Abstract
Background:
Frailty may occur at younger ages among HIV+ populations. We evaluated associations of frailty status with self-reported single and recurrent falls in the Women’s Interagency HIV Study (WIHS).
Methods:
Frailty status was defined using the Fried Frailty Phenotype (FFP) among 897 HIV+ and 392 HIV- women; median age 53 years. Women were classified as robust (FFP 0), prefrail (FFP 1–2) and frail (FFP 3–5). Stepwise logistic regression models adjusting for HIV status and study site were fit to evaluate associations of FFP with self-reported single (1 vs. 0) and recurrent falls (≥2 vs. 0) over the prior 12 months.
Results:
HIV+ women were less likely to be frail (9% vs.14% vs. p=0.009), but frequency of falls did not differ by HIV status. In multivariate analyses, recurrent falls were more common among prefrail [adjusted odds ratio (AOR) 2.23, 95%CI: 1.40 – 3.57, p=0.0008] and frail (AOR 3.61, 95%CI: 1.90 – 6.89, p<0.0001) than robust women. Among HIV+ women, single (AOR 2.88, 95%CI: 1.16–7.20, p=0.023) and recurrent falls (AOR 3.50, 95%CI: 1.24–9.88, p= 0.018) were more common among those who were frail; recurrent, but not single falls, were more common among prefrail than robust HIV+ women (AOR 2.00, 95%CI: 1.03–3.91, p= 0.042).
Conclusions:
HIV+ women were less likely to be frail. Compared to robust women, prefrail and frail women with and without HIV were more likely to experience single or recurrent falls within a 12-month period. Additional studies are needed to develop interventions that decrease development of frailty and reduce risk of recurrent falls among HIV+ women.
Keywords: Fall, frailty, HIV, aging, women
INTRODUCTION
Falls are a growing and significant public health problem in the U.S, with one of four U.S. residents aged ≥65 years sustaining a fall annually, leading to approximately 7 million fall injuries and 3 million fall-related emergency department visits annually.1 Falls are the leading cause of fatal and nonfatal injuries among persons aged ≥65 years2 and alarmingly, national trends show that between 2007 to 2016 the rate of deaths from falls increased by 30%.3 Women are more likely to report falling than men, and nonfatal fall-related injuries including fracture also disproportionately affect women.2,4
Frailty reflects a decrease in physiologic reserves leading to increased vulnerability to stressors and has been frequently defined in cohort studies by use of a frailty phenotype, characterized by slowness, weakness, weight loss, fatigue, and low physical activity.5–7 Risk factors for falls, such as reduced muscle strength and mobility, share characteristics of frailty.8 Prevalence of frailty increases with age, is higher among women, and has been associated with greater risk of falls9,10 and recurrent falls in community-dwelling older men11 and women.12
As the lifespan of people with HIV (PWH) in the U.S. approaches that of uninfected persons, PWH are experiencing a high burden of conditions associated with aging, including geriatric syndromes such as falls, frailty, and sarcopenia.13 Emerging data show an unexpectedly high rate of falls among middle-aged PWH, who appear to have similar rates of falls as uninfected persons who are decades older.14–16 Women with HIV appear to be at greater risk for falls than men with HIV after taking into account other risk factors for falling.17 We previously reported that among middle-aged women in the Women’s Interagency HIV Study (WIHS), (median age 42 years HIV+ and 39 years HIV-), frailty independently predicted recurrent, but not single, falls occurring over a two-year period 10 years after frailty assessment.18 We now undertake the current study to determine whether the Fried Frailty Phenotype (FFP) is associated with short-term (12 months) frequency of falls among participants in the WIHS, including associations between individual frailty components and falls.
METHODS
Study Population
The WIHS is an ongoing, multicenter cohort study of the natural and treated history of HIV in women. Enrollment occurred initially at six consortia (Bronx/Manhattan NY, Brooklyn NY, Chicago IL, Washington DC, San Francisco CA, and Los Angeles, CA) in 1994–95, and subsequently in 2001–02 and 2011–12. In 2014–15, the WIHS added four southern U.S. sites: Atlanta GA, Chapel Hill NC, Miami FL, and Birmingham AL/Jackson MI. WIHS methods and baseline cohort characteristics have been described previously.19 At semiannual visits, participants completed face-to-face interviews and physical examinations, and provided biological specimens. Written informed consent was obtained using procedures approved by the investigational review board at each of the collaborating institutions.
Frailty Definition
Frailty was defined using the FFP, which has been utilized in numerous population-based studies and cohorts including the WIHS.5,7,20 The FFP is comprised of five components: 1) slow gait, 2) reduced grip strength, 3) exhaustion, 4) unintentional weight loss of 10 pounds within 6 months, and 5) low physical activity. Gait speed was measured with a timed four-meter walk and the faster of two attempts was used for analysis. Grip strength was measured using a hand-held Jamar dynamometer in the dominant hand and the highest value (greatest strength in kg) of three attempts was used for analysis. Cut points for slow gait (adjusted for body height) and reduced grip strength [adjusted for body mass index (BMI)] from the Cardiovascular Health Study (CHS).5,7 The presence of exhaustion, weight loss, and low physical activity were based on self- report.20,21 A participant was categorized as frail with 3–5 FFP components, pre-frail with 1–2 FFP components, and robust with no FFP components (FFP 0).22
Falls Ascertainment
Beginning in 2014, WIHS participants reported occurrence of falls within the prior 6 months at each semiannual study visit. The current analyses included participants who completed frailty assessments and falls questionnaires from 2 semiannual visits over a 12-month period (falls questionnaires were completed at the same visit in which frailty assessment occurred as well as at the preceding WIHS study visit, from October 2017 to March 2018). A fall was defined as “an unexpected event, including a slip or trip, in which you lost your balance and landed on the floor, ground or lower level, or hit an object like a table or chair.” Participants were instructed not to include falls resulting from a major medical event (for example, a stroke or seizure) or an overwhelming external hazard (for example, hit by a truck or pushed).23 Participants reporting at least one fall were then asked whether they had “1” or “2 or more” falls in the prior 6 months, sought medical attention for any of these falls, or if any of these falls resulted in a fracture.
Statistical Analyses
Demographics were measured at WIHS enrollment; other covariates were time invariant and measured at the visit when frailty was assessed (referred to as the index visit). Candidate covariates for multivariable models included sociodemographics, substance use factors, comorbid conditions, central nervous system (CNS) active medications, and HIV-related factors, as follows: Sociodemographics: age per 10 years, race/ethnicity (White, Hispanic/Other, vs. Black [reference]), annual household income ≤$12,000; having graduated from high school; and year of WIHS enrollment (2001–2, 2011–14, vs. 1994–5). Substance use: tobacco use (current smoker, former smoker, vs. never smoker [reference]); cocaine, crack, and/or heroin use (current, former, vs. never [reference]); marijuana use (current, former, vs. never [reference]); and recent (six month) alcohol use: heavy (≥14 drinks/week), moderate (3–13 drinks/week), or light (<3 drinks/week) vs. none [reference]). CNS active medication classes: anticonvulsants, antidepressants, antipsychotics, sedatives (including benzodiazepines, barbiturates, and non- benzodiazepine sleep aids), and muscle relaxants; number of CNS active medications were summed and analyzed as 0, 1, 2, or 3+ medications. Comorbid conditions: peripheral neuropathy (self-report of numbness, tingling, or burning sensations in arms, legs, hands or feet lasting for more than two weeks); obesity (BMI >30 kg/m2); subjective cognitive complaints (self-report of major problems with memory or concentration that interfered with normal everyday activities and lasted for more than two weeks, or self-report of confusion, getting lost in a familiar place or inability to perform routine mental tasks); depressive symptoms [modified Center for Epidemiology Studies Depression (CES-D) score ≥15,18,24,25 modified by excluding these two symptoms that overlap with the FFP: ‘this past week I could not get going’ (overlaps with low physical activity) and ‘this past week everything was an effort’ (overlaps with exhaustion)], diabetes mellitus as previously operationalized in WIHS;26 renal dysfunction (estimated glomerular filtration rate (eGFR) <60 ml/min using the Modification of Diet in Renal Disease calculation);27 hypertension (self-reported hypertension with systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of an antihypertensive medication);28 and Hepatitis C Virus (HCV) infection (HCV antibody positive with detectable RNA).
Additional HIV disease specific covariates included: nadir CD4+ count (lowest CD4 measured at or prior to index visit) and current (at index visit) CD4+ count, current log10 HIV RNA level, current suppressed (<80 copies/mL) HIV RNA, current/prior AIDS-defining illness (ADI), current antiretroviral therapy (ART) use.
Medians, interquartile ranges, and proportions summarized study variables. Chi-square tests compared proportions of categorized variables between HIV-positive (HIV+) and HIV- seronegative (HIV-) women and proportions between those with no fall, single fall (defined as one fall during the 12-month period), and recurrent falls (defined as 2 or more falls during the 12- month period). Bivariate analyses also evaluated each factor in association with single fall (vs. no fall) and recurrent falls (vs. no fall), for HIV+ and HIV- participants together and separately using univariate logistic regression.
Stepwise logistic regression models determined independent associations with odds of falling over 12 months for a single fall (1 vs. 0) or recurrent falls (≥2 vs. 0), allowing other variables shown in Table 1 (except FFP components) to enter and remain in the model if p<0.05. Primary outcomes were report of single fall and report of recurrent falls over 12 months, compared with no fall. HIV status and frailty status (the primary exposures of interest) were forced into models, and all models were additionally adjusted for study site. Separate multivariable models restricted to women with HIV were constructed similarly and additionally evaluated the contribution of ART and disease-specific characteristics on odds of falls. To evaluate the associations of individual FFP components and falls, we constructed logistic regression models excluding FFP and instead allowed each of the five frailty characteristics to enter the stepwise regression.22,29,30
Table 1.
Characteristics of 897 HIV+ and 332 HIV- WIHS Participants at Index Visit
| Characteristic, N (%) | HIV-infected (N=897) | HIV-uninfected (N=332) | P value* |
|---|---|---|---|
| Age at index visit, years, mean ±SD | 52.7 ±7.2 | 52.9 ± 7.8 | 0.84 |
| Education level high school or greater | 593 (66%) | 218 (66%) | 0.86 |
| Annual Income ≥$12,000/yr | 445 (50%) | 158 (48%) | 0.54 |
| Enrollment year | |||
| 94–95 | 288 (32%) | 113 (34%) | 0.61 |
| 01–02 | 213 (24%) | 83 (25%) | |
| 11–15 | 396 (44%) | 136 (41%) | |
| WIHS Site | 0.043 | ||
| Bronx/Manhattan | 121 (14%) | 67 (20%) | |
| Brooklyn | 117 (13%) | 36 (11%) | |
| Washington DC | 137 (15%) | 48 (15%) | |
| Chicago | 138 (15%) | 51 (15%) | |
| San Francisco | 107 (12%) | 46 (14%) | |
| Southern sites | 277 (31%) | 84 (25%) | |
| Race | 0.005 | ||
| White | 140 (16%) | 28 (8.4%) | |
| Black | 666 (74%) | 265 (80%) | |
| Hispanic/Other | 91 (10%) | 39 (12%) | |
| Smoking status | 0.006 | ||
| Never | 292 (33%) | 78 (24%) | |
| Past | 262 (29%) | 102 (31%) | |
| Current | 343 (38%) | 152 (46%) | |
| Cocaine, crack, or heroin use | < 0.0001 | ||
| Never | 388 (43%) | 105 (32%) | |
| Past | 451 (50%) | 183 (55%) | |
| Current | 58 (6.5%) | 44 (13%) | |
| Marijuana use | 0.062 | ||
| Never | 270 (30%) | 79 (24%) | |
| Past | 438 (49%) | 169 (51%) | |
| Current | 189 (21%) | 84 (25%) | |
| Recent alcohol use | <0.0001 | ||
| None | 499 (56%) | 134 (40%) | |
| Light (< 3 drinks/wk) | 342 (38%) | 158 (48%) | |
| Moderate (3–13 drinks/wk) | 22 (3%) | 9 (3%) | |
| Heavy (≥14 drinks/wk) | 34 (4%) | 31 (9%) | |
| Comorbidities | |||
| Hepatitis C Virus infection | 153 (17%) | 43 (13%) | 0.081 |
| Diabetes Mellitus | 227 (25%) | 96 (29%) | 0.20 |
| Hypertension | 507 (57%) | 186 (56%) | 0.88 |
| Renal dysfunction (eGFR <60) | 120 (13%) | 23 (7%) | 0.002 |
| Depressive symptoms (modified CESD ≥15) | 243 (27%) | 110 (33%) | 0.038 |
| Peripheral neuropathy | 175 (20%) | 54 (16%) | 0.19 |
| Obesity (≥30kg/m2) | 461 (51%) | 188 (57%) | 0.092 |
| Subjective cognitive complaints | 92 (10%) | 39 (12%) | 0.45 |
| CNS active medication currently used | |||
| Anticonvulsants | 158 (18%) | 41 (12%) | 0.026 |
| Antidepressants | 247 (28%) | 74 (22%) | 0.063 |
| Antipsychotics | 101 (11%) | 47 (14%) | 0.17 |
| Benzodiazepines and other sedatives | 105 (12%) | 46 (14%) | 0.31 |
| Muscle relaxants | 50 (6%) | 24 (7%) | 0.28 |
| Number of current CNS active medication types | 0.19 | ||
| 0 | 512 (57%) | 204 (61%) | |
| 1 | 201 (22%) | 57 (17%) | |
| 2 | 109 (12%) | 46 (14%) | |
| ≥3 | 75 (8%) | 25 (8%) | |
| Fall status | 0.20 | ||
| No fall | 665 (74%) | 238 (72%) | 0.43 |
| One fall | 117 (13%) | 42 (13%) | |
| More than one fall | 115 (13%) | 52 (16%) | |
| Frailty score | 0.024 | ||
| 0 | 362 (40%) | 135 (41%) | |
| 1–2 | 456 (51%) | 151 (46%) | |
| 3–5 | 79 (9%) | 46 (14%) | |
| Components of Frailty Index | |||
| Slow gait | 109 (13%) | 52 (16%) | 0.11 |
| Reduced grip strength | 193 (24%) | 62 (21%) | 0.31 |
| Exhaustion | 263 (29%) | 108 (33%) | 0.28 |
| Unintentional weight loss | 70 (8%) | 25 (8%) | 0.87 |
| Low physical activity | 257 (29%) | 115 (35%) | 0.042 |
| HIV disease related characteristics | |||
| AIDS defining illness ever | 332 (37%) | N/A | N/A |
| Current CD4+ cell count (cells/μl), mean ± SD | 720 ± 365 | N/A | N/A |
| Nadir CD4+ cell count (cells/μl), mean ± SD | 359 ± 246 | N/A | N/A |
| Suppressed HIV RNA viral load (<80c/mL) | 634 (71%) | N/A | N/A |
| Current ART use at index | 831 (93%) | N/A | N/A |
From Chi-square test. Percentages may not add up to 100% due to rounding.
All p-values reported are two-sided, from chi-square (for unadjusted proportions), Wilcoxon (for unadjusted continuous variables), and Wald tests (for logistic regression). Results were considered significant at p<0.05. SAS version 9.4 (Cary, IN) was used.
RESULTS
All 1,229 participants (897 HIV+ and 332 HIV-) who completed the frailty assessment (index visit) and two falls interviews (at the index and previous semiannual visit) were included in these analyses. Selected demographic, clinical, and substance use characteristics at index visit were stratified by HIV status and are shown in Table 1. Women with HIV had lower BMI, were more often postmenopausal, and more likely co-infected with HCV. Women with HIV were also less likely to report history of cocaine use, heavy alcohol consumption, or current smoking. Among 897 HIV+ women with HIV, 37% reported a prior ADI, 93% reported current ART use, and 71% had a suppressed HIV RNA viral load.
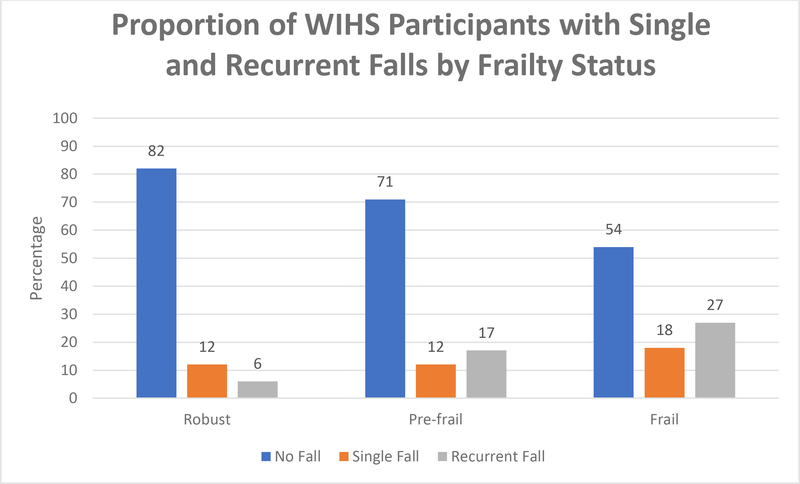
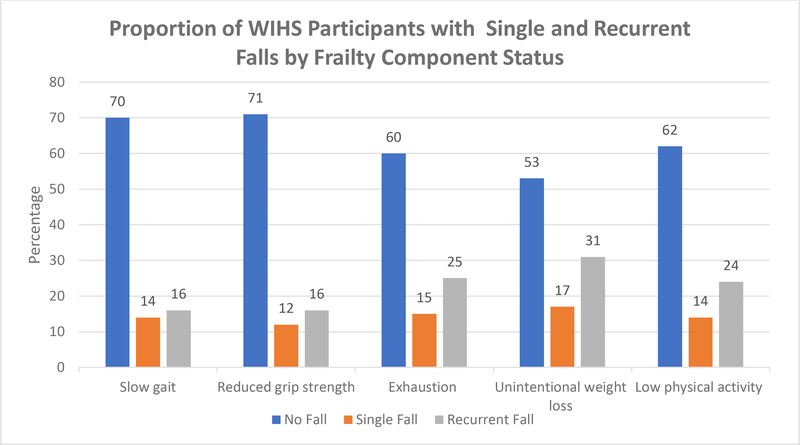
Of the 897 women with HIV, 232 (26%) reported at least one fall over the previous 12- month period, with 117 (13%) reporting a single fall and 115 (13%) recurrent (>1) falls. Of the 332 women without HIV, 94 (28%) had at least one fall over the 12-month period, with 42 (13%) reporting a single fall and 52 (16%) recurrent falls. Women with HIV were less likely to be frail compared to women without HIV and less likely to report low physical activity (Table 1). Overall, 18% of robust, 29% of pre-frail, and 46% of frail women had recurrent falls over 12 months (P < 0.001). Figures 1 and 2 show the proportion of women with falls by frailty status and frailty components. Among robust women, 12% reported single fall and 6% experienced recurrent falls; 12% of pre-frail women experienced single fall and 17% recurrent falls; among frail women, 18% had single fall and 27% recurrent falls (Fig 1). When evaluating individual frailty components, a single fall was reported in 14% of women with slow gait, 12% of women with reduced grip strength, 15% with self- reported exhaustion, 17% with unintentional weight loss and 14% with low physical activity. Recurrent falls were reported by 16% of women with slow gait, 17% with reduced grip strength, 25% who had self-reported exhaustion, 31% with unintentional weight loss, and 21% with low physical activity (Fig 2).
Figure 1.
Figure 2.
Association between FFP and Falls in Women with and without HIV
Compared with robust women, being pre-frail (adjusted odds ratio, AOR 0.97; 95%CI: 0.66 – 1.43, p=0.87) or frail (AOR 1.56; 95%CI: 0.86 – 2.85, p=0.15) was not statistically associated with report of a single fall in the prior 12 months in multivariable models. However, being pre-frail (AOR 2.23; 95%CI: 1.40 – 3.57, p=0.0008) and frail (AOR 3.61; 95%CI: 1.90 −6.89, p<0.0001) were associated with a greater odds of reporting recurrent falls within 12 months (Table 2).
Table 2.
Overall Frailty Score and Other Factors Independently Associated with Single and Recurrent Falls Over 12 Months
| Single (vs. 0) Falls | Recurrent (vs. 0) Falls | |||||
|---|---|---|---|---|---|---|
| Adjusted Odds Ratio* | 95% CI* | P value* | Adjusted Odds Ratio* | 95% CI* | P value* | |
| HIV Positive | 1.05 | 0.70 – 1.57 | 0.81 | 0.95 | 0.63 – 1.44 | 0.82 |
| Frailty Status | ||||||
| Pre-frail (frailty score 1–2) | 0.97 | 0.66 – 1.43 | 0.87 | 2.23 | 1.40 – 3.57 | 0.0008 |
| Frail (frailty score 3–5) | 1.56 | 0.86 – 2.85 | 0.15 | 3.61 | 1.90 – 6.89 | <0.0001 |
| Age (per 10 years) | - | - | - | 1.33 | 1.01 – 1.75 | 0.041 |
| Marijuana use (ref=never) | ||||||
| Former | 1.52 | 0.96 – 2.41 | 0.077 | 1.61 | 0.96 – 2.68 | 0.069 |
| Current | 2.26 | 1.34 – 3.82 | 0.0021 | 2.53 | 1.44 – 4.43 | 0.0012 |
| Hypertension | - | - | - | 0.61 | 0.41– 0.90 | 0.014 |
| Cognitive complaints | - | - | - | 2.02 | 1.23 – 3.32 | 0.0058 |
| Depressive symptoms (modified CESD ≥15) | 1.75 | 1.18 – 2.60 | 0.0055 | 2.51 | 1.69 – 3.72 | <0.0001 |
| # (up to 3) CNS active medication classes | 1.29 | 1.07 – 1.54 | 0.0062 | 1.43 | 1.20 – 1.71 | <0.0001 |
From multivariate logistic regression model that also includes study site.
Association between FFP Components and Falls in Women with and without HIV
Individual FFP components were not associated with single falls in multivariable models (Table 3). However, in models assessing relationships between FFP components and recurrent falls, reduced grip strength (AOR 1.75, 95%CI: 1.07–2.87, p=0.025), exhaustion (AOR 1.89, 95%CI: 1.19–2.99, p=0.0069), and low physical activity (AOR 1.88, 95%CI: 1.19–2.79, p=0.0067) were independently associated with report of recurrent falls within the prior 12 months (Table 3).
Table 3:
Frailty Components Associated with Single and Recurrent Falls over 12 Months
| Single (vs. 0) Falls | Recurrent (vs. 0) Falls | |||||
|---|---|---|---|---|---|---|
| Adjusted Odds Ratio* | 95% CI* | P value* | Adjusted Odds Ratio* | 95% CI* | P value* | |
| HIV Positive | 1.03 | 0.65 – 1.61 | 0.91 | 0.82 | 0.52 – 1.30 | 0.41 |
| Reduced grip strength | - | - | - | 1.75 | 1.07 – 2.87 | 0.025 |
| Exhaustion | - | - | - | 1.89 | 1.19 – 2.99 | 0.0069 |
| Low physical activity | - | - | - | 1.88 | 1.19 – 2.97 | 0.0067 |
| Marijuana use (ref=never) | ||||||
| Former | - | - | - | 1.71 | 0.93 – 3.13 | 0.084 |
| Current | - | - | - | 2.70 | 1.38 – 5.26 | 0.0035 |
| Diabetes Mellitus | 0.58 | 0.36 – 0.96 | 0.033 | |||
| Neuropathy | 2.12 | 1.33 – 3.37 | 0.0016 | 2.26 | 1.32 – 3.86 | 0.002 |
| Cognitive complaints | - | - | - | 2.19 | 1.24 – 3.86 | 0.0071 |
| Depressive symptoms (modified CESD ≥15) | 2.22 | 1.45 – 3.40 | 0.0003 | 1.96 | 1.25– 3.09 | 0.0036 |
| # (up to 3) CNS active medication classes | 1.36 | 1.12 – 1.65 | 0.0021 | 1.45 | 1.19 – 1.77 | 0.0002 |
From multivariate logistic regression model that also includes study site.
Relationship between FFP and Falls among Women with HIV
In multivariate analyses limited to women with HIV, being frail was independently associated with a greater odds of reporting a single fall (AOR 2.88, 95%CI: 1.16–7.20, p=0.023) and recurrent falls (AOR 3.50, 95%CI: 1.24–9.88, p=0.018) in the prior 12 months compared with robust women (Table 4). Pre-frailty was independently associated with recurrent falls (AOR 2.00, 95%CI: 1.03–3.91, p=0.042) but not with single fall over the prior 12 months. Women with HIV who were receiving ART were less likely to report recurrent falls than those not on ART (AOR 0.26, 95%CI: 0.10–0.66, p=0.0046). HIV serostatus and other HIV-specific factors such as current ART class, current or ever efavirenz use, or ever use of zidovudine, stavudine, or didanosine were not associated with falls.
Table 4.
Overall Frailty Score and Other Factors Independently Associated with Single and Recurrent Falls Over 12 Months Among Women with HIV Only
| Single (vs. 0) Falls | Recurrent (vs. 0) Falls | |||||
|---|---|---|---|---|---|---|
| Adjusted Odds Ratio* | 95% CI* | P value* | Adjusted Odds Ratio* | 95% CI* | P value* | |
| Frailty status | ||||||
| Pre-frail (frailty score 1–2) | 1.01 | 0.56 – 1.82 | 0.98 | 2.00 | 1.03 – 3.91 | 0.042 |
| Frail (frailty score 3–5) | 2.88 | 1.16 – 7.20 | 0.023 | 3.50 | 1.24 – 9.88 | 0.018 |
| Annual income ≥$12,000 | 1.81 | 1.02 – 3.19 | 0.042 | - | - | - |
| Smoking status | ||||||
| Former | - | - | - | 2.52 | 1.14 – 5.56 | 0.022 |
| Current | - | - | - | 1.12 | 0.55 – 2.27 | 0.76 |
| Cocaine, crack, or heroin use | ||||||
| Former | 3.11 | 1.53 – 6.32 | 0.0017 | - | - | - |
| Current | 2.57 | 0.85 – 7.76 | 0.093 | - | - | - |
| Marijuana use | ||||||
| Former | 1.19 | 0.53 – 2.67 | 0.67 | - | - | - |
| Current | 2.76 | 1.18 – 6.46 | 0.019 | - | - | - |
| Neuropathy | - | - | - | 2.06 | 1.07 – 3.98 | 0.031 |
| Cognitive complaints | 3.10 | 1.41 – 6.81 | 0.0050 | - | - | - |
| #CNS active medication classes | 1.35 | 1.04 – 1.76 | 0.026 | 1.33 | 1.00 – 1.77 | 0.046 |
| ART use at index visit | - | - | - | 0.26 | 0.10 – 0.66 | 0.0046 |
From multivariate logistic regression model that also includes study site.
DISCUSSION
Ours is the first study to characterize the short-term relationship of falls with FFP and its individual components among women with and without HIV. Among a diverse cohort of older women with and without HIV, pre-frail and frail women were more likely to have experienced recurrent falls over a 12-month time period when compared to those who were robust. Of the FFP components, self-reported exhaustion, reduced grip strength, and low physical activity were independently associated with greater odds of recurrent falls. However, neither the FFP, nor its components, were associated with short-term odds of a single fall. Although HIV serostatus was not associated with odds of either single or recurrent falls, antiretroviral therapy use was associated with lower odds of having sustained recurrent falls among women with HIV.
We found that women who repeatedly fall within a one-year time frame were likely to be prefrail or frail, whereas single falls have little relationship to frailty. In the predominantly male AIDS Clinical Trials Group A5322 observational study, Tassiopoulos et al. found that prefrailty (AOR 3.80, 95% CI:1.87, 7.72) and frailty (AOR 17.3, 95% CI:7.03, 42.6) were independent predictors of recurrent falls among PWH over a 12-month period, as were weak grip strength and slow gait.22,31 The overall relationships between frailty and falls are consistent across the ACTG and WIHS, despite significant differences between the composition of the cohorts in terms of sex, race, and HIV serostatus. However, the magnitude of the odds ratios between prefrailty and falls, and particularly for frailty and falls, are smaller in our study than those reported in the ACTG, which lacks an HIV-seronegative comparison group and has a low proportion of female participants. The overall prevalence of frailty was higher in women without HIV, as has been reported previously in WIHS by Fatukasi et al, who also found that the most common combinations of frailty components were similar by serostatus, and included low physical activity and exhaustion for pre-frail women, in combination with one other component for frail women.7 We found that low physical activity and exhaustion were associated with recurrent falls, as was reduced grip strength, suggesting that these particular aspects of the frailty phenotype may drive the risk of falls among women with and without HIV as they age.
In HIV- seronegative populations, frailty is associated with older age,5,32–34 and occurs more frequently among women than men,5,34–37 yet in a meta-analysis of studies of community- dwelling older adults, the fall risk associated with frailty was higher in men than in women.9 Future studies examining frailty and falls among older PWH should include large sample size of both men and women, as well as a seronegative comparison group who are otherwise similar to participants with HIV, in order to understand how these relationships and their clinical implications differ by sex, age, and HIV serostatus.
In the general population, most published studies on frailty include only persons age 65 and older,5,38 with some also including those between age 50–65.39–43 Despite published associations of frailty with mortality in those younger than age 65,44 some have urged caution in applying the concept of frailty to younger and middle-aged adulthood, and suggested that perhaps the biology as well as clinical implications of frailty phenotype differ substantially between younger and older persons.44 Our findings suggest that prefrailty and frailty have clinical relevance among women with and without HIV, despite a median age of approximately 50 years. Our data therefore add to an improved understanding of the occurrence and consequences of frailty at younger ages among a potentially susceptible group. To understand the impact of HIV infection and its treatment, comorbid conditions, and the role of chronological age on the development and impact of frailty across a longer period of the adult lifespan, additional research would benefit from both inclusion of middle-aged PWH and the growing numbers of PWH over age 65 years,
The associations of the FFP and recurrent falls observed in this study appear to be incremental, such that the lowest odds of recurrent falls are among robust women, the greatest odds of recurrent falls occur among frail women, with the odds of recurrent falls among the prefrail being intermediate. These findings are consistent with studies among older HIV- seronegative persons, reporting associations between prefrailty (as well as report of exhaustion) and falls,45 and meta-analyses demonstrating the greatest risk for falls among frail elderly, followed by those prefrail,10,46 and associations of frailty with recurrent falls;10 others have reported non-significant associations between prefrailty and fall risk.9 Thus, our findings suggest that prefrailty is an important precursor to frailty and is predictive of adverse outcomes such as falls among women with and without HIV. Use of the FFP holds promise for the identification of those at increased risk of falls, particularly women with complex medical conditions including HIV.
While prefrailty may occur earlier47 and then progress to frailty,5 this progression may also be reversible, allowing people with prefrailty to subsequently achieve a robust state and, although less likely, frail persons may improve to a prefrail state.48 Among non-disabled community-dwelling older adults, frailty has been shown to be a dynamic process, however transitions to states of greater frailty occur almost twice as often as transitions to states of lesser frailty over 54 weeks (up to 43% vs 23%) and transition from frail to non-frail states is uncommon (<1%).48 Studies conducted among predominantly49or exclusively6 male PWH have shown that frailty status was largely stable over 12 months49 and that transition from non-frail (including robust and prefrail) to frailty was uncommon; however prefrailty transitions were not examined, nor were transitions to states of lesser frailty, and further limited by a six-month interval between frailty measures rather than the 18-month interval commonly used in geriatric studies of frailty. 6 While less is known about frailty progression among women with HIV, among middle-aged PWH attending a metabolic clinic in Northern Italy, using a 31-item frailty index to measure frailty status over four years, frailty status improved in 53% of PWH, remained unchanged in 19%, and worsened in 28%; female sex, higher current and nadir CD4 cell counts, and fewer smoking pack years were protective.50 We were unable to examine the effects of frailty progression in this study. Additional research on factors associated with frailty progression among women and men with HIV with longer observation time are needed, as well as studies examining the extent to which frailty and prefrailty are modifiable.
This study has several strengths. The WIHS is a racially and ethnically diverse cohort that includes HIV-seronegative women with similar demographic and behavioral characteristics to women with HIV. Participants are well characterized with regard to not only frailty, but also comorbid medical conditions that affect fall risk. Moreover, because women are at greater risk for developing geriatric syndromes such as frailty and falls than are men, yet are underrepresented among studies of PWH, our study contributes to a greater understanding of risks of age-related conditions faced women with and at-risk for HIV.
Our study also has several limitations. First, because our study includes measurement of frailty at one time point only, with falls reported in the preceding 12 months, we are unable to determine the direction of associations between falls and FFP. It is possible that repeated falls lead to prefrailty and frailty; women who are prefrail or frail may be at greater risk for subsequent recurrent falls, or these relationships may be bidirectional. Moreover, although the FFP was measured once, frailty status may change over time. Future studies should examine how falls affect functional status and development and progression of frailty, and vice-versa. An additional limitation of our study was that falls in the prior six-months were assessed by self-report, which may be subject to recall bias, and women with impaired memory may not accurately report events that occurred six-months earlier. Because complete medication count data were not available, we were unable to evaluate the contribution of polypharmacy on risk of falls, or how it influences the relationship between frailty and falls. Lastly, our study includes only women, and findings may not be generalizable to men, and as a convenient sample, WIHS may not be representative of women with or without HIV at large.
Taken together, our study suggests that the FFP identifies adults younger than age 65 years who are at increased risk of falls, particularly women with complex medical conditions such as HIV. Because prefrailty is highly prevalent and may be modifiable, it may represent a potential intervention target; however further research is still needed to develop and test interventions to prevent the onset of frailty, slow its progression, and mitigate its adverse effects such as falls in persons above and below age 65 with and without HIV.
Acknowledgments
Conflicts of Interest and Source of Funding: For all authors, there are no conflicts of interest. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS), now that MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR).
MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
AS has received funding from Gilead Sciences, Inc.
References
- 1.Bergen G, Stevens MR, Burns ER. Falls and Fall Injuries Among Adults Aged ≥65 Years - United States, 2014. MMWR Morbidity and mortality weekly report 2016;65:993–8. [DOI] [PubMed] [Google Scholar]
- 2.Bergen G, Stevens MR, Burns ER. Falls and Fall Injuries Among Adults Aged >/=65 Years - United States, 2014. MMWR Morbidity and mortality weekly report 2016;65:993–8. [DOI] [PubMed] [Google Scholar]
- 3.Burns E, Kakara R. Deaths from Falls Among Persons Aged >/=65 Years - United States, 2007–2016. MMWR Morbidity and mortality weekly report 2018;67:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens JA, Sogolow ED. Gender differences for non-fatal unintentional fall related injuries among older adults. Injury prevention : journal of the International Society for Child and Adolescent Injury Prevention 2005;11:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 6.Althoff KN, Jacobson LP, Cranston RD, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. The journals of gerontology Series A, Biological sciences and medical sciences 2014;69:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fatukasi TV, Edmonds A, Gustafson DR, et al. Prevalence and 1-year incidence of frailty among women with and without HIV in the Women’s Interagency HIV Study. Aids 2019;33:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas 2013;75:51–61. [DOI] [PubMed] [Google Scholar]
- 9.Kojima G Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Journal of the American Medical Directors Association 2015;16:1027–33. [DOI] [PubMed] [Google Scholar]
- 10.Cheng MH, Chang SF. Frailty as a Risk Factor for Falls Among Community Dwelling People: Evidence From a Meta-Analysis. Journal of nursing scholarship : an official publication of Sigma Theta Tau International Honor Society of Nursing 2017;49:529–36. [DOI] [PubMed] [Google Scholar]
- 11.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. Journal of the American Geriatrics Society 2009;57:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Archives of internal medicine 2008;168:382–9. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins KL, Brown TT, Margolick JB, Erlandson KM. Geriatric syndromes: new frontiers in HIV and sarcopenia. Aids 2017;31 Suppl 2:S137–s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Hoover DR, Shi Q, et al. Falls among middle-aged women in the Women’s Interagency HIV Study. Antiviral therapy 2016;21:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Hoover DR, Shi Q, et al. Longitudinal study of falls among HIV-infected and uninfected women: the role of cognition. Antiviral therapy 2018;23:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erlandson KM, Plankey MW, Springer G, et al. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV medicine 2016;17:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlandson KM, Allshouse AA, Jankowski CM, et al. Risk factors for falls in HIV-infected persons. Journal of acquired immune deficiency syndromes (1999) 2012;61:484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Hoover DR, Shi Q, et al. Frailty as a predictor of falls in HIV-infected and uninfected women. Antiviral therapy 2019;24:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacon MC vWV, Alden C, et al. The Women’s Interagency HIV Study: an Observational Cohort Brings Clinical Sciences to the Bench. Clin Diagn Lab Immunol 2005;12:1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terzian AS, Holman S, Nathwani N, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. Journal of women’s health (2002) 2009;18:1965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gustafson DR, Shi Q, Thurn M, et al. Frailty and Constellations of Factors in Aging HIV-infected and Uninfected Women--The Women’s Interagency HIV Study. The Journal of frailty & aging 2016;5:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassiopoulos K, Abdo M, Wu K, et al. Frailty is strongly associated with increased risk of recurrent falls among older HIV-infected adults. Aids 2017;31:2287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamb SEJ-SE, Hauer K, et al. Prevention of Falls Network E, Outcomes Consensus G. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618–22. [DOI] [PubMed] [Google Scholar]
- 24.LS R The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977;1:385–401. [Google Scholar]
- 25.Gustafson DR, Shi Q, Holman S, et al. Predicting death over 8 years in a prospective cohort of HIV-infected women: the Women’s Interagency HIV Study. BMJ open 2017;7:e013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tien PCSM, Cox C, et al. Association of HIV infection with Incident Diabetes Mellitus: Impact of using Hemoglobin A1C as a Criterion for Diabetes. Journal of acquired immune deficiency syndromes (1999) 2012;61:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey ASBJ, Lewis JB., Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 28.Khalsa AKR, Mack WJ, et al. Correlates of prevalent hypertension in a large cohort of HIV- infected women: Women’s Interagency HIV Study. AIDS (London, England) 2007;21:2539–41. [DOI] [PubMed] [Google Scholar]
- 29.Provencher V, Beland F, Demers L, et al. Are frailty components associated with disability in specific activities of daily living in community-dwelling older adults? A multicenter Canadian study. Archives of gerontology and geriatrics 2017;73:187–94. [DOI] [PubMed] [Google Scholar]
- 30.Bouillon K, Sabia S, Jokela M, et al. Validating a widely used measure of frailty: are all sub- components necessary? Evidence from the Whitehall II cohort study. Age 2013;35:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlandson KM, Perez J, Abdo M, et al. Frailty, Neurocognitive Impairment, or Both in Predicting Poor Health Outcomes Among Adults Living With Human Immunodeficiency Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2019;68:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. Journal of the American Geriatrics Society 2005;53:1321–30. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 34.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. The journals of gerontology Series A, Biological sciences and medical sciences 2004;59:1310–7. [DOI] [PubMed] [Google Scholar]
- 35.Puts MT, Lips P, Deeg DJ. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. Journal of the American Geriatrics Society 2005;53:40–7. [DOI] [PubMed] [Google Scholar]
- 36.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. Journal of the American Geriatrics Society 2010;58:681–7. [DOI] [PubMed] [Google Scholar]
- 37.Collerton J, Martin-Ruiz C, Davies K, et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ Study. Mechanisms of ageing and development 2012;133:456–66. [DOI] [PubMed] [Google Scholar]
- 38.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet (London, England) 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chode S, Malmstrom TK, Miller DK, Morley JE. Frailty, Diabetes, and Mortality in Middle-Aged African Americans. The journal of nutrition, health & aging 2016;20:854–9. [DOI] [PubMed] [Google Scholar]
- 40.Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA. A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC geriatrics 2010;10:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitnitski A, Song X, Rockwood K. Trajectories of changes over twelve years in the health status of Canadians from late middle age. Experimental gerontology 2012;47:893–9. [DOI] [PubMed] [Google Scholar]
- 42.Ravindrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: results from the European Male Aging Study (EMAS). Archives of gerontology and geriatrics 2013;57:360–8. [DOI] [PubMed] [Google Scholar]
- 43.Diaz de Leon Gonzalez E, Gutierrez Hermosillo H, Martinez Beltran JA, et al. Validation of the FRAIL scale in Mexican elderly: results from the Mexican Health and Aging Study. Aging clinical and experimental research 2016;28:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. The Lancet Public health 2018;3:e323–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umegaki H, Makino T, Uemura K, et al. Falls in community-dwelling prefrail older adults. Health & social care in the community 2020;28:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fhon JR, Rodrigues RA, Neira WF, Huayta VM, Robazzi ML. Fall and its association with the frailty syndrome in the elderly: systematic review with meta-analysis. Revista da Escola de Enfermagem da U S P 2016;50:1005–13. [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 2011;183:E487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community- living older persons. Archives of internal medicine 2006;166:418–23. [DOI] [PubMed] [Google Scholar]
- 49.Escota GV, Patel P, Brooks JT, et al. Short communication: The Veterans Aging Cohort Study Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS research and human retroviruses 2015;31:313–7. [DOI] [PubMed] [Google Scholar]
- 50.Brothers TD, Kirkland S, Theou O, et al. Predictors of transitions in frailty severity and mortality among people aging with HIV. PloS one 2017;12:e0185352–e. [DOI] [PMC free article] [PubMed] [Google Scholar]