Graphical abstract
Abbreviations: Ozone, O3; Protective Equipment, PPE
Keywords: Ozone, Decontamination, SARS-CoV-2, Protective equipment, COVID-19
Abstract
COVID-19 pandemic created a global shortage of medical protective equipment. Here, we considered ozone (O3) a disinfectant alternative due to its potent oxidative activity against biological macromolecules. The O3 decontamination assays were done using SARS-CoV-2 obtained from patients to produce artificial contamination of N95 masks and biosecurity gowns. The quantification of SARS-CoV-2 was performed before and after exposing the samples to different ozone gas concentrations for times between 5 and 30 min. Viral loads as a function of the O3 exposure time were estimated from the data obtained by the RT-PCR technique. The genetic material of the virus was no longer detected for any tested concentrations after 15 min of O3 exposure, which means a disinfection Concentration-Time above 144 ppm min. Vibrational spectroscopies were used to follow the modifications of the polymeric fibers after the O3 treatment. The results indicate that the N95 masks could be safely reused after decontamination with treatments of 15 min at the established O3 doses for a maximum of 6 cycles.
1. Introduction
The shortage of medical protective equipment (PPE) observed during the recent acute respiratory syndrome coronavirus outbreak (SARS-CoV-2) [1], [2], [3], [4], [5] limited their availability for the hospital personnel leading to a high percentage of infected personnel worldwide [6]. One possible stratagem for mitigating the demand for PPEs (N95) is their reuse after adequate decontamination. The decontamination process needs to be easy to implement, safe, able to inactivate any potentially infectious material on their surfaces, and preserve the filtration efficiency.
Different methods have been proposed to achieve the proper decontamination of N95 [7], [8], [9], [10], [11], [12], [13], [14], [15] facemasks, but few have been officially approved [16], [17], [18]. Ozone (O3), which has a strong oxidation potential and documented antimicrobial properties [19], [20], [21], [22], [23], [24], has not been widely evaluated [25], and it could be a simple alternative for decontamination. However, considering that the PPEs are composed of 4–6 layers of non-woven polymeric fibers and polymers are degraded by oxidative species, such as O3 [26], [27], the integrity of the materials should be evaluated. This work aimed to demonstrate whether O3 can be used as a disinfectant for high-demand PPEs and determine their lifetime based on the joint evaluation of the molecular integrity of the polymers using vibrational techniques, the mechanical properties of the straps and the filtration efficiency.
2. Materials and methods
Circles (1 cm in diameter) of the outer surface of N95 and biosafety gowns (B.G.) were inoculated with 50 μL of SARS-CoV-2 diluted in viral preservation media (DOUBANGTM disposable, Biocomma at 1:1, 1:10, 1:100, 1:1000, and 1:10000). Subsequently, inoculums were incubated at room temperature for 40 min for their total absorption in the materials. This artificial contamination test simulates a “real” contamination situation through bioaerosols since health personnel is exposed to them during the management of COVID-19 patients. Decontamination assays were performed using a portable domestic O3 generator. The samples were placed in a stainless-steel tray and were introduced in an airtight glass chamber 8 mm thick with a 15.6 L capacity kept at room temperature (25 °C). Ozone was injected through a 4.8 mm internal diameter hose at different exposure times (5, 10, 15, and 30 min) using a constant dose of 600 mg/h. Assuming an uniform distribution of the gas, the O3 concentrations achieved in the chamber after the injection times are 3.2, 6.4, 9.6 and 19.2 ppm, respectively. A glass Petri dish with gauze soaked with sterile distilled water was introduced to maintain a humid environment (40% R.H.) inside the chamber (Fig. S1 and S2). Atmospheric oxygen was injected using a fish tank pump during the same exposure times for the control group. Control and contaminated samples were subjected to detection of SARS-CoV-2 using RT-PCR to quantify viral loads. The molecular integrity of the polymeric materials was evaluated using Raman and FTIR as a function of O3 exposure time and compare with an estimate of the filtration efficiency (F.E.) [28]. More experimental details are in the supplementary file.
3. Results and discussion
Fig. 1 shows the Log reduction in viral load as the O3 exposure time increases for the different dilutions.
Fig. 1.
Logarithmic reduction in the viral loads after exposure to the ozone for A. the N95 facemask and B. the biosafety gown. The standard deviations obtained from three replications are not visible. The numeric values are present in Tables S1 and S2.
The SARS-CoV-2 virus is not detected after O3 decontamination for all viral loads tested in both N95 masks and B.G. at exposure times equal and above 15 min, i. e, a 100 % percent reduction value (PRV) was achieved. For shorter exposure times, 5 and 10 min, the virus is not detected only for the higher dilutions. Meanwhile, for the most concentrated samples: undiluted and 1:10 dilution, the virus is still detected at 5 and 10 min (Table S1). Nevertheless, the PRV is above 95 %. This simulated contamination experiment allows us to determine that 15 min of exposure or “Concentration × Times (CT)” [29] above 144 ppm min were sufficient to accomplish the undetectability of SARS-CoV-2 even at the highest viral concentrations. These CT values are larger than those reported for viral disinfection experiments of water [30], but much lower than when ozone gas is tested for room decontamination using surrogate viruses (4800 ppm min) [31].
Tseng and Li [24] evaluated the effects of O3 concentrations (0.6–1.2 ppm) and exposure times (5–120 min) on the structure of the viral capsid of four bacteriophages used as indicators of polioviruses, enteroviruses, enveloped viruses (such as SARS-CoV-2) and HIV. The viruses required above 47 min of O3 (1.2 ppm) to be 99% inactivated. Those times are larger than the 15 min demonstrated in this work to inhibit SARS-CoV-2. Lee et al. [25] exposed N95 and KF94 masks contaminated with human coronavirus (HCoV-229E), finding that the virus lost its infectivity after 1 min at 120 ppm of O3, explained as due to damage to the viral envelope. In agreement with Tizaoui [23], who showed that O3 could attack the proteins and lipids of the virus's envelope, particularly amino acids, such as tryptophan, methionine, cysteine, and fatty acids.
Limitations of the present study are that the null detection of the virus by RT-PCR is not a test of the virus infectivity and further studies should be performed to measure the real distribution of O3 in the chamber and determine the effect of humidity.
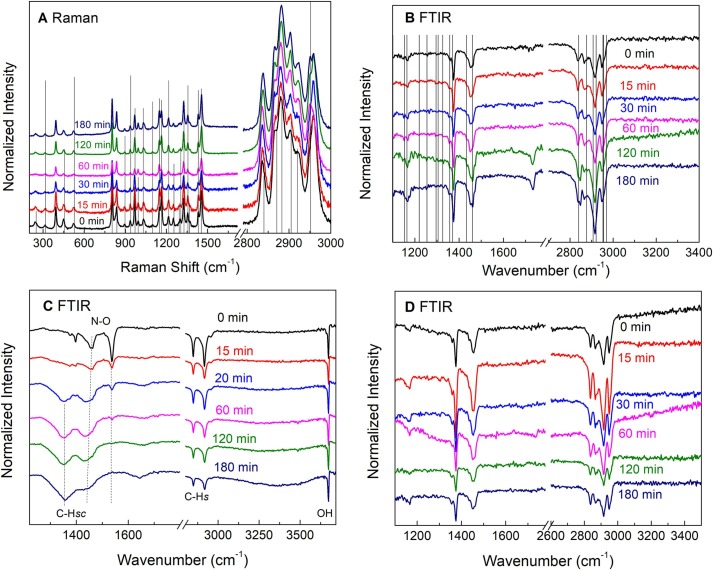
Vibrational spectroscopies were used to determine the molecular integrity of the materials for O3 exposure periods up to 180 min, which we consider as equivalent to 12 disinfection cycles of 15 min each. N95 masks and B.G. are made of polypropylene (P.P.) fibers and the elastic straps of a thermoelastic material. Fig. 2 A and B show the Raman and FTIR spectra of the N95 masks, respectively, including the lines from the P.P. reference spectra [26]. For the PP, the degradation is characterized by changes in the hydroxyl (OH) (3400 cm−1), carbonyl (C = O, 1650–1850 cm−1), and unsaturated (C = C) groups (890 and 1648 cm−1) [26], [27]. Fig. 2A and B indicate no significant degradation of the P.P. fibers at shorter exposure times. However, the appearance of the carbonyl group signal in the FTIR spectra for the samples treated at 120 (8 cycles) and 180 min indicate the outset of degradation [32], [33], [34], [35].
Fig. 2.
A. Raman spectra of the N95 facemasks as a function of the O3 exposure time. B. ATR-FTIR spectra of the N95 facemasks. Carbonyl (1700 cm−1) groups due to the ozonolytic degradation are observed after 120 min. C. FTIR spectra of the elastic straps of the N95 facemasks. Carbonyl and hydroxyl degradation signals and the scission of the C.H. bonds are observed. D. FTIR spectra of a piece of the biosafety gowns.
Fig. 2C shows the FTIR of the N95 elastic bands. The main functional groups identified in the untreated samples are C.H. stretching (s) and scissoring (sc), N-O, and free O.H. stretching. Here, essential changes can be observed from the 15 min of O3 application. The N-O band disappears, and the C.H. modes are broader and shifted to lower wavenumbers, indicating chain scission [36]. Fig. 2D shows the FTIR spectra of the B.G. samples that did not show any signs of degradation, probably because it was a single P.P. layer.
A proxy (Fig. S3) of the effect of the O3 treatment time in the filtration efficiency (F.E.) is shown in Fig. 3 A. The data obtained using a homemade equipment suggest that the F.E. reduces slightly with the O3 treatment time for the smaller particles (0.3 μm), but it is below 95% only after 180 min.
Fig. 3.
Summary of the stress–strain curves of the elastic bands before and after the O3 treatment. A. Tensile strength, B. Elongation at the break.
Fig. 3 B, C shows that the maximum tensile strength (σmax) - and elongation at break (ε) of the elastic straps remain nearly unchanged for treatment times below 120 min. At and above this time, σmax and ε increased, which means more extensive deformation for the elastic straps exposed to 8 - O3 cycles for the same applied strength. Such increased deformation has a negative effect on the fitting of the facemask, suggesting that less than 8 disinfection cycles should be used to preserve the protection. Kumar et al.[15] compared different disinfection methods, finding that the F.E. is less prone to failures than the fitting test.
4. Conclusions
The microbiological evidence indicated that the use of O3 (144 ppm min) is an alternative for decontamination for polypropylene-based N95 masks and biosafety gowns. The results showed that the N95 facemask could stand the O3 treatment up to 120 min (8 cycles), but taking a safe margin; we suggest that the N95 masks could be reused up to 6 cycles. The data from the F.E. test indicates that the number of recommended cycles is consistent with those reported by the FDA for the approved decontamination tests.
CRediT authorship contribution statement
G. Ibáñez-Cervantes: Investigation, Conceptualization, Funding acquisition. G.E. Lugo-Zamudio: Investigation. C. Cruz-Cruz: Investigation. E.M. Durán-Manuel: Investigation. J.C. Bravata-Alcántara: Investigation. E. García-Moncada: Investigation. M. Mata-Rocha: Investigation. L. Delgado-Balbuena: Investigation. M.A. Cureño-Díaz: Investigation. C.R. Ramírez-Cortina: Investigation. G. León-Ávila: Investigation. B. Nogueda-Torres: Investigation. J.M. Hernández-Hernández: Investigation. S.E. Rodil: Investigation. J.M. Bello-López: Investigation, Project administration, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
“Sistema Nacional de Investigadores (SNI)” from CONACyT. Projects CONACyT 313771 and SECTEI 096/2020.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.matlet.2021.131554.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nogee D., Tomassoni A.J. Infect. Control Hosp. Epidemiol. 2020;41(8):958. doi: 10.1017/ice.2020.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tirupathi R., Bharathidasan K., Palabindala V., Salim S.A., Al-Tawfiq J.A. Infez Med. 2020;28(suppl 1):57–63. [PubMed] [Google Scholar]
- 3.Srinivasan S., Peh W. Malays Orthop J. 2020;14(2):16–22. doi: 10.5704/MOJ.2007.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H.L., Huang J., Zhang C.J.P., He Z., Ming W.K. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranney M.L., Griffeth V., Jha A.K. N Engl J Med. 2020;382(18) doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 6.Adams J.G., Walls R.M. JAMA. 2020;323(15):1439–1440. doi: 10.1001/jama.2020.3972. [DOI] [PubMed] [Google Scholar]
- 7.T. Huber, O. Goldman, A.E. Epstein, G. Stella, T.P. Sakmar, Biophysical Journal. [DOI] [PMC free article] [PubMed]
- 8.Batéjat C., Grassin Q., Manuguerra J.-C., Leclercq I. Journal of Biosafety and Biosecurity. 2021;3(1):1–3. doi: 10.1016/j.jobb.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao L., Xiao W., Zhao M., Yu X., Wang H., Wang Q., Chu S., Cui Y. ACS Nano. 2020;14(5):6348–6356. doi: 10.1021/acsnano.0c03597. [DOI] [PubMed] [Google Scholar]
- 10.Lindsley W.G., Martin S.B., Jr., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., Noti J.D. J Occup Environ Hyg. 2015;12(8):509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkis-Onofre R., Borges R.D.C., Demarco G., Dotto L., Schwendicke F., Demarco F.F. J Dent. 2021;104 doi: 10.1016/j.jdent.2020.103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibáñez-Cervantes G., Bravata-Alcántara J.C., Nájera-Cortés A.S., Meneses-Cruz S., Delgado-Balbuena L., Cruz-Cruz C., Durán-Manuel E.M., Cureño-Díaz M.A., Gómez-Zamora E., Chávez-Ocaña S., Sosa-Hernández O., Aguilar-Rojas A., Bello-López J.M. Am. J. Infect. Control. 2020;48(9):1037–1041. doi: 10.1016/j.ajic.2020.06.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderegg L., Meisenhelder C., Ngooi C.O., Liao L., Xiao W., Chu S., Cui Y., Doyle J.M. PLoS ONE. 2020;15(7) doi: 10.1371/journal.pone.0234851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith J.S., Hanseler H., Welle J., Rattray R., Campbell M., Brotherton T., Moudgil T., Pack T.F., Wegmann K., Jensen S., Jin J., Bifulco C.B., Prahl S.A., Fox B.A., Stucky N.L. J. Clin. Transl. Sci. 2021;5(1) doi: 10.1017/cts.2020.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A., Kasloff S.B., Leung A., Cutts T., Strong J.E., Hills K., Gu F.X., Chen P., Vazquez-Grande G., Rush B., Lother S., Malo K., Zarychanski R., Krishnan J. PLoS ONE. 2020;15(12) doi: 10.1371/journal.pone.0243965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ECDC, Options for the decontamination and reuse of respirators in the context of the COVID-19 pandemic, European Centre for Disease Prevention and Control. , Stockholm, 2020.
- 17.FDA, Decontamination System EUAs for Personal Protective Equipment, 2021.
- 18.Kobayashi L.M., Marins B.R., Costa P., Perazzo H., Castro R. Infect Control Hosp Epidemiol. 2020;41(11):1364–1366. doi: 10.1017/ice.2020.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gérard C., Franssen F., La Carbona S., Monteiro S., Cozma-Petruţ A., Utaaker K.S., Režek Jambrak A., Rowan N., Rodríguez-Lazaro D., Nasser A., Tysnes K., Robertson L.J. Trends Food Sci. Technol. 2019;91:12–23. [Google Scholar]
- 20.Zanet S., Battisti E., Alciati R., Trisciuoglio A., Cauda C., Ferroglio E. J. Apic. Res. 2019;58(1):62–66. [Google Scholar]
- 21.Ding W., Jin W., Cao S., Zhou X., Wang C., Jiang Q., Huang H., Tu R., Han S.-F., Wang Q. Water Res. 2019;160:339–349. doi: 10.1016/j.watres.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Kadoya S.-S., Nishimura O., Kato H., Sano D. Water Research X. 2021;11 doi: 10.1016/j.wroa.2021.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tizaoui C. Ozone Sci. Eng. 2020;42(5):378–385. [Google Scholar]
- 24.Tseng C., Li C. J Environ Health. 2008;70(10):56–62. [PubMed] [Google Scholar]
- 25.J. Lee, C. Bong, P.K. Bae, A.T. Abafogi, S.H. Baek, Y.-B. Shin, M.S. Bak, S. Park, medRxiv (2020) 2020.04.26.20080317.
- 26.Andreassen E. In: Polypropylene: An A-Z reference. Karger-Kocsis J., editor. Springer; Netherlands, Dordrecht: 1999. Infrared and Raman spectroscopy of polypropylene; pp. 320–328. [Google Scholar]
- 27.Koenig J.L. Appl. Spectrosc. Rev. 1971;4(2):233–305. [Google Scholar]
- 28.Drewnick F., Pikmann J., Fachinger F., Moormann L., Sprang F., Borrmann S. Aerosol Sci. Technol. 2021;55(1):63–79. [Google Scholar]
- 29.Chick H. Journal of Hygiene. 1908;8(1):92–158. doi: 10.1017/s0022172400006987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston-Enriquez J.A., Haas C.N., Jacangelo J., Gerba C.P. Water Res. 2005;39(15):3650–3656. doi: 10.1016/j.watres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Franke G., Knobling B., Brill F.H., Becker B., Klupp E.M., Belmar Campos C., Pfefferle S., Lütgehetmann M., Knobloch J.K. J Hosp Infect. 2021;112:108–113. doi: 10.1016/j.jhin.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlsson D.J., Wiles D.M. Macromolecules. 1969;2(6):597–606. [Google Scholar]
- 33.J. Almond, P. Sugumaar, M.N. Wenzel, G. Hill, C. Wallis, e-Polymers 20(1) (2020) 369-381.
- 34.He G.-J., Zheng T.-T., Ke D.-M., Cao X.-W., Yin X.-C., Xu B.-P. RSC Adv. 2015;5(55):44115–44120. [Google Scholar]
- 35.de Carvalho C.L., Silveira A.F., Rosa D.D.S. SpringerPlus. 2013;2(1):623. doi: 10.1186/2193-1801-2-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.C. Beachell, S.P. Nemphos, Oxidative Degradation of Polymers in Presence of Ozone, OZONE CHEMISTRY AND TECHNOLOGY, AMERICAN CHEMICAL SOCIETY1959, pp. 168-175.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.