-aryl sulfonamides. J. Org. Chem. 2021, 86, 9084–9095. 10.1021/acs.joc.1c01065. [DOI] [PubMed] [Google Scholar]
- Wen H.; Luo N.; Zhu Q.; Luo R. Amide Iridium complexes as catalysts for transfer hydrogenation reduction of N-sulfonylimine. J. Org. Chem. 2021, 86, 3850–3859. 10.1021/acs.joc.0c02680. [DOI] [PubMed] [Google Scholar]
- a Feng Y.; Zhang Z.; Fu Q.; Yao Q.; Huang H.; Shen J.; Cui X. Ir-catalyzed regiospecific mono-sulfamidation of arylquinazolinones. Chin. Chem. Lett. 2020, 31, 58–60. 10.1016/j.cclet.2019.05.013. [DOI] [Google Scholar]; b Zhu S.; Jin G.; He P.; Xu Y.; Huang Q. Unexpected formation of the novel fluorinated diazenes. Tetrahedron Lett. 2003, 44, 8717–8719. 10.1016/j.tetlet.2003.09.174. [DOI] [Google Scholar]; c Liskamp R. M.; Brouwer A. J.; Merkx R.; Dabrowska K.; Rijkers D. T. S. Synthesis and applications of β-aminoethanesulfonyl azides. Synthesis 2006, 3, 455–460. 10.1055/s-2006-926273. [DOI] [Google Scholar]; d Chow S. Y.; Stevens M. Y.; Odell L. R. Sulfonyl azides as precursors in ligand-free palladium-catalyzed synthesis of sulfonyl carbamates and sulfonyl ureas and synthesis of sulfonamides. J. Org. Chem. 2016, 81, 2681–2691. 10.1021/acs.joc.5b02755. [DOI] [PubMed] [Google Scholar]
- Paz N. R.; Rodríguez-Sosa D.; Valdés H.; Marticorena R.; Melián D.; Copano M. B.; González C. C.; Herrera A. J. Chemoselective intramolecular functionalization of methyl groups in nonconstrained molecules promoted by N-Iodosulfonamides. Org. Lett. 2015, 17, 2370–2373. 10.1021/acs.orglett.5b00866. [DOI] [PubMed] [Google Scholar]
- Bosnidou A. E.; Muñiz K. Intermolecular radical C(sp3)-H amination under Iodine catalysis. Angew. Chem., Int. Ed. 2019, 58, 7485–7489. 10.1002/anie.201901673. [DOI] [PubMed] [Google Scholar]
- Wu F.; Ariyarathna J. P.; Kaur N.; Alom N.-E.; Kennell M. L.; Bassiouni O. H.; Li W. Halogen-bond-induced consecutive Csp3-H aminations via hydrogen atom transfer relay strategy. Org. Lett. 2020, 22, 2135–2140. 10.1021/acs.orglett.0c00081. [DOI] [PubMed] [Google Scholar]
- Gang F.-l.; Zhu F.; Li X.-t.; Wei J.-l.; Wu W.-j.; Zhang J.-w. Synthesis and bioactivities evaluation of L-pyroglutamic acid analogues from natural product lead. Bioorg. Med. Chem. 2018, 26, 4644–4649. 10.1016/j.bmc.2018.07.041. [DOI] [PubMed] [Google Scholar]
- Rigo B.; El Ghammarti S.; Gautret P.; Couturier D. Studies on pyrrolidinones. An improved synthesis of pyroglutamoyl chloride. Synth. Commun. 1994, 24, 2597–2607. 10.1080/00397919408010572. [DOI] [Google Scholar]
- Hughes D. L.; Reamer R. A.; Bergan J. J.; Grabowski E. J. J. A mechanistic study of the Mitsunobu esterification reaction. J. Am. Chem. Soc. 1988, 110, 6487–6491. 10.1021/ja00227a032. [DOI] [Google Scholar]
- Liu H.; Pang Z.; Hao L.; Sun J.; Zhang Z.; Wen F.; Xia C. Sulfonylimination of proline with sulfonylazides involving aldehyde-induced decarboxylation coupling. Org. Lett. 2021, 23, 1234–1238. 10.1021/acs.orglett.0c04187. [DOI] [PubMed] [Google Scholar]
- Sun J.; Sun H.; Hao L.; Liu H.; Zhang Z.; Wen F.; Li H.; Duan G.; You G.; Xia C. Metal-free synthesis of pyrrole-imidazole alkaloids via a tandem C-N, C-C coupling protocol. Adv. Synth. Catal. 2021, 363, 1882–1886. 10.1002/adsc.202001441. [DOI] [Google Scholar]
- Hao L.; Wang G.; Sun J.; Xu J.; Li H.; Duan G.; Xia C.; Zhang P. From phenylhydrazone to 1H-1, 2, 4-triazoles via nitrification, reduction and cyclization. Adv. Synth. Catal. 2020, 362, 1657–1662. 10.1002/adsc.201901563. [DOI] [Google Scholar]
- Hao L.; Liu H.; Zhang Z.; Wen F.; Xia C.; Pang Z. Cascade reaction to 1H-pyrazoles from hydrazones via sodium nitrite promoted dual C–C/C–N formation, annulation and aromatization with 1, 2-dichloroethane. Chin. Chem. Lett. 2021, 32, 2309–2312. 10.1016/j.cclet.2021.02.025. [DOI] [Google Scholar]
- Xia C.; Wei Z.; Yang Y.; Yu W.; Liao H.; Shen C.; Zhang P. Palladium-catalyzed thioetherification of quinolone derivatives via decarboxylative C–S cross-couplings. Chem.—Asian J. 2016, 11, 360–366. 10.1002/asia.201500808. [DOI] [PubMed] [Google Scholar]
- Cai J.; Chen S.; Zhang W.; Wei Y.; Lu J.; Xing J.; Dong Y. Proteomic analysis of differentially expressed proteins in 5-fluorouracil-treated human breast cancer MCF-7 cells. Clin. Transl. Oncol. 2014, 16, 650–659. 10.1007/s12094-013-1127-9. [DOI] [PubMed] [Google Scholar]
- Ono N.; Yamada T.; Saito T.; Tanaka K.; Kaji A. A convenient procedure for esterification of carboxylic acids. Bull. Chem. Soc. 1978, 51, 2401–2404. 10.1246/bcsj.51.2401. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.