Abstract
Background & Aims
Studies have shown decreased response to coronavirus disease 2019 (COVID-19) vaccinations in some populations. In addition, it is possible that vaccine-triggered immune activation could trigger immune dysregulation and thus exacerbate inflammatory bowel diseases (IBD). In this population-based study we used the epi-Israeli IBD Research Nucleus validated cohort to explore the effectiveness of COVID-19 vaccination in IBD and to assess its effect on disease outcomes.
Methods
We included all IBD patients insured in 2 of the 4 Israeli health maintenance organizations, covering 35% of the population. Patients receiving 2 Pfizer-BioNTech BNT162b2 vaccine doses between December 2020 and June 2021 were individually matched to non-IBD controls. To assess IBD outcomes, we matched vaccinated to unvaccinated IBD patients, and response was analyzed per medical treatment.
Results
In total, 12,109 IBD patients received 2 vaccine doses, of whom 4946 were matched to non-IBD controls (mean age, 51 ± 16 years; median follow-up, 22 weeks; interquartile range, 4–24). Fifteen patients in each group (0.3%) developed COVID-19 after vaccination (odds ratio, 1; 95% confidence interval, 0.49–2.05; P = 1.0). Patients on tumor necrosis factor (TNF) inhibitors and/or corticosteroids did not have a higher incidence of infection. To explore IBD outcomes, 707 vaccinated IBD patients were compared with unvaccinated IBD patients by stringent matching (median follow-up, 14 weeks; interquartile range, 2.3–20.4). The risk of exacerbation was 29% in the vaccinated patients compared with 26% in unvaccinated patients (P = .3).
Conclusions
COVID-19 vaccine effectiveness in IBD patients is comparable with that in non-IBD controls and is not influenced by treatment with TNF inhibitors or corticosteroids. The IBD exacerbation rate did not differ between vaccinated and unvaccinated patients.
Keywords: SARS-CoV-2, Vaccination, Crohn’s Disease, Ulcerative Colitis
Abbreviations used in this paper: CD, Crohn’s disease; CI, confidence interval; COVID-19, coronavirus disease 2019; epi-IIRN, epi-Israeli IBD Research Nucleus; HMO, health maintenance organization; IBD, inflammatory bowel disease; IQR, interquartile range; OR, odds ratio; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SMD, standardized mean difference; TNF, tumor necrosis factor; UC, ulcerative colitis
What You Need to Know.
Background
Although Pfizer COVID-19 vaccine is extremely effective at preventing infection, questions have arisen regarding its effectiveness in inflammatory bowel disease (IBD) patients on immunosuppressive medications. In addition, its effect on IBD outcomes has not been assessed.
Findings
In a large population-based study, Pfizer COVID-19 vaccine was equally effective at preventing infection in IBD patients, including those on immunosuppressive medication, as in non-IBD subjects. Vaccinated IBD patients had no more disease exacerbations after vaccination than unvaccinated IBD patients.
Implications for patient care
The study demonstrates that the Pfizer COVID-19 vaccine provided excellent protection for IBD patients on immunosuppression for as long as 22 weeks, and that no worsening of IBD outcomes occurred after vaccination.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus has caused more than 200 million confirmed cases of coronavirus disease 2019 (COVID-19) globally as of mid-2021 and more than 4.3 million deaths.1 Mass vaccination is the most effective strategy for managing the pandemic. Various factors may interfere with host response to vaccination and potentially compromise vaccine effectiveness, including advanced age2 and various types of immune suppression such as immunosuppressive medications.3 Indeed, decreased seroconversion rates to vaccines other than COVID-19 have been demonstrated in inflammatory bowel disease (IBD) patients treated with tumor necrosis factor (TNF) inhibitors.4, 5, 6 Recent reports have suggested impaired serologic response to COVID-19 infection in patients treated with TNF inhibitors and immunomodulators7; serologic response to vaccination has also been found to be impaired.8 , 9 However, because most of these patients do seroconvert,10 , 11 it is unclear whether this translates into higher infection rates.8 , 12 , 13 Accordingly, concerns have been expressed as to whether the new COVID-19 vaccines are as effective in IBD patients, especially in those treated with immunosuppressive medications. One real-world study from Israel14 and one prospective cohort study9 have suggested similar effectiveness of the COVID-19 vaccine as in non-IBD subjects, but follow-up in these studies was short.
An additional concern is that immune activation due to COVID-19 vaccination would trigger IBD exacerbation. It is theoretically plausible that the immune activation initiated by COVID-19 vaccination might trigger IBD exacerbations through an immune-mediated dysregulation of the mucosal immune system. The effect of COVID-19 vaccination on IBD activity has only been assessed thus far for short follow-up periods (up to 4 weeks).9 , 12 For routinely administered vaccines other than COVID-19, no vaccine has yet been demonstrated to cause IBD flares, but there are no controlled studies specifically exploring their effect on disease outcomes in IBD.
In the present population-based controlled study, we aimed to explore both the effectiveness of COVID-19 vaccination in preventing SARS-CoV-2 infection, specifically in patients treated with TNF inhibitors and corticosteroids, and its effect on IBD course.
Methods
For this study, we used the administrative database of the validated Israeli IBD Research Nucleus (epi-IIRN) cohort. The epi-IIRN includes all IBD patients in Israel, identified by validated case ascertainment algorithms, with 3 non-IBD controls (identified by the algorithms as not having IBD) matched to each patient on the basis of age, sex, jurisdiction, and health maintenance organization (HMO). The previously published development and validation process of the case ascertainment algorithms to identify patients with IBD within the HMOs showed high accuracy (99% specificity, 89% sensitivity, 92% positive predictive value, and 99% negative predictive value).15 , 16
We included subjects from 2 of the 4 national HMOs, covering 35% of the Israeli population. The HMOs are fully computerized and maintain electronic records on all health contacts, diagnoses (International Classification of Diseases, Ninth Revision), medications, laboratory test results, and utilization of other ambulatory health services, linked online to a central server since 2000–2003 (depending on the HMO). Medication data are accurate because the Israeli health care system provides the drugs via the HMOs while covering their costs. SARS-CoV-2 polymerase chain reaction (PCR) data are extremely accurate, because PCR results in Israel have been universally recorded in the HMO electronic health records since the start of the pandemic. During the COVID-19 pandemic, the Israeli Ministry of Health required central online daily registration of COVID-19 vaccination and SARS-CoV-2 PCR results, enabling highly accurate assessment of vaccination impact.17
On December 11, 2020, the U.S. Food and Drug Administration issued an emergency use authorization for the Pfizer-BioNTech BNT162b2 COVID-19 vaccine. The vaccination program began in Israel toward the end of December 2020, and by June 2021, 56% of Israeli residents had received 2 vaccine doses.17 The follow-up period for the present study was thus from December 1, 2020 to June 30, 2021. During this period, the only available vaccine in Israel was the Pfizer-BioNtech BNT162b2 vaccine. The unparalleled rapidity of the Israeli vaccination campaign and use of only one vaccine brand, alongside the epi-IIRN validated national longitudinal IBD database, offer a unique opportunity to explore the effects of vaccination on a large IBD population-based cohort with exact matching.
A portion of our data was included in the previous study by Ben-Tov et al14 on vaccine effectiveness in IBD patients. Here, we added a second HMO, used more stringent matching, lengthened the follow-up period, and, most importantly, explored the novel question of whether the vaccine influences IBD activity.
COVID-19 Vaccine Effectiveness
For this analysis, we excluded subjects who had confirmed SARS-CoV-2 infection or positive serology at any time before the second vaccine and those who had received only 1 vaccine dose. Each vaccinated IBD patient was individually matched to a vaccinated non-IBD subject by using the following variables: year of birth, sex, jurisdiction of residence, HMO, and dates of the first vaccination with a caliper of ±3 days.
A biased higher or lower response to vaccination may be a result of a different background infection rate between individuals with and without IBD. To explore this potential bias, we individually matched each unvaccinated IBD patient to a non-IBD unvaccinated control by year of birth, sex, jurisdiction of residence, and HMO. Comorbidities that according to the U.S. Centers for Disease Control and Prevention may impact COVID-19 severity (Appendix 1) were compared between the matched groups to ensure balanced distribution.
To assess the influence of immunosuppressive medications on vaccine effectiveness, we performed a subanalysis using propensity score matching to compare the SARS-CoV-2 infection rate among IBD patients treated with TNF inhibitors alone (infliximab, adalimumab, golimumab, certolizumab pegol), systemic corticosteroids alone, or combined TNF inhibitors and steroids at the time of vaccination with (1) all other IBD patients and with (2) patients treated with other biologics or small molecules (vedolizumab, ustekinumab, tofacitinib). To calculate propensity scores for both comparisons, a logistic regression model was applied, and matching was performed using the nearest neighbor with a caliper of .1. Variables included in the propensity score model were IBD subtype (Crohn’s disease [CD]/ulcerative colitis [UC]), age at diagnosis, year of birth, sex, preexisting conditions score, HMO, jurisdiction of residence, and time of the first and second vaccines (with a caliper of ±3 days). As an additional exact matching variable designed to correct for disease severity, we formed severity subgroups through hierarchical clustering machine-learning methods based on the patients’ blood work (hemoglobin, C-reactive protein, erythrocyte sedimentation rate, albumin, platelets, white blood cell count) performed during the preceding year (Supplementary Table 1, Appendix 3). These subgroups categorize patients with heterogeneous available data to assist in accounting for disease severity.
Inflammatory Bowel Disease Activity After Vaccination
To explore the impact of vaccination on IBD disease course, we matched vaccinated (2 doses) to unvaccinated IBD patients by sex, jurisdiction of residence, IBD type (CD or UC), disease severity clusters based on blood work (as defined above), number of disease flares during the preceding 2 years (defined as any event of medication escalation, all-cause hospitalization, or steroid use), and age at IBD diagnosis (with a caliper of ±1 year) (Supplementary Table 1, Appendix 2). We excluded subjects who had confirmed SARS-CoV-2 infection or positive serology at any time before the second vaccine and, in matched controls, before the vaccinated date of the matched patient.
To further reduce the possibility of a confounding effect stemming from disease severity, the interval from the most recent flare to the first vaccine was used as an additional matching variable. The duration of follow-up of each IBD vaccinated–IBD unvaccinated pair was from the date of the second vaccination of the case until the earliest of the following: vaccination date of the unvaccinated individual, SARS-CoV-2 positivity of any one of the pair, death, or June 30, 2021. In the event that a control was vaccinated, he/she was converted to a case and was rematched accordingly.
The outcome of IBD exacerbation was defined as treatment escalation, commencement of corticosteroids or enema, or hospitalization (Appendix 2). In addition, a sensitivity analysis was performed using a narrow definition of commencement of corticosteroids only.
Statistics
Variables are presented as mean ± standard deviation or median (interquartile range [IQR]) for continuous and categorical variables, respectively. Comparisons between groups were made by Student t test, Wilcoxon rank-sum test, one-way analysis of variance, and χ2, as appropriate. Because of the large sample size, P values are presented along with standardized mean differences (SMDs), in which a SMD greater than 0.1 was considered meaningful. Odds ratios (ORs) were calculated using the Haldane-Anscombe correction to express the association between the exposure (eg, IBD patients vs non-IBD patients) and the outcome (eg, positive PCR test). Times to positive SARS-CoV-2 PCR test and to IBD flares are presented using Kaplan-Meier survival curves and compared using the log-rank test with robust variance estimator18 to account for the individual matching. Analyses were performed using R; P < .05 was considered significant. The study was approved by the local ethics committee.
Results
COVID-19 Vaccine Effectiveness
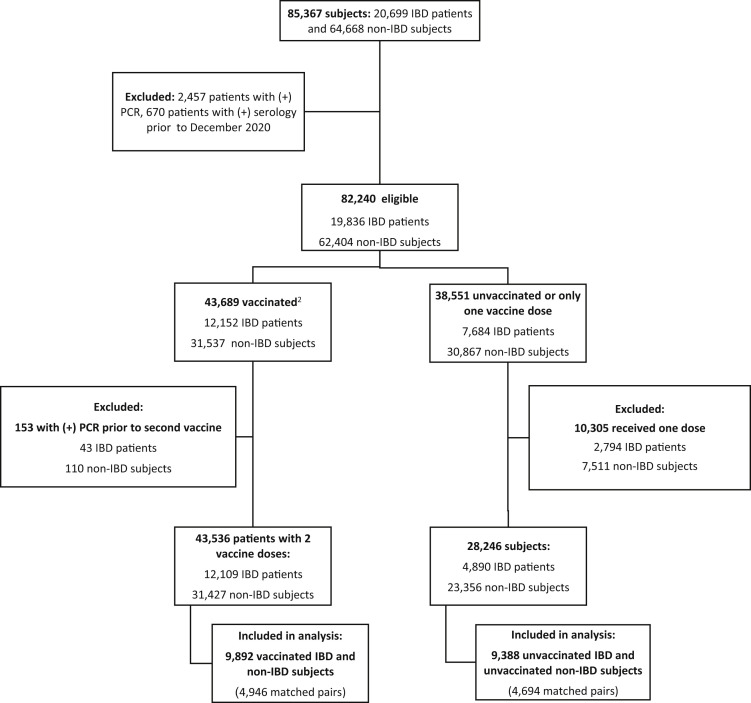
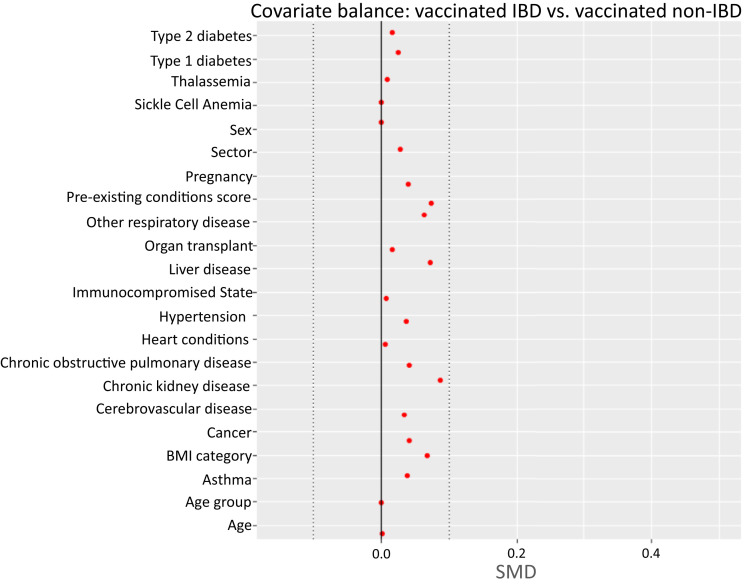
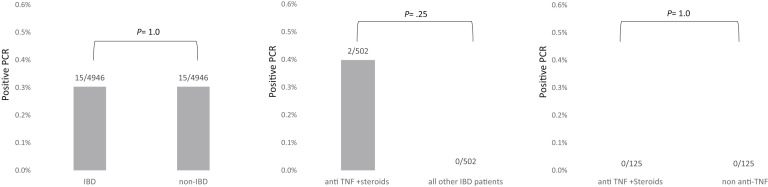
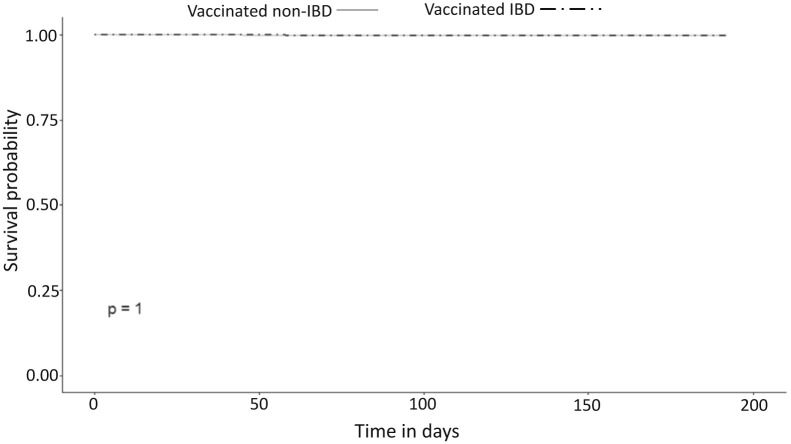
Between December 1, 2020 and June 30, 2021, 12,109 IBD patients and 31,427 non-IBD controls without previous recorded SARS-CoV-2 infection received 2 vaccine doses (Figure 1, Supplementary Table 3 ). The cohort included 4946 pairs, matched for year of birth, sex, jurisdiction of residence, HMO, and vaccination dates. The groups were well-balanced, with SMD <0.1 for all demographic and disease characteristic variables (Table 1 , Supplementary Figure 1). The post-vaccination SARS-CoV-2 infection rate was identical between vaccinated IBD patients (15/4946, 0.3%) and vaccinated non-IBD controls (15/4946, 0.3%; OR, 1; 95% confidence interval [CI], 0.49–2.05; P = 1.0) (Figure 2 ). Similarly, time to positive SARS-CoV-2 PCR showed no difference between the groups (Supplementary Figure 2).
Figure 1.
Included patients from the epi-IIRN cohort. 1Non-IBD controls were matched by age, sex, HMO, and jurisdiction of residence. 2Included individuals were vaccinated with 2 doses of Pfizer-BioNTech BNT126b2 from December 2020 to June 2021.
Table 1.
Basic Characteristics of Vaccinated Patients With and Without IBD in the Matched Cohort
| Non-IBD (n = 4946) | IBD (n = 4946) | P value | SMD | |
|---|---|---|---|---|
| Age (y) | 51 ± 16 | 51 ± 16 | 1 | <0.001 |
| <18 | 20 (0.4%) | 20 (0.4%) | ||
| 18–39 | 1288 (26%) | 1288 (26%) | ||
| 40–59 | 2047 (41%) | 2047 (41%) | ||
| 60–69 | 823 (17%) | 823 (17%) | ||
| 70–79 | 627 (13%) | 627 (13%) | ||
| 80+ | 141 (3%) | 141 (3%) | ||
| Sex, male | 2412 (49%) | 2412 (49%) | 1 | <0.001 |
| Duration of follow-up after second vaccine (weeks) | 22 (4–24) | 22 (4–24) | 1 | <0.001 |
| IBD type | ||||
| CD | — | 2447 (49%) | — | — |
| UC | — | 2499 (51%) | — | — |
| Disease duration (y) | — | 12 (1–24) | — | — |
| Treatment during the preceding year | ||||
| Mesalamine | — | 1441 (29%) | <.001 | 0.91 |
| Corticosteroid | — | 203 (4%) | <.001 | 0.29 |
| Immunomodulator | — | 294 (6%) | <.001 | 0.36 |
| Anti-TNF | — | 487 (10%) | <.001 | 0.47 |
| Vedolizumab | — | 185 (4%) | <.001 | 0.28 |
| Ustekinumab | — | 96 (2%) | <.001 | 0.2 |
| Tofacitinib | — | 28 (1%) | <.001 | 0.11 |
| IBD hospitalization ever | — | 2329 (47%) | <.001 | 1.33 |
| IBD surgery ever | — | 668 (14%) | <.001 | 0.56 |
| Corticosteroids therapy ever | — | 2722 (55%) | <.001 | 1.57 |
| Preexisting conditions scorea | .011 | 0.073 | ||
| 0 | 2099 (42%) | 2001 (41%) | ||
| 1 | 1388 (28%) | 1352 (27%) | ||
| 2 | 696 (14%) | 736 (15%) | ||
| 3 | 396 (8%) | 404 (8%) | ||
| ≥4 | 367 (7%) | 453 (9%) |
NOTE. Count (%), mean ± standard deviation, or medians (IQR) are presented as appropriate.
CD, Crohn’s disease; IBD, inflammatory bowel diseases; SMD, standardized mean difference; TNF, tumor necrosis factor; UC, ulcerative colitis.
Count of total number of preexisting conditions defined by the Centers for Disease Control as risk factors (Appendix 1).
Supplementary Figure 1.
Covariate balance for vaccinated IBD patients vs vaccinated non-IBD subjects. BMI, body mass index.
Figure 2.
SARS-CoV-2 infection rate in (A) vaccinated IBD patients vs vaccinated non-IBD subjects; (B) vaccinated IBD patients treated with anti-TNF and/or corticosteroid vs all other IBD patients; and (C) vaccinated IBD patients treated with anti-TNF and/or corticosteroid vs other biologics.
Supplementary Figure 2.
Survival curves for time to first positive PCR for IBD vs non-IBD.
Effect of Medical Therapy on Vaccine Effectiveness
Of the 536 vaccinated IBD patients receiving TNF inhibitors and/or corticosteroids at the time of vaccination, 2 (0.4%) had a positive SARS-CoV-2 PCR during the study period, compared with 36/11,573 (0.3%) vaccinated IBD patients who did not receive these medications (P = 1.0). Propensity score matching was successful for 502 pairs and showed similar infection rates (2/502, 0.4% for TNF inhibitors/steroids vs 0/502 for all others; P = .48; Figure 2).
To further reduce confounding, we compared patients on TNF inhibitors/corticosteroids with patients on any biologics other than TNF inhibitors to capture a group with likely greater disease severity, with similar results (2/536, 0.4% for TNF inhibitors/steroids vs 0/189 for all other biologics; P = .97; Figure 2). Finally, the latter groups were compared by propensity score after more stringent matching by a variety of IBD and demographic variables, including exact matching of IBD severity (Supplementary Figure 3). The matched analysis compared 125 patients in each group; no patients from either group tested positive for SARS-CoV-2 (Figure 2).
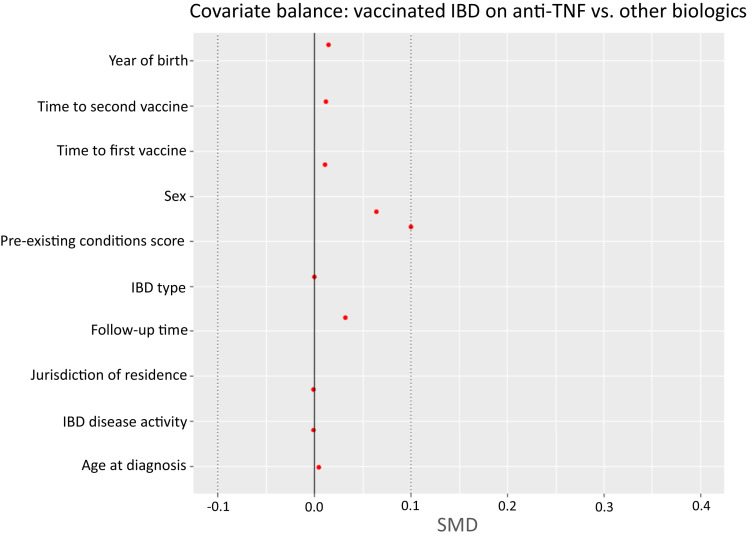
Supplementary Figure 3.
Covariate balance for vaccinated IBD patients on anti-TNF vs other biologics.
Background SARS-CoV-2 Infection Rates in Unvaccinated Inflammatory Bowel Disease Patients and Unvaccinated Non–Inflammatory Bowel Disease Controls
We considered the possibility that IBD patients may have exercised greater caution in their attempts to avoid exposure to SARS-CoV-2, thus decreasing the background infection rate compared with non-IBD subjects and confounding the estimate of vaccine effectiveness. Therefore, we determined the SARS-CoV-2 infection rate during the study period among 4694 unvaccinated IBD patients matched to 4694 unvaccinated non-IBD controls. Infection rates were slightly higher in the unvaccinated IBD patients (461/4694, 9.8%) than in the matched unvaccinated non-IBD individuals (362/4694, 7.7%; OR, 1.3; 95% CI, 1.13–1.51; P = .0003). The observed difference was in the opposite direction from the hypothesis, and thus it does not confound the conclusion that the vaccine is at least as effective in the IBD population as in non-IBD controls.
Effect of Vaccination on IBD Disease Activity
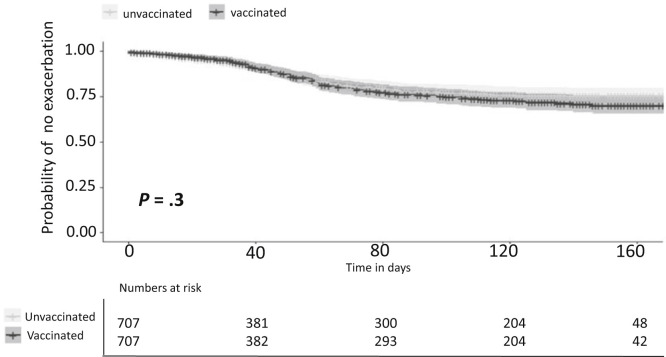
For this analysis, 2108 vaccinated IBD patients were matched to unvaccinated IBD patients by sex, jurisdiction of residence, IBD type (CD or UC), and disease severity according to blood work clusters. Median follow-up was 12 weeks (IQR, 2.4–20.6). No difference in disease outcome was seen during the first 40 days after the second vaccination, but thereafter, time to flare was shorter in vaccinated compared with unvaccinated IBD patients (Supplementary Figure 4). Overall, 44% of vaccinated and 34% of unvaccinated patients experienced an exacerbation or treatment escalation (P < .0001; number needed to harm = 10).
Supplementary Figure 4.
Time to exacerbation in vaccinated vs unvaccinated IBD patients matched for disease severity.
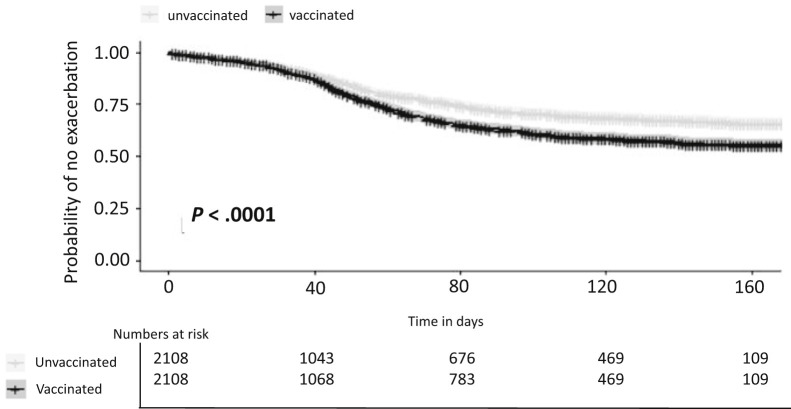
Considering the possibility that despite the comprehensive matching the model was still unable to fully account for baseline disease severity, we applied more stringent matching criteria including number of exacerbations during the previous 2 years and time interval from the last exacerbation (defined as in the outcome). This cohort included 707 pairs of vaccinated and unvaccinated IBD patients with similar baseline characteristics (Table 2 ) and a median follow-up of 14 weeks (IQR, 2.3–20.4). No substantive difference in disease outcomes was found between the groups (Figure 3 ); the overall risk of exacerbation was 29% in vaccinated patients and 26% in unvaccinated patients (P = .3).
Table 2.
Basic Characteristics of Vaccinated and Unvaccinated IBD Patients in the Matched Cohort
| Non-vaccinated (n = 707) | Vaccinated (n = 707) | P value | SMD | |
|---|---|---|---|---|
| Age (y) | 31 ± 13.0 | 31 ± 13.0 | 1 | <0.001 |
| Sex, male | 358 (50.6%) | 358 (50.6%) | 1 | <0.001 |
| Duration of follow-up after second vaccine (weeks) | 14 (2.3–20.4) | 14 (2.3–20.4) | 1 | <0.001 |
| IBD type | ||||
| CD | 485 (69%) | 485 (69%) | 1 | 1 |
| UC | 222 (31%) | 222 (31%) | 1 | 1 |
| Disease duration (y) | 8.6 (5.4–12) | 8.6 (5.4–12) | 1 | <0.001 |
| Treatment over last year | ||||
| Mesalamine | 94 (13.3%) | 114 (16.1%) | .154 | 0.080 |
| Corticosteroid | 23 (3.3%) | 16 (2.3%) | .330 | 0.060 |
| Immunomodulator | 25 (3.5%) | 35 (5.0%) | .235 | 0.070 |
| Anti-TNF | 95 (13.4%) | 107 (15.1%) | .403 | 0.049 |
| Vedolizumab | 18 (2.5%) | 30 (4.2%) | .106 | 0.094 |
| Ustekinumab | 8 (1.1%) | 12 (1.7%) | .499 | 0.048 |
| Tofacitinib | 1 (0.1%) | 2 (0.3%) | 1.0 | 0.031 |
| IBD hospitalization ever | 271 (38.3%) | 304 (43.0%) | .083 | 0.095 |
| IBD surgery ever | 65 (9.2%) | 94 (13.3%) | .018 | 0.130 |
| Corticosteroids therapy ever | 350 (49.5%) | 376 (53.2%) | .183 | 0.074 |
| Disease activity groupa,b | 1 | <0.001 | ||
| 1 | 76 (12%) | 76 (12%) | ||
| 2 | 508 (83%) | 508 (83%) | ||
| 3 | 32 (5.2%) | 32 (5.2%) |
NOTE. Count (%), mean ± standard deviation, or medians (IQR) are presented as appropriate.
CD, Crohn’s disease; IBD, inflammatory bowel diseases; SMD, standardized mean difference; TNF, tumor necrosis factor; UC, ulcerative colitis.
Disease activity group calculated by hierarchical clustering of laboratory results (Supplementary Table 1, Appendix 3).
91 pairs had no laboratory results and could not be assigned to a disease activity group.
Figure 3.
Time to exacerbation in vaccinated vs unvaccinated IBD patients matched for disease severity, number of pre-vaccine flares, and recentness of last flare.
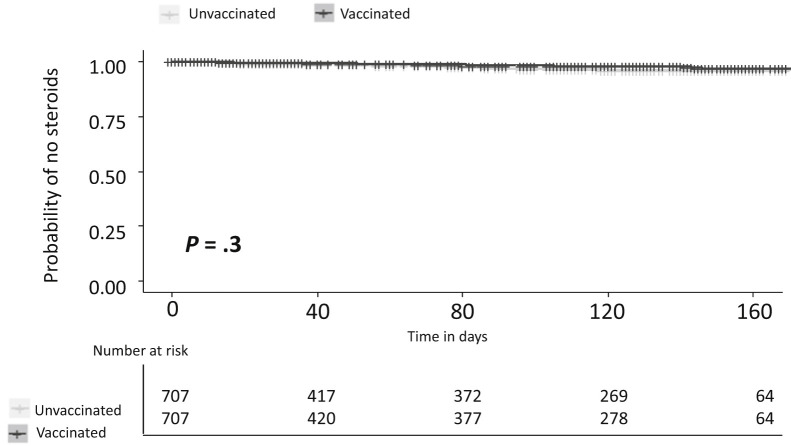
Finally, in light of the relatively high exacerbation rate, we performed a sensitivity analysis on the same cohort using a narrow definition of commencement of corticosteroids only. Here too, no difference was found between the groups (Figure 4 ); the overall risk of exacerbation was 1.4% in vaccinated patients and 2.1% in unvaccinated patients (P = .3).
Figure 4.
Time to steroid administration in vaccinated vs unvaccinated IBD patients matched for disease severity, number of pre-vaccine flares, and recentness of last flare.
Discussion
In this population-based study of all patients from 2 of the 4 national HMOs in Israel, we found that the overall COVID-19 vaccine effectiveness was similar between IBD patients and matched non-IBD controls. Focusing on medical therapy, we found that patients on TNF inhibitors and/or corticosteroids did not have a higher SARS-CoV-2 infection rate, even after precise matching for demographics, underlying diseases, and IBD severity.
Our initial comparison revealed that vaccination was associated with increased risk of IBD exacerbation from 40 days onward, despite exact matching of demographics, laboratory markers of disease severity, and number of exacerbations in the preceding 2 years. However, when we included in the analysis the recentness of the last exacerbation before baseline, the difference was attenuated and was no longer significant. This underscores the challenge of accounting for disease severity in administrative databases and the importance of stringent matching of disease severity when assessing the influence of an exposure on disease course.
The effect of COVID-19 vaccination on short-term (4 weeks) IBD course was assessed by 2 groups, who reported no clinical and laboratory exacerbation compared with pre-vaccination baseline in a prospective cohort of 185 patients with IBD stratified according to treatment9 and no increase in corticosteroid prescription 1 month after vaccination in a large retrospective cohort compared with a matched unvaccinated cohort.12 However, accurate capture of IBD exacerbation in a retrospective study is challenging. Furthermore, ruling out an effect of vaccination on IBD activity likely requires more than a month of follow-up. Our data enabled a more comprehensive definition of exacerbation, including hospitalizations, treatment escalation, and commencement of corticosteroid or enema. The broad definition and the longer follow-up period (median, 14 weeks) enabled improved capture of IBD exacerbation. To address the possibility that our broad definition could have potentially overdiagnosed exacerbation, we performed a sensitivity analysis defining exacerbation as steroid commencement only and still found no difference between the groups.
The finding that TNF inhibitors did not affect vaccine efficacy requires further discussion in light of existing literature showing decreased serologic response in patients on TNF inhibitors for various vaccines including hepatitis A,4 hepatitis B,5 and influenza6 vaccines.
Regarding response to COVID-19 vaccination in TNF inhibitor–treated patients, data are conflicting. Dailey et al19 found a robust response to 2 vaccine doses in a small prospective cohort, and all 33 vaccinated IBD patients in the study seroconverted. Khan et al20 found that BNT162b2 and Moderna mRNA-1273 vaccines were effective in a large retrospective cohort of IBD patients, irrespective of medications, but follow-up was brief, and the study did not include non-IBD controls. In contrast, in a large retrospective study (CLARITY IBD), Kennedy et al8 found lower antibody levels in infliximab-treated patients compared with vedolizumab-treated patients, although patients in this study received only 1 vaccine dose (Pfizer-BioNTech BNT162b2 or Astra-Zeneca ChAdOx1). Edelman-Klapper et al9 prospectively followed a group of 185 IBD patients after 2 doses of BNT162b2 vaccine and found that although all patients on anti-TNF medication seroconverted, antibody levels were significantly lower and neutralizing and inhibitory functions were similarly lower in this patient group. These studies raise concern for reduced durability of vaccine efficacy. Two recent serologic studies, the PREVENT-COVID study and the CORALE-IBD study, found that the large majority of IBD patients seroconverted, although levels in TNF-inhibitor patients were somewhat lower.10 , 11 Our findings support the notion that although post-vaccine antibody levels and function are both reduced in anti–TNF-treated patients, they are nonetheless sufficient to protect from infection for at least a 22-week median follow-up period.
Our findings support those of the two previous studies that addressed real-world COVID-19 vaccine effectiveness for preventing infection in patients on anti-TNF medication; neither study found increased COVID-19 incidence in these patients. However, Hadi et al12 did not specify length of follow-up, and Ben-Tov et al14 followed the cohort for a median of 10 weeks. In the current study, we have shown that vaccine effectiveness in IBD patients on TNF inhibitors and corticosteroids continues to be unimpaired for up to 22 weeks.
The strengths of our study include a large population-based cohort, as well as rigorous individual and propensity score matching to reduce the inevitable confounders inherent in retrospective research using observational data. Because COVID-19 prevalence fluctuated greatly during the study period, exact matching of vaccination date enabled the comparison of subject pairs during the same time period, with identical background COVID-19 prevalence. The large population of IBD patients who received the vaccine in a relatively short period enabled us to retain sufficient homogenous numbers for meaningful analyses even after stringent matching. Furthermore, all vaccinated individuals in the study received the same vaccine (BNT162b2), contributing to the uniformity of the comparison. Rigorous pre-vaccine disease adjustment allowed accurate detection of the vaccine effect on IBD activity and led to the likely conclusion that the vaccine is not associated with increased IBD exacerbations. Finally, the follow-up period enabled better capture of SARS-CoV-2 infections.
The reason for our finding of higher COVID-19 incidence in unvaccinated IBD patients compared with unvaccinated individuals without IBD is not clear, though it should be noted that the IBD patients had a generally higher prevalence of a variety of underlying medical conditions than the non-IBD group among both unvaccinated as well as vaccinated individuals (Supplementary Table 2). This finding serves to increase certainty that the low infection rate in vaccinated IBD patients was not biased by a lower background rate and strengthens the significance of our finding that the vaccine protected IBD patients equally as well as those without IBD.
The main limitations of the study relate to its retrospective analysis of data obtained from an administrative database. It is possible that some hidden confounding variables were still not properly addressed and that some of the data were biased by misclassification. Nonetheless, case ascertainment of IBD in the epi-IIRN database is one of the most accurate globally, and registration of medications and COVID-19–related data are very accurate by virtue of the function of the Israeli health care system. The low infection rates in vaccinated subjects limit our statistical power to prove equivalent effectiveness, but the fact that the infection rate was so low in a very large cohort clearly shows that the vaccine was highly effective in both groups, including those on anti-TNF therapy.
Although the number of matched patients available for analysis was inevitably much lower than the number in the total cohort because of rigorous matching, comparison of the group that participated in the effectiveness analysis with the entire cohort revealed minimal differences in most demographic parameters (Supplementary Table 4).
In conclusion, we found that COVID-19 BNT162b2 vaccine was equally effective in IBD patients and in the non-IBD population, including those on TNF inhibitors and corticosteroids, and likely did not increase the risk of IBD exacerbation. The former finding supports previous short-term follow-up data. The present study is the first large controlled study to address the latter conclusion using a broad definition of exacerbation and provides further reassurance regarding safety of the COVID-19 vaccine in IBD patients.
Acknowledgments
CRediT Authorship Contributions
Raffi Lev-Tzion, MD (Conceptualization: Equal; Formal analysis: Equal; Methodology: Equal; Project administration: Equal; Writing – original draft: Lead; Writing – review & editing: Equal)
Gili Focht, MBA, MSc (Conceptualization: Equal; Formal analysis: Equal; Funding acquisition: Supporting; Methodology: Equal; Project administration: Equal; Supervision: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Rona Lujan, MA (Conceptualization: Supporting; Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Writing – original draft: Supporting; Writing – review & editing: Equal)
Adi Mendelovici, MSc (Conceptualization: Supporting; Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Writing – review & editing: Equal)
Chagit Friss, BSc (Conceptualization: Supporting; Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Writing – review & editing: Equal)
Shira Greenfeld, MD (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Equal)
Revital Kariv, MD (Conceptualization: Supporting; Writing – review & editing: Equal)
Amir Ben-Tov, MD (Conceptualization: Supporting; Writing – review & editing: Equal)
Eran Matz, MD, MHA (Conceptualization: Supporting; Data curation: Supporting; Writing – review & editing: Equal)
Daniel Nevo, PhD (Conceptualization: Equal; Formal analysis: Supporting; Methodology: Equal; Writing – review & editing: Equal)
Yuval Barak-Corren, MD, MS (Formal analysis: Supporting; Writing – review & editing: Equal)
Iris Dotan, MD (Conceptualization: Supporting; Methodology: Equal; Writing – review & editing: Equal)
Dan Turner, MD, PhD (Conceptualization: Lead; Formal analysis: Supporting; Funding acquisition: Lead; Methodology: Equal; Project administration: Equal; Supervision: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest These authors disclose the following: DT received consultation fee, research grant, royalties, or honorarium from Janssen, Pfizer, Ferring, AbbVie, Takeda, Atlantic Health, Shire, Celgene, Lilly, Roche, Thermo Fisher, BMS, SorrisoPharma, and Cytoreason. RK received consultation fee, research grant, royalties, or honorarium from Takeda and Pfizer. ID received consultation fee, research grant, royalties, or honorarium from Janssen, Pfizer, Ferring, AbbVie, Takeda, Celgene/BMS, Roche/Genentech Janssen, Arena, Neopharm, Gilead, Galapagos, Celltrion, Rafa Laboratories, Falk Pharma, MSD, Cambridge Healthcare, Sublimity, Nestle, Wild Biotech, Food Industries Organization, Integra Holdings, Abbott, Athos, Peer Voice, Medscape, Mediahuset, and GSK. The remaining authors disclose no conflicts.
Funding The epiIIRN work is supported by an educational grant from The Leona M. and Harry B. Helmsley Charitable Trust.
Note: To access the supplementary material accompanying this article, please click here.
Appendix 1. Centers for Disease Control Preexisting Conditions Codes
| Variable | Value | Definitions | Timing |
|---|---|---|---|
| Body mass index (kg/m2) | Overweight: 2–29.9 Obese: 30–39.99 Severely obese: ≥40 |
Closest value to vaccine period taken in the past 5 years and not taken during pregnancy | |
| Pregnancy | 0/1 | Pregnancy determined from February 2, 2020 | |
| Cancer | 0/1 | General ICD9 codes: 174, 175, 153, 154, 162, 188, 183, 182, 157, 191, 192, 151, 172, 201, 200, 202.4, 204, 205, 206, 207.1, 208.1, 189, 160, 161, 180, 140, 141, 142, 143, 144, 145, 150, 155, 156, 170, 171, 176, 184, 186, 187, 203, 152, 158, 159, 163, 164, 165, 190, 196, 197, 198, 199, "V10.5", "V10.6", "V10.1", "V10.4" Specific ICD9 codes: 233.0, "V10.3", 185, "V10.46", "V10.51", "V10.43", 179, "V10.42", "V10.85", "V10.04", "V10.82", "V10.52", 164.0, 195.0, "V10.21", "V10.22", "V10.41", "V10.03", ”V10.07", "V10.81", 193, "V10.87", 273.3, 181, 192.8 Procedure ICD9 code: 85.4 |
In the past 5 years |
| Chronic kidney disease | 0/1 | General ICD9 codes: 585, 581, 582, 583, 588, 589 Specific ICD9 codes: 996.81, "V42.0", 403.01, 403.11, 403.21, 403.31, 403.41, 403.51, 403.61, 403.71, 403.81, 403.91, 404.02, 404.12, 404.22, 404.32, 404.42, 404.52, 404.62, 404.72, 404.82, 404.92, 404.03, 404.13, 404.23, 404.33, 404.43, 404.53, 404.63, 404.73, 404.83, 404.93, 586, 250.4, 274.1, 440.1, 587 Procedure ICD9 codes: 39.95, 54.98, 55.6 |
Ever |
| Chronic obstructive pulmonary disease | 0/1 | General ICD9 codes: 491, 492 Specific ICD9 codes: 496 |
Ever |
| Heart conditions | 0/1 | General ICD9 codes: 410, 411, 413, 414, 428, 425, 416 Specific ICD9 codes: 412, 413, 414, 429.2, 429.7, "V45.81", "V45.82", 398.91, 402.01, 402.11, 402.21, 402.31, 402.41, 402.51, 402.61, 402.71, 402.81, 402.91, 404.01, 404.11, 404.21, 404.31, 404.41, 404.51, 404.61, 404.71, 404.81, 404.91, 404.03, 404.13, 404.23, 404.33, 404.43, 404.53, 404.63, 404.73, 404.83, 404.93, 416.9, 514 |
Ever |
| Solid organ transplant | 0/1 | Specific ICD9 codes: 996.81, "V42.0", "V42.7", "V42.1", "V43.2", "V42.83", "V42.6" Procedure ICD9 codes: 55.6, 50.5, 37.5, 52.8, 33.5, 33.6 |
Ever |
| Sickle cell disease | 0/1 | Specific ICD9 code: 282.6 | Ever |
| Type 1 diabetes | 0/1 | General ICD9 codes: 250.01, 250.11, 250.21, 250.31, 250.41, 250.51, 250.61, 250.71, 250.81, 250.91, 250.03, 250.13, 250.23, 250.33, 250.43, 250.53, 250.63, 250.73, 250.83, 250.93 | Ever |
| Type 2 diabetes | 0/1 | General ICD9 codes: 250 Specific ICD9 codes: 357.2, 362.0 Exclude ICD9 codes: 250.01, 250.11, 250.21, 250.31, 250.41, 250.51, 250.61, 250.71, 250.81, 250.91, 250.03, 250.13, 250.23, 250.33, 250.43, 250.53, 250.63, 250.73, 250.83, 250.93 |
Ever |
| Asthma | 0/1 | Specific IBD9 code: 493 | Ever |
| Cerebrovascular disease | 0/1 | General ICD9 codes: 432, 433, 434, 435, 436, 438 Specific codes ICD9: 362.34, 430, 431 |
Ever |
| Other respiratory disease | 0/1 | General ICD9 codes: 494, 277.0 Specific ICD9 codes: 515 |
Ever |
| Hypertension | 0/1 | General ICD9 codes: 401, 402, 403, 404, 405 | Ever |
| Immunocompromised state | 0/1 | General ICD9 codes: "042", "043", "044", "V42.8", "V42.8" Specific ICD9 codes: "795.71", "V08" Procedure ICD9 codes: 41.0 |
Ever |
| Neurologic conditions | 0/1 | General ICD9 codes: 290, 294, 331, 358, 345, 343, 334, 356, 335, 730.7, 331.3, 333, 334, 336, 335.1, 237.7, 742.81, 742.82 Specific ICD9 codes: 310.1, "332.0", 332.1, 340, 333.4, 138, "V12.02", 228.02, 307.23, 330.9, 331.4, 337, 335.1, "359.0", 359.21, "357.0", 742.81, 742.82 |
Ever |
| Liver disease | 0/1 | General ICD9 codes: 571, 572 Specific ICD9 codes: "070.22", "070.23", "070.32", "070.33", "070.44", "070.54", "V02.61", "V02.62", 275.1, 277.4, 452, "453.0", 571.8, 571.9 |
Ever |
| Thalassemia | 0/1 | General ICD9 code: “282.4” | Ever |
ICD9, International Classification of Diseases, Ninth Revision.
Appendix 2. Method Used for Defining a Flare
Each one of the following counted as a flare for assessing disease outcomes in the IBD patients:
-
1.Escalation of IBD medical treatment
-
a.Any medication switch except for changes considered to be de-escalation (ie, change from biologics to immunomodulators or mesalamine and change from an immunomodulator to mesalamine) as well as change of one anti-TNF to another anti-TNF.
-
b.Combo therapy: defined as 60 days or more of both immunomodulator and biological treatment.
-
a.
-
2.
Commencement of corticosteroid (including budesonide) or enema.
-
3.
Hospitalizations – all-cause hospitalizations: each billing record of hospitalization with a gap of more than 7 days to the next billing date was considered one hospitalization.
Appendix 3. Methods Used for Disease Severity Clustering
To create an indication of disease activity based on laboratory data that were collected as part of routine practice with a high percentage of missing data, agglomerative hierarchical clustering was used to categorize the IBD patients into disease severity groups based on the laboratory results closest to the vaccination date. Hierarchical clustering is an unsupervised statistical method for data exploration and partitioning into groups termed clusters where elements are grouped into a hierarchy of clusters according to their similarities.1 An advantage of this method is that a patient could be categorized on the basis of at minimum one lab result. For example, if a patient has only a hemoglobin result value in the 2 years before the vaccine period, that patient will be included in the clustering process. Patients without any laboratory results in the 2-year period before the vaccination period were excluded from the clustering analysis and termed "no known laboratory results" for the matching process. The clustering enabled another estimation of the patient’s disease activity during the year before vaccination. Laboratory results included in the clustering analysis were C-reactive protein, erythrocyte sedimentation rate, platelets, hemoglobin, albumin, and white blood count and were standardized, as appropriate, by dividing the test result by the age and sex adjusted upper/lower normal limit.
For hierarchical clustering, a dissimilarity matrix on the standardized variables with Gower’s distance was computed in R package cluster. 2 The dissimilarity matrix measures whether objects can be clustered on the basis of how similar or distant they are.3 The cluster package was chosen to handle missing data, ie, NA characters.3 Next, agglomerative hierarchical clustering, a bottom-up process, was performed by using Ward’s method, which was chosen because it produces clusters that minimize within-group variance4 and because it produced a large agglomerative coefficient (0.99) that describes the strength of the clustering structure. The optimal number of clusters, between 4 and 6, was chosen using the elbow method. The order of the clusters from mild to extreme disease activity was determined by observing the mean values of the laboratory results. Importantly, this method is purely descriptive, and no outcome data were used in the process; thus any statistical assessment of differences in outcomes between the clusters remains independent.
Supplementary Table 1.
Hierarchical Clustering of Laboratory Results of All IBD Patients, Vaccinated and Unvaccinated
| Laboratory test/disease activity group | 1 (N =2908) | 2 (N = 10,822) | 3 (N = 2766) | 4 (N = 557) | 5 (N = 141) | 6 (N = 3426) |
|---|---|---|---|---|---|---|
| CRP (mg/dL) | 0.72 ± 1.38 | 0.74 ± 1.15 | 2.96 ± 3.5 | 8.8 ± 7.9 | 21.8 ± 14.1 | — |
| Missing (%) | 902 (31%) | 1979 (18%) | 518 (19%) | 101 (18%) | 16 (11%) | 3426 (100%) |
| ESR (mm/h) | 0.66 ± 0.53 | 0.8 ± 0.7 | 1.44 ± 1.03 | 1.76 ± 1.07 | 3.14 ± 2.09 | — |
| Missing (%) | 2055 (71%) | 7378 (68%) | 1758 (64%) | 360 (65%) | 80 (57%) | 3426 (100%) |
| Platelets (10∗3/μL) | 0.54 ± 0.15 | 0.56 ± 0.12 | 0.7 ± 0.2 | 0.79 ± 0.33 | 0.95 ± 0.54 | — |
| Missing (%) | 31 (1%) | 80 (0.7%) | 12 (0.5%) | 4 (0.7%) | 3 (2.1%) | 3426 (100%) |
| WBC (k/μL) | 0.68 ± 0.19 | 0.65 ± 0.2 | 0.75 ± 0.3 | 0.81 ± 0.52 | 9.03 ± 67.1 | — |
| Missing (%) | 25 (0.9%) | 73 (0.7%) | 9 (0.3%) | 3 (0.5%) | 3 (2.1%) | 3426 (100%) |
| Hemoglobin (g/dL) | 1.18 ± 0.06 | 1.05 ± 0.065 | 0.95 ± 0.1 | 0.86 ± 0.13 | 0.8 ± 0.18 | — |
| Missing (%) | 25 (0.9%) | 73 (0.7%) | 9 (0.3%) | 3 (0.5%) | 3 (2.1%) | 3426 (100%) |
| Albumin (g/dL) | 1.26 ± 0.08 | 1.19 ± 0.07 | 1.09 ± 0.09 | 0.99 ± 0.14 | 0.9 ± 0.18 | — |
| Missing (%) | 314 (11%) | 1740 (16%) | 298 (11%) | 36 (6.5%) | 17 (12%) | 3426 (100%) |
NOTE. Disease activity is ranked from 1 to 5, 1 is mild and 5 is extreme, 6 is no known laboratory results in the 2 years before the vaccination. Count (%) or mean ± standard deviation are presented as appropriate.a
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood count.
Values were standardized, as appropriate, by dividing the test result by the age and sex adjusted upper/lower normal limit.
Supplementary Table 2.
Basic Characteristics of Vaccinated Individuals With and Without IBD and Unvaccinated Individuals With and Without IBD in Unmatched Cohort
| Not vaccinated |
Vaccinated |
P value | SMD | |||||
|---|---|---|---|---|---|---|---|---|
| IBD (N = 4890) | Non-IBD (N = 23,356) | P value | SMD | IBD (N = 12,109) | Non-IBD (N = 31,427) | |||
| Age (y) | 52 ± 24 | 51 ± 23 | <.001 | 0.054 | 48 ± 18 | 48 ± 17 | .026 | 0.024 |
| Age group (y) | <.001 | 0.155 | <.001 | 0.066 | ||||
| <18 | 279 (5.7%) | 990 (4.2%) | 138 (1.1%) | 298 (0.9%) | ||||
| 18–39 | 1451 (30%) | 7553 (32%) | 4008 (33%) | 10,499 (33%) | ||||
| 40–49 | 838 (17%) | 3723 (16%) | 2262 (19%) | 6400 (20%) | ||||
| 50–59 | 626 (13%) | 3140 (13%) | 2165 (18%) | 5811 (19%) | ||||
| 60–69 | 426 (8.7%) | 2609 (11%) | 1737 (14%) | 4207 (13%) | ||||
| 70–79 | 430 (8.8%) | 2216 (9.5%) | 1263 (10%) | 3016 (10%) | ||||
| 80+ | 840 (17.2%) | 3125 (13%) | 536 (4.4%) | 1196 (3.8%) | ||||
| Sex, male | 2500 (51%) | 12,256 (53%) | .088 | 0.027 | 6030 (50%) | 15,372 (49%) | .1 | 0.018 |
| Disease duration (y) | 11 (5.7–17) | — | 12 (6.1–18) | — | ||||
| Weeks from second vaccine | — | — | 21 (18–23) | 20 (17–22) | <.001 | 0.21 | ||
| BMI category | <.001 | 0.409 | <.001 | 0.502 | ||||
| Overweight (25–29.9 kg/m2) | 174 (3.6%) | 253 (1.1%) | 734 (6.1%) | 736 (2.3%) | ||||
| Obese (30–39.99 kg/m2) | 196 (4.0%) | 538 (2.3%) | 930 (7.7%) | 2096 (6.7%) | ||||
| Severe obesity (>40 kg/m2) | 67 (1.4%) | 313 (1.3%) | 330 (2.7%) | 1184 (3.8%) | ||||
| Pregnancy | 117 (2.4%) | 438 (1.9%) | .02 | 0.036 | 199 (1.6%) | 623 (2.0%) | .022 | 0.025 |
| Treatment over last year | ||||||||
| Anti-TNF | 0 (0%) | 0 (0%) | 1 | <0.001 | 452 (3.7%) | 0 (0%) | <.001 | 0.519 |
| Corticosteroids | 167 (3.4%) | 0 (0%) | <.001 | 0.266 | 105 (0.9%) | 0 (0%) | <.001 | 0.278 |
| Immunomodulators | 187 (3.8%) | 0 (0%) | <.001 | 0.282 | 217 (1.8%) | 0 (0%) | <.001 | 0.336 |
| Mesalamine | 553 (11%) | 0 (0%) | <.001 | 0.505 | 738 (6.1%) | 0 (0%) | <.001 | 0.386 |
| Vedolizumab | 88 (1.8%) | 0 (0%) | <.001 | 0.191 | 122 (1.0%) | 0 (0%) | <.001 | 0.292 |
| Ustekinumab | 55 (1.1%) | 0 (0%) | <.001 | 0.151 | 76 (0.6%) | 0 (0%) | <.001 | 0.213 |
| Tofacitinib | 11 (0.2%) | 0 (0%) | <.001 | 0.067 | 9 (0.1%) | 0 (0%) | <.001 | 0.096 |
| IBD surgery ever | 736 (15%) | 0 (0%) | <.001 | 0.595 | 1689 (14%) | 0 (0%) | <.001 | 0.569 |
| IBD hospitalization ever | 2668 (55%) | 0 (0%) | <.001 | 1.55 | 5909 (49%) | 0 (0%) | <.001 | 1.381 |
| Corticosteroids use ever | 2705 (55%) | 0 (0%) | <.001 | 1.574 | 6922 (57%) | 0 (0%) | <.001 | 1.634 |
| Preexisting conditions scorea | <.001 | 0.461 | <.001 | 0.145 | ||||
| 0 | 2053 (42%) | 14988 (64%) | 4922 (41%) | 14,761 (47%) | ||||
| 1 | 1142 (23%) | 3584 (15%) | 3412 (28%) | 8545 (27%) | ||||
| 2 | 571 (12%) | 1712 (7.3%) | 1664 (14%) | 3756 (12%) | ||||
| 3 | 367 (7.5%) | 1180 (5.1%) | 935 (7.7%) | 2109 (6.7%) | ||||
| 4+ | 757 (16%) | 1892 (8.1%) | 1176 (10%) | 2256 (7.2%) | ||||
| Preexisting conditions | ||||||||
| Cancer | 250 (5.1%) | 766 (3.3%) | <.001 | 0.092 | 582 (4.8%) | 1201 (3.8%) | <.001 | 0.048 |
| Chronic kidney disease | 399 (8.2%) | 942 (4%) | <.001 | 0.173 | 578 (4.8%) | 922 (2.9%) | <.001 | 0.096 |
| Chronic obstructive pulmonary disease | 382 (7.8%) | 854 (3.7%) | <.001 | 0.179 | 548 (4.5%) | 1123 (3.6%) | <.001 | 0.048 |
| Heart conditions | 857 (18%) | 2373 (10%) | <.001 | 0.214 | 1314 (11%) | 2728 (8.7%) | <.001 | 0.073 |
| Organ transplant | 13 (0.3%) | 24 (0.1%) | .008 | 0.038 | 29 (0.2%) | 34 (0.1%) | .002 | 0.032 |
| Sickle cell | 0 (0%) | 0 (0%) | 1 | <0.001 | 0 (0%) | 0 (0%) | 1 | <0.001 |
| Type II diabetes | 705 (14%) | 2461 (11%) | <.001 | 0.118 | 1641 (14%) | 3954 (13%) | .007 | 0.029 |
| Asthma | 1038 (21%) | 2385 (10%) | <.001 | 0.306 | 2717 (22%) | 5474 (17%) | <.001 | 0.126 |
| Cerebrovascular disease | 527 (11%) | 1446 (6.2%) | <.001 | 0.165 | 927 (7.7%) | 1932 (6.1%) | <.001 | 0.06 |
| Other respiratory disease | 106 (2.2%) | 126 (0.5%) | <.001 | 0.141 | 186 (1.5%) | 279 (0.9%) | <.001 | 0.059 |
| Hypertension | 1427 (29%) | 4573 (20%) | <.001 | 0.225 | 3234 (27%) | 8315 (27%) | .606 | 0.006 |
| Immunocompromised state | 19 (0.4%) | 59 (0.3%) | .134 | 0.024 | 40 (0.3%) | 73 (0.2%) | .09 | 0.019 |
| Type I diabetes | 115 (2.4%) | 371 (1.6%) | <.001 | 0.055 | 252 (2.1%) | 540 (1.7%) | .012 | 0.027 |
| Liver disease | 513 (11%) | 1188 (5.1%) | <.001 | 0.203 | 1634 (14%) | 3309 (11%) | <.001 | 0.091 |
| Thalassemia | 48 (1.0%) | 96 (0.4%) | <.001 | 0.069 | 108 (0.9%) | 247 (0.8%) | .297 | 0.012 |
NOTE. Count (%), mean ± standard deviation, or medians (IQR) are presented as appropriate.
BMI, body mass index.
Number of preexisting conditions defined by the Centers for Disease Control as risk factors (Appendix 1).
Supplementary Table 3.
Basic Characteristics of Vaccinated Individuals With and Without IBD and Unvaccinated Individuals With and Without IBD in Matched Cohort
| Not vaccinated |
Vaccinated |
Non-IBD (N = 4946) | P value | SMD | ||||
|---|---|---|---|---|---|---|---|---|
| IBD (N = 4694) | Non-IBD (N = 4694) | P value | SMD | IBD (N = 4946) | ||||
| Age (y) | 52 ± 24 | 52 ± 24 | .973 | 0.002 | 51 ± 16 | 51 ± 16) | .979 | 0.001 |
| Age group (y) | 1 | <0.001 | 1 | <0.001 | ||||
| <18 | 267 (5.7%) | 267 (5.7%) | 20 (0.4%) | 20 (0.4%) | ||||
| 18–39 | 1407 (30%) | 1407 (30%) | 1288 (26%) | 1288 (26%) | ||||
| 40–49 | 816 (17%) | 816 (17%) | 1015 (21%) | 1015 (21%) | ||||
| 50–59 | 603 (13%) | 603 (13%) | 1032 (21%) | 1032 (21%) | ||||
| 60–69 | 411 (8.8%) | 411 (8.8%) | 823 (17%) | 823 (17%) | ||||
| 70–79 | 416 (8.9%) | 416 (8.9%) | 627 (13%) | 627 (13%) | ||||
| 80+ | 774 (17%) | 774 (17%) | 141 (2.9%) | 141 (2.9%) | ||||
| Sex, male | 2414 (51%) | 2414 (51%) | 1 | <0.001 | 2412 (49%) | 2412 (49%) | 1 | <0.001 |
| Disease duration (y) | 11 (5.7–17) | — | 12 (1–24) | — | ||||
| Weeks from second vaccine | 22 (4–22) | 22 (4–22) | 1 | <0.001 | ||||
| BMI category | <.001 | 0.215 | .008 | 0.069 | ||||
| Overweight (25–29.9 kg/m2) | 163 (3.5%) | 32 (0.7%) | 69 (1.4%) | 64 (1.3%) | ||||
| Obese (30–39.99 kg/m2) | 192 (4.1%) | 137 (2.9%) | 320 (6.5%) | 340 (6.9%) | ||||
| Severe obesity (>40 kg/m2) | 67 (1.4%) | 99 (2.1%) | 143 (2.9%) | 203 (4.1%) | ||||
| Pregnancy | 122 (2.6%) | 109 (2.3%) | .424 | 0.018 | 75 (1.5%) | 59 (1.2%) | .069 | 0.039 |
| Treatment over last year | ||||||||
| Anti-TNF | 319 (6.8%) | 0 (0%) | <.001 | 0.382 | 42 (0.8%) | 0 (0%) | <.001 | 0.467 |
| Corticosteroid | 162 (3.5%) | 0 (0%) | <.001 | 0.267 | 10 (0.2%) | 0 (0%) | <.001 | 0.293 |
| Immunomodulator | 178 (3.8%) | 0 (0%) | <.001 | 0.281 | 13 (0.3%) | 0 (0%) | <.001 | 0.356 |
| Mesalamine | 537 (11%) | 0 (0%) | <.001 | 0.508 | 90 (1.8%) | 0 (0%) | <.001 | 0.91 |
| Vedolizumab | 86 (1.8%) | 0 (0%) | <.001 | 0.193 | 11 (0.2%) | 0 (0%) | <.001 | 0.28 |
| Ustekinumab | 55 (1.2%) | 0 (0%) | <.001 | 0.154 | 8 (0.2%) | 0 (0%) | <.001 | 0.2 |
| Tofacitinib | 10 (0.2%) | 0 (0%) | .004 | 0.065 | 2 (0.0%) | 0 (0%) | <.001 | 0.11 |
| IBD surgery ever | 705 (15%) | 0 (0%) | <.001 | 0.595 | 668 (14%) | 0 (0%) | <.001 | 1.33 |
| IBD hospitalization ever | 2540 (54%) | 0 (0%) | <.001 | 1.536 | 2329 (47%) | 0 (0%) | <.001 | 0.56 |
| Corticosteroids use ever | 2586 (55%) | 0 (0%) | <.001 | 1.566 | 2722 (55%) | 0 (0%) | <.001 | 1.57 |
| Preexisting conditions scorea | <.001 | 0.283 | .011 | 0.073 | ||||
| 0 | 1987 (42%) | 2619 (56%) | 2001 (41%) | 2099 (42%) | ||||
| 1 | 1106 (24%) | 915 (20%) | 1352 (27%) | 1388 (28%) | ||||
| 2 | 543 (12%) | 433 (9.2%) | 736 (15%) | 696 (14%) | ||||
| 3 | 344 (7.3%) | 278 (5.9%) | 404 (8.2%) | 396 (8.0%) | ||||
| 4+ | 714 (15%) | 449 (10%) | 453 (9.2%) | 367 (7.4%) | ||||
| Preexisting conditions | ||||||||
| Cancer | 247 (5.3%) | 197 (4.2%) | .017 | 0.05 | 282 (5.7%) | 236 (4.8%) | .042 | 0.042 |
| Chronic kidney disease | 376 (8.0%) | 234 (5.0%) | <.001 | 0.123 | 228 (4.6%) | 145 (2.9%) | <.001 | 0.088 |
| Chronic obstructive pulmonary disease | 358 (7.6%) | 181 (3.9%) | <.001 | 0.163 | 220 (4.4%) | 180 (3.6%) | .047 | 0.041 |
| Heart conditions | 805 (17%) | 574 (12%) | <.001 | 0.139 | 520 (10.5%) | 509 (10.3%) | .742 | 0.007 |
| Organ transplant | 13 (0.3%) | 4 (0.1%) | .052 | 0.045 | 8 (0.2%) | 5 (0.1%) | .579 | 0.017 |
| Sickle cell | 0 (0%) | 0 (0%) | 1 | <0.001 | 0 (0%) | 0 (0%) | 1 | <0.001 |
| Type II diabetes | 665 (14%) | 597 (13%) | <.001 | 0.244 | 703 (14%) | 732 (15%) | .424 | 0.017 |
| Asthma | 994 (21%) | 571 (12%) | <.001 | 0.306 | 892 (18%) | 820 (17%) | .059 | 0.038 |
| Cerebrovascular disease | 500 (11%) | 320 (6.8%) | <.001 | 0.136 | 390 (7.9%) | 346 (7.0%) | .099 | 0.034 |
| Other respiratory disease | 99 (2.1%) | 23 (0.5%) | <.001 | 0.143 | 80 (1.6%) | 45 (0.9%) | .002 | 0.063 |
| Hypertension | 1339 (29%) | 1124 (24%) | <.001 | 0.104 | 1380 (28%) | 1462 (30%) | .072 | 0.037 |
| Immunocompromised state | 19 (0.4%) | 14 (0.3%) | .485 | 0.018 | 15 (0.3%) | 13 (0.3%) | .85 | 0.008 |
| Type I diabetes | 107 (2.3%) | 82 (1.7%) | .078 | 0.038 | 107 (2.2%) | 89 (1.8%) | .22 | 0.026 |
| Liver disease | 492 (11%) | 308 (6.6%) | <.001 | 0.141 | 726 (15%) | 603 (12%) | <.001 | 0.073 |
| Thalassemia | 47 (1.0%) | 25 (0.5%) | .013 | 0.054 | 42 (0.8%) | 38 (0.8%) | .736 | 0.009 |
NOTE. Count (%), mean ± standard deviation, or medians (IQR) are presented as appropriate.
BMI, body mass index.
Count of total number of preexisting conditions defined by the Centers for Disease Control as risk factors (Appendix 1).
Supplementary Table 4.
Comparison of Matched IBD Patients Included in Effectiveness Analysis With Entire Unmatched Cohort
| Demographic/vaccine variables | Entire cohort (not matched) (N = 12,105) | Included (matched) (N = 4946) | SMD |
|---|---|---|---|
| Sex | 0.02 | ||
| Male | 6027 (49.8%) | 2412 (48.8%) | |
| Female | 6078 (50.2%) | 2534 (51.2%) | |
| Year of birth | 1972 (17.8) | 1970 (16.1) | 0.135 |
| HMOs | 0.544 | ||
| HMO 2 | 9690 (80.0%) | 4787 (96.8%) | |
| HMO 4 | 2274 (18.8%) | 159 (3.2%) | |
| District | 0.393 | ||
| A | 1396 (11.5%) | 408 (8.2%) | |
| B | 3696 (30.5%) | 1907 (38.6%) | |
| C | 1036 (8.6%) | 191 (3.9%) | |
| D | 1329 (11.0%) | 429 (8.7%) | |
| E | 437 (3.6%) | 55 (1.1%) | |
| F | 613 (5.1%) | 86 (1.7%) | |
| G | 3598 (29.7%) | 1870 (37.8%) | |
| Time to first vaccine | 38.0 (30–64) | 36.0 (29–59) | 0.208 |
| Time between vaccine doses | 3.00 (3.00–3.00) | 3.00 (3.00–3.00) | 0.234 |
NOTE. Count (%), mean ± standard deviation, or medians (IQR) are presented as appropriate.
References
- 1.WHO COVID-19 Dashboard Geneva: World Health Organization. 2020. https://covid19.who.int/ Available at:
- 2.Wiedermann U., Garner-Spitzer E., Wagner A. Primary vaccine failure to routine vaccines: why and what to do? Hum Vaccin Immunother. 2016;12:239–243. doi: 10.1080/21645515.2015.1093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papp K.A., Haraoui B., Kumar D., et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J Cutan Med Surg. 2019;23:50–74. doi: 10.1177/1203475418811335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park S.H., Yang S.K., Park S.K., et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:69–74. doi: 10.1097/01.MIB.0000437736.91712.a1. [DOI] [PubMed] [Google Scholar]
- 5.Andrade P., Santos-Antunes J., Rodrigues S., et al. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2015;30:1591–1595. doi: 10.1111/jgh.13001. [DOI] [PubMed] [Google Scholar]
- 6.Hagihara Y., Ohfuji S., Watanabe K., et al. Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J Crohns Colitis. 2014;8:223–233. doi: 10.1016/j.crohns.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy N.A., Goodhand J.R., Bewshea C., et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy N.A., Lin S., Goodhand J.R., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021 doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 9.Edelman-Klapper H., Zittan E., Bar-Gil Shitrit A., et al. Lower serologic response to COVID-19 mRNA vaccine in patients with inflammatory bowel diseases treated with anti-TNFα. Gastroenterology. 2022;162:454–467. doi: 10.1053/j.gastro.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melmed G.Y., Botwin G.J., Sobhani K., et al. Antibody responses after SARS-CoV-2 mRNA vaccination in adults with inflammatory bowel disease. Ann Intern Med. 2021 doi: 10.7326/M21-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappelman M.D., Weaver K.N., Boccieri M., et al. Humoral immune response to messenger RNA COVID-19 vaccines among patients with inflammatory bowel disease. Gastroenterology. 2021;161:1340–1343.e2. doi: 10.1053/j.gastro.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadi Y.B., Thakkar S., Shah-Khan S.M., et al. COVID-19 vaccination is safe and effective in patients with inflammatory bowel disease: analysis of a large multi-institutional research network in the United States. Gastroenterology. 2021;161:1336–1339.e3. doi: 10.1053/j.gastro.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong S.Y., Dixon R., Martinez Pazos V., et al. Serologic response to messenger RNA coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021;161:715–718.e4. doi: 10.1053/j.gastro.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben-Tov A., Banon T., Chodick G., et al. BNT162b2 messenger RNA COVID-19 vaccine effectiveness in patients with inflammatory bowel disease: preliminary real-world data during mass vaccination campaign. Gastroenterology. 2021;161:1715–1717.e1. doi: 10.1053/j.gastro.2021.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stulman M.Y., Asayag N., Focht G., et al. Epidemiology of inflammatory bowel diseases in Israel: a nationwide Epi-Israeli IBD Research Nucleus Study. Inflamm Bowel Dis. 2021;27:1784–1794. doi: 10.1093/ibd/izaa341. [DOI] [PubMed] [Google Scholar]
- 16.Friedman M.Y., Leventer-Roberts M., Rosenblum J., et al. Development and validation of novel algorithms to identify patients with inflammatory bowel diseases in Israel: an epi-IIRN group study. Clin Epidemiol. 2018;10:671–681. doi: 10.2147/CLEP.S151339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israel Ministry of Health COVID-19 Dashboard. https://datadashboard.health.gov.il/COVID-19/general Available at:
- 18.Borgan O. In: Modeling survival data: extending the cox model. Therneau T.M.G.P., editor. Springer-Verlag; Berlin/Heidelberg, Germany: 2000. Multiple events per subject; pp. 169–229. [Google Scholar]
- 19.Dailey J., Kozhaya L., Dogan M., et al. Antibody responses to SARS-CoV-2 after infection or vaccination in children and young adults with inflammatory bowel disease. Inflamm Bowel Dis. 2021 doi: 10.1093/ibd/izab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan N., Mahmud N. Effectiveness of SARS-CoV-2 vaccination in a Veterans Affairs cohort of patients with inflammatory bowel disease with diverse exposure to immunosuppressive medications. Gastroenterology. 2021;161:827–836. doi: 10.1053/j.gastro.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Galili T. Dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics. 2015;31:3718–3720. doi: 10.1093/bioinformatics/btv428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maechler M, Rousseuw P, Struyf A, et al. Cluster: cluster analysis basics and extensions. R package version 2.1.2: for new features, see the 'Changelog' file (in the package source). 2021.
- 3.Struyf A., Hubert M., Rousseeuw P. Clustering in an object-oriented environment. Journal of Statistical Software. 1997;1:1–30. [Google Scholar]
- 4.Murtagh F., Legendre P. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? Journal of Classification. 2014;31:274–295. [Google Scholar]