Abstract
A major barrier to the emergence of distant metastases is the survival of circulating tumor cells (CTCs) within the vasculature. Lethal stressors including shear forces from blood flow, anoikis arising from cellular detachment, and exposure to natural killer cells, combine to subvert the ability of primary tumor cells to survive and ultimately seed distant lesions. Further attenuation of this rate-limiting process via therapeutic intervention offers a very attractive opportunity for improving cancer patient outcomes, in turn prompting the need for a deeper understanding of the molecular and cellular mechanisms underlying CTC viability. MUC4 is a very large and heavily glycosylated protein expressed at the apical surfaces of the epithelia of a variety of tissues, is involved in cellular growth signaling and adhesiveness, and contributes to the protection and lubrication of cellular linings. Analysis of patient-matched breast tumor specimens has demonstrated that MUC4 protein levels are upregulated in metastatic lesions relative to primary tumor among all breast tumor subtypes, pointing to a possible selective advantage for MUC4 overexpression in metastasis. Analysis of a genetically engineered mouse model of HER2-positive breast cancer has demonstrated that metastatic efficiency is markedly suppressed with MUC4 deletion, and MUC4-knockout tumor cells poorly associate with platelets and white blood cells known to support CTC viability. In this review we discuss the diverse roles of MUC4 in tumor progression and metastasis, and propose that intervening in MUC4 intercellular interactions with binding partners on blood-borne aggregating cells could potentially thwart breast cancer metastatic efficiency.
Keywords: breast, metastasis, circulating tumor cell, mucin, Muc4
Introduction
Breast cancer metastasis.
Despite significant advances in the detection and treatment of breast tumors over the last few decades, metastatic disease remains the primary cause of breast cancer-related deaths (Weigelt et al., 2005). Metastasis is a multi-step process involving primary tumor cell invasion into the stromal compartment, intravasation into the blood or lymphatic systems, extravasation from capillaries at secondary sites, and colonization and outgrowth of metastatic lesions (Talmadge and Fidler, 2010, Lambert et al., 2017). Metastasizing tumor cells exhibit unique characteristics including altered cellular adhesion, evasion of programmed cell death, induction of the epithelial-to-mesenchymal transition (EMT), and association with cells in circulation (Lambert et al., 2017). Important intermediaries of the distant metastatic cascade are circulating tumor cells (CTCs), which exit primary tumors as single cells or clusters and travel to distant sites to seed metastatic lesions (Lambert et al., 2017, Micalizzi et al., 2017). It has been estimated that only ~0.01% of cells that enter circulation survive to seed metastatic lesions (Reymond et al., 2013), prompting questions concerning the mechanisms by which CTCs are able to shield themselves from the harsh conditions of transit.
While our understanding of the molecular underpinnings governing breast cancer metastasis have improved in recent years, our ability to successfully treat metastatic disease lags far behind. Novel methods and reliable markers to predict the probability of metastasis together with new approaches to therapeutically intervene could provide significant clinical benefit to the patients. Currently employed anti-metastatic therapeutic strategies closely align with those employed in the treatment of primary breast tumors, and typically involve a combination of chemotherapeutics, targeted therapeutics, and immunotherapies (Scully et al., 2012, Ganesh and Massagué, 2021). A significant challenge to this approach is that metastatic lesions often accumulate genetic alterations that are molecularly distinct from primary tumors, rendering such therapeutic avenues ineffective (Fidler and Kripke, 1977, Ganesh and Massagué, 2021). Thus, increasing effort has been dedicated toward identifying new prognostic markers for metastasis and developing strategies to target processes that contribute to aggressive disease, such as angiogenesis, cell motility, the metastatic microenvironment, metastatic dormancy, and circulating tumor cells (Steeg, 2016; Fontebasso and Dubinett, 2015).
Mucin proteins.
Mucins are large, heavily glycosylated cell surface proteins that normally function to lubricate and protect epithelial and vascular surfaces. The human mucin family is comprised of at least twenty distinct members that fall into two categories, secreted and membrane-bound. Both classes are characterized by the presence of a highly O-glycosylated variable number of tandem repeat (VNTR) domain, which contributes to cell protection by forming a large, protruding and negatively-charged structure that helps physically shield the cell surface from external assaults (Van Klinken et al., 1995). However, each class is also distinguished by its own distinct domains and functions. The secreted mucins (including MUC2, MUC5AC, MUC5B, MUC6, MUC7, and MUC19) contain trypsin inhibitor-like (TIL), von Willebrand factor type D (vWD), and C-terminal cysteine knot domains (Perez-Vilar and Hill, 1999) (Figure 1A). Importantly, secreted mucins oligomerize through their cysteine-rich vWD and C-terminal cysteine-knot domains (Perez-Vilar and Hill, 1999), directly contributing to their ability to coat epithelial and vasculature structures, and protect against outside infection and physical or chemical damage.
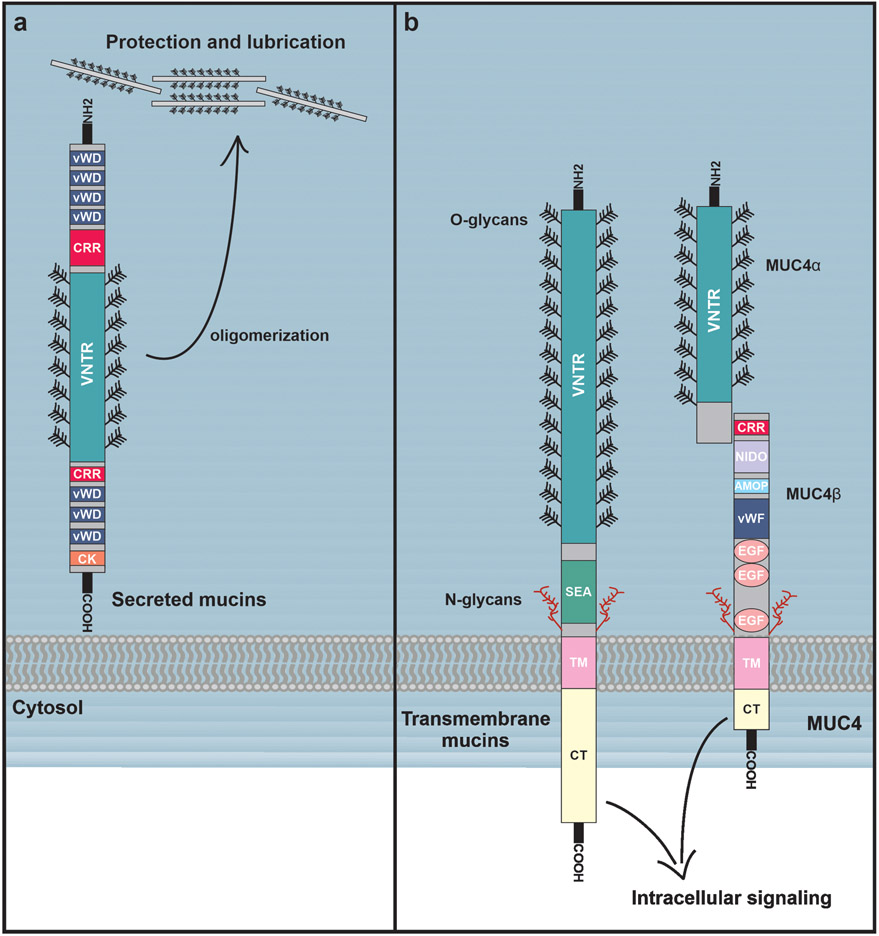
Figure 1.
Structures of secreted and membrane-bound mucins. (A) Secreted mucins contain multiple vWD (von Willebrand factor D) domains, cysteine-rich regions (CRRs), as well as a single C-terminal cysteine-knot (CK) domain. The middle regions of the secreted mucins consist of a highly O-glycosylated VNTR domain which contains cysteine-rich regions dispersed throughout. Secreted mucins oligomerize through their CRR and CK domains, forming large highly viscous aggregates that contribute to the lubrication and protection of epithelial surfaces. (B) Membrane-bound mucins are structurally similar to secreted mucins, and contain vWD domains, CRRs, and O-glycosylated VNTR domains, but are distinguished by a single transmembrane domain that tethers them to the cell surface. Membrane-bound mucins also contain SEA domains as well as some N-glycosylated regions. Notably, in MUC4 the SEA domain is replaced with NIDO and AMOP domains. MUC4 also contains three EGF-like domains, and is proteolytically cleaved to produce noncovalently-linked MUC4ɑ and MUC4β subunits. While membrane-bound mucins are also critical to the protection and lubrication epithelial surfaces, they can simultaneously play extensive roles in cellular signaling, often through their cytoplasmic tails.
Membrane-bound mucins (including, MUC1, MUC3, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, and MUC20) also contain cysteine-rich regions similar to vWD domains and C-terminal cysteine knot domains (Bansil and Turner, 2018), but are distinguished from secreted mucins by the presence of a single transmembrane domain that tethers them to the cell surface. All membrane-bound mucins contain a sea urchin sperm protein, enterokinase, and agrin (SEA) domain, with the exception of MUC4, which instead contains a nidogen domain and an adhesion-associated (AMOP) domain (Kufe, 2009). Many of the transmembrane mucins also contain EGF-like domains important for interactions with other proteins (Figure 1B). While transmembrane mucins also contribute to coating and protection of epithelial surfaces, they also appear to play additional roles in cell signaling (Carraway et al., 2003). Importantly, numerous studies have demonstrated that aberrant expression of membrane-bound mucins, most notably MUC1 and MUC4, confer aggressive characteristics to tumors by promoting cellular proliferation, motility, and survival (the contributions of MUC1 to malignancy are reviewed in Nath and Mukherjee, 2014; Chen et al., 2021). MUC4 has been widely implicated as a prominent contributor to breast cancer progression and metastasis (Workman et al., 2009a, Rowson-Hodel et al., 2018) and thus will be the focus of this review.
Membrane mucin MUC4.
Mucin-4 (MUC4), a membrane-bound mucin, has been extensively studied in a diverse collection of normal tissues and solid tumors. Many of the foundational studies on MUC4 structure and expression patterns were performed in rat tissues over two decades ago, and key observations have been subsequently confirmed in human tissues following the cloning of the human gene (Moniaux et al., 1999). While MUC4 has been assigned diverse functions across many cell and tumor types, and function may be context-dependent in some cases, its central role as a mediator of metastasis is beginning to emerge. In this regard, MUC4 has been implicated as a regulator of several processes critical to metastasis, including cell adhesion (Komatsu et al., 1997), EMT and invasion (Moniaux et al., 2007), tumor cell docking (Senapati et al., 2012), therapeutic resistance (Price-Schiavi et al., 2002), and cell survival (Carraway et al., 2001; Komatsu et al., 2001). Because of its recent identification as an important regulator of metastasis by aiding CTC survival in circulation by associating with blood cells (Rowson-Hodel et al., 2018), we will provide an overview of MUC4 characteristics, emphasizing its role as a mediator of CTC-blood cell associations, and discuss the implications of these observations in cancer progression and the development of anti-metastatic therapeutic strategies.
Regulation of MUC4 expression
MUC4 expression is regulated through a collection of diverse mechanisms throughout both transcription and translation. The MUC4 5’ promoter region contains tissue-specific as well as positive and negative regulatory elements, underscoring the complex regulation of MUC4 by a plethora of signaling pathways (Price-Schiavi et al., 2000a, Perrais et al., 2001). For example, in rat mammary adenocarcinoma cells, the transcription factor PEA3 directly binds the MUC4 promoter and mediates MUC4 transcription via Erk and SAPK/JNK signaling (Perez et al., 2003). Others have reported that, in human epithelial cancer cells, MUC4 expression is spatiotemporally regulated by various transcription factors including HNF-1/-4, FOXA1/A2, GATA-4/-5/-6 and CDX-1/-2 (Jonckheere et al., 2007). Similarly, β-catenin can directly bind to the promoter of MUC4 and alter transcript and protein levels in colorectal and pancreatic cancer cells in a Wnt-dependent manner (Pai et al., 2016a, Pai et al., 2016b). MUC4 is also regulated by insulin and IGF-1 in an Erk-dependent manner in rat mammary epithelial cells (Zhu et al., 2000), and the pro-inflammatory cytokine IL-6 engages the gp130/STAT3 signaling pathway to stimulate the direct binding of phosphorylated-STAT3 to the MUC4 promoter in gastric cancer cells (Mejías-Luque et al., 2008).
Evidence suggests that MUC4 is also regulated epigenetically. In pancreatic cancer cells, the MUC4 5’UTR is methylated at CpG sites, and inhibition of histone deacetylation alters MUC4 expression (Vincent et al., 2008). A similar study confirmed that methylation in the MUC4 promoter region regulates MUC4 expression, and that the pattern of DNA methylation correlates with MUC4 expression across a number of solid tumor types (Yamada et al., 2009).
MUC4 is transcriptionally induced in both normal and cancer tissues via hormonal and cellular transformation inputs, respectively. For example, in the rat uterine epithelium, MUC4 is transcriptionally regulated by ovarian hormones, such as estrogen and progesterone, where MUC4 expression patterns are important for proper blastocyst implantation (McNeer et al., 1998). On the other hand, MUC4 expression is elevated during pancreatic carcinogenesis by K-ras to engage MAPK, NF-κB, and RalB signaling pathways (Vasseur et al., 2015), and by the FXR/FAK/c-Jun signaling axis to promote pancreatic tumor progression and metastasis (Joshi et al., 2016).
It has also been observed that MUC4 expression is altered through the direct binding of multiple regulators to MUC4 mRNA in the 3’ UTR. In mouse epithelial tissues, galectin-3 regulates MUC4 mRNA stability and expression through the intermediate hbRNP-L (Coppin et al., 2017). Moreover, Srivastava et al. (Srivastava et al., 2011) demonstrated that miRNA-150 directly binds to the 3’ UTR of MUC4 mRNA to reduce MUC4 expression, resulting in decreased growth, clonogenicity, migration and invasion, but increased cell-cell adhesion of pancreatic cancer cells. Similarly, miR-219-1-3p negatively regulates MUC4 expression in pancreatic cancer cells by binding to the MUC4 3’UTR to decrease cell proliferation and downstream activation of Akt and Erk (Lahdaoui et al., 2015). In cervical cancer cells, miR-211 directly binds the MUC4 3’ UTR to decrease MUC4 expression and cancer cell invasion, likely via reversal of EMT phenotypic properties (Xu et al., 2017).
TGFβ has been identified as a major regulator of MUC4 at multiple levels. Price-Shiavi et al. (Price-Schiavi et al., 2000b) found that in the rat mammary epithelial cells, but not in tumor cells, MUC4 is post-transcriptionally upregulated by TGFβ during pregnancy (Price-Schiavi et al., 1998), while in rat uterine luminal epithelial cells, regulation of MUC4 protein is controlled by TGFβ expressed in the stroma and may be antagonized by estrogen (Idris and Carraway, 2000). Mechanistically, it has been observed that TGFβ targets the MUC4 precursor protein for proteasomal degradation (Lomako et al., 2009, Price-Schiavi et al., 1998). In pancreatic tumor cells, retinoic acid alters MUC4 through direct regulation of TGFβ expression (Choudhury et al., 2000). MUC4 was also found to be transcriptionally upregulated by TGFβ in pancreatic cancer cells, and Smad2/Smad4 activation at the MUC4 promoter was negatively regulated by Smad7 and c-Ski (Jonckheere et al., 2004). Similarly, SMAD2 regulates TGFβ-induced MUC4 expression in mammary epithelial cells, whereas IFN-gamma, through induction of STAT1 activation and upregulation of expression of the inhibitory Smad7, inhibits the effect of TGF-β on MUC4 expression (Soto et al., 2003). Together, these studies underscore the remarkably complex regulatory mechanisms governing MUC4 expression in both normal and cancer contexts.
MUC4 structure and function
MUC4 structure.
MUC4 was identified as sialomucin complex (SMC) in a highly metastatic rat adenocarcinoma (Sherblom et al., 1980a; Sherblom et al., 1980b). Human MUC4 is located on chromosome 3q29 (Porchet et al., 1991; Gross et al., 1992), varies in length from 4468 to 8468 amino acid residues, and is highly similar in structure to rat MUC4 (Moniaux et al., 1999). The MUC4 precursor is synthesized in the endoplasmic reticulum where it is N-glycosylated, folds, and is then proteolytically cleaved to produce the two non-covalently associated subunits, MUC4α and MUC4β (Sheng et al., 1990; Sherblom and Carraway, 1980; Helm and Carraway, 1981; Soto et al., 2006). MUC4α contains the variable tandem repeat domain, which is subject to extensive O-glycosylation following cleavage of the precursor (Spielman et al., 1987, Nollet et al., 1998). O-glycans primarily contribute to the bulky and protruding structure of MUC4, which is important for the protection and lubrication functions of the complex. While the structure of the alpha subunit is quite similar between rat and human, human MUC4 contains a much more extensive variable tandem repeat domain, making the molecule significantly larger (Moniaux et al., 1999). The membrane-associated subunit MUC4β shares a 60-70% amino acid sequence identity between rat and human (Moniaux et al., 1999). MUC4β consists of a ~120 kDa extracellular region, a single hydrophobic transmembrane domain and a very short (~20 residue) cytoplasmic tail. The extracellular region contains N-glycosylation sites and two epidermal growth factor (EGF)-like domains (Sheng et al., 1992, Moniaux et al., 1999) that confer important roles to the MUC4β subunit in cell signaling (Carraway et al., 1999; Wu et al., 1994).
MUC4 expression patterns.
MUC4 is expressed in numerous normal epithelial tissues during development and in adult (Price-Schiavi et al., 2000b; Rossi et al., 1996; Idris and Carraway, 1999; Zhang et al., 2005). In adult tissues, MUC4 is expressed in endothelial cells lining the vasculature (Zhang et al., 2005) and on the apical surfaces of epithelial cells, where it may serve as a marker of differentiation (Li et al., 2001). While early observations with a rat mammary adenocarcinoma model suggested that MUC4 may be overexpressed in tumors relative to normal tissues (Rossi et al., 1996), subsequent findings with patient samples indicate that MUC4 is aberrantly expressed across diverse solid tumor types, and is often, but not always, correlated with worsened prognosis or advanced disease (Table 1). MUC4 expression is increased in the primary tumors of various breast tumor subtypes (Komatsu et al., 1999; Rakha et al., 2005; Mukhopadhyay et al., 2013; Shet et al., 2013; Mercogliano et al., 2017b), upper aerodigestive tract squamous cell carcinomas (Weed et al., 2001), ovarian tumors (Chauhan et al., 2006), pancreatic adenocarcinomas (Mimeault et al., 2010; Kaur et al., 2014), lung adenocarcinomas (Gao et al., 2014), the “columnar type” of mucin-producing bile duct tumors (Shibahara et al., 2004b), glioblastomas (Li et al., 2014b), cervical tumors (Xu et al., 2017), and intrahepatic cholangiocarcinomas (Shibahara et al., 2004a) compared to normal tissues. In TCGA datasets, high MUC4 expression is correlated with poor patient survival in pancreatic cancer, bladder cancer, colon cancer, lung adenocarcinoma, lung squamous carcinoma, ovarian cancer, skin cancer, and stomach cancer. In these tumors, MUC4 expression is linked to genes involved in cell adhesion, cell-cell junctions, glycosylation, and cell signaling (Jonckheere and Van Seuningen, 2018).
Table 1.
Muc4 expression in primary tumors and metastatic lesions. NR, not reported; ICC, immunocytochemistry; IHC, immunohistochemistry; WB, western blotting.
| Cancer Type | Muc4 Expression Patterns (Primary Tumor) | Muc4 Expression Patterns (Metastases) | Correlation with Prognostic Factor or Disease Stage | Method of Detection | Reference |
|---|---|---|---|---|---|
| Breast Cancer | Expressed in 95% of primary breast tumors | NR | Muc4 expression correlates with tumor grade | IHC | (Rakha 2005) |
| Overexpressed in primary invasive triple-negative tumors compared to normal | NR | NR | IHC | (Mukhopadhyay 2013) | |
| Expressed in the majority of secretory carcinomas of the breast | No increased expression in metastatic lesions | NR | IHC | (Shet 2013) | |
| Decreased expression in primary tumor compared to patient-matched normal | Increased expression in patient-matched lymphnode metastases | NR | WB/IHC | (Workman 2009) | |
| Expressed in majority of patient breast cancer samples | NR | High frequency of Muc4 expression in malignant cells in body fluids | ICC | (Komatsu 1999) | |
| Highly expressed in invasive micropapillary carcinoma of the breast | NR | Muc4 expression correlates with shorter death free survival and poor response to standard of care | IHC | (Mercogliano 2017) | |
| Aerodigestive Tract Squamous Cell Cancer | Expressed in primary tumors | Expression persists in metastatic lesions | No correlation with patient prognosis | ICC/WB | (Weed 2001) |
| Ovarian Cancer | Overexpressed in primary tumor compared to normal tissue | NR | No correlation with patient survival | IHC | (Chauhan 2006) |
| Pancreatic Cancer | Overexpressed in primary tumor compared to normal tissue | NR | NR | IHC | (Mimeault 2013) |
| Expressed in primary tumors | NR | Muc4 expression correlates with disease progression | IHC | (Kaur 2014) | |
| Lung Cancer | Highly expressed in primary tumor | Decreased expression in lymph node metastases | Correlates with risk of lymph node metastasis | WB | (Gao 2014) |
| Glioblastoma | Upregulated in tumor compared to normal tissue | NR | NR | WB/IHC | (Li 2014) |
| Cervical Cancer | Overexpressed in primary tumor compared to normal tissue | NR | Strongly associated with lymph node metastases | IHC/gene microarray | (Xu 2017) |
| Cholangiocarcinoma | Expressed in "columnar type" primary tumors | NR | No correlation with patient prognosis | IHC | (Shibahara 2004b) |
| Overexpressed in intrahepatic type compared to normal tissue | NR | Correlates with patient prognosis | IHC | (Shibahara 2004a) |
In human breast tumors, MUC4 appears to undergo significant changes in expression throughout the tumor progression process. We previously assessed MUC4 protein abundance using tissue microarrays containing patient-matched normal breast tissue, primary breast tumor tissue, and lymph node metastases. In contrast to other studies, we observed decreased MUC4 expression in the primary tumors compared to matched normal tissues, followed by a recovery of MUC4 expression in lymph node metastases (Workman et al., 2009a). Low MUC4 expression in the primary tumor is consistent with the role of MUC4 as a marker of differentiation in epithelial cells (Li et al., 2001); as tumors develop, the cellular transition from a differentiated to a de-differentiated state decreases MUC4 expression (Gabbert et al., 1985). However, the increased expression of MUC4 in lymph node metastases suggests that MUC4 may confer an advantage to cells attempting to metastasize. These MUC4 expression trends were independent of ER/PR and HER2 status, pointing to a possible universal role of MUC4 in promoting breast cancer malignancy. Collectively, MUC4 expression in various cancer types and stages of disease is highly diverse, suggesting that MUC4 function is likely dependent on the biological context.
MUC4 as a mediator of tumor cell adhesion and cell-cell interactions.
A major function of MUC4 is the promotion of anti-adhesiveness. The extensively modification of MUC4α by O-linked glycans creates a bulky and rigid structure that contributes to steric hindrance and the approach of foreign pathogens, bodies and molecules (Komatsu et al., 1997). Early studies in rat tissues suggest that overexpression of MUC4 disrupts both cell-cell and cell-substrate interactions, the degree to which is dependent on the number of repeats in the VNTR region and extent of O-glycosylation of the rat MUC4α subunit (Komatsu et al., 1997). N-glycosylation of the MUC4β extracellular domain can also contribute to the steric hindrance properties of MUC4, enabling the protein to mask the cell surface and prevent the binding of molecules to their substrates (Komatsu et al., 1997). In normal polarized human mammary epithelial cells, overexpression relocalizes MUC4 from the apical surface to the lateral surfaces of epithelial cells, resulting in disruption of adherens junctions and impairing cell-cell attachments (Pino et al., 2006). Additionally, MUC4 overexpression in pancreatic tumor cells disrupts tumor cell-extracellular matrix (ECM) interactions by inhibiting integrin-mediated cell adhesion (Chaturvedi et al., 2007), and inhibits cell adhesion and aggregation by preventing interactions between MUC4-expressing tumor cells (Singh et al., 2004). Importantly, loss of MUC4 in melanoma cells results in a rapid reversal from a non-adherent to an adherent state (Komatsu et al., 1997), a process critical to metastasis. In rat mammary adenocarcinoma, MUC4 expression on the cell surface aids in tumor cell evasion of immune killing by masking antigens for immune cell recognition through steric disruption of interactions between tumor cells and cytotoxic immune cells (Sherblom and Moody, 1986, Komatsu et al., 1999). While MUC4 is an important mediator of anti-adhesiveness, the ability of MUC4 to directly engage molecules on the surface of other cells appears to promote adhesion in some biological contexts. In human pancreatic cancer cells, surface MUC4 interacts with galectin-3 on endothelial cells to support the attachment and docking of CTCs to the endothelium, a process essential for extravasation and seeding of metastatic colonies (Senapati et al., 2012). Together, these studies demonstrate that MUC4 mediates a diverse array of cell-cell adhesion and cellular interactions that promote metastasis.
MUC4 contribution to cellular survival mechanisms.
MUC4 also contributes to anti-apoptotic signals in cancer cells to promote tumor growth and metastasis through its membrane-associated beta subunit. Overexpression of rat MUC4 in human melanoma cells promotes xenografted tumor growth through suppression of cell death rather than alteration of cellular proliferation rate by directly regulating cell survival signals (Carraway et al., 2001, Komatsu et al., 2001). In human breast cancer cells, MUC4 suppresses apoptosis through mechanisms both dependent and independent of the HER2 (ErbB2) receptor tyrosine kinase, in some contexts signaling through the PI3K-Akt pathway (Workman et al., 2009b). Additionally, increased MUC4 expression in melanoma cells decreases the expression of the cell cycle inhibitor p27kip, inactivates the proapoptotic protein Bad, and increases expression of the prosurvival protein Bcl-xL (Jepson et al., 2002, Workman et al., 2009b). Similar effects were observed in pancreatic tumor cells, where upregulation of MUC4 expression leads to increased cell proliferation and decreased cell death (Chaturvedi et al., 2007). Importantly, regulation of cell survival signals by MUC4 was observed in response to a variety of insults, including chemotherapeutic agents, lack of serum factors, and loss of adhesion (Workman et al., 2009b). In contrast, loss of MUC4 reduces ErbB2/HER2-mediated signaling, induces FOXO1 transcription, and promotes caspase 3-mediated apoptosis in ovarian cancer cells (Bae et al., 2017). These combined observations support a role for MUC4 as a potent regulator of programmed cell death through diverse signals, a feature critical for successful metastatic dissemination and survival of CTCs in circulation.
MUC4 contribution to therapeutic resistance.
Consistent with the anti-adhesive and cell survival properties ascribed to the MUC4α and MUC4β subunits, MUC4 has also been implicated in mediating therapeutic resistance through steric hindrance and regulation of programmed cell death. Human ER+/HER+ breast cancer cells orthotopically transplanted into mice exhibit upregulated MUC4 expression after therapeutic intervention with tamoxifen, an ER-targeted therapy (Chen et al., 2012). Additionally, ErbB2/HER2 receptor tyrosine kinase expression and signaling are elevated post tamoxifen treatment, implying that MUC4 contributes to a phenotypic switch to ErbB2/HER2 dependence and renders ER-targeted therapeutics ineffective (Chen et al., 2012). Moreover, it appears that MUC4 directly blocks therapeutic efficacy through steric hindrance at the cell surface. Indeed, cell surface MUC4 in melanoma and breast cancer cells reduced binding of anti-HER2 antibodies through steric hindrance and the formation of MUC4-ErbB2/HER2 complexes, which prevents binding of the anti-HER2 antibody Herceptin to its target (Price-Schiavi et al., 2002). Consistent with these findings, MUC4 expression is significantly higher in the Herceptin-resistant breast cancer cell line JIMT-1 compared with Herceptin-sensitive lines, and correlates with less binding of Herceptin to HER2. Importantly, RNAi-mediated MUC4 suppression restores Herceptin binding (Nagy et al., 2005). The mechanisms by which MUC4 is upregulated to promote therapeutic resistance appear varied and highly context-dependent. Studies by Mercogliano et al. suggest that the upregulation of MUC4 and reduction of Herceptin binding and efficacy in HER2-positive breast cancers may be TNFα-dependent (Mercogliano et al., 2017a). However, others have observed that breast and gastric cancer cells treated with Herceptin exhibit STAT3-dependent upregulation of MUC1 and MUC4, blocking Herceptin binding to HER2 to mediate therapy resistance (Li et al., 2014a).
The ability of MUC4 to regulate programmed cell death and cell survival provides yet another mechanism by which MUC4 mediates therapeutic resistance. In pancreatic tumor cells, MUC4 promotes both cell survival and resistance to apoptosis, rendering the tumor cells resistant to gemcitabine, a commonly employed chemotherapeutic, and shRNA-mediated MUC4 knockdown re-sensitizes tumor cells to the cytotoxicity (Mimeault et al., 2010). Mechanistically, overexpression of MUC4 in pancreatic tumor cells appears to engage an ErbB2/HER2-Erk signaling axis that results in the inactivation of Bad and resistance to apoptosis (Bafna et al., 2009). Together, these studies demonstrate that MUC4 engages multiple signaling pathways and mechanisms to mediate therapeutic resistance to both HER2-targeted and other therapeutics, enabling tumor cells to adapt and progress to malignancy.
MUC4 Signaling
Signaling through ErbB2/HER2.
Over two decades of studies point to a role for MUC4 as an intramembrane binding partner for ErbB2 (human form referred to as HER2). MUC4β was shown to directly interact with the extracellular domain of ErbB2/HER2 via one of its EGF-like domains (Carraway et al., 1999). Subsequent studies demonstrated that MUC4 induces ErbB2/HER2 phosphorylation on tyrosine residues 1139 and 1248 (Jepson et al., 2002; Ramsauer et al., 2003; Ramsauer et al., 2006), and that ectopic MUC4 potentiates neuregulin-1-mediated ErbB2/HER2 signaling (Carraway et al., 1999). Investigation of the complex revealed that MUC4 alters the subcellular localization of ErbB2/HER2 in diverse cellular contexts. For example, in the developing rat mammary gland, MUC4 and ErbB2/HER2 are localized to the apical and basolateral membranes, respectively, and expression of ErbB2/HER2 and MUC4 is controlled through distinct regulatory mechanisms. However, during late pregnancy and lactation, MUC4 and ErbB2/HER2 form a complex that is localized to apical cell surfaces in the rat mammary gland epithelia. Similarly, MUC4 promotes the re-localization of phosphorylated ErbB2/HER2 from the basolateral surface to the apical surface of epithelial cells without altering cell polarity (Ramsauer et al., 2003). The apical localization of ErbB2/HER2 may serve to segregate it from its dimerization partner ErbB3/HER3 and activating ligands to alter signaling and downstream outcomes. Despite segregation, ErbB2/HER2 signals through p38-MAPK and Akt, providing evidence that MUC4 independently stimulates ErbB2/HER2 signaling (Ramsauer et al., 2006).
In melanoma and breast cancer cells, MUC4 expression promotes PI3K recruitment to ErbB3/HER3 at the plasma membrane and potentiates ErbB2/HER2-ErbB3/HER3-PI3K signaling (Funes et al., 2006). However, MUC4 expression significantly increases Neuregulin-1 ligand binding to ErbB2/HER2 and ErbB3/HER3 in these cells but does not impact receptor protein levels (Funes et al., 2006). These findings suggest that MUC4 potentiates ErbB2/HER2 signaling by trafficking both receptors to the plasma membrane from intracellular compartments and by suppressing receptor internalization (Funes et al., 2006). In ovarian cancer cells, MUC4 increases both ErbB2/HER2 expression and signaling through FAK-Akt-Erk (Ponnusamy et al., 2008).
In pancreatic and ovarian cancer cells MUC4 appears to stabilize ErbB2/HER2 and drive signaling through the FAK, MAPK, and JNK pathways, contributing to cancer cell proliferation, migration, and invasion (Chaturvedi et al., 2008; Ponnusamy et al., 2011; Jonckheere et al., 2012). Consistently, stabilization of ErbB2/HER2 by MUC4 in pancreatic cancer cells results in PI3K-dependent activation of Akt, increased signaling through NF-κB, and elevation of the expression of Lipocalin2, a multifunctional glycoprotein that may serve as a potential biomarker during tumor progression (Kaur et al., 2014). Additionally, MUC4 increases ErbB2/HER2-FAK-Src signaling, which results in the lysosome-dependent degradation of E-Cadherin and β-Catenin-mediated Wnt-signaling, driving pancreatic cancer cell proliferation and metastasis, as well as angiogenesis (Zhi et al., 2014).
MUC4 signaling through other axes.
MUC4 can also signal through other EGFR family proteins, including EGFR and ErbB3/HER3. In triple-negative breast cancer, MUC4 increases expression of EGFR and ErbB3/HER3, activating Erk1/2, PKC, and FAK to drive cell proliferation, motility, and invasiveness in vitro, as well as increased tumor growth and metastasis in vivo (Mukhopadhyay et al., 2013). In pancreatic cancer, MUC4 interacts with ErbB3/HER3 in the absence of ErbB2/HER2 to mediate signaling through PI3K/Erk/c-Myc to increase cell proliferation, and signaling through FAK/Src to mediate cell motility (Lakshmanan et al., 2015). In glioblastoma, MUC4 mediates higher cell proliferation and invasiveness through regulation of EGFR expression (Li et al., 2014b). In pancreatic cancer cells, overexpression of MUC4/Y, a splice variant that lacks much of the alpha subunit, enhances both angiogenic and metastatic properties of cells in vitro and in vivo through activation of Notch3 signaling, and correlates with a decreased overall survival in vivo (Tang et al., 2016).
MUC4 is a mediator of cell motility events critical to metastasis in a variety of solid tumor types (Moniaux et al., 2007; Chaturvedi et al., 2007; Ponnusamy et al., 2010; Ponnusamy et al., 2008). Mechanistically, MUC4-mediated motility is driven by reorganization of the actin cytoskeleton, including the formation of microspikes, lamellipodia, and filopodia-like structures (Chaturvedi et al., 2007, Ponnusamy et al., 2008). Further support for a role of MUC4 in promoting metastatic behavior comes from studies demonstrating that MUC4 may be a critical regulator of EMT in multiple tumor types. In ovarian cancer cells, MUC4 overexpression was observed to drive a phenotypic shift from an epithelial-like cellular morphology to a mesenchymal-like cellular morphology, decreased expression of epithelial markers such as E-cadherin and cytokeratin-18, and increased expression of mesenchymal markers including N-cadherin and vimentin (Ponnusamy et al., 2008). Further investigation demonstrated that these expression changes were mediated by the EMT-inducing transcription factors Twist1, Twist2 and Snail (Ponnusamy et al., 2010). MUC4-mediated induction of EMT was also observed in pancreatic cancer and lung adenocarcinoma cells via an FGFR1-β-catenin dependent mechanism (Rachagani et al., 2012, Gao et al., 2014).
MUC4 Involvement in Circulating Tumor Cell Survival
Circulating Tumor Cells.
CTCs are a heterogenous population of cells that leave the primary tumor and seed metastases through the acquisition of characteristics that support their exit from primary lesions, entry into the vasculature, survival in circulation, and ability to colonize and proliferate at distant tissues. CTCs can access the vasculature through either passive shedding from the primary tumor or through local invasion and intravasation. Tumors cells are able to invade from the primary tumor through multiple modes of migration including single cell invasion, where single cells undergo EMT and acquire a mesenchymal and invasive phenotype (Pantel and Speicher, 2016), and collective invasion, where cells enter circulation collectively and transit the vasculature as clusters (VanderVorst et al., 2019). While CTCs largely exist as single cells, Aceto et al. reported that in mouse models of breast cancer, CTCs form clusters of 2-50 cells in circulation. While rare compared to single CTCs, these clusters are 20 to 50-fold more metastatic than single CTCs (Aceto et al., 2014). Indeed, the presence of CTC clusters in the bloodstream of breast cancer patients is correlated with poor clinical outcomes (Wang et al., 2017).
CTCs and CTC clusters must also survive the harsh environment of circulation by withstanding shear forces, evading immune attack, and suppressing anoikis. Through acquired mesenchymal and stem-like features, often driven by stemness-associated gene upregulation via transcription factor binding site hypomethylation, breast CTC clusters achieve enhanced anchorage-independent growth and metastasize more effectively than single CTCs (Gkountela et al., 2019). Furthermore, studies in lung and breast cancer suggest that association with stromal cells (e.g., cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and endothelial cells) from primary tumors increases CTC viability both in the bloodstream and at metastatic sites by conferring shear stress resistance (Duda et al., 2010), and by stimulating angiogenesis and EMT (Matsumura et al., 2019), respectively.
While the characterization of CTCs is crucial to our understanding of metastasis, their very low abundance in the bloodstream and heterogeneity makes both identification and isolation difficult. Thus, much effort has been put into improving the detection and enrichment of CTCs in circulation through selection techniques based on biological and physical properties (reviewed in (Zhu et al., 2018)). Emerging advancements in CTC technologies and analysis will further our understanding of their involvement in metastatic disease and hopefully augment their potential as biomarkers of disease progression and therapeutic response.
MUC4 involvement in CTC viability.
Initial observations that MUC4 protein is more abundant in metastatic lesions compared to patient-matched primary tumors led to speculation that MUC4 may aid in successful metastatic dissemination (Workman et al., 2009a). Our studies employing MUC4-deficient mice crossed into the NDL (Neu DeLetion mutant) murine model of ErbB2/HER2-induced mammary tumorigenesis revealed that MUC4 ablation significantly reduces the number of metastatic lesions in the lungs (Rowson-Hodel et al., 2018). Importantly, no differences in primary tumor growth rates or tumor burden are observed between MUC4WT/NDL and MUC4KO/NDL animals, nor is any effect of MUC4 knockout on NDL tumor cell migration in vitro. A tail-vein mouse model of metastasis using MUC4WT/NDL and MUC4KO/NDL tumor-derived cells recapitulated MUC4 impact on lung metastatic colonization, demonstrating that differences could not be attributed to unequal access to the vasculature. Combined with the observation that MUC4 ablated tumor-derived cells are less viable in suspension in vitro, these findings support a role for MUC4 in promoting cell survival in circulation (Rowson-Hodel et al., 2018).
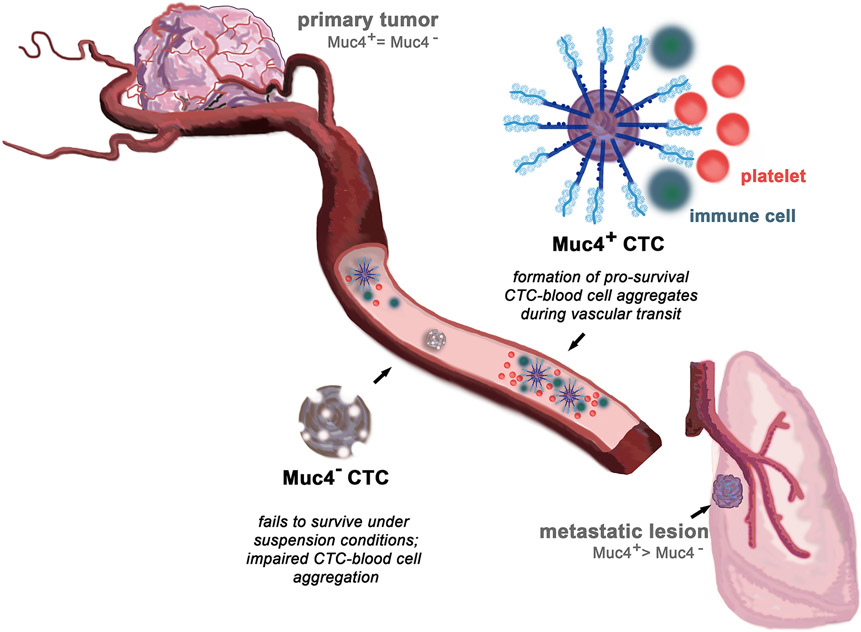
CTCs are known to form aggregates in circulation with other tumor cells, immune cells and platelets (Gay and Felding-Habermann, 2011). Loss of MUC4 in NDL tumor-derived CTCs exhibited significantly impaired abilities to interact with platelets compared to their MUC4-expressing counterparts both in vitro and in vivo, suggesting that MUC4 may aid in the metastatic process through this mechanism. In this model, MUC4-expressing cells promote cell-cell aggregates with platelets and immune cells in circulation, aiding CTC survival in the vasculature and more efficiently seeding metastatic lesions. On the other hand, MUC4-negative cells are less able to form CTC-blood cell aggregates, thus rendering them unable to survive non-adherent conditions, and impairing their successful colonization of metastatic lesions (Figure 2).
Figure 2.
Muc4 mediates CTC survival in circulation. Muc4-positive CTCs form pro-survival cell aggregates with blood-borne cells during vascular transit, enabling them to more efficiently seed metastatic lesions at distant organs. On the other hand, Muc4-negative CTCs fail to survive the harsh conditions of transit, succumbing to shear forces and undergoing anoikis. These cells are unable to form CTC-blood cell aggregates, impairing their ability to successfully colonize metastatic lesions (Rowson-Hodel et al., 2018).
CTCs have a short survival time in circulation, necessitating the acquisition of survival advantages during transit. Association of tumor cells with blood cells is a long-recognized means by which CTCs are bolstered against harsh bloodstream conditions (Gasic et al., 1968). Since Gasic and colleagues first observed metastatic suppression via thrombocytopenia more than 50 years ago (Gasic et al., 1968), researchers have identified essential roles for platelets in CTC immune evasion, cell survival, and invasiveness (Nieswandt et al., 1999). While aggregation of CTCs with platelets is variable and largely dependent on the tumor type, this process is generally thought to enhance metastatic capabilities. Mechanistically, enhanced metastatic potential is mediated by diverse drivers including the induction of tumor cell proliferation, the facilitation of tumor cell extravasation, and the enhancement of tumor cell interactions with the extracellular matrix (Tsuruo et al., 1986). Importantly, CTC-platelet aggregation occurs within minutes of entry into the bloodstream, and plays a critical role in evasion of immune surveillance by “cloaking” CTCs from natural killer (NK) cells and in promoting survival in the face of harsh environmental factors (Nieswandt et al., 1999; Palumbo et al., 2005). CTCs have also been observed to interact with circulating immune cells such as neutrophils, which mediate cell cycle progression and increased CTC adhesion at secondary sites to facilitate metastatic seeding (Huh et al., 2010).
Given the contribution of CTC-platelet interaction to metastatic dissemination, significant effort has been put into identifying mechanisms to intervene in this association to clinically address metastasis. Currently, platelet inhibitors (Lou et al., 2015), platelet-mimicking drug delivery nanovehicles (Hu et al., 2015), and genetic engineering of platelets (Li et al., 2016) are being investigated to determine if disruption of CTC-platelet interactions might provide significant clinical benefit to cancer patients. Findings by Rowson-Hodel et al. that MUC4 is an essential component of platelet-CTC interactions suggest that MUC4 may be a viable therapeutic target for eliminating CTCs in the vasculature (Rowson-Hodel et al., 2018). Further evidence supporting the targeting of mucins during CTC transit in the vasculature comes from observations that sialic acid-containing glycans mediate tumor cell-platelet aggregation (Bastida et al., 1987), and a portion of mucin glycan side chains terminate in negatively-charged sialic acids (Linden et al., 2008). These observations implicate transmembrane mucins, such as MUC4, as important mediators of CTC-blood cell interactions critical to successful metastatic dissemination and highlight the need for a better understanding of the molecular mechanisms underlying CTC-blood cell interactions to identify druggable targets for clinically addressing metastasis.
Potential Therapeutic Strategies
Because CTC viability is a remarkably stringent barrier to metastatic efficiency; squeezing that bottleneck tighter by suppressing MUC4-mediated interactions of CTCs with other blood-borne cells offers a tremendous opportunity to intervene in metastatic efficiency. A MUC4/CTC-based strategy would necessarily involve the systemic delivery of an anti-MUC4 agent to CTCs via the vasculature, and such an agent would likely be a component of maintenance therapy following primary treatment. However, development of such a strategy will likely be hampered by high MUC4 expression in intestinal epithelial and vascular endothelial cells, each of which could serve as a competing sink for small molecule or biologics in delivery to tumors. Targeting transcriptional regulators of MUC4 expression is another possible path to therapeutic intervention. However, because such transcriptional regulators control broad expression programs in multiple tissue types, the advantage of tumor-specific MUC4 targeting is lost with this approach. Likewise, strategies to provoke cytokine-induced MUC4 degradation in CTCs also suffer from specificity issues, and strategies to deliver MUC4-targeting knockdown or knockout agents via CTC-directed nanoparticles could be rendered ineffective by the reported ability of highly-expressed MUC4 to interfere with the binding of large molecules and complexes to the cell surface (Nagy et al., 2005, Komatsu et al., 1997, Price-Schiavi et al., 2002). Thus, the most promising avenue for therapeutic intervention will likely involve the identification of the MUC4 binding components on blood-borne cells, and the inhibition of those contacts to reduce CTC viability. In this regard, it is reasonable to suspect that MUC4 is simply a major presenter of tumor cell-enriched glycan motifs recognized by lectins and other carbohydrate-binding proteins, and that glycomic studies with CTCs could provide key insight into the nature of intravascular cell-cell interactions as well as inform the development of novel therapeutic approaches.
Conclusions and Future Directions
Based on its structure, expression patterns, and function, MUC4 is beginning to emerge as an attractive target for therapeutic intervention into metastatic cancer. A large and growing body of evidence points to roles for aberrant MUC4 expression in the progression of a variety of solid tumor types. At the same time, studies of the full knockout mouse demonstrate that MUC4 is not essential for development, viability or fertility, and that its ablation has minimal impact on adult tissue morphology and function, strongly suggesting that systemic approaches for targeting MUC4 could elicit minimal side effects. While MUC4 appears to harbor an array of functions that might benefit the progression of a growing tumor, its role in promoting CTC viability is particularly intriguing. For example, observations from the study of patient-matched breast cancer patient samples and a genetically engineered mouse model of breast cancer indicate that the re-expression of MUC4 during the transition from primary to metastatic tumor drives metastatic efficiency at least in part by promoting CTC viability in circulation. This conclusion then raises the possibility that the process of MUC4-mediated CTC survival may be targeted for the benefit of breast cancer patients whose tumors are at risk of distant metastasis.
While the role of MUC4 in CTC-mediated breast cancer metastasis is most well-characterized, it is likely that MUC4 plays roles in CTC stabilization of other carcinomas as well. The immediate availability of a MUC4 knockout mouse with few discernable developmental phenotypes will facilitate analogous studies with genetically engineered mouse models of other tumor types. As with breast cancer, such studies will be most impactful when coupled with MUC4 expression surveys of patient-matched primary and metastatic lesions. For these studies it will be essential to employ immunohistochemical or other protein-based methods, as it has been demonstrated that MUC4 protein stability is highly dependent on factors produced by the microenvironment (Price-Schiavi et al., 1998; Price-Schiavi et al., 2000b; Lomako et al., 2009). Finally, a particularly interesting question concerns the role of MUC4 in CTC self-association, an issue not previously addressed in the MUC4 knockout study (Rowson-Hodel et al., 2018). Because clusters are 1-2 orders of magnitude more potent in seeding metastases than single CTCs, the identification of homotypic cell-cell contacts is an issue of immediate interest.
Funding
This work was supported by the NIH grants CA230742 and CA250211 (KLC), and NIH fellowship CA246900 (CAD).
Footnotes
Declaration of interest
The authors declare no conflicts of interest.
References
- ACETO N, BARDIA A, MIYAMOTO DT, DONALDSON MC, WITTNER BS, SPENCER JA, YU M, PELY A, ENGSTROM A, ZHU H, et al. 2014. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell, 158, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAE JS, LEE J, PARK Y, PARK K, KIM JR, CHO DH, JANG KY & PARK SH 2017. Attenuation of MUC4 potentiates the anticancer activity of auranofin via regulation of the Her2/Akt/FOXO3 pathway in ovarian cancer cells. Oncol Rep, 38, 2417–2425. [DOI] [PubMed] [Google Scholar]
- BAFNA S, KAUR S, MOMI N & BATRA SK 2009. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer, 101, 1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANSIL R & TURNER BS 2018. The biology of mucus: Composition, synthesis and organization. Adv Drug Deliv Rev, 124, 3–15. [DOI] [PubMed] [Google Scholar]
- BASTIDA E, ALMIRALL L, JAMIESON GA & ORDINAS A 1987. Cell surface sialylation of two human tumor cell lines and its correlation with their platelet-activating activity. Cancer Res, 47, 1767–70. [PubMed] [Google Scholar]
- CARRAWAY CC, KOMATSU M, JEPSON S, ARANGO M & CARRAWAY K 2001. Muc4\sialomucin Complex, A Specific Intramembrane Modulator of ERBB2/HER2/NEU, Potentiates Primary Tumor Growth and Suppresses Apoptosis in A Xenotransplanted Melanoma. ScientificWorldJournal, 1, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRAWAY KL 3RD, ROSSI EA, KOMATSU M, PRICE-SCHIAVI SA, HUANG D, GUY PM, CARVAJAL ME, FREGIEN N, CARRAWAY CA & CARRAWAY KL 1999. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem, 274, 5263–6. [DOI] [PubMed] [Google Scholar]
- CARRAWAY KL, RAMSAUER VP, HAQ B & CAROTHERS CARRAWAY CA 2003. Cell signaling through membrane mucins. Bioessays, 25, 66–71. [DOI] [PubMed] [Google Scholar]
- CHATURVEDI P, SINGH AP, CHAKRABORTY S, CHAUHAN SC, BAFNA S, MEZA JL, SINGH PK, HOLLINGSWORTH MA, MEHTA PP & BATRA SK 2008. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res, 68, 2065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATURVEDI P, SINGH AP, MONIAUX N, SENAPATI S, CHAKRABORTY S, MEZA JL & BATRA SK 2007. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res, 5, 309–20. [DOI] [PubMed] [Google Scholar]
- CHAUHAN SC, SINGH AP, RUIZ F, JOHANSSON SL, JAIN M, SMITH LM, MONIAUX N & BATRA SK 2006. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod Pathol, 19, 1386–94. [DOI] [PubMed] [Google Scholar]
- CHEN AC, MIGLIACCIO I, RIMAWI M, LOPEZ-TARRUELLA S, CREIGHTON CJ, MASSARWEH S, HUANG C, WANG YC, BATRA SK, GUTIERREZ MC, et al. 2012. Upregulation of mucin4 in ER-positive/HER2-overexpressing breast cancer xenografts with acquired resistance to endocrine and HER2-targeted therapies. Breast Cancer Res Treat, 134, 583–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN W, ZHANG Z, ZHANG S, ZHU P, KO JK & YUNG KK 2021. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. International Journal of Molecular Sciences, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUDHURY A, SINGH RK, MONIAUX N, EL-METWALLY TH, AUBERT JP & BATRA SK 2000. Retinoic acid-dependent transforming growth factor-beta 2-mediated induction of MUC4 mucin expression in human pancreatic tumor cells follows retinoic acid receptor-alpha signaling pathway. J Biol Chem, 275, 33929–36. [DOI] [PubMed] [Google Scholar]
- COPPIN L, VINCENT A, FRÉNOIS F, DUCHÊNE B, LAHDAOUI F, STECHLY L, RENAUD F, VILLENET C, VAN SEUNINGEN I, LETEURTRE E, et al. 2017. Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci Rep, 7, 43927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDA DG, DUYVERMAN AM, KOHNO M, SNUDERL M, STELLER EJ, FUKUMURA D & JAIN RK 2010. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A, 107, 21677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIDLER I & KRIPKE M 1977. Metastasis results from preexisting variant cells within a malignant tumor. Science, 197, 893–895. [DOI] [PubMed] [Google Scholar]
- FONTEBASSO Y & DUBINETT SM 2015. Drug Development for Metastasis Prevention. Crit Rev Oncog, 20, 449–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUNES M, MILLER JK, LAI C, CARRAWAY KL 3RD & SWEENEY C 2006. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem, 281, 19310–9. [DOI] [PubMed] [Google Scholar]
- GABBERT H, WAGNER R, MOLL R & GERHARZ CD 1985. Tumor dedifferentiation: an important step in tumor invasion. Clin Exp Metastasis, 3, 257–79. [DOI] [PubMed] [Google Scholar]
- GANESH K & MASSAGUÉ J 2021. Targeting metastatic cancer. Nat Med, 27, 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO L, LIU J, ZHANG B, ZHANG H, WANG D, ZHANG T, LIU Y & WANG C 2014. Functional MUC4 suppress epithelial-mesenchymal transition in lung adenocarcinoma metastasis. Tumour Biol, 35, 1335–41. [DOI] [PubMed] [Google Scholar]
- GASIC GJ, GASIC TB & STEWART CC 1968. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A, 61, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAY LJ & FELDING-HABERMANN B 2011. Contribution of platelets to tumour metastasis. Nat Rev Cancer, 11, 123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GKOUNTELA S, CASTRO-GINER F, SZCZERBA BM, VETTER M, LANDIN J, SCHERRER R, KROL I, SCHEIDMANN MC, BEISEL C, STIRNIMANN CU, et al. 2019. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell, 176, 98–112.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS MS, GUYONNET-DUPERAT V, PORCHET N, BERNHEIM A, AUBERT JP & NGUYEN VC 1992. Mucin 4 (MUC4) gene: regional assignment (3q29) and RFLP analysis. Ann Genet, 35, 21–6. [PubMed] [Google Scholar]
- HELM RM & CARRAWAY KL 1981. Evidence for the association of two cell surface glycoproteins of 13762 mammary ascites tumor cells. Concanavalin A-induced redistribution of peanut agglutinin-binding proteins. Exp Cell Res, 135, 418–24. [DOI] [PubMed] [Google Scholar]
- HU Q, SUN W, QIAN C, WANG C, BOMBA HN & GU Z 2015. Anticancer Platelet-Mimicking Nanovehicles. Adv Mater, 27, 7043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUH SJ, LIANG S, SHARMA A, DONG C & ROBERTSON GP 2010. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res, 70, 6071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDRIS N & CARRAWAY KL 1999. Sialomucin complex (Muc4) expression in the rat female reproductive tract. Biol Reprod, 61, 1431–8. [DOI] [PubMed] [Google Scholar]
- IDRIS N & CARRAWAY KL 2000. Regulation of sialomucin complex/Muc4 expression in rat uterine luminal epithelial cells by transforming growth factor-beta: implications for blastocyst implantation. J Cell Physiol, 185, 310–6. [DOI] [PubMed] [Google Scholar]
- JEPSON S, KOMATSU M, HAQ B, ARANGO ME, HUANG D, CARRAWAY CA & CARRAWAY KL 2002. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene, 21, 7524–32. [DOI] [PubMed] [Google Scholar]
- JONCKHEERE N, PERRAIS M, MARIETTE C, BATRA SK, AUBERT JP, PIGNY P & VAN SEUNINGEN I 2004. A role for human MUC4 mucin gene, the ErbB2 ligand, as a target of TGF-beta in pancreatic carcinogenesis. Oncogene, 23, 5729–38. [DOI] [PubMed] [Google Scholar]
- JONCKHEERE N, SKRYPEK N, MERLIN J, DESSEIN AF, DUMONT P, LETEURTRE E, HARRIS A, DESSEYN JL, SUSINI C, FRÉNOIS F, et al. 2012. The mucin MUC4 and its membrane partner ErbB2 regulate biological properties of human CAPAN-2 pancreatic cancer cells via different signalling pathways. PLoS One, 7, e32232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONCKHEERE N & VAN SEUNINGEN I 2018. Integrative analysis of the cancer genome atlas and cancer cell lines encyclopedia large-scale genomic databases: MUC4/MUC16/MUC20 signature is associated with poor survival in human carcinomas. J Transl Med, 16, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONCKHEERE N, VINCENT A, PERRAIS M, DUCOUROUBLE MP, MALE AK, AUBERT JP, PIGNY P, CARRAWAY KL, FREUND JN, RENES IB, et al. 2007. The human mucin MUC4 is transcriptionally regulated by caudal-related homeobox, hepatocyte nuclear factors, forkhead box A, and GATA endodermal transcription factors in epithelial cancer cells. J Biol Chem, 282, 22638–50. [DOI] [PubMed] [Google Scholar]
- JOSHI S, CRUZ E, RACHAGANI S, GUHA S, BRAND RE, PONNUSAMY MP, KUMAR S & BATRA SK 2016. Bile acids-mediated overexpression of MUC4 via FAK-dependent c-Jun activation in pancreatic cancer. Mol Oncol, 10, 1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR S, SHARMA N, KRISHN SR, LAKSHMANAN I, RACHAGANI S, BAINE MJ, SMITH LM, LELE SM, SASSON AR, GUHA S, et al. 2014. MUC4-mediated regulation of acute phase protein lipocalin 2 through HER2/AKT/NF-κB signaling in pancreatic cancer. Clin Cancer Res, 20, 688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMATSU M, CARRAWAY CA, FREGIEN NL & CARRAWAY KL 1997. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem, 272, 33245–54. [DOI] [PubMed] [Google Scholar]
- KOMATSU M, JEPSON S, ARANGO ME, CAROTHERS CARRAWAY CA & CARRAWAY KL 2001. Muc4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene, 20, 461–70. [DOI] [PubMed] [Google Scholar]
- KOMATSU M, YEE L & CARRAWAY KL 1999. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res, 59, 2229–36. [PubMed] [Google Scholar]
- KUFE DW 2009. Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer, 9, 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAHDAOUI F, DELPU Y, VINCENT A, RENAUD F, MESSAGER M, DUCHÊNE B, LETEURTRE E, MARIETTE C, TORRISANI J, JONCKHEERE N, et al. 2015. miR-219-1-3p is a negative regulator of the mucin MUC4 expression and is a tumor suppressor in pancreatic cancer. Oncogene, 34, 780–8. [DOI] [PubMed] [Google Scholar]
- LAKSHMANAN I, SESHACHARYULU P, HARIDAS D, RACHAGANI S, GUPTA S, JOSHI S, GUDA C, YAN Y, JAIN M, GANTI AK, et al. 2015. Novel HER3/MUC4 oncogenic signaling aggravates the tumorigenic phenotypes of pancreatic cancer cells. Oncotarget, 6, 21085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERT AW, PATTABIRAMAN DR & WEINBERG RA 2017. Emerging Biological Principles of Metastasis. Cell, 168, 670–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI G, ZHAO L, LI W, FAN K, QIAN W, HOU S, WANG H, DAI J, WEI H & GUO Y 2014a. Feedback activation of STAT3 mediates trastuzumab resistance via upregulation of MUC1 and MUC4 expression. Oncotarget, 5, 8317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J, SHARKEY CC, WUN B, LIESVELD JL & KING MR 2016. Genetic engineering of platelets to neutralize circulating tumor cells. J Control Release, 228, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI P, PRICE-SCHIAVI SA, RUDLAND PS & CARRAWAY KL 2001. Sialomucin complex (rat Muc4) transmembrane subunit binds the differentiation marker peanut lectin in the normal rat mammary gland. J Cell Physiol, 186, 397–405. [DOI] [PubMed] [Google Scholar]
- LI W, WU C, YAO Y, DONG B, WEI Z, LV X, ZHANG J & XU Y 2014b. MUC4 modulates human glioblastoma cell proliferation and invasion by upregulating EGFR expression. Neurosci Lett, 566, 82–7. [DOI] [PubMed] [Google Scholar]
- LINDEN SK, SUTTON P, KARLSSON NG, KOROLIK V & MCGUCKIN MA 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol, 1, 183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMAKO WM, LOMAKO J, SOTO P, CARRAWAY CA & CARRAWAY KL 2009. TGFbeta regulation of membrane mucin Muc4 via proteosome degradation. J Cell Biochem, 107, 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOU XL, SUN J, GONG SQ, YU XF, GONG R & DENG H 2015. Interaction between circulating cancer cells and platelets: clinical implication. Chin J Cancer Res, 27, 450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMURA Y, ITO Y, MEZAWA Y, SULIDAN K, DAIGO Y, HIRAGA T, MOGUSHI K, WALI N, SUZUKI H, ITOH T, et al. 2019. Stromal fibroblasts induce metastatic tumor cell clusters via epithelial-mesenchymal plasticity. Life Sci Alliance, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNEER RR, CARRAWAY CA, FREGIEN NL & CARRAWAY KL 1998. Characterization of the expression and steroid hormone control of sialomucin complex in the rat uterus: implications for uterine receptivity. J Cell Physiol, 176, 110–9. [DOI] [PubMed] [Google Scholar]
- MEJÍAS-LUQUE R, PEIRÓ S, VINCENT A, VAN SEUNINGEN I & DE BOLÓS C 2008. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim Biophys Acta, 1783, 1728–36. [DOI] [PubMed] [Google Scholar]
- MERCOGLIANO MF, DE MARTINO M, VENTURUTTI L, RIVAS MA, PROIETTI CJ, INURRIGARRO G, FRAHM I, ALLEMAND DH, DEZA EG, ARES S, et al. 2017a. TNFα-Induced Mucin 4 Expression Elicits Trastuzumab Resistance in HER2-Positive Breast Cancer. Clin Cancer Res, 23, 636–648. [DOI] [PubMed] [Google Scholar]
- MERCOGLIANO MF, INURRIGARRO G, DE MARTINO M, VENTURUTTI L, RIVAS MA, CORDO-RUSSO R, PROIETTI CJ, FERNÁNDEZ EA, FRAHM I, BARCHUK S, et al. 2017b. Invasive micropapillary carcinoma of the breast overexpresses MUC4 and is associated with poor outcome to adjuvant trastuzumab in HER2-positive breast cancer. BMC Cancer, 17, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICALIZZI DS, MAHESWARAN S & HABER DA 2017. A conduit to metastasis: circulating tumor cell biology. Genes Dev, 31, 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMEAULT M, JOHANSSON SL, SENAPATI S, MOMI N, CHAKRABORTY S & BATRA SK 2010. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett, 295, 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONIAUX N, CHATURVEDI P, VARSHNEY GC, MEZA JL, RODRIGUEZ-SIERRA JF, AUBERT JP & BATRA SK 2007. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer, 97, 345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONIAUX N, NOLLET S, PORCHET N, DEGAND P, LAINE A & AUBERT JP 1999. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J, 338 (Pt 2), 325–33. [PMC free article] [PubMed] [Google Scholar]
- MUKHOPADHYAY P, LAKSHMANAN I, PONNUSAMY MP, CHAKRABORTY S, JAIN M, PAI P, SMITH LM, LELE SM & BATRA SK 2013. MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS One, 8, e54455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGY P, FRIEDLÄNDER E, TANNER M, KAPANEN AI, CARRAWAY KL, ISOLA J & JOVIN TM 2005. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res, 65, 473–82. [PubMed] [Google Scholar]
- NATH S & MUKHERJEE P 2014. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends in Molecular Medicine, 20, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIESWANDT B, HAFNER M, ECHTENACHER B & MÄNNEL DN 1999. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res, 59, 1295–300. [PubMed] [Google Scholar]
- NOLLET S, MONIAUX N, MAURY J, PETITPREZ D, DEGAND P, LAINE A, PORCHET N & AUBERT JP 1998. Human mucin gene MUC4: organization of its 5'-region and polymorphism of its central tandem repeat array. Biochem J, 332 (Pt 3), 739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAI P, RACHAGANI S, DHAWAN P, SHEININ YM, MACHA MA, QAZI AK, CHUGH S, PONNUSAMY MP, MALLYA K, POTHURAJU R, et al. 2016a. MUC4 is negatively regulated through the Wnt/β-catenin pathway via the Notch effector Hath1 in colorectal cancer. Genes Cancer, 7, 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAI P, RACHAGANI S, LAKSHMANAN I, MACHA MA, SHEININ Y, SMITH LM, PONNUSAMY MP & BATRA SK 2016b. The canonical Wnt pathway regulates the metastasis-promoting mucin MUC4 in pancreatic ductal adenocarcinoma. Mol Oncol, 10, 224–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALUMBO JS, TALMAGE KE, MASSARI JV, LA JEUNESSE CM, FLICK MJ, KOMBRINCK KW, JIROUSKOVÁ M & DEGEN JL 2005. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood, 105, 178–85. [DOI] [PubMed] [Google Scholar]
- PANTEL K & SPEICHER MR 2016. The biology of circulating tumor cells. Oncogene, 35, 1216–24. [DOI] [PubMed] [Google Scholar]
- PEREZ-VILAR J & HILL RL 1999. The structure and assembly of secreted mucins. J Biol Chem, 274, 31751–4. [DOI] [PubMed] [Google Scholar]
- PEREZ A, BARCO R, FERNANDEZ I, PRICE-SCHIAVI SA & CARRAWAY KL 2003. PEA3 transactivates the Muc4/sialomucin complex promoter in mammary epithelial and tumor cells. J Biol Chem, 278, 36942–52. [DOI] [PubMed] [Google Scholar]
- PERRAIS M, PIGNY P, DUCOUROUBLE MP, PETITPREZ D, PORCHET N, AUBERT JP & VAN SEUNINGEN I 2001. Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinflammatory cytokines for its regulation in pancreatic cancer cells. J Biol Chem, 276, 30923–33. [DOI] [PubMed] [Google Scholar]
- PINO V, RAMSAUER VP, SALAS P, CAROTHERS CARRAWAY CA & CARRAWAY KL 2006. Membrane mucin Muc4 induces density-dependent changes in ERK activation in mammary epithelial and tumor cells: role in reversal of contact inhibition. J Biol Chem, 281, 29411–20. [DOI] [PubMed] [Google Scholar]
- PONNUSAMY MP, LAKSHMANAN I, JAIN M, DAS S, CHAKRABORTY S, DEY P & BATRA SK 2010. MUC4 mucin-induced epithelial to mesenchymal transition: a novel mechanism for metastasis of human ovarian cancer cells. Oncogene, 29, 5741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONNUSAMY MP, SESHACHARYULU P, VAZ A, DEY P & BATRA SK 2011. MUC4 stabilizes HER2 expression and maintains the cancer stem cell population in ovarian cancer cells. J Ovarian Res, 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONNUSAMY MP, SINGH AP, JAIN M, CHAKRABORTY S, MONIAUX N & BATRA SK 2008. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer, 99, 520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORCHET N, DUFOSSE J, AUDIE JP, DUPERAT VG, PERINI JM, NGUYEN VC, DEGAND P & AUBERT JP 1991. Structural features of the core proteins of human airway mucins ascertained by cDNA cloning. Am Rev Respir Dis, 144, S15–8. [DOI] [PubMed] [Google Scholar]
- PRICE-SCHIAVI SA, CARRAWAY CA, FREGIEN N & CARRAWAY KL 1998. Post-transcriptional regulation of a milk membrane protein, the sialomucin complex (Ascites sialoglycoprotein (ASGP)-1/ASGP-2, rat muc4), by transforming growth factor beta. J Biol Chem, 273, 35228–37. [DOI] [PubMed] [Google Scholar]
- PRICE-SCHIAVI SA, JEPSON S, LI P, ARANGO M, RUDLAND PS, YEE L & CARRAWAY KL 2002. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer, 99, 783–91. [DOI] [PubMed] [Google Scholar]
- PRICE-SCHIAVI SA, PEREZ A, BARCO R & CARRAWAY KL 2000a. Cloning and characterization of the 5' flanking region of the sialomucin complex/rat Muc4 gene: promoter activity in cultured cells. Biochem J, 349, 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRICE-SCHIAVI SA, ZHU X, AQUININ R & CARRAWAY KL 2000b. Sialomucin complex (rat Muc4) is regulated by transforming growth factor beta in mammary gland by a novel post-translational mechanism. J Biol Chem, 275, 17800–7. [DOI] [PubMed] [Google Scholar]
- RACHAGANI S, MACHA MA, PONNUSAMY MP, HARIDAS D, KAUR S, JAIN M & BATRA SK 2012. MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis, 33, 1953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAKHA EA, BOYCE RW, ABD EL-REHIM D, KURIEN T, GREEN AR, PAISH EC, ROBERTSON JF & ELLIS IO 2005. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol, 18, 1295–304. [DOI] [PubMed] [Google Scholar]
- RAMSAUER VP, CARRAWAY CA, SALAS PJ & CARRAWAY KL 2003. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, translocates ErbB2 to the apical surface in polarized epithelial cells. J Biol Chem, 278, 30142–7. [DOI] [PubMed] [Google Scholar]
- RAMSAUER VP, PINO V, FAROOQ A, CAROTHERS CARRAWAY CA, SALAS PJ & CARRAWAY KL 2006. Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell, 17, 2931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYMOND N, D'ÁGUA BB & RIDLEY AJ 2013. Crossing the endothelial barrier during metastasis. Nat Rev Cancer, 13, 858–70. [DOI] [PubMed] [Google Scholar]
- ROSSI EA, MCNEER RR, PRICE-SCHIAVI SA, VAN DEN BRANDE JM, KOMATSU M, THOMPSON JF, CARRAWAY CA, FREGIEN NL & CARRAWAY KL 1996. Sialomucin complex, a heterodimeric glycoprotein complex. Expression as a soluble, secretable form in lactating mammary gland and colon. J Biol Chem, 271, 33476–85. [DOI] [PubMed] [Google Scholar]
- ROWSON-HODEL AR, WALD JH, HATAKEYAMA J, O'NEAL WK, STONEBRAKER JR, VANDERVORST K, SALDANA MJ, BOROWSKY AD, SWEENEY C & CARRAWAY KL 3RD 2018. Membrane Mucin Muc4 promotes blood cell association with tumor cells and mediates efficient metastasis in a mouse model of breast cancer. Oncogene, 37, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCULLY OJ, BAY B-H, YIP G & YU Y 2012. Breast Cancer Metastasis. Cancer Genomics - Proteomics, 9, 311–320. [PubMed] [Google Scholar]
- SENAPATI S, GNANAPRAGASSAM VS, MONIAUX N, MOMI N & BATRA SK 2012. Role of MUC4-NIDO domain in the MUC4-mediated metastasis of pancreatic cancer cells. Oncogene, 31, 3346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHENG Z, WU K, CARRAWAY KL & FREGIEN N 1992. Molecular cloning of the transmembrane component of the 13762 mammary adenocarcinoma sialomucin complex. A new member of the epidermal growth factor superfamily. J Biol Chem, 267, 16341–6. [PubMed] [Google Scholar]
- SHENG ZQ, HULL SR & CARRAWAY KL 1990. Biosynthesis of the cell surface sialomucin complex of ascites 13762 rat mammary adenocarcinoma cells from a high molecular weight precursor. J Biol Chem, 265, 8505–10. [PubMed] [Google Scholar]
- SHERBLOM AP, BUCK RL & CARRAWAY KL 1980a. Purification of the major sialoglycoproteins of 13762 MAT-B1 and MAT-C1 rat ascites mammary adenocarcinoma cells by density gradient centrifugation in cesium chloride and guanidine hydrochloride. J Biol Chem, 255, 783–90. [PubMed] [Google Scholar]
- SHERBLOM AP & CARRAWAY KL 1980. A complex of two cell surface glycoproteins from ascites mammary adenocarcinoma cells. J Biol Chem, 255, 12051–9. [PubMed] [Google Scholar]
- SHERBLOM AP, HUGGINS JW, CHESNUT RW, BUCK RL, OWNBY CL, DERMER GB & CARRAWAY KL 1980b. Cell surface properties of ascites sublines of the 13762 rat mammary adenocarcinoma. Relationship of the major sialoglycoprotein to xenotransplantability. Exp Cell Res, 126, 417–26. [DOI] [PubMed] [Google Scholar]
- SHERBLOM AP & MOODY CE 1986. Cell surface sialomucin and resistance to natural cell-mediated cytotoxicity of rat mammary tumor ascites cells. Cancer Res, 46, 4543–6. [PubMed] [Google Scholar]
- SHET T, VALSANGAR S & DHENDE S 2013. Secretory carcinoma of breast: pattern of MUC 2/MUC 4/MUC 6 expression. Breast J, 19, 222–4. [DOI] [PubMed] [Google Scholar]
- SHIBAHARA H, TAMADA S, GOTO M, ODA K, NAGINO M, NAGASAKA T, BATRA SK, HOLLINGSWORTH MA, IMAI K, NIMURA Y, et al. 2004a. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol, 28, 327–38. [DOI] [PubMed] [Google Scholar]
- SHIBAHARA H, TAMADA S, HIGASHI M, GOTO M, BATRA SK, HOLLINGSWORTH MA, IMAI K & YONEZAWA S 2004b. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology, 39, 220–9. [DOI] [PubMed] [Google Scholar]
- SINGH AP, MONIAUX N, CHAUHAN SC, MEZA JL & BATRA SK 2004. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res, 64, 622–30. [DOI] [PubMed] [Google Scholar]
- SOTO P, PRICE-SCHIAVI SA & CARRAWAY KL 2003. SMAD2 and SMAD7 involvement in the post-translational regulation of Muc4 via the transforming growth factor-beta and interferon-gamma pathways in rat mammary epithelial cells. J Biol Chem, 278, 20338–44. [DOI] [PubMed] [Google Scholar]
- SOTO P, ZHANG J & CARRAWAY KL 2006. Enzymatic cleavage as a processing step in the maturation of Muc4/sialomucin complex. J Cell Biochem, 97, 1267–74. [DOI] [PubMed] [Google Scholar]
- SPIELMAN J, ROCKLEY NL & CARRAWAY KL 1987. Temporal aspects of O-glycosylation and cell surface expression of ascites sialoglycoprotein-1, the major cell surface sialomucin of 13762 mammary ascites tumor cells. J Biol Chem, 262, 269–75. [PubMed] [Google Scholar]
- SRIVASTAVA SK, BHARDWAJ A, SINGH S, ARORA S, WANG B, GRIZZLE WE & SINGH AP 2011. MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis, 32, 1832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEEG PS 2016. Targeting metastasis. Nat Rev Cancer, 16, 201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALMADGE JE & FIDLER IJ 2010. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res, 70, 5649–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG J, ZHU Y, XIE K, ZHANG X, ZHI X, WANG W, LI Z, ZHANG Q, WANG L, WANG J, et al. 2016. The role of the AMOP domain in MUC4/Y-promoted tumour angiogenesis and metastasis in pancreatic cancer. J Exp Clin Cancer Res, 35, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUO T, KAWABATA H, IIDA H & YAMORI T 1986. Tumor-induced platelet aggregation and growth promoting factors as determinants for successful tumor metastasis. Clin Exp Metastasis, 4, 25–33. [DOI] [PubMed] [Google Scholar]
- VAN KLINKEN BJ, DEKKER J, BÜLLER HA & EINERHAND AW 1995. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol, 269, G613–27. [DOI] [PubMed] [Google Scholar]
- VANDERVORST K, DREYER CA, KONOPELSKI SE, LEE H, HO HH & CARRAWAY KL 3RD 2019. Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis. Cancer Res, 79, 1719–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VASSEUR R, SKRYPEK N, DUCHÊNE B, RENAUD F, MARTÍNEZ-MAQUEDA D, VINCENT A, PORCHET N, VAN SEUNINGEN I & JONCKHEERE N 2015. The mucin MUC4 is a transcriptional and post-transcriptional target of K-ras oncogene in pancreatic cancer. Implication of MAPK/AP-1, NF-κB and RalB signaling pathways. Biochim Biophys Acta, 1849, 1375–84. [DOI] [PubMed] [Google Scholar]
- VINCENT A, DUCOUROUBLE MP & VAN SEUNINGEN I 2008. Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. Faseb j, 22, 3035–45. [DOI] [PubMed] [Google Scholar]
- WANG C, MU Z, CHERVONEVA I, AUSTIN L, YE Z, ROSSI G, PALAZZO JP, SUN C, ABU-KHALAF M, MYERS RE, et al. 2017. Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat, 161, 83–94. [DOI] [PubMed] [Google Scholar]
- WEED DT, GOMEZ-FERNANDEZ C, BONFANTE E, LEE TD, PACHECO J, CARVAJAL ME, GOODWIN WJ & CARRAWAY KL 2001. MUC4 (sialomucin complex) expression in salivary gland tumors and squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Head Neck Surg, 124, 127–41. [DOI] [PubMed] [Google Scholar]
- WEIGELT B, PETERSE JL & VAN'T VEER LJ 2005. Breast cancer metastasis: markers and models. Nature Reviews Cancer, 5, 591–602. [DOI] [PubMed] [Google Scholar]
- WORKMAN HC, MILLER JK, INGALLA EQ, KAUR RP, YAMAMOTO DI, BECKETT LA, YOUNG LJ, CARDIFF RD, BOROWSKY AD, CARRAWAY KL, et al. 2009a. The membrane mucin MUC4 is elevated in breast tumor lymph node metastases relative to matched primary tumors and confers aggressive properties to breast cancer cells. Breast Cancer Res, 11, R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORKMAN HC, SWEENEY C & CARRAWAY KL 3RD 2009b. The membrane mucin Muc4 inhibits apoptosis induced by multiple insults via ErbB2-dependent and ErbB2-independent mechanisms. Cancer Res, 69, 2845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU K, SALAS PJ, YEE L, FREGIEN N & CARRAWAY KL 1994. Tissue and tumor expression of a cell surface glycoprotein complex containing an integral membrane glycoprotein activator of p185neu. Oncogene, 9, 3139–47. [PubMed] [Google Scholar]
- XU D, LIU S, ZHANG L & SONG L 2017. MiR-211 inhibits invasion and epithelial-to-mesenchymal transition (EMT) of cervical cancer cells via targeting MUC4. Biochem Biophys Res Commun, 485, 556–562. [DOI] [PubMed] [Google Scholar]
- YAMADA N, NISHIDA Y, TSUTSUMIDA H, GOTO M, HIGASHI M, NOMOTO M & YONEZAWA S 2009. Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer, 100, 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG J, PEREZ A, YASIN M, SOTO P, RONG M, THEODOROPOULOS G, CAROTHERS CARRAWAY CA & CARRAWAY KL 2005. Presence of MUC4 in human milk and at the luminal surfaces of blood vessels. J Cell Physiol, 204, 166–77. [DOI] [PubMed] [Google Scholar]
- ZHI X, TAO J, XIE K, ZHU Y, LI Z, TANG J, WANG W, XU H, ZHANG J & XU Z 2014. MUC4-induced nuclear translocation of β-catenin: a novel mechanism for growth, metastasis and angiogenesis in pancreatic cancer. Cancer Lett, 346, 104–13. [DOI] [PubMed] [Google Scholar]
- ZHU X, PRICE-SCHIAVI SA & CARRAWAY KL 2000. Extracellular regulated kinase (ERK)-dependent regulation of sialomucin complex/rat Muc4 in mammary epithelial cells. Oncogene, 19, 4354–61. [DOI] [PubMed] [Google Scholar]
- ZHU Z, QIU S, SHAO K & HOU Y 2018. Progress and challenges of sequencing and analyzing circulating tumor cells. Cell Biol Toxicol, 34, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]