Abstract
Objective:
Type 1 diabetes (T1D) is characterized by autoimmune β-cell destruction, but exocrine pancreas abnormalities may also play a role in the disease pathophysiology. Herein, we review the current evidence of exocrine damage in T1D and discuss its underlying pathophysiology, clinical evaluation, and treatment.
Method:
Extensive literature search was performed for “type 1 diabetes” and “exocrine dysfunction” on PubMed and Google Scholar databases.
Results:
T1D pancreata are significantly smaller than controls, both in weight and volume. T cells, dendritic cells, neutrophils, and products of complement activation are seen in T1D exocrine tissues. Exocrine pancreas fibrosis, arteriosclerosis, fatty infiltration, and acinar atrophy are also observed on histology. Pancreatic exocrine insufficiency (PEI) can be assessed through direct exocrine testing, fecal elastase concentration, and measurement of serum exocrine enzymes. The prevalence of PEI in T1D varies by modality and study but is consistently greater than controls. The clinical relevance of PEI in T1D is debatable, as many patients with laboratory evidence of PEI are asymptomatic. However, in PEI-symptomatic patients reported benefits of pancreatic enzyme replacement therapy (PERT) include relief of gastrointestinal symptoms, improved quality of life, better glycemic control, and optimal nutrition.
Conclusion:
Exocrine pancreas abnormalities often occur in T1D. Whether exocrine dysfunction occurs simultaneously with β-cell destruction, as a result of β-cell loss, or as a combination of both remains to be definitively answered. In T1D with gastrointestinal complaints, PEI should be evaluated, usually via fecal elastase measurements. PERT is recommended for T1D patients with symptoms and laboratory evidence of PEI.
INTRODUCTION
Type 1 diabetes mellitus (T1D) is an autoimmune endocrine disorder characterized by T-cell–mediated destruction of insulin-producing β-cells in the pancreatic islets. The underlying etiology has yet to be fully elucidated, but evidence supports an environmental nidus with genetic predisposition (1). Pancreatic endocrine deficiency in T1D leads to hallmark hyperglycemia, and while subclinical for most patients, exocrine function may also be affected. Nearly a century has passed since the well-known discovery of insulin, but observations related to potential exocrine dysfunction in T1D predate even the groundbreaking effort by Banting et al (2). Famed pathologist, Dr. Russell Cecil, noted interacinar inflammation and decreased pancreas size in the diabetic pancreas in 1909 (3). In 1925, exocrine dysfunction in T1D patients was described by Harvard gastroenterologist Dr. Chester Jones (4). Since then, additional abnormalities in the exocrine pancreas in T1D have been observed, including reductions in volume, fibrosis, arteriosclerosis, immune cell infiltration, acinar atrophy, and reduced amylase, lipase, and trypsinogen concentrations (3,5–33). This review summarizes the evidence documenting pancreatic exocrine dysfunction in T1D, explores possible causes, and integrates clinical relevance for endocrine practitioners. Finally, we discuss the treatment of pancreatic insufficiency in T1D patients and areas for future research.
EMBRYOLOGY AND ANATOMY OF THE PANCREAS
The human pancreas is formed at week 5 of gestation and starts as separate ventral (comprising part of the head and uncinate region) and dorsal anlagen (comprising remaining part of the head, and entire body and tail) before rotation and merging during week 7 (34). Both exocrine and endocrine pancreata arise from a common progenitor of the foregut endoderm (35). The exocrine compartment of the pancreas accounts for over 95% of pancreatic mass. The base exocrine unit is called the acinus, comprised of acinar, centroacinar, and ductal epithelial cells. A lobule consists of hundreds of acini connected through intercalated ducts. Lobules drain via intralobular ducts, which come together at interlobular ducts and empty into the main pancreatic duct. The main pancreatic duct joins the accessory pancreatic duct and common bile duct to enter the duodenum at the ampulla of Vater (34). Acinar cells in immediate proximity to the islets are called peri-islets; those that are distal are termed tele-islets. Islets are intralobular and highly vascularized, receiving up to 20% of arterial blood supply to the pancreas despite representing approximately 2% of its mass. Islet efferent vessels drain into lobular capillaries or veins, creating an isletacinar portal system (35). Neurons of the sympathetic nervous system innervate the blood vessels of the pancreas via the splanchnic nerves and lead to vasoconstriction as well as reduced exocrine pancreas blood flow when stimulated (36).
NORMAL PHYSIOLOGY OF THE EXOCRINE PANCREAS
Acinar cells produce and store digestive (pro)enzymes, including proteases (trypsinogen, chymotrypsin, elastase, etc.), amylase, lipase, and nucleases, in zymogens. Fatty and amino acids in chyme stimulate intestinal release of cholecystokinin (CCK), a neuro-enteroendocrine hormone. CCK activates the vagus nerve leading to cholinergic stimulation of acinar cells and consequent release of zymogen granules (35,37). Secretin and a decrease in duodenal pH from chyme cause ductal cells to release sodium, chloride, bicarbonate, and water into the pancreatic ducts via aquaporin and cystic fibrosis transmembrane conductance regular channels (37,38).
The endocrine pancreas may also influence its exocrine counterpart, presumably by the aforementioned islet-acinar vascular axis. Islets are composed of endocrine cells, divided into five primary types. Over 75% of islet cells in the dorsal pancreatic lineage are β-cells, which produce insulin and amylin (while islets in the ventral lineage are mostly γ-cells). α-Cells produce glucagon, δ-cells produce somatostatin, γ-cells (also termed PP or F cells) produce pancreatic polypeptide (PP), and ε-cells produce ghrelin (34,39). As β-cells are the largest contributor to the islet-acinar axis, insulin may regulate exocrine function. High local insulin concentrations potentiate overall pancreatic growth (40). This may explain the histologic peri-insular “halos” comprised of acinar cells with larger size, zymogen granules, and nuclei (17,41,42). Insulin binds to receptors on acinar cells that stimulate growth, amylase synthesis, and secretion (43,44). PP, somatostatin, ghrelin, and many other lesser-known pancreatic hormones (adrenomedullin, neuropeptide Y, peptide YY, etc.) inhibit exocrine secretion (35). Glucagon’s effect on the exocrine pancreas is more complex, depending on the chronicity of stimulation and whether stimuli are received in a fasting versus fed state. Chronic glucagon exposure may lead to acinar atrophy (45). In a fasting or starved state (such as that of untreated diabetes), glucagon inhibits exocrine secretion but may stimulate secretion during satiation via amino acid stimulation (40,46). Thus the altered hormone profile in T1D likely impedes effective exocrine function.
THE EXOCRINE PANCREAS IN PATIENTS WITH DIABETES
The pancreata in all types of diabetes have been extensively studied (Table 1). The size of the pancreas in individuals with T1D is significantly smaller than controls despite β-cells only comprising a small percentage of pancreatic mass (14). Compared to age-matched controls, the weight of postmortem T1D pancreata is reduced by 35 to 45% (6,20,22,33). Imaging studies of those living with T1D have demonstrated that the volume of the pancreas is reduced 18 to 52% compared to controls, depending on method (i.e., ultrasound, computed tomography, or magnetic resonance imaging) (5,7,9,13,15,21,24,28,31,32). These data provide strong evidence to support the hypothesis that T1D is not just a disease of the endocrine compartment but also affects the pancreas at large.
Table 1.
Changes in the Exocrine Pancreas and Enzymes in T1D
| Changes | Significant findings | Source (Reference) |
|---|---|---|
| Reduced pancreas sizea. | • 35%, mean weight at autopsy aged 14 and over • 52%, median area via ultrasound • 37%, mean volume at autopsy • 32%, mean PVI via CT • 31%, mean PVI via MRI • 45%, mean weight from cadaveric organ donors • 26%, mean PVIc via MRI • 33%, mean PVI via CT • 27%, mean volume via MRI • 47%, mean volume via MRI or CT; 4/25 T1D with ~6% progressive volume loss per year • 41%, median volume via MRI; 7.2% relative volume loss over first year of new onset T1D • 18%, mean transverse area via ultrasound • 22%, mean RPV of recent onset via MRI • 45%, mean weight from cadaveric organ donors |
MacLean 1959 (22) Fonseca 1985 (9) Löhr 1987 (20) Goda 2001 (15) Gaglia 2011 (13) Campbell-Thompson 2012 (6) Williams 2012 (31) Lu 2016 (21) Regnell 2016 (24) Virostko 2016 (28) Virostko 2019 (32) Augustine 2019 (5) Campbell-Thompson 2019 (7) Wright 2020 (33) |
| Acinar atrophy | • Compressed, atrophic glandular acini; many acini replaced with connective tissue • Small acini with depleted zymogens in prolonged diabetes • Severe atrophy with smaller acinar cells • Acinar atrophy in 21% • Overall acinar area reduced compared to controls and AAb+ • Less acinar cells, but similar in size |
Cecil 1908 (3) Foulis 1986 (10) Löhr 1987 (20) Waguri 1997 (29) Tang 2020c Wright 2020 (33) |
| Fatty infiltration & Fibrosis | • Adipose infiltration of stroma; adipose tissue replaced acini in 11%; fibrosis around vessels and ducts; 24% significant sclerosis, worse with age • Intra-lobular and peri-lobular sclerosis, worse with chronicity • Fatty changes in 32%; Inter-acinar fibrosis in 66% • Fatty degeneration and fibrosis, worse with age, irrespective of T1D duration • Increased fibrosis: thickened inter-acinar septa and intraparenchymal deposits of connective tissue |
Cecil 1908 (3) Gepts 1965 (14) Waguri 1997 (29) Bonnet-Serrano 2018 (46) Wright 2020 (33) |
| Immune histopathology | • Lymphoid, eosinophil, plasma, and PMNs • PMNs in 6/119; diffuse lymphocytes in 9/119 cases • Peri-insular lymphocytes, reticular cells, PMNs • Lymphocytic infiltration in 47% • Increased C4d (product of complement) in vascular endothelium and extracellular matrix • Neutrophils in exocrine pancreas in new onset and chronic cases • High numbers of cytotoxic T-cells, helper T-cells, and monocytes |
Cecil 1908 (3) Foulis 1986 (10) Gepts 1965 (14) Waguri 1997 (29) Rowe 2013 (26) Valle 2013 (27) Rodriguez-Calvo 2014 (25) |
| Vascular changes | • Thickening of pancreatic vessels with small arteriole destruction, worse with age • Arteriosclerosis, worse with chronicity • Microvasculature has smaller diameters & increased density in insulin negative islets, but no difference in exocrine vessel diameter/density |
Cecil 1908 (3) Gepts 1965 (14) Canzano 2019 (51) |
| Decreased exocrine enzymesb | • 54% ↓ amylase (DEF) • 65% ↓ amylase, 80% ↓ trypsin (DEF) • 78% ↓ trypsin (DEF) • 36% ↓ amylase, 37% ↓ lipase, 26% ↓ trypsin (DEF) • Significantly lower trypsinogen levels in T1D and multiple AAb+ subjects |
Pollard 1943 (23) Frier 1976 (12) Frier 1980 (11) Lankisch 1982 (18) Li 2017 (19) |
Abbreviations: AAb+ = autoantibody positive; CT = computed tomography; DEF = direct exocrine function testing; IDD = insulin-dependent diabetic; MRI = magnetic resonance imaging; PVI = pancreatic volume index (volume/body surface area or volume/body weightc); PMN = polymorphonuclear leukocyte; RPV = relative pancreatic volume (volume/body mass index); T1D = type 1 diabetes.
Percentage reduced compared to control subjects.
Percentages of T1D patients with decreased levels.
Unpublished.
Immune cells have been found in the exocrine pancreas in T1D, supporting a model of generalized pancreatic inflammation. T cells may contribute to destruction of the endocrine pancreas; however, along with dendritic cells, T cells are also reported to be present in exocrine tissues (3,10,14,25,29,30,39). Neutrophils and products of complement activation have also been found throughout the tissues of some T1D pancreata, suggesting evidence of innate immune system involvement (3,10,14,16,26,27,39). Other histologic changes shown in nonendocrine pancreatic tissue in T1D include fibrosis, fatty infiltration, and arteriosclerosis (3,10,14,20,29,33,47). Fibrosis is usually present in an inter-acinar fashion and associated with concomitant arteriosclerosis (3,33). Fibrosis and arteriosclerosis may be mediated by mesenchymal pancreatic stellate cells that potentiate fibrosis in the setting of hyperglycemia and hypoxia (48,49). Acinar atrophy is also observed in patients with prolonged duration of diabetes (10,20,29,33,50). Most recently, acinar cells were found to have similar size and proliferation but overall decreased number in T1D donors, regardless of time of diabetes onset (33). These observations provide further evidence in support of the assertion that exocrine dysfunction is an important, though often unrecognized, component of T1D pathophysiology.
Studies comparing T1D pancreata to those in type 2 diabetes, autoantibody positive (AAb+) (pre-T1D), and autoantibody negative (AAb−) nondiabetic controls may provide additional clues to the genesis of the disease processes. We have shown that T1D donors lacking insulin-positive islet cells had reduced acinar tissue area compared to both AAb+ subjects and AAb− controls, suggesting that decreased acinar size may not occur until after the onset of symptomatic T1D (X. Tang, unpublished data under review, 2020). In contrast, significantly smaller pancreata were found in all first-degree relatives of T1D patients (smallest in AAb+) as compared to controls, positing that a baseline smaller pancreas is a risk factor for T1D from a shared genetic or environmental background (7). In another study, we demonstrated that serum trypsinogen levels, a reflection of exocrine function, were low in T1D individuals, with multiple AAb+ individuals also having reduced values compared to single AAb+ subjects and AAb− controls. These data suggest that exocrine dysfunction may evolve as progression to T1D occurs but may not be clinically apparent until the late pre-T1D phase (19).
UNDERLYING MECHANISMS OF EXOCRINE DYSFUNCTION
It remains to be determined whether T1D is a disorder of primarily β-cell loss (an effect of losing β-cells and insulin production) or associated with disease of the entire pancreas (from a shared primary mechanism).
Argument for Exocrine Dysfunction Secondary to β-Cell Loss
The effects of primary β-cell loss leading to subsequent exocrine damage can be divided into the categories of (1) endocrine dysfunction, (2) vascular damage, and (3) autonomic neuropathy:
Endocrine dysfunction: Insulin produced by β-cells may act as a trophic factor to acini within the isletacinar portal system (40). In the absence of high local insulin concentrations, acinar growth and enzyme production are reduced. This is supported by the observation that there is a greater reduction in body and tail size of the pancreas in T1D, compared to the head, where PP cells dominate islets instea d of β-cells (51). Unopposed glucagon and somatostatin release further contribute to inhibiting exocrine secretion (35).
Vascular damage: Islets receive 10 times more pancreatic blood flow than exocrine pancreatic tissues. In longstanding T1D, β-cell death and subsequent hyperglycemia further diminishes pancreatic blood supply through arteriosclerosis (3,14). This may lead to disruption of the islet-acinar portal system and in turn, acinar ischemia. The microvasculature of insulin-negative islets in T1D have smaller diameters and increased density compared to controls yet have similar peri-islet exocrine vessel characteristics (52). However, pericytes, mural cells that surrounds islet capillary endothelium, also control local blood flow. As β-cells release insulin, ATP is released, causing pericytes to relax and capillaries to dilate (53). While further studies are needed, it can be hypothesized that in the absence of β-cells, pericytes remain contracted, reducing overall flow of pancreatic capillaries and decreasing perfusion.
Autonomic neuropathy: As cholinergic input is vital for the cephalic phase of digestion to stimulate acini to release zymogens, disruption in this pathway may contribute to exocrine dysfunction in T1D (37). Islets have a complex autonomic innervation network that also interacts with acinar cells, though those details are beyond the scope of this review (54). As hyperglycemia creates oxidative stress and can therefore induce nerve damage, the vagus network, the neuronal stimulus for zymogen release, can be blunted by diabetes (55). Gastrointestinal autonomic neuropathy may, therefore, coexist with exocrine insufficiency.
Argument for Primary Pancreatic Disorder Causing Exocrine Dysfunction and Diabetes
Inflammatory cells seen in both the islets and exocrine pancreas of T1D patients support more generalized pancreatic involvement prior to T1D onset (10,25). Exocrine AAb have also been observed in T1D. AAb to exocrine proteins including lactoferrin, carbonic anhydrase II (in ductal cells), amylase alpha-2A, pancreatic cytokeratin, and bile salt-dependent lipase (BSDL) are reported significantly more often in T1D compared to controls (56–59). Panicot et al (57) found that BSDL AAb were also present in T1D first-degree relatives and those with prediabetes even before GAD65, IA-2, and insulin AAb were detected. Islet cell autoantigen (ICA69) is present in islets and also in acinar and ductal tissues (60). Whether exocrine AAb are pathogenic or more likely, a marker of inflammation, still needs to be explored. Significant destruction of β-cells with generalized pancreatic inflammation may occur due to a genetic predisposition for cellular fragility (61). Polymorphisms in genes that control apoptosis, DNA repair, and mitochondrial function have been identified in T1D patients and could result in reduced β-cell survivability in inflammatory states (62,63). T1D may, thus, occur in the setting of both exocrine and endocrine immunemediated inflammation but with pronounced, persistent destruction of especially fragile β-cells in high-risk genotypes (61).
Heterogeneity of Diabetes and Exocrine Dysfunction: Endotypes
The endotype hypothesis combines all of the suspected etiologies by acknowledging that T1D actually encompasses a heterogeneous group of pathologies (61,64). An endotype refers to a subtype of T1D that has a unique pathophysiology, such as cohorts with different human leukocyte antigen haplotypes or groups with distinct types of CD20 infiltration (64). A delineation of specific endotypes is beyond the scope of this review, but the concept is important to note, as each may affect the exocrine pancreas differently. As such, there may be variation in the severity, causes, and effects of exocrine dysfunction in T1D.
EVALUATION AND MANAGEMENT OF EXOCRINE INSUFFICIENCY IN T1D
The true prevalence of pancreatic exocrine insufficiency (PEI) in T1D patients is difficult to assess. Jones et al (4) first reported decreased enzyme activity after a cream meal in 49% (69% in those under 30 years of age) of patients with diabetes. Later studies of PEI prevalence by direct testing has been reported in up to 80% of T1D patients based on trypsin measurement (12). Stimulated tests, such as secretin or cholecystokinin testing, are the gold standard for diagnosis of PEI. However, these difficult tests require duodenal intubation for hours (8,11,12,18,23,39,65,66). Newer, less-invasive testing has been developed to assess PEI involving enzyme levels in serum, saliva, urine, or stool, with fecal elastase concentration (FEC) being the most studied and clinically available (67–71). The standard cut-off for PEI in FEC is 200 μg/g, which allows for a sensitivity of 96% and specificity of 88% based on pooled results from 6 studies (72). Of note, using FEC for PEI can result in false positives in the presence of other disease states, such as diarrhea from infection or celiac disease, necessitating that other causes are simultaneously evaluated and ruled out (73).
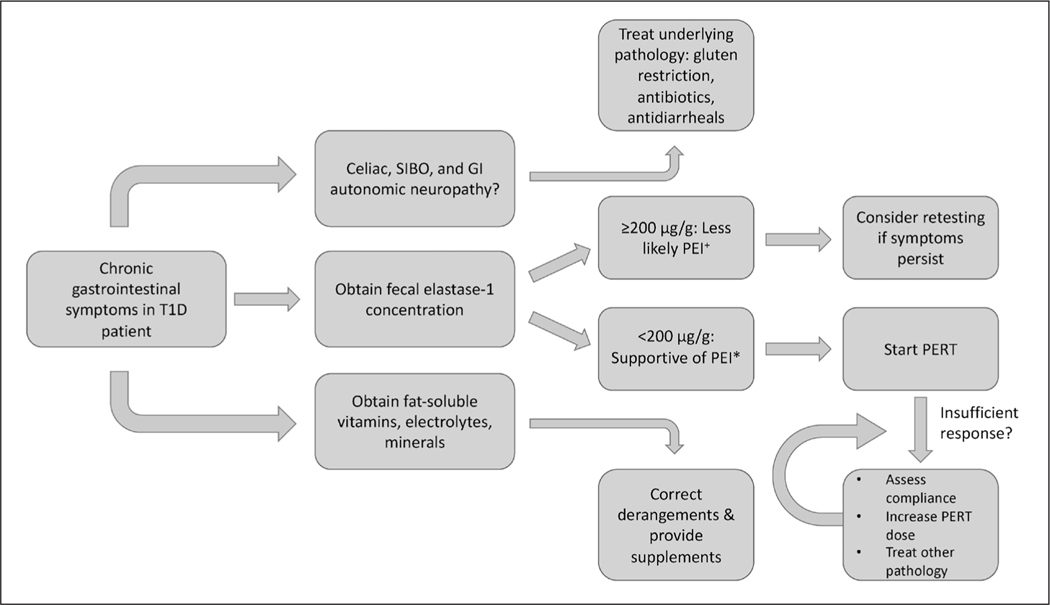
PEI in T1D clinically presents with diarrhea, bloating, cramping, and/or weight loss, largely from steatorrhea (74). However, these symptoms can be attributed to other complications of diabetes, such as gastrointestinal autonomic neuropathy (i.e., gastroparesis), steatorrhea from celiac disease, or bacterial overgrowth of the proximal small bowel (65,67). As such, the evaluation of gastrointestinal symptoms in T1D patients often requires further testing. Evaluation for celiac disease is especially warranted, as the risk is about 5 times greater in T1D patients than the general population. Celiac disease can be differentiated from PEI by elevated anti-tissue transglutaminase antibodies (or other celiac autoantibodies) and definitively diagnosed by small bowel biopsy (75). The clinical relevance of PEI in T1D is still largely unknown, as many patients with laboratory evidence of PEI are actually asymptomatic (67). One study found 42% of patients with diabetes (type 1 and 2) with gastrointestinal symptoms had low FEC-1, but they did not report if there was treatment with significant symptom improvement (74). Studies demonstrating benefits in treating asymptomatic T1D patients are lacking; thus, testing for PEI via FEC-1 should be reserved for those with chronic gastrointestinal symptoms (Fig. 1). Interestingly, in a follow-up study of 13 patients with a normal secretin-pancreozymin test, only 2 (~15%) developed abnormal results on repeat testing 11 years later, suggesting that if PEI is absent early in the course of T1D, it is unlikely to develop in longstanding disease (8).
Fig. 1.
Proposed algorithm for evaluation and treatment of pancreatic exocrine insufficiency (PEI) in type 1 diabetes (T1D).
+Mild PEI may still have fecal elastase concentration over 200 μg/g.
*False positives may result from other diarrheal diseases that dilute stool.
GI = gastrointestinal; PERT = pancreatic enzyme replacement therapy; SIBO = small intestinal bacterial overgrowth.
The treatment of PEI is pancreatic enzyme replacement therapy (PERT) given as pancrelipase or pancreatin, a combination of protease, amylase, and lipase (Fig. 1). Possible benefits of treatment include resolution of gastrointestinal symptoms, improved quality of life, better glycemic control, decreased maldigestion-induced hypoglycemia, and optimal absorption of fat soluble vitamins (A, D, E, K) (76–79). Replacement of any vitamin or mineral deficiencies should occur simultaneously with PERT. Dosing of PERT was largely derived from cystic fibrosis patients and is represented as lipase units. Recommended starting doses are 1,000 lipase units/kg/meal for children under 4 years of age, 500 units/kg/meal for children older than 4 years of age, and 20,000 to 40,000 units per meal for adults, titrated to clinical response to a maximum of 10,000 units/kg/day or 4,000 units per gram of fat consumed per day (77,80). The most common side effect of PERT is constipation, but large fluctuations in blood sugar and insulin requirements may occur when starting PERT in T1D patients due to improved digestion of starch (77,81). Continuous glucose monitors may be especially useful to curtail such variations and establish a new insulin regimen safely and efficiently.
CONCLUSION
The past century has revealed more about the intricacies of diabetes as advances in pathology, immunology, and radiology have resulted in better modalities of studying the pancreas. As our knowledge has progressed, additional questions into the pathophysiology of diabetes continue to arise. The exocrine component of the pancreas is significantly affected in T1D, both histologically and clinically. However, as discussed in this article, the sequence of pancreatic insults is debated, and more longitudinal studies are needed to further characterize how exocrine dysfunction occurs in T1D. As evidence of clear benefits of PERT in patients with subclinical PEI is lacking, stool studies should be reserved for patients with clinical symptoms. Unknowingly combining unique endotypes of T1D patients into one homogenous disease group clouds the picture of the intricate relationship between the exocrine and endocrine pancreas in T1D. The future of diabetes care may involve identification of a patient’s specific endotype with associated risk of concomitant exocrine disease, tailoring treatment to the individual while striving for a means to prevent and cure the disease.
ACKNOWLEDGMENT
This study was supported by the National Institutes of Health (NIH) (P01 AI-42288, DP3 DK101120–01, OT2 OD023861, 1R01 DK122160); the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID-SCR_014541), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018–557-Q-R), and The Leona M. & Harry B. Helmsley Charitable Trust (Grant# 2018PG-T1D053); the Jeffrey Keene Family Professorship, and the McJunkin Family Charitable Foundation. The Type 1 Diabetes TrialNet Study Group is a clinical trials network currently funded by the NIH through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01DK061010, U01DK061034, U01DK061042, U01DK061058, U01DK085461, U01DK085465, U01DK085466, U01DK085476, U01DK085499, U01DK085509, U01DK103180, U01DK103153, U01DK103266, U01DK103282, U01DK106984, U01DK106994, U01DK107013, U01DK107014, UC4DK106993, and UC4DK117009.
Abbreviations:
- AAb+
autoantibody positive
- AAb−
autoantibody negative
- FEC
fecal elastase concentration
- PEI
pancreatic exocrine insufficiency
- PERT
pancreatic enzyme replacement therapy
- PP
pancreatic polypeptide
- T1D
type 1 diabetes
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014; 383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banting FG, Best CH, Collip JB, Macleod JJ, Noble EC. The effect of pancreatic extract (insulin) on normal rabbits. Am J Physiol-Legacy Content. 1922;62:162–176. [Google Scholar]
- 3.Cecil RL. A study of the pathological anatomy of the pancreas in ninety cases of diabetes mellitus. J Exp Med. 1909;11:266–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones CM, Castle WB, Mulholland HB, Bailey F. Pancreatic and hepatic activity in diabetes mellitus: the alterations with some observations on the etiology of the disease. Arch Intern Med. 1925;35:315–336. [Google Scholar]
- 5.Augustine P, Gent R, Louise J, et al. Pancreas size and exocrine function is decreased in young children with recent-onset type 1 diabetes. Diabet Med. 2020;37:1340–1343. [DOI] [PubMed] [Google Scholar]
- 6.Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308:2337–2339. [DOI] [PubMed] [Google Scholar]
- 7.Campbell-Thompson ML, Filipp SL, Grajo JR, et al. Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care. 2019;42:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creutzfeldt W, Gleichmann D, Otto J, Stöckmann F, Maisonneuve P, Lankisch PG. Follow-up of exocrine pancreatic function in type-1 diabetes mellitus. Digestion. 2005;72:71–75. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca V, Berger LA, Beckett AG, Dandona P. Size of pancreas in diabetes mellitus: a study based on ultrasound. Br Med J (Clin Res Ed). 1985;291:1240–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type I (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29:267–274. [DOI] [PubMed] [Google Scholar]
- 11.Frier BM, Adrian TE, Saunders JH, Bloom SR. Serum trypsin concentration and pancreatic trypsin secretion in insulin-dependent diabetes mellitus. Clin Chim Acta. 1980;105:297–300. [DOI] [PubMed] [Google Scholar]
- 12.Frier BM, Saunders JH, Wormsley KG, Bouchier IA. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut. 1976;17:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaglia JL, Guimaraes AR, Harisinghani M, et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–633. [DOI] [PubMed] [Google Scholar]
- 15.Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, Hahafusa T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta Diabetol. 2001;38:145–149. [DOI] [PubMed] [Google Scholar]
- 16.Korsgren S, Molin Y, Salmela K, Lundgren T, Melhus A, Korsgren O. On the etiology of type 1 diabetes: a new animal model signifying a decisive role for bacteria eliciting an adverse innate immunity response. Am J Pathol. 2012;181:1735–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusmartseva I, Beery M, Hiller H, et al. Temporal analysis of amylase expression in control, autoantibody-positive, and type 1 diabetes pancreatic tissues. Diabetes. 2020;69:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lankisch PG, Manthey G, Otto J, et al. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion. 1982;25:211–216. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Campbell-Thompson M, Wasserfall CH, et al. Serum trypsinogen levels in type 1 diabetes. Diabetes Care. 2017;40: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Löhr M, Klöppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30:757–762. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Hou X, Pang C, et al. Pancreatic volume is reduced in patients with latent autoimmune diabetes in adults. Diabetes Metab Res Rev. 2016;32:858–866. [DOI] [PubMed] [Google Scholar]
- 22.Maclean N, Ogilvie RF. Observations on the pancreatic islet tissue of young diabetic subjects. Diabetes. 1959;8:83–91. [DOI] [PubMed] [Google Scholar]
- 23.Pollard HM, Miller L, Brewer WA. The external secretion of the pancreas and diabetes mellitus. Am J Dig Dis. 1943;10:20–23. [Google Scholar]
- 24.Regnell SE, Peterson P, Trinh L, et al. Pancreas volume and fat fraction in children with type 1 diabetes. Diabet Med. 2016;33:1374–1379. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe P, Wasserfall C, Croker B, et al. Increased complement activation in human type 1 diabetes pancreata. Diabetes Care. 2013;36:3815–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valle A, Giamporcaro GM, Scavini M, et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62:2072–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virostko J, Hilmes M, Eitel K, Moore DJ, Powers AC. Use of the electronic medical record to assess pancreas size in type 1 diabetes. PLoS One. 2016;11:e0158825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waguri M, Hanafusa T, Itoh N, et al. Histopathologic study of the pancreas shows a characteristic lymphocytic infiltration in Japanese patients with IDDM. Endocr J. 1997;44:23–33. [DOI] [PubMed] [Google Scholar]
- 30.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AJK, Thrower SL, Sequeiros IM, et al. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E2109–E2113. [DOI] [PubMed] [Google Scholar]
- 32.Virostko J, Williams J, Hilmes M, et al. Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care. 2019;42:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JJ, Saunders DC, Dai C, et al. Decreased pancreatic acinar cell number in type 1 diabetes. Diabetologia. 2020;63: 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longnecker DS.Anatomy and Histology of the Pancreas. Pancreapedia: The Exocrine Pancreas Knowledge Base; 2014. [Google Scholar]
- 35.Pandiri AR. Overview of exocrine pancreatic pathobiology. Toxicol Pathol. 2014;42:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babic T, Travagli RA.Neural Control of the Pancreas.Pancreapedia: The Exocrine Pancreas Knowledge Base; 2016. [Google Scholar]
- 37.Hart P, Conwell D.Secretion of the Human Exocrine Pancreas in Health and Disease. Pancreapedia: The Exocrine Pancreas Knowledge Base; 2017. [Google Scholar]
- 38.Arsenijevic T, Perret J, Van Laethem JL, Delporte C. Aquaporins involvement in pancreas physiology and in pancreatic diseases. Int J Mol Sci. 2019;20:5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M. Abnormalities of the exocrine pancreas in type 1 diabetes. Curr Diab Rep. 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut. 1981;22:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cosnier J. Peculiar image of the exocrine peri-insular zones in the white rat, the peri-insular halo [in French]. C R Seances Soc Biol Fil. 1955;149:263–265. [PubMed] [Google Scholar]
- 42.Aughsteen AA, Kataoka K. Morphometric studies on the juxta-insular and tele-insular acinar cells of the pancreas in normal and streptozotocin-induced diabetic rats. J Electron Microsc (Tokyo). 1993;42:79–87. [PubMed] [Google Scholar]
- 43.Söling HD, Unger KO. The role of insulin in the regulation of -amylase synthesis in the rat pancreas. Eur J Clin Invest. 1972;2:199–212. [DOI] [PubMed] [Google Scholar]
- 44.Mössner J, Logsdon CD, Williams JA, Goldfine ID. Insulin, via its own receptor, regulates growth and amylase synthesis in pancreatic acinar AR42J cells. Diabetes. 1985;34:891–897. [DOI] [PubMed] [Google Scholar]
- 45.Salter JM, Davidson IW, Best CH. The pathologic effects of large amounts of glucagon. Diabetes. 1957;6:248–255. [DOI] [PubMed] [Google Scholar]
- 46.Shaw HM, Heath TJ. The effect of glucagon on the formation of pancreatic juice and bile in the rat. Can J Physiol Pharmacol. 1973;51:1–5. [DOI] [PubMed] [Google Scholar]
- 47.Bonnet-Serrano F, Diedisheim M, Mallone R, Larger E. Decreased α-cell mass and early structural alterations of the exocrine pancreas in patients with type 1 diabetes: an analysis based on the nPOD repository. PLoS One. 2018;13:e0191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nomiyama Y, Tashiro M, Yamaguchi T, et al. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas. 2007;34: 364–372. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe S, Nagashio Y, Asaumi H, et al. Pressure activates rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1175–G1181. [DOI] [PubMed] [Google Scholar]
- 50.Owyang C. Endocrine changes in pancreatic insufficiency. The Pancreas: Biology, Pathobiology and Disease. New York, NY: Raven; 1993: 8803–8813. [Google Scholar]
- 51.Campbell-Thompson ML, Kaddis JS, Wasserfall C, et al. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canzano JS, Nasif LH, Butterworth EA, Fu DA, Atkinson MA, Campbell-Thompson M. Islet microvasculature alterations with loss of beta-cells in patients with type 1 diabetes. J Histochem Cytochem. 2019;67:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab. 2018;27:630–644.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barreto SG, Carati CJ, Toouli J, Saccone GT. The isletacinar axis of the pancreas: more than just insulin. Am J Physiol Gastrointest Liver Physiol. 2010;299:G10–G22. [DOI] [PubMed] [Google Scholar]
- 55.Feldman EL, Nave KA, Jensen TS, Bennett DL. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Endo T, Takizawa S, Tanaka S, Takahashi M, Fujii H, Kamisawa T, Kobayashi T. Amylase alpha-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant type 1 diabetes. Diabetes. 2009;58:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Panicot L, Mas E, Thivolet C, Lombardo D. Circulating antibodies against an exocrine pancreatic enzyme in type 1 diabetes. Diabetes. 1999;48:2316–2323. [DOI] [PubMed] [Google Scholar]
- 58.Taniguchi T, Okazaki K, Okamoto M, et al. High prevalence of autoantibodies against carbonic anhydrase II and lactoferrin in type 1 diabetes: concept of autoimmune exocrinopathy and endocrinopathy of the pancreas. Pancreas. 2003;27:26–30. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi T, Nakanishi K, Kajio H, et al. Pancreatic cytokeratin: an antigen of pancreatic exocrine cell autoantibodies in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33: 363–370. [DOI] [PubMed] [Google Scholar]
- 60.Mally MI, Cirulli V, Hayek A, Otonkoski T. ICA69 is expressed equally in the human endocrine and exocrine pancreas. Diabetologia. 1996;39:474–480. [DOI] [PubMed] [Google Scholar]
- 61.Vecchio F, Messina G, Giovenzana A, Petrelli A. New evidence of exocrine pancreatopathy in pre-symptomatic and symptomatic type 1 diabetes. Curr Diab Rep. 2019;19:92. [DOI] [PubMed] [Google Scholar]
- 62.Dooley J, Tian L, Schonefeldt S, et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet. 2016;48:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexandre-Heymann L, Mallone R, Boitard C, Scharfmann R, Larger E. Structure and function of the exocrine pancreas in patients with type 1 diabetes. Rev Endocr Metab Disord. 2019;20:129–149. [DOI] [PubMed] [Google Scholar]
- 66.Diamond JS, Siegel SA, Kantor JL. The secretin test in the diagnosis of pancreatic diseases with a report of one hundred thirty tests. Am J Dig Dis. 1940;7:435–442. [Google Scholar]
- 67.Cavalot F, Bonomo K, Fiora E, Bacillo E, Salacone P, Chirio M, et al. Does pancreatic elastase-1 in stools predict steatorrhea in type 1 diabetes? Diabetes Care. 2006;29:719–721. [DOI] [PubMed] [Google Scholar]
- 68.Hardt PD, Hauenschild A, Nalop J, et al. High prevalence of exocrine pancreatic insufficiency in diabetes mellitus. Pancreatology. 2003;3:395–402. [DOI] [PubMed] [Google Scholar]
- 69.Icks A, Haastert B, Giani G, Rathmann W. Low fecal elastase-1 in type I diabetes mellitus. Z Gastroenterol. 2001;39:823–830. [DOI] [PubMed] [Google Scholar]
- 70.Canaway S, Phillips I, Betts P. Pancreatic exocrine insufficiency and type 1 diabetes mellitus. Br J Nurs. 2000;9:2030–2032. [DOI] [PubMed] [Google Scholar]
- 71.Larger E, Philippe MF, Barbot-Trystram L, et al. Pancreatic exocrine function in patients with diabetes. Diabet Med. 2012;29:1047–1054. [DOI] [PubMed] [Google Scholar]
- 72.Vanga RR, Tansel A, Sidiq S, El-Serag HB, Othman MO. Diagnostic performance of measurement of fecal elastase-1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1220–1228.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol. 2011;8:405–415. [DOI] [PubMed] [Google Scholar]
- 74.Cummings MH, Chong L, Hunter V, Kar PS, Meeking DR, Cranston IC. Gastrointestinal symptoms and pancreatic exocrine insufficiency in type 1 and type 2 diabetes. Pract Diabetes. 2015;32:54–58. [Google Scholar]
- 75.Type Goodwin G. 1 diabetes mellitus and celiac disease: distinct autoimmune disorders that share common pathogenic mechanisms. Horm Res Paediatr. 2019;92:285–292. [DOI] [PubMed] [Google Scholar]
- 76.Ewald N, Bretzel RG, Fantus IG, et al. Pancreatin therapy in patients with insulin-treated diabetes mellitus and exocrine pancreatic insufficiency according to low fecal elastase 1 concentrations. Results of a prospective multi-Centre trial. Diabetes Metab Res Rev. 2007;23:386–391. [DOI] [PubMed] [Google Scholar]
- 77.Löhr J-M, Oliver MR, Frulloni L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United European Gastroenterol J. 2013;1:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Working Party of the Australasian Pancreatic Club, Smith RC, Smith SF, et al. Summary and recommendations from the Australasian guidelines for the management of pancreatic exocrine insufficiency. Pancreatology. 2016;16:164–180. [DOI] [PubMed] [Google Scholar]
- 79.Piciucchi M, Capurso G, Archibugi L, Delle Fave MM, Capasso M, Delle Fave G. Exocrine pancreatic insufficiency in diabetic patients: prevalence, mechanisms, and treatment. Int J Endocrinol. 2015;2015:595649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee CKK. Drug dosages. In: Hughes H, Kahl L, eds. The Harriet Lane Handbook: A Manual for Pediatric House Officers. 21st ed. Philadelphia, PA: Elsevier; 2018: 1004–1005. [Google Scholar]
- 81.O’Keefe SJ, Cariem AK, Levy M. The exacerbation of pancreatic endocrine dysfunction by potent pancreatic exocrine supplements in patients with chronic pancreatitis. J Clin Gastroenterol. 2001;32:319–323. [DOI] [PubMed] [Google Scholar]