Abstract
BACKGROUND
Initial studies on COVID-19 in pregnancy have demonstrated a range of neutralizing activity, but little has been published on the full profile of SARS CoV-2 related antibodies in maternal and cordblood.
OBJECTIVE
This study aimed to describe the profile and specificity of maternal and neonatal cord blood antibody profiles in response to SARS-CoV-2 virus exposure.
STUDY DESIGN
This was a prospective cohort study of delivering patients at Thomas Jefferson University Hospital from April 2020 to February 2021. The primary objective was to describe unique maternal and fetal antibody epitope titers and specificity in patients with COVID-19 history. Serologic profile was assessed with a multiplex platform. Antigens used were hemagglutinin trimer influenza A (Hong Kong H3); spike trimers for SARS-CoV-2, SARS-CoV-1, Middle East respiratory syndrome coronavirus, and betacoronaviruses HKU-1 and OC43; and spike N-terminal domain, spike receptor-binding domain, and nucleocapsid protein (full length) for SARS-CoV-2.
RESULTS
Here, 112 maternal samples and 101 maternal and cord blood pairs were analyzed. Of note, 37 patients had a known history of COVID-19 (positive polymerase chain reaction test) during pregnancy. Of 36 patients, 16 (44%) were diagnosed with COVID-19 within 7 days of delivery. Moreover, 15 of the remaining 76 patients (20%) without a known diagnosis had positive maternal serology. For those with a history of COVID-19, we identified robust immunoglobulin G response in maternal blood to CoV-2 nucleocapsid, spike (full length), and spike (receptor-binding domain) antigens with more modest responses to the spike (N-terminal domain) antigen. In contrast, the maternal blood immunoglobulin M response seemed more specific to spike (full length) epitopes than nucleocapsid, spike (receptor-binding domain), or spike (N-terminal domain) epitopes. There were significantly higher maternal and cord blood immunoglobulin G responses not only to CoV-2 spike (127.1-fold; standard deviation, 2.0; P<.00001) but also to CoV-1 spike (21.1-fold higher; standard deviation, 1.8; P<.00001) and Middle East respiratory syndrome spike (6.9-fold higher; standard deviation, 2.5; P<.00001). In contrast, maternal immunoglobulin M responses were more specific to CoV-2 spike (15.8-fold; standard deviation, 2.1; P<.00001) but less specific to CoV-1 (2.5-fold higher; standard deviation, 0.71; P<.00001) and no significant difference for Middle East respiratory syndrome. Maternal and cord blood immunoglobulin G antibodies were highly correlated for both spike and nucleocapsid (R2=0.96 and 0.94, respectively).
CONCLUSION
Placental transfer was efficient, with robust nucleocapsid and spike responses. Both nucleocapsid and spike antibody responses should be studied for a better understanding of COVID-19 immunity. Immunoglobulin G antibodies were cross-reactive with related CoV-1 and Middle East respiratory syndrome spike epitopes, whereas immunoglobulin M antibodies, which cannot cross the placenta to provide neonatal passive immunity, were more SARS-CoV-2 specific. Neonatal cord blood may have significantly different fine specificity than maternal blood, despite the high efficiency of immunoglobulin G transfer.
Key words: COVID-19, immunity, passive immunity, pregnancy, serology
AJOG Global Reports at a Glance.
Why was this study conducted?
Although several studies have documented passive placental transfer of SARS-CoV-2 antibodies, there has not been a comprehensive serologic profile that includes antibody specificity in maternal vs. neonatal cord blood antibody profiles.
Key findings
Maternal COVID-19 exposure was associated with specific maternal and cord blood antibody signatures that relate to latency from exposure. There was highly efficient transplacental transfer in maternal to cord blood immunoglobulin (Ig)G, with IgG response to nucleocapsid showing the highest specificity for evidence of infection. IgM, which cannot cross the placenta to provide neonatal passive immunity, was more SARS-CoV-2 specific, although both maternal and cord blood IgG antibodies were cross-reactive with related CoV-1 and Middle East respiratory syndrome spike epitopes. Maternal titers were positively correlated with latency from infection and placental vascular pathology.
What does this add to what is known?
Although COVID-19 vaccines, monoclonal antibody therapy, and serologic studies have focused on SARS-CoV-2 spike antibodies, our results highlighted the potential import of nucleocapsid antibodies in SARS-CoV-2 immunity and target for therapy. Although there is a highly efficient placental transfer of IgG, the notable specificity of IgM compared with IgG suggested that cord blood, which is effectively depleted of the highly specific maternal IgM response, may have significantly different fine specificity than maternal blood, despite the high efficiency of IgG transfer.
Introduction
Pregnant women were found to be particularly vulnerable to respiratory pathogens. Moreover, they are more likely to need intensive care and have higher mortality because of SARS-CoV-2 infection.1, 2, 3 SARS-CoV-2 infection (COVID-19) is associated with higher rates of preterm birth,4 preeclampsia,5,6 and placental pathology.7
Pregnancy significantly alters many elements of adaptive immunity in a gestational age-specific manner.8, 9, 10 These adaptations have been studied in viral infection, for example, in influenza A infection.11 The effect of immunologic adaptations in SARS-CoV-2 infection during pregnancy has not been delineated. Initial studies on patients with COVID-19 highlighted several findings: CoV-2–specific antibody responses are measurable 2 to 3 weeks after the onset of symptoms,12,13 and almost everyone who recovers from SARS-CoV-2 infection develops antibody responses14 that show a wide range of neutralization activity.15 However, many questions remain, such as the durability of these responses, the isotype profile at different stages of infection, and the determinants of neonatal passive immunity. The presence and timing of neonatal immunity after maternal exposure can be a key consideration in repeat vaccination in pregnancy. As such, research into placental transfer of maternal antibodies and production of fetus-derived antibodies have aided in developing guidelines for timing pertussis and flu vaccinations for maximal maternal and neonatal benefit.16,17 A study of serologic positive pregnant women found that immunoglobulin (Ig)G responses were higher in symptomatic women vs asymptomatic women, maternal IgM and IgG peaked 15 to 30 days after the onset of symptoms, and passive IgG immunity was found in approximately three-quarters of neonates and associated with maternal IgG levels.18 Another study identified a high placental transfer ratio of antibodies, which increased with latency between diagnosis and delivery.19 These studies did not evaluate the range of SARS-CoV-2 antibody epitopes or cross-reactivity with related viruses, limiting our understanding of the breadth of SARS-CoV-2 antibody response and our ability to select optimal epitopes to follow in future research studies.
This study aimed to describe the profile and specificity of maternal serum and neonatal cord blood antibody responses to maternal SARS-CoV-2 virus exposure.
Materials and Methods
This study was approved by Thomas Jefferson University Institutional Review Board as minimal risk, and each participant provided written consent. This study followed the “Strengthening of Reporting of Observational Studies in Epidemiology” reporting guidelines.
Cohort selection
This was a prospective cohort of pregnant patients who consented to the collection of samples at delivery, including maternal blood on admission and cord blood at delivery as part of an ongoing delivery cohort biorepository. Patients were consented either on admission for delivery or as an outpatient before planned delivery at our hospital. Per the study protocol, additional maternal blood was collected on admission to the hospital, and cord blood was collected at delivery. This cohort included participants from April 2020 to February 2021; however, because of COVID-19–related research restrictions, most participants were recruited from November 2020 to February 2021. Participants were included if they were consented and had maternal and/or cord blood samples available for this study. Those with previous COVID-19 vaccination were excluded. Participants were split into 2 groups: (1) patients with COVID-19, which was indicated by either documented SARS-CoV-2 polymerase chain reaction (PCR) test at any time in pregnancy or positive maternal spike IgG or IgM at delivery based on assays below or (2) patients without COVID-19, which indicated no history of COVID-19 and a negative SARS-CoV-2 PCR test on admission to the hospital.
Data collection
Electronic medical records were reviewed for demographic, medical, and obstetrical history; date of first positive SARS-CoV-2 PCR test; date of delivery; antenatal complications; and delivery outcomes. The latency between COVID-19 diagnosis and delivery was categorized both continuously and as a binary outcome: within 7 days of delivery or >7 days from delivery. The standard of care at our institution includes a universal SARS-CoV-2 PCR test on admission to the hospital and a neonatal SARS-CoV-2 PCR test if the mother has a positive diagnosis on admission for delivery.
Assays
Serologic assessment
For assessing antibody specificity, a multiplex testing platform (Meso Scale Diagnostics, Rockville, MD): antigens manufactured in a mammalian expression system (Expi293F) are printed onto 10-plex plates. The antigens used were hemagglutinin trimer influenza A (Hong Kong H3); the spike (soluble ectodomain with T4 trimerization domain) trimers for SARS-CoV-2, SARS-CoV-1, Middle East respiratory syndrome coronavirus (MERS-CoV), and betacoronaviruses HKU-1 and OC43; and the spike N-terminal domain (NTD, Q14-L303 of the SARS-CoV-2 spike sequence), receptor-binding domain (RBD, R319-F541 of the SARS-CoV-2 spike sequence), and nucleocapsid protein (full length) for SARS-CoV-2 and bovine serum albumin as negative control. Assays were performed following the manufacturer's instructions. In brief, plates were blocked using Blocker A Solution and incubated at room temperature (RT) for 1 hour on a plate shaker, shaking at 700 rpm. The plates were washed 3 times with 1 × Meso Scale Discovery (MSD) Wash Buffer. Sera were diluted to 1:1000 dilution with Diluent 100. Positive samples (pooled human serum from patients with COVID-19) and negative samples (pooled prepandemic human serum) were used as controls. Plates were sealed and incubated at RT for 2 hours on a plate shaker, shaking at 700 rpm, and then washed 3 times with 1 × MSD Wash Buffer. The detection antibody, SULFO-TAG, with either antihuman IgG antibody or antihuman IgM antibody, was diluted to 2 µg/mL in Diluent 100 (MSD) and added to the wells and incubated at RT for 1 hour on a plate shaker. After washing, MSD GOLD Read Buffer B was added to each well, and immediately, the plates were read on the MESO QuickPlex SQ 120 (MSD).
Placental histopathology
Placental histopathology was conducted as per routine clinical indications, which included COVID-19 at our institution. Clinical indications for sending placenta for pathology included COVID-19 in pregnancy; maternal medical comorbidity, such as hypertension or diabetes mellitus; antenatal complication, such as chorioamnionitis; preterm delivery; and nonreassuring fetal heart rate tracing. Placental findings were categorized as described in the Supplemental Table.
Outcomes
The primary outcome was to describe maternal antibody epitope profile and specificity related to COVID-19 exposure and correlate with cord blood levels. All antibody responses were analyzed after log transformation of the mean luminescence intensity readout of the MSD assay. Seropositivity for the CoV-2 spike protein antigen was defined on the basis of cutoffs of 8.85 for IgM and 8.96 for IgG, in log transformation, as previously described.20 Additional serologic outcomes included the correlation between epitope levels, the relation between antibody epitopes, and the latency to delivery.
Statistical analysis
Statistical analysis was performed using SPSS (version 26.0, SPSS IBM, New York, NY) and R (http://www.R-project.org). Continuous variables were compared with Student t test, categorical with Pearson chi-square analysis. Differences in antibody levels between participants with previous COVID-19 exposure and those without were expressed as a fold change difference between median antibody levels for each group, with standard deviations (SDs) expressed as a fold change based on the median of the group with previous COVID-19 exposure and group without COVID-19 exposure. The correlation between factors was assessed with bivariate correlation and reported with Pearson correlation coefficient (r). Comparisons of paired maternal and cord blood serology were carried out using linear regression to determine the slope, correlation coefficient, and R2. The relationship between latency (>7 or <7 days) and antibody titers was evaluated with simple binary logistic regression. A P value of <.05 was considered significant for all analyses. The figures were generated using the stats, ggplot2, and corrplot packages in R.
Results
During the study period, there were 112 maternal samples, including 101 maternal and cord blood pairs collected. Of note, 36 patients had a known history of COVID-19 (positive PCR test) in the pregnancy. Of the 36 patients, 16 (44%) were diagnosed with COVID-19 within 7 days of delivery. Moreover, 15 of the remaining 76 patients without a known diagnosis had a positive maternal serology (IgG or IgM to SARS-CoV-2 spike); this was reflected in positive cord blood IgG as well. This represented a 20% seroprevalence rate among study participants.
Baseline characteristics
Baseline characteristics are described in Table 1. There were 51 in the group with COVID-19 (40 with maternal and cord blood paired samples available). Black and Hispanic patients were disproportionately represented in the group with COVID-19. The severity of COVID-19 illness and symptoms was documented for 32 of 36 known cases with most patients (n=30) being asymptomatic or having mild severity of illness and 2 having moderate severity of illness.
Table 1.
Comparison of baseline characteristics and pregnancy outcomes in pregnant women with and without polymerase chain reaction or serologic evidence of COVID-19
| Demographics | Negative maternal COVID-19 PCR and serology (n=61) | Positive maternal COVID-19 PCR or serology (n=51) | P value |
|---|---|---|---|
| Race | .03 | ||
| White (non-Hispanic) | 35 (57) | 18 (35) | |
| Black (non-Hispanic) | 19 (31) | 23 (45) | |
| Asian | 2 (3) | 0 (0) | |
| Hispanic | 5 (8) | 10 (29) | |
| Chronic hypertension | 7 (12) | 8 (16) | .56 |
| Pregestational diabetes mellitus | 0 (0) | 2 (4) | .21 |
| Primiparous | 33 (54) | 30 (59) | .62 |
| Preterm birth (<37 wk) | 4 (7) | 10 (20) | .04 |
| Preeclampsia | 16 (26) | 14 (28) | .88 |
Data are presented as number (percentage), unless otherwise indicated.
PCR, polymerase chain reaction.
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
Maternal SARS-CoV-2 serology
Of the 36 patients with PCR-confirmed SARS-CoV-2 infection, 33 (92%) had positive spike IgM, and 33 (92%) had positive spike IgG. We found that participants with reported COVID-19 diagnosis have high CoV-2 spike protein–specific IgM and IgG levels in maternal blood and high IgG but low IgM levels in cord blood, consistent with the dogma that IgM does not cross the placenta (Figure 1). Among participants with no previous COVID-19 diagnosis, 15 of 76 (20%) were seropositive for CoV-2 spike protein, which is consistent with serosurveillance studies.21
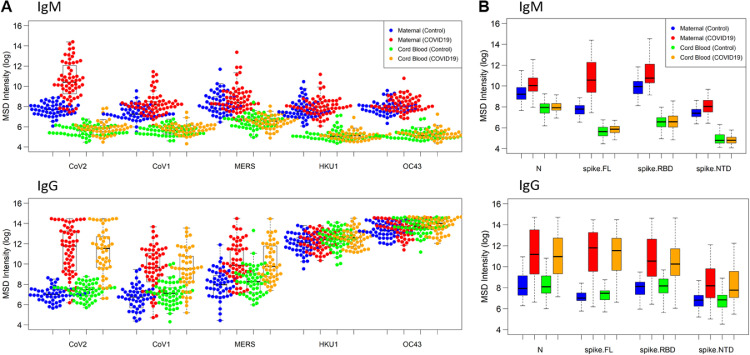
Figure 1.
Maternal and cordblood SARS CoV-2 serological profile at delivery
Maternal and cord blood COVID-19 refers to samples from patients with known COVID-19 history (polymerase chain reaction confirmed) or positive maternal serology. Data are reported as natural log-transformed luminescence signal. A, CoV-specific IgM responses (top) and CoV-specific IgG responses (bottom) in maternal sera and cord blood samples to the spike proteins of SARS-CoV-1, SARS-CoV-2, MERS-CoV, HKU-1, and OC43. Participants with previous COVID-19 history had significantly higher responses not only to CoV-2 spike (P<10−18) but also to CoV-1 spike (P<10−9) and MERS spike (P<10−8). In contrast, for IgM responses in maternal blood, participants with previous COVID-19 history showed a significantly higher response to CoV-2 (P<10−19) but a lesser response to CoV-1 (P<10−5) and no significant difference to MERS. B, Fine specificity of SARS-CoV-2–specific IgM (top) and IgG (bottom) responses in maternal sera and cord blood samples to SARS-CoV-2 epitopes, that is, N protein, the full-length S protein, and its functional subdomains, that is, RBD and NTD. Participants with previous COVID-19 had robust IgG response in maternal blood to CoV-2 N, S (full length), and S (RBD) antigens that were approximately 40-fold, 130-fold, and 15-fold higher, respectively, than what was found in participants without previous COVID-19, with more modest responses to the S (NTD) antigen. In contrast, maternal blood IgM response to S (full length) was approximately 15-fold higher in participants with COVID-19 than in participants with no previous COVID-19 history but only 2- to 2-fold higher for N or S (RBD).
IgG, immunoglobulin G; IgM, immunoglobulin M; MERS, Middle East respiratory syndrome; N, nucleocapsid; NTD, N-terminal domain; RBD, receptor-binding domain; S, spike.
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
In terms of the magnitude and epitope specificity of the CoV-2 antibody response, we found that participants with previous COVID-19 had robust IgG response in maternal blood to CoV-2 nucleocapsid, spike (full length), and spike (RBD) antigens that were 39.5-fold (SD, 3.2; P<.00001), 127.1-fold (SD, 2.0; P<.00001), and 13.6-fold higher (SD, 0.88; P<.00001), respectively, than what was found in participants without previous COVID-19, with more modest responses to the spike (NTD) antigen (Figure 1, B). In contrast, the maternal blood IgM response seemed more specific to spike (full length) epitopes than nucleocapsid, spike (RBD), or spike (NTD) epitopes. For example, maternal blood IgM response to spike (full length) was 15.8-fold (SD, 2.1; P<.00001) higher in participants with COVID-19 than in participants with no previous COVID-19 history, but only 2.2-higher for nucleocapsid (SD, 0.83; P<.00001) and 2.3-fold higher for spike (RBD) (SD, 0.81; P<.00001) (Figure 1, B).
SARS-CoV-2 positive and relation cross-reactivity to related viruses
We evaluated maternal IgG and IgM responses to different coronavirus spike proteins to assess the cross-reactivity of these antibody responses across coronaviruses. For IgG responses on maternal and cord blood, we found that participants with previous COVID-19 history had significantly higher responses not only to CoV-2 spike (as described above) but also to CoV-1 spike (21.1-fold higher; SD, 1.8; P<.00001) and MERS spike (6.9-fold higher; SD, 2.5; P<.00001), suggesting a largely cross-reactive IgG response. In contrast, for IgM responses in maternal blood, we found the response more specific to CoV-2, with participants with previous COVID-19 history showing a significantly higher response to CoV-2 (as described above) but a lesser response to CoV-1 (2.5-fold higher; SD, 0.71; P<.00001) and no significant difference for MERS (Figure 1, A).
Cord blood SARS-CoV-2 serology
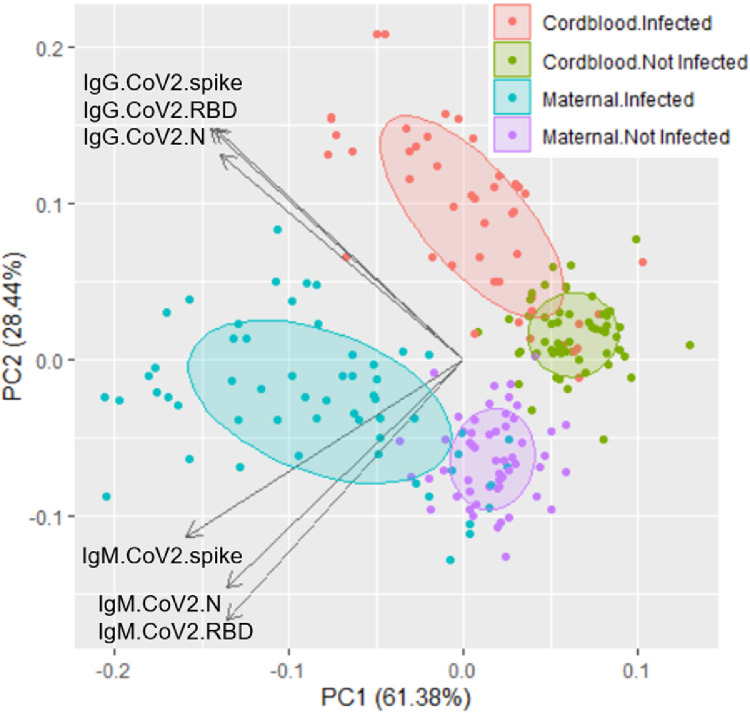
Cord blood responses largely mirrored maternal blood responses for IgG concerning the magnitude and epitope specificity (Figure 1). As expected, IgM responses in cord blood were approximately 50- to 400-fold lower than their corresponding IgM responses in maternal blood. Finally, the principal component analysis plot of IgG and IgM responses to CoV-2 antigens in the panel showed that samples with previous COVID-19 exposure are distinguishable from samples without previous exposure and maternal samples are distinguishable from cord blood samples (Figure 2).
Figure 2.
Principal component analysis of antibody responses
IgM and IgG responses to CoV-2 antigens N protein, spike, and RBD distinguish between previous COVID-19 cases from maternal and cord blood.
IgG, immunoglobulin G; IgM, immunoglobulin M; N, nucleocapsid; NTD, N-terminal domain; RBD, receptor-binding domain
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
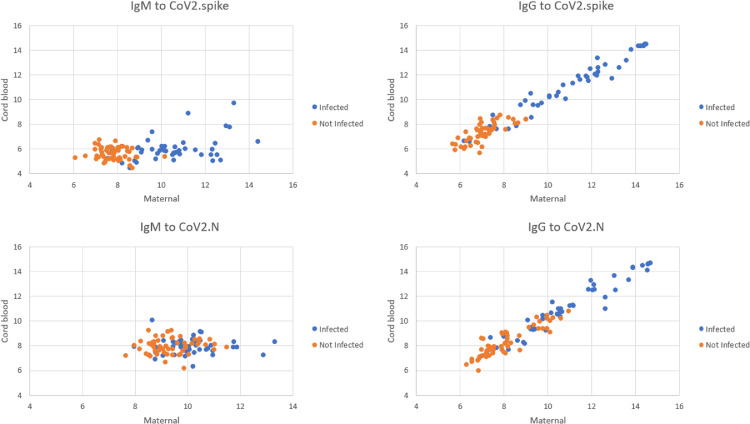
We found a high correlation of IgG (R2=0.96 and R2=0.94) but not IgM responses (R2=0.13 and R2=0.01) between paired maternal and cord blood samples for CoV-2 spike and nucleocapsid antigens, respectively (Figure 3). A linear fit between maternal and cord blood IgG responses showed a slope of 1.01 for both CoV-2 spike (SD, 0.010; P<10−15) and CoV-2 nucleocapsid antigens (SD, 0.006; P<10−15).
Figure 3.
Correlation between maternal and cord blood antibody titers
Correlation between maternal and cord blood IgG and IgM antibodies to full-length spike and N epitopes. A linear fit between maternal and cord blood IgG responses showed a slope of 1.01 for both CoV-2 spike and CoV-2 N protein antigens.
IgG, immunoglobulin G; IgM, immunoglobulin M; N, nucleocapsid.
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
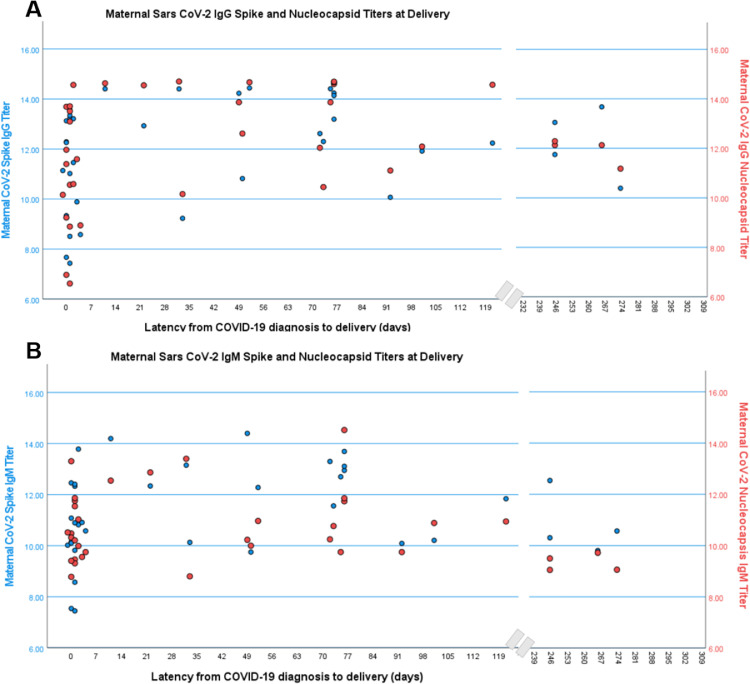
SARS-CoV-2 serology and latency
In a cross-sectional evaluation of examining titers by latency, titers of SARS-CoV-2 spike IgG and IgM rose rapidly within the first 7 days of infection, whereas nucleocapsid IgM was similar across time points (Figure 4). In evaluating maternal and cord blood serology in relation to latency, COVID-19 >7 days from delivery was specifically positively associated with maternal spike IgG (odds ratio (OR)=1.6 [95% confidence interval (CI), 1.1–2.4]; P=.02), nucleocapsid IgG (OR=1.7 [95% CI, 1.1–2.6]; P=.01), RBD IgG (OR=1.5 [95% CI, 1.0–2.2]; P=.03), NTD IgG (OR=1.6 [95% CI, 0.99–2.5]; P=.05), spike IgM (OR=1.7 [95% CI, 1.1–2.7]; P=.03), and RBD IgM (OR=1.9 [95% CI, 1.03–3.4]; P=.04), but not NTD IgM (OR=1.1 [95% CI, 0.5–2.2]; P=.87) or nucleocapsid IgM (OR=1.2 [95% CI, 0.7–2.0]; P=.42).
Figure 4.
Maternal SARS CoV-2 titers at delivery and latency from infection
Data were taken from 36 documented COVID-19 cases. Data are reported as natural log-transformed luminescence signal.
IgG, immunoglobulin G.
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
SARS-CoV-2 serology and placental pathology
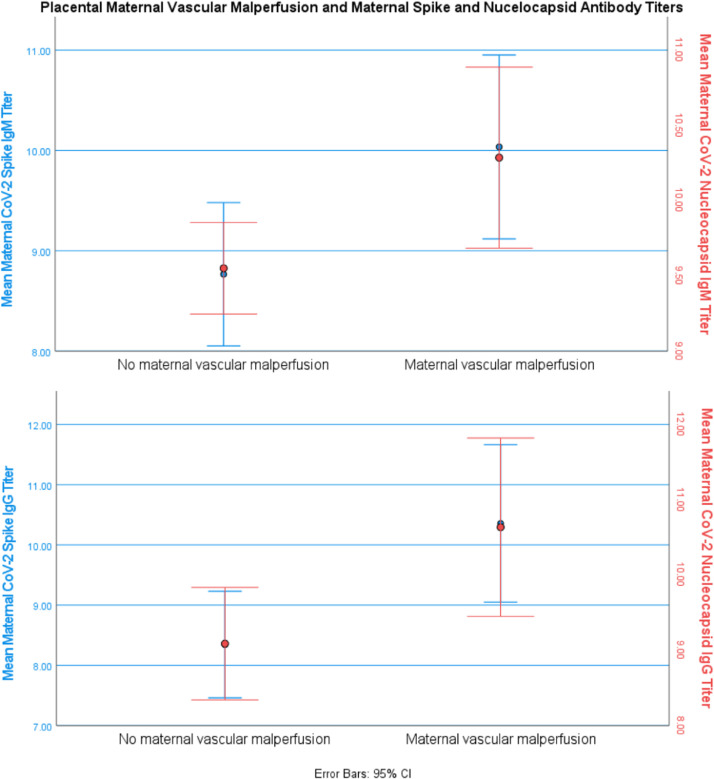
Placental histopathology was available for 52 participants (27 without COVID-19 and 25 with COVID-19). It is described in Table 2. Mean maternal nucleocapsid IgM and spike IgM were significantly higher in participants with maternal vascular malperfusion (10.3±1.5 vs 9.6±0.8 [mean difference (MD), 0.7 (95% CI, 0.07–1.4); P=.03] and 10.0±2.0 vs 8.8±1.9 [MD, 1.3 (95% CI, 0.1–2.4); P=.02], respectively), as were nucleocapsid IgG and spike IgG (10.6±2.7 vs 9.1 ±2.0 [MD, 1.5 (95% CI, 0.2–2.9); P=.02] and 10.4±2.9 vs 8.3±2.4 [MD, 2.0 (95% CI, 0.5–3.6); P=.01], respectively) (Figure 5). In examining the subset of COVID-19–exposed individuals, those with placental maternal vascular malperfusion generally exhibited higher titers, maternal spike IgG (12.1±2.0 vs 11.0±1.9; MD, 1.2 [95% CI, −0.5 to 2.8]; P=.15), nucleocapsid IgG (12.0±2.2 vs 11.1±1.8; MD, 0.90 [95% CI, −0.8 to 2.6]; P=.29), and spike IgM (11.2 vs 10.6; MD, 0.5 [95% CI, −0.9 to 2.0]; P=.45), but this was only statistically significant for maternal nucleocapsid IgM (10.7±1.3 vs 9.8; MD, 0.9 [95% CI, 0.1–1.7]; P=.03).
Table 2.
Placental pathologic findings associated with COVID-19 exposure
| Variable | Negative maternal COVID-19 PCR and serology (n=27) | Positive maternal COVID-19 PCR or serology (n=25) | P value |
|---|---|---|---|
| Pregnancy characteristics | |||
| Race | .41 | ||
| White, non-Hispanic | 14 (52) | 9 (36) | |
| Black, non-Hispanic | 8 (30) | 9 (36) | |
| Asian | 1 (4) | 0 (0) | |
| Hispanic | 4 (15) | 7 (28) | |
| Previous full-term delivery | 13 (48) | 15 (60) | .39 |
| Previous preterm birth | 3 (11) | 1 (4) | .34 |
| Chronic hypertension | 7 (36) | 4 (16) | .38 |
| Pregestational diabetes mellitus | 0 (0) | 1 (4) | .48 |
| Preeclampsia or gestational hypertension | 10 (37) | 7 (28) | .49 |
| Placental pathology | |||
| Placenta unremarkable | 6 (22) | 1 (4) | .10 |
| Acute inflammatory pathology | 7 (26) | 9 (36) | .43 |
| Chronic inflammatory pathology | 1 (4) | 0 (0) | .33 |
| Maternal vascular malperfusion | 8 (30) | 14 (56) | .05 |
| Fetal vascular malperfusion | 1 (4) | 0 (0) | .33 |
| Intervillous thrombus | 3 (11) | 3 (12) | .92 |
Data are presented as number (percentage), unless otherwise indicated.
PCR, polymerase chain reaction
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
Figure 5.
Maternal SARS CoV-2 antibody titers and placental pathology
Data were taken from 22 patients with maternal vascular malperfusion documented on placental histopathology and 30 patients with no evidence of maternal vascular malperfusion. Data are reported as natural log-transformed luminescence signal. Mean maternal nucleoprotein (N)-IgM and spike (S)-IgM were significantly higher in patients with maternal vascular malperfusion (10.3±1.5 vs 9.6±0.8 [P=.03] and 10.0±2.0 vs 8.8±1.9 [P=.02], respectively), as were N-IgG and S-IgG (10.6±2.7 vs 9.1 ±2.0 [P=.02] and 10.4±2.9 vs 8.3±2.4 [P=.01], respectively).
CI, confidence interval; IgG, immunoglobulin G; IgM, immunoglobulin M.
Boelig. Serologic profile of maternal and cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022.
Discussion
Principal findings
There was highly efficient transfer in maternal to cord blood IgG, with IgG response to nucleocapsid and spike and IgM to spike (full length) showing the highest specificity. IgG antibodies were cross-reactive with related CoV-1 and MERS spike epitopes, whereas IgM antibodies, which largely do not cross the placenta, were highly SARS-CoV-2 specific. Our results suggested that (1) both nucleocapsid and full-length spike IgG and IgM antibody epitopes should be included in evaluating serologic responses in future studies of disease or vaccine development, (2) serologic profile functions as a proxy for latency from disease exposure and in the absence of known disease (ie, lack of access to testing), and (3) cord blood, which is effectively depleted of the highly specific maternal IgM response, may have significantly different fine specificity than maternal blood, despite the high efficiency of IgG transfer.
Results in the context of what is known
This study has added to existing studies through a comprehensive evaluation of maternal and cord blood serologic responses. Of note, 1 study that included 83 mother and baby dyads examined only spike RBD and IgG and IgM and similarly identified a strong correlation between mother and cord blood and latency to delivery.19 Another study of 88 mother and baby dyads identified that IgM peaked at 15 days and that IgG peaked at 30 days, which is consistent with our findings regarding maternal and cord blood nucleocapsid IgG titers and latency of >7 days from delivery, although that testing platform was semiquantitative using a combination of spike RBD and nucleocapsid antigen to report “total” IgG and IgM titer responses and thus did not provide granular detail on antibody epitopes and specificity.18 A third study22 looked at 63 mother and baby pairs and examined anti-RBD and antinucleocapsid IgG. The limited positive serology reported (65%–70% of mothers with positive PCR results) demonstrated the limitations of examining isolated antibody epitopes and specifically of RBD-only epitopes.
SARS-CoV-2 immunity
Although previous studies have focused on SARS-CoV-2 spike protein, we evaluated a range of epitopes. Our results demonstrated the high sensitivity of our platform with a detection of spike (full-length) IgM and IgG in approximately 90% of documented PCR-positive infections. Similarly in nonpregnant patients, we found high levels of nucleocapsid IgG and IgM titers in those with a history of COVID-19.23 Studies in nonpregnant individuals identified IgM peaks in nucleocapsid and spike epitopes in the second week of infection.24 We found that maternal nucleocapsid antibodies demonstrated the highest specificity for documented SARS-CoV-2 infection and were highly expressed in both maternal and cord blood samples. Nucleocapsid proteins of many coronaviruses were highly immunogenic and highly expressed during acute infection.25 Although the focus of vaccine and monoclonal antibody therapeutics has been on spike antigen, these results, consistent with other studies in nonpregnant adults, highlighted the potential import of nucleocapsid antibodies in SARS-CoV-2 immunity and target for therapy. Finally, similar to the reports cited above,18,19,22 we found a high degree of correlation and a linear fit with a slope of 1.0 between maternal and cord blood IgG antibodies and cord blood IgG concentrations, indicating a highly efficient transfer of CoV-2 IgG antibodies. Finally, in examining serology across latency, we found that antibody titers rise rapidly after 7 days, persist >100 days, and are associated with increased antibody responses, specifically against nucleocapsid and spike epitopes, demonstrating that these antibody responses are durable rather than short lived.
Unique to our study, we also evaluated the cross-reactivity to related viruses by comparing baseline (COVID-19–negative) antibody response against related coronavirus spike antigen to the antibody response in those with COVID-19. We found that although maternal IgG antibodies were cross-reactive with related MERS and CoV-1 spike epitopes, IgM antibodies were highly specific for CoV-2 and had a similar response to MERS and CoV-1 as those without COVID-19. Given that placental antibody transfer is IgG limited, this suggested that cord blood antibody responses may be more cross-reactive and lack some of the CoV-2–specific responses found in the IgM of maternal blood. Previous work on adult patients from the Republic of Korea indicated that IgM and IgG have distinct antibody profiles.20 Moreover, there was an indication that the profile of the IgM vs IgG response is indicative of functional activities and subsequent disease severity.26 Cross-reactivity among the different coronaviruses has been a subject of debate. Because the antibody profiles of IgM and IgG differed and were associated with distinct biologic functions, the lack of IgM in the cord blood may likely fail to transfer at least some of the protective immunity from the mother to the child. This was further highlighted by recent work demonstrating the importance of IgM in SARS-CoV-2 neutralizing activity in adults27,28 and another study comparing pediatric and adult responses that found that pediatric response was predominantly antispike IgG in contrast to adult IgG, IgM, and IgA against both spike and nucleocapsid epitopes and had reduced neutralizing activity compared with adults.29 These systematic differences in isotype and epitope specificity raised the possibility that cord blood, which is effectively depleted of the highly specific maternal IgM response, may have significantly different fine specificity and may therefore have different neutralizing activity than maternal blood, despite the high efficiency of IgG transfer.
SARS-CoV-2 serology and perinatal outcomes
Similar to previous studies,30 we have identified an increased rate of preterm in the setting of COVID-19 exposure, even in this small cohort. Moreover, we identified higher SARS-CoV-2 antibody titers in those with placental maternal vascular malperfusion. This placental finding with COVID-19 has been previously reported,31,32 and our finding of higher titers related to this finding rather than just COVID-19 exposure suggested that time from illness and potentially chronic or downstream effects from COVID-19 lead to placental pathology rather than the acute infection (ie, within 7 days from infection).
Strengths and limitations
This study has several strengths. Other evaluations of COVID-19 serology in the mother and baby dyad focused on limited antibody epitopes. We have provided a comprehensive evaluation of maternal and neonatal cord blood serologic responses looking across the array of SARS-CoV-2 antibody epitopes and specificity of response through evaluation of cross-reactivity to related viruses. This was a unique examination of serologic profile not only in the mother, but also in what is passively acquired by the neonate and the implications for passive immunity. Although a single institution, our population was diverse, improving external validity.
This study has limitations as well. The number of mother and baby dyads limited our ability to provide a more detailed evaluation of serologic changes over time. There may be antibody epitope correlations that we were not powered to detect. Finally, although we identified differences in maternal and cord blood antibody signatures that could impact immunity, we did not evaluate functional activity.
Conclusion
Maternal COVID-19 exposure was associated with specific maternal and cord blood antibody signatures, with nucleocapsid and full-length spike epitopes demonstrating the highest specificity in distinguishing exposed vs nonexposed individuals. Serologic profile was related to latency from exposure. There was a highly efficient transfer in maternal to cord blood IgG antibodies. IgG antibodies were cross-reactive with related CoV-1 and MERS spike epitopes, whereas IgM antibodies, which cannot cross the placenta to provide neonatal passive immunity, were highly SARS-CoV-2 specific, suggesting there may be important qualitative distinctions between maternal immunity and neonatal passive immunity.
Acknowledgments
We would like to acknowledge our senior research coordinator Brandy Firman and the Thomas Jefferson University Hospital Labor and Delivery teams who assisted in sample collection. We would like to thank Ms Jessica Bolton (Walter Reed Army Institute of Research) for technical assistance in the serologic analysis.
Footnotes
R.C.B. is supported by the PhRMA Faculty Development Award. This study was funded, in part, by a National Institutes of Health (NIH) grant (grant number 3R21HD101127-01S1; principal investigator [PI]: R.C.B.) and a pilot grant (Z.H.A.) through an Institutional Development award from the National Institute of General Medical Sciences of the NIH (grant number U54GM104941; PI: Z.H.A.).
The authors report no conflict of interest.
The material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human participants as prescribed in AR 70-25. This paper has been approved for public release with unlimited distribution.
Cite this article as: Boelig RC, Chaudhury S, Aghai ZH, et al. Comprehensive serologic profile and specificity of maternal and neonatal cord blood SARS-CoV-2 antibodies. Am J Obstet Gynecol Glob Rep 2022;2:100046.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xagr.2021.100046.
Appendix. Supplementary materials
References
- 1.Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.04.030. 109.e1-109.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodworth KR, Olsen EO, Neelam V, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223 doi: 10.1016/j.ajog.2020.04.014. 111.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbloom JI, Raghuraman N, Carter EB, Kelly JC. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2021;224:623–624. doi: 10.1016/j.ajog.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghaeepour N, Ganio EA, Mcilwain D, et al. An immune clock of human pregnancy. Sci Immunol. 2017;2:eaan2946. doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraus TA, Engel SM, Sperling RS, et al. Characterizing the pregnancy immune phenotype: results of the viral immunity and pregnancy (VIP) study. J Clin Immunol. 2012;32:300–311. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Gars M, Seiler C, Kay AW, et al. Pregnancy-induced alterations in NK cell phenotype and function. Front Immunol. 2019;10:2469. doi: 10.3389/fimmu.2019.02469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Gars M, Kay AW, Bayless NL, et al. Increased proinflammatory responses of monocytes and plasmacytoid dendritic cells to influenza A virus infection during pregnancy. J Infect Dis. 2016;214:1666–1671. doi: 10.1093/infdis/jiw448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1930–1934. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 15.Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu Raya B, Edwards KM, Scheifele DW, Halperin SA. Pertussis and influenza immunisation during pregnancy: a landscape review. Lancet Infect Dis. 2017;17:e209–e222. doi: 10.1016/S1473-3099(17)30190-1. [DOI] [PubMed] [Google Scholar]
- 17.Cuningham W, Geard N, Fielding JE, et al. Optimal timing of influenza vaccine during pregnancy: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2019;13:438–452. doi: 10.1111/irv.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubiak JM, Murphy EA, Yee J, et al. Severe acute respiratory syndrome coronavirus 2 serology levels in pregnant women and their neonates. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2021.01.016. 73.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175:594–600. doi: 10.1001/jamapediatrics.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhury S, Hutter J, Bolton JS, et al. Serological profiles of pan-coronavirus-specific responses in COVID-19 patients using a multiplexed electro-chemiluminescence-based testing platform. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannery DD, Gouma S, Dhudasia MB, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5:eabd5709. doi: 10.1126/sciimmunol.abd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlow AG, Li JZ, Collier AY, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun B, Feng Y, Mo X, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng W, Liu G, Ma H, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527:618–623. doi: 10.1016/j.bbrc.2020.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Liang B, Chen C, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12:1813. doi: 10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klingler J, Weiss S, Itri V, et al. Role of immunoglobulin M and A antibodies in the neutralization of severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2021;223:957–970. doi: 10.1093/infdis/jiaa784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasser R, Cloutier M, Prévost J, et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villar J, Ariff S, Gunier RB, et al. Maternaland neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menter T, Mertz KD, Jiang S, et al. Placental pathology findings during and after SARS-CoV-2 infection: features of villitis and malperfusion. Pathobiology. 2021;88:69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.