Abstract
Background
Magnesium sulphate has been used to inhibit preterm labour to prevent preterm birth. There is no consensus as to the safety profile of different treatment regimens with respect to dose, duration, route and timing of administration.
Objectives
To assess the efficacy and safety of alternative magnesium sulphate regimens when used as single agent tocolytic therapy during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 September 2015) and reference lists of retrieved studies.
Selection criteria
Randomised trials comparing different magnesium sulphate treatment regimens when used as single agent tocolytic therapy during pregnancy in women in preterm labour. Quasi‐randomised trials were eligible for inclusion but none were identified. Cross‐over and cluster trials were not eligible for inclusion. Health outcomes were considered at the level of the mother, the infant/child and the health service.
Intervention: intravenous or oral magnesium sulphate given alone for tocolysis.
Comparison: alternative dosing regimens of magnesium sulphate given alone for tocolysis.
Data collection and analysis
Two review authors independently assessed trial eligibility and quality and extracted data.
Main results
Three trials including 360 women and their infants were identified as eligible for inclusion in this review. Two trials were rated as low risk of bias for random sequence generation and concealment of allocation. A third trial was assessed as unclear risk of bias for these domains but did not report data for any of the outcomes examined in this review. No trials were rated to be of high quality overall.
Intravenous magnesium sulphate was administered according to low‐dose regimens (4 g loading dose followed by 2 g/hour continuous infusion and/or increased by 1 g/hour hourly until successful tocolysis or failure of treatment), or high‐dose regimens (4 g loading dose followed by 5 g/hour continuous infusion and increased by 1 g/hour hourly until successful tocolysis or failure of treatment, or 6 g loading dose followed by 2 g/hour continuous infusion and increased by 1 g/hour hourly until successful tocolysis or failure of treatment).
There were no differences seen between high‐dose magnesium sulphate regimens compared with low‐dose magnesium sulphate regimens for the primary outcome of fetal, neonatal and infant death (risk ratio (RR) 0.43, 95% confidence interval (CI) 0.12 to 1.56; one trial, 100 infants). Using the GRADE approach, the evidence for fetal, neonatal and infant death was considered to be VERY LOW quality. No data were reported for any of the other primary maternal and infant health outcomes (birth less than 48 hours after trial entry; composite serious infant outcome; composite serious maternal outcome).
There were no clear differences seen between high‐dose magnesium sulphate regimens compared with low‐dose magnesium sulphate regimens for the secondary infant health outcomes of fetal death; neonatal death; and rate of hypocalcaemia, osteopenia or fracture; and secondary maternal health outcomes of rate of caesarean birth; pulmonary oedema; and maternal self‐reported adverse effects. Pulmonary oedema was reported in two women given high‐dose magnesium sulphate, but not in any of the women given low‐dose magnesium sulphate.
In a single trial of high and low doses of magnesium sulphate for tocolysis including 100 infants, the risk of respiratory distress syndrome was lower with use of a high‐dose regimen compared with a low‐dose regimen (RR 0.31, 95% CI 0.11 to 0.88; one trial, 100 infants). Using the GRADE approach, the evidence for respiratory distress syndrome was judged to be LOW quality. No difference was seen in the rate of admission to the neonatal intensive care unit. However, for those babies admitted, a high‐dose regimen was associated with a reduction in the length of stay in the neonatal intensive care unit compared with a low‐dose regimen (mean difference ‐3.10 days, 95% confidence interval ‐5.48 to ‐0.72).
We found no data for the majority of our secondary outcomes.
Authors' conclusions
There are limited data available (three studies, with data from only two studies) comparing different dosing regimens of magnesium sulphate given as single agent tocolytic therapy for the prevention of preterm birth. There is no evidence examining duration of therapy, timing of therapy and the role for repeat dosing.
Downgrading decisions for our primary outcome of fetal, neonatal and infant death were based on wide confidence intervals (crossing the line of no effect), lack of blinding and a limited number of studies. No data were available for any of our other important outcomes: birth less than 48 hours after trial entry; composite serious infant outcome; composite serious maternal outcome. The data are limited by volume and the outcomes reported. Only eight of our 45 pre‐specified primary and secondary maternal and infant health outcomes were reported on in the included studies. No long‐term outcomes were reported. Downgrading decisions for the evidence on the risk of respiratory distress were based on wide confidence intervals (crossing the line of no effect) and lack of blinding.
There is some evidence from a single study suggesting a reduction in the length of stay in the neonatal intensive care unit and a reduced risk of respiratory distress syndrome where a high‐dose regimen of magnesium sulphate has been used compared with a low‐dose regimen. However, given that evidence has been drawn from a single study (with a small sample size), these data should be interpreted with caution.
Magnesium sulphate has been shown to be of benefit in a wide range of obstetric settings, although it has not been recommended for tocolysis. In clinical settings where health benefits are established, further trials are needed to address the lack of evidence regarding the optimal dose (loading dose and maintenance dose), duration of therapy, timing of therapy and role for repeat dosing in terms of efficacy and safety for mothers and their children. Ongoing examination of different regimens with respect to important health outcomes is required.
Plain language summary
Different magnesium sulphate regimens given to mothers to prevent preterm labour
Babies born early ‐ before 37 weeks' estimated gestation ‐ are at an increased risk of dying or being seriously unwell, especially if they are born very early. Various drugs have been given to women to try and stop babies being born too soon. Magnesium sulphate has been one of the drugs used when women go into labour too early.
Although it has now been shown that magnesium sulphate does not help prevent babies being born too soon, it is important to know the safest and best way to give magnesium sulphate if it is used for mothers in preterm labour. Particular concerns about high doses of magnesium sulphate for women in preterm labour, including increased risk of deaths of babies, have been raised. (Magnesium sulphate has been shown to help prevent and treat eclampsia in women with high blood pressure during pregnancy, and in mothers at risk of preterm birth, low doses can protect the baby's brain and improve long‐term outcomes for the infant. These uses are covered in other Cochrane reviews.)
This review identified three trials (involving 360 women and their infants), but one trial did not provide any relevant data. The trials were small and were assessed as being at a low or unclear risk of bias. The trials did not report many outcomes of relevance to this review. We did find limited evidence to suggest that when magnesium sulphate was given to mothers in preterm labour, differences in the dose (high‐dose versus low‐dose) did not impact on the number of babies that died (very low quality evidence). There were no data to assess other important outcomes: birth less than 48 hours after entry to the trial, or serious outcomes for mothers or their babies.
The included trials provided very few data for other outcomes relevant to this review (overall, we were only able to examine eight of the 45 outcomes we wanted to examine).
One trial did find that the rate of newborn respiratory distress syndrome (low quality evidence) and the length of stay in the neonatal intensive care unit were reduced with high‐dose magnesium sulphate (compared to the babies born to the group of women who were given low‐dose magnesium sulphate). However, this result is based on evidence from one small study and should therefore be interpreted with caution.
The rate of caesarean birth did not differ between those women given high‐dose and those women given low‐dose magnesium sulphate. Nor were there any differences between groups in terms of the number of babies that died before birth or during the subsequent month or the number of babies with low levels of calcium in their blood, low bone density or bone fractures. The frequency of self‐reported adverse effects in mothers including flushing, headache (two trials, 248 women), or nausea and vomiting (one trial, 100 women) did not differ between high‐dose and low‐dose magnesium sulphate groups. Pulmonary oedema was reported in two mothers given high‐dose magnesium sulphate, and in none of the mothers given low‐dose magnesium sulphate.
No trials have looked at different durations of treatment, timing and other ways of giving magnesium sulphate to mothers going in to labour too early.
Further trials are needed to address the lack of evidence regarding the best dose, duration of therapy, timing of therapy and role for repeat dosing in terms of efficacy and safety for mothers and their children.
Summary of findings
Summary of findings for the main comparison. Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour.
| Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour | ||||||
| Patient or population: Women in preterm labour Intervention: High‐dose magnesium sulphate Comparison: Low‐dose magnesium sulphate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with low‐dose (as defined by the trialist) magnesium sulphate | Risk with high‐dose (as defined by the trialist) magnesium sulphate | |||||
| Fetal, neonatal and infant death | Study population | RR 0.43 (0.12 to 1.56) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 140 per 1000 | 60 per 1000 (17 to 218) | |||||
| Respiratory distress syndrome | Study population | RR 0.31 (0.11 to 0.88) | 100 (1 RCT) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 260 per 1000 | 81 per 1000 (29 to 229) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: Randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Evidence is based on a single trial
2 Evidence of wide confidence intervals crossing the line of no effect
3 No evidence of blinding of participants, personnel or outcome assessors
Background
Description of the condition
Preterm birth, defined as birth prior to 37 weeks' estimated gestation (WHO 1992), is a leading cause of perinatal mortality (Beck 2010). Preterm infants are at significant risk of short‐term and long‐term morbidity (How 2006). The costs to individual families and to the community are great, and the burden on modern healthcare systems is significant. Spontaneous preterm labour contributes to 40% to 50% of all preterm birth (Goldenberg 2008). The prevention of spontaneous preterm labour using tocolysis has been a focus of obstetric research (Tsatsaris 2004).
Description of the intervention
Various pharmacological agents have been used in an attempt to arrest spontaneous preterm labour and therefore prolong pregnancy. Betamimetics (Neilson 2014), calcium channel blockers (Flenady 2014b), magnesium sulphate (Crowther 2014) and oxytocin receptor antagonists (Flenady 2014a) have each been the subject of Cochrane systematic reviews. Other drugs advocated for tocolysis include the prostaglandin inhibitor, indomethacin (Klauser 2012), selective COX‐2 inhibitors, rofecoxib and celecoxib (Borna 2007; McWhorter 2004), progesterone (Borna 2008), nitrates and ethanol. Controversy remains as to which agent is preferable. A meta‐analysis of 55 randomised controlled trials of tocolytic therapy (Haas 2012) found prostaglandin inhibitors and magnesium sulphate to have the highest probability of delaying birth by 48 hours. However, prostaglandin inhibitors and calcium channel blockers were most likely to be the best class of therapy in terms of effectiveness, maternal side‐effect profile and neonatal outcomes. The American College of Obstetricians and Gynecologists has supported ‘first‐line tocolytic treatment with beta‐adrenergic receptor agonists, calcium channel blockers, or non‐steroidal anti‐inflammatories (NSAIDs) for short‐term prolongation of pregnancy’ (ACOG 2012).
Magnesium sulphate when given for tocolysis has not been demonstrated to significantly prolong pregnancy (risk ratio 0.87; 95% confidence interval 0.61 to 1.24, nine trials). Its tocolytic efficacy has been found to be similar to that of calcium channel blockers and betamimetics (Crowther 2014). The side‐effect profile of magnesium sulphate when given for tocolysis is greater than that of other tocolytic agents such as the calcium channel blocker, nifedipine (Lyell 2007).
There has been limited evaluation of the efficacy and safety of alternative treatment regimens of magnesium sulphate given for tocolysis. One systematic review has included treatment regimens given for tocolysis in an evaluation of different antenatal magnesium sulphate regimens with respect to maternal adverse effects (Bain 2013).
Alternatively, magnesium sulphate is used widely in other obstetric and perinatal contexts. Magnesium sulphate has an established role in the prevention and treatment of eclampsia (Duley 2010a; Duley 2010b; Duley 2010c), and in fetal neuroprotection for women at risk of preterm birth (Doyle 2009). In each setting, the efficacy and safety of different treatment regimens have been examined in Cochrane systematic reviews (Duley 2010d and Bain 2012 respectively).
Clinical trials have evaluated magnesium sulphate administered for both acute tocolysis (Crowther 2014) and maintenance tocolysis (Han 2013). Treatment regimens have differed with respect to dose, route, duration, and timing of administration (James 2010).
Dosing of magnesium sulphate when given for tocolysis has varied between clinical trials. Low‐dose regimens have typically included a loading dose of 4 g followed by an infusion of 2 g per hour. Early trials limited maximum dosing according to total dose administered in 24 hours (usually 10 g to 12 g). More aggressive regimens have included loading doses of 6 g (Glock 1993; Haghighi 1999; Larmon 1999; Morales 1993; Schorr 1997), and maintenance infusion rates with incremental rate increases (usually by 0.5 g at 30‐ or 60‐minute intervals) until cessation of contractions is achieved or a maximum hourly rate of up to 5 g is reached. The current obstetric standard for use in preterm labour indicates administration of intravenous magnesium sulphate with a loading dose of 4 to 6 g, followed by a maintenance infusion of 2 g per hour until uterine quiescence is established (James 2010).
Timing of administration has varied between treatment regimens. Loading doses have been given either as a bolus, over 15 minutes or over 30 minutes. Duration of therapy has extended from six hours to 24 hours, or until tocolysis has been achieved.
Different routes of administration have been evaluated. Oral magnesium therapy has been trialled for maintenance tocolysis either according to study protocol or when it has been established that preterm labour has been arrested (Han 2013). Intramuscular magnesium therapy has been limited in its use for tocolysis by the potential for adverse reaction at the injection site (Bain 2013).
Monitoring of magnesium sulphate therapy has been performed clinically or by evaluating serum magnesium levels. Magnesium sulphate toxicity can allow appropriate administration of calcium gluconate as an antidote (Tsatsaris 2004). Concerns have been raised over the utility of serum monitoring (Lurie 2004): monitoring is resource‐expensive; and serum levels increase slowly and unpredictably after intramuscular administration.
Clinical adverse side effects of magnesium sulphate have been evaluated extensively. For the mother, the most common adverse side effect reported after drug administration is flushing. Other maternal adverse side effects include gastrointestinal disturbance, muscle weakness, thirst, headache, drowsiness and confusion. Serious cardiorespiratory events including pulmonary oedema and cardiac or respiratory arrest have been reported (James 2010).
Common neonatal side effects include increased time to established respiration, lethargy and poor sucking (Klauser 2012). An association between the administration of high‐dose magnesium sulphate and an increased total death rate (fetal, neonatal and infant) has been reported (Mittendorf 1997).
Additionally, it has been suggested that prolonged administration of magnesium sulphate affects fetal calcium metabolism, increasing the risk of hypocalcaemia (Nassar 2006). In May 2013, the US Food and Drug Administration (FDA) issued a statement warning against prolonged (more than five to seven days), continuous administration of magnesium sulphate for the prevention of preterm birth given the risk of fetal hypocalcaemia, osteopenia and fractures (FDA 2013).
While magnesium sulphate has been deemed to be safe when used for the prevention and treatment of eclampsia (FDA 2013), and for fetal neuroprotection in the setting of preterm birth (Doyle 2009), many clinicians and researchers have cautioned against use of magnesium sulphate for tocolysis (Grimes 2006). Tocolytic regimens typically involve high‐dose and prolonged intravenous administration of magnesium sulphate (Pryde 2009). While low‐dose regimens of magnesium sulphate offer fetal neuroprotection (Doyle 2009), high‐dose regimens have been associated with an increased fetal, neonatal and infant death rate (Mittendorf 1997) and fetal osteopenia (Nassar 2006). It has been suggested that this may reflect a dose‐response relationship and a potential 'therapeutic window' (Pryde 2009). Further delineation of this dose‐response relationship is required.
How the intervention might work
Magnesium sulphate was first described for use as a tocolytic agent in 1977 (Steer 1977). An association between magnesium sulphate and uterine quiescence has long been established (Abarbanel 1945; Hall 1957). However, the mechanism of action of magnesium sulphate tocolysis remains incompletely defined. Evidence suggests the tocolytic effect of magnesium has both an intracellular and extracellular component (Lurie 2004). It is suggested that magnesium alters calcium uptake, binding and distribution in uterine smooth muscle cells, thereby reducing the frequency of cell depolarisation and inhibiting myometrial contraction (Lewis 2005). More recent evidence suggests the tocolytic effect of magnesium might have an anti‐inflammatory component (Dowling 2012; Tam Tam 2011).
Why it is important to do this review
Magnesium sulphate has been trialled as a tocolytic agent in the context of threatened preterm labour for the prevention of preterm birth. Controversy exists regarding the benefits of magnesium sulphate therapy when given for tocolysis (Grimes 2006). There has been limited evaluation of the efficacy and safety of alternative regimens of magnesium sulphate when given for tocolysis. In order to determine the optimal dose, duration, route and timing of administration, evaluation of different regimens of magnesium sulphate given for tocolysis is warranted. Given its widespread availability and use in other obstetric contexts, further review of the safety profile of magnesium sulphate is of value.
Objectives
This review assesses the efficacy and safety of alternative magnesium sulphate regimens when used as single agent tocolytic therapy during pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials comparing different magnesium sulphate regimens when administered to women as the only tocolytic therapy in the setting of threatened preterm labour were included. Quasi‐randomised trials were eligible for inclusion but none were identified. Cross‐over and cluster‐randomised trials were not eligible for inclusion. Conference abstracts were included.
Types of participants
Women thought to be in preterm labour with a singleton or multiple pregnancy and administered magnesium sulphate for the prevention of preterm birth were included. Women who had a planned preterm birth or preterm induction of labour were not included.
Types of interventions
Intervention: intravenous or oral magnesium sulphate given alone for tocolysis.
Comparison: alternative dosing regimens of magnesium sulphate given alone for tocolysis.
Trials examining treatment regimens including magnesium sulphate co‐administered with alternative tocolytic agents were not included.
Types of outcome measures
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been pre‐specified following consultation with the editors and authors of the individual reviews.
Consensus was reached on a set of six ‘core’ outcomes, which are highlighted below. These have been included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
For the infant/child
Fetal, neonatal and infant death
Birth less than 48 hours after trial entry
Composite serious infant outcome (defined as death or chronic lung disease [oxygen requirement at 28 days of life or later]; intraventricular haemorrhage (IVH) [grade three or four] or periventricular leucomalacia (PVL); major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient or intelligence quotient less than two standard deviations below the mean])
For the mother
Composite serious maternal outcome (defined as maternal death, cardiac arrest, respiratory arrest or admission to intensive care unit)
Secondary outcomes
For the infant
Fetal death
Neonatal death
Preterm birth (less than 37 weeks)
Very preterm birth (less than 34 weeks)
Extremely preterm birth (less than 28 weeks)
Interval between trial entry and birth
Gestational age at birth
Apgar score less than seven at five minutes
Active resuscitation at birth (assisted ventilation via an endotracheal tube)
Use of respiratory support (mechanical ventilation or continuous positive airways pressure)
Air leak syndrome
Respiratory distress syndrome
Chronic lung disease (need for supplemental oxygen at 28 days of life or later)
Use of postnatal corticosteroids
IVH
Grade 3 or 4 IVH
PVL
Necrotising enterocolitis
Proven neonatal infection
Hypocalcaemia, osteopenia, or fracture(s)
For the child
Cerebral palsy (mild, moderate or severe, evaluated separately)
Developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below the mean)
Legal blindness
Sensorineural deafness requiring hearing aids
For the mother
Death
Cardiac arrest
Respiratory arrest
Antepartum haemorrhage
Postpartum haemorrhage
Need for blood transfusion
Mode of delivery
Any adverse effect(s) of therapy
Hypotension
Tachycardia
Reduced or absent tendon reflexes
Hypocalcaemia
Pulmonary oedema
Self‐reported adverse effects or symptoms attributed to magnesium sulphate therapy (including discomfort at the infusion site, blurred vision, dizziness, headache, mouth dryness, muscle weakness, nausea or vomiting, drowsiness, sweating, flushing, other)
Discontinuation of therapy
Satisfaction with therapy
Health services outcomes
Admission to intensive care unit for the mother
Length of postnatal hospitalisation for the mother
Admission to neonatal intensive care unit for the infant
Length of stay in neonatal intensive care unit for the infant
Length of neonatal hospitalisation for the infant
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2015).
For full search methods used to populate the PCG Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly: the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full texts of all relevant trial reports identified through the searching activities described above are reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that review authors then fully account for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third author. The data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes. We selected up to a maximum of seven outcomes for the mother and the infant.
For the infant/child
Fetal, neonatal and infant death
Birth less than 48 hours after trial entry
Composite serious infant outcome (defined as death or chronic lung disease [oxygen requirement at 28 days of life or later]; IVH [grade three or four] or PVL; major neurosensory disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay/intellectual impairment [defined as developmental quotient or intelligence quotient less than two standard deviations below the mean])
Respiratory distress syndrome
For the mother
Composite serious maternal outcome (defined as maternal death, cardiac arrest, respiratory arrest or admission to intensive care unit)
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Fetal, neonatal and infant death
Birth less than 48 hours after trial entry
Composite serious infant outcome
Composite serious maternal outcome
Respiratory distress syndrome
The GRADEpro Guideline Development Tool was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Women are the unit of analysis for maternal outcomes. For trials involving multiple pregnancies, we planned that the unit of analysis would be per infant for fetal, neonatal and child outcomes. As infants from multiple pregnancies are not independent, we planned to use cluster‐trial methods in the analyses, where the data allowed and where multiples made up a substantial proportion of the trial population, to account for non‐independence of variables (Gates 2004). Multiple pregnancies did not make up a substantial proportion of the trial population in each included study. Additionally, the presentation of data in the included studies did not allow use of cluster‐trial methods in the analyses. In future updates, if identified studies include sufficient multiple pregnancy data, cluster‐trial methods will be used for analyses to account for non‐independence of variables.
In future updates, where a trial has multiple arms (more than two comparisons) and is included in the same meta‐analysis, the number of events and the sample size from one of the groups will be divided by the number of arms compared in the trial. This will ensure that no participants are double counted. If a subgroup analysis is being undertaken then the overall summary statistic will not be reported.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number of participants randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as present if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. In future updates, if there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
Where random‐effects analyses are used, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to perform comparisons for different types of regimens; including differences in route of administration, loading and maintenance doses, duration of treatment, timing of treatment and the possibility of repeat dosing. Given the data available in included studies was limited, we performed a comparison of different regimens with respect to dose (high‐dose versus low‐dose). If we identify studies including data that allow further comparisons to be made, these will be included in future updates.
We did not identify substantial heterogeneity. However, if we identify substantial heterogeneity in future updates, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if so, use random‐effects analysis to produce it.
Maternal and pregnancy characteristics are likely to affect health outcomes of both mother and child.
We will carry out planned subgroup analyses, where sufficient data are available, based on:
significant factor(s) contributing to or precipitating preterm labour: including presence or absence of preterm prelabour rupture of membranes;
multiple versus singleton pregnancy;
gestational age at time of randomisation and treatment: less than 28 weeks', 28 weeks' to less than 34 weeks', 34 weeks' to less than 37 weeks' or 37 weeks' or more;
use of antenatal corticosteroids for fetal lung maturation: in 50% or more of the study population or in less than 50% of the study population.
Subgroup analysis will be restricted to the review's primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014), and we will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, sensitivity analysis will be performed where necessary to investigate the effect of trial quality, as defined by allocation concealment and other 'Risk of bias' components, by excluding studies determined as 'high risk of bias' for these components. Sensitivity analyses will be restricted to primary outcomes examined.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved seven reports relating to seven studies (see:Figure 1). We included three studies (Behrad 2003; Soguk 2004; Terrone 2000) and excluded three studies (Martin 1990; Martin 1998; Zygmunt 2003). One study is ongoing (Namazi 2013).
1.
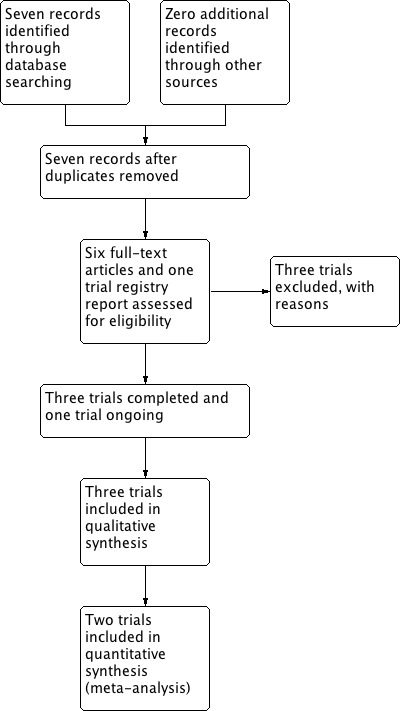
Study flow diagram.
Included studies
Three trials (involving 360 women) comparing different treatment regimens of magnesium sulphate given for tocolysis were included in the review (Behrad 2003; Soguk 2004; Terrone 2000), of which only two trials reported clinically meaningful data (Behrad 2003; Terrone 2000). The third trial did not report data for any of the outcomes examined in this review (Soguk 2004).
The trials were conducted in Iran (Behrad 2003), Turkey (Soguk 2004), and the United States of America (Terrone 2000).
Women with both singleton and twin pregnancies were included in the trials. One trial included twin gestations in 3% of cases (5/148) (Terrone 2000). A second trial included twin gestations in 4% of cases (4/100) (Behrad 2003). Gestational age at recruitment was similar in two trials at 24 to 35 weeks' and 24 to 34 weeks' respectively (Behrad 2003; Terrone 2000). One trial recruited women from 28 to 34 weeks' gestation (Soguk 2004).
The treatment regimens of magnesium sulphate given for tocolysis varied. In the trial conducted by Terrone, all women received a fixed loading dose of 4 g, followed by a continuous infusion of 2 g/hour in the low‐dose group and 5 g/hour in the high‐dose group. In both the low‐dose and high‐dose groups, where women continued to exhibit signs of labour, the infusion rate was increased on an hourly basis by 1 g/hour until successful tocolysis or treatment failure (Terrone 2000).
In the trial conducted by Behrad, women received a loading dose of 4 g in the low‐dose group and 6 g in the high‐dose group. All women then received a continuous infusion of 2 g/hour. In the high‐dose group, for women with continued contractions or cervical dilatation or effacement, the maintenance dose was increased on an hourly basis by 1 g/hour until successful tocolysis or treatment failure (Behrad 2003).
In the trial conducted by Soguk, women in the low‐dose group did not receive a loading dose. A maintenance dose of magnesium sulphate was administered via continuous infusion at the rate of 1 g/hour. Women in the high‐dose group ('standard dose group') received a loading dose of 4 g followed by a continuous infusion of 2 g/hour (Soguk 2004).
In two trials, women received corticosteroids for accelerating fetal lung maturation and prophylactic antibiotics where Group B streptococcus had been identified antenatally. Alternative tocolytic therapies and maintenance tocolytic therapies were not used in either trial (Behrad 2003; Terrone 2000).
One study examining different treatment regimens of intravenous magnesium sulphate in preterm labour pain management was ongoing at the time of completion of this review (Namazi 2013). See Characteristics of ongoing studies.
Excluded studies
Three trials were excluded because they did not compare different treatment regimens of magnesium sulphate given as single agent tocolytic therapy during pregnancy.
Two trials compared preparations of oral magnesium gluconate and magnesium chloride (Martin 1990; Martin 1998). A third trial compared different preparations of magnesium sulphate (ready to use infusion solution versus infusion solution concentrate diluted in carrier solution) administered according to the same treatment regimen (Zygmunt 2003).
Risk of bias in included studies
The summary of risk of bias can be referred to in Figure 2; and Figure 3.
2.
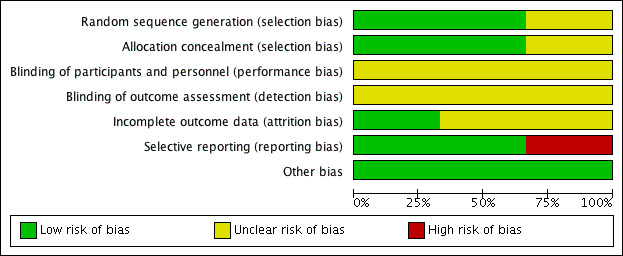
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
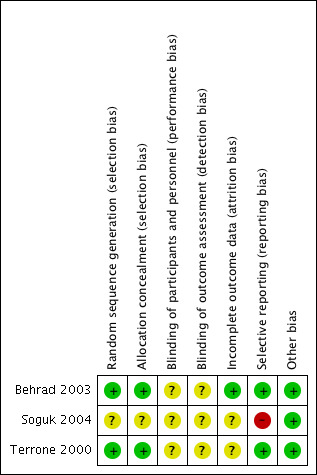
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation using computer‐generated random number tables was reported in two trials (Behrad 2003; Terrone 2000), and these were assessed as low risk of bias. The third trial reported that the participants were randomly assigned but gave no further details and this trial was assessed as unclear risk of bias (Soguk 2004).
Two trials reported allocation concealment using sealed numbered opaque envelopes (low risk of bias) (Terrone 2000; Behrad 2003). No details for allocation concealment were reported by one trial (unclear risk of bias) (Soguk 2004).
Blinding
No information was given on blinding of participants and research personnel in any of the included trials (Behrad 2003; Soguk 2004; Terrone 2000). Consequently, all three studies were assessed as being at unclear risk for performance and detection bias.
Incomplete outcome data
In one trial, 12 out of 160 women (eight in the low‐dose group and four in the high‐dose group) were excluded from analysis because of treatment failure and subsequent birth. The authors reported that the reason for exclusion of these women was that the time to successful tocolysis could not be assessed (Terrone 2000).
Losses to follow‐up were not reported for any of the included trials (Behrad 2003; Soguk 2004; Terrone 2000).
Selective reporting
There was no obvious evidence of selective reporting in two of the trials (Behrad 2003; Terrone 2000). One trial did not pre‐specify any of the outcomes reported (high risk of bias) (Soguk 2004).
Other potential sources of bias
There was no evidence of other bias.
Effects of interventions
See: Table 1
Magnesium sulphate high‐dose regimen versus magnesium sulphate low‐dose regimen
Primary outcomes
For the Infant/child
Fetal, neonatal and infant death was reported in one trial involving 100 women (Behrad 2003). No difference in the risk of fetal, neonatal and infant death for high‐dose compared with low‐dose magnesium sulphate was seen in this trial (risk ratio (RR) 0.43, 95% confidence interval (CI) 0.12 to 1.56; one trial, 100 infants) (Analysis 1.1). Using the GRADE approach the quality of the evidence was judged to be VERY LOW.
1.1. Analysis.
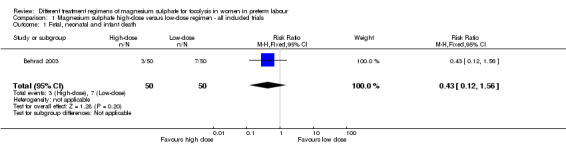
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 1 Fetal, neonatal and infant death.
No data were reported for birth less than 48 hours after trial entry or for composite serious infant outcome in any of the included trials.
For the mother
None of the included trials reported data for composite serious maternal outcome.
Secondary outcomes
For the infant/child
Data from one trial (Behrad 2003, reporting data on 100 infants) were available for four of this review's secondary outcomes.
No differences were seen in the rate of fetal death (RR 1.00, 95% CI 0.06 to 15.55; Analysis 1.2), neonatal death (RR 0.33, 95% CI 0.07 to 1.57; Analysis 1.3), or rate of hypocalcaemia, osteopenia or fracture (RR 0.33, 95% CI 0.01 to 7.99; Analysis 1.5). The risk of respiratory distress syndrome was lower with use of high‐dose magnesium sulphate compared with low‐dose magnesium sulphate (RR 0.31, 95% CI 0.11 to 0.88; one trial, 100 infants) (Analysis 1.4). Using the GRADE approach the evidence was considered to be LOW quality.
1.2. Analysis.
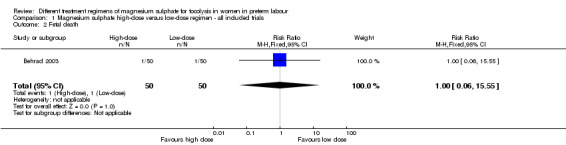
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 2 Fetal death.
1.3. Analysis.
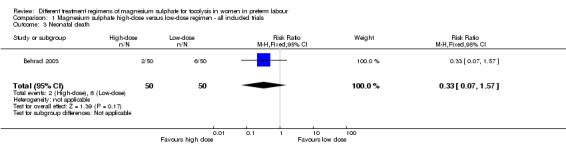
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 3 Neonatal death.
1.5. Analysis.
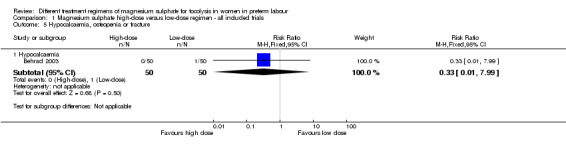
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 5 Hypocalcaemia, osteopenia or fracture.
1.4. Analysis.
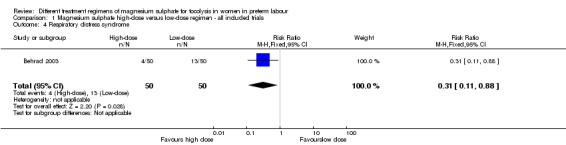
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 4 Respiratory distress syndrome.
No data were reported for preterm birth (< 37 weeks'), very preterm birth (< 34 weeks'), extremely preterm birth (< 28 weeks'), birth < 48 hours after trial entry, interval between trial entry and birth, gestational age at birth, Apgar score less than seven at five minutes, active resuscitation at birth, use of respiratory support, air leak syndrome, chronic lung disease, use of postnatal corticosteroids, intraventricular haemorrhage (IVH), Grade 3 or 4 IVH, periventricular leucomalacia (PVL), necrotising enterocolitis, proven neonatal infection, cerebral palsy, developmental delay, legal blindness or sensorineural deafness.
For the mother
Maternal data were reported in two trials (Behrad 2003; Terrone 2000).
There was no difference in the rate of caesarean birth in women given a high‐dose regimen compared with those given a low‐dose regimen of magnesium sulphate (RR 0.90, 95% CI 0.59 to 1.37; two trials, 248 women; Analysis 1.6). Pulmonary oedema was reported in two women receiving high‐dose treatment. There were no cases of pulmonary oedema reported in women receiving low‐dose treatment (RR 4.49, 95% CI 0.22 to 92.03; one trial, 148 women; Analysis 1.7) (Terrone 2000).
1.6. Analysis.
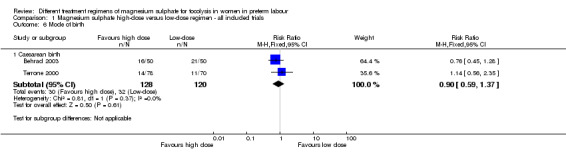
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 6 Mode of birth.
1.7. Analysis.
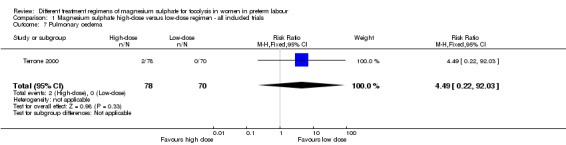
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 7 Pulmonary oedema.
There were no differences in the frequency of self‐reported adverse effects including flushing (average RR 1.64, 95% CI 0.89 to 3.03; two trials, 248 women; I2 = 60%, Tau2 = 0.12; Analysis 1.8), headache (average RR 1.78, 95% CI 0.95 to 3.31; two trials, 248 women; Analysis 1.8), or nausea and vomiting (average RR 1.27, 95% CI 0.73 to 2.20; one trial, 100 women; Analysis 1.8).
1.8. Analysis.
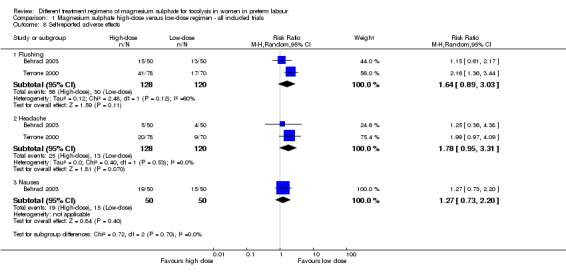
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 8 Self‐reported adverse effects.
No data were reported for maternal death, cardiac arrest, respiratory arrest, antepartum haemorrhage, postpartum haemorrhage, need for blood transfusion, any adverse effects of therapy, hypotension, tachycardia, reduced/absent tendon reflexes, hypocalcaemia, discontinuation of therapy or satisfaction with therapy.
Health services outcomes
Minimal data were reported for health services outcomes. One trial examined the rate of admission to the neonatal intensive care unit and found no difference in those treated with a high‐dose magnesium sulphate regimen compared with a low‐dose regimen (RR 0.46, 95% CI 0.19 to 1.12; one trial, 100 infants; Analysis 1.9). A high‐dose regimen of magnesium sulphate was associated with a reduction in length of stay in the neonatal intensive care unit (mean difference (MD) ‐3.10 days, 95% CI ‐5.48 to ‐0.72; one study, 100 infants; Analysis 1.10). No data were reported for maternal admission to the intensive care unit, length of postnatal hospitalisation or length of neonatal hospitalisation.
1.9. Analysis.
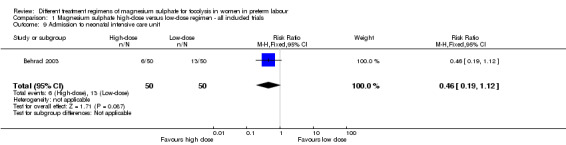
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 9 Admission to neonatal intensive care unit.
1.10. Analysis.
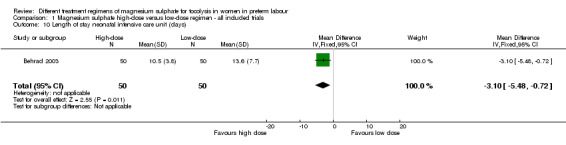
Comparison 1 Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials, Outcome 10 Length of stay neonatal intensive care unit (days).
Discussion
Summary of main results
Three trials (involving 360 women) comparing different treatment regimens of magnesium sulphate given for tocolysis were included in this review (although one trial did not report data for any of the pre‐specified outcomes examined). There were no differences seen between high‐dose magnesium sulphate regimens compared with low‐dose magnesium sulphate regimens for the primary outcome of fetal, neonatal and infant death. No data were reported for any of the other primary maternal and infant health outcomes (birth less than 48 hours after trial entry; composite serious infant outcome; composite serious maternal outcome). No data were reported for childhood outcomes. The data are limited by volume and the outcomes reported. Only eight of our 45 pre‐specified primary and secondary maternal and infant health outcomes were reported on in the included studies. No long‐term outcomes were reported. No trials were rated to be of high quality overall.
Although the need for admission to a neonatal intensive care unit was not different, for babies that were admitted, a high‐dose regimen of magnesium sulphate was associated with a reduced length of stay compared with a low‐dose regimen. Similarly, there was some evidence to suggest that a high‐dose regimen was associated with a reduced risk of respiratory distress syndrome compared to babies born to women in the low‐dose regimen. However, this evidence was based on a single trial including 100 infants and therefore should be interpreted with caution.
Pulmonary oedema was reported in two women given high‐dose magnesium sulphate, but not in any of the women given low‐dose magnesium sulphate. This difference did not reach significance. There were no differences between regimens for maternal self‐reported adverse effects associated with treatment including flushing, headache or nausea and vomiting.
Overall completeness and applicability of evidence
Despite the fact that magnesium sulphate has been used widely for tocolysis for many years, only three small trials have compared different treatment regimens of magnesium sulphate to determine optimal efficacy and safety when given as single‐agent tocolytic therapy.
Quality of the evidence
Of the three trials included, two trials reported randomisation via the use of numbered envelopes. The third trial reported no details for allocation concealment. There was no evidence of blinding of participants, research staff or outcome assessors in any of the trials. Losses to follow‐up were not reported or unclear. No trials were rated to be of high quality overall.
The quality of the evidence was downgraded (three times) for our primary outcome of fetal, neonatal and infant death, based on wide confidence intervals (crossing the line of no effect), lack of blinding and a limited number of studies. Evidence relating to our secondary outcome of respiratory distress syndrome was downgraded twice due to wide confidence intervals crossing the line of no effect and lack of blinding.
Potential biases in the review process
Given the small number of mothers and infants in the three trials included, these data should be interpreted with caution. One of the three trials did not report on any of the outcomes specified for this review.
Agreements and disagreements with other studies or reviews
There was no evidence of benefit in terms of fetal or neonatal mortality with the use of either high‐ or low‐dose magnesium sulphate treatment regimens. Previously, it has been reported that high‐dose magnesium sulphate therapy may be associated with an increase in the fetal and paediatric death rate (Crowther 2014). In this review, no increased risk was observed when high‐dose treatment regimens were compared to low‐dose treatment regimens.
Magnesium sulphate has been widely used in obstetric contexts as a tocolytic, for neuroprotection of the fetus for mothers at risk of preterm birth, and for the prevention and treatment of eclampsia. To date, a consensus has not been reached regarding the optimal treatment regimen in terms of efficacy and safety in each setting (Bain 2012; Duley 2010d).
Magnesium sulphate has not been shown to prevent or delay preterm birth. However, examination of different treatment regimens with respect to dose, duration and timing of administration may provide useful information with respect to the safety and tolerability of magnesium sulphate when given in pregnancy.
Authors' conclusions
Implications for practice.
Magnesium sulphate has been used widely as a tocolytic agent in clinical settings even though magnesium sulphate has not been shown to prevent or delay preterm birth. However, magnesium sulphate has an established role in neuroprotection of the fetus when given to women at risk of very preterm birth.
There is insufficient evidence to assess the efficacy and safety of alternative magnesium sulphate regimens when used as single agent tocolytic therapy during pregnancy. We identified three small studies (with data from only two studies with small sample sizes). The majority of this review's outcomes were not reported in the included studies.
No studies have examined the optimal duration of therapy, timing of therapy or the role for repeat dosing.
Implications for research.
Magnesium sulphate has been shown to be of benefit in a wide range of obstetric settings, although it has not been recommended for tocolysis. In clinical settings where health benefits are established, further trials are needed to address the lack of evidence regarding the optimal dose (loading dose and maintenance dose), duration of therapy, timing of therapy and role for repeat dosing in terms of efficacy and safety for mothers and their children. Ongoing examination of different regimens with respect to important health outcomes is required.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Magnesium sulphate high‐dose versus low‐dose regimen ‐ all included trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fetal, neonatal and infant death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.56] |
| 2 Fetal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 3 Neonatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.57] |
| 4 Respiratory distress syndrome | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.88] |
| 5 Hypocalcaemia, osteopenia or fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hypocalcaemia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 6 Mode of birth | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Caesarean birth | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 7 Pulmonary oedema | 1 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 92.03] |
| 8 Self‐reported adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Flushing | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.89, 3.03] |
| 8.2 Headache | 2 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.95, 3.31] |
| 8.3 Nausea | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.73, 2.20] |
| 9 Admission to neonatal intensive care unit | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.12] |
| 10 Length of stay neonatal intensive care unit (days) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐5.48, ‐0.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Behrad 2003.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: Imam Reza’s Hospital, Iran. Participants: women (n = 100; 50 in the low‐dose group, 50 in the high‐dose group) between 24‐35 weeks' gestation with spontaneous preterm labour. Singleton and twin pregnancies were included. Definition of preterm labour: uterine contractions of more than 4 contractions per 20 minutes plus 1 of: cervical dilatation of at least 1 cm but less than 5 cm diameter, cervical effacement ≥ 80%, and/or progressive cervical dilatation and effacement. Exclusion criteria: higher‐order multiple gestations, rupture of membranes, non‐reassuring fetal assessment (abnormalities of the fetal heart rate pattern), evidence of intrauterine infection (temperature ≥ 38°C, leucocytosis, uterine tenderness, malodorous discharge), vaginal bleeding, patients with a history of diabetes mellitus, myasthenia gravis, any other neuromuscular diseases, impaired renal function (serum creatinine > 1.2 mg/dL), hypotension (mean arterial pressure < 70 mm Hg), maternal bradycardia (heart rate < 60 beats per minute), atrioventricular block, inability or refusal to provide informed consent. |
|
| Interventions | All women
Low‐dose group
High‐dose group
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | Mean age of women: 23.8 ± 5.2 years in the low‐dose group; 24 ± 4.4 years in the high‐dose group. Twin gestations: 3/50 in the low‐dose group; 1/50 in the high‐dose group. Approval for this study was granted from the institutional review board of the University of Mashhad Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by computer‐generated number allocation. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details were given regarding blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details were given regarding blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selecting reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
Soguk 2004.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: Sekai Tahir Burak Women Health Education and Research Hospital, Ankara, Turkey. Participants: women (n = 100; 50 in the low‐dose group, 50 in the high‐dose group) between 28‐34 weeks' gestation with preterm labour that was unresponsive to intravenous fluid therapy and sedation. Definition of preterm labour: not stated. Exclusion criteria: not stated. |
|
| Interventions | Low‐dose group
High‐dose group ('standard dose group')
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants received low‐dose or standard dose therapy 'randomly'. |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was given on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make the judgement. |
| Selective reporting (reporting bias) | High risk | No outcomes were listed a priori. It is unclear if the outcomes reported were the pre‐specified primary and secondary outcomes. |
| Other bias | Low risk | No obvious risk of other bias. |
Terrone 2000.
| Methods | Randomised controlled trial. | |
| Participants |
Setting: University of Mississippi Medical Center, Mississippi, USA. Participants: women (n = 160; 78 in the low‐dose group, 82 in the high‐dose group) between 24‐34 weeks' gestation with spontaneous preterm labour. Singleton and twin pregnancies were included. Definition of preterm labour: advancement seen on cervical examination with uterine contractions while the patient was admitted to the triage unit OR dilatation of 2 cm and effacement of 80% with ≥ 6 uterine contractions per hour. Exclusion criteria: higher‐order multiple gestations, rupture of membranes, non reassuring fetal assessment, evidence of intrauterine infection, treatment with any tocolytic agent before maternal transport, women who could not tolerate high doses of magnesium sulphate (for example, women with renal failure), inability or refusal to provide informed consent. |
|
| Interventions | All women
Low‐dose group
High‐dose group
|
|
| Outcomes | Primary
Secondary
|
|
| Notes | Mean age of women: 24 ± 5.1 years in the low‐dose group; 24 ± 4.8 years in the high‐dose group. Twin gestations: 2/70 in the low‐dose group; 3/78 in the high‐dose group. Approval for this study was granted from the institutional review board of The University of Mississippi Medical Centre. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number allocation, assigned by selection of the next numbered envelope. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was given on blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was given on blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 women excluded from analysis due to treatment failure and subsequent birth (8 in the low‐dose group and 4 in the high‐dose group). No losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No obvious risk of other bias. |
°C: degrees Celsius cm: centimetre(s) g: gram(s) g/h: gram(s) per hour mg: milligram(s) mg/dL: milligrams per decilitre mm Hg: millimetres of mercury
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Martin 1990 | Randomised controlled trial. Setting: University of Mississippi Medical Center, Mississippi, USA. Participants: women (n = 44; 23 magnesium gluconate, 21 magnesium chloride) with preterm labour. Definition of preterm labour: not stated. Intervention: oral magnesium gluconate compared with oral magnesium chloride given for maintenance tocolysis after the successful arrest of labour with parenteral magnesium sulphate. Reason for exclusion This study did not examine different treatment regimens of parenteral magnesium sulphate as single agent tocolytic therapy for the arrest of preterm labour. Rather, it examined the use of different oral magnesium preparations for maintenance tocolysis given after the successful arrest of labour with parenteral magnesium sulphate. |
| Martin 1998 | Randomised controlled trial. Setting: University of Mississippi Medical Center, Mississippi, USA. Participants: women (n = 47; 25 magnesium gluconate, 22 magnesium chloride) with preterm labour. Definition of preterm labour: presence of regular uterine contractions with a change in cervical exam. Intervention: oral magnesium gluconate compared with oral magnesium chloride given for maintenance tocolysis after the successful arrest of labour with parenteral magnesium sulphate. Reason for exclusion This study did not examine different treatment regimens of parenteral magnesium sulphate as single agent tocolytic therapy for the arrest of preterm labour. Rather, it examined the use of different oral magnesium preparations for maintenance tocolysis given after the successful arrest of labour with parenteral magnesium sulphate. |
| Zygmunt 2003 | Open‐label, randomised, parallel‐group, actively controlled study. Setting: three study centres in Germany (Hessian, Giessen, Heidelberg). Participants: women (n = 46; 23 in treatment group) with preterm labour with indication for single agent tocolysis therapy with magnesium sulphate. Definition of preterm labour: not stated. Intervention: loading dose of 4 g magnesium sulphate administered over 30 minutes. Maintenance dose of 1‐2 g/h magnesium sulphate up to 21 days via (1) ready to use infusion solution with 24 g magnesium sulphate per 500 mL OR (2) infusion solution concentrate, diluted in carrier solution before administration 20 g/500 mL. Reason for exclusion This study did not examine different treatment regimens of parenteral magnesium sulphate as single agent tocolytic therapy for the arrest of preterm labour. All women were given the same treatment regimen using two different preparations of magnesium sulphate (ready to use infusion solution versus infusion solution concentrate diluted in carrier solution). |
g: gram(s) g/h: gram(s) per hour mL: millilitre(s)
Characteristics of ongoing studies [ordered by study ID]
Namazi 2013.
| Trial name or title | Comparison of maintenance therapy and continuous intravenous therapy with magnesium sulphate in preterm labour pain management at 24‐36 weeks' gestation: a randomized controlled trial. |
| Methods | Single‐arm, single‐blinded, randomised controlled trial. |
| Participants |
Setting: Shariati Hospital, Bandar Abbas, Hormozgan, Iran. Participants: women (n = 70) with preterm labour pains, between 24‐36 weeks’ gestation. Inclusion criteria: presence of ≥ 4 uterine contractions during 20 minutes, cervical dilatation less than 4 cm, effacement less than 80%. Exclusion criteria: co‐existing medical disease (including diabetes mellitus, asthma, cardiovascular disease), pre‐eclampsia, uterine bleeding, rupture of membranes, placenta decolman, intrauterine infection, urinary tract infection, fetal anomalies, previous preterm labour pain, lack of decreasing uterine contractions during first 2 hours after administration of magnesium sulphate, patient requiring administration of magnesium sulphate after 24 hours. |
| Interventions | Low‐dose group (Group A)
High‐dose group (Group B)
|
| Outcomes | Primary
Secondary
|
| Starting date | 2013‐03‐20. |
| Contact information | Seyed Shojaeddin Namazi. |
| Notes | Approval for this study was granted from the Student Research Committee, Hormozgan University of Medical Sciences. |
cc: cubic centimetre(s) cm: centimetre(s) g: gram(s) g/h: gram(s) per hour
Differences between protocol and review
Birth less than 48 hours after trial entry is no longer included as both a primary infant outcome and a secondary infant outcome. It is listed as a primary infant outcome only.
We have used the GRADE approach to assess the quality of the body of evidence and as detailed in 'Table 1'.
Contributions of authors
Helen McNamara wrote the first draft of the review, with Julie Brown and Caroline Crowther making comments and contributing to subsequent drafts. Helen McNamara and Julie Brown assessed each study for inclusion and risk of bias, and extracted data for meta‐analysis. Caroline Crowther acted as the third author, resolving discrepancies where they arose.
Sources of support
Internal sources
No sources of support supplied
External sources
National Health and Medical Research Council, Australia.
Declarations of interest
Helen C McNamara: none known
Caroline A Crowther: none known
Julie Brown: none known
New
References
References to studies included in this review
Behrad 2003 {published data only}
- Behrad V, Moossavifar N, Mojtahedzadeh M, Esmalli H, Moghtadeli P. A prospective, randomized, controlled trial of high and low doses of magnesium sulfate for acute tocolysis. Acta Medica Iranica 2003;41(2):126‐31. [Google Scholar]
Soguk 2004 {published data only}
- Soguk C, Tapisiz OL, Mungan T. Low dose treatment protocol in magnesium sulfate tocolysis. International Journal of Gynecology & Obstetrics 2004;86:37‐8. [DOI] [PubMed] [Google Scholar]
Terrone 2000 {published data only}
- Terrone DA, Rinehart BK, Kimmel ES, May WL, Larmon JE, Morrison JC. A prospective, randomized, controlled trial of high and low maintenance doses of magnesium sulfate for acute tocolysis. American Journal of Obstetrics and Gynecology 2000;182(6):1477‐82. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Martin 1990 {published data only}
- Martin RW, Perry KG, Martin JN, Hess LW, Morrison JC. Oral magnesium for tocolysis: a comparison of magnesium gluconate and enteric‐coated magnesium chloride. Proceedings of 37th Annual Meeting of the Society for Gynecologic Investigation; 1990 March 21‐24; St Louis, USA. 1990:167.
Martin 1998 {published data only}
- Martin RW, Perry KGJ, Martin JN, Seago DP, Roberts WE, Morrison JC. Oral magnesium for tocolysis: a comparison of magnesium gluconate and enteric‐coated magnesium chloride. Journal of the Mississippi State Medical Association 1998;39(5):180‐2. [PubMed] [Google Scholar]
Zygmunt 2003 {published data only}
- Zygmunt M, Heilmann L, Berg C, Wallwiener D, Grischke E, Munstedt K, et al. Local and systemic tolerability of magnesium sulphate for tocolysis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;107(2):168‐75. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Namazi 2013 {published data only}
- Namazi SS. Comparison of maintenance therapy and continuous intravenous therapy with magnesium sulfate in preterm labor pain management at 24‐36 weeks gestation: a randomized controlled trial. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 10 January 2015].
Additional references
Abarbanel 1945
- Abarbanel, AR. The spasmolysant action of magnesium ions on the tetanically contracting human gravid uterus. American Journal of Obstetrics and Gynecology 1945;49(4):473‐83. [Google Scholar]
ACOG 2012
- ACOG. ACOG Practice Bulletin. Clinical management guidelines for obstetrician‐gynecologists. Number 127, June 2012. Management of preterm labor. Obstetrics & Gynecology 2012; Vol. 119:1308‐17.
Bain 2012
- Bain E, Middleton P, Crowther CA. Different magnesium sulphate regimens for neuroprotection of the fetus for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009302.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bain 2013
- Bain ES, Middleton PF, Crowther CA. Maternal adverse effects of different antenatal magnesium sulphate regimens for improving maternal and infant outcomes: a systematic review. BMC Pregnancy and Childbirth 2013;13(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2010
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bulletin of the World Health Organization 2010;88(1):31‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borna 2007
- Borna S, Saeidi FM. Celecoxib versus magnesium sulfate to arrest preterm labor: randomized trial. Journal of Obstetrics and Gynaecology Research 2007;33(5):631‐4. [DOI] [PubMed] [Google Scholar]
Borna 2008
- Borna S, Sahabi N. Progesterone for maintenance tocolytic therapy after threatened preterm labour: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(1):58‐63. [DOI] [PubMed] [Google Scholar]
Crowther 2014
- Crowther CA, Brown J, McKinlay CJD, Middleton P. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD001060.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowling 2012
- Dowling O, Chatterjee PK, Gupta M, Tam Tam HB, Xue X, Lewis D, et al. Magnesium sulfate reduces bacterial LPS‐induced inflammation at the maternal‐fetal interface. Placenta 2012;33(5):392‐8. [DOI] [PubMed] [Google Scholar]
Doyle 2009
- Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004661.pub3] [DOI] [PubMed] [Google Scholar]
Duley 2010a
- Duley L, Gülmezoglu AM, Henderson‐Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD000025.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2010b
- Duley LJ, Henderson‐Smart D, Chou D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000128.pub2] [DOI] [PubMed] [Google Scholar]
Duley 2010c
- Duley L, Henderson‐Smart DJ, Walker GJA, Chou D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD000127.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2010d
- Duley L, Matar HE, Almerie MQ, Hall DR. Alternative magnesium sulphate regimens for women with pre‐eclampsia and eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD007388.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2013
- FDA, U.S. Food, Drug Administration. FDA Recommends against prolonged use of magnesium sulfate to stop pre‐term labour due to bone changes in exposed babies. Drug Safety Communications, U.S. Food and Drug Administration 2013.
Flenady 2014a
- Flenady V, Reinebrant HE, Liley HG, Tambimuttu EG, Papatsonis DNM. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD004452.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flenady 2014b
- Flenady V, Wojcieszek AM, Papatsonis DNM, Stock OM, Murray L, Jardine LA, et al. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glock 1993
- Glock JL, Morales WJ. Efficacy and safety of nifedipine versus magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;169(4):960‐4. [DOI] [PubMed] [Google Scholar]
Goldenberg 2008
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2006
- Grimes DA, Nanda K. Magnesium sulfate tocolysis: time to quit. Obstetrics & Gynecology 2006;108(4):986‐9. [DOI] [PubMed] [Google Scholar]
Haas 2012
- Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta‐analysis. BMJ 2012;345(7879):e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haghighi 1999
- Haghighi L. Prevention of preterm delivery: nifedipine or magnesium sulfate. International Journal of Gynaecology and Obstetrics 1999;66(3):297‐8. [DOI] [PubMed] [Google Scholar]
Hall 1957
- Hall DG. Serum magnesium in pregnancy. Obstetrics & Gynecology 1957;9(2):158‐62. [PubMed] [Google Scholar]
Han 2013
- Han S, Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000940.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
How 2006
- How HY, Zafaranchi L, Stella CL, Recht K, Maxwell RA, Sibai B, et al. Tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled pilot study. American Journal of Obstetrics and Gynecology 2006;194(4):976‐81. [DOI] [PubMed] [Google Scholar]
James 2010
- James MF. Magnesium in obstetrics. Best Practice and Research Clinical Obstetrics and Gynaecology 2010;24(3):327‐37. [DOI] [PubMed] [Google Scholar]
Klauser 2012
- Klauser CK, Briery CM, Keiser SD, Martin RW, Kosek MA, Morrison JC. Effect of antenatal tocolysis on neonatal outcomes. Journal of Maternal Fetal & Neonatal Medicine 2012;25(12):2778‐81. [DOI] [PubMed] [Google Scholar]
Larmon 1999
- Larmon JE, Ross BS, May WL, Dickerson GA, Fischer RG, Morrison JC. Oral nicardipine versus intravenous magnesium sulfate for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1999;181(6):1432‐7. [DOI] [PubMed] [Google Scholar]
Lewis 2005
- Lewis DF. Magnesium sulfate: the first‐line tocolytic. Obstetrics and Gynecology Clinics of North America 2005;32(3):485‐500. [DOI] [PubMed] [Google Scholar]
Lurie 2004
- Lurie S, Gur D, Sadan O, Glezerman M. Relationship between uterine contractions and serum magnesium levels in patients treated for threatened preterm labour with intravenous magnesium sulphate. Journal of Obstetrics and Gynaecology 2004;24(3):247‐8. [DOI] [PubMed] [Google Scholar]
Lyell 2007
- Lyell DJ, Pullen K, Campbell L, Ching S, Druzin ML, Chitkara U, et al. Magnesium sulfate compared with nifedipine for acute tocolysis of preterm labor: a randomized controlled trial. Obstetrics & Gynecology 2007;110(1):61‐7. [DOI] [PubMed] [Google Scholar]
McWhorter 2004
- McWhorter J, Carlan SJ, O'Leary TD, Richichi K, O'Brien WF. Rofecoxib versus magnesium sulfate to arrest preterm labor: a randomized trial. Obstetrics & Gynecology 2004;103(5 Pt 1):923‐30. [DOI] [PubMed] [Google Scholar]
Mittendorf 1997
- Mittendorf R, Covert R, Boman J, Khoshnood B, Lee K, Siegler M. Is tocolytic magnesium sulphate associated with increased total paediatric mortality?. Lancet 1997;350(9090):1517‐8. [DOI] [PubMed] [Google Scholar]
Morales 1993
- Morales WJ, Madhav H. Efficacy and safety of indomethacin compared with magnesium sulfate in the management of preterm labor: a randomized study. American Journal of Obstetrics and Gynecology 1993;169(1):97‐102. [DOI] [PubMed] [Google Scholar]
Nassar 2006
- Nassar AH, Sakhel K, Maarouf H, Naassan GR, Usta IM. Adverse maternal and neonatal outcome of prolonged magnesium sulfate tocolysis. Acta Obstetricia et Gynecologica Scandinavica 2006;85(9):1099‐103. [DOI] [PubMed] [Google Scholar]
Neilson 2014
- Neilson JP, West HM, Dowswell T. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD004352.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pryde 2009
- Pryde PG, Mittendorf R. Contemporary usage of obstetric magnesium sulfate: indication, contraindication, and relevance of dose. Obstetrics & Gynecology 2009;114(3):669‐73. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schorr 1997
- Schorr SJ. Ketorolac is a safe and effective drug for acute tocolysis. Acta Diabetologica Latina 1997;176:S7. [Google Scholar]
Steer 1977
- Steer CM, Petrie RH. A comparison of magnesium sulfate and alcohol for the prevention of premature labor. American Journal of Obstetrics and Gynecology 1977;129(1):1‐4. [DOI] [PubMed] [Google Scholar]
Tam Tam 2011
- Tam Tam HB, Dowling O, Xue X, Lewis D, Rochelson B, Metz CN. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. American Journal of Obstetrics and Gynecology 2011;204(4):364.e1‐364.e8. [DOI] [PubMed] [Google Scholar]
Tsatsaris 2004
- Tsatsaris V, Cabrol D, Carbonne B. Pharmacokinetics of tocolytic agents. Clinical Pharmacokinetics 2004;43(13):833‐44. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. Pregnancy, childbirth and the puerperium. http://www.who.int/classifications/icd/en/ [accessed 24 May 2011] 1992.


