Abstract
Background and aims
We highlight the peripheral neurologic complications of coronavirus disease 2019 (COVID-19) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an ongoing global health emergency.
Methods
We evaluated twenty-five patients admitted to the COVID-19 Recovery Unit (CRU) at New York-Presbyterian Weill Cornell University Medical Center after intensive care hospitalization with confirmed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), whom neurology was consulted for weakness and/or paresthesias. All patients were clinically evaluated by a neuromuscular neurologist who performed electrodiagnostic (EDX) studies when indicated. Magnetic resonance imaging (MRI) of the affected regions, along with nerve and muscle biopsies were obtained in select patients to better elucidate the underlying diagnosis.
Results
We found fourteen out of twenty-five patients with prolonged hospitalization for COVID-19 infection to have peripheral neurological complications, identified as plexopathies, peripheral neuropathies and entrapment neuropathies. The other eleven patients were not found to have peripheral neurologic causes for their symptoms. Patients with peripheral neurological complications often exhibited more than one type of concurrently. Specifically, there were four cases of plexopathies, nine cases of entrapment neuropathies, and six cases of peripheral neuropathies, which included cranial neuropathy, sciatic neuropathy, and multiple mononeuropathies.
Conclusions
We explore the possibility that the idiopathic peripheral neurologic complications could be manifestations of the COVID-19 disease spectrum, possibly resulting from micro-thrombotic induced nerve ischemia.
Keywords: COVID-19, SARS-CoV-2, Brachial plexopathy, Peripheral neuropathy, Entrapment neuropathy, RA02
1. Introduction
Many neurological complications of the coronavirus disease 2019 (COVID-19) pandemic, such as encephalitis, ischemic stroke, and acute inflammatory demyelinating polyneuropathy (AIDP) have been documented in the literature [1,2]. However, the full spectrum of peripheral neurological complications of COVID-19 and risk factors have not been well described. Here we present a single center experience as a case series of peripheral neurological complications observed in patients hospitalized with COVID-19.
2. Materials and methods
From March to June of two thousand twenty-one, the neurology service evaluated twenty-five patients admitted to the COVID-19 Recovery Unit (CRU) at New York-Presbyterian Weill Cornell University Medical Center after intensive care hospitalization with confirmed severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), whom neurology was consulted for weakness and/or paresthesias. All patients were clinically evaluated by a neuromuscular neurologist who performed electrodiagnostic (EDX) studies when indicated. Magnetic resonance imaging (MRI) of the affected regions, along with nerve and muscle biopsies were obtained in select patients to better elucidate the underlying diagnosis.
3. Results
Table 1 describes key demographic patient data, comorbidities, inpatient complications, and relevant peak lab values organized by type of peripheral neurological complication. Table 2 summarizes results EDX studies, MRIs, and muscle and nerve biopsies by peripheral neurologic complication diagnosis. There were ultimately fourteen patients that were found to have peripheral neurological complications from the original twenty-five patients we were asked to evaluate (the remaining eleven were not found to have peripheral causes for their symptoms).
Table 1.
Demographics, characteristics, and peripheral neurological complication
| Total patients (14) | Peripheral neurological complication |
|||
|---|---|---|---|---|
| Plexopathy (n = 4) | Peripheral neuropathy (n = 6) | Entrapment neuropathy (n = 10) | ||
| Median age, range (years) | 57, 33–82 | 54, 43–66 | 51, 33–71 | 56, 33–82 |
| Male no. (%) | 14 (100) | 4 (100) | 6 (100) | 9 (100) |
| White no. (%) | 6 (43) | 1 (25) | 4 (67) | 3 (33) |
| Hispanic/Latino no. (%) | 6 (43) | 3 (75) | 2 (33) | 4 (44) |
| Asian no. (%) | 2 (14) | 0 (0) | 0 (0) | 2 (22) |
| COVID-19 status no. (%) | 13 (93) | 4 (100) | 5 (83) | 9 (100) |
| Diabetes mellitus no. (%) | 6 (43) | 1 (25) | 1 (17) | 6 (67) |
| Obesity no. (%) | 4 (29) | 1 (25) | 1 (17) | 4 (44) |
| Intubation for COVID-19 no. (%) | 14 (100) | 4 (100) | 6 (100) | 9 (100) |
| Paralysis during hospitalization no. (%) | 6 (43) | 2 (50) | 4 (67) | 3 (33) |
| Pronation for ARDS no. (%) | 4 (29) | 2 (50) | 2 (33) | 3 (33) |
| Hydroxychloroquine no. (%) | 13 (93) | 4 (100) | 6 (100) | 8 (89) |
| Corticosteroids use no. (%) | 6 (43) | 1 (25) | 4 (67) | 3 (33) |
| Remedesivir no. (%) | 4 (29) | 0 (0) | 3 (50) | 2 (22) |
| Rhabdomyolysis no. (%) | 1 (7) | 1 (25) | 1 (17) | 1 (11) |
| Cutaneous rash no. (%) | 2 (14) | 1 (25) | 1 (17) | 1 (11) |
| VTE no. (%) | 5 (36) | 2 (50) | 2 (33) | 3 (33) |
| RRT no. (%) | 5 (36) | 2 (50) | 2 (33) | 3 (33) |
| Vasopressor support no. (%) | 11 (79) | 4 (100) | 5 (83) | 7 (78) |
| Bacteremia no. (%) | 7 (50) | 3 (75) | 3 (50) | 5 (56) |
| COVID-19 diagnosis to neurological symptoms onset (days) | 35 | 37 | 33 | 35 |
| Length of hospitalization (days) | 52 | 53 | 53 | 48 |
| Length of rehabilitation (days) | 22 | 28 | 24 | 23 |
| Average peak values | ||||
| CPK (U/L) | 1958 | 2517 | 2461 | 2710 |
| D-dimer (ng/mL) | 4916 | 4820 | 6427 | 3255 |
| CRP (mg/dL) | 128 | 93 | 126 | 119 |
| ESR (mm/h) | 106 | 101 | 113 | 100 |
| Ferritin (ng/mL) | 2358 | 1875 | 2794 | 2467 |
Abbreviations: n = total cases of neuropathy in each category. ARDS: acute respiratory distress syndrome. VTE: venous thromboembolism. RRT: renal replacement therapy. CPK: creatine phosphokinase. CRP: C-reactive protein. ESR: erythrocyte sedimentation rate.
Table 2.
Clinical summary
| Patient no. | Peripheral neurologic complication | Electrodiagnostic studies | Muscle & nerve biopsies |
|---|---|---|---|
| Magnetic resonance imaging | |||
| 1 | Brachial plexopathy | L. brachial plexopathy with sparing of axillary n. and suprascapular n. fibers | Not done |
| L. brachial plexus showed asymmetric T2-hyperintensity of plexus trunks, divisions, and cords | |||
| 2 | Brachial plexopathy | L. brachial plexopathy with sparing of axillary n. fibers | Not done |
| Diffuse L. brachial plexitis with serratus anterior muscle T2-hyperintense signal and enhancement reflecting acute denervation edema | |||
| 3 | Brachial plexopathy | Normal | Not done |
| L. brachial plexus showed increased T2 signal of the left C5, C6 and C7 nerve roots extending to the upper and middle trunks | |||
| 4 | Peripheral neuropathy | Bilateral sciatic neuropathies | L. PB. m biopsy: severe myofiber atrophy, rare myonecrosis |
| Brachial plexopathy | Bilateral upper trunk brachial plexopathies | L. sural n. biopsy: severe, active axonal loss | |
| Entrapment neuropathy | Triceps-sparing L. radial neuropathy | Bilateral brachial plexi showed increased T2 signal in the C5/C6 roots | |
| 5 | Peripheral neuropathy | Multiple mononeuropathies: R. radial n., musculocutaneous n., median n. | Not done |
| Normal MRI of the R. brachial plexus | |||
| 6 | Peripheral neuropathy | Bilateral sciatic neuropathies | R. PB m. biopsy: moderate m. atrophy |
| Entrapment neuropathy | Bilateral cubital tunnel syndromes; R. PIN entrapment |
R. medial antebrachial cutaneous n. biopsy: myelinated axon loss with numerous phagocytosing macrophages within the endoneurium | |
| MRI R. forearm showed T2-hyperintense posterior interosseous nerve in the Arcade of Frohse | |||
| 7 | Peripheral neuropathy | L. sciatic neuropathy | Not done |
| MRI L. lumbosacral plexus without contrast showed an enlarged, edematous L. sciatic nerve behind the L. greater trochanter, distal to the sciatic notch | |||
| 8 | Peripheral neuropathy | Bilateral sciatic neuropathies | R. SPN biopsy: severe axonal neuropathy without vasculitis or thrombosis |
| R. PB m. biopsy: extensive m. atrophy | |||
| MRI not done (normal CT pelvis without contrast) | |||
| 9 | Peripheral neuropathy | Complete R. facial axonotmesis | Not done |
| MRI brain shows long segment enhancement of the R. facial nerve from the fundus of the intra-canalicular segment through the mastoid | |||
| 10 | Entrapment neuropathy | Not done; clinical diagnosis of bilateral common peroneal entrapment neuropathies at the fibular heads | Not done |
| Not done | |||
| 11 | Entrapment neuropathy | Bilateral common peroneal (axonotmetic) neuropathies at the fibular head | Not done |
| Not done | |||
| 12 | Entrapment neuropathies | L. common peroneal neuropathy at fibular head; R. median neuropathy at the wrist | Not done |
| Not done | |||
| 13 | Entrapment neuropathy | R. ulnar neuropathy at cubital tunnel | Not done |
| Not done | |||
| 14 | Entrapment neuropathies | R. ulnar neuropathy at Guyon's canal; L. common peroneal neuropathy at the fibular head | Not done |
| MRI R. wrist showed R. ulnar nerve enlargement and increased signal in Guyon's canal |
Abbreviations: L = left, R = right, PB = peroneus brevis muscle, m. biopsy = muscle biopsy, SPN = superficial peroneal nerve, n. biopsy = nerve biopsy, PIN = posterior interosseous nerve.
Of the four patients with sciatic neuropathies, one with bilateral sciatic neuropathies was explained by rhabdomyolysis induced bilateral gluteal compartment syndromes, with a peak creatine phosphokinase levels of greater than 80,000 (U/L), and edema with enhancement of the bilateral quadratus femoris muscles on pelvic MRI. Peroneus brevis muscle biopsy demonstrated severe myofiber atrophy, multifocal myofibrillar derangement and rare myonecrosis. There have been several cases of rhabdomyolysis complicating COVID-19 infection, including one as a presenting symptom [3], and the other as a later complication [4]. Neither of these cases had muscle biopsy performed. Of the four patients with brachial plexopathy, one had been managed with respiratory proning therapy while intubated and sedated who rapidly recovered indicating a compressive neurapraxic plexopathy caused by shoulder hyperabduction during proning, a well described complication of proning therapy in critically ill patients, before and after the advent of COVID-9 [5,6]. None of the remaining three patients with brachial plexopathies, one of whom underwent proning, nor the remaining three patients with bilateral sciatic neuropathies and the patient with upper extremity mononeuritis multiplex experienced any recovery during their hospitalization. MRI findings of these idiopathic brachial plexopathies and sciatic neuropathies are shown in Fig. 1 .
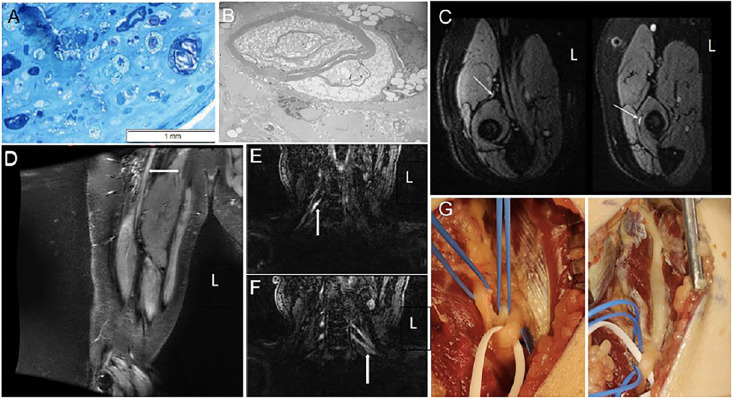
Fig. 1.
Nerve biopsy, MRI findings, and intraoperative photos.
Nerve biopsy, MRI findings, and intraoperative photos: (A) Epoxy resin section of the nerve, magnification x400. Axonal loss associated with numerous macrophages engulfing degenerating nerve fibers. (B) Electron microscopy, original magnification x5000. A macrophage appears to be stripping myelin sheath. (C) MRI FLAIR: normal intensity of the posterior interosseous branch of the radial nerve prior to the supinator tunnel, left, and increased intensity within the supinator tunnel, right. (D) MRI neurogram demonstrates enlargement and increased T2 hyperintensity of right sciatic nerve (white arrow). (E, F) MRI neurogram demonstrates increased T2 hyperintensity seen in the roots of the brachial plexus bilaterally (white arrows) in the same patient. (G) Intraoperative photos pre- (left) and post-surgical decompression (right) of the posterior interosseous branch of the radial nerve at the Arcade of Frohse.
Abbreviations: FLAIR - fluid-attenuated inversion recovery
4. Discussion
Of the ten patients with entrapment neuropathies, one was found to have posterior interosseous nerve entrapment (PIN) at the arcade of Frohse in the supinator channel, which has not been described as a complication of prolonged immobilization due to sedation [7]. The patient underwent right posterior interosseous neurolysis with biopsy of the right medial antebrachial cutaneous nerve, due to clinical numbness in this nerve's territory with an absent right medial antebrachial cutaneous sensory response. The involvement of this nerve along with the posterior interosseous does suggest possible neuralgic amyotrophy or Parsonage Turner syndrome which has been described as a cause of posterior interosseous neuropathy [8]. There have been several cases of Parsonage Turner syndrome, including one that was pure sensory, associated with COVID-19 infection [9]. The medial antebrachial cutaneous biopsy was done to clarify the underlying neuropathology and etiology of this unusual neuropathy in this critically ill patient with COVID-19 infection. It revealed significant axonal loss and numerous phagocytosing macrophages present within the endoneurium. Electron microscopy showed remaining myelinated fibers were entirely or partially engulfed by macrophages, with some macrophages appearing to penetrate loosened myelin loops, occasionally exhibiting features of vesicular degeneration. Naked axons were also present in the widened and edematous endoneurium. Fig. 1 shows these nerve biopsy findings and intraoperative photographs of pre- and post-release of the posterior interosseous branch of the radial nerve at the Arcade of Frohse. We felt these biopsy findings were suggestive of microvascular thrombotic ischemia, however the findings were not robust.
We observed ten patients to have typical peripheral neurological complications of prolonged hospitalization [10], including entrapment neuropathies of the median, ulnar, radial and peroneal nerves. None of our patients had critical illness polyneuropathy or critical illness myopathy. Of note, five patients had systemic thrombotic complications, including deep venous thrombosis and pulmonary embolism. Two patients were diagnosed with COVID-19 cutaneous rashes, which have been suggested to be complement-mediated and caused by microscopic angiopathy from an underlying systemic process [11]. There was no clear correlation between the systemic thrombotic complications and a specific peripheral neurologic complication. No patient had premorbid plexopathy, peripheral neuropathy, or entrapment neuropathy to confound our observations.
Certain peripheral neurologic complications secondary to prolonged hospitalizations and ICU care are expected in patients with COVID-19, namely median, ulnar and peroneal entrapment neuropathies, critical illness polyneuropathy and critical illness myopathy [11]. The underlying cause may be lack of frequent position changes while hospitalized, such as prolonged extension of the knee for peroneal nerve injury and prolonged flexion of the elbow for cubital tunnel syndrome. These entrapment neuropathies are preventable and may be under-recognized. Preventative measures such as avoiding shoulder hyperabduction in prone patients, wedge pillows to maintain some knee flexion, extension bracing and padding at the elbows to prevent ulnar neurapraxias, with frequent physical therapy using passive range of motion, are crucial adjunctive therapies in COVID-19 patients to reduce the risk of developing peripheral neurological complications from prolonged hospitalization.
We also found patients with peripheral neurological complications that are not typical of prolonged hospitalization, specifically three cases of bilateral sciatic neuropathies, three patients with brachial plexopathies, and one ipsilateral median, musculocutaneous and radial mononeuritis multiplex patient. One possible mechanism for these findings may be the result of uncontrolled systemic inflammation triggering microthrombotic angiopathy involving the vasa nervorum. In support of this, we found complete clinical and electromyographic sparing of axillary and infraspinatus fascicles in two cases of otherwise severe brachial panplexopathies that showed complete clinical and electromyographic denervation of the musculocutaneous and radial fascicles in the upper trunks that anatomically carry these fascicles all together, suggesting a patchy microvascular injury to the epineural vasa nervorum supplying individual nerve fascicles of the upper brachial plexus trunks in these two patients. Coronaviruses such as severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) have previously been postulated to cause muscle and nerve injury through multiple mechanisms, including direct neuronal invasion, systemic inflammation and prolonged illness [12,13]. However, we did not find supporting pathological evidence for this on nerve biopsies, albeit from superficial peroneal nerves far distal from the injured sciatic nerves.
Direct neuronal invasion, complement-mediated inflammation, and endothelial dysfunction have been proposed as the underlying mechanisms for COVID-19 neurological injury [1,2,13,14]. Viral entry into the central nervous system (CNS) via angiotensin-converting enzyme-2 (ACE2) receptors has been suggested to cause CNS pathologies such as ischemic strokes and encephalitis [14]. It is possible that SARS-CoV-2 may disrupt endothelial cell function in a similar ACE2 receptor-mediated mechanism involving the blood-nerve barrier, which could trigger the release of thrombogenic ultra-large von Willebrand factor (ULVWF) multimers and cause micro-thrombotic injury in the peripheral nerves. Under normal conditions, ULVWF multimers are cleaved by the protease ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) to prevent microthrombi formation [15]. COVID-19 patients have also been found to have moderately reduced serum level of ADAMTS-13, and subsequent excessive microthrombi in vasa nervorum could cause nerve ischemia and fascicular infarction in the setting of a refractory hypercoagulable state, not fully mitigated by current anti- inflammatory, anticoagulation, and antithrombotic therapies [15,16]. Alternative potential therapies could include monoclonal antibodies which inhibit complement activation, and plasmapheresis, which has been shown to reduce von Willebrand Factor concentration in plasma [16], potentially reducing the burden of micro-thrombotic injury in COVID-19.
Limitations of our observations include our small sample size and all male patient population. Furthermore, nerve biopsies could not be performed on the sciatic nerve or brachial plexus. Given the recent nature of the pandemic, we are likely only seeing glimpses of the long-term complications from COVID-19. Larger observational and longitudinal studies following the course and recovery of COVID-19 patients are crucial for better understanding of the underlying disease mechanisms.
5. Conclusions
We present the main clinical and laboratory findings of patients with COVID-19 in our single institution who were found to have peripheral neurological complications. We further explore the possibility that these could be manifestations of the COVID-19 disease spectrum, hypothesizing that they could be related to micro-thrombotic induced nerve ischemia.
Financial disclosures
None of the authors have conflicts of interest to disclose.
Study funding
No targeted funding reported.
Publication history
The manuscript is not under publication consideration elsewhere.
Ethical publication statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consents
Verbal informed consents were obtained from all patients at the initial clinical encounter. Institutional review board approval was obtained for this study.
Acknowledgements
We acknowledge Dr. Matthew Fink for his critical review.
Appendix A. Abbreviations
| COVID-19 | coronavirus disease 2019 |
| AIDP | acute inflammatory demyelinating polyneuropathy |
| CRU | COVID-19 Recovery Unit |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus-2 |
| EDX | electrodiagnostic |
| CIM | critical illness myopathies |
| CIPN | critical illness polyneuropathy |
| MRI | magnetic resonance imaging |
| SARS | severe acute respiratory syndrome |
| MERS | Middle East respiratory syndrome |
| CNS | central nervous system |
| ACE2 | angiotensin-converting enzyme-2 |
| ULVWF | ultra-large von Willebrand factor |
| ADAMST13 | a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 |
| CT | computerized tomography |
| L | left |
| R | right |
| m. biopsy | muscle biopsy |
| n. biopsy | nerve biopsy |
References
- 1.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain-Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020 Jun 25;382(26):2574–2576. doi: 10.1056/NEJMc2009191. 32302082 Epub 2020 Apr 17. PMC7182017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suwanwongse K., Shabarek N. Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus. 2020;12(4):e7561. doi: 10.7759/cureus.7561. 32382463 Apr 6. PMC7202588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg. Infect. Dis. 2020 Jul;26(7):1618–1620. doi: 10.3201/eid2607.200445. 32197060 Epub 2020 Jun 21. PMC7323559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez-Soblechero A., García C.A., Sáez Ansotegui A., Fernández-Lorente J., Catalina-Álvarez I., Grandas F., Muñoz-Blanco J.L. Upper trunk brachial plexopathy as a consequence of prone positioning due to SARS-CoV-2 acute respiratory distress syndrome. Muscle Nerve. 2020 Nov;62(5):E76–E78. doi: 10.1002/mus.27055. 32875575 Epub 2020 Sep 10. [DOI] [PubMed] [Google Scholar]
- 6.O’Sullivan Joel, Miller Caroline, Jeffrey Jack, Power Dominic. Brachial plexus neuropathies during the COVID-19 pandemic: a retrospective case series of 15 patients in critical care. Phys. Ther. 2021;101(1):1–8. doi: 10.1093/ptj/pzaa191. 2021-01-04, Vol.101 (1). Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang Alan C., Rodner Craig M. Unusual compression neuropathies of the forearm, part I: radial nerve. J. Hand Surg. (American Ed.) 2009:1906–1914. doi: 10.1016/j.jhsa.2009.10.016. 34.10. [DOI] [PubMed] [Google Scholar]
- 8.Akane Mao, Iwatsuki Katsuyuki, Tatebe Masahiro, Nishizuka Takanobu, Kurimoto Shigeru, Yamamoto Michiro, Hirata Hitoshi. Anterior interosseous nerve and posterior interosseous nerve involvement in neuralgic amyotrophy. Clin. Neurol. Neurosurg. 2016;151:108–112. doi: 10.1016/j.clineuro.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Cacciavillani Mario, Salvalaggio Alessandro, Briani Chiara. Pure sensory neuralgic amyotrophy in COVID-19 infection. Muscle Nerve. 2021;63(1):E7–E8. doi: 10.1002/mus.27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. J. Lab. Clin. Med. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobson-Webb Lisa D., Juel Vern C. Common entrapment neuropathies. CONTINUUM: Lifelong Learn. Neurol. 2017;23(2):487–511. doi: 10.1212/CON.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 12.Tsai L.-K., Hsieh S.-T., Chao C.-C., et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 13.Arabi Y.M., Harthi A., Hussein J., et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 15.Morici N., Bottiroli M., Fumagalli R., Marini C., Cattaneo M. Role of von Willebrand factor and ADAMTS-13 in the pathogenesis of thrombi in SARS-CoV-2 infection: time to rethink. Thromb. Haemost. 2020:20–23. doi: 10.1055/s-0040-1713400. [DOI] [PubMed] [Google Scholar]
- 16.Zachariah U., Nair S.C., Goel A., et al. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: consider low volume plasma exchange and low dose steroid. Thromb. Res. 2020;192:2. doi: 10.1016/j.thromres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]