Abstract
Malignant bone tumors are aggressive neoplasms which arise from bone tissue or as a result of metastasis. The most prevalent types of cancer, such as breast, prostate, and lung cancer, all preferentially metastasize to bone, yet the role of the bone niche in promoting cancer progression remains poorly understood. Tissue engineering has the potential to bridge this knowledge gap by providing 3D in vitro systems that can be specifically designed to mimic key properties of the bone niche in a more physiologically relevant context than standard 2D culture. Elucidating the crucial components of the bone niche that recruit metastatic cells, support tumor growth, and promote cancer-induced destruction of bone tissue would support efforts for preventing and treating these devastating malignancies. In this review, we summarize recent efforts focused on developing in vitro 3D models of primary bone cancer and bone metastasis using tissue engineering approaches. Such 3D in vitro models can enable the identification of effective therapeutic targets and facilitate high-throughput drug screening to effectively treat bone cancers.
Keywords: Tissue engineering, Biomaterials, Bone cancer, Bone metastasis
1. Introduction
Malignant bone tumors can arise as a result of primary bone cancer or develop from cancers that have metastasized to the bones. Primary bone cancers, such as osteosarcoma and Ewing’s sarcoma, are aggressive malignancies that affect about 3500 people each year and typically arise during childhood [1]. About 30% of patients with primary bone cancer die within 5 years as a result of poor response to therapy. In contrast to primary bone cancers, only 10% of patients with bone metastases survive after 5 years due to a lack of curative therapies. Bone metastases arise from prevalent cancer types such as breast, prostate, lung, and kidney cancers and often result in severe pain, spinal cord compression, and bone fractures [2].
Tissue engineering design has been traditionally geared towards mimicking the cellular, physical, and soluble microenvironment of healthy tissues to promote regeneration in vivo. More recently, these strategies have been employed to recapitulate abnormal tissue microenvironments in vitro to elucidate mechanisms of disease progression such as those found in cancer. These 3D in vitro mimics of the tumor microenvironment have shown great promise in recapitulating in vivo tumor growth, invasion, and resistance to drug therapy [3]. Emerging efforts have included models of soft tissue tumors, such as brain [4–9], breast [10–12], kidney [13], lung [14–16], bladder [17], ovarian [18–20], pancreatic [21], and colorectal cancer [22–24]. Despite these encouraging developments, the majority of 3D models have focused on mimicking the tissue environment of soft tumors. These tumor models primarily rely on suspension cultures or embedding within hydrogel matrices, which are limited by the range of stiffnesses that they can achieve (1–150 kPa) [25]. There remains a need for customized materials that can specifically mimic the properties of native bone (Fig. 1), which is orders of magnitude stiffer (10–15 MPa) than conventional hydrogel systems. In addition to stiffness, bone tissue has unique matrix composition consisting of 70% minerals and 30% collagen matrix [26]. This highly specialized matrix maintains homeostasis in healthy bone tissue, yet the role of bone extracellular matrix (ECM) in driving cancer progression remains unknown. The bone environment is also comprised of complex and dynamic interactions between resident cells, such as osteoblasts, osteoclasts, endothelial, immune, and hematopoietic cells [27], all of which have been implicated in the pathogenesis of bone cancers [28–31]. To address these needs, tissue engineering methods have been employed to develop customized 3D systems that mimic the physicochemical and cellular environment of the bone tumor niche.
Fig. 1.
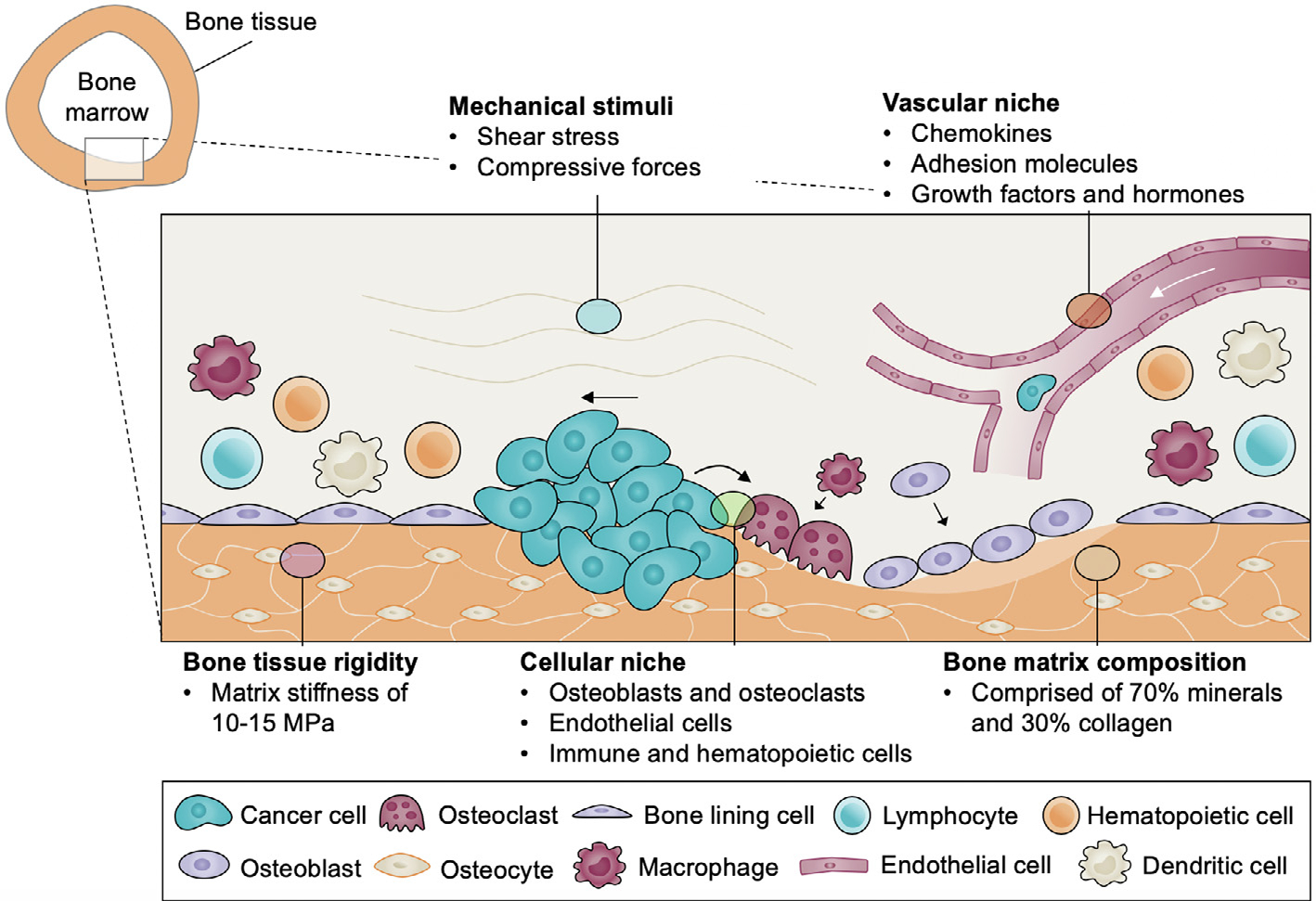
Schematic of multifactorial bone cancer niche, highlighting the cell-cell and cell-matrix interactions at the bone marrow/long bone interface. Bone is composed of highly rigid and specialized extracellular matrix composed primarily of minerals and collagen type I. This complex tissue is maintained through dynamic interactions with resident cells, including osteoblasts, osteoclasts, endothelial cells, immune, and hematopoietic cells. Bone tissue is continuously exposed to mechanical stimuli, such as shear and compressive forces, which further modulate bone homeostasis. How this complex niche promotes cancer progression remains poorly understood.
In this review, we summarize emerging efforts harnessing tissue engineering strategies for modeling primary bone cancer using tumor spheroids and biomaterials-based platforms. For bone metastases, we survey recent work focused on modeling (i) cancer cell recruitment to bone, (ii) the role of the bone matrix in modulating the cancer cell phenotype, and (iii) cancer cell-driven destruction of bone tissue using breast cancer and prostate cancer metastases as model systems. We further discuss future directions in guiding the design of tissue engineered models for studying bone cancer pathogenesis and response to therapy.
2. Modeling primary bone cancer in vitro and in vivo
2.1. Types of primary bone cancer
Primary bone cancers are rare neoplasms comprised of cells that have undergone malignant transformation within bone tissue. The most frequently diagnosed primary bone cancers are osteosarcoma, chondrosarcoma, and Ewing’s sarcoma [32]. Of these, osteosarcoma (OS) and Ewing’s sarcoma (EWS) occur predominantly in children and young adults [1,33]. Osteosarcomas arise from transformed cells of mesenchymal origin, and as a result, can possess phenotypes that resemble osteoblasts, chondroblasts, or fibroblasts, but have in common the malignant production of osteoid [34]. In contrast, Ewing’s sarcoma belongs to a family of tumors derived from primitive neuroectoderm which form aggressive tumors that develop most frequently in the bones but can also arise in extraosseous tissue [35,36]. In both osteosarcoma and Ewing’s sarcoma, patients who respond poorly to the standard chemotherapy regimen or have recurrent and/or metastatic disease have a 5-year survival rate below 20% [37,38]. In addition, primary bone cancers remain largely understudied due to their low prevalence, and as a result, the standard treatment for these tumors has not seen improvement for over 30 years. These outcomes highlight an urgent need to (i) uncover the mechanisms of bone cancer pathogenesis, (ii) develop improved, targeted therapies for bone sarcomas and (iii) improve screening methods to predict patient response to available therapeutics.
2.2. Types of primary bone cancer models
Drug discovery and development rely heavily on findings from in vitro and in vivo models. Culturing cancer cells in 2D monolayers on tissue culture plastic is the current gold standard for in vitro cancer modeling due to its ease of use and low cost. Monolayer culture allows for studies to be performed under completely controlled conditions in a highly reproducible manner and can typically yield results within shorter experimental timescales than in vivo models, making them suitable for high-throughput screening of drug candidates. However, cancer cells grown in 2D culture can differ substantially from those in vivo in terms of their morphology, protein signaling, and drug response [39]. Recent studies have shown that cancer treatment outcomes do not solely rely on cancer cell-intrinsic properties, but that these are also dependent on 3D cell-ECM and cell-cell interactions between cancer cells and their native niche [40,41], which are absent in 2D culture.
In vivo models are generally used to validate findings after initial testing has been performed in 2D culture, due to their higher cost and lower-throughput. In vivo models have the advantage of providing a relevant physiological context of tumor growth allowing them to more accurately predict tumor development and drug response than 2D culture. Subcutaneous xenograft tumor models, in which cancer cells are injected directly under the skin and allowed to develop into a tumor, are among the most common type of in vivo model used in cancer research due to their simplicity and low cost [39]. However, subcutaneous models lack the key mineralization cues and other cell types present in the bone niche, which are important modulators of the cancer cell phenotype [42–44]. Recent studies have shown that OS cell phenotype cannot be fully retained in mouse subcutaneous models, which lack interactions with native bone [45]. Orthotopic models of bone cancer, in which cancer cells are directly injected into a bone marrow cavity such as that of the femur or the tibia, have been shown to better retain OS cell phenotype [45]. Due to the pivotal role of the bone niche in modulating cancer cell phenotype, orthotopic bone cancer models will be critical for in vivo validation of in vitro findings.
Recent studies have demonstrated that bone cancer cells utilize paracrine signals from mesenchymal stem cells (MSCs), osteoblasts, osteoclasts, and immune cells present in the bone niche to favor their own growth advantage and survival [46–48]. In addition, extracellular matrix cues can promote growth and decrease bone tumor sensitivity to therapeutics [49]. While in vivo models are necessary for validation, these have the disadvantage of providing poor control over study parameters and as a result, elucidating specific mechanisms by which the bone niche modulates bone cancer progression and drug response is often not possible. Tissue-engineered, 3D in vitro bone cancer models can help to bridge the gap between preclinical in vitro screens and in vivo models by providing a more physiologically representative context for assessing drug efficacy. Recent studies have shown that 3D culture can better mimic the cancer cell phenotype than 2D culture [39], as these can incorporate key features of the bone tumor niche, such as 3D cell-cell and cell-ECM interactions (Table 1). Tissue-engineered bone cancer models have the potential to identify specific microenvironmental drivers of chemoresistance, which could be instrumental in developing improved methods of sensitizing cancer cells to therapeutics. In this section, we summarize emerging efforts in developing 3D in vitro models of osteosarcoma and Ewing’s sarcoma and discuss their potential for recapitulating tumor drug resistance and interactions with the native bone niche (Table 2).
Table 1.
Comparison of models used to study primary bone cancer development.
| Model | 2D Monolayers | 3D Models | Animal models |
|---|---|---|---|
|
| |||
| Tunability | ++ | ++ | + |
| Physiological Relevance | + | ++ | +++ |
| High-throughput | +++ | ++ | + |
| Low Cost | +++ | ++ | + |
Table 2.
Summary of previously reported 3D models of primary bone cancer using different biomaterials and bone cancer cell types.
| 3D Model | Biomaterial | Cancer | Refs. |
|---|---|---|---|
|
| |||
| Spheroids Soft biomaterials | – | Ewing’s sarcoma | [57] |
| – | Osteosarcoma | [56] | |
| Matrigel | Osteosarcoma | [63,64] | |
| Collagen | Ewing’s sarcoma | [61] | |
| Osteosarcoma | [60] | ||
| Hyaluronic acid | Ewing’s sarcoma | [61] | |
| Poly(ethylene glycol) diacrylate | Osteosarcoma | [65] | |
| Stiff biomaterials | Porous silk sponge | Osteosarcoma | [74] |
| Decellularized bone | Ewing’s sarcoma | [70,71] | |
| Poly(ε-caprolactone) (PCL) | Ewing’s sarcoma | [39,69,77] | |
| Poly(propylene fumarate) (PPF) | Ewing’s sarcoma | [78] | |
2.2.1. Spheroid models
Initial efforts in transitioning 2D cultures into 3D relied on the use of tumor spheroids, whereby a 3D structure is formed in suspension through the spontaneous aggregation of cells [50]. Tumor spheroids have been widely used in cancer research as they can be induced through simple and well-established techniques such as hanging drop, liquid overlay, and spinner flask methods [51]. Tumor spheroids are capable of providing 3D cell-cell and cell-ECM interactions similar to those found in vivo [52,53]. In addition, large spheroids (400–600 μm) can form oxygen and nutrient gradients which lead to the formation of proliferation gradients and zonal heterogeneity. Such heterogeneity leads to the formation of proliferative, quiescent, and necrotic zones and a hypoxic core which closely resemble those found in vivo [52,54]. As a result, the gene and protein expression signature of tumor spheroids are much closer to those of in vivo tumors than 2D culture models in terms of tumor proliferation, metabolism, angiogenesis, and drug resistance. [55]. In addition, multicellular spheroids allow for the study of bone cancer cell interactions with other cell types in the bone niche. OS spheroids have been shown to be more resistant to chemotherapeutics such as doxorubicin, cisplatin, taurolidine, and taxol than their 2D counterparts [56], making them an attractive platform for studying drug resistance. Similar results have been observed in spheroid models of EWS for drugs such as doxorubicin, etoposide, and carboplatin [57]. Multicellular spheroids of osteosarcoma cells and MSCs have demonstrated that MSC crosstalk with OS cells recapitulate cancer cell dormancy in vitro and can provide a relevant platform for studying drug resistance in dormant cancer cells [58]. OS spheroids have also been used in coculture with endothelial cells grown in 2D monolayers to study the role of 3D OS culture on angiogenesis in vitro [59]. Nigris et al. found that OS cells cultured in 3D upregulated expression of HIF and VEGF, which promoted tube formation by endothelial cells. Interestingly, this model facilitated the discovery of the role of CXCR4/YY1 signaling in promoting tumor angiogenesis in vivo [59]. Although tumor spheroids can recapitulate in vivo drug responses and incorporate interactions with stromal cells, these are challenging to employ for mechanistically studying the role of the bone niche in cancer progression, as they provide limited control over cell-matrix and cell-cell interactions. As a result, such models are not suitable for studying cancer cell invasion and interactions with various tissue interfaces.
2.2.2. Modeling primary bone cancer using soft biomaterials
Following the advent of spheroid culture, soft biomaterials such as hydrogels (1–150 kPa) were employed for modeling cancer in 3D. In contrast to spheroids, hydrogels can be endowed with a broad range of biochemical and biophysical properties and allow for spatial patterning of cellular and matrix cues. Hydrogels have been widely used to study cancer cell invasion, matrix remodeling, angiogenesis, and responses to matrix properties such as stiffness and composition in soft tissue tumors [3]. Hydrogel-based models of primary bone cancer have been developed using naturally-derived polymers such as collagen type I [60,61], hyaluronic acid [61], alginate [62], and Matrigel [63,64] and synthetic materials such as poly(ethylene glycol diacrylate) (PEGDA) [65]. Although hydrogel models do not mimic the stiffness range of bone tissue, these have the advantage that they can be designed to mimic bone composition. For instance, OS cells cultured in a 3D collagen matrix display differential responses to phosphatide inositol-3 kinase (PI3K) pathway inhibition, a promising anti-cancer target which regulates cell growth, migration, and survival, compared to cells grown in 2D. Moreover, collagen gel stiffness further modulates PI3K inhibition responses [60].
Hydrogels have also been used extensively in combination with hydroxyapatite, the main mineral component in bone, to promote bone formation in vitro and in vivo [66]. Findings from previous work in bone tissue engineering have demonstrated that incorporation of hydroxyapatite can modulate stem cell phenotype towards osteogenic differentiation, yet the role of hydroxyapatite in modulating bone cancer progression remains unknown. The overall effect of scaffold composition on bone cancer phenotype also remains unstudied and will require the use of side-by-side comparisons to identify the role of biochemical cues in tumor progression.
In addition, 3D matrix stiffness has been found to be vital in the maintenance of cancer stem cell (CSC) and epithelial-mesenchymal transition (EMT) markers for osteosarcoma. Using PEGDA hydrogels of varying stiffness, Jabbari et al. demonstrated that the optimal stiffness for maintaining CSC and EMT marker expression was different for osteosarcoma, breast cancer, and colorectal cancer, such that OS cells required higher stiffness (50 kPa) while breast cancer required lower stiffness (5 kPa) hydrogels to maintain this phenotype [65]. These results highlight the need to develop customized biomaterials for modeling primary bone cancer in vitro.
2.2.3. Modeling primary bone cancer using stiff biomaterials
In addition to hydrogels, 3D bone cancer models can be developed using stiff biomaterials (MPa range), such as poly(esters) and ceramics. Given that stiff matrices have traditionally been regarded to promote bone tissue formation, many tissue-engineered systems which were originally designed to promote bone regeneration in vivo can serve as promising platforms for modeling the bone cancer niche in vitro. For example, a recent study employed electrospun poly(ε-caprolactone) (PCL) scaffolds to study the effects of 3D culture on EWS cell phenotype (Fig. 2A) [39]. Compared to 2D culture, PCL scaffolds promote EWS tumor growth kinetics, protein expression patterns, and response to doxorubicin therapy that more closely mimic subcutaneous tumor models in mice. (Fig. 2B, C) [39]. IGF-1R/mTOR pathway, a promising therapeutic target in EWS, was found to be inactive in 2D culture (Fig. 2B), demonstrating that 2D culture cannot be reliably used to predict the drug response to anti-IGF1R therapies. In contrast, PCL scaffolds supported retention of IGF-1R signaling in EWS cells to comparable levels of in vivo models.
Fig. 2.
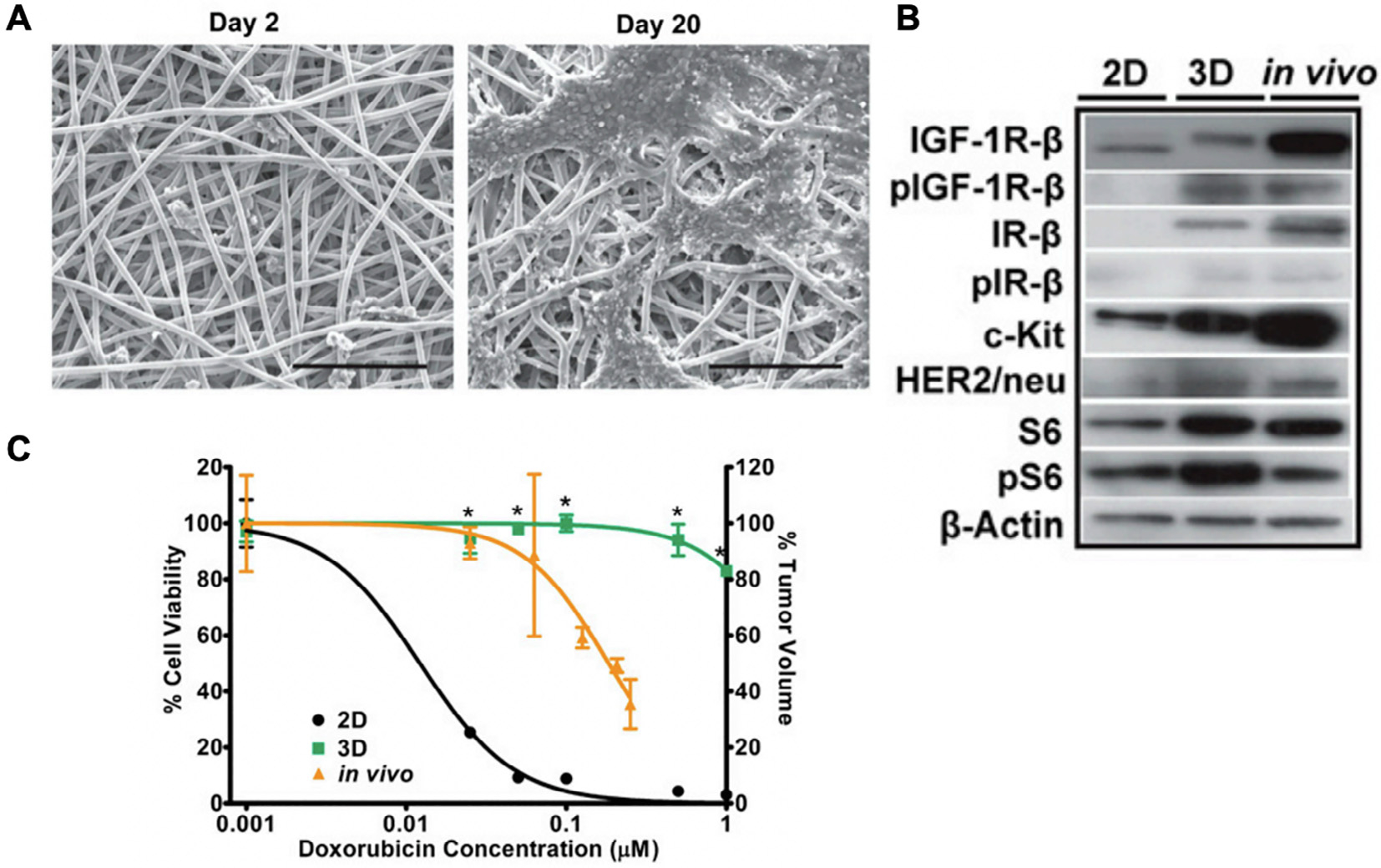
A 3D model of Ewing’s sarcoma (ES) using poly(ε-caprolactone) (PCL) scaffolds. (A) Scanning electron micrographs of ES cells in PCL scaffolds on day 2 and day 20. Scale bar: 200 μm. (B) Expression of IGF-1R/mTOR pathway-related proteins in ES cells cultured in 2D, 3D PCL scaffolds, and in vivo tumors. (C) ES cell dose response to doxorubicin therapy in 2D culture (black, IC50 = 0.0122 μM), 3D PCL scaffolds (green, IC50 = 2.738 μM), and in vivo (yellow, IC50 = 0.184 μM). Reproduced with permission from [39].
Cancer cell-stromal cell interactions in the bone niche can also enhance cancer resistance to therapy [58,67,68]. Further developments using PCL scaffolds demonstrated that coculture of EWS cells with MSCs dramatically enhances IGF-1 secretion by EWS cells through MSC activation of IL-6 and Stat3, which increased tumor cell resistance to IGF-1R blockade with dalotuzumab [69]. To mimic the ECM composition of native bone, decellularized bone scaffolds have been utilized as 3D models of EWS [70,71]. Decellularized bone scaffolds promoted the retention of hypoxic and glycolytic markers in EWS and coculture of EWS cells with osteoblasts and monocyte-derived osteoclasts enhanced expression of oncogenic markers, EWS/FLI1 and NKX2.2, and downregulation of bone markers OPN, BSP, and TRAP [70]. Osteosarcoma tumors are also known to promote angiogenesis to support tumor growth and metastasis [72,73]. Silk scaffolds have been used to model OS cell-induced angiogenesis in vitro [74]. OS cells cultured in silk scaffolds showed dramatic increases in expression of angiogenic factors secreted by tumor cells, such as FGF, HIF-1α, IL-8, and VEGF-A compared to 2D culture. Future angiogenesis studies could leverage such platforms to elucidate osteosarcoma-endothelial cell crosstalk in 3D through coculture. Future studies could further harness such tissue-engineered models to elucidate the crosstalk between OS cells and endothelial cells in 3D co-culture. Given this emerging data demonstrating the importance of stromal cells in bone cancer progression and drug resistance, in vitro strategies incorporating these cell types will be critical for developing new therapies.
Biomaterials also provide in vitro 3D platforms for studying the role of mechanical stimuli in bone cancer progression. Bone tissue is continuously exposed to mechanical forces in vivo, and such forces have been shown to be vital for bone development and homeostasis [75,76], yet their role in bone cancer is largely unknown. Shear stress in bone is generated by interstitial fluid flow caused by compressive loading during physical activity. To study the role of shear stress in bone cancer, EWS cell-laden PCL scaffolds were exposed to flow perfusion in a bioreactor and examined for the response to therapy [77]. Flow perfusion significantly impaired EWS cell sensitivity to IGF-1R blockade by enhancing secretion of IGF-1. To capture the heterogeneity of shear stresses that tumor cells experience within the native bone environment, Trachtenberg et al. developed 3D-printed poly(propylene fumarate) scaffolds with pore size gradients which, when cultured under flow perfusion, exposed EWS cells to a gradient of shear stresses (Fig. 3A, B) [78]. Flow perfusion within layers of highest shear stress promoted higher rates of EWS cell proliferation when compared to low shear stress regions and this effect correlated with secretion of IGF-1 by EWS cells (Fig. 3C). Such 3D culture models with gradient properties have promising applications for studying tumor heterogeneity within the bone niche and its effect on responses to drug therapy.
Fig. 3.
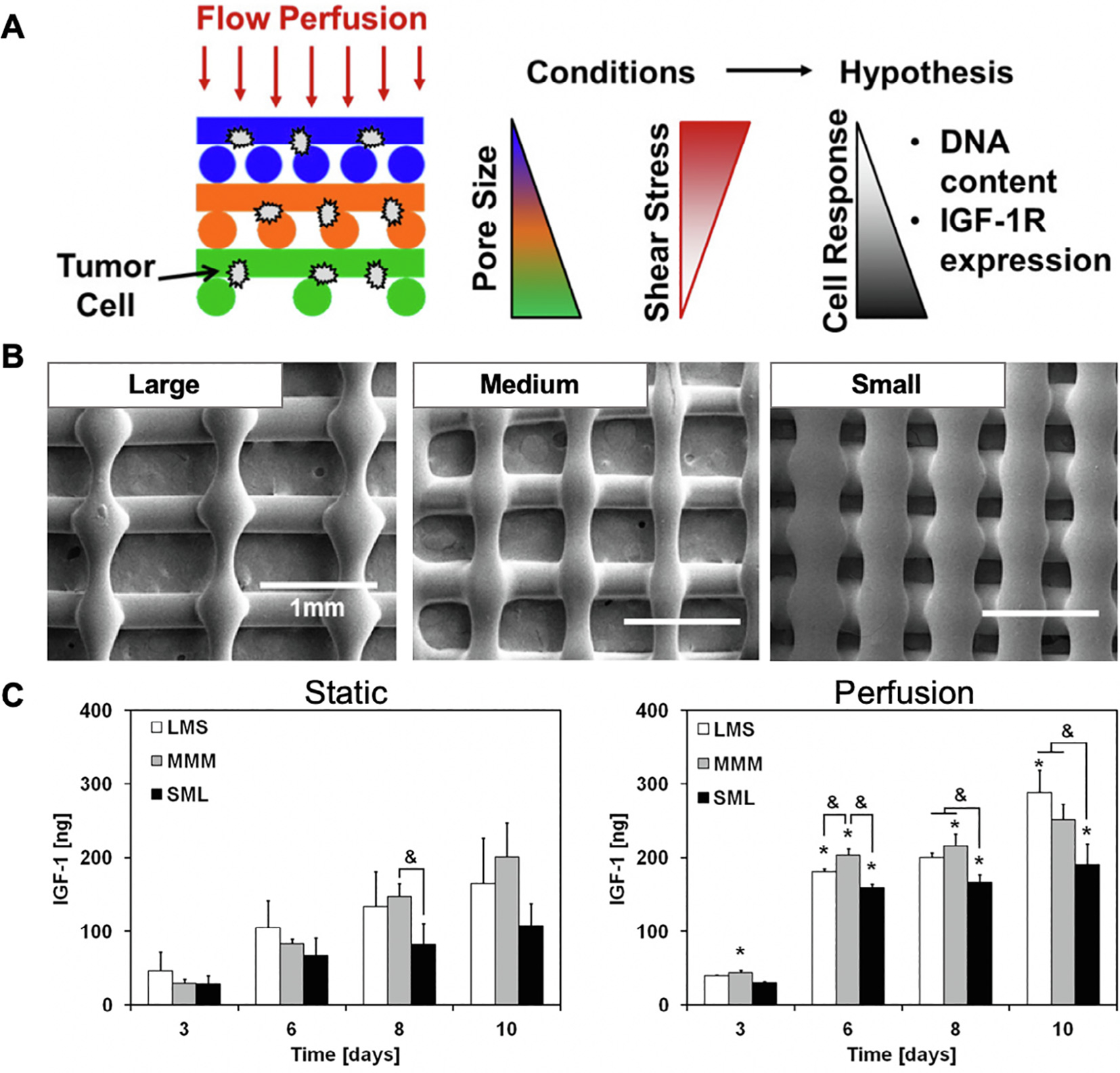
Poly(propylene fumarate) (PPF) scaffolds with varying pore size for elucidating the effect of shear stress gradients on Ewing’s sarcoma (ES) cells. (A) Schematic representation of experimental approach. (B) Scanning electron micrographs of large, medium, and small pore size regions within PPF scaffolds. (C) Quantification of IGF-1 secretion by ES cells cultured in PPF scaffolds under static and perfusion conditions. Reproduced with permission from [78].
Emerging work on developing tissue engineered 3D in vitro models of primary bone cancer has identified the role of 3D cell-cell interactions, matrix stiffness, cancer-stromal interactions, and shear stress in modulating cancer progression and drug response. As such, these properties should be incorporated in future studies. In addition, the effect of matrix composition and the role of other cell types in the niche, such as immune cells, remain to be evaluated in 3D models and will likely be of importance in guiding drug development.
3. Modeling cancer metastasis to bone
3.1. Basic bone metastasis biology
In contrast to primary bone cancer, where the malignant cells are native to the bone environment and are derived from bone progenitor cells, bone metastases develop through interactions that inherently involve cells of different tissue origin. To develop effective therapies for treating bone metastasis, the following key questions remain to be answered: (1) What components of the bone niche attract certain cancer cells to preferentially metastasize to bone? (2) How do bone niche cues modulate the phenotype of metastatic cancer cells from other tissue origins? (3) What are the mechanisms that metastatic cancer cells employ to destroy bone tissue? and (4) How does bone resorption further impact cancer metastasis?
Bone is the most frequent metastatic site for common tumors such as breast cancer, prostate cancer, and lung cancer [2], yet why these cancer cells preferentially metastasize to bone remains largely unclear. Pediatric primary bone cancers also frequently metastasize to the bones [79]. Recent in vitro and in vivo findings have shown that chemokine signaling may play a key role in promoting cancer cell homing to the bone niche [80,81]. Further studies have suggested that integrin binding with bone ECM proteins, such as osteopontin (OPN) and bone sialoprotein (BSP) [82–84] may promote cancer cell adhesion to the bone matrix. Once in the bone, metastatic cancer cells proliferate, invade, and disrupt normal bone homeostasis through complex and dynamic interactions with the native niche. In healthy bone tissue, homeostasis is maintained through the process of bone remodeling, whereby bone resorption by osteoclasts is counterbalanced by the stimulation of new bone formation by osteoblasts. When cancer cells are present, however, this balance becomes disrupted, resulting in abnormal bone tissue formation and/or dysregulated bone resorption. As such, bone metastases generally contain a combination of lesion sites that are either osteoblastic (bone forming) or osteolytic (bone resorbing) in nature [30,85]. In both of these cases, bone integrity and stability is compromised, resulting in severe pain, fractures, spinal cord compression, and ultimately, death [30]. Finally, how cancer cells induce bone resorption and the role of bone resorption in promoting bone metastasis progression remain poorly understood. One current hypothesis stipulates that bone breakdown promotes tumor growth through the release of growth factors, such as IGF-1 and TGF-β, by cleaving them from their respective binding proteins within bone matrix [85]. Elucidating the unknown driving mechanisms in bone metastasis progression would guide development of therapeutics that specifically target these steps.
One of the major challenges for elucidating the processes that drive bone metastasis is the difficulty in modulating specific cell-matrix or cell-cell interactions using in vivo models. Tissue engineering represents an exciting new avenue for uncovering unknown biology in bone metastasis by providing 3D in vitro culture models with tunable complexity that mimic cell-matrix and cell-cell interactions in the bone niche. Because bone metastases develop through a complex, multistep process, the 3D in vitro platform of choice and its requirements will be defined by the specific stage of the metastatic cascade being studied. To mimic different stages of bone metastasis development, 3D in vitro models have harnessed existing tissue engineering tools such as microfluidic devices, natural and synthetic biomaterial scaffolds, and decellularized bone.
Elucidating the mechanisms by which the bone niche promotes metastasizing cancer cells to preferentially colonize the bones requires the use of platforms which can mimic cancer cell intravasation, movement through the circulation, and subsequent extravasation into bone. Microfluidic devices are an attractive platform for studying these early stages of the metastatic cascade as they provide perfusable microenvironments that support vascular network formation and cell culture in 3D using conventional hydrogels that can be perfused and crosslinked within the device [86,87]. Microfluidic devices can also be engineered to provide mimics of more than one type of tissue within separate chambers, allowing for the study of organ-specific colonization to be performed within a single device. While microfluidic devices are suitable for studying bone colonization by metastatic cancer cells, they are limited in the types of biomaterials that they can incorporate. For example, fluids with high viscosities and harsh solvents are not compatible with flow in microfluidic devices. Macroporous scaffolds, such as those previously developed for bone tissue engineering, would also not be compatible with such platforms since most of these require prefabrication using methods that cannot be performed within a microfluidic device. Finally, microfluidic devices provide limited volumetric throughput of samples to be harvested for downstream analyses.
To examine the role of bone niche cues in modulating metastatic bone cancer cell phenotype, recent studies have leveraged biomaterials which have been previously used successfully for bone tissue engineering. These include naturally-derived biomaterials such as chitosan [44], gelatin [88], and hyaluronic acid [89], and synthetic materials including polyurethane [90], PCL [91–93], and poly(lactide-co-glycolide) (PLG) [43]. In addition, emerging studies have incorporated the use of hydroxyapatite coatings to study the effect of bone minerals on the cancer cell phenotype [42–44,88,94–96], which are suitable for elucidating the role of bone matrix on tumor progression. In order to elucidate the underlying interactions between the bone niche and cancer cells, biomaterials with tunable parameters such as stiffness and biochemical composition are highly desirable as these would allow the user to systematically vary individual parameters and identify their role in tumor progression. Thus far, only the effect of hydroxyapatite has been explored. Furthermore, the effect of other bone matrix proteins such as OPN and BSP has only been tested in 2D coatings [82,83] and has yet to be studied using 3D scaffolds. Decellularized bone scaffolds have also been employed to study bone metastasis [97]. While these have the advantage of recapitulating bone composition and structure, they do not provide control over matrix cues, and as a result cannot be used to decouple the role of matrix components in bone metastasis. However, decellularized bone would be highly advantageous for studies of bone destruction. The destruction of bone tissue after bone metastasis inherently involves interactions between cancer cells and cells in the native bone niche. Therefore, elucidating how cancer cells destroy bone tissue will require 3D coculture platforms which include osteoblasts, osteoclasts, and/or their progenitors. Previous studies have incorporated coculture of osteoblasts with cancer cells [86,87,90,92,96], but the effect of direct coculture with osteoclasts remains unknown. Using breast cancer- and prostate cancer-derived bone metastases as model diseases, here we review recent progress in developing tissue engineered 3D in vitro models towards answering some of the questions above (Table 3).
Table 3.
Summary of previously reported 3D models to elucidate key biological steps involved in bone metastases using different biomaterials.
| Question | Cancer model | Biomaterials | Refs. |
|---|---|---|---|
|
| |||
| How does bone tissue recruit metastatic cancer cells? | Breast cancer | Fibrin | [87,102] |
| Prostate Cancer | PCL | [91] | |
| How does the bone matrix modulate cancer phenotype? | Breast cancer | Chitosan + Hydroxyapatite | [44] |
| Poly-ether-urethane | [90] | ||
| PLG + Hydroxyapatite | [42,43] | ||
| PEGDA + Hydroxyapatite | [94] | ||
| Gelatin + Hydroxyapatite | [88] | ||
| Prostate cancer | PCL | [91] | |
| Collagen + Hydroxyapatite | [95,121] | ||
| PCL-TCP + PEGDA | [92] | ||
| Hyaluronan | [89] | ||
| Decellularized bone | [97] | ||
| PCL/gelatin | [93] | ||
| Silk | [119] | ||
| How do cancer cells destroy bone tissue? | Breast Cancer | PLG + Hydroxyapatite | [42,43] |
| Gelatin + Hydroxyapatite | [88] | ||
| Prostate Cancer | PCL | [91] | |
| Decellularized bone | [97] | ||
| Silk | [119] | ||
PCL: poly(ε-caprolactone); PLG: poly(lactide-co-glycolide); PEGDA: poly(ethylene glycol) diacrylate; TCP: Tricalcium phosphate.
3.2. Tissue-engineered in vitro models of breast cancer bone metastasis
3.2.1. Breast cancer metastasis to bone
Breast cancer is the most common female cancer in the US and the second most frequent cause of cancer-related death in women [1]. About 75% of patients with advanced breast cancer develop bone metastases and 87% of these die within 5 years of diagnosis [30,98,99]. The destruction of healthy bone tissues by breast cancer cells is one of the major causes of patient morbidity and death [30,98]. In breast cancer-derived bone metastasis, the bones are destroyed through lesions that are predominantly osteolytic in nature, although osteoblastic lesions can also be present [85]. In this section, we survey recent tissue-engineered, 3D in vitro models designed to elucidate the role of the bone niche in promoting breast cancer cell recruitment, growth, and destruction of bone tissue.
3.2.2. How does bone tissue recruit metastatic breast cancer cells?
Chemokine signaling is vital for maintaining homeostasis in healthy tissues through recruitment of cells during processes such as inflammation and tissue regeneration. Previous work using 2D culture models suggest that bone tissues can attract breast cancer (BrCa) cells by chemokine signaling or adhesion molecules. For example, MSCs in the bone niche secrete CXCL12, which bind to CXCR4 expressed on the BrCa cell surface. CXCR4 overexpression on BrCa cells has also been reported to be associated with increased risk for bone metastases [80,100]. In addition to chemokine signaling, BrCa cells also express αvβ3 and αvβ5 integrins, which bind to bone ECM proteins such as osteopontin, bone sialoprotein, and vitronectin [82–84]. In addition, BrCa cells express E-cadherin which can engage N-cadherin on osteoblast cells [101]. Interestingly, BrCa cells can also secrete OPN and BSP, and this has been associated with poor prognosis and higher potential for metastasis to bone [83,84].
To mimic the process of breast cancer-bone metastasis through a blood vessel network, Bersini et al. developed a 3D microfluidic model with endothelial cell-lined microchannels mimicking vasculature embedded in tissue engineered bone niche [102]. Specifically, human bone marrow-derived MSCs were first embedded in a collagen hydrogel and differentiated towards bone lineage, then lined up with endothelial cells, offering a 3D tissue engineered bone model for studying BrCa extravasation towards bone. Compared to collagen-only control, tissue engineered bone models significantly enhanced recruitment of BrCa cells, as shown by higher extravasation, migration distance, and subsequent development of micrometastases. The authors further demonstrated that recruitment of BrCa cells towards the tissue engineered bone was mediated through CXCL5, a chemokine secreted by osteoblasts which binds to CXCR2 expressed by BrCa cells. To study organ-specific colonization of metastatic breast cancer cells, Jeon et al. further expanded the application of this model to mimic the microenvironment of bone and muscle (Fig. 4A), and compared the extravasation of BrCa towards either a bone or muscle-mimicking niche [87]. Consistent with clinical observations, BrCa cells demonstrated much higher extravasation towards the bone-mimicking niche compared to the muscle-mimicking niche (Fig. 4B, C). These results validate the physiological relevance of such tissue engineered models for mimicking organ-specific colonization of metastatic breast cancer cells.
Fig. 4.
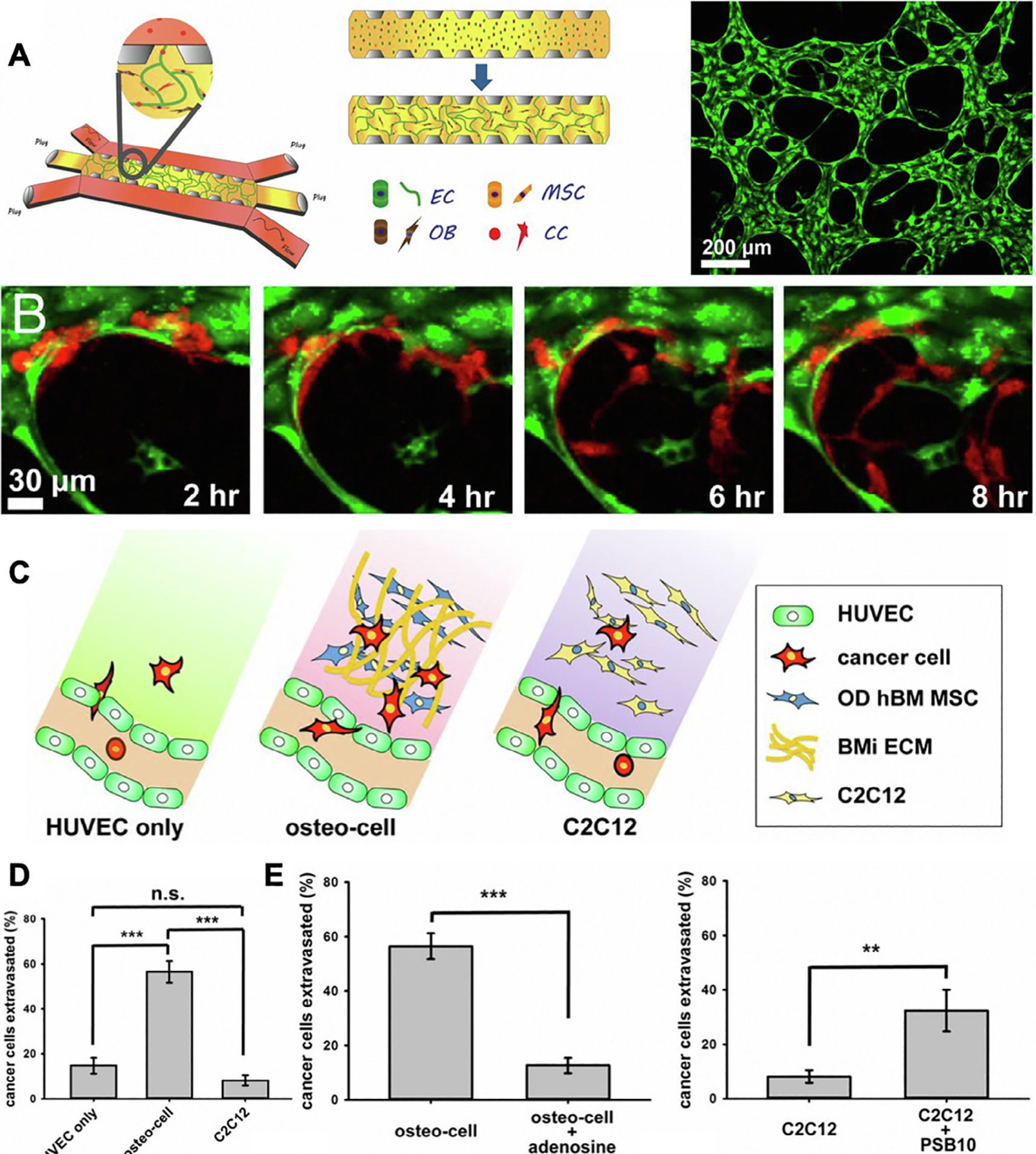
A microfluidic-based model to mimic breast cancer cell extravasation within a microvascularized, bone-mimicking niche. (A) Triculture of endothelial cells (ECs), MSCs, and osteoblasts (OBs) initially seeded in a fibrin gel surrounded by two perfusable media channels. Cancer cells (CC) were introduced on day 4. ECs formed microvascular networks with branched structures (Right Panel). (B) Cancer cells (red) extravasated through engineered vasculature (green) into the surrounding bone-mimicking environment. (C) Schematic representation of cancer cell extravasation observed in HUVEC only, osteo-cell, and C2C12 (muscle-mimicking) environments. (D) Percentage of cancer cell extravasation into HUVEC only, osteo-cell, and C2C12 environments. (E) Percent extravasation of cancer cells into osteo-cell and C2C12 environments after treatment with adenosine and PSB10 (A3AR antagonist). Reproduced with permission from Ref. [87].
In addition to studying organ-specific colonization, such tissue engineered models can also provide a tool for testing the role of specific signaling in driving or inhibiting cancer cell metastasis, allowing discovery of potential new therapeutic targets for blocking undesirable metastasis. Given that muscle-mimicking niche inhibited breast cancer migration and highly expressed A3 adenosine, the authors hypothesized that this may be a mechanism that muscle cells employ to inhibit BrCa metastasis towards muscle. Indeed, blocking A3 adenosine receptors on BrCa cells increased extravasation into muscle-mimicking matrices. In contrast, overexpressing A3 adenosine in bone niche cells decreased BrCa metastasis (Fig. 4D, E). Together, these results suggest adenosine signaling is involved in BrCa cell metastasis. While this study shows the promise of using tissue engineered 3D models for uncovering unknown mechanisms involved in driving cancer cell migration, the 3D matrix used was collagen gel alone, which does not mimic the stiffness or mineral components present in the bone niche. Future research on designing 3D biomaterials that better mimics the biochemical and mechanical properties of bone niche will be necessary to provide a better understanding of how bone-specific niche cues, such as mineralization, contribute to recruit BrCa cell colonization in bone.
3.2.3. How does the bone matrix modulate breast cancer phenotype?
When circulating breast cancer cells migrate and adhere to the bone surface, they are presented with a biochemical and physical environment that is drastically different from breast tissues. While breast tissue is soft and lacks minerals, bone is the stiffest tissue in the human body and is composed of 70% inorganic minerals. Using mineral-containing biomaterials as 3D scaffold for growing BrCa cells, researchers have sought to elucidate the role of bone minerals in modulating breast cancer phenotype in 3D. In a study by Pathi et al., PLG scaffolds were coated with hydroxyapatite (HA), the main mineral component of bone to mimic bone niche [43]. HA-containing PLG scaffolds significantly enhanced adhesion and proliferation of BrCa cells compared to PLG scaffold without HA. Using 3D scaffolds coated with varying size of HA, recent studies suggest BrCa phenotype can be further modulated by the specific properties of HA particles. In native bone tissue, HA particle size and crystallinity can vary during bone remodeling. PLG scaffolds coated with smaller, less-crystalline HA particles have been shown to promote breast cancer cell adhesion and growth by enhancing the adsorption of serum proteins onto the scaffold surface [42]. The role of HA crystallinity has also been investigated using chitosan scaffolds. Breast cancer cells cultured with 10% nanocrystalline HA showed significantly higher adhesion and proliferation when compared to 20% nanocrystalline, 20% microcrystalline, and amorphous HA groups [44]. These findings suggest a potential role for bone remodeling in promoting breast cancer growth through interactions with HA.
In addition to cell-matrix interactions, tissue engineered 3D models involving multiple cell types have also been developed to elucidate the role of cell-cell interactions in promoting breast cancer proliferation and drug resistance to chemotherapy. Towards this goal, Zhou et al. utilized hydroxyapatite-containing gelatin scaffolds for co-culturing BrCa cells with cell types present in bone niche such as osteoblasts and MSCs [88]. Co-culture of BrCa cells with either osteoblasts or MSCs promoted BrCa cell proliferation and secretion of VEGF. Co-culture BrCa cells with MSCs also induced BrCa cell resistance to fluorouracil, a chemotherapeutic drug for treating breast cancer [94]. Similarly, another study using polyurethane scaffolds showed co-culture of BrCa cells with osteoblasts increased E-cadherin expression in BrCa cells and tumor aggregates [90]. Together, these results validate the importance of cell-cell interactions in driving BrCa metastasis. For future studies, it would be important to design biomaterials that better mimic the bone matrix compositions, and incorporate other key cell types in bone niche, such as osteoclasts, to mimic the complex cell-cell interactions in bone niche.
3.2.4. How do breast cancer cells destroy bone tissue?
Bone destruction is one of the main causes of bone metastasis-associated pain and morbidity [30,85,98]. Breast cancer cells that have invaded into bone secrete PTH-rP, IL-8, and IL-11 which can stimulate differentiation and subsequent activation of osteoclasts, leading to bone resorption [30,98]. Breast cancer-derived bone metastases are predominantly osteolytic, whereby bone resorption occurs at much higher rates than new bone formation, resulting in a significant loss of bone mass [85]. Emerging efforts in tissue engineering have used bone-mimetic scaffolds to examine the effect of bone minerals on breast cancer cell secretion of osteoclastogenic factors. For example, mineralized PLG scaffolds were found to enhance IL-8 secretion by breast cancer cells when compared to a non-mineralized control [43]. Furthermore, media conditioned by breast cancer cells cultured within mineralized PLG scaffolds stimulated osteoclast differentiation in vitro. When coated with HA particles of varying crystallinity, PLG scaffolds containing more crystalline HA stimulated breast cancer cell expression of IL-8 [42]. Recently, it has been hypothesized that in addition to promoting osteoclast activity, breast cancer cells also further tip the scale towards bone loss by actively suppressing new bone formation [103,104]. Findings using 3D printed gelatin/HA scaffolds to model breast cancer interactions with osteoblasts and MSCs have provided support for this hypothesis by demonstrating that breast cancer cells inhibit the proliferation and alkaline phosphatase (ALP) activity of these cells when cocultured within 3D bone-like matrices [88].
3.3. Tissue-engineered in vitro models of prostate cancer-bone metastasis
3.3.1. Prostate cancer metastasis to bone
Bone is the most frequent site for prostate cancer (PCa) metastasis [31]. More than 80% of advanced PCa patients are diagnosed with bone metastases, reducing patient survival to 25% within 5 years [105]. Currently, there are no treatment options for PCa-bone metastases besides palliative care [106]. Mechanisms owing to the high propensity and recruitment of PCa to bone are not well understood but are generally attributed to chemotaxis, cancer cell-endothelium interactions, and the “seed and soil” hypothesis [106,107]. The progression and metastatic behavior of PCa is complex and dynamic as it depends on the roles of the host microenvironment stromal cells, immune cells, endothelial cells, growth regulatory factors, and the ECM [106]. PCa cells also perturb the bone remodeling processes to promote the formation of osteoblastic lesions, resulting in dysregulated deposition of woven bone, which is mechanically weak and dysfunctional compared to mature, lamellar bone [108]. Here, we discuss tissue engineering approaches to create biomimetic, 3D cancer models as tools for elucidating the mechanisms that allow the bone matrix to recruit prostate cancer cells, modulate cancer cell behavior and progression, and ultimately lead to bone destruction.
3.3.2. How does bone tissue recruit metastatic prostate cancer cells?
The cellular and molecular mechanisms believed to drive metastatic PCa cell homing to the bone matrix include a complex assortment of chemokine signaling, adhesion molecules, crosstalk between PCa cells and the bone environment, and attraction to the hematopoietic stem cell (HSC) niche [109]. Given 2D cell culture models often fail to mimic disease phenotype, previous research have relied heavily on mouse models for mechanistic studies [109,110]. Previous in vivo studies have suggested that several factors are involved in PCa homing to bone, including the chemokine signaling pathway CXCL12/CXCR7 [111] and adhesion molecules like Annexin2 [112] and cadherin-11 [109,113]. In addition, tumor cells secrete factors that stimulate bone stroma cells such as osteoblasts and osteoclasts, and bone stroma cells respond by secreting factors that drive tumor growth and proliferation. This crosstalk and eventual establishment of an autocrine loop can ultimately lead to an aggressive disease [109]. Some factors and molecules involved in this crosstalk, as elucidated through in vivo models, include BMP, RANKL [114,115], and transcription factor RUNX2 (which in PCa cells have been shown to promote activity of secreted osteolytic factors) [116]. PCa cells were also shown to target the HSC niche during metastasis, and compete with HSCs for occupancy [117]. While these mouse models have provided great tools to reveal cellular interactions and mechanisms involved in PCa cell homing, they are limited by high cost and low throughput, and also face several inherent limitations. First, there are fundamental differences between mouse and human tumorigeneses, and species-specific interactions cannot be replicated using mouse models [91,106,118]. Second, the physiological complexity of mouse models, while useful for enhancing physiological relevance, also makes it difficult to assess the role of individual niche components in driving tumor metastasis. To overcome the challenges associated with mouse models, tissue engineered in vitro 3D models can provide an alternative tool to elucidate the homing mechanisms of prostate cancer towards bone.
Compared to in vivo models, tissue engineered in vitro models are low cost, support the use of human cells, and allow for defined and tunable niche inputs to facilitate mechanistic studies [106]. As an example, Holzapfel et al. developed tissue-engineered bone using MSC seeded polycaprolactone (PCL) scaffolds, which were transplanted into a mouse subcutaneous model (Fig. 5A–E). Such ectopic tissue engineered bone was shown to attract PCa cell homing inoculated via the left ventricle of the murine host and support macro-metastases (Fig. 5F–I) [91]. These results validate tissue engineered bone constructs provide some essential components that support the mimicry of PCa-bone homing in vivo. Future studies may harness tissue engineered bone tissues as in vitro models to study the role of individual bone niche cues in recruiting PCa cells.
Fig. 5.

Modeling prostate cancer-bone metastasis in vivo using tissue-engineered bone implanted in a mouse subcutaneous model as a target for metastasis. (A) Gross morphology of polycaprolactone (PCL) scaffolds. (B) Scanning electron micrographs of PCL scaffolds. (C) Actin (red) and nuclear (blue) staining and (D) Live/dead staining of human mesenchymal progenitor cells (hMPCs) seeded on PCL scaffolds. (E) Subcutaneous implantation of PCL scaffolds in mice. (F) Hematoxylin and eosin staining of engineered bone constructs. (G) TRAP staining showed activated osteoclasts within tissue-engineered bone. (H) Immunohistochemical staining showed prostate cancer cells infused through mouse left cardiac ventricle were detected inside tissue-engineered bone implanted in a mouse subcutaneous model. (I) Bioluminescence imaging confirmed prostate cancer cells metastasized to tissue-engineered bone. (J) Micro-computed tomography showing bone resorption in tissue-engineered bone containing prostate cancer metastases. (K) High magnification image of TRAP+ cells within tissue engineered bone with metastasis compared to control. Reproduced with permission from [91].
Unlike animal models, tissue engineered 3D in vitro cancer models allow more precise control for cell-matrix or cell-cell interactions. This makes it possible to assess the role of individual components within the bone niche for recruiting PCa cells to bone, which are very difficult to study using complex animal models. While harnessing tissue-engineered bone models to study PCa-bone metastasis are emerging, this is generally an unexplored field with little research completed. In the future, tissue-engineered bone models may be used to elucidate the role of chemotactic factors (e.g. CXCL12, TGF-β, BMPs, RUNX2), adhesion molecules or specific cell-matrix ligand interactions in driving cancer cell recruitment to bone. Apart from physical factors in the bone, models can also be established to test the role of hypoxia, acidic pH, and calcium in recruiting PCa cells to the bone [109]. Given the low cost and ease of fabricating tissue engineered cancer models, they could also facilitate high throughput drug testing to evaluate therapeutics identified from mechanistic studies.
3.3.3. How does the bone matrix modulate prostate cancer phenotype?
The bone matrix contains ~70% minerals and ~30% proteins, most of which is type I collagen [26]. Biomaterials have been used to recapitulate key bone matrix components and investigate how bone matrix cues modulate PCa cell phenotype. For example, a decellularized bone matrix produced from human primary osteoblasts was used to study PCa cell interactions with specific components in the bone niche [97]. The osteoblast-derived matrix supported PCa cell adhesion, proliferation, upregulated genes associated with increased invasive phenotype, and osteolysis by PCa cells [97]. When cultured in 3D decellularized bone matrix, PCa cells exhibited osteomimetic phenotype, as shown by increased osteonectin expression and elevated production of parathyroid hormone-related protein (PTHrP) [97].
Using biomaterials with tunable compositions and topographical cues, researchers have developed tissue engineered models to examine the role of cell-matrix interactions in PCa-bone metastasis. Hartman et. al. assessed the role of varying physical cues (fiber diameter, surface morphology, and porosity) or biochemical cues (with or without perlecan domain IV heparan sulfate proteoglycan) on supporting PCa cell phenotype retention using electrospun PCL scaffolds [93]. In another example, 3D silk scaffolds functionalized with BMP-2 was shown to stimulate PCa cell migration and allowed for osteomimicry, which enables PCa cells to survive, proliferate and invade in the bone [119]. These studies validate biomaterials can provide useful 3D in vitro models to elucidate the role of bone niche (biochemical and/or physical) cues in modulating PCa bone metastases, and such mechanistic studies can yield new insights in identification of new therapeutic targets.
In addition to cell-matrix interactions, tissue-engineered models can also be harnessed to elucidate the role of cell-cell interactions in driving PCa bone metastases. In one example, PCa cells were mixed with osteoblasts, and co-encapsulated in 3D MMP-degradable hyaluronic acid hydrogels. This coculture model allows cell-cell interactions through both direct cell-cell contact and paracrine signal exchange and was shown to support a co-stimulatory PCa-osteoblast relationship that mimics in vivo phenotype [89]. In another study, Sieh, et. al. encapsulated human PCa cells in PEGDA hydrogels, which was attached to a tissue engineered bone construct fabricated using human osteoblasts in a PCL-tricalcium phosphate scaffold. This model separated the two cell types physically but allowed exchange of paracrine signals. Paracrine signal exchange alone led to enhanced osteomimicry and modulated the expression of androgen-responsive genes in PCa cells [92]. Since androgen-deprivation therapy (ADT) is a standard of care for advanced PCa patients, this finding suggests that paracrine signals from bone tissues may induce androgen receptor activation, resulting in decreased therapeutic efficacy of ADT [95,120]. Overall, these studies validate that 3D co-culture models can be useful for elucidating the role of cell-cell interactions in driving PCa-bone metastasis.
Tissue-engineered models can also be used to assess the efficacy of new potential therapeutic candidates for treating PCa bone metastases. When PCa cells were cultured in 3D collagen I based scaffolds, they exhibited increased drug resistance to docetaxel treatment compared to 2D culture, which better mimics the in vivo drug response [95]. In a follow up study, PCa cells were co-cultured with osteoblasts in collagen-nHA scaffolds, for assessing efficacy of potential therapeutics using nanoparticle-mediated siRNA delivery [121]. One advantage of using tissue engineered in vitro cancer models for drug screening is low cost and ease for high-throughput screening to test the dosage responses or combinatorial drug effects. It can also provide a cost-effective platform to facilitate high-throughput drug screening using patient-specific cancer cells to help optimize treatment options.
3.3.4. How do prostate cancer cells destroy bone tissue?
While still at infant stage, recent emerging efforts have started to explore harnessing tissue engineered models to elucidate how prostate cancer cells destroy bone tissues upon invasion. Prostate cancer bone metastases are generally known to cause osteoblastic lesions [108]. However, when cultured on a decellularized bone matrix, PCa cells showed PTHrP induction, which is involved in activating osteoclast-initiated bone resorption [97]. These results suggest PCa cells can destroy bone through both osteoblastic and osteoclastic lesions. In another study, PCa cells were seeded in 3D silk scaffold modified with BMP-2, which also supported osteolytic activity in PCa cells [119]. As highlighted above, a tissue-engineered bone using MSC seeded polycaprolactone (mPCL) scaffolds were transplanted into a mouse subcutaneous model to determine if PCa cells could home, proliferate and develop metastases at this engineered site. Holzapfel et al. successfully demonstrated that PCa were able to infiltrate into the tissue-engineered bone, develop extensive osteolytic lesions, and stain for activated osteoclasts degrading within tissue engineered bone (Fig. 5J, K) [91]. However, tissue-engineered models demonstrating osteoblastic lesion or “woven” bone formation for prostate cancer bone metastases have yet to be seen.
4. Summary and future directions
The field of developing tissue engineered 3D models to mimic primary and metastatic bone cancer progression remains at its infancy. While extensive work has been done in developing 3D cancer models of soft tissues such as brain and breast cancer, little work has been done to design biomaterials to specifically mimic the bone niche, which is fundamentally distinct in both stiffness and composition. There remains a critical need to develop better tissue-engineered bone cancer models to bridge the technological gap between 2D culture and animal models by providing low cost and tunable in vitro systems that better recapitulate the 3D physicochemical and cellular environments found in native bone tissues.
Emerging tissue engineered 3D platforms for modeling primary bone cancer have enabled the study of the role of bone matrix, stromal cell interactions, and shear stress in modulating cancer progression and resistance to drug therapies. Future work can further leverage biomaterials design to elucidate remaining unknown questions such as the role of bone minerals or other matrix cues in modulating the primary bone cancer phenotype. Recent findings have shown that 3D models better capture the bone cancer phenotype compared to 2D culture, yet the role of the choice of biomaterial on the cancer phenotype remains to be studied. Further work performing side-by-side comparisons of biomaterials against an in vivo control can be used to identify the optimal biomaterials design for mimicking in vivo tumor progression. In addition, such biomaterials platforms can also be integrated with perfusion systems to interrogate the synergistic effects of mechanical stimuli and bone matrix. For bone metastasis, tunable tissue engineered 3D platforms are needed to decouple the roles of cell-cell signaling and bone matrix in promoting colonization of bone tissue by metastatic cancer cells. Recent work has demonstrated that interactions with the bone niche promote cancer cell production of osteoclastogenic factors, thereby stimulating bone resorption [30,85], yet the role of bone resorption on cancer progression has not been addressed in previous models. Future 3D models should integrate additional cell types that mimic specific aspects of the microenvironment. For instance, incorporation of osteoclasts or their progenitors in 3D coculture with cancer cells could shed light on the mechanisms of cancer cell-driven bone resorption and its subsequent effects on cancer growth and spreading. Due to the crucial role of angiogenesis in tumor development, coculture models including endothelial cells may more closely mimic the in vivo bone niche. Such biomaterials platforms can be leveraged to examine the effect of the bone niche on modulating cancer cell drug response and can provide a means for identifying functional biomarkers in a physiologically relevant context.
We envision tissue engineered in vitro models specifically customized to mimic the bone niche can significantly advance the field of bone cancer research and future drug development in several ways. First, by providing precise control over bone-mimicking niche cues and cell-cell interactions among multiple cell types in the bone, such bone cancer models can enable discovery of the mechanisms that drive bone cancer progression, which may guide development and validation of therapeutics that target to disrupt such signaling pathways. Second, 3D in vitro bone cancer models may expedite drug discovery by providing low cost, high-throughput screening platforms that yield more physiologically relevant results than conventional 2D models. These capabilities are especially useful for developing therapies that target tumor-stroma interactions, such as those that target integrins [122,123], cell-cell interactions [124], and paracrine signals [46]. Finally, tissue-engineered models can guide therapeutic strategies in a personalized manner by incorporating patient-specific cells for functional characterization and drug screening. One limitation of using tissue-engineered scaffolds is that they are currently more labor and time intensive to generate than 2D cultures. Advances in automatic robotics and fabrication technologies such as 3D bioprinting could help reduce the time and further increase the high-throughput workflows. Together, these advances could accelerate the adoption of biomaterials and tissue engineered 3D bone cancer models for both research discovery and clinical translation of personalized medicine in cancer therapy.
Statement of Significance.
Biomaterials-based 3D culture have been traditionally used for tissue regeneration. Recent research harnessed biomaterials to create 3D in vitro cancer models, with demonstrated advantages over conventional 2D culture in recapitulating tumor progression and drug response in vivo. However, previous work has been largely limited to modeling soft tissue cancer, such as breast cancer and brain cancer. Unlike soft tissues, bone is characterized with high stiffness and mineral content. Primary bone cancer affects mostly children with poor treatment outcomes, and bone is the most common site of cancer metastasis. Here we summarize emerging efforts on engineering 3D bone cancer models using tissue engineering approaches, and future directions needed to further advance this relatively new research area.
Acknowledgements
The authors acknowledge NIH R01DE024772 (F.Y.), NSF CAREER award CBET-1351289 (F.Y.), California Institute for Regenerative Medicine Tools and Technologies Award RT3–07804 (F.Y.), the Stanford Bio-X Interdisciplinary Initiative Program (F.Y.), the Stanford Child Health Research Institute Faculty Scholar Award (F.Y.), the NSF Graduate Research Fellowship (E.C.G.D) for support.
Abbreviations:
- BMP
Bone Morphogenetic Protein
- BSP
Bone Sialoprotein
- CXCL12
C-X-C Motif Chemokine Ligand 12
- CXCL5
C-X-C Motif Chemokine Ligand 5
- CXCR2
C-X-C Motif Chemokine Receptor 2
- CXCR4
C-X-C Motif Chemokine Receptor 4
- CXCR7
C-X-C Motif Chemokine Receptor 7
- FLI1
Friend Leukemia Integration 1
- FGF
Fibroblast Growth Factor
- HIF
Hypoxia-Inducible Factor
- IGF-1
Insulin-Like Growth Factor 1
- IGF-1R
Insulin-Like Growth Factor Receptor 1
- IL-6
Interleukin 6
- IL-8
Interleukin 8
- IL-11
Interleukin 11
- MMP
Matrix Metalloproteinase
- mTOR
Mammalian Target of Rapamycin
- OPN
Osteopontin
- PTH-rP
Parathyroid Hormone-Related Protein
- RANKL
Receptor Activator of Nuclear Factor Kappa-B Ligand
- RUNX2
Runt-Related Transcription Factor 2
- Stat3
Signal Transducer and Activator of Transcription 3
- TGF-β
Transforming Growth Factor Beta
- TRAP
Tartrate-Resistant Acid Phosphatase
- VEGF
Vascular Endothelial Growth Factor
- YY1
Yin Yang 1
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J. Clin (2019), 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- [2].Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, Gonçalves F, Bone metastases: an overview, Oncol. Rev (2017), 10.4081/oncol.2017.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Park KM, Lewis D, Gerecht S, Bioinspired hydrogels to engineer cancer microenvironments, Annu. Rev. Biomed. Eng (2017), 10.1146/annurev-bioeng-071516-044619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lv D, Yu S-C, Ping Y-F, Wu H, Zhao X, Zhang H, Cui Y, Chen B, Zhang X, Dai J, Bian X-W, Yao X-H, A three-dimensional collagen scaffold cell culture system for screening anti-glioma therapeutics, Oncotarget (2016), 10.18632/oncotarget.10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma NKL, Lim JK, Leong MF, Sandanaraj E, Ang BT, Tang C, Wan ACA, Collaboration of 3D context and extracellular matrix in the development of glioma stemness in a 3D model, Biomaterials (2016), 10.1016/j.biomaterials.2015.11.031. [DOI] [PubMed] [Google Scholar]
- [6].Pedron S, Becka E, Harley BAC, Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid, Biomaterials. (2013), 10.1016/j.biomaterials.2013.06.024. [DOI] [PubMed] [Google Scholar]
- [7].Munson JM, Bellamkonda RV, Swartz MA, Interstitial flow in a 3d microenvironment increases glioma invasion by a cxcr4-dependent mechanism, Cancer Res. (2013), 10.1158/0008-5472.CAN-12-2838. [DOI] [PubMed] [Google Scholar]
- [8].Wang C, Tong X, Yang F, Bioengineered 3D brain tumor model to elucidate the effects of matrix stiff ness on glioblastoma cell behavior using PEG-based hydrogels, Mol. Pharm (2014). [DOI] [PubMed] [Google Scholar]
- [9].Wang C, Tong X, Jiang X, Yang F, Effect of matrix metalloproteinase-mediated matrix degradation on glioblastoma cell behavior in 3D PEG-based hydrogels, J. Biomed. Mater. Res. A (2017), 10.1002/jbm.a.35947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen L, Xiao Z, Meng Y, Zhao Y, Han J, Su G, Chen B, Dai J, The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs, Biomaterials 33 (2012) 1437–1444, 10.1016/j.biomaterials.2011.10.056. [DOI] [PubMed] [Google Scholar]
- [11].Dunne LW, Huang Z, Meng W, Fan X, Zhang N, Zhang Q, An Z, Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments, Biomaterials (2014), 10.1016/j.biomaterials.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [12].Maguire SL, Peck B, Wai PT, Campbell J, Barker H, Gulati A, Daley F, Vyse S, Huang P, Lord CJ, Farnie G, Brennan K, Natrajan R, Three-dimensional modelling identifies novel genetic dependencies associated with breast cancer progression in the isogenic MCF10 model, J. Pathol (2016), 10.1002/path.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller CP, Tsuchida C, Zheng Y, Himmelfarb J, Akilesh S, A 3D human renal cell carcinoma-on-a-chip for the study of tumor angiogenesis, Neoplasia (United States) (2018), 10.1016/j.neo.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu Z, Gao Y, Hao Y, Li E, Wang Y, Zhang J, Wang W, Gao Z, Wang Q, Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer, Biomaterials (2013), https:// 10.1016/j.biomaterials.2013.02.045. [DOI] [PubMed] [Google Scholar]
- [15].Simon KA, Mosadegh B, Minn KT, Lockett MR, Mohammady MR, Boucher DM, Hall AB, Hillier SM, Udagawa T, Eustace BK, Whitesides GM, Metabolic response of lung cancer cells to radiation in a paper-based 3D cell culture system, Biomaterials (2016), 10.1016/j.biomaterials.2016.03.002. [DOI] [PubMed] [Google Scholar]
- [16].Stratmann AT, Fecher D, Wangorsch G, Göttlich C, Walles T, Walles H, Dandekar T, Dandekar G, Nietzer SL, Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model, Mol. Oncol (2014), 10.1016/j.molonc.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ringuette Goulet C, Bernard G, Chabaud S, Couture A, Langlois A, Neveu B, Pouliot F, Bolduc S, Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery, Biomaterials (2017), 10.1016/j.biomaterials.2017.08.041. [DOI] [PubMed] [Google Scholar]
- [18].Myungjin Lee J, Mhawech-Fauceglia P, Lee N, Cristina Parsanian L, Gail Lin Y, Andrew Gayther S, Lawrenson K, A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro, Lab. Investig (2013), 10.1038/labinvest.2013.41. [DOI] [PubMed] [Google Scholar]
- [19].Shin CS, Kwak B, Han B, Park K, Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug, Mol. Pharm (2013), 10.1021/mp300595a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Loessner D, Rizzi SC, Stok KS, Fuehrmann T, Hollier B, Magdolen V, Hutmacher DW, Clements JA, A bioengineered 3D ovarian cancer model for the assessment of peptidase-mediated enhancement of spheroid growth and intraperitoneal spread, Biomaterials (2013), 10.1016/j.biomaterials.2013.06.009. [DOI] [PubMed] [Google Scholar]
- [21].Chiellini F, Puppi D, Piras AM, Morelli A, Bartoli C, Migone C, Modelling of pancreatic ductal adenocarcinoma: In vitro with three-dimensional microstructured hydrogels, RSC Adv. (2016), 10.1039/c6ra08420f. [DOI] [Google Scholar]
- [22].Piccoli M, D’Angelo E, Crotti S, Sensi F, Urbani L, Maghin E, Burns A, De Coppi P, Fassan M, Rugge M, Rizzolio F, Giordano A, Pilati P, Mammano E, Pucciarelli S, Agostini M, Decellularized colorectal cancer matrix as bioactive microenvironment for in vitro 3D cancer research, J. Cell. Physiol (2018), 10.1002/jcp.26403. [DOI] [PubMed] [Google Scholar]
- [23].Jeppesen M, Hagel G, Glenthoj A, Vainer B, Ibsen P, Harling H, Thastrup O, Jørgensen LN, Thastrup J, Short-term spheroid culture of primary colorectal cancer cells as an in vitro model for personalizing cancer medicine, PLoS One (2017), 10.1371/journal.pone.0183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nyga A, Loizidou M, Emberton M, Cheema U, A novel tissue engineered three-dimensional in vitro colorectal cancer model, Acta Biomater. (2013), 10.1016/j.actbio.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tsou Y-H, Khoneisser J, Huang P-C, Xu X, Hydrogel as a bioactive material to regulate stem cell fate, Bioact. Mater 1 (2016) 39–55, 10.1016/J.BIOACTMAT.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Feng X, Chemical and biochemical basis of cell-bone matrix interaction in health and disease, Curr. Chem. Biol (2012), 10.2174/2212796810903020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marksjr S, Odgren P, Structure and development of the skeleton, Princ. Bone Biol (2007), 10.1016/b978-012098652-1/50103-7. [DOI] [Google Scholar]
- [28].Alfranca A, Martinez-Cruzado L, Tornin J, Abarrategi A, Amaral T, De Alava E, Menendez P, Garcia-Castro J, Rodriguez R, Bone microenvironment signals in osteosarcoma development, Cell. Mol. Life Sci (2015), 10.1007/s00018-015-1918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bussard KM, Gay CV, Mastro AM, The bone microenvironment in metastasis; what is special about bone?, Cancer Metast Rev. (2008), 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- [30].Weilbaecher KN, Guise TA, McCauley LK, Cancer to bone: a fatal attraction, Nat. Rev. Cancer 11 (2011) 411–425. nrc3055 [pii] 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Croucher PI, McDonald MM, Martin TJ, Bone metastasis: the importance of the neighbourhood, Nat. Rev. Cancer 16 (2016) 373–386, 10.1038/nrc.2016.44. [DOI] [PubMed] [Google Scholar]
- [32].Gibbs CP, Weber K, Scarborough MT, Malignant bone tumors, J. Bone Joint Surg 83 (2001) 1728–1745. [Google Scholar]
- [33].Heare T, Hensley MA, Dell’Orfano S, Bone tumors: osteosarcoma and Ewing’s sarcoma, Curr. Opin. Pediatr 21 (2009) 365–372, 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Rosenberg AE, Bone-forming tumors, Surg. Pathol. Clin (2017), 10.1016/j.path.2017.04.006. [DOI] [PubMed] [Google Scholar]
- [35].De Alava E, Gerald WL, Molecular biology of the Ewing’s sarcoma/primitive neuroectodermal tumor family, J. Clin. Oncol (2000). [DOI] [PubMed] [Google Scholar]
- [36].Angervall L, Enzinger FM, Extraskeletal neoplasm resembling Ewing’s sarcoma, Cancer (1975), . [DOI] [PubMed] [Google Scholar]
- [37].Vasquez L, Tarrillo F, Oscanoa M, Maza I, Geronimo J, Paredes G, Silva JM, Sialer L, Analysis of prognostic factors in high-grade osteosarcoma of the extremities in children: a 15-year single-institution experience, Front. Oncol (2016), 10.3389/fonc.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bosma SE, Ayu O, Fiocco M, Gelderblom H, Dijkstra PDS, Prognostic factors for survival in Ewing sarcoma: a systematic review, Surg. Oncol 27 (2018) 603–610, 10.1016/J.SURONC.2018.07.016. [DOI] [PubMed] [Google Scholar]
- [39].Fong ELS, Lamhamedi-Cherradi S-E, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK, Farach-Carson MC, Vishwamitra D, Demicco EG, Menegaz BA, Amin HM, Mikos AG, Ludwig JA, Modeling Ewing sarcoma tumors in vitro with 3D scaffolds, Proc. Natl. Acad. Sci 110 (2013) 6500–6505, 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bissell MJ, Radisky D, Putting tumours in context, Nat. Rev. Cancer 1 (2001) 46–54, 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Quail DF, Joyce JA, Microenvironmental regulation of tumor progression and metastasis, Nat. Med (2013), 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pathi SP, Lin DDW, Dorvee JR, Estroff LA, Fischbach C, Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis, Biomaterials 32 (2011) 5112–5122, 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C, A novel 3-D mineralized tumor model to study breast cancer bone metastasis, PLoS One 5 (2010), 10.1371/journal.pone.0008849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu W, Wang M, Fu Y, Castro NJ, Fu SW, Zhang LG, Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study, Acta Biomater. 14 (2015) 164–174, 10.1016/j.actbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- [45].Guijarro MV, Ghivizzani SC, Gibbs CP, Animal models in osteosarcoma, Front. Oncol 4 (2014) 189, 10.3389/fonc.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kansara M, Teng MW, Smyth MJ, Thomas DM, Translational biology of osteosarcoma, Nat. Rev. Cancer 14 (2014) 722–735, 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- [47].Tu B, Zhu J, Liu S, Wang L, Fan Q, Hao Y, Fan C, Tang T-T, Mesenchymal stem cells promote osteosarcoma cell survival and drug resistance through activation of STAT3, Oncotarget 7 (2016) 48296–48308, 10.18632/oncotarget.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Redini F, Heymann D, Bone tumor environment as a potential therapeutic target in Ewing sarcoma, Front. Oncol (2015), 10.3389/fonc.2015.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Harisi R, Dudas J, Nagy-Olah J, Timar F, Szendroi M, Jeney A, Extracellular matrix induces doxorubicin-resistance in human osteosarcoma cells by suppression of p53 function, Cancer Biol. Ther (2007). [DOI] [PubMed] [Google Scholar]
- [50].Mueller-Klieser W, Multicellular spheroids – A review on cellular aggregates in cancer research, J. Cancer Res. Clin. Oncol (1987). doi: 10.1007/BF00391431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Horst EN, Ward MR, Raghavan S, Mehta P, Rowley KR, Mehta G, Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity, Oncotarget (2016), 10.18632/oncotarget.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA, Multicellular tumor spheroids: an underestimated tool is catching up again, J. Biotechnol (2010), 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- [53].Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, De Boer J, Spheroid culture as a tool for creating 3D complex tissues, Trends Biotechnol. (2013), 10.1016/j.tibtech.2012.12.003. [DOI] [PubMed] [Google Scholar]
- [54].Gottfried E, Kunz-Schughart LA, Andreesen R, Kreutz M, Brave little world: spheroids as an in vitro model to study tumor-immune-cell interactions, Cell Cycle (2006), 10.4161/cc.5.7.2624. [DOI] [PubMed] [Google Scholar]
- [55].Nath S, Devi GR, Three-dimensional culture systems in cancer research: Focus on tumor spheroid model, Pharmacol. Ther (2016), 10.1016/j.pharmthera.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rimann M, Laternser S, Gvozdenovic A, Muff R, Fuchs B, Kelm JM, Graf-Hausner U, An in vitro osteosarcoma 3D microtissue model for drug development, J. Biotechnol 189 (2014) 129–135, 10.1016/j.jbiotec.2014.09.005. [DOI] [PubMed] [Google Scholar]
- [57].Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PHB, E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase, Cancer Res. (2007), 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cortini M, Massa A, Avnet S, Bonuccelli G, Baldini N, Tumor-activated mesenchymal stromal cells promote osteosarcoma stemness and migratory potential via IL-6 secretion, PLoS One (2016), 10.1371/journal.pone.0166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].de Nigris F, Crudele V, Giovane A, Casamassimi A, Giordano A, Garban HJ, Cacciatore F, Pentimalli F, Marquez-Garban DC, Petrillo A, Cito L, Sommese L, Fiore A, Petrillo M, Siani A, Barbieri A, Arra C, Rengo F, Hayashi T, Al-Omran M, Ignarro LJ, Napoli C, CXCR4/YY1 inhibition impairs VEGF network and angiogenesis during malignancy, Proc. Natl. Acad. Sci (2010), 10.1073/pnas.1008256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fallica B, Maffei JS, Villa S, Makin G, Zaman M, Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels, PLoS One (2012), 10.1371/journal.pone.0048024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Marturano-Kruik A, Villasante A, Yaeger K, Ambati SR, Chramiec A, Raimondi MT, Vunjak-Novakovic G, Biomechanical regulation of drug sensitivity in an engineered model of human tumor, Biomaterials 150 (2018) 150–161, 10.1016/j.biomaterials.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Akeda K, Nishimura A, Satonaka H, Shintani K, Kusuzaki K, Matsumine A, Kasai Y, Masuda K, Uchida A, Three-dimensional alginate spheroid culture system of murine osteosarcoma, Oncol. Rep (2009), 10.3892/or_00000527. [DOI] [PubMed] [Google Scholar]
- [63].Suzuki Y, Nishida Y, Naruse T, Gemba T, Ishiguro N, Pericellular matrix formation alters the efficiency of intracellular uptake of oligonucleotides in osteosarcoma cells, J. Surg. Res (2009), 10.1016/j.jss.2008.02.037. [DOI] [PubMed] [Google Scholar]
- [64].Techavichit P, Gao Y, Kurenbekova L, Shuck R, Donehower LA, Yustein JT, Secreted Frizzled-Related Protein 2 (sFRP2) promotes osteosarcoma invasion and metastatic potential, BMC Cancer. (2016), 10.1186/s12885-016-2909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jabbari E, Sarvestani SK, Daneshian L, Moeinzadeh S, Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells, PLoS One (2015), 10.1371/journal.pone.0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA, Mineralization of hydrogels for bone regeneration, Tissue Eng. Part B. Rev 16 (2010) 577–585, 10.1089/ten.teb.2010.0462. [DOI] [PubMed] [Google Scholar]
- [67].Cortini M, Avnet S, Baldini N, Mesenchymal stroma: Role in osteosarcoma progression, Cancer Lett. (2017), 10.1016/j.canlet.2017.07.024. [DOI] [PubMed] [Google Scholar]
- [68].Volchenboum SL, Andrade J, Huang L, Barkauskas DA, Krailo M, Womer RB, Ranft A, Potratz J, Dirksen U, Triche TJ, Lawlor ER, Gene expression profiling of Ewing sarcoma tumors reveals the prognostic importance of tumor-stromal interactions: a report from the Children’s Oncology Group, J. Pathol. Clin. Res 1 (2015) 83–94, 10.1002/cjp2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Santoro M, Menegaz BA, Lamhamedi-Cherradi S-E, Molina ER, Wu D, Priebe W, Ludwig JA, Mikos AG, Modeling stroma-induced drug resistance in a tissue-engineered tumor model of Ewing sarcoma, Tissue Eng. Part A 23 (2017) 80–89, 10.1089/ten.tea.2016.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Villasante A, Marturano-Kruik A, Robinson ST, Liu Z, Guo XE, Vunjak-Novakovic G, Tissue-engineered model of human osteolytic bone tumor, Tissue Eng. Part C Methods 23 (2017) 98–107, 10.1089/ten.tec.2016.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Villasante A, Marturano-Kruik A, Vunjak-Novakovic G, Bioengineered human tumor within a bone niche, Biomaterials 35 (2014) 5785–5794, 10.1016/j.biomaterials.2014.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xie L, Ji T, Guo W, Anti-angiogenesis target therapy for advanced osteosarcoma, Oncol. Rep 38 (2017) 625–636, 10.3892/or.2017.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mikulić D, Ilić I, Cepulić M, Orlić D, Giljević JS, Fattorini I, Seiwerth S, Tumor angiogenesis and outcome in osteosarcoma, Pediatr. Hematol. Oncol 21 (2004) 611–619. http://www.ncbi.nlm.nih.gov/pubmed/15626017 (accessed March 3, 2019). [DOI] [PubMed] [Google Scholar]
- [74].Tan PHS, Aung KZ, Toh SL, Goh JCH, Nathan SS, Three-dimensional porous silk tumor constructs in the approximation of in vivo osteosarcoma physiology, Biomaterials 32 (2011) 6131–6137, 10.1016/j.biomaterials.2011.04.084. [DOI] [PubMed] [Google Scholar]
- [75].Stoltz J-F, Magdalou J, George D, Chen Y, Li Y, De Isla N, He X, Remond Y, Influence of mechanical forces on bone: introduction to mechanobiology and mechanical adaptation concept, J. Cell. Immunother 4 (2018) 10–12, 10.1016/J.JOCIT.2018.09.003. [DOI] [Google Scholar]
- [76].Williams JL, Iannotti JP, Ham A, Bleuit J, Chen JH, Effects of fluid shear stress on bone cells, Biorheology 31 (1994) 163–170. http://www.ncbi.nlm.nih.gov/pubmed/8729478 (accessed March 3, 2019). [DOI] [PubMed] [Google Scholar]
- [77].Santoro M, Lamhamedi-Cherradi S-E, Menegaz BA, Ludwig JA, Mikos AG, Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma, Proc. Natl. Acad. Sci 112 (2015) 10304–10309, 10.1073/pnas.1506684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Trachtenberg JE, Santoro M, Williams C, Piard CM, Smith BT, Placone JK, Menegaz BA, Molina ER, Lamhamedi-Cherradi S-E, Ludwig JA, Sikavitsas VI, Fisher JP, Mikos AG, Effects of shear stress gradients on Ewing sarcoma cells using 3D printed scaffolds and flow perfusion acsbiomaterials.6b00641, ACS Biomater. Sci. Eng (2017), 10.1021/acsbiomaterials.6b00641. [DOI] [PubMed] [Google Scholar]
- [79].Jeffree GM, Price CHG, Sissons HA, The metastatic patterns of osteosarcoma, Br. J. Cancer (1975), 10.1038/bjc.1975.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A, Involvement of chemokine receptors in breast cancer metastasis, Nature 410 (2001) 50–56, 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- [81].Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK, Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone, Cancer Res. 62 (2002) 1832–1837 (accessed March 3, 2019). [PubMed] [Google Scholar]
- [82].Sung V, Stubbs JT, Fisher L, Aaron AD, Thompson EW, Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the avb3 and avb5 integrins, J. Cell. Physiol 176 (1998) 482–494, . [DOI] [PubMed] [Google Scholar]
- [83].Adwan H, Bäuerle T, Najajreh Y, Elazer V, Golomb G, Berger M, Decreased levels of osteopontin and bone sialoprotein II are correlated with reduced proliferation, colony formation, and migration of GFP-MDA-MB-231 cells, Int. J. Oncol 24 (2004) 1235–1244, 10.3892/ijo.24.5.1235. [DOI] [PubMed] [Google Scholar]
- [84].Wang J, Wang L, Xia B, Yang C, Lai H, Chen X, BSP gene silencing inhibits migration, invasion, and bone metastasis of MDA-MB-231BO human breast cancer cells, PLoS One 8 (2013), 10.1371/journal.pone.0062936 e62936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mundy GR, Metastasis: Metastasis to bone: causes, consequences and therapeutic opportunities, Nat. Rev. Cancer 2 (2002) 584–593, 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- [86].Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD, A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone, Biomaterials 35 (2014) 2454–2461, 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD, Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation, Proc. Natl. Acad. Sci 112 (2015) 214–219, 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhou X, Zhu W, Nowicki M, Miao S, Cui H, Holmes B, Glazer RI, Zhang LG, 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study, ACS Appl. Mater. Interfaces 8 (2016) 30017–30026, 10.1021/acsami.6b10673. [DOI] [PubMed] [Google Scholar]
- [89].Fong ELS, Wan X, Yang J, Morgado M, Mikos AG, Harrington DA, Navone NM, Farach-Carson MC, A 3D in vitro model of patient-derived prostate cancer xenograft for controlled interrogation of in vivo tumor-stromal interactions, Biomaterials 77 (2016) 164–172, 10.1016/j.biomaterials.2015.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Angeloni V, Contessi N, De Marco C, Bertoldi S, Tanzi MC, Daidone MG, Farè S, Polyurethane foam scaffold as in vitro model for breast cancer bone metastasis, Acta Biomater. 63 (2017) 306–316, 10.1016/j.actbio.2017.09.017. [DOI] [PubMed] [Google Scholar]
- [91].Holzapfel BM, Wagner F, Loessner D, Holzapfel NP, Thibaudeau L, Crawford R, Ling MT, Clements JA, Russell PJ, Hutmacher DW, Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone, Biomaterials 35 (2014) 4108–4115, 10.1016/j.biomaterials.2014.01.062. [DOI] [PubMed] [Google Scholar]
- [92].Sieh S, Taubenberger AV, Lehman ML, Clements JA, Nelson CC, Hutmacher DW, Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis, Bone 63 (2014) 121–131, 10.1016/j.bone.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [93].Hartman O, Zhang C, Adams EL, Farach-Carson MC, Petrelli NJ, Chase BD, Rabolt JF, Biofunctionalization of electrospun PCL-based scaffolds with perlecan domain IV peptide to create a 3-D pharmacokinetic cancer model, Biomaterials 31 (2010) 5700–5718, 10.1016/j.biomaterials.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhu W, Holmes B, Glazer RI, Zhang LG, 3D printed nanocomposite matrix for the study of breast cancer bone metastasis, Nanomed. Nanotechnol. Biol. Med 12 (2016) 69–79, 10.1016/j.nano.2015.09.010. [DOI] [PubMed] [Google Scholar]
- [95].Fitzgerald KA, Guo J, Tierney EG, Curtin CM, Malhotra M, Darcy R, O’Brien FJ, O’Driscoll CM, The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics, Biomaterials 66 (2015) 53–66, 10.1016/j.biomaterials.2015.07.019. [DOI] [PubMed] [Google Scholar]
- [96].Fitzgerald KA, Guo J, Raftery RM, Castaño IM, Curtin CM, Gooding M, Darcy R, O’ Brien FJ, O’ Driscoll CM, Nanoparticle-mediated siRNA delivery assessed in a 3D co-culture model simulating prostate cancer bone metastasis, Int. J. Pharm (2016), 10.1016/j.ijpharm.2016.07.079. [DOI] [PubMed] [Google Scholar]
- [97].Reichert JC, Quent VMC, Burke LJ, Stansfield SH, Clements JA, Hutmacher DW, Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment, Biomaterials 31 (2010) 7928–7936, 10.1016/j.biomaterials.2010.06.055. [DOI] [PubMed] [Google Scholar]
- [98].Chen Y-C, Sosnoski DM, Mastro AM, Breast cancer metastasis to the bone: mechanisms of bone loss, Breast Cancer Res. 12 (2010) 215, 10.1186/bcr2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kirkinis MN, Spelman T, May D, Choong PFM, Metastatic bone disease of the pelvis and extremities: rationalizing orthopaedic treatment, ANZ J. Surg (2017), 10.1111/ans.13615. [DOI] [PubMed] [Google Scholar]
- [100].Hung C-S, Su H-Y, Liang H-H, Lai C-W, Chang Y-C, Ho Y-S, Wu C-H, Ho J-D, Wei P-L, Chang Y-J, High-level expression of CXCR4 in breast cancer is associated with early distant and bone metastases, Tumor Biol. 35 (2014) 1581–1588, 10.1007/s13277-013-1218-9. [DOI] [PubMed] [Google Scholar]
- [101].Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J, Lee B, Westbrook TF, Wong STC, Jin X, Rosen JM, Osborne CK, Zhang XH-F, The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells, Cancer Cell. 27 (2015) 193–210, 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD, A microfluidic 3D invitro model for specificity of breast cancer metastasis to bone, Biomaterials 35 (2014) 2454–2461, 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mendoza-Villanueva D, Zeef L, Shore P, Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFb-dependent expression of the Wnt antagonist, sclerostin, Breast Cancer Res. 13 (2011) R106, 10.1186/bcr3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nakajima K, Kho DH, Yanagawa T, Harazono Y, Gao X, Hogan V, Raz A, Galectin-3 inhibits osteoblast differentiation through notch signaling, Neoplasia 16 (2014) 939–949, 10.1016/J.NEO.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sturge J, Caley MP, Waxman J, Bone metastasis in prostate cancer: Emerging therapeutic strategies, Nat. Rev. Clin. Oncol (2011), 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- [106].Ziaee S, Chu GC, Huang JM, Sieh S, Chung LW, Prostate cancer metastasis: roles of recruitment and reprogramming, cell signal network and three-dimensional growth characteristics, Transl. Androl. Urol (2015), 10.3978/j.issn.2223-4683.2015.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Reichert JC, Quent VMC, Burke LJ, Stansfield SH, Clements JA, Hutmacher DW, Mineralized human primary osteoblast matrices as a model system to analyse interactions of prostate cancer cells with the bone microenvironment, Biomaterials (2010), 10.1016/j.biomaterials.2010.06.055. [DOI] [PubMed] [Google Scholar]
- [108].Hensel J, Thalmann GN, Biology of bone metastases in prostate cancer, Urology (2016), 10.1016/j.urology.2015.12.039. [DOI] [PubMed] [Google Scholar]
- [109].Mishra A, Shiozawa Y, Pienta KJ, Taichman RS, Homing of cancer cells to the bone, Cancer Microenviron. (2011), 10.1007/s12307-011-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Simmons JK, Hildreth BE, Supsavhad W, Elshafae SM, Hassan BB, Dirksen WP, Toribio RE, Rosol TJ, Animal models of bone metastasis, Vet. Pathol. (2015), 10.1177/0300985815586223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS, The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer, J. Biol. Chem (2008), 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- [112].Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS, Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer, J. Cell. Biochem (2008), 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zurita AJ, Yu-Lee L-Y, Cheng C-J, Chen D-T, Lee Y-C, Zhang S, Logothetis CJ, Lin S-H, Hu MC-T, Yeh ET, Chu K, Ye X, Cadherin-11 promotes the metastasis of prostate cancer cells to bone, Mol. Cancer Res (2008), 10.1158/1541-7786.mcr-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Virk MS, Petrigliano FA, Liu NQ, Chatziioannou AF, Stout D, Kang CO, Dougall WC, Lieberman JR, Influence of simultaneous targeting of the bone morphogenetic protein pathway and RANK/RANKL axis in osteolytic prostate cancer lesion in bone, Bone (2009), 10.1016/j.bone.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, Shirai T, Matrisian LM, Futakuchi M, MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL, Cancer Cell (2005), 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [116].Akech J, Wixted JJ, Bedard K, Van Der Deen M, Hussain S, Guise TA, Van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB, Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions, Oncogene (2010), 10.1038/onc.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS, Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow, J. Clin. Invest. (2011), 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Rangarajan A, Weinberg RA, Comparative biology of mouse versus human cells: modelling human cancer in mice, Nat. Rev. Cancer (2003), 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- [119].Kwon H, Kim HJ, Rice WL, Subramanian B, Park S-H, Georgakoudi I, Kaplan DL, Development of an in vitro model to study the impact of BMP-2 on metastasis to bone, J. Tissue Eng. Regen. Med 4 (2010) 590–599, 10.1002/term.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Lonergan PE, Tindall DJ, Androgen receptor signaling in prostate cancer development and progression, J. Carcinog (2011), 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Fitzgerald KA, Guo J, Raftery RM, Castaño IM, Curtin CM, Gooding M, Darcy R, O’ Brien FJ, O’ Driscoll CM, Nanoparticle-mediated siRNA delivery assessed in a 3D co-culture model simulating prostate cancer bone metastasis, Int. J. Pharm 511 (2016) 1058–1069, 10.1016/j.ijpharm.2016.07.079. [DOI] [PubMed] [Google Scholar]
- [122].Lasinska I, Mackiewicz J, Integrins as a new target for cancer treatment, Anticancer. Agents Med. Chem 18 (2018), 10.2174/1871520618666181119103413. [DOI] [PubMed] [Google Scholar]
- [123].Hamidi H, Pietilä M, Ivaska J, The complexity of integrins in cancer and new scopes for therapeutic targeting, Br. J. Cancer 115 (2016) 1017, 10.1038/BJC.2016.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Simon M, Stefan N, Plückthun A, Zangemeister-Wittke U, Epithelial cell adhesion molecule-targeted drug delivery for cancer therapy, Expert Opin. Drug Deliv 10 (2013) 451–468, 10.1517/17425247.2013.759938. [DOI] [PubMed] [Google Scholar]


