Abstract
Compared to the growing body of literature on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection and quantification in sewage, there are limited studies reporting on correlations between the viral loads in sewage and the prevalence of infected patients. The present work is a part of the regular monitoring effort for SARS-CoV-2 in wastewater influents from seven wastewater treatment plants (WWTPs) in Tehran, Iran, starting from late September 2020 until early April 2021. These facilities cover ~64% of the metropolis serving >5000,000 M individuals. The study set out to track the trends in the prevalence of COVID-19 in the community using wastewater based epidemiology (WBE) and to investigate whether these measurements correlate with officially reported infections in the population. Composite sewage samples collected over 16 h were enriched by polyethylene glycol precipitation and the corresponding threshold cycle (Ct) profiles for CDC ‘N’ and ‘ORF1ab’ assays were derived through real time RT-qPCR. Monte Carlo simulation model was employed to provide estimates of the disease prevalence in the study area. RNA from SARS-CoV-2 was detectable in 100% (‘N’ assay) and 81% (‘ORF1ab’ assay) of totally 91 sewage samples, with viral loads ranging from 40 to 45,000 gene copies/L. The outbreak of COVID-19 positively correlated (R2 = 0.80) with the measured viral load in sewage samples. Furthermore, sewage SARS-CoV-2 RNA loads preceded infections in the population by 1 to 2 days, which were in line with public adherence with and support for government instructions to contain the pandemic. Given the transient presence of human host-restricted infections such as SARS-CoV-2, these results provide evidence for assessment of the effectiveness of coordinated efforts that specifically address public health responses based on wastewater-based disease surveillance against not only COVID-19 but also for future infectious outbreaks.
Keywords: Monte Carlo simulation, Public health, SARS-CoV-2, Sewage surveillance, Wastewater-based epidemiology
Graphical abstract

1. Introduction
The ongoing coronavirus disease (COVID-19) has caused the world to undergo unprecedented changes in a short space of time. According to recent updates (8 September 2021), 222,042,024 confirmed cases and 4,588,950 deaths have been reported globally (CSSE, 2021). Several lines of evidence show that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent for the disease, does not only affect the upper respiratory tract and the lungs but also the ilium and colon, resulting in diverse gastrointestinal manifestations (Amereh et al., 2021; Foladori et al., 2020; Wu et al., 2020a). A significant number of infected diagnosed patients (15–83% of cases) shed viral fragments for a prolonged time, ranging from 14 to 21 days (Lodder and de Roda Husman, 2020; Wu et al., 2020a; Xu et al., 2020), with 102 to 108 RNA copies per gram of stool on average (Lescure et al., 2020; Pan et al., 2020; Woelfel et al., 2020); and the reported shedding period and magnitude varying greatly among cases. The prolonged incubation time (~5 days) and virus shedding even from asymptomatic individuals allow the virus to spread quickly without medical detection and containment, which subsequently contribute to the development of infection as a whole (Li et al., 2020). The virus, in turn, can be released into wastewater and onsite sanitation systems.
The detection of SARS-CoV-2 RNA in feces and untreated wastewater points towards the potential of wastewater surveillance to capture a near real-time picture of the viral disease burden within a community and also to gain clues on outbreaks within a catchment or a specific region (Wu et al., 2020b; Wurtzer et al., 2020). The method offers a scalable and cost-effective way to anonymously track population-level infection and disease prevalence (Bivins et al., 2020; Coccia, 2020; Keshaviah, 2017; Kumar, 2021).
Concomitantly with the emergence of COVID-19, several countries - including Australia (Ahmed et al., 2020a), Spain (Randazzo et al., 2020), France (Wurtzer et al., 2020), Sweden (Saguti et al., 2021), Italy (La Rosa et al., 2020), India (Kumar et al., 2020b) and Pakistan (Yaqub et al., 2021) - quickly pivoted existing sewage surveillance programs, designed to monitor illicit drugs and other viral pathogens, to follow SARS-CoV-2 by quantifying the virus genetic materials in consecutive wastewater samples. Based on these early successes in detecting and quantifying the novel coronavirus, hundreds of sewage systems all over the world - particularly in developed nations - began testing their wastewater for SARS-CoV-2 (Gonzalez et al., 2020; Martin et al., 2020; Prado et al., 2020; Torii et al., 2021; Wu et al., 2020b). However, knowledge about the serious and emerging problem of wastewater surveillance for the presence of SARS-CoV-2 from resource-poor regions and least-developed nations is presently lacking. Analysis of sewage samples in a municipal wastewater treatment plant (WWTP) in Istanbul, Turkey (Kocamemi et al., 2020) confirmed the presence of novel coronavirus in primary sludge. Detection of SARS-CoV-2 RNA and its variation along the sewer network has also been previously demonstrated in the vicinity wastewaters of a COVID-19 isolation center in Bangladesh (Ahmed et al., 2021), reinforcing the use of this approach for community surveillance by wastewater-based epidemiology (WBE) as a new paradigm in public health. It is supported by the limited and cost-intensive testing capacity of these communities as compared with developed resource-rich nations, where the number and proportion of affected cases are mainly determined through individual testing and laboratory-based bio-molecular diagnostics (Abu-Ali et al., 2021; Jeremijenko et al., 2020; Saththasivam et al., 2021).
On 19 February 2020, Iran reported its first confirmed cases of infections in Qom - a religious city in central Iran, just virtually 93 days after its emergence in Wuhan city, China. Two days later (21 February), Tehran, the capital city of the country confirmed its first case. Both cities perform competently in the entry flow of transmissible diseases into the country. Despite sustained efforts to contain the virus exploiting various policies, it is still posing a serious national challenge today and the country is facing the so-called 5th epidemic wave of COVID-19. As of September 8, 2021, the number of officially reported confirmed cases has reached more than 4,000,000 (representing 3% of the 222,042,024 cases reported globally), and over 100,000 COVID-19 confirmed deaths have been officially reported by the Iranian Ministry of Health (IMoHaM, 2020). At time of submission, Iran is the third country in worldwide ranking with the largest number of confirmed cases within the last 28 days (CSSE, 2021). The ministry has also reported 351,641 confirmed cases of COVID-19 to date, with 23,643 death cases in Tehran (IMoHaM, 2020).
In Iran, the pandemic has been exacerbated by cultural and societal norms, access and allocation of Covid-19 vaccines, lack and cost of clinical kits, and inability to track affected individuals in a timely and comprehensive manner. The situation, in tune with other communities, is indeed compounded by asymptomatic or pre-symptomatic infections (Wu et al., 2020a; Lodder and de Roda Husman, 2020; Xu et al., 2020). In recognition of this gap, National Institute for Medical Research Development (NIMAD) has funded wastewater testing in Tehran, which provides a cost-effective strategy for measuring the population-level prevalence of not only recent pandemic but also of future infectious disease, chronic disease, and drug epidemics. Here, a seven-month comprehensive surveillance of SARS-CoV-2 in seven municipal WWTPs in Tehran metropolitan, which is still ongoing, was performed to delve into whether these measurements mirror the number of clinically diagnosed infections in the population. Further, temporal variations in virus-specific RNA loadings quantified via WBE in studied WWTPs during the lockdown and implementation of precautionary intervention periods were analyzed to address the practical needs and timelines of concerned authorities and policymakers.
2. Materials and methods
2.1. Study area
The present study was conducted in Tehran, the second-largest metropolitan area in the Middle East and the most populous city in Iran (Fig. 1 ). The city occupies an area of 730 km2 and encompasses a resident population of about 8.694 million according to the 2016 national census and day-time population of over 12 million because of the massive commute from outlying areas (Iran SCo, 2016). Land use is predominantly residential, with some industrial and commercial activity. The climate is semi-arid with an annual mean temperature of 18.5 °C and average annual precipitation of about 220 mm. The city has been highly impacted by the COVID-19 pandemic. The number of daily PCR diagnoses was limited to just about 1100 in the early months of the outbreak in the city, whereas it reached up to 12,000 during each additional wave of COVID-19.
Fig. 1.
Location of WWTPs sampled during this study, representing virtually ~5.561 Million individuals (64% of Tehran's total population).
Tehran's sewage project, as has been defined in the urban master plan, has a collection pipe network of almost 9000-km-long; in which already 7000 km is in operation and about 684 × 103 cubic meters of sewage is daily collected in the city. So far, just about 64% of Tehran inhabitants (approximately 5.561 million) have access to the public sewerage system while the remaining sewage goes underground or flows over the surface inadvertently creating environmental hazards including surface and groundwater pollution. Nevertheless, the operational performances of treatment plants are satisfactory and meet the basic criteria prescribed by national standards for secondary treatment for discharge into streams. Thus far, the presence of SARS-CoV-2 in wastewater samples achieved in Iran has been limited (Gholipour et al., 2021; Nasseri et al., 2021; Tanhaei et al., 2021), including our previous work (Rafiee et al., 2021). To the best of our knowledge, this is the first study to correlate SARS-CoV-2 RNA loads in wastewater with COVID-19 disease burden in the country. The work is a part of a regular monitoring effort for novel coronavirus in Tehran sanitary sewerage that was initiated on 30 September 2020.
2.2. Sample collection and pre-treatment
Sewage samples were collected from six medium-sized WWTPs (Shahrak-e Gharb (A), Ekbatan (B), Zargandeh (C), Gheytarieh (D), Sahebgharanieh (E), and Mahllati (F)), all representing urban catchments. Further, Tehran's South wastewater treatment plant (Southern Tehran WWTP) (G) that serves a large part of the metropolitan was also included in the sampling scheme (Table 1 ). This plant has been planned with a treatment capacity of 4.2 × 106 people. The total number of inhabitants served by these WWTPs, the percent coverage of the total population, and treatment capacity of wastewater have also been summarized in Table 1. The plants also differed in their connected sewershed area characteristics as well as demographic density. The locations of these plants also are shown in Fig. 1. About 8 L composite samples of untreated urban wastewater entering each WWTP were collected by sampling wastewater every 60 min in a flow-proportional mode (~500 mL per 1000 m3 influent wastewater), which was subsequently pooled.
Table 1.
Summarized properties of wastewater treatment plants (WWTPs) sampled during the study.
| WWTP | Nominal no. of connected residentsb | Capacity (m3/day) | Approximate coverage of total population (%) | Sampling technique |
|---|---|---|---|---|
| Shahrak-e Gharb | 1,000,000 | 513,000 | 11 | Manual |
| Ekbatan | 630,000 | 15,000 | 7 | Manual |
| Zargandeh | 180,000 | 3000 | 2 | Manual |
| Gheytarieh | 150,000 | 410 | 1.6 | Manual |
| Sahebgharanieh | 21,000 | 120,000 | 0.23 | Manual |
| Mahllati | 190,000 | 5000 | 2 | Manual |
| Southern Tehran | 3,500,000 | 650,000 | 40 | Auto sampler |
The sampling was performed from late September 2020 until early April 2021 between 8:00 to 24:00, biweekly. The operators of WWTPs at medium-sized units collected the samples, while storing them at approximately 4 °C during sampling; whereas, 24-h composite samples were collected using an auto-sampler in the large-sized Southern Tehran WWTP. Samples were transported on melting ice to the laboratory and stored at about 4 °C until further analysis. In total, 13 rounds of samples were taken from 30th September 2020 to 8th April 2021. Various physicochemical properties (including BOD5, TSS, TN, TP, pH, and temperature) of raw sewage entering each WWTP during the study period were determined. It is worth mentioning that there was no significant rainfall or snowmelt infiltration into the sewer collections systems during sewage sampling.
2.3. Sample processing for isolation and quantification of viral RNA
Samples were immediately concentrated upon receipt in the Research Institute for Gastroenterology and Liver Diseases lab, followed by molecular processing within 24 h of sample collection.
2.3.1. Sample concentration
Polyethylene glycol (PEG) precipitation was applied for virus enrichment in sewage samples, as described by Jones and Johns (2009) with modifications outlined previously (Rafiee et al., 2021). An analysis of the data available in the literature already confirms the effectiveness of PEG precipitation for SARS-CoV-2 in sewage and surface water samples (Ahmed et al., 2020b; Haramoto et al., 2020; Torii et al., 2021). The method began with centrifugation of sewage subsamples (50 mL) at 4000 × g for 10 min. The top aqueous layer (40 mL) was then recovered carefully and mixed with PEG 8000 50% (w/v) and NaCl (1.5 M). The resultant mixture was incubated overnight at 4 °C (300 rpm) and centrifuged at 15,000 ×g for 30 min at 4 °C. The supernatant was discarded and the resulting pellets were re-suspended in ~300 μL RNase-free water. RNA was directly extracted from this sample.
2.3.2. RNA extraction and SARS-CoV-2 specific RT-qPCR
RNA was extracted from virus concentrates using a QIAamp® Viral RNA mini kit (Qiagen Company, Hilden, Germany - Catalog No. 52904) following the manufacturer's instructions. All RNA extracts were stored at −80 °C and subjected to reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays for detection of SARS-CoV-2 within 24 h of sample extraction. First, we employed qualitative measurement, and hence, the absence or presence of the virus based on threshold cycles (Ct). Amplification curves were manually inspected using quality control measures and those with threshold cycles beyond 40 were discarded (i.e., Ct = 40 was the detection limit). Preliminary testing of samples with ORF1ab and N genes of SARS-CoV-2 as well as RNase P (internal process control) using TaqPath™ Covid-19 RT-PCR Kit (Sansure Biotech Inc., China) demonstrated the best detection and least variance with N gene. Relative numbers of viral genomes in wastewater samples (gene copies/volume of sample) were then calculated from Ct values determined for each sample in relation to an assay-specific standard quantitative calibration curve, generated by five 10-fold serial dilutions of 2019-nCoV_N positive control plasmid (ID WB4920E, SARS-CoV-2 N1 (provided by Shinegene, China)). The SARS-CoV-2 signal in the samples was assessed using a singleplex one-step RT-qPCR targeting the N1 gene region of SARS-CoV-2 genome. Reactions consisted of 5 μL of RNA template, 500 nM of each of forward and reverse primers (Metabion, Germany) in 4 × CAPITAL one-step qRT-PCR Probe Mastermix (biotechrabbit, Germany) with 200 nM probe (Metabion, Germany) in a final volume of 20 μL. Reverse transcription (RT) was performed at 50 °C for 10 min followed by RT inactivation and initial denaturation at 95 °C for 3 min. This was followed by 45 cycles of denaturation at 95 °C for 10 s and annealing/extension at 55 °C for 30 s with a Rotor-Gene Q MDx thermal cycler (QIAGEN Hilden, Germany). Nuclease free water was used as no template control in our study (Sequences of primers and probe was provided in Supplementary information (SI) - Table S1).
All RNA samples obtained from sewage were tested in triplicate reactions and the average quantities were reported in viral gene copies/L ± standard deviation (SD). The assay limit of detection (ALOD, ≥95% detection probability) was assessed and determined to be approximately 3 copies/reaction. Likewise, the assay limit of quantification (ALOQ, CV = 35%) was approximately 3.1 copies/reaction. The standard curve used to quantify SARS-CoV-2 in field samples showed a slope, R-squared and primer efficiency value of −3.335, 1 and 0.99, respectively. More precisely, gene copy numbers were calculated as follows, considering the well-established principle of 3.32 Ct change in correspondence to 10-fold change: Gene copy number = 10((40-Ct)/(10/3.32)) (Kumar et al., 2020a). Viral concentrations were recorded as gene copies per 1000 mL (gc/L), given the volumes of sample, concentrate (eluate), nucleic acid extracts, and RT-qPCR reaction (Eq. (1)).
| (1) |
2.4. Assessment of viral recovery
To evaluate the efficacy of virus concentration and extraction methods, 15 μL of 4.5 × 105 gc of enveloped avian coronavirus (IBV) was seeded into 40 mL of seven wastewater samples from different WWTPs and three 10-fold serial dilutions were tested to determine the method's limit of detection. Positive and negative nucleic acid control extractions of Nuclease-free water with or without the same quantity of IBV spike-in were used to quantify IVB recovery by RT-PCR. Enrichment, extraction and detection/quantification were all performed as previously outlined. To obtain a broader picture, the results were used to determine cross-contamination during the nucleic acid extraction process or RT-PCR assay setup and also the effect of sewage on virus recovery. The RNA was isolated from independently processed samples and RT-PCR was performed. The efficacy of viral recovery was calculated using the following equation (Eq. (2)):
| (2) |
The mean and standard deviation was calculated.
2.5. Population normalized infection prevalence
The prevalence of infected individuals within each sewer catchment was estimated through Monte Carlo simulation given the total number of SARS-CoV-2 RNA copies in wastewater each day, as measured by RT-qPCR, and the number of viral RNA copies excreted daily in stool by an infected person as the following equation (Eq. (3)):
| (3) |
where, C and Q stand for measured viral load in wastewater and corresponding daily influent flow, respectively. Monte Carlo simulation was conducted using the Oracle Crystal Ball (Version 11.1.2.4.850 Oracle©) Excel add-on to account for the variability in some of the factors in Eq. (3). Per person virus load to the sewage was modeled as a log-uniform distribution from 2.56 to 7.67 by considering the shedding rates of SARS-CoV-2 RNA copies/g of feces during the periods of heaviest shedding among mild cases of COVID-19, as reported elsewhere (Woelfel et al., 2020). Hereby, the daily per capita loads of stool was modeled as a normal distribution with a range of 100–400 g feces/person according to Hart and Halden (2020) and Rose et al. (2015). It is worth mentioning that the number of viral RNA copies found in stool varies from patient to patient and varies within a single patient as the disease progresses. According to Li et al. (2021), from pooled stool samples, the mean shedding magnitude was about 104.52 copies/g with a 95% confident interval ranging from 104.26 to 104.78 copies/g. The daily flow rates of wastewater were provided by Tehran sewerage Co. and the average per capita wastewater rate was calculated as of 220 L/person/day. Likewise, published census data coupled with WWTPs design capacities were used to estimate the inhabitant population of each catchment area.
To improve the accuracy of the prediction model in estimating the number of people infected, the viral load shed per mL of urine in infected persons, as well as the recovery percentage of viral particles in wastewater was added. The urinary viral load was taken to be 2.50 Log10 per mL (Peng et al., 2020). The daily volume of urine per capita was modeled as a log-uniform distribution with a minimum of 2.78 and a maximum of 3.76 (Lemann et al., 1996).
In the present study, recovery efficiency was 42.55 ± 12.45% of IVB spiked into untreated wastewater. Therefore, the recovery efficiency was modeled as a uniform distribution with a minimum recovery of 30.1% and a maximum of 55%. The revised model was employed to estimate the total number of infected people through the following equation (Eq. (4)):
| (4) |
It is worth noting here that along with some of the uncertainties touched upon above, RNA losses in the sewer may undermine the accuracy of prevalence back-estimation through WBE. Despite the transient infectivity of SARS-CoV-2 in wastewater environment, some have argued that its RNA is significantly more persistent, reaching 3–33 days (Wu et al., 2020b). Owing to the lack of sufficient information, the RNA losses in the sewer were ignored in our study, knowing that this approach can result in an underestimation of the infected population (Kapo et al., 2017; Weidhaas et al., 2021). In addition to the fact that only 64% of the inhabitants have access to the public sewage system, there is currently insufficient information to quantify to what extent that WBE under or overestimates the viral load. As such, the study examines whether trends observed in wastewater correlate with clinical data, which is possible and important as a starting point for further research.
2.6. Statistical analysis
The announced numbers of COVID-19 cases (discharged, inpatients, and dead) in Tehran were derived from a database on clinical surveillance maintained by the Ministry of Health and Medical Education (MOHME) (IMoHaM, 2020).
All statistical analyses and figures elaboration were carried out using GraphPad Prism v.8.4.1 (GraphPad Software, San Diego, CA, USA). All tests were done considering a statistical significance level of p < 0.05.
3. Results and discussion
3.1. Confirmed COVID-19 cases in the study area
As of 8 September 2021, Tehran had reported 351,641 COVID-19 cases and 23,643 deaths, accounting for 8% of the 4,833,135 cases and 22% of the 104,716 deaths reported nationwide. The number of daily diagnosed cases in the city at the beginning of the present study (30 September) was 339 patients, which increased to as high as 408 cases per day as of 6 April 2021. Fig. 2 illustrates new cases of infection and the number of deaths from coronavirus pandemic in Tehran from September 2020 to May 2021, announced by the Ministry of Health. Some have argued that the number of cases reported all over the country in the early months may represent only about 20% of the real number due mainly to the limited surveillance and lack of test kits as not all cases were identified (IMoHaM, 2020). There are also unofficial reports demonstrating that the actual number of deaths is at least 2.5 to 3 folds (or even much more than that) of the reported number. At the metropolis level, the first wave of the epidemic occurred in mid-March 2020. By the end of June 2020, Tehran had not yet declared the peak of its second wave. A later increase in the number of COVID-19 diagnosed cases in July is further attributed to increased detection capacity. In line with facing a second wave of cases, the city reported increases of 10 to 4602% in the mean number of new cases per day being reported. As of the first November 2020, the metropolitan was experiencing its third wave of outbreaks. The city lockdown and early implementation of precautionary measures bought time for authorities to boost their response capacity. On 4th April 2021, at the beginning of the fourth wave, Tehran confirmed 315 cases and in the next 14 days with the daily average of 620. The fourth wave of COVID-19 in Tehran, similar to other parts of the country, has had severe consequences in the form of more clinically confirmed cases attributed to the emergence of new more virulent variants and a reduction in the supply of essential treatments (such as limitations of hospital capacity, provision of antiviral drug, etc). As can be seen, there was a clear trend of separation between either new cases of infection or the number of deaths from CODID-19 over this month and those reported for the preceding two months, which could not only be assigned to facing new and more transmissible variants of the virus but also lack of public adherence with and support for precautionary measures and preventive guidelines to reduce transmission of SARS-CoV-2. Specially, social and family gatherings during New Year bulk purchases, crowds of people to provide essential needs (in particular chicken queues), national holidays in conjunction with new year (Nowruz) celebration all increased, in parallel to reductions in follow-up instructions against COVID-19 transmission risks (adherence to social distancing, using masks in public places, regular washing of hands, among others). Since mid-March 2021, the public adherence rate to precautionary measures and health protocols declined rapidly and reached 34–40%, which was the lowest level of compliance since the early pandemic. To date of submission of this work, Tehran sees the risk of the fifth wave of cases fed by lineage B.1.617, and the sub-lineage B.1.617.2, commonly referred to as the Delta variant.
Fig. 2.
Cases of infection from coronavirus pandemic in Tehran from September 2020 to May 2021 (IMoHaM, 2020).
3.2. Optimization of RT-qPCR assays
Development of the N (nucleocapcid), ORF1ab (Open reading frame 1ab), E (envelope), M (membrane), S (spike), and RdRP (RNA-dependent RNA polymerase) assays, paved the way for rapid, sensitive and accurate strain-level detection using RT-PCR. These assays have extensively been employed for SARS-CoV-2 RNA detection in raw wastewater and treated effluent samples (Hillary et al., 2021; Medema et al., 2020; Rimoldi et al., 2020; Saguti et al., 2021; Wu et al., 2021). All samples (91 out of 91) were SARS-CoV-2 positive with RT-qPCR assays using ‘N’ target gene, whereas just 74 (81%) were positive for ‘ORF1ab’ and in 17 remaining samples led to false negatives (See SI - Table S2). The results throw light on the relative merits of ‘N’ as compared with ‘ORF1ab’. Given the Ct value differences, these results suggest that ‘N’ is more sensitive (~1 log10) for detection of SARS-CoV-2 in sewage, as compared with ‘ORF1ab’. A number of studies in raw and treated wastewater samples have, in agreement with our findings, reported that CDC N primer/probe sets outperform RdRP and E genes for detecting beta coronaviruses, including 2019-nCoV (Corman et al., 2020). Three ‘N’ gene assays (N1, N2, and N3) each targeting a different region of the nucleocapsid (N) gene have been proposed by US CDC and their specificity against other viruses, comprising human coronaviruses, has been reported (Amereh et al., 2021; Corman et al., 2020). The ‘ORF1ab’ gene assay has also been considered as confirmatory testing (specific to SARS-CoV-2) in some studies to estimate the infection incidence within the population. The expression of ‘ORF1ab’ requires ribosomal frame shifting, implying that it is produced at significantly lower levels as compared to N-encoded functions and sub-genomic RNA (Barra et al., 2020). Therefore, in the infected samples, the ‘ORF1ab’ copy number is expected to be lower than ‘N’. In contrast, the ‘N’ target is highly expressed because its sequence is present in almost all sub-genomic RNA (Barra et al., 2020; Kim et al., 2020).
The method (in terms of concentration, extraction and detection) used herein showed a 42.55 ± 12.45% % (average and standard deviation) recovery efficiency for IVB, a surrogate for SARS-CoV-2 from untreated wastewater. This recovery was comparable to those reported by Ahmed et al. (2020b) and outperformed others demonstrating 26.7 ± 15.3% and 31 to 32% recovery efficiencies for the murine hepatitis virus from wastewater (Ahmed et al., 2020b) and human adenovirus from river water (Ahmed et al., 2015), respectively. PEG precipitation seems to be an efficient method to recover SARS-CoV-2 RNA from various environmental matrices and has previously been used for the enrichment of viruses from aqueous solutions (Gyawali et al., 2019; Kumar et al., 2020a; Kumar et al., 2020b; Kumar, 2021; Mull and Hill, 2012; Wu et al., 2020a) and more recently for the concentration of SARS-CoV-2 RNA from untreated wastewater (Ahmed et al., 2020a; Kocamemi et al., 2020; Medema et al., 2020). It appears to be a promising approach for virus concentration because both the liquid and solid fractions of wastewater are incorporated for enrichment of viruses. Nevertheless, PEG precipitation is in the throes of the co-concentration of PCR inhibitors. This idea supports Kocamemi et al. (2020) findings which reported a 1–1.5 log10 reduction in detection following PEG precipitation and RNA isolation. Therefore, the presence of inhibitors in wastewater samples needs to be investigated, and if present, efforts should be taken to minimize overall inhibition. To achieve this, it is recommend that each wastewater sample be seeded with a surrogate virus as a whole-process control to obtain information on the surrogate virus and RNA recovery as well as RT-PCR inhibition for the entire process starting from sample concentration to RT-PCR detection. Accordingly, we used IBV as a whole process control in our study. This method, however, has been reported to provide improved recovery of viral particulates compared to ultrafiltration and other methods (Ahmed et al., 2020b). Further research is required to establish and standardize protocols for virus extraction in order to compare studies conducted all over the world; nevertheless, it is worth noting here that a variety of methods have given comparable trends during an inter-laboratory comparison study of 36 methods (Pecson et al., 2021). Moreover, the measurement of plausible losses during SARS-CoV-2 virus concentration and thus the actual detection efficiency may differ from surrogate viruses and even an inactivated form of SARSCoV-2 itself. This might be related to the dissimilarity of surrogate viruses (e.g., bovine coronavirus (BCoV), bovine respiratory syncytial virus (BRSV), bacteriophage Phi6, human coronavirus OC43,5 human coronavirus HCoV-E, murine hepatitis virus (MHV), F-specific RNA phages,23 and vesicular stomatitis virus (VSV)) to SARS-CoV-2 in structure and morphology. To date, a detailed comparison between different surrogate viruses and SARS-CoV-2 in the concentration, extraction and detection from wastewater is still lacking, which requires further investigations (Kantor et al., 2021). Subsequently, variation in the viral recoveries can represent not only differences in the concentration methods but also in the structure, genetic components, as well as the behavior of different surrogate viruses.
3.3. Concentration of SARS-CoV-2 RNA in wastewater samples
A total of 91wastewater samples were collected every 2 weeks from seven major municipal WWTPs (cumulative inlet loading >1.5 × 106 m3 of wastewater per day). Sewage sampling was conducted from September 2020 until April 2021 during the pandemic outbreak of SARS-CoV-2 in Tehran to investigate the presence and titers of virus-specific RNA in untreated sewage samples. All tested samples were positive according to SARS-CoV-2 RNA Nucleocapsid (N) genes assay, and within the cycle quantification value (Ct values below 40 cycles) (See SI - Table S2). RNase P gene detection was positively identified in all samples tested, confirming successful isolation of human as well as viral nucleic acids. It is worth mentioning that RNase P target is amplified as a quality control for the extraction method and to corroborate the absence of PCR-inhibitors in the sample (CDC, 2020).
Ct profiles for the tested influent wastewater samples varied from 28.8 to 38.9. Congruently, viral titers in sewage samples collected from the seven WWTPs correspond to a range of 37.74 to 45,607.77 RNA copies/L using the N gene target, implying a huge variation in gene copies of SARS-CoV-2. In a similar way, Ct values for the ORF1ab marker ranged from 31.2 to 39.13, which correspond to 33.75 to 8544.34 RNA copies/L using the ORF1ab gene target. Smaller Ct values of SARS-CoV-2 RNA copies found for N-genes might possibly be attributed to higher sensitivity of markers targeting the N region as compared with ORF1ab, which is in agreement with the growing body of research (Jung et al., 2020; Zhou et al., 2020). Comparison of virus loadings observed in our study with those of other studies confirms similar SARS-CoV-2 wastewater loads (Sherchan et al., 2020), but broadly differ from estimates of viral load in the state of Massachusetts in late March 2020, where they reported high counts ranging between 50 and 300 gene copies/mL in sewage samples (Wu et al., 2020a). Likewise, Wurtzer et al. (2020) reported viral loads as high as 3.2 × 106 genome units/L in Paris (France), which is the highest SARS-CoV-2 concentration ever reported in wastewater influent. They also reported a significant positive association between SARS-CoV-2 concentrations in WWTP influents and the number of infected individuals in their study region. It is important to caveat a lack of standardization in calculating from copies per reaction based on a standard curve to gene copies/L of SARS-CoV-2 which may significantly impact the range of values reported, and therefore comparisons. However, it is encouraging to compare these findings with those reported for a few Middle East countries like Turkey (Kocamemi et al., 2020), Qatar (Saththasivam et al., 2021), United Arab Emirates (Hasan et al., 2021), and Pakistan (Yaqub et al., 2021), whose wastewater viral concentrations have been published. We recognize stark distinction between SARS-CoV-2 titers in our study and what other communities, in particular countries in the region, are reporting. Differences between studies are also abundant, and can include variability in the severity and load of fecal shedding, the efficiency of RNA recovery from sewage samples, and RNA losses in sewage pipes and during experimental procedures, among others. To verify the above viewpoints, the maximum SARS-CoV-2 RNA concentrations reported in raw sewage samples ranged from 1.2 × 103 to 3.2 × 106 gene copies/L (Ahmed et al., 2020a; La Rosa et al., 2020; Nemudryi et al., 2020; Randazzo et al., 2020; Wurtzer et al., 2020).
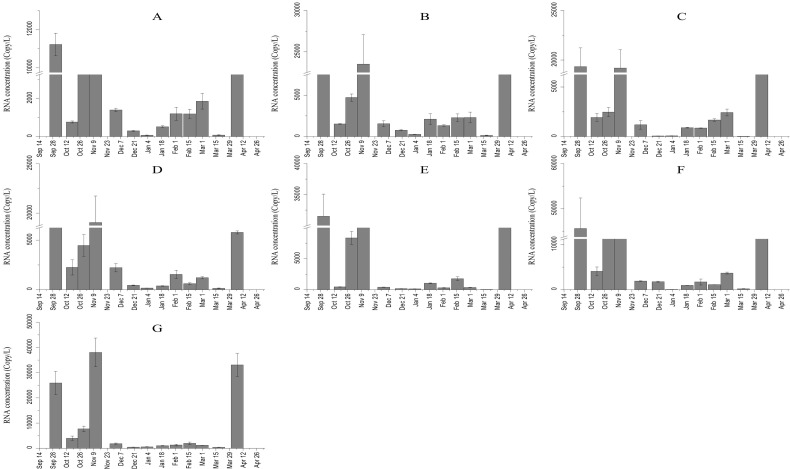
Timeline variations in viral RNA concentration in wastewater point to changes in the prevalence of shedders in a sewer catchment, which could be associated with a combination of pandemic response initiatives across the city. Since the announcement of the first cases of infection, the metropolitan in tune with national policies has established some preventive measures targeting restrictions on interprovincial traveling, closures for schools and universities, workplace closures, limitation of employees' capacity at estate workplaces, cancellations of public events, restrictions on public transportation, stay at home and shelter in place requirements, as well as traffic restrictions within the city from 10:00 p.m. to 3:00 a.m. The possible impact of these measures was more evident during December 2020 and March 2021. The Ct values coupled with corresponding viral loads throughout the 29-week study period are comparable among WWTPs (Fig. 3 ), with an increase in SARS-CoV-2 viral load from mid-October. Ct values decreased to <31.27 (Fig. 4 ), which coincides with the population-wide COVID-19 resurgence as observed in Fig. 5 (the so-called third wave), demonstrating the necessity for applying timely preventive measures. From late November onwards, the city has surpassed the outbreak as supported by decreasing the daily new confirmed case counts in the city within the following 20 days of the survey in which the number of new cases reduced approximately 7 fold, 82 in the case of 10 December 2020. Further, from 25th January to 22nd March when the corresponding curve of daily diagnosed COVID-19 cases was flattening (Fig. 5), it was also noticed that the measured virus load numbers in sewage also declined in all WWTPs (~ 84%). Again, measurements of the viral load in wastewater kept an increasing trend until peaked on 6 April 2021 in studied sewage plants (about 110,000 gene copies/L), which were also in line with climbing SARS-CoV-2 diagnosed cases. This observation also coincides with lifting lockdowns, reduced public adherence to COVID-19 mitigation strategies, and prevalence of Alpha variant (B.1.1.7).
Fig. 3.
SARS-CoV-2 RNA (‘N’ target) concentration in the influent of seven major WWTPs (Shahrak-e Gharb (A), Ekbatan (B), Zargandeh (C), Gheytarieh (D), Sahebgharanieh (E), Mahllati (F), and Tehran's South wastewater treatment plant (G)) in Tehran. Standard deviations (SD) shown in the bar charts were based on triplicate measurements.
Fig. 4.
SARS-CoV-2 spread in different areas of Tehran according to wastewater treatment plants (WWTPs).
Fig. 5.
Cumulative COVID-19 positive cases as reported in Tehran (IMoHaM, 2020) and estimated SARS-CoV-2 shedding individuals in sewersheds based on the developed model.
Viral RNA titers were assessed by 3.32-Ct intervals from 25 to 40, which permits broad comparisons to be made between differing situations. It is worthwhile that this interval is the exact counterpart of ~1 log10 gc decrease. For a clearer comparison, low Ct values (equivalent to high SARS-CoV-2 RNA titers) for all seven WWTPs of Tehran were concentrated between early October and mid-November 2020 and also on early April 2021 which is mirrored by the period when the city registered the highest numbers of infections and related deaths. We observed the lowest Ct values (corresponding to the highest viral loads) among biweekly tested samples on first October 2020 when it reached 28.8 Ct corresponding to a viral load of 45,607.78 gene copies/L, with 516 reported COVID-19 cases four days later on 4th October. Overall, Southern Tehran WWTP seems to carry the highest amount of virus in wastewater followed by Mahallati (see also Fig. 3), but no statistically significant difference was noticed between the median viral loads obtained among treatment plants. The dynamic profile of viral loading relevant for all WWTPs with peaks approximately every four weeks and preceding variations in the number of newly confirmed cases by 19 to 21 days as observed in Fig. 3, has important implications in managing COVID-19 spread. In line with the third wave of daily reported SARS-CoV-2 positive cases in Tehran, ascending trends of SARS-CoV-2 measurements were also noticed in all the WWTPs (approximately 140,000 gene copies/L). An increasing trend was also observed in early April 2021 coinciding with the fourth wave (about 110,000 gene copies/L). From late January to mid-March 2021, all WWTPs reported increasing viral loads, with the exception of Sahebgharanieh WWTP which remained constant and comparatively lower than other sampled sites. This inconsistency may be explained by the rather discrete location of Sahebgharanieh catchment, which is located in the north of the city and is characterized as a built up area of the city with relatively low population density. One of the benefits that emerges from wastewater testing and in particular the spatial variations of viral load numbers is that tracking large-scale infectious disease through sewage analyses offers a stylized view of the main workings of the epidemic than the total number of infected individuals in the community. This is particularly important in light of the variability in the severity and shedding duration among the population (Saguti et al., 2021; Hasan et al., 2021). Taken together, our results confirm that SARs-CoV-2 concentrations in wastewater closely coincide with or precede confirmed virological laboratory testing, as has been previously reported (Medema et al., 2020; Saguti et al., 2021; Trottier et al., 2020; Wurtzer et al., 2020).
3.4. Prevalence estimate of SARS-CoV-2
Several studies have reported that the quantity of SARS-CoV-2 in a sewer catchment is correlated with the actual number of infected individuals in that sampled area (Fongaro et al., 2021; Kitamura et al., 2021; Saththasivam et al., 2021). Herein, viral load numbers in sewage samples were converted to the number of infections based on the analysis of Monte Carlo simulation to explore whether these measurements mirror infections in the population.
Taking into account the normal distribution of cases, it is only possible to compare the daily positive cases against the total estimated infected population of the studied WWTPs. It is important to bear in mind that the city is served in part (64%) by these sewage works. The daily estimated number of SARS-CoV-2 shedding individuals was thus compared with the stated fraction of confirmed COVID-19 cases. The spearman correlation, however, did not reach statistical significance (p > 0.05). We repeated the linear regression analyses adjusting for the virus shedding period and asymptomatic cases. Given that SARS-CoV-2 shedding in stool of infected patients lasts approximately 22 days (with an interquartile range between 17 and 31 days) (Zheng et al., 2020), the measured viral load at the influent of each WWTP reflects a cumulative amount shed by affected individuals during the relevant window. Thus, it can be deduced that the measured concentration accounts for infected individuals, symptomatic or not – those that have and have not been clinically identified - infected several days prior to each sampling day. Congruently, a cumulative estimate of the relevant window was used as a reference in our study. This preconceived idea had also been demonstrated in COVID-19 outbreak monitoring very recently using a similar approach in Qatar (Saththasivam et al., 2021). In line with a generally assumed long incubation period of SARS-CoV-2 (Li et al., 2021), the number of infections on any given date was roughly calculated by taking the sum of officially declared confirmed cases on the day of sampling, 10 days before the sampling date, and 11 days following each sampling date. It implies that the summation did account for the patients already diagnosed and those not yet diagnosed but already positive within the population.
The possible number of the infected population estimated through the WBE model is much higher than the daily reported positive cases, demonstrating that the number of actual infections is likely underestimated. However, most studies have presented the association of the SARS-CoV-2 RNA levels in wastewater and clinically confirmed cases, although this typically encompasses only a proportion of those with COVID-19 and who typically have symptoms, as mass testing of all of a population is rare. To get a more refined picture of the relationship, the estimated infections from Monte Carlo simulations on any given sampling date were compared to the corrected 22 days of cumulative COVID-19 caseloads (Fig. 5). Overall, the estimated number of individuals shedding SARS-CoV-2 within sewersheds was found to be linearly correlated with the cumulative diagnosed positive cases (R 2 = 0.80, p < 0.001) (Fig. 6 ). Moreover, it can be inferred that the estimated infected population against the officially declared case counts is 353:1. More specifically, the estimated sum of the infected population is more than the sum of the confirmed cases during the time course of sewage monitoring. While theoretically it is expected to be a 1:1 ratio, this result is corroborated by prior research (Albastaki et al., 2021; Wu et al., 2021) and could be attributed to the inclusion of both the diagnosed and undiagnosed cases in sewage viral loads. La Rosa et al. (2020) speculated that a substantial number of the population are either asymptomatic or paucisymptomatic and would otherwise remain clinically undetected. Likewise, earlier studies also noted a lack of symptoms in up to 70% of infections (Byambasuren et al., 2021; Oran and Topol, 2021). Furthermore, Meyerowitz et al. (2021) found a range of pre-symptomatic or asymptomatic COVID-19 cases averaging 43 ± 28%. As yet, limited parallel research has definitely established strong correlations between viral loads in sewage and the prevalence of clinically positive patients. While variable correlations do exist in the global literature ranging from 0.18–0.81 (Greenwald et al., 2021; Randazzo et al., 2020; Weidhaas et al., 2021). However, it is important to note that correlations with wastewater are typically carried out based on populations of individuals which test positive on a given day, and largely ignores the fact that mass testing is not carried out frequently. Therefore, positive cases typically represent the proportion of the population which has symptoms and ignores large societal inequality and variabilities which impact testing including availability of testing, affordability, personal biases and opinions, institutional distrust, etc. More studies of comparable study populations are thus needed to address which associations remain consistent.
Fig. 6.
Estimated SARS-CoV-2 infected individuals (derived from the developed model based on wastewater analyses) in sewersheds over the study period compared to the confirmed COVID-19 cases.
3.5. Limitations of study and future perspectives
An important limitation of this study is that the exact population being served by each WWTP is not known. As a starting point, this study examined the relationship between SARS-CoV-2 RNA loads in sewage with officially reported COVID-19 prevalence in the community, and highlights what is possible with collaboration among different governmental organizations. Although this study demonstrates the application of WBE as a new paradigm in public health, it is recommended to have long-term and daily monitoring to improve its resolution. In general, the changes in SARS-CoV-2 RNA concentrations in wastewater corroborated with the announced daily cases; however, we did not attempt to measure the lag between wastewater data and public testing data, since the sewage surveillance results were documented for biweekly obtained samples while the public test results were reported in daily basis. Likewise, the study had some other limitations, including the lack of solid analysis in wastewater to consider the partitioning of SARS-CoV-2 via adsorption to solids. There were also uncertainties due to limited knowledge about the infection of gastrointestinal tract, the concentration of viral RNA in stool over the time-course of the illness, the variability in viral dynamics in individuals and fecal shedding. Finally, in this study the normalization was not performed, though consensus on what approach to use for normalization is still ongoing.
4. Conclusions
Despite international efforts to contain SARS-CoV-2 through the implementation of various regional and continent specific policies, it is still posing a serious global challenge. The long incubation period, viral shedding and transmission by asymptomatic and pre-symptomatic infected individuals, and virus mutation add to the difficulties in managing the outbreak. Wastewater-based disease surveillance, as a new paradigm in public health, has been suggested for monitoring community outbreaks; however, comparably little is known about the interrelationships between wastewater viral titers and the abundance of recorded clinical cases within the community, although this has greatly improved over ~21 months since the pandemic began. Various methodological challenges associated with WBE would affect the accuracy of prevalence estimation. To date, the overall uncertainty of WBE and the impact of each step on the prevalence estimation are largely unknown. In the other words, the differences between the estimated number of cases infected by SARS-CoV-2 (based on the viral load of wastewater) and the officially reported cases of COVID-19 may be the result not only of the lack of a more extensive laboratory diagnosis but also of the uncertainties considered in this work within the model for estimating the number of infected people.
Wastewater viral titers were found to be linearly correlated with the diagnosed caseloads, however, considerable differences were observed between the estimated SARS-CoV-2 shedders and officially reported cases of COVID-19. As mass testing is not a thing, this is literally something that affects every WBE study. Overall, this study reiterates the effectiveness of precautionary efforts in the fight against not only COVID-19 but also future infectious outbreaks using wastewater analysis.
CRediT authorship contribution statement
Fatemeh Amereh: Investigation, Processing experimental data, Data curation, Software, Performing the analysis, Designing the figures, Mapping, Writing-Original draft preparation. Mahsa Jahangiri-rad: Reviewing and Editing. Anoushiravan Mohseni-Bandpei: Reviewing and Editing. Seyed Reza Mohebbi: Methodology. Hamid Asadzadeh-Aghdaei: Reviewing and Editing. Hossein Dabiri: Reviewing and Editing. Akbar Eslami: Reviewing and Editing. Kasra Roostaei: Sampling. Rahim Aali: Methodology. Parisa Hamian: Mapping. Mohammad Rafiee: Conceptualization, Supervision, Writing-Original draft preparation, Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Institute for Medical Research Development (NIMAD) under Grant No. 994141. We would like to acknowledge Tehran Sewerage Co. for assistance with sample collection. We would also like to acknowledge Research Institute for Gastroenterology and Liver Diseases that generously provided us with their laboratories. The authors also thank anonymous reviewers for their valuable comments on this manuscript.
Editor: Warish Ahmed
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.152597.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Harwood V., Gyawali P., Sidhu J., Toze S. Comparison of concentration methods for quantitative detection of sewage-associated viral markers in environmental waters. Appl. Environ. Microbiol. 2015;81:2042–2049. doi: 10.1128/AEM.03851-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Ali H., Yaniv K., Bar-Zeev E., Chaudhury S., Shaga M., Lakkakula S., et al. Tracking SARS-CoV-2 RNA through the wastewater treatment process. ACS EST Water. 2021;1:1161–1167. doi: 10.1021/acsestwater.0c00216. [DOI] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., et al. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Islam M.A., Kumar M., Hossain M., Bhattacharya P., Islam M.T., et al. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: variation along the sewer network. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albastaki A., Naji M., Lootah R., Almeheiri R., Almulla H., Almarri I., et al. First confirmed detection of SARS-COV-2 in untreated municipal and aircraft wastewater in Dubai, UAE: the use of wastewater based epidemiology as an early warning tool to monitor the prevalence of COVID-19. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amereh F., Negahban-Azar M., Isazadeh S., Dabiri H., Masihi N., Jahangiri-rad M., et al. Sewage systems surveillance for SARS-CoV-2: identification of knowledge gaps, emerging threats, and future research needs. Pathogens. 2021;10:946. doi: 10.3390/pathogens10080946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra G.B., Santa Rita T.H., Mesquita P.G., Jácomo R.H., Nery L.F.A. Analytical sensitivity and specificity of two RT-qPCR protocols for SARS-CoV-2 detection performed in an automated workflow. Genes. 2020;11:1183. doi: 10.3390/genes11101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., et al. ACS Publications; 2020. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. [DOI] [PubMed] [Google Scholar]
- Byambasuren O., Dobler C.C., Bell K., Rojas D.P., Clark J., McLaws M.-L., et al. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2019-Novel Coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes. 2020. https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf Available from:
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- <collab>CSSE J.collab. COVID-19 dashboard by the Center for Systems Science and Engineering(CSSE) at Johns Hopkins University (JHU) 2021. https://coronavirus.jhu.edu/map.html.Education
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., et al. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., Stoco P.H., Souza D.S.M., Grisard E.C., Magri M.E., Rogovski P., et al. The presence of SARS-CoV-2 RNA in human sewage in Santa Catarina, Brazil, November 2019. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour S., Mohammadi F., Nikaeen M., Shamsizadeh Z., Khazeni A., Sahbaei Z., et al. COVID-19 infection risk from exposure to aerosols of wastewater treatment plants. Chemosphere. 2021;273 doi: 10.1016/j.chemosphere.2021.129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R., Curtis K., Bivins A., Bibby K., Weir M.H., Yetka K., et al. COVID-19 surveillance in southeastern Virginia using wastewater-based epidemiology. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald H.D., Kennedy L.C., Hinkle A., Whitney O.N., Fan V.B., Crits-Christoph A., et al. Tools for interpretation of wastewater SARS-CoV-2 temporal and spatial trends demonstrated with data collected in the San Francisco Bay Area. Water Res. X. 2021;12 doi: 10.1016/j.wroa.2021.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali P., Croucher D., Ahmed W., Devane M., Hewitt J. Evaluation of pepper mild mottle virus as an indicator of human faecal pollution in shellfish and growing waters. Water Res. 2019;154:370–376. doi: 10.1016/j.watres.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., Ibrahim Y., Daou M., Kannout H., Jan N., Lopes A., et al. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary L.S., Farkas K., Maher K.H., Lucaci A., Thorpe J., Distaso M.A., et al. Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMoHaM 2020. https://behdasht.gov.ir/
- Iran SCo Population and housing censuses. 2016. https://www.amar.org.ir/Portals/1/cen-sus/2016/results/general/population/census-2016-general-results-population-t1.xls
- Jeremijenko A., Chemaitelly H., Ayoub H.H., Abdulla M.A.H., Abou-Samra A.B., Al Ajmi J.A.A., et al. Evidence for and level of herd immunity against SARS-CoV-2 infection: the ten-community study. MedRxiv. 2020 doi: 10.1101/2020.09.24.20200543. [DOI] [Google Scholar]
- Jones T.H., Johns M.W. Improved detection of F-specific RNA coliphages in fecal material by extraction and polyethylene glycol precipitation. Appl. Environ. Microbiol. 2009;75:6142–6146. doi: 10.1128/AEM.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.-m., Akhmetzhanov A.R., Hayashi K., Linton N.M., Yang Y., Yuan B., et al. Real-time estimation of the risk of death from novel coronavirus (COVID-19) infection: inference using exported cases. J. Clin. Med. 2020;9:523. doi: 10.3390/jcm9020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor R.S., Nelson K.L., Greenwald H.D., Kennedy L.C. Challenges in measuring the recovery of SARS-CoV-2 from wastewater. Environ. Sci. Technol. 2021;55:3514–3519. doi: 10.1021/acs.est.0c08210. [DOI] [PubMed] [Google Scholar]
- Kapo K.E., Paschka M., Vamshi R., Sebasky M., McDonough K. Estimation of US sewer residence time distributions for national-scale risk assessment of down-the-drain chemicals. Sci. Total Environ. 2017;603:445–452. doi: 10.1016/j.scitotenv.2017.06.075. [DOI] [PubMed] [Google Scholar]
- Keshaviah A. Mathematica Policy Research; Washington: 2017. The potential of wastewater testing for public health and safety. [Google Scholar]
- Kim S., Kim D.-M., Lee B. 2020. Insufficient sensitivity of RNA dependent RNA polymerase gene of SARS-CoV-2 viral genome as confirmatory test using Korean COVID-19 cases. [Google Scholar]
- Kitamura K., Sadamasu K., Muramatsu M., Yoshida H. Efficient detection of SARS-CoV-2 RNA in the solid fraction of wastewater. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Sait A., Kadi H., Sarac F., Aydin I., et al. Routine SARS-CoV-2 wastewater surveillance results in Turkey to follow Covid-19 outbreak. medRxiv. 2020 doi: 10.1101/2020.12.21.20248586. [DOI] [Google Scholar]
- Kumar M. 2021. Prevalence of SARS-CoV-2 in communities through wastewater surveillance-a potential approach for estimation of disease burden. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., et al. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., et al. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;141326 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemann J., Jr., Pleuss J.A., Worcester E.M., Hornick L., Schrab D., Hoffmann R.G. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49:200–208. doi: 10.1038/ki.1996.27. [DOI] [PubMed] [Google Scholar]
- Lescure F.-X., Bouadma L., Nguyen D., Parisey M., Wicky P.-H., Behillil S., et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect. Dis. 2020;20:697–706. doi: 10.1016/S1473-3099(20)30200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Su Y.-Y., Zhi S.-S., Huang J., Zhuang C.-L., Bai W.-Z., et al. Virus shedding dynamics in asymptomatic and mildly symptomatic patients infected with SARS-CoV-2. 2020;26:1556. doi: 10.1016/j.cmi.2020.07.008. e1-1556. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021;129039 doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol. Hepatol. 2020;5:533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Klapsa D., Wilton T., Zambon M., Bentley E., Bujaki E., et al. Tracking SARS-CoV-2 in sewage: evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses. 2020;12:1144. doi: 10.3390/v12101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E.A., Richterman A., Bogoch I.I., Low N., Cevik M. Towards an accurate and systematic characterisation of persistently asymptomatic infection with SARS-CoV-2. Lancet Infect. Dis. 2021;21(6):e163–e169. doi: 10.1016/S1473-3099(20)30837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mull B., Hill V.R. Recovery of diverse microbes in high turbidity surface water samples using dead-end ultrafiltration. J. Microbiol. Methods. 2012;91:429–433. doi: 10.1016/j.mimet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri S., Yavarian J., Baghani A.N., Azad T.M., Nejati A., Nabizadeh R., et al. The presence of SARS-CoV-2 in raw and treated wastewater in 3 cities of Iran: Tehran, Qom and anzali during coronavirus disease 2019 (COVID-19) outbreak. J. Environ. Health Sci. Eng. 2021;19:573–584. doi: 10.1007/s40201-021-00629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., et al. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann. Intern. Med. 2021;174:655–662. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecson B.M., Darby E., Haas C.N., Amha Y.M., Bartolo M., Danielson R., et al. Reproducibility and sensitivity of 36 methods to quantify the SARS-CoV-2 genetic signal in raw wastewater: findings from an interlaboratory methods evaluation in the US. Environ. Sci. Water Res. Technol. 2021;7:504–520. doi: 10.1039/d0ew00946f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Liu J., Xu W., Luo Q., Chen D., Lei Z., et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. https://onlinelibrary.wiley.com/doi/epdf/10.1002/jmv.25936?src=getftr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. Preliminary results of SARS-CoV-2 detection in sewerage system in Niterói municipality, Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2020:115. doi: 10.1590/0074-02760200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee M., Isazadeh S., Mohseni-Bandpei A., Mohebbi S.R., Jahangiri-Rad M., Eslami A., et al. Moore swab performs equal to composite and outperforms grab sampling for SARS-CoV-2 monitoring in wastewater. Sci. Total Environ. 2021;148205 doi: 10.1016/j.scitotenv.2021.148205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguti F., Magnil E., Enache L., Churqui M.P., Johansson A., Lumley D., et al. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021;189 doi: 10.1016/j.watres.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saththasivam J., El-Malah S.S., Gomez T.A., Jabbar K.A., Remanan R., Krishnankutty A.K., et al. COVID-19 (SARS-CoV-2) outbreak monitoring using wastewater-based epidemiology in Qatar. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., et al. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanhaei M., Mohebbi S.R., Hosseini S.M., Rafieepoor M., Kazemian S., Ghaemi A., et al. The first detection of SARS-CoV-2 RNA in the wastewater of Tehran, Iran. Environ. Sci. Pollut. Res. 2021;1–8 doi: 10.1007/s11356-021-13393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii S., Furumai H., Katayama H. Applicability of polyethylene glycol precipitation followed by acid guanidinium thiocyanate-phenol-chloroform extraction for the detection of SARS-CoV-2 RNA from municipal wastewater. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.143067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., Darques R., Mouheb N.A., Partiot E., Bakhache W., Deffieu M.S., et al. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., et al. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Mueller M.A., et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. MedRxiv. 2020 doi: 10.1101/2020.03.05.20030502. [DOI] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. Medrxiv. 2020 doi: 10.1101/2020.06.15.20117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., et al. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. Msystems. 2020;:5. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., et al. Wastewater surveillance of SARS-CoV-2 across 40 US states from February to June 2020. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J., Maday Y., Teyssou R., Richard E., et al. Evaluation of lockdown effect on SARS-CoV-2 dynamics through viral genome quantification in waste water, greater Paris, France, 5 March to 23 April 2020. Eurosurveillance. 2020;25:2000776. doi: 10.2807/1560-7917.ES.2020.25.50.2000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqub T., Nawaz M., Shabbir M.Z., Ali M.A., Altaf I., Raza S., et al. A longitudinal survey for genome-based identification of SARS-CoV-2 in sewage water in selected lockdown areas of Lahore City, Pakistan: a potential approach for future smart lockdown strategy. Biomed. Environ. Sci. 2021;34(9):729–733. doi: 10.3967/bes2021.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(883–890) doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material