Abstract
Serum samples obtained from healthy, asymptomatic dogs in areas of Wisconsin and northern Illinois where Lyme disease is endemic or nonendemic were assayed for antibodies to Borrelia burgdorferi by enzyme-linked immunosorbent assay (ELISA), and positive results were confirmed by immunoblot assay. We found that 56.9% (562 of 1,077) of the samples were positive by ELISA and 82.0% (461 of 562) were positive by immunoblotting. A logistic regression model was developed to distinguish between nonvaccinated dogs naturally infected with B. burgdorferi from areas where the disease is endemic and dogs from areas where the disease is nonendemic that were vaccinated against Lyme disease. Of the 18 protein bands analyzed, 8 were found to be significantly different (P < 0.05) between the two groups. p93, p34, p31, and p28 occurred with increased frequency in vaccinated dogs, while p58, p37, p35, and p30 occurred more frequently in naturally infected dogs. The logistic regression equation obtained was used to determine the probability of natural infection among vaccinated dogs residing in areas where the disease is endemic. Of 125 samples, 87.2% had a very low probability of natural infection and only 2.4% were highly likely to be infected. Logistic regression is a useful method for distinguishing between vaccinated and naturally infected dogs and predicting the serological status of vaccinated dogs from areas where Lyme disease is endemic.
Since Borrelia burgdorferi was found to be the causative agent of Lyme disease, various methods have been employed for the determination of antibodies to the spirochete in humans and in domestic and wild animals. The enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent-antibody assay (IFA) have been used to screen serum, and immunoblotting techniques have been used to confirm positive results (1, 5, 10, 15, 21, 22, 31). Various studies have determined the type and number of bands that must be present for a sample to be considered positive (5, 17, 24, 33) and to distinguish between the early and late stages of Lyme disease in humans (32). Band patterns may differ according to the duration of infection and the type of B. burgdorferi strain affecting an individual. In addition, antigens for serologic analysis are prepared from cultured spirochetes, which may express different proteins than spirochetes transmitted through natural infection. Therefore, the number and type of bands present in positive immunoblots can be highly variable and the diagnostic criteria used to identify positive immunoblots are still controversial.
Immunoblotting has also been used to diagnose canine Lyme disease; however, serologic diagnosis is complicated by the presence of heterologous antibodies due to oral Treponema infection and Leptospira vaccination (19, 23, 26) and vaccination with whole-cell Lyme disease bacterins (Fort Dodge Laboratories, Fort Dodge, Iowa). In areas where Lyme disease is endemic and the vaccine is used extensively, it is difficult to determine whether a vaccinated dog exhibiting symptoms of Lyme disease was infected prior to vaccination or whether the dog acquired a natural infection despite vaccination. Jacobson et al. (12) reported that vaccinated dogs developed strong antibody responses to OspA (p31) and OspB (p34) and usually did not develop responses to p30, p28, and p19. Wittenbrink et al. (31) documented the presence of six major bands, p93, p75, p60, p41, p39, and p31, with vaccinated dogs reacting to a smaller number of bands. In another canine study (9), different immunoblot patterns were found among four B. burgdorferi strains, especially in the 45- to 34-kDa and 26- to 15-kDa ranges. No definitive criteria have been established to distinguish naturally infected, unvaccinated dogs from vaccinated dogs that may also be harboring an active infection. Vaccines may induce the presence of bands in immunoblots comparable in number and intensity to those present in natural infection, thus obfuscating serologic test results. Dogs are not routinely screened for antibodies to B. burgdorferi prior to vaccination in clinical settings; thus, baseline information on the serologic status of dogs is generally not available.
Serologic analyses of dog sera by immunoblot assay are also important for epidemiologic studies. Dogs are at higher risk for Lyme disease than are humans in areas where it is endemic (16, 18) and can act as sentinels to determine the regional risk of Lyme disease. Serological analyses of dog sera from veterinary clinics have shown positive correlations between the prevalence of antibodies to B. burgdorferi and the distribution of tick vectors. However, as with serologic diagnosis in the clinical setting, vaccination may confound the results of canine serosurveys conducted to aid in the preparation of regional disease risk maps.
The primary purpose of this study was to compare the band patterns of immunoblots of the sera of naturally infected dogs from areas where the disease is endemic and vaccinated dogs from areas where the disease is nonendemic in the upper midwestern United States. The bands that were significantly different between these two groups were determined using logistic regression analysis, and a final model was developed that best distinguished between vaccinated and naturally infected dogs. This model could then be used to compute the probability of natural infection among vaccinated dogs from areas where the disease is endemic.
MATERIALS AND METHODS
Canine serum samples were obtained from 25 counties in Wisconsin and 7 counties in northern Illinois to determine the distribution of B. burgdorferi seropositivity. Participating clinics submitted serum samples drawn from clinically normal canines for routine diagnosis, such as for heartworm. The owner and veterinarian together completed a questionnaire which included the following information on each dog: age, sex, breed, residential address, vaccination status, history of tick exposure, history of travel outside the county, and history of symptoms consistent with Lyme disease. Each clinic submitted 20 samples, with one to five clinics participating per county. Only dogs that had not traveled outside of their county of residence were included in the analysis presented here.
An ELISA similar to those described by Magnarelli et al. (22) and Lindenmayer et al. (15) was used to detect serum antibodies to B. burgdorferi as follows. Sera were diluted 1:320 to reduce cross-reactivity with heterologous antibodies (15, 19, 20, 26, 31). The antigen used was a heat-killed, sonicated B. burgdorferi spirochete suspension (Kirkegaard & Perry, Gaithersburg, Md.) diluted 1:100 in carbonate-bicarbonate coating buffer (27.6 mM Na2CO3, 19 mM NaHCO3, pH 9.6). Test sera were diluted in blocking buffer (5% [wt/vol] blotting grade dehydrated milk [Bio-Rad, Hercules, Calif.] in phosphate-buffered saline). The detecting antibody was a peroxidase-labeled goat anti-dog immunoglobulin G (IgG [heavy and light chains]; Kirkegaard & Perry) diluted 1:8,000 in blocking buffer. The detecting substrate was TMB-ELISA (tetramethyl benzidine; Life Technologies, Gaithersburg, Md.). Reactions were stopped after incubation for 30 min with 2 N H2SO4, and the optical density (OD) of wells was read at 450 nm using an ELISA plate reader (Molecular Devices). Samples were considered positive when the ODs were greater than or equal to 3 standard deviations above the mean OD of negative controls. The endpoint titers of positive sera were determined with twofold serial dilutions. Positive control sera with high titers (≥1:1,280) were from a site in northern Michigan (30) or were obtained courtesy of L. Magnarelli of the Connecticut Agricultural Experiment Station (New Haven). Negative control canine sera were from dogs with the following prerequisites: no history of vaccination for Lyme disease, no history of tick bite, no suspected diagnosis of Lyme disease, no periodontal disease, previously negative by IFA for antibodies to B. burgdorferi (<1:160), and not resident of an area where the disease is endemic (30). We used six positive and six negative control sera on each microtiter plate, and all sera were tested in duplicate.
Western blot analyses were performed on all samples positive by ELISA using the Lyme Immunostrip IgG Assay kit (Immunetics, Inc., Cambridge, Mass.). Aliquots (10 μl) of undiluted canine serum samples were added to channels containing the test strips and 1 ml of dilution buffer. Antigens on membranes of this kit were separated by the manufacturer and include the following 18 bands: p93, p66, p60, p58, p45, p41, p39, p37, p35, p34, p31, p30, p28, p23, p18, p17, p15, and p14. Visualization of specific protein bands indicated the presence of serum IgG antibodies against B. burgdorferi-derived antigens. Samples were classified as positive or negative in accordance with the criteria established by Immunetics, Inc. Positive samples had two or more bands present in the p30 to p14 region, and negative samples had less than two bands present in this region.
For this analysis, dogs were classified as naturally infected if they had a positive immunoblot, had no history of vaccination, and resided in the high- to moderate-risk Wisconsin counties of Chippewa, Eau Claire, Washburn, Sawyer, Lincoln, Clark, and Marathon (2). Dogs were classified as vaccinated if they had a positive immunoblot result, if the owner and veterinarian reported vaccination with the Lyme bacterin, and if they resided in the low- to no-risk counties of DeKalb, Boone, LaSalle, and Green in Illinois and Green, Columbia, Dodge, Fond du Lac, Outagamie, Rock, Walworth, Sauk, Oconto, Racine, and Shawano in Wisconsin (2). The band frequencies of the immunoblots from these canine samples were tabulated. A univariate analysis was performed using the chi-square or Fisher exact test (EPI-INFO, Atlanta, Ga.) to compare the frequencies of bands in sera classified as vaccinated or naturally exposed. To determine whether any band or group of bands was significantly associated with vaccination or natural infection status, we performed a logistic regression (SPSS, Chicago, Ill.) in which the dichotomous outcome variable was naturally infected dogs from areas where Lyme disease is endemic (coded as one) and vaccinated dogs from areas of low to no risk (coded as zero). The independent variables were coded as the presence (code one) or absence (code zero) of bands corresponding to the 18 antigens present on the immunoblot strips. All independent variables were included in the initial model. Variables (P < 0.10) were removed one at a time from the regression model in a backward, stepwise fashion until only those bands contributing significantly (P < 0.05) to the model remained. The final logistic regression equation obtained was used to compute the probability of infection among the vaccinated dogs from areas where the disease is endemic.
RESULTS
A total of 1,077 samples with accompanying completed questionnaires was received from the participating veterinary clinics. Of these, 562 (52.2%) were positive by ELISA. Western blot analyses performed on these samples revealed 101 negatives and 461 positives. Of the 461 immunoblot-positive samples, 89 were considered naturally infected and 234 were considered vaccinated by the previously stated criteria. These samples were selected for the logistic regression analysis. The remaining samples included those from 125 vaccinated dogs residing in areas where the disease is endemic.
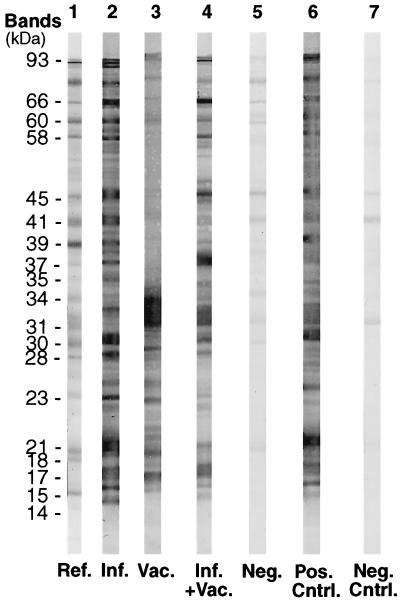
Figure 1 shows representative samples of immunoblots obtained from this study. The immunoblots from the vaccinated (lane 3) dog from an area where the disease is nonendemic and the naturally infected (lane 2) dog from an area where the disease is endemic differ mainly in the number of bands present within the higher-molecular-weight region. The status of these dogs is fairly easy to determine in accordance with previously established criteria. However, the only difference between the immunoblots for infected (lane 2) and vaccinated (lane 4) dogs from areas where the disease is endemic was the presence of the p31 and p34 bands in samples from the vaccinated dog. This illustrates the difficulty in interpreting the status of the immunoblot in lane 4.
FIG. 1.
Representative immunoblots from the Immunetics IgG test kit. Lanes: 1, reference strip from Immunetics with bands identified; 2, naturally exposed dog from an area where the disease is endemic; 3, vaccinated dog from an area where the disease is nonendemic; 4, vaccinated dog from an area where the disease is endemic (all significant bands are present); 5, negative dog; 6, positive control; 7, negative control. Abbreviations: Ref., reference; Inf., infected; Vac., vaccinated; Neg., negative; Pos., positive; Cntrl., control.
The contingency table analysis of band frequencies of samples from naturally infected and vaccinated dogs (Table 1) showed that all of the bands differed significantly in frequency between these two groups (P < 0.05), except for bands p28, p21, and p17. However, there were no bands that were uniquely present in one group. Using logistic regression analysis, the final model (Table 2) indicated that eight bands were significantly different between the vaccinated and naturally infected groups. p58, p37, p35, and p30 were found to occur more frequently in infected, nonvaccinated dogs, and p93, p34, p31, and p28 appeared more often in vaccinated dogs. Differences in protein expression between spirochetes that are transmitted to hosts via ticks and spirochetes that are grown in culture may explain the differences in band frequencies, as demonstrated in several studies. Schwan et al. (28) reported that spirochetes in unfed ticks express OspA (p31) on their surface, which is down-regulated as ticks begin to feed. Fikrig et al. (6) showed that antibodies to p35 and p37 were found in mice infected with B. burgdorferi via ticks but not in mice infected with killed spirochetes.
TABLE 1.
Band frequency and chi-square analysis of serum samples from naturally infected and vaccinated dogs
| Band | % Band frequency
|
χ2 | P value | |
|---|---|---|---|---|
| Infected | Vaccinated | |||
| p93 | 94 | 70 | 22.81 | <0.001 |
| p66 | 94 | 68 | 24.18 | <0.001 |
| p60 | 98 | 65 | 36.04 | <0.001 |
| p58 | 84 | 31 | 74.42 | <0.001 |
| p45 | 97 | 57 | 44.48 | <0.001 |
| p41 | 94 | 65 | 28.37 | <0.001 |
| p39 | 88 | 47 | 43.76 | <0.001 |
| p37 | 97 | 18 | 164.00 | <0.001 |
| p35 | 79 | 6 | 180.00 | <0.001 |
| p34 | 30 | 97 | 166.48 | <0.001 |
| p31 | 17 | 99 | 247.00 | <0.001 |
| p30 | 91 | 82 | 4.27 | 0.038 |
| p28 | 72 | 79 | 1.87 | 0.17 |
| p23 | 47 | 62 | 5.00 | 0.03 |
| p21 | 71 | 72 | 0.03 | 0.86 |
| p18 | 64 | 76 | 5.69 | 0.02 |
| p17 | 71 | 61 | 2.61 | 0.11 |
| p15 | 71 | 51 | 9.99 | 0.002 |
| p14 | 54 | 36 | 8.68 | 0.003 |
TABLE 2.
Significant bands in the logistic regression model
| Band | Regression coefficient | Significance level |
|---|---|---|
| p93 | −3.62 | 0.02 |
| p58 | 2.10 | 0.03 |
| p37 | 4.37 | 0.003 |
| p35 | 2.68 | 0.009 |
| p34 | −2.90 | 0.006 |
| p31 | −6.84 | <0.001 |
| p30 | 3.60 | 0.01 |
| p28 | −2.55 | 0.02 |
The logistic regression model included the seven bands as significant (P < 0.05) predictors of infection status and the intercept term 2.85. The regression equation (10) assuming z = log odds, was: z = 2.85 − 3.62(p93) + 2.10(p58) + 4.37(p37) + 2.70(p35) − 2.90(p34) − 6.84(p31) + 3.60(p30) − 2.55(p28). The Hosmer and Lemeshow (11) goodness-of-fit test indicated no significant deviation of observed from expected values in the model (χ2 = 6.50, P = 0.48). The overall fit of the model, as measured by the log-likelihood statistic (G), was highly significant (χ2 = 332.70, P < 0.0001). No significant interactions between bands were obtained from the model.
The probability that a vaccinated dog is also infected can be calculated as follows. The value of z is estimated by multiplying the coefficient of each independent variable by 1 if the band is present in the immunoblot and by 0 if the band is absent. The z value is then entered into the following equation (13): P(x) = 1/[1 + e−(Z)], where P(x) is the probability of natural infection, ranging from 0 to 1. For example, in an immunoblot having only the p31 and p34 bands (i.e., those bands most frequently present in vaccinated, uninfected dogs), the probability that the dog is also naturally infected is only 0.10%. In a sample containing all of the bands except p31 and p34, the probability of natural infection is very high (99.9%).
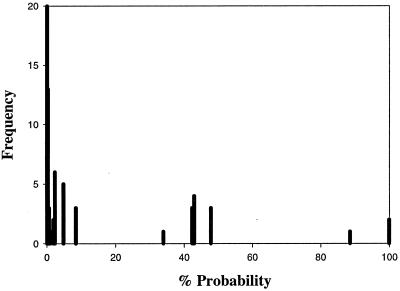
To test the predictive ability of the model, the immunoblot results for the remaining 125 samples of vaccinated dogs from areas where the disease is endemic were entered into the equation to determine the probability of natural infection. Among the samples, 87.2% had a probability of less than 10% of being naturally infected (Fig. 2). There were two small, well-defined clusters at 30 to 50% probability and at greater than 80% probability of natural infection. The model was able to separate adequately the vaccinated group from the other two groups having an increased probability of infection. These samples would have been considered positive for infection by previously derived criteria. However, the model determined that only 2.4% were highly likely to be infected and 10.4% were possibly infected.
FIG. 2.
Histogram of the probability of natural infection among vaccinated dogs from areas where Lyme disease is endemic.
DISCUSSION
Detection of antibodies corresponding to particular B. burgdorferi antigens, as revealed by immunoblot assay, is useful in at least two settings. In clinical practice, it is important to determine if a dog that has symptoms resembling Lyme disease and shows variable antibodies in an immunoblot assay, as a consequence of either vaccination, natural exposure to B. burgdorferi, or the presence of heterologous antibodies (20), is actually infected. In the epidemiologic setting, when dog sera are used in serosurveys to measure exposure to B. burgdorferi by tick bite in a given region (16, 18), researchers need to know whether the antibodies they detect by ELISA or IFA are a consequence of natural exposure, vaccination, or both. Vaccination confounds the interpretation of results of serologic and immunoblot assays in both settings, as no definitive criteria are available for separating naturally infected from vaccinated dogs. Here, we analyzed canine sera submitted by veterinary clinics in areas where Lyme disease is endemic or nonendemic, and where vaccination records were reliably available, in order to determine if such criteria could be derived through statistical analysis.
Previous studies have characterized serological responses in natural and experimental infections in dogs. Barthold et al. (1) reported variable immunoblot patterns in natural infections, where the most frequent reactions were against p41, p39, and p22 (possibly OspC). Naturally infected dogs have also been found to have strong antibody responses to p41, p39, and various high-molecular-weight proteins (14). For naturally exposed dogs, Greene et al. (10) reported the presence of at least 15 bands on immunoblots. Major bands included 83 (i.e., 93 by Immunetics immunoblot assay), 66, 61 to 60, 41 to 39, 31 to 29, 17, and 15 kDa. Samples from dogs experimentally infected with live spirochetes exhibited the p31 and p34 bands, with usually six or fewer other bands present. Samples from dogs vaccinated with killed spirochetes also reacted strongly and predominantly to the p31 and p34 antigens (1, 4).
The logistic regression model showed that vaccinated dogs exhibited increased frequencies of antibodies to p31 and p34, consistent with previous studies (1), but also had greater frequencies of the p28 (possibly OspF, a surface lipoprotein) and p93 bands. The p41 band could not be used to distinguish between the two groups, and it was also consistently present in immunoblot-negative samples from dogs that had been vaccinated against leptospirosis. It is important to emphasize that p23 (OspC) was not found to be a reliable marker separating vaccinated and naturally infected dogs, even though other studies have suggested that it should be present in natural infections (5, 7, 25).
The model showed that among those dogs determined to be naturally infected, the only bands exhibiting significantly increased frequencies were p58, p37, p35, and p30. Bands p58 and p30 are considered significant bands in the human diagnostic criteria for Lyme disease by the Centers for Disease Control and Prevention (3). p35 and p37 have been shown to be markers of natural infection in mice by Fikrig et al. (6). The p39 band has been reported as a possible marker for natural infection in dogs and mice (1, 10, 29). However, Chu et al. (4) reported that vaccinated dogs had antibodies to p39 in addition to p31 and p34 and Gauthier and Mansfield (8) found p39 antibodies present in 100% of naturally infected dogs and in 60% of recently vaccinated dogs. In our model, p39 antibodies were present in both vaccinated and naturally infected dogs.
When a vaccinated dog from an area where the disease is endemic is exhibiting symptoms of Lyme disease and has been previously vaccinated, it is important to determine if the dog is also harboring an active infection. The logistic regression model was used to determine if certain antibodies to B. burgdorferi antigens that are present in naturally infected dogs were significantly different from those elicited through vaccination. The model was able to determine that eight bands were significantly different between the two groups. Gauthier and Mansfield (8) found specific bands that were unique to each status; however, in the present study, individual bands did not seem to be uniquely present in vaccinated or naturally infected canines. The logistic regression model allows the simultaneous inclusion and evaluation of the bands for significance and interaction. An adequate sample size (n = 269) relative to the number of independent variables (n = 19) ensured sufficient power to detect the significant differences in bands between the two groups. The logistic regression equation was derived, and the presence or absence of significant bands was used to classify the immunoblot results into the vaccinated or infected status. The model was then used to compute the probability of a superimposed natural infection among the vaccinated dogs from areas where the disease is endemic and predict their serological status.
The bands that were statistically significant in the model were also found to be biologically significant, as supported by the referenced literature. However, this model may reflect the band patterns seen in our study area. Since band patterns may vary regionally, depending on possible strain variations of B. burgdorferi and different laboratory protocols, individual laboratories with adequate sample numbers can develop a model using their own immunoblots to distinguish between natural infection and the vaccinated state. Using a quantitative approach for the analysis of immunoblots can aid veterinarians in the diagnosis of canine Lyme disease and epidemiologists in a more accurate determination of canine B. burgdorferi prevalence rates, since vaccination has become a widely accepted method of Lyme disease prevention and its use will likely continue.
ACKNOWLEDGMENTS
This study was funded by NIH grant AI36917.
We thank L. Greeley and M. Segre for their assistance with serology and J. Piesman and R. Weigel for their helpful comments on the preparation of the manuscript.
REFERENCES
- 1.Barthold S W, Levy S A, Fikrig E, Bockenstedt L K, Smith A L. Serologic responses of dogs naturally exposed to or vaccinated against Borrelia burgdorferi infection. J Am Vet Med Assoc. 1995;207:1435–1440. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Methods used for creating a national Lyme disease risk map. Morb Mortal Wkly Rep. 1999;48(RR07):21–24. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 4.Chu H J, Chavez L G, Jr, Blumer B M, Sebring R W, Wasmoen T L, Acree W M. Immunogenicity and efficacy study of a commercial Borrelia burgdorferi bacterin. J Am Vet Med Assoc. 1992;201:403–411. [PubMed] [Google Scholar]
- 5.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 6.Fikrig E, Barthold S W, Sun W, Feng W, Telford S R, 3rd, Flavell R A. Borrelia burgdorferi P35 and P37 proteins, expressed in vivo, elicit protective immunity. Immunity. 1997;6:531–539. doi: 10.1016/s1074-7613(00)80341-6. [DOI] [PubMed] [Google Scholar]
- 7.Fung B P, McHugh G L, Leong J M, Steere A C. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62:3213–3221. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauthier D T, Mansfield L S. Western immunoblot analysis for distinguishing vaccination and infection status with Borrelia burgdorferi (Lyme disease) in dogs. J Vet Diagn Investig. 1999;11:259–265. doi: 10.1177/104063879901100309. [DOI] [PubMed] [Google Scholar]
- 9.Greene R T, Walker R L, Burgess E C, Levine J F. Heterogeneity in immunoblot patterns obtained by using four strains of Borrelia burgdorferi and sera from naturally exposed dogs. J Clin Microbiol. 1988;26:2287–2291. doi: 10.1128/jcm.26.11.2287-2291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene R T, Levine J F, Breitschwerdt E B, Walker R L, Berkhoff H A, Cullen J, Nicholson W L. Clinical and serologic evaluations of induced Borrelia burgdorferi infection in dogs. Am J Vet Res. 1988;49:752–757. [PubMed] [Google Scholar]
- 11.Hosmer D W, Lemeshow S. Applied logistic regression. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 12.Jacobson R H, Chang Y F, Shin S J. Lyme disease: laboratory diagnosis of infected and vaccinated symptomatic dogs. Semin Vet Med Surg (Small Anim) 1996;11:172–182. doi: 10.1016/s1096-2867(96)80030-2. [DOI] [PubMed] [Google Scholar]
- 13.Kleinbaum D G. Logistic regression: a self-learning text. New York, N.Y: Springer-Verlag; 1994. [Google Scholar]
- 14.Levy S A, Lissman B A, Ficke C M. Performance of a Borrelia burgdorferi bacterin in borreliosis-endemic areas. J Am Vet Med Assoc. 1993;202:1834–1838. [PubMed] [Google Scholar]
- 15.Lindenmayer J, Weber M, Bryant J, Marquez E, Onderdonk A. Comparison of indirect immunofluorescent-antibody assay, enzyme-linked immunosorbent assay, and Western immunoblot for the diagnosis of Lyme disease in dogs. J Clin Microbiol. 1990;28:92–96. doi: 10.1128/jcm.28.1.92-96.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenmayer J, Durwood M, Onderdonk A. Dogs as sentinels for Lyme disease in Massachusetts. Am J Public Health. 1991;81:1448–1455. doi: 10.2105/ajph.81.11.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma B, Christen D, Leung D, Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992;30:370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnarelli L, Anderson J, Kaufman A, Lieberman L, Whitney G. Borreliosis in dogs from southern Connecticut. J Am Vet Med Assoc. 1985;186:955–959. [PubMed] [Google Scholar]
- 19.Magnarelli L, Anderson J, Schreier A, Ficke C. Clinical and serological studies of canine borreliosis. J Am Vet Med Assoc. 1987;191:1089–1094. [PubMed] [Google Scholar]
- 20.Magnarelli L A, Anderson J F, Johnson R C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 21.Magnarelli L A, Flavell R A, Padula S J, Anderson J F, Fikrig E. Serologic diagnosis of canine and equine borreliosis: use of recombinant antigens in enzyme-linked immunosorbent assays. J Clin Microbiol. 1997;35:169–173. doi: 10.1128/jcm.35.1.169-173.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnarelli L A, Meegan J M, Anderson J F, Chappell W A. Comparison of an indirect fluorescent-antibody test with an enzyme-linked immunosorbent assay for serological studies of Lyme disease. J Clin Microbiol. 1984;20:181–184. doi: 10.1128/jcm.20.2.181-184.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnarelli L A, Miller J N, Anderson J F, Riviere G R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990;28:1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pachner A R, Ricalton N S. Western blotting in evaluating Lyme seropositivity and the utility of a gel densitometric approach. Neurology. 1992;42:2185–2192. doi: 10.1212/wnl.42.11.2185. [DOI] [PubMed] [Google Scholar]
- 25.Padula S J, Sampieri A, Dias F, Szczepanski A, Ryan R W. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993;61:5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schillhorn van Veen T, Murphy A, Colmeri B. False positive Borrelia burgdorferi antibody titers associated with periodontal disease in dogs. Vet Rec. 1993;132:512. doi: 10.1136/vr.132.20.512. [DOI] [PubMed] [Google Scholar]
- 27.Schott S. Logistic regression and discriminant analysis. J Am Vet Med Assoc. 1991;198:1902–1903. [PubMed] [Google Scholar]
- 28.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson W J, Burgdorfer W, Schrumpf M E, Karstens R H, Schwan T G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991;29:236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker E D, Stobierski M G, Poplar M L, Smith T W, Murphy A J, Smith P C, Schmitt S M, Cooley T M, Kramer C M. Geographic distribution of ticks (Acari: Ixodidae) in Michigan, with emphasis on Ixodes scapularis and Borrelia burgdorferi. J Med Entomol. 1998;35:872–882. doi: 10.1093/jmedent/35.5.872. [DOI] [PubMed] [Google Scholar]
- 31.Wittenbrink M M, Failing K, Krauss H. Enzyme-linked immunosorbent assay and immunoblot analysis for detection of antibodies to Borrelia burgdorferi in dogs. The impact of serum absorption with homologous and heterologous bacteriae. Vet Microbiol. 1996;48:257–268. doi: 10.1016/0378-1135(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 32.Zoller L, Burkard S, Shafer H. Validity of Western immunoblot patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991;29:174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoller L, Cremer J, Faulde M. Western blot as a tool in the diagnosis of Lyme borreliosis. Electrophoresis. 1993;14:937–944. doi: 10.1002/elps.11501401149. [DOI] [PubMed] [Google Scholar]