Abstract
Background
Malnutrition is highly prevalent in hospitalized patients but seldom recognized and treated. Malnutrition poses several adverse events, such as increased infection rates, length of hospital stay, and mortality, as well as costs. Early nutrition interventions have been shown to decrease the associated malnutrition burdens, leading to relevant savings. Thus, this study aims to evaluate the cost‐effectiveness of nutrition therapy, including oral supplements to at‐risk or malnourished adult inpatients admitted to the Brazilian Public System (SUS) hospitals.
Method
A cost‐effectiveness model, encompassing a 1‐year period and regarding total costs, length of hospital stay, readmissions, and mortality related to malnutrition, was developed, having the provision of early nutrition therapy as the intervention variable. The number of avoided hospitalization days, prevented hospital readmissions, and prevented deaths defined the effectiveness of the model. All the costs were estimated based on the SUS database.
Results
Early nutrition therapy provided to all at‐risk or malnourished patients would represent cost‐effectiveness of US $92.24, US $544.59, US $1848.12, and US $3698.92, for each day of hospitalization avoided, for additional patients having access to hospitalization, for preventing readmission, and for prevented death, respectively. The highest impact on savings was represented by the mean reduction in the length of hospital stay.
Conclusion
Early oral nutrition intervention for patients malnourished or at risk of malnutrition resulted in overall reduced hospital costs. These findings provide a rationale to tackle the implementation of educational programs focusing on the care of inpatients with malnutrition or its risk.
Keywords: cost‐effectiveness, enteral nutrition, malnutrition, medical inpatients, nutrition therapy, oral supplements, parenteral nutrition
Clinical Relevant Statement
Early nutrition therapy to at‐risk or malnourished patients is cost‐effective, and this was the goal of the current study.
Introduction
Malnutrition is highly prevalent in hospitals worldwide. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 In Brazil, the prevalence of malnutrition has been reported to be about 50%, and when specific groups of patients are assessed, such as those with cancer or on the waiting list for a liver transplant, the rates reach 70%. 14 , 15 There are several risk factors related to malnutrition, such as socioeconomic problems, particularly poverty. Depressive and emotional variations, poor oral health, and polypharmacy, especially in elderly patients, also contribute to malnutrition. However, the most relevant risk factor is the disease per se, which may lead to anorexia, changes in nutrient metabolism (absorption, distribution, storage, utilization, and excretion), drug‐nutrient interactions, increased requirements, and losses, as well as metabolic derangements. 2
Patients with malnutrition present increased chances of medical complications, mostly infectious, which can, in turn, require intensified care and lead to delay in recovery, thus causing extended hospitalization time. 16 , 17 , 18 , 19 Furthermore, malnourished patients are at higher risk of hospital readmissions and mortality. 8 , 20 , 21 , 22 , 23 On the other hand, nutrition interventions—oral nutritional supplements (ONSs), enteral nutrition (EN), and parenteral nutrition (PN)—have been shown to lead to clinical improvement and may reduce the economic burden related to malnutrition. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 However, nutrition therapy is underrecognized and underprescribed, as demonstrated by the Inquérito Brasileiro de Avaliação Nutricional (IBRANUTRI) study 13 and by other authors worldwide. 40 Despite the malnutrition prevalence of 48.7%, there were few registries suggesting awareness about the nutrition status, and only a few patients were receiving nutrition therapy (6.1% EN, 4.0% oral supplements, and 1.2% PN). 13
The economic impact of malnutrition has been addressed by a few authors who have highlighted the increased related costs, and interventions have demonstrated positive cost‐effectiveness and clinical outcomes. 41 , 42 , 43 , 44 , 45 , 46 , 47 In Brazil, a model study derived from the IBRANUTRI indicated that for every dollar invested in the treatment of malnutrition, there would be a savings of US $4. 48 Therefore, the goal of the current study is to provide a pharmacoeconomic model to estimate the efficiency (costs apropos of effects) related to the provision of early nutrition therapy, including oral supplements, to patients malnourished or at risk of malnutrition, considering the Brazilian Public System (SUS) reimbursement system.
Methods
To set up the economic model, at‐risk or malnourished adult nonsurgical, non‐oncologic hospitalized patients were considered. Critically ill patients were excluded. All the analyses were carried out based on a 1‐year perspective and considering the dollar value for the year 2017. The assessed variables relied on the nutrition therapy offered by SUS. In this regard, 6.9% of the SUS hospitals are qualified to receive reimbursement and only EN and PN are covered, leaving ONSs without coverage.
The model was developed as if patients who were malnourished or at risk of malnutrition were started on nutrition therapy on the first day of hospitalization. The assessed health outcomes were avoided days of hospitalization, potential new hospitalizations, prevented readmissions, and prevented deaths. These outcomes were also used to calculate the cost‐effectiveness per day of avoided hospitalization, cost‐effectiveness for potential new hospitalization, cost‐effectiveness due to avoided readmission, and the cost‐effectiveness due to avoided deaths. Second and third alternative scenarios were also modeled. The former considered the same variables, but with nutrition therapy starting after the sixth day of hospitalization, and the latter with nutrition therapy initiated after 2 weeks of hospitalization.
A decision tree (Figure 1), according to the DATASUS admission data in the year 2017, was set up. Therefore, the data represent hospitalized patients in 2017 regarding the number of patients, number of days of hospitalization, hospitalization costs, and deaths. Since these data do not describe whether patients were nutritionally at risk or malnourished and because there was no information regarding any type of nutrition therapy, the comparator group of the decision tree was the prevalence of nutritionally at‐risk or malnourished patients reported by a previous study. 49 It is important to highlight that the overall prevalence of malnutrition of the latter study was lower than in data from 2 previous studies carried out in the country, in which the majority of the patients were admitted to public hospitals. 13 , 49 , 50 After that, we plotted what would happen if all these patients received nutrition therapy (Figure 1). For this purpose, we established a mean length of hospital stay (LOS) reduction of 0.35 days if the patients received early nutrition therapy, including oral supplements, based on a previous study with a similar population. 51 As for nutrition therapy, we assumed that 50% of the patients, who were receiving EN, could be receiving ONSs, whereas the other 50% could be receiving tube feeding (currently, SUS does not cover oral supplements). Also, for those patients receiving both EN and PN, we assumed, based on clinical expertise, that on 80% of the days, they are receiving EN and, on 20% of the days, receiving PN. The length of time receiving nutrition therapy was estimated to be 80% of the total LOS (Table 1).
Figure 1.
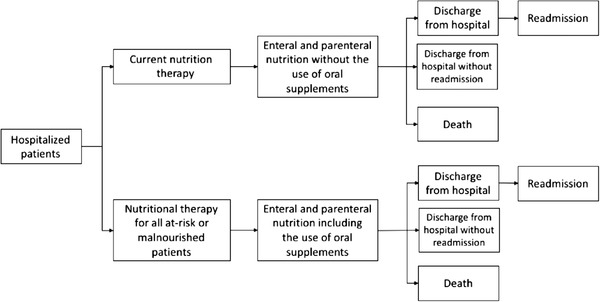
Structure of the model.
Table 1.
Assumed Proportion of Nutrition Therapy, According to the Type
| Type of nutrition therapy | Proportion of use | Reference |
|---|---|---|
| Percent enteral | 69.10% | Clinical experts’ input |
| Percent parenteral | 10.63% | Clinical experts’ input |
| Percent enteral + parenteral | 20.27% | Clinical experts’ input |
To estimate readmissions, once the SUS data do not contemplate this variable, data from a literature meta‐analysis (submitted) were assessed. The latter indicated that, on average, at‐risk or malnourished patients have a 31.3% risk of hospital readmission in 30 days compared with 19.7% for well‐nourished individuals. Furthermore, it was hypothesized that if nutrition therapy were offered early, there would be a 6.0% reduction in hospital readmission risk over the course of 30 days. Mortality, having the same meta‐analysis as support, was assumed to be decreased by 12% of patients if early nutrition were provided.
The costs of LOS are based on SUS reimbursement (supplementary material S1), and in Table 2, there are data regarding the costs of nutrition therapy.
Table 2.
Costs of Nutrition Therapy According to Government Reimbursement for Enteral and Parenteral Nutrition and Market Price for Oral Nutritional Supplements
Sensitivity analysis, using a second‐order Monte Carlo simulation of 10,000 iterations, was used to investigate the effects of uncertainty in the model. The Palisade's @risk software was used for this analysis. The results are shown as a cost‐effectiveness plane. One‐way deterministic sensitivity analyses were carried out, whereby input values were individually adjusted to plausible upper and lower bounds, as shown in Table 3, with 95% confidence intervals (CIs). The remaining values retained their baseline value. Because, in Brazil, there is neither an official cost‐effectiveness threshold 52 nor a consistent assessment of quality‐adjusted life‐years (QALYs) for malnutrition, thresholds of US $9821.41 and US $29,464.23, corresponding to 1 and 3 per‐capita gross domestic product, were used. 53
Table 3.
One‐Way Deterministic Sensitivity Analyses and Probabilistic Sensitivity Analysis
| Parameters | Base case | Min value | Max value | Distribution |
|---|---|---|---|---|
| Percent of patients at nutrition risk 51 | 37.25% | 26.25% | 48.24% | β |
| Reduction in the number of hospital days 51 | −0.35 | −1.04 | 0.34 | Normal |
| Percent of days with nutrition therapya | 80.00% | 70.00% | 90.00% | β |
| Percent of days without nutrition therapya | 20.00% | 10.00% | 30.00% | β |
| Percent enteral usea | 69.10% | 57.00% | 80.00% | β |
| Percent parenteral usea | 10.63% | 10.00% | 10.00% | β |
| Percent enteral + parenteral usea | 20.27% | 10.00% | 33.00% | β |
| Percent of enteral use among those who used enteral + parenterala | 80% | 70.00% | 90.00% | β |
| Percent parenteral use among those who used enteral + parenterala | 20% | 10.00% | 30.00% | β |
| Enteral nutrition costb | R$30 | R$30.00 | R$45.00 | Log‐normal |
| Oral supplement costb | R$30 | R$30.00 | R$45.00 | Log‐normal |
| Parenteral nutrition costb | R$60 | R$60.00 | R$90.00 | Log‐normal |
| Risk of readmission in patients with nutrition risk or malnourishedc | 31.28% | 23.16% | 39.41% | β |
| Risk of readmission in patients without nutrition riskc | 19.73% | 14.76% | 24.71% | β |
| Readmission risk reduction in patients receiving nutrition therapy 51 | 0.94 | 0.79 | 1.12 | Log‐normal |
| Reduction of the risk of mortality in patients that were at nutrition risk or malnourished and received nutrition therapyc | 0.88 | 0.64 | 1.21 | Log‐normal |
max, maximum; min, minimum.
a Clinical expertise.
bReimbursement and market value (in real, Brazilian money).
cLiterature meta‐analysis (submitted).
Costs and health outcomes were not discounted, as the time horizon was only 1 year.
Results
The use of early ONSs, EN, or PN would result in 420,658 avoided days of hospitalization, 71,252 new potential admissions, 20,996 avoided readmissions, and 10,491 deaths, with an increased cost of US $38,803,768.73 compared with the current SUS configuration (Table 4). Table 4 also depicts the comparison of cost‐effectiveness in case the supplementation started on the 6th and 14th day of hospitalization. The cost‐effectiveness results become progressively worse as there is a delay in the initiation of supplementation.
Table 4.
Cost‐Effectiveness According to the Different Modeled Scenarios
| Parameter | Incremental cost | Effectiveness | Cost‐effectiveness |
|---|---|---|---|
| Early nutrition intervention | |||
| Prevented hospitalizations | US $38,803,768.73 | 420,658 | US $92.24 |
| Potential new admissions | US $38,803,768.73 | 71,252 | US $544.59 |
| Avoidable readmissions | US $38,803,768.73 | 20,996 | US $1848.12 |
| Preventable deaths | US $38,803,768.73 | 10,491 | US $3698.92 |
| Nutrition therapy after the sixth day of hospitalization | |||
| Prevented hospitalizations | US $35,311,541.65 | 111,452 | US $316.83 |
| Potential new admissions | US $35,311,541.65 | 18,878 | US $1870.51 |
| Avoidable readmissions | US $35,311,541.65 | 5314 | US $6645.43 |
| Preventable deaths | US $35,311,541.65 | 4237 | US $8333.46 |
| Nutrition therapy after the 14th day of hospitalization | |||
| Preventable hospitalizations | US $19,881,290.66 | 33,671 | US $590.47 |
| Potential new admissions | US $19,881,290.66 | 5703 | US $3485.99 |
| Avoidable readmissions | US $19,881,290.66 | 1568 | US $12,683.16 |
| Preventable deaths | US $19,881,290.66 | 1522 | US $13,066.17 |
The results of the 1‐way deterministic sensitivity analysis are shown in the tornado diagrams in Figure 2, in which the most influential driver of the cost‐effectiveness is at the top and the least important driver is at the bottom. The mean reduction in the LOS has the highest influence on the incremental cost‐effectiveness ratio (ICER) for all 4 cost‐effectiveness outcomes. The model responds less to changes in any other values, including the percentage of days receiving nutrition therapy, readmission risk reduction, and mortality risk decrease for at‐risk or malnourished patients who receive interventions.
Figure 2.
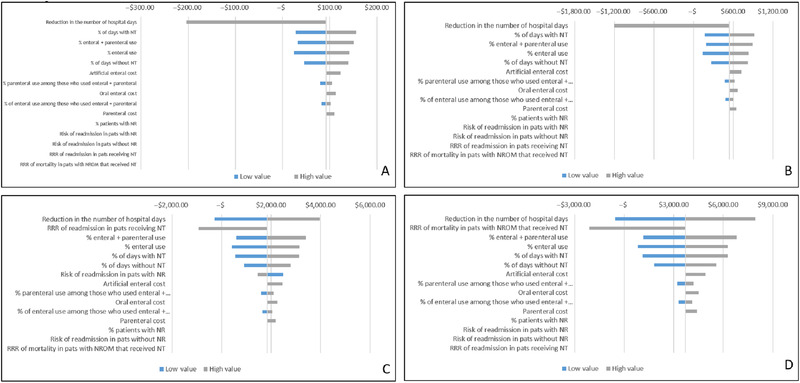
Tornado diagrams showing the 1‐way deterministic sensitivity analysis of the model simulation for the base case. (A) Preventable hospitalization. (B) Potential new admissions. (C) Avoidable readmission. (D) Preventable deaths. NR, nutrition risk; NROM, nutritional risk or malnourished; NT, nutrition therapy; pats, patients; RRR, Risk ratio reduction.
The cost‐effectiveness acceptability planes (Figure 3) display the distribution of results regarding the probabilistic sensitivity analysis for all 4 cost‐effectiveness outcomes. They demonstrate the high probability for all outcomes (avoidable hospitalization [85.7%], decreased potential new admissions [83.7%], avoidable readmissions [73.3%], and preventable deaths [70.7%]) if nutrition therapy were prescribed to all malnourished or at‐risk patients admitted to SUS hospitals, implying a cost‐per‐outcome threshold of less than US $9821.41.
Figure 3.
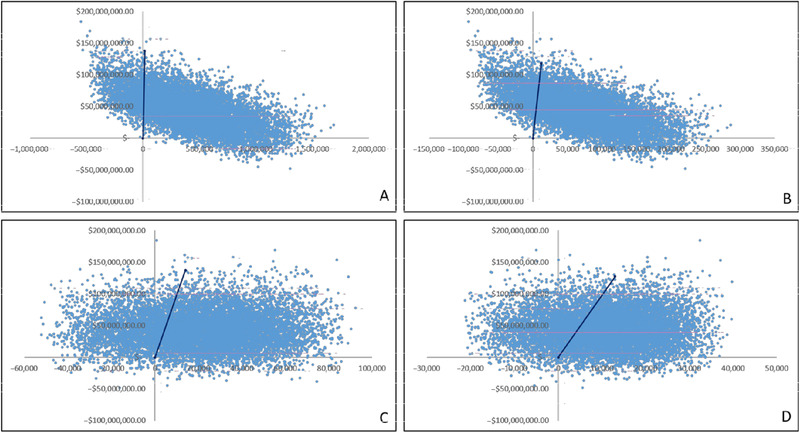
Cost‐effectiveness planes displaying the results of the Monte Carlo simulation. (A) Avoidable hospitalization. (B) Potential new admissions. (C) Avoidable readmission avoided (D) Preventable deaths.
Discussion
Malnutrition is a highly prevalent syndrome in the hospital setting, and it impacts worse outcomes, mortality, and costs 8 , 16 , 42 , 47 , 54 , 55 , 56 . Despite this well‐known scenario, malnutrition awareness is low 12 , 13 , 40 , 57 , nutrition therapy is still underprescribed 13 , 40 , and its value for money is less commonly studied in the era of pharmacoeconomics.
Mitchell and Porter, in 2016, assessed the literature to establish the cost‐effectiveness of identifying and treating hospital malnutrition. They reviewed data on adult patients with or at risk of malnutrition. They assessed 1174 published manuscripts, and 19 were potentially eligible, but they only included 3 in the systematic review, which highlighted the absence of high‐quality data regarding the topic and limited their conclusions. 41 More recently, a few other authors have addressed the topic, and a study from Colombia showed that if patients received early nutrition therapy, there would be a 35.8% savings per nutrition‐treated patient, and taken broadly, the potential cost savings from a nutrition care program for patients at malnutrition risk would be US $862.6 million per year. 47
The impact of hospital malnutrition on healthcare costs is multifactorial; however, the longer LOS was the variable that influenced the ICER for all 4 areas of cost‐effectiveness in our model. It is well known that malnourished patients remain in the hospital for longer periods. 3 , 8 , 16 The IBRANUTRI study 16 showed that malnourished patients had a mean LOS of 16.7 ± 24.5 days vs 10.1 ± 11.7 days of the well‐nourished individuals. Longer LOS not only impacts costs but is also directly related to the availability of more beds, which are essential in countries with fewer resources and where it is common for hospitals to be short on availability.
Malnutrition is multifactorial, and if there are risk factors that cannot be immediately resolved by the system, such as socioeconomic imbalances, there are others that can be minimized by adequate interventions. In this regard, providing early treatment to at‐risk populations, like elderly and sick patients, will decrease the burden imposed by these risk factors. Pan et al conducted a multicenter study with cancer patients to assess the influence of nutrition therapy (EN and PN) on the outcomes of those who were malnourished, and they were able to identify that patients who received EN or PN had a decreased relative risk of adverse events (EN, 0.08 [95% CI, 0.01–0.62] and PN, 0.56 [95% CI, 0.33–0.96]) 58 . Sun et al, in a randomized clinical trial with patients who were undergoing major abdominal operations and received either early postoperative oral nutrition or conventional care, showed that the intervention group had a better overall recovery. Mainly, these patients presented with better nutrient intake in the first week after the operation, and time of bowel sounds, flatus, and defecation was shortened in the individuals receiving early ONS. Moreover, these patients remained in the hospital 2 days less, and the total costs were significantly decreased. 59 Sriram et al tested the effects of a nutrition‐focused quality‐improvement program (QIP) on readmissions and LOS in 4 North American hospitals. The QIP consisted of malnutrition risk screening by nurses at admission; early initiation (within 24 hours) of ONSs for at‐risk patients, which were maintained post discharge; nutrition therapy; and postdischarge nutrition instructions and follow‐up by telephone calls. Thirty‐day readmissions and LOS were significantly decreased for at‐risk or malnourished patients. 25
Health decision makers must be aware of the negative impact of malnutrition and the benefits of treating it early with either oral supplements, EN, or PN. Improving patient nutrition status may contribute to the efficiency and financial sustainability of the health systems. Unfortunately, few studies have addressed the value for money, the willingness‐to‐pay threshold, and the cost per QALY, commonly used in healthcare, regarding nutrition therapy. 60
Our cost‐effectiveness model used the data from hospitalized patients in the public health sector in Brazil in 2017. Although there was no information regarding the nutrition status, interventions, and readmissions and although only a few hospitals were, in fact, allowed to be reimbursed for nutrition therapy, our model was able to provide a projection of what would have happened in terms of LOS, readmissions, and mortality if all nutritionally at‐risk or malnourished patients were treated (including oral supplements, EN, and PN), and for this, a decision tree was used. To run the model, we assumed that 37.25% of the individuals were at risk or malnourished, 51 a value below the prevalence rates reported by previous studies in the country and the world 8 , 12 , 13 , 15 , 40 , 50 , 61 , 62 , 63 , 64 , 65 , 66 . Therefore, this limitation, in fact, represents a potential benefit considering that many more individuals would be treated. For the other included missing SUS variables, we assumed values that have been published either in Brazil or in the world 13 , 44 , 46 , 47 or relied on clinical expertise.
The model showed that an increase in nutrition therapy reimbursement with the addition of oral supplements (which currently is not reimbursed in the country) generates 2.2% fewer days of hospitalization, which is in line with several other published data. 42 , 67 , 68 , 69 Moreover, analysis of the 2 other scenarios (the patients would start nutrition therapy after the 6th and 14th days of hospitalization), which represent the routine practice in most hospitals, showed that the early initiation of nutrition therapy is more cost‐effective. Our model also revealed that the number of readmissions may be reduced by 3%, similar to what has been shown by other authors. 67 , 68 , 70 Sharma et al, in Australia, assessed 11,750 readmissions within 6 months, with 2897 (11%) early and 8853 (33.8%) late readmissions. Malnourished patients had a higher risk of both early (odds ratio [OR], 1.39; 95% CI, 1.12–1.73) and late readmissions (OR, 1.23; 95% CI, 1.06–128). 70
Our study presents some further limitations. First, individual‐level cost data were not collected because we used a population‐based approach often used in public health, which is contrary to the high‐risk approach that focuses on individuals. Whereas the former targets “vulnerable” population segments, as the hospitalized malnourished patients, the latter identifies the individual as having elevated risk of a particular outcome assessed in terms of the intervention. In this regard, to run randomized nutrition interventions to malnourished individuals to assess cost‐effectiveness would be absolutely unethical. Therefore, the intersection of these 2 concepts seems appropriate and helpful in public health. 71
Second, although Brazil is a continental country with various cultural and educational nuances, this model did not account for variability and inequality in the practice of nutrition therapy, which could affect clinical and cost outcomes.
In conclusion, according to our model, the prescription of early nutrition therapy (ONS, EN, or PN) to adult patients nutritionally at risk or malnourished can decrease the LOS and hospital readmissions. Thus, there will be more available hospital beds, which would benefit more patients in need of hospitalization. Also, early nutrition therapy has shown to be cost‐effective. In other words, the effects are large enough to justify the cost. This study highlights how proper medical inpatient nutrition care is of utmost importance in the quality of healthcare delivery, bringing clinically and economically significant outcomes. Furthermore, our study highlights that nutrition therapy is a valuable intervention for the healthcare system, especially for the Brazilian public health system.
Funding Information
This work has been funded by Grupo Estratégico de Nutrição Especializada (GENE). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Conflicts of Interest
M. I. Toulson Davisson Correia is a speaker for Abbott Nutrition, Baxter International, Danone Nutrition, Fresenius Kabi, and Nestlé Nutrition. She is in the educational/scientific board for Baxter Nutrition, Fresenius Kabi, and Abbott. She is the receiver of a grant by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). D. de Oliveira Toledo is a speaker for Danone Nutrition, Fresenius Kabi, and Nestlé Nutrition. M. Castro is a speaker for Abbott Nutrition, Fresenius Kabi, and Nestlé Nutrition. M. C. M. Fonseca received a grant from Grupo Estratégico de Nutrição Especializada (GENE) to develop the hospital malnutrition model; he is the scientific director for Axia.Bio Life Sciences International, a consultancy company that provides assistance to distinct healthcare stakeholders.
Statement of Authorship
M. I. Toulson Davisson Correia, D. de Oliveira Toledo, G. Tannus Branco de Araújo, and M. C. M. Fonseca contributed to the study design; D. Sansone, D. Farah, and T. R. de Morais Andrade contributed to the data collection and data figures; G. Tannus Branco de Araújo, M. C. M. Fonseca, D. Sansone, D. Farah, and T. R. de Morais Andrade contributed to the data analysis; M. I. Toulson Davisson Correia, D. de Oliveira Toledo, M. Castro, G. Tannus Branco de Araújo, and M. C. M. Fonseca contributed to the data interpretation; M. I. Toulson Davisson Correia contributed to the writing and final editing of the manuscript; D. de Oliveira Toledo, M. Castro, D. Sansone, D. Farah, T. R. de Morais Andrade, and M. C. M. Fonseca contributed to the drafting of the manuscript. All authors contributed to the final approval of the manuscript.
Supporting information
Supporting Information
Acknowledgments
We thank Grupo Estratégico de Nutrição Especializada (GENE) for funding this publication.
References
- 1. Casanova MJ, Chaparro M, Molina B, et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J Crohns Colitis. 2017;11(12):1430‐9. [DOI] [PubMed] [Google Scholar]
- 2. Correia M, Perman MI, Waitzberg DL. Hospital malnutrition in Latin America: A systematic review. Clin Nutr. 2017;36(4):958‐67. [DOI] [PubMed] [Google Scholar]
- 3. Correia MI, Hegazi RA, Diaz‐Pizarro Graf JI, et al. Addressing Disease‐Related Malnutrition in Healthcare: A Latin American Perspective. JPEN J Parenter Enteral Nutr. 2016;40(3):319‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allard JP, Keller H, Jeejeebhoy KN, et al. Malnutrition at Hospital Admission‐Contributors and Effect on Length of Stay: A Prospective Cohort Study From the Canadian Malnutrition Task Force. JPEN J Parenter Enteral Nutr. 2016;40(4):487‐97. [DOI] [PubMed] [Google Scholar]
- 5. Gavriilidou NN, Pihlsgård M, Elmståhl S. High degree of BMI misclassification of malnutrition among Swedish elderly population: Age‐adjusted height estimation using knee height and demispan. Eur J Clin Nutr. 2015;69(5):565‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hecht C, Weber M, Grote V, et al. Disease associated malnutrition correlates with length of hospital stay in children. Clin Nutr. 2014;34(1):53‐59. [DOI] [PubMed] [Google Scholar]
- 7. Corkins MR, Guenter P, DiMaria‐Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186‐95. [DOI] [PubMed] [Google Scholar]
- 8. Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3‐year mortality. Clin Nutr. 2012;31(3):345‐50. [DOI] [PubMed] [Google Scholar]
- 9. Burgos R, Sarto B, Elío I, et al. Prevalence of malnutrition and its etiological factors in hospitals. Nutr Hosp. 2012;27(2):469‐76. [DOI] [PubMed] [Google Scholar]
- 10. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734‐8. [DOI] [PubMed] [Google Scholar]
- 11. Elia M, Russell CA, Stratton RJ. Malnutrition in the UK: policies to address the problem. Proc Nutr Soc. 2010;69(4):470‐6. [DOI] [PubMed] [Google Scholar]
- 12. Correia MI, Campos AC, Study EC. Prevalence of hospital malnutrition in Latin America: the multicenter ELAN study. Nutrition. 2003;19(10):823‐5. [DOI] [PubMed] [Google Scholar]
- 13. Waitzberg DL, Caiaffa WT, Correia MI. Hospital malnutrition: the Brazilian national survey (IBRANUTRI): a study of 4000 patients. Nutrition. 2001;17(7‐8):573‐80. [DOI] [PubMed] [Google Scholar]
- 14. Mauricio SF, Xiao J, Prado CM, Gonzalez MC, Correia M. Different nutritional assessment tools as predictors of postoperative complications in patients undergoing colorectal cancer resection. Clin Nutr. 2017;37(5):1505‐1511. [DOI] [PubMed] [Google Scholar]
- 15. Ferreira LG, Anastácio LR, Lima AS, Correia MI. Malnutrition and inadequate food intake of patients in the waiting list for liver transplant. Article in Portuguese. Rev Assoc Med Bras. 2009;55(4):389‐93. [DOI] [PubMed] [Google Scholar]
- 16. Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235‐9. [DOI] [PubMed] [Google Scholar]
- 17. Correia MITD, Perman MI, Waitzberg DL. Hospital malnutrition in Latin America: A systematic review. Clin Nutr. 2017;36(4):958‐67. [DOI] [PubMed] [Google Scholar]
- 18. Cangelosi MJ, Rodday AM, Saunders T, Cohen JT. Evaluation of the economic burden of diseases associated with poor nutrition status. JPEN J Parenter Enteral Nutr. 2014;38(2_suppl):35S‐41S. [DOI] [PubMed] [Google Scholar]
- 19. Giraldo NA, Vásquez Velásquez J, Roldán Cano PA, Ospina Astudillo C, Sosa Cardona YP. Cost‐effectiveness of early nutritional therapy in malnourished adult patients in a high complexity hospital. Nutr Hosp. 2015;32(6):2938‐47. [DOI] [PubMed] [Google Scholar]
- 20. Chung AS, Hustedt JW, Walker R, Jones C, Lowe J, Russell GV. Increasing severity of malnutrition is associated with poorer 30‐day outcomes in patients undergoing hip fracture surgery. J Orthop Trauma. 2018;32(4):155‐60. [DOI] [PubMed] [Google Scholar]
- 21. Mogensen KM, Horkan CM, Purtle SW, et al. Malnutrition, critical illness survivors, and postdischarge outcomes: a cohort study. JPEN J Parenter Enteral Nutr. 2018;42(3):557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bally MR, Blaser Yildirim PZ, Bounoure L, et al. Nutritional support and outcomes in malnourished medical inpatients: a systematic review and meta‐analysis. JAMA Intern Med. 2016;176(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 23. Hiller LD, Shaw RF, Fabri PJ. Difference in composite end point of readmission and death between malnourished and nonmalnourished veterans assessed using academy of nutrition and dietetics/american society for parenteral and enteral nutrition clinical characteristics. JPEN J Parenter Enteral Nutr. 2017;. 41(8):1316‐1324. [DOI] [PubMed] [Google Scholar]
- 24. Cereda E, Klersy C, Andreola M, et al. Cost‐effectiveness of a disease‐specific oral nutritional support for pressure ulcer healing. Clin Nutr. 2017;36(1):246‐52. [DOI] [PubMed] [Google Scholar]
- 25. Sriram K, Sulo S, VanDerBosch G, et al. A comprehensive nutrition‐focused quality improvement program reduces 30‐day readmissions and length of stay in hospitalized patients. JPEN J Parenter Enteral Nutr. 2017;41(3):384‐91. [DOI] [PubMed] [Google Scholar]
- 26. Bounoure L, Gomes F, Stanga Z, et al. Detection and treatment of medical inpatients with or at‐risk of malnutrition: Suggested procedures based on validated guidelines. Nutrition. 2016;32(7‐8):790‐8. [DOI] [PubMed] [Google Scholar]
- 27. Ravasco P. Nutritional approaches in cancer: relevance of individualized counseling and supplementation. Nutrition. 2015;31(4):603‐4. [DOI] [PubMed] [Google Scholar]
- 28. Bowrey DJ, Baker M, Halliday V, et al. A randomised controlled trial of six weeks of home enteral nutrition versus standard care after oesophagectomy or total gastrectomy for cancer: report on a pilot and feasibility study. Trials. 2015;16:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sánchez‐Lara K, Turcott JG, Juárez‐Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non‐small cell lung cancer: randomised trial. Clin Nutr. 2014;33(6):1017‐23. [DOI] [PubMed] [Google Scholar]
- 30. de van der Schueren M, Elia M, Gramlich L, et al. Clinical and economic outcomes of nutrition interventions across the continuum of care. Ann N Y Acad Sci. 2014;1321:20‐40. [DOI] [PubMed] [Google Scholar]
- 31. Hollander FM, van Pierre DD, de Roos NM, van de Graaf EA, Iestra JA. Effects of nutritional status and dietetic interventions on survival in Cystic Fibrosis patients before and after lung transplantation. J Cyst Fibros. 2014;13(2):212‐8. [DOI] [PubMed] [Google Scholar]
- 32. van der Meij BS, Langius JA, Spreeuwenberg MD, et al. Oral nutritional supplements containing n‐3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: an RCT. Eur J Clin Nutr. 2012;66(3):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Langer G, Grossmann K, Fleischer S, et al. Nutritional interventions for liver‐transplanted patients. Cochrane Database Syst Rev. 2012(8):CD007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430‐41. [DOI] [PubMed] [Google Scholar]
- 35. Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non‐small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24(5):431‐40. [DOI] [PubMed] [Google Scholar]
- 36. van der Meij BS, Langius JA, Smit EF, et al. Oral nutritional supplements containing (n‐3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non‐small cell lung cancer during multimodality treatment. J Nutr. 2010;140(10):1774‐80. [DOI] [PubMed] [Google Scholar]
- 37. Guida B, Trio R, Laccetti R, et al. Role of dietary intervention on metabolic abnormalities and nutritional status after renal transplantation. Nephrol Dial Transplant. 2007;22(11):3304‐10. [DOI] [PubMed] [Google Scholar]
- 38. Gonçalves Dias MC, de Fátima Nunes Marucci M, Nadalin W, Waitzberg DL. Nutritional intervention improves the caloric and proteic ingestion of head and neck cancer patients under radiotherapy. Nutr Hosp. 2005;20(5):320‐5. [PubMed] [Google Scholar]
- 39. Muscaritoli M, Grieco G, Capria S, Iori AP, Rossi Fanelli F. Nutritional and metabolic support in patients undergoing bone marrow transplantation. Am J Clin Nutr. 2002;75(2):183‐90. [DOI] [PubMed] [Google Scholar]
- 40. Tobert CM, Mott SL, Nepple KG. Malnutrition Diagnosis during Adult Inpatient Hospitalizations: Analysis of a Multi‐Institutional Collaborative Database of Academic Medical Centers. J Acad Nutr Diet. 2018;118(1):125‐31. [DOI] [PubMed] [Google Scholar]
- 41. Mitchell H, Porter J. The cost‐effectiveness of identifying and treating malnutrition in hospitals: a systematic review. J Hum Nutr Diet. 2016;29(2):156‐64. [DOI] [PubMed] [Google Scholar]
- 42. Kruizenga HM, Van Tulder MW, Seidell JC, Thijs A, Ader HJ, Van Bokhorst‐de van der Schueren MA. Effectiveness and cost‐effectiveness of early screening and treatment of malnourished patients. Am J Clin Nutr. 2005;82(5):1082‐9. [DOI] [PubMed] [Google Scholar]
- 43. Rypkema G, Adang E, Dicke H, et al. Cost‐effectiveness of an interdisciplinary intervention in geriatric inpatients to prevent malnutrition. J Nutr Health Aging. 2004;8(2):122‐7. [PubMed] [Google Scholar]
- 44. Waitzberg DL, Correia MITD. Custos e benefıcios da nutrição parenteral e parenteral na assistência integral à saúde. Rev Bras Nutr Clin. 1999;14:213‐9. [Google Scholar]
- 45. Philipson TJ, Snider JT, Lakdawalla DN, Stryckman B, Goldman DP. Impact of oral nutritional supplementation on hospital outcomes. Am J Manag Care. 2013;19(2):121‐8. [PubMed] [Google Scholar]
- 46. Correia MITD, Perman MI, Pradelli L, Omaralsaleh A, Waitzberg DL. Economic burden of hospital malnutrition and the cost‐benefit of supplemental parenteral nutrition in critically ill patients in Latin America. J Med Econom. 2018;21(. 11):1047‐1056. [DOI] [PubMed] [Google Scholar]
- 47. Buitrago G, Vargas J, Sulo S, et al. Targeting malnutrition: Nutrition programs yield cost savings for hospitalized patients. Clin Nutr. 2020;39(9):2896‐2901. [DOI] [PubMed] [Google Scholar]
- 48. Waitzberg DL, Correia MITD. Custos e benefícios da nutrição enteral e parenteral na assistência integral à saúde. Rev Bras Nutr Clin; 1999: 213‐9. [Google Scholar]
- 49. Fonseca M, Farah D, D Toledo, Correia I. Prevalence of disease related malnutrition in Brazil and Latin America. Value Health. 2019;22:S45. [Google Scholar]
- 50. Brito PA, de Vasconcelos Generoso S, Correia MI. Prevalence of pressure ulcers in hospitals in Brazil and association with nutritional status–a multicenter, cross‐sectional study. Nutrition. 2013;29(4):646‐9. [DOI] [PubMed] [Google Scholar]
- 51. Farah D, Sansone D, Andrade TRM, Araujo GTB, Fonseca M. Prevalence of disease‐related malnutrition in Brazil and Latin America. Value Health. 2019;22:S44. [Google Scholar]
- 52. Bridges JF, Onukwugha E, Mullins CD. Healthcare rationing by proxy: cost‐effectiveness analysis and the misuse of the $50,000 threshold in the US. Pharmacoeconomics. 2010;28(3):175‐84. [DOI] [PubMed] [Google Scholar]
- 53. Instituto Brasileiro de Geografia e Estatística. 2017. https://www.ibge.gov.br/en/ibge-search.html?searchphrase=all&searchword=GDP. Accesed August 15, 2018.
- 54. Ruiz AJ, Buitrago G, Rodriguez N, et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin Nutr. 2019;38(3):1310‐6. [DOI] [PubMed] [Google Scholar]
- 55. Gomes F, Emery PW, Weekes CE. Risk of Malnutrition Is an Independent Predictor of Mortality, Length of Hospital Stay, and Hospitalization Costs in Stroke Patients. J Stroke Cerebrovasc Dis. 2016;25(4):799‐806. [DOI] [PubMed] [Google Scholar]
- 56. Mosquera C, Koutlas NJ, Edwards KC, et al. Impact of malnutrition on gastrointestinal surgical patients. J Surg Res. 2016;205(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 57. Ray S, Laur C, Douglas P, et al. Nutrition education and leadership for improved clinical outcomes: training and supporting junior doctors to run ‘Nutrition Awareness Weeks’ in three NHS hospitals across England. BMC Med Educ. 2014;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pan H, Cai S, Ji J, et al. The impact of nutritional status, nutritional risk, and nutritional treatment on clinical outcome of 2248 hospitalized cancer patients: a multi‐center, prospective cohort study in Chinese teaching hospitals. Nutr Cancer. 2013;65(1):62‐70. [DOI] [PubMed] [Google Scholar]
- 59. Sun DL, Li WM, Li SM, et al. Comparison of multi‐modal early oral nutrition for the tolerance of oral nutrition with conventional care after major abdominal surgery: a prospective, randomized, single‐blind trial. Nutr J. 2017;16(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walzer S, Droeschel D, Nuijten M, Chevrou‐Severac H. Health economics evidence for medical nutrition: are these interventions value for money in integrated care? Clinicoecon Outcomes Res. 2014;6:241‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Toulson Davisson Correia MI. Addressing the Hidden Burden of Malnutrition for Hospitalized Patients. J Acad Nutr Diet. 2018;118(1):37‐9. [DOI] [PubMed] [Google Scholar]
- 62. Waitzberg DL, De Aguilar‐Nascimento JE, Dias MCG, Pinho N, Moura R, Correia MITD. Hospital and homecare malnutrition and nutritional therapy in Brazil. Strategies for alleviating it: a position paper. Nutr Hosp. 2017;34(4):969‐75. [DOI] [PubMed] [Google Scholar]
- 63. Mauricio SF, Ribeiro HS, Correia MI. Nutritional Status Parameters as Risk Factors for Mortality in Cancer Patients. Nutr Cancer. 2016;68(6):949‐57. [DOI] [PubMed] [Google Scholar]
- 64. Fontes D, SeV Generoso, Toulson Davisson Correia MI. Subjective global assessment: a reliable nutritional assessment tool to predict outcomes in critically ill patients. Clin Nutr. 2014;33(2):291‐5. [DOI] [PubMed] [Google Scholar]
- 65. Maurício SF, da Silva JB, Bering T, Correia MI. Relationship between nutritional status and the Glasgow Prognostic Score in patients with colorectal cancer. Nutrition. 2013;29(4):625‐9. [DOI] [PubMed] [Google Scholar]
- 66. Ryan AM, Power DG, Daly L, et al. Cancer‐associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199‐211. [DOI] [PubMed] [Google Scholar]
- 67. Hudson L, Chittams J, Griffith C, Compher C. Malnutrition identified by academy of nutrition and dietetics/american society for parenteral and enteral nutrition is associated with more 30‐day readmissions, greater hospital mortality, and longer hospital stays: a retrospective analysis of nutrition assessment data in a major medical center. JPEN J Parenter Enteral Nutr. 2018;42(5):892‐7. [DOI] [PubMed] [Google Scholar]
- 68. Budzynski J, Tojek K, Czerniak B, Banaszkiewicz Z. Scores of nutritional risk and parameters of nutritional status assessment as predictors of in‐hospital mortality and readmissions in the general hospital population. Clin Nutr. 2016;35(6):1464‐71. [DOI] [PubMed] [Google Scholar]
- 69. Tangvik RJ, Tell GS, Eisman JA, et al. The nutritional strategy: four questions predict morbidity, mortality and health care costs. Clin Nutr. 2014;33(4):634‐41. [DOI] [PubMed] [Google Scholar]
- 70. Sharma Y, Miller M, Kaambwa B, et al. Factors influencing early and late readmissions in Australian hospitalised patients and investigating role of admission nutrition status as a predictor of hospital readmissions: a cohort study. BMJ Open. 2018;8(6):e022246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McLaren L. In defense of a population‐level approach to prevention: why public health matters today. Can J Public Health. 2019;110(3):279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. DATASUS. MdS . Sistema de Gerenciamento da Tabela de Procedimentos, Medicamentos e OPM do SUS (SIGTAP/DATASUS). 2017. http://sigtap.datasus.gov.br/tabela-unificada/app/sec/inicio.jsp. Accesed August 15, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


