Abstract
The value of rates of change in FEV1 and DLCO to predict disease progression, and initiation of mTOR inhibitor therapy has not been evaluated.
In 84 pre-menopausal LAM patients, individual rates of change in FEV1 and DLCO and 95% confidence intervals (CI), were used to derive subsequent lowest values of FEV1 and DLCO that would prompt initiation of sirolimus therapy. These treatment criteria were compared with one based on FEV1 or DLCO ≤ 70 % predicted. In 12 patients undergoing sirolimus therapy both methods for determining the optimal point for initiation of therapy were evaluated.
Twenty-seven and 35 patients, respectively, who experienced rates of change in FEV1 and DLCO greater than expected, would have been excluded from therapy based on an FEV1 or DLCO > 70 % predicted. Twenty-five of the 84 patients were eventually treated but only when FEV1 or DLCO were ≤ 70 %. Applying such treatment criteria to 12 patients undergoing sirolimus therapy, would have delayed treatment for many years.
Pre-menopausal women in whom FEV1 or DLCO are declining at rates above the expected based on their individual rates of decline, should be considered for sirolimus therapy before the FEV1 or DLCO falls to ≤ 70 %.
Introduction
Lymphangioleiomyomatosis (LAM) is a rare disease that affects predominantly women and involves the lungs, causing cystic destruction, recurrent pneumothoraces and dyspnea, the thoraco-abdominal and pelvic lymphatics, leading to lymphatic masses, i. e., lymphangioleiomyomas, and chylous effusions, and the kidneys, where it may present as mixed mesenchymal tumors named angiomyolipomas (AML) [1]. LAM may occur sporadically and in subjects with an autosomal dominant neurocutaneous disorder, tuberous sclerosis complex (TSC) [1].
LAM cells contain mutations in the TSC genes TSC1 and TSC2, that encode the proteins hamartin and tuberin, respectively [2-4], which exist as a cytosolic complex. Tuberin is a GTPase-activating protein for Ras homolog enriched in brain (Rheb) [5]. Loss of hamartin or tuberin activity causes accumulation of active Rheb-GTP and stimulation of its effector, mechanistic target of rapamycin complex 1 (mTORC1), which results in increased cell size and proliferation [5]. This finding lead to the concept that mTOR inhibitors, such as sirolimus, could be used to treat patients with LAM, and was followed by the Multicenter International Lymphangioleiomyomatosis Efficacy and Safety of Sirolimus (MILES) double-blind controlled study of sirolimus in patients with LAM [6].
The MILES trial showed that sirolimus stabilizes lung function in patients with FEV1 ≤70 % predicted [6]. Isolated reduction in DLCO ≤70 % was not a criteria for enrollment. Accordingly, the MILES trial criteria for initiation of mTOR inhibitor therapy excluded patients with FEV1 >70 % who, by the time their FEV1 declined to 70 % predicted or less, might have lost one third or more of their lung function.
Although there is general agreement that LAM patients with chylous effusions and lymphangioleiomyomas, and angiomyolipomas benefit from m-TOR inhibitor therapy [1], no criteria based on FEV1 or DLCO and their rates of decline , except those derived from the MILES trial, have been proposed to identify LAM patients at risk for disease progression who may benefit from earlier initiation of mTOR inhibitor therapy. The problem with identifying such patients at an earlier date is that rates of decline of FEV1 and DLCO are variable [7-9]. Johnson and Tattersfield [8] reported a mean (±SD) rate of FEV1 decline of 118±142 ml per year in 43 patients and a rate of DLCO decline of 0.905±1.54 ml/min/mmHg in 33 patients. They noted that the greater the number of tests employed in deriving the slopes of FEV1 and DLCO per year, the less the variation between measurements. Another group [9] reported mean (SEM) rates of FEV1 decline of 75±9 ml (1.7±0.4 % predicted) and a reduction in DLCO of 0.69±0.07 ml/min/mmHg (2.4±0.4 % predicted) per year. This study however comprised post-menopausal patients, and patients with LAM-TSC, who may have had milder lung disease [9]. Other studies, reported higher rates of decline in FEV1 in patients with reduced DLCO [10], patients with FEV1 ≤ 70 % predicted [11], patients presenting with dyspnea instead of pneumothorax [12], and patients with a positive response to bronchodilators [13], suggesting that such patients may warrant earlier sirolimus therapy. Given these data, we questioned whether waiting to start mTOR inhibitor therapy until patients have experienced reductions in FEV1 or DLCO to ≤ 70 % predicted was a justifiable criteria for therapy.
Apart from the European Respiratory Society and American Thoracic Society/Japanese Respiratory Society consensus statements suggesting that sirolimus may be considered for patients with rapid decline in lung function [14,15], no guidelines for initiation of mTOR inhibitor therapy or enrollment in clinical trials based on accelerated loss of function have been established. Such a determination depends on whether a decline in FEV1 or DLCO corresponds to true progression of disease or is a random variation without clinical significance; several other factors such as the reproducibility of the test and the quality of the testing need to be considered.
Although LAM may have varying clinical courses and different factors may affect rates of disease progression, the aim of our study was to assess the value of past rates of functional decline in predicting future FEV1 or DLCO values to provide a method for potentially anticipating therapy with sirolimus prior to greater losses in lung function. We focused our research on pre-menopausal patients only and defined a rapid decline in FEV1 or DLCO as one that is greater than the greater 95 % confidence interval for the individual rates of decline based on four tests performed within 18 months. Accordingly, the aims of our study were to determine whether measurement of four or more FEV1 or DLCO performed over 18 months could predict future declines in lung function, which might prompt earlier initiation of mTOR inhibitor therapy. The rationale to also evaluate a treatment criteria based on rates of decline of DLCO and a DLCO ≤ 70 % predicted was prompted by those patients who have FEV1 above 70 % predicted , and experience persistent decline in DLCO . Inasmuch as such patients were not the subject of the MILES trial, we thought that excluding LAM patients with reduced DLCO from this analysis appeared to be inappropriate.
Material and Methods
Patient populations.
From a cohort of 173 patients participating in a LAM natural history and pathogenesis protocol (NHLBI Protocol 95-H-0186), that was approved by the National Heart, Lung, and Blood Institute Institutional Review Board, we identified 84 pre-menopausal patients seen within a span of five years who are the subject of this study. The demographic features of the 84 patients are shown on Table 1. Patients were diagnosed with LAM based on clinical, radiographic, and histopathologic criteria (Table 1) [14,15]. Eighteen patients had TSC-LAM and the remaining 66 had sporadic LAM. Twenty-five patients were eventually treated with sirolimus but all data presented here were obtained before therapy.
Table 1.
Demographic features of 84 pre-menopausal lymphangioleiomyomatosis patients
| Number of patients | 84 |
| Age of LAM diagnosis | 34.7±7.1 |
| Age of first symptoms | 32.0±7.2 |
| History of pneumothorax | 48 (57 %) |
| History of TSC | 18 (21 %) |
| History of chylothorax | 19 (22 %) |
| History of lymphangioleiomyomas | 26 (31 %) |
| History of angiomyolipoma | 49 (58 %) |
| History of anti-estrogen therapy | 29 (34 %) |
| History of pneumonia | 7 (8 %) |
| Initial presentation | |
| Dyspnea | 25 (30 %) |
| Pneumothorax | 33 (39 %) |
| Hemoptysis | 7 (8 %) |
| Abdominal pain | 5 (6 %) |
| Cough | 4 (5 %) |
| Chest pain | 3 (3 %) |
| No symptoms | 7 (8 %) |
| Mode of diagnosis | |
| Lung biopsy | 43 (51 %) |
| Abdominal mass biopsy | 6 (7 %) |
| Characteristic CT and TSC | 10 (12 %) |
| Characteristic CT and lymphangioleiomyoma | 8 (10 %) |
| Characteristic CT and angiomyolipoma | 7 (8 %) |
| Characteristic CT and chylous effusion | 1 (1 %) |
| Characteristic CT scan and elevated serum VEGF-D levels | 2 (2 %) |
To characterize the pattern of lung function decline in patients who eventually were begun on mTOR inhibitor therapy, we also examined lung function data from a cohort of 118 LAM patients undergoing therapy with mTOR inhibitors. Among those patients we identified 12 without evidence of lymphatic disease, who were started on sirolimus therapy because of an FEV1 and/or DLCO≤ 70 % predicted and for whom lung function studies comprising many years of observation prior to therapy, made possible to assess pre-therapy patterns of lung function decline. The aim of this component of our research was to determine how many years and what rates of decline were experienced prior to initiation of treatment based either on a FEV1 ≤ 70 % predicted or a DLCO ≤ 70 % predicted. This group of patients were tested every six to every twelve months. Clinical, laboratory tests, chest X rays, and pulmonary function were obtained at each visit. Exercise tests and imaging studies were obtained respectively, yearly and every two years. All FEV1 values reported in the manuscript refer to pre-bronchodilator data.
Pulmonary function testing.
Lung volumes and flow rates were measured using a Master Screen PFT, Erich Jaeger; (Wuerzburg, Germany) system according to American Thoracic Society/European Respiratory Society standards [16-18].
Study design.
We estimated individual yearly rates of change of FEV1 and DLCO in 84 subjects seen within a time-span of five years, who were tested at least five times, where the most recent test was within eighteen months of the previous one. The most recent visit was excluded from the derivation of the rates of change (slope) of FEV1 and DLCO. The remaining visits were used to derive the slopes and 95 % confidence interval for each subject (9). Then, for each subject, we determined the expected lower values of FEV1 and DLCO using their individual rates of change, the higher limit of the 95% CI, the last measurement used in the derivation of slopes, and the time elapsed from this visit until the next measurement of FEV1 and DLCO.
Although the reproducibility of DLCO measurements is considered to be less than that of FEV1, there are LAM patients in whom FEV1 is well preserved and DLCO is reduced and declines progressively. We suggest that in these patients, a decline in DLCO, indicates progression of lung disease that may be confirmed by exercise testing that may uncover worsening exercise-induced hypoxemia, and by quantitative computed tomography grading of cystic lung disease severity. We believe that patients with a percent-predicted FEV1 above 70 % and a declining DLCO should be considered for sirolimus therapy
We hypothesized that if the last actual % predicted FEV1 or DLCO measured were lower than the expected lower limit calculated from the individual rates of decline of % predicted FEV1 or % predicted DLCO, treatment with mTOR inhibitors would be considered. That is, the calculated expected lower limit of FEV1 or DLCO was compared to the last actual measurement not used in the derivation of slopes. If these values were lower than the expected, patients were considered to have rapid decline in function. These expected lower limits of FEV1 or DLCO were taken to be a threshold below which treatment would be considered.
Three algorithms were used to assess whether treatment would be considered or not: 1) if the last actual % predicted FEV1 was lower than its expected lower limit calculated above, treatment would be considered. 2) if the last actual % predicted DLCO was lower than its expected lower limit calculated above, treatment would also be considered. The criteria for treatment based on the above analytic methods were compared to 3) conventional treatment criteria based on % predicted FEV1 or DLCO ≤70%.
Results
Demographics.
The mean age of the 84 pre-menopausal patients was 38.5±6.6 (19-46) years. Age of diagnosis and age of first LAM-related symptoms were respectively, 34.7±7.1 (18.7-45.5) and 32.0±7.2 (16.7-44.2) years. Demographic features of these patients are shown on Table 1. The mean age of the group of 12 patients who were eventually started on sirolimus was 37.8±9.4 (range, 24-53) years at the time of the first visit, and 51.4±9.8 (range, 35-68) years at the time of initiation of mTOR inhibitor therapy.
Indications for mTOR inhibitor therapy based on individual rates of change of FEV1 and DLCO and its 95 % confidence intervals.
The 84 pre-menopausal patients underwent a total of 526 tests. All pulmonary function data were collected over a period of five years [8]. The mean (SEM) yearly rate of change of FEV1 was −72±13 ml (95% confidence interval −98, −46 ml) or −1.93±0.46 percent predicted (95% confidence interval −2.84, −1.03 % predicted). The mean (SEM) yearly rate of change in DLCO was −0.49±0.11 ml/min/mmHg (95% confidence interval −0.70, −0.27 ml/min/mmHg) or −1.97± 0.51 percent predicted (95% confidence interval- 2.98, −1.03 % predicted).
Among the 84 patients (Table 1) we identified 27 (32.1 %) with decline in FEV1 and 35 (41.7 %) with decline in DLCO greater than expected. The average rates of change in FEV1 and DLCO in 35 pre-menopausal patients with FEV1 ≤ 70% and 39 patients with DLCO ≤70 % predicted were respectively, −112±19 ml (95 % confidence interval −151, −73 ml) or −3.59 ±0.67 % predicted (95% confidence interval −4.91, −2.27 % predicted), and −0.63±0.14 ml/l/mmHg (confidence interval −0.91, −0.3 ml/min/mmHg) or −2.75±0.70 % predicted (95% confidence interval −4.12, −1.38 % predicted). Of these, 15 and 24, respectively, also met the FEV1≤70 % and the DLCO ≤70 % criteria for mTOR inhibitor therapy. However,12 patients with decline in FEV1 greater than expected, and 11 with declines in DLCO greater than expected would not have been treated if the criteria used for treatment was a % predicted FEV1 or a % predicted DLCO ≤70 %.
The expected median interquartile rate (IQR) time to get to a % predicted FEV1 ≤ 70 % in subjects with FEV1 decline greater than expected was 7.4 years (4.0, 13.4 years). For the DLCO the expected median (IQR) time to get to a % predicted DLCO ≤70 % in subjects with greater than expected decline was 13.6 years (2.3, 24.4 years). We used median instead of mean values because the distribution is skewed.
We also focused on how long it would take in patients with FEV1 or DLCO > 70 % predicted for these functional parameters to be reduced to ≤ 70 % predicted. We identified 16 patients with FEV1 > 70 % predicted who were followed for 8.3±4.5 years. FEV1 was reduced from 87±13 % predicted to 68±2 % predicted (p<0.001) at an annual rate of 88±11 ml/year or 2.4 ±0.3 % predicted per year (Table 1S). In 20 patients with DLCO > 70 % predicted followed for 7.4±4.8 years, DLCO declined from 83±13 % predicted to 67 ±3 % predicted at an annual rate of 0.74±0.09 ml/min/mmHg, or 2.9±0.4 % predicted per year . Off these patients only eight and six, respectively, were eventually treated with sirolimus but not before FEV1 or DLCO had fallen to ≤ 70 % predicted.
Indications for mTOR inhibitor therapy based on individual rates of change in FEV1 or DLCO and its 95 % confidence intervals in patients with FEV1 or DLCO > 70 % predicted.
The average rates of change in FEV1 and DLCO in pre-menopausal patients with FEV1 or DLCO > 70 % predicted were respectively, −63±25 (95 % confidence interval −39, 164 ml) or −0.75 ±0.58 % predicted (95% confidence interval −1.94, 0.44 % predicted) and −0.20±0.11 ml/min/mmHg (95 % confidence interval −0.66, 0.26 mlin/mmHg) or −1.29±0.74 % predicted (95% confidence interval −2.75, 0.18 % predicted). Of this sub-group of patients (Table 3), 12 (24.5 %) had lower than the expected FEV1, and 11 (24.4 %) had lower than the expected DLCO (Table 3). Two of the 23 patients had greater than expected rates of decline in FEV1 and DLCO. The remaining patients had a decline in at least one of the two criteria.
Table 3.
Analysis of potential treatment of LAM patients based on a method derived from rates of decline in pre-menopausal patients with FEV1 or DLCO > 70 % predicted *
| Treated Based on Analytical Method |
|||
|---|---|---|---|
| Yes | No | Total | |
| FEV1> 70 % | 12 (24.5) | 37 (75.5) | 49 (100.0) |
| DLCO> 70 % | 11 (24.4) | 34 (75.6) | 45 (100.0) |
Columns show the numbers of patients to be treated with mTOR inhibitors when the observed FEV1 and DLCO were lower than the expected values derived from rates of decline in FEV1 and DLCO. Percentages of patients are shown within parenthesis.
Time course and rates of change in FEV1 and DLCO preceding therapy in 12 LAM patients treated with mTOR inhibitors.
Initial mean FEV1 and DLCO were respectively, 2.8±0.5 liters (97±10 % predicted) and 20.6±3.9 ml/min/mmHg (89.1±11.5 % predicted) (Table 4). We measured the rates of change of FEV1 and DLCO using four tests and compared FEV1 and DLCO values from the fourth test with values from tests performed 9.8±4.1 months later. We found that during this period of time FEV1 and DLCO changed by −3.3 and −5.3 % predicted respectively and % predicted FEV1 and DLCO values were significantly lower than those observed on the last test utilized to estimate the rates of change (Table 4). By the time the patients were began on therapy, FEV1 and DLCO had declined even further, to 1.7±0.4 liters (68.4±19.2 % predicted) and 12.4 ± 2.8 ml/min/mmHg (60.8±11.8 % predicted) respectively.
Table 4.
Lung function tests on first visit, time of estimation of rates of FEV1 and DLCO declines, the next test, and before and after sirolimus therapy in 12 patients with LAM.
| First test | Last slope test | Next test | Pre-sirolimus | Post-sirolimus | |
|---|---|---|---|---|---|
| Age (years) | 35.6±9.4 | 41.2±9.3 | 42.0±9.2 | 49.4±9.6 | 53.3±10.3 |
| TLC (liters) | 5.1±0.5 | 5.2±0.7 | 5.2±0.6 | 5.2±0.8 | 4.6±0.8 |
| TLC (% predicted) | 100.6±9.1 | 101.5±11.2 | 100.8±10.3 | 105.2±17.4 | 93.0±12.3 |
| FVC (liters) | 3.6±0.6 | 3.6±0.4 | 3.5±0.5 | 3.1±0.7 | 3.0±0.6 |
| FVC (% predicted) | 100.8±13.7 | 104±12.3 | 101.4±13.8 | 89.3±36 | 94±15.1 |
| FEV1 (liters) | 2.8±0.44 | 2.45±0.23 | 2.28±0.28 | 1.72±0.48 | 1.62±0.4 |
| FEV1 (% predicted) | 97±10.1 | 90.2±11.1 | 85±13 | 68.4±19.2 | 67.6±17.9 |
| DLCO (ml/min/mmHg/year) | 20.6±3.9 | 16.45±2.3 | 15.1±1.9 | 12.4±2.8 | 11.2±3.4 |
| DLCO (% predicted) | 89.1±11.5 | 77.6±7.9 | 71.6±7 | 60.8±11.8 | 56.5±14.7 |
| Change in FEV1 (ml/year) | - | −105±17 | −40±24 | ||
| Change in FEV1 (% /year) | −3.7±1.3 | −3.3 | −3.0±0.5 | −1±0.8 | |
| Change in DLCO (ml/min/mmHg) | - | −0.66±0.06 | −0.05±0.14 * | ||
| Change in DLCO (% /year) | −2.6±1.5 | −5.3 | −2.6±0.3 | 0.02±0.6 * | |
| Time since first test (years) | - | 5.9±2.8 | 6.7±2.8 | 14.1±4.6 | 18.0±4.8 |
p=0.001
Discussion
This is the first study where an analysis of rates of decline in FEV1, and DLCO, predicts greater loss of function before patients cross the 70 % predicted FEV1 threshold, that based on the MILES study became an accepted standard for initiation of sirolimus therapy. In the current study we show that in 84 pre-menopausal LAM patients, using individual rates of functional decline in FEV1 or DLCO, and 95 % confidence intervals, an analytical method using estimated expected % predicted FEV1 or DLCO based on past rates of FEV1 or DLCO declines identifies patients with faster loss of lung function who had not yet crossed a therapeutic threshold of % predicted FEV1 or DLCO ≤ 70 % predicted. These patients are at risk of experiencing a progressive loss of function before being considered for therapy.
Currently, mTOR inhibitor therapy may be recommended for subjects with lymphatic disease, and size or complications of angiomyolipomas, and those patients, who based on the MILES study enrollment criteria, have % predicted FEV1≤ 70 %. Two consensus statements have suggested also that subjects with rapid disease progression should be considered for therapy, but rapid progression of disease was not defined [14,15].
In our study we found that 23 of 49 pre-menopausal patients with FEV1 or DLCO >70 % predicted, also experienced changes in FEV1 or DLCO greater than the higher 95 % confidence interval. These data suggest that some pre-menopausal LAM patients with % predicted FEV1 or DLCO > 70 %, may experience declines in lung function that in a given time will cause reductions in FEV1 or DLCO to ≤ 70 % predicted. Our data suggest that such patients should undergo frequent lung function testing to calculate rates of decline of FEV1 and DLCO over time. Subsequent testing will determine whether FEV1 or DLCO values are lower than the expected values derived from the lower limit of the 95% confidence interval.
It should be noted however, that it has not yet been established whether a patient whose lung function is falling but still has an FEV1 or DLCO above 70 % predicted should receive mTOR inhibitor therapy. Our data suggest that at a minimum, these patients are at additional risk for progression of lung disease and warrant close follow-up with frequent monitoring of disease by lung function testing and estimation of rates of FEV1 and DLCO decline. In the case of DLCO, disease progression may also be confirmed by other means such as new onset of exercise-induced hypoxemia, or quantitative grading of lung disease by computed tomography, which if confirmed, may further ascertain the need for initiation of therapy. The alternative to this approach, waiting to initiate of mTOR inhibitor therapy until either FEV1 or DLCO have fallen to or under 70 % predicted, may result in greater loss in lung function. This was confirmed by data from 12 patients who eventually were begun on sirolimus therapy, because of reductions in FEV1 and/or DLCO from values at or above 80 % predicted to values ≤ 70 % predicted (Table 4). Indeed, after measuring rates of decline in lung function employing four tests, we found that a fifth test performed within a year showed further losses in lung function. Treatment with mTOR inhibitors should have been started at that point instead of much later, when patients had already lost more than 20 % of the FEV1 and 15 % of the DLCO (Table 4). We suggest that a case can be made for initiation of therapy for those patients in whom tests done after estimating the rate of decline in function demonstrate persistent large declines in FEV1 or DLCO. Such decision must be balanced against the risks of drug toxicity. Perhaps in patients who have not yet reached the 70% predicted FEV1 or DLCO threshold, low dose sirolimus therapy could be considered [19].
We compared using the individual 95 % confidence of rates of change in FEV1 or DLCO, versus the confidence interval for the entire group mean rates of decline in FEV1 or DLCO. A lesser number of patients who would be potential candidates for mTOR inhibitor therapy was identified when individual rates of decline were employed. Indeed, the analysis based on group rates performed better than one based on individual rates and 95 % confidence limits. However, similar data from relatively large LAM patient population are not available to practicing physicians. Therefore, we suggest using individual rates and a minimum of four tests within a period of 18 months to estimate the changes in FEV1 and DLCO. If subsequent testing yields FEV1 or DLCO values lower than the expected lower limit, and in absence of an obvious cause for this change such as a recent pneumothorax, pleurodesis or intercurrent respiratory infection, initiation of mTOR inhibitor therapy should strongly be considered.
In our experience, the reproducibility of FEV1 measurements in LAM patients is generally good, but an occasional patient may experience differences that may reach 7 to 8 % between two measurements (online supplemental Figure 1S). In the case of DLCO the problem is more complex, because of the greater intra-individual variability of this test [17]. In this case, exercise testing and imaging studies, may assist in determining whether a measured reduction in DLCO parallels new onset of exercise-induced hypoxemia or increase in lung cysts.
A major concern with this approach is the timing of initiation of therapy in patients in whom lung function is declining at a steady, yet slow, rate. Let us take a patient in whom FEV1 or DLCO declines every year but at a slow rate of 1-2 % per year. Should treatment be started when their values reach under 80 % predicted? It remains also to be defined if earlier initiation of mTOR inhibitors, including in those with normal FEV1 and DLCO or FEV1 or DLCO above 100 % predicted, would be beneficial for patients with LAM. We suggest however, that evaluation of the rate of change in FEV1 and DLCO may have a special importance in those patients in whom values of FEV1 or DLCO are above 100% of predicted. In this population, to wait until FEV1 and DLCO reach 70% of predicted to initiate the use of sirolimus would be more deleterious because it results into a 30 % loss of lung function. Measurement of cyst scores by computed tomography may help defining progression of disease [20]. At present, definite answers to these questions can not be provided.
Based on our data, we recommend that pre-menopausal LAM patients in whom lung function is declining steadily at rates well above the expected based on their estimated rates of decline, should be followed closely and be strongly considered for mTOR inhibitor therapy before the FEV1 or DLCO falls to ≤ 70 % predicted.
Supplementary Material
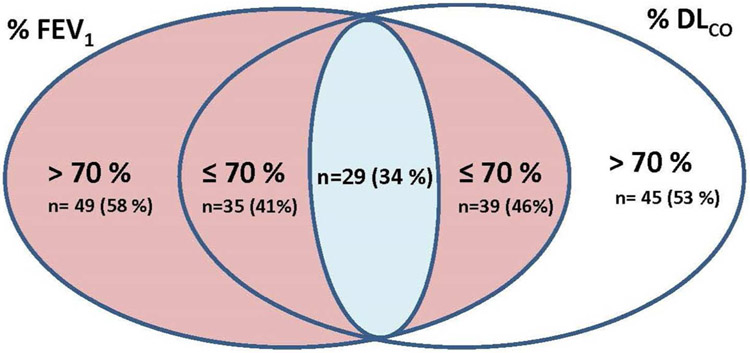
Figure 1.
Venn diagram representing the FEV1 and DLCO profile of 84 LAM pre-menopausal patients. Of the 84 patients, 49 and 45 respectively, had FEV1 or DLCO above 70 % predicted. Thirty-five patients had an FEV1 ≤ 70 % predicted and 39 had a DLCO ≤ 70 % predicted. Twenty-nine patients had both FEV1 and DLCO ≤ 70 % predicted. Twelve of the 49 patients with FEV1> 70 % predicted and 11 of the 45 patients with DLCO> 70 % predicted had greater than expected declines in in FEV1 and DLCO, respectively.
Table 2.
Comparator analysis of treatment of 84 pre-menopausal LAM patients based on lung function criteria versus an analytical method derived from individual rates of functional decline and confidence intervals *
| Percent predicted FEV1 N (%) | Treated Based on Analytical Method |
|||
|---|---|---|---|---|
| Yes | No | Total | ||
| FEV1 ≤70 % | Yes | 15 (17.9) | 20 (23.8) | 35 (41.7) |
| No | 12 (14.3) | 37 (44.0) | 49 (58.3) | |
| Total | 27 (32.1) | 57 (67.9) | 84 (100) | |
| Percent predicted DLCO N (%) | Yes | No | Total | |
| DLCO ≤70 % | Yes | 24 (28.6) | 15 (17.9) | 39 (46.4) |
| No | 11 (13.1) | 34 (40.5) | 45 (53.6) | |
| Total | 35 (41.7) | 49 (58.3) | 84 (100) | |
Rows show the number of patients who would be treated with mTOR inhibitors if FEV1≤70 % predicted or DLCO ≤70 % predicted. Columns show the number of patients to be treated with mTOR inhibitors when the observed FEV1 or DLCO was lower than the expected values derived from rates of decline in FEV1 and DLCO. Percentages of patients are shown within parenthesis.
Take home message:
Past rates of FEV1 or DLCO decline are indicators of future lung function loss and initiation of sirolimus therapy.
Acknowledgments
This study was supported by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute.
Footnotes
This is an original manuscript that has never been published nor is it being considered for publication elsewhere. The manuscript has been seen and approved by all authors. All authors take responsibility for the integrity of the data and the data analysis, and for the integrity of the submission. The objectives and procedures undertaken are honestly disclosed. None of the authors has any financial conflicts of interest.
References
- 1.Johnson SR, Taveira-DaSilva AM, Moss J. Lymphangioleiomyomatosis. Clin Chest Med 2016; 37:389–403. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Astrinidis A, Henske EP. Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis. Am J Respir Crit Care Med 2001;164:1537–1540. [DOI] [PubMed] [Google Scholar]
- 3.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet 1998;62:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoncu R, Efeyan A, Sabatini DM. mTOR. from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd, Goldberg HJ, Young LR, Kinder BW, Downey GP, Sullivan EJ, Colby TV, McKay RT, Cohen MM, Korbee L, Taveira-DaSilva AM, Lee HS, Krischer JP, Trapnell BC; National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011;364:1595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med 1995; 151:527–533 [DOI] [PubMed] [Google Scholar]
- 8.Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med 1999; 160:628–633. [DOI] [PubMed] [Google Scholar]
- 9.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest 2004;126:1867–1874. [DOI] [PubMed] [Google Scholar]
- 10.Lazor R, Valeyre D, Lacronique J, Wallaert B, Urban T, Cordier J-F. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires. Low initial KCO predicts rapid FEV1 decline in pulmonary lymphangioleiomyomatosis. Respir Med 2004; 98:536–541. [DOI] [PubMed] [Google Scholar]
- 11.Hayashida M, Yasuo M, Hanaoka M, Seyama K, Inoue Y, Tatsumi K, Mishima M; Respiratory Failure Research Group of the Ministry of Health, Labour, and Welfare, Japan .Reductions in pulmonary function detected in patients with lymphangioleiomyomatosis: An analysis of the Japanese National Research Project on Intractable Diseases database. Respir Investig 2016;54:193–200. [DOI] [PubMed] [Google Scholar]
- 12.Hayashida M, Seyama K, Inoue Y, Fujimoto K, Kubo K; Respiratory Failure Research Group of the Japanese Ministry of Health, Labor, and Welfare. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology 2007;12:523–530. [DOI] [PubMed] [Google Scholar]
- 13.Taveira-DaSilva AM, Hedin C, Stylianou MP, Travis WD, Matsui K, Ferrans VJ, Moss J. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am J Respir Crit Care Med 2001;164:1072–1076. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, Bonetti F, Kingswood C; Review Panel of the ERS LAM Task Force European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 2010; 35:14–26. [DOI] [PubMed] [Google Scholar]
- 15.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, Steagall WK, Johnson SR, Sahn SA, Ryu JH, Strange C, Seyama K, Sullivan EJ, Kotloff RM, Downey GP, Chapman JT, Han MK, D'Armiento JM, Inoue Y, Henske EP, Bissler JJ, Colby TV, Kinder BW, Wikenheiser-Brokamp KA, Brown KK, Cordier JF, Meyer C, Cottin V, Brozek JL, Smith K, Wilson KC, Moss J; ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis Diagnosis and Management. Am J Respir Crit Care Med. 2016;194:748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 17.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CPM, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hanhinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. [DOI] [PubMed] [Google Scholar]
- 19.Ando K, Kurihara M, Kataoka H, Ueyama M, Togo S, Sato T, Doi T, Iwakami S, Takahashi K, Seyama K, Mikami Ml. Efficacy and safety of low-dose sirolimus for treatment of lymphangioleiomyomatosis. Respir Investig. 2013;51:175–183. [DOI] [PubMed] [Google Scholar]
- 20.Yao J, Taveira-DaSilva AM, Colby TV, Moss J CT grading of lung disease in lymphangioleiomyomatosis. AJR Am J Roentgenol 2012;199:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.