Abstract
Powdery mildew, caused by Sphaerotheca sp., annually causes severe losses in yield and quality in Rosa roxburghii production areas of southwest China. In this study, the role of the co-application of allicin and chitosan in the resistance of R. roxburghii against powdery mildew and its effects on growth, yield and quality of R. roxburghii were investigated. The laboratory toxicity test results show that allicin exhibited a superior antifungal activity against Sphaerotheca sp. with EC50 value of 148.65 mg kg−1. In the field, the foliar application of allicin could effectively enhance chitosan against powdery mildew with control efficacy of 85.97% by spraying 5% allicin microemulsion (ME) 100–time liquid + chitosan 100–time liquid, which was significantly (p < 0.01) higher than 76.70% of allicin, 70.93% of chitosan and 60.23% of polyoxin. The co-application of allicin and chitosan effectively enhanced the photosynthetic rate and chlorophyll of R. roxburghii compared with allicin, chitosan or polyoxin alone. Moreover, allicin used together with chitosan was more effective than allicin or chitosan alone in enhancing R. roxburghii plant growth and fruit yield as well as improving R. roxburghii fruit quality. This work highlights that the co-application of allicin and chitosan can be used as a green, cost-effective and environmentally friendly alternative strategy to conventional antibiotics for controlling powdery mildew of R. roxburghii.
Keywords: allicin, chitosan, Sphaerotheca sp., antibiotic, Rosa roxburghii
1. Introduction
Rosa roxburghii Tratt., an edible and medicinal fruit rich in vitamin C, flavonoids, superoxide dismutase (SOD) and various minerals, has high medicinal and nutritional values [1,2,3,4,5]. Recently, the R. roxburghii industry has developed rapidly in southwest China, especially in Guizhou Province, where the planting areas reached 170,000 hm2 in 2020 [3,6]. Powdery mildew, caused by Sphaerotheca sp., is the most serious disease regarding R. roxburghii production [1]. In Guizhou Province of southwest China, powdery mildew seriously affects the growth, yield, and quality of R. roxburghii, and often causes 30~40% economic losses [7]. Although some chemical fungicides (triadimefon, myclobutanil, azoxystrobin and tebuconazole) [8] and conventional antibiotics (polyoxin and kasugamycin) [9] are frequently used to control powdery mildew, their residuals inevitably affect the environment, wildlife, and human beings [10]. Moreover, these chemicals and antibiotics easily generate resistance to pathogens with the increase in the use frequency [11,12]. Therefore, there is an urgent need to develop an alternative, cost-effective and environmentally friendly control strategy against powdery mildew of R. roxburghii.
It is generally believed that natural products are mild and basically harmless compared with chemical fungicides and conventional antibiotics. Although this view is not completely accurate, it has been suggested as one of the reasons for the growing preference of natural products by consumers, and their increasingly popular use in agriculture [13,14]. For instance, Yan et al. [8] reported that 6% ascorbic acid aqueous solutions could induce R. roxburghii against powdery mildew with the control effect of 61.45%. Chitosan, a natural resource substance for sustainable agriculture, can be used as a resistance inductor and biofungicide for controlling plant diseases and as a promoter for enhancing plant growth [15,16,17,18,19,20]. In our previous study, the foliar application of 1.0~1.5% chitosan could effectively control powdery mildew of R. roxburghii with an inducing control efficacy of 69.30~72.87%, and could notably induce the systemic disease resistance of R. roxburghii, as well as reliably enhancing its photosynthesis, growth, yield, and quality [7]. Although chitosan can be used as an effective, safe and economical inductor for controlling powdery mildew, its control effect is still relatively inferior. Thus, natural products enhancing chitosan against powdery mildew of R. roxburghii are worthy of further exploration and development.
Allicin, an oxygenated sulfur natural compound, was isolated and identified from garlic in 1944 by Cavallito and Bailey [21]. Since then, allicin has been widely used in agricultural plant protection and medical therapy due to its superior antimicrobial activity and ecofriendly advantage [13,14,22,23,24,25]. Allicin has a prominent reactivity, antioxidant activity and membrane permeability, and can undergo thiol–disulphide exchange reactions with free thiol groups of proteins in microorganisms [13,14,26,27,28]. In agriculture, allicin has been demonstrated to have satisfactory bioactivity against many plant-pathogenic fungi, such as Plectospherella cucumerina, Botrytis cinerea, Phytophthora infestans, Xanthomonas axonopodis, Magnaporthe grisea and Alternaria brassicicola [13,29]. However, to date, there are no documentations available about the application of allicin for controlling powdery mildew of R. roxburghii caused by Sphaerotheca sp. Moreover, whether allicin can be used as an adjuvant to enhance chitosan against powdery mildew of R. roxburghii. is worth further attention.
In this work, the bioactivity of allicin, chitosan and conventional antibiotics against Sphaerotheca sp. was firstly determined. Subsequently, the field control efficacy of the co-application of allicin and chitosan for powdery mildew of R. roxburghii was evaluated. Moreover, the effects of the co-application of allicin and chitosan on the powdery mildew resistance, growth, yield and quality of R. roxburghii were investigated. This study provides a green, cost-effective and environmentally friendly alternative strategy to conventional antibiotics for controlling powdery mildew of R. roxburghii.
2. Materials and Methods
2.1. Fungicides
5% allicin microemulsion (ME) was produced from Ciyuan Biotechnology Co. Ltd. (Xian, China). Chitosan (deacetylation ≥90.00%) was obtained from Huarun Bioengineering Co. Ltd. (Zhenzhou, China). Additionally, 3% polyoxin wettable powder (WP) and 6% kasugamycin WP were provided by Lvdun Biological Products Co. Ltd. (Xian, China).
2.2. Field Site
The field experiments were carried out in 2020 in an orchard of R. roxburghii with a 7-year-old ‘Guinong 5’ cultivar in Chaxiang village, Gujiao Town, Longli country, Guizhou Province, China (26°54′36″ N, 106°95′13″ E). The planting density of R. roxburghii trees was 106 plants per 666.7 m2. The annual rainfall, mean temperature, annual sunshine duration, frostless season and mean altitude of field site were about 1100 mm, 13.9 °C, 1265 h, 280 days and 1384 m, respectively. The physical and chemical characteristics of planting soils are shown in Table 1.
Table 1.
The physical and chemical characteristics of planting soils of R. roxburghii.
| Parameters | Content | Parameters | Content |
|---|---|---|---|
| Organic matter | 13.17 g·kg−1 | Exchangeable calcium | 18.32 cmol·kg−1 |
| Total nitrogen | 1.37 g kg−1 | Exchangeable magnesium | 305.37 mg·kg−1 |
| Total phosphorus | 1.72 g kg−1 | Available zinc | 0.63 mg·kg−1 |
| Total potassium | 1.11 g kg−1 | Available iron | 6.42 mg·kg−1 |
| Available nitrogen | 57.43 mg·kg−1 | Available manganese | 15.33 mg·kg−1 |
| Available phosphorus | 4.21 mg·kg−1 | Available boron | 0.14 mg·kg−1 |
| Available potassium | 26.75 mg·kg−1 | pH | 6.89 |
2.3. In Vitro Toxicity Tests
The Sphaerotheca sp. pathogens of powdery mildew on R. roxburghii leaves were brushed into sterile water to produce a spore suspension with a concentration of about 100 spores per field of vision under a low power microscope. The healthy young leaves of R. roxburghii were washed, and their surface water was air-dried. No pathogen spores were found after microscopic examination. The healthy leaves were made into leaf discs with a diameter of 6 mm using a hole punch. The leaf discs were, respectively, immersed in the five gradient concentration solution of each tested fungicide for 10 s, and then placed in a Petri dish. Ten leaf discs were inoculated in each treatment with four replicates. Powdery mildew spores were inoculated on the front side of leaf discs by spray method, and the medium was sterile water. They were cultured in an artificial climate chamber at 20 °C for 7 days, with 10,000 lx of light intensity, 16 h/d of light duration and 70% relative humidity. The formula for calculating the inhibition rate of pathogens was as Equation (1):
| Inhibition rate (%) = 100 × (1 − Diseased leaf counts in treatment/Diseased leaf counts in control dish) | (1) |
EC50 (effective concentration of 50% inhibition rate) values were calculated statistically by a SPSS 18.0 software (SPSS Inc., Chicago, IL, USA).
2.4. Field Control Experiment of Powdery Mildew of R. roxburghii
The control experiment of powdery mildew of R. roxburghii was carried out using the foliar spray method. The experimental treatments included 5% allicin ME 100–time dilution liquid + chitosan 100–time dilution liquid, 5% allicin ME 100–time dilution liquid, chitosan 100–time dilution liquid, 3% polyoxin WP 100–time dilution liquid and clear water (control). A total of twenty plots were arranged randomly with four replicates and each plot had nine trees. Five trees on the diagonal of each plot were used for determination. Considering powdery mildew mainly damages the fresh young leaves and stems, as well as flower buds, flowers, and young fruits of R. roxburghii, about 1.50 L of fungicide dilution liquid was sprayed on each R. roxburghii plant (including leaves, stems, flowers and buds) on 31 March and 29 April 2020.
2.5. Investigation of Control Effect of Powdery Mildew of R. roxburghii, and Determination of Its Resistance Parameters, Photosynthetic Rate and Chlorophyll
The control effect of tested fungicides for powdery mildew of R. roxburghii was investigated on May 30 in 2020 according to Li et al. [7]. The incidence rate, disease index, and control effect of tested fungicides for powdery mildew of R. roxburghii were calculated according to Equations (2)–(4), respectively. The incidence degree: 0 = no incidence, 1 = 1~2 diseased lobules with thin hyphae, 2 = 3~4 diseased lobules with thick hyphae, 3 = 5~6 diseased lobules with dense hyphae, 4 = more than 7 diseased lobules with dense hyphae.
| Incidence rate (%) = 100 × Number of diseased leaves/Total number of leaves | (2) |
| Disease index = 100 × ∑ (Disease grade value × Number of leaves within each grade)/(Total number of leaves × the highest grade) | (3) |
| Control effect (%) = 100 × (1 − Disease index of treatment/Disease index of control) | (4) |
The resistance parameters of R. roxburghii leaves, such as proline (Pro), soluble sugar, malonaldehyde (MDA), flavonoid, SOD activity and PPO activity, were determined on 30 May 2020 as described by Zhang et al. [30,31]. The photosynthetic rate (Pn) of leaves in R. roxburghii was monitored using a portable LI-6400XT photosynthesis measurement system (LI-COR Inc., Lincoln, NE, USA) at 8:00–10:00 a.m. on 30 May. Chlorophyll content of R. roxburghii leaves was determined by a UV-5800PC spectrophotometer at 645 nm (OD645) and 663 nm (OD663) with an acetone–ethanol (v/v, 2:1) extraction.
2.6. Determination of Yield and Quality of R. roxburghii
Fruits of R. roxburghii were randomly collected on 2 September in 2020, and then single fruit weight, yield per plant and quality of each plot were determined. The weighing method was used for determining single fruit weight and fruit yield per plant. Fruit quality of R. roxburghii, including vitamin C, soluble solid, soluble sugar, total acidity, soluble protein, flavonoid and SOD activity, were also determined as described by Zhang et al. [30,31].
2.7. Statistical Analyses
The mean ± standard deviation (SD) of four replicates were exhibited. SPSS 18.0 was used for analyses. Significant differences were determined by a one-way analysis of variance (ANOVA). Origin 10.0 was used for drawing the chart.
3. Results
3.1. Toxicity of Allicin and Chitosan against Sphaerotheca sp.
The toxicity of allicin, chitosan, polyoxin and kasugamycin against Sphaerotheca sp. is shown in Table 2. The 5% allicin ME treatment exhibited an outstanding toxicity against Sphaerotheca sp. of R. roxburghii with EC50 value of 148.65 mg kg−1, which was higher by 2.80- 1.24- and 6.95-fold than chitosan, 3% polyoxin WP and 6% kasugamycin WP, respectively. Although chitosan had a relatively inferior toxicity against Sphaerotheca sp., its EC50 value was 2.48-fold higher than that of 6% kasugamycin WP. The results here indicate that allicin possessed a superior antimicrobial activity compared to conventional antibiotics including polyoxin and kasugamycin.
Table 2.
The toxicity of allicin, chitosan, polyoxin and kasugamycin against Sphaerotheca sp.
| Treatments | Regression Equation | Determination Coefficient (R2) | EC50 (mg kg−1) |
|---|---|---|---|
| 5% Allicin ME | y = 2.3339 + 1.2274 x | 0.9626 | 148.65 |
| Chitosan | y = 2.5343 + 0.9413 x | 0.9748 | 416.21 |
| 3% Polyoxin WP | y = 2.9799 + 0.8922 x | 0.9937 | 183.68 |
| 6% Kasugamycin WP | y = 2.4254 + 0.8542 x | 0.9406 | 1032.88 |
y and x indicate the inhibition rate and fungicide concentration, respectively.
3.2. Field Control Effect of Allicin and Chitosan against Powdery Mildew of Rosa roxburghii
The field control effect of allicin + chitosan, allicin, chitosan and polyoxin against powdery mildew in R. roxburghii are shown in Table 3. Allicin + chitosan, allicin, chitosan and polyoxin significantly (p < 0.01) decreased the incidence rate and disease index of powdery mildew of R. roxburghii, and allicin + chitosan was the most effective. The control effect of allicin + chitosan against powdery mildew was 85.97%, which was significantly (p < 0.01) higher than 76.70% of allicin, 70.93% of chitosan and 60.23% of polyoxin. These results indicate that the co-application of allicin and chitosan effectively controlled powdery mildew of R. roxburghii, whose control effect was significantly better than that of allicin, chitosan, or polyoxin alone.
Table 3.
The control effect of allicin and chitosan against powdery mildew of R. roxburghii.
| Treatments | Incidence Rate (%) | Disease Index | Control Effect (%) |
|---|---|---|---|
| Allicin + Chitosan | 11.00 ± 1.00 cC | 2.14 ± 0.18 dD | 85.97 ± 1.16 aA |
| Allicin | 14.33 ± 1.53 cC | 3.53 ± 0.22 cC | 76.70 ± 1.10 bB |
| Chitosan | 16.00 ± 3.61 cC | 4.42 ± 0.10 cC | 70.93 ± 2.12 cB |
| Polyoxin | 26.67 ± 1.53 bB | 6.04 ± 0.19 bB | 60.23 ± 4.17 dC |
| Control | 45.67 ± 4.51 aA | 15.26 ± 1.12 aA |
Values indicate the mean ± SD of three replicates. Different small letters indicate significant differences at 5% level (p < 0.05), and different capital letters indicate significant differences at 1% level (p < 0.01).
3.3. Effects of Allicin and Chitosan on Resistance Parameters of R. roxburghii Leaves
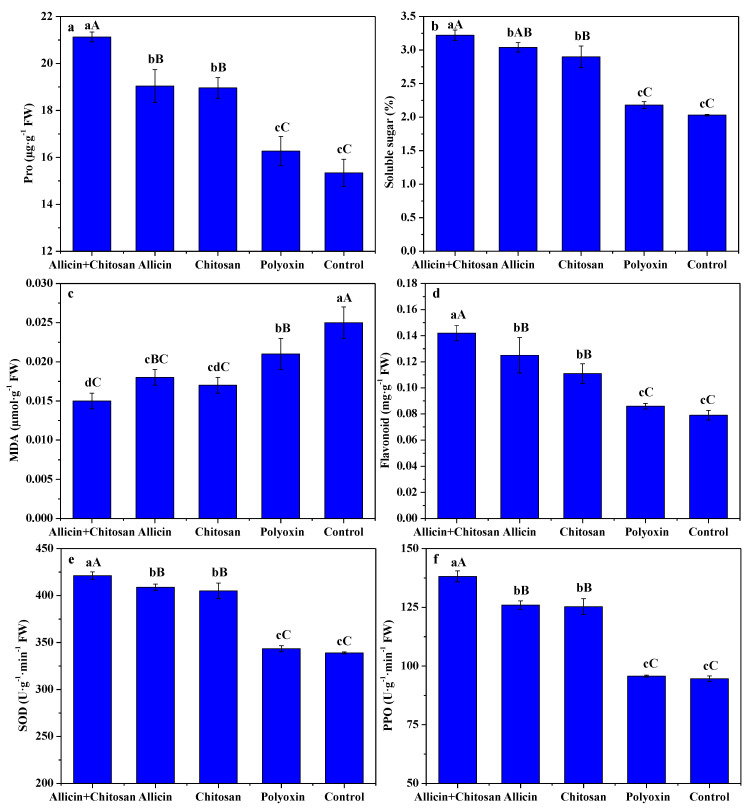
Figure 1 depicts the effects of allicin + chitosan, allicin, chitosan and polyoxin on the Pro, soluble sugar, MDA, flavonoid, SOD activity and PPO activity of leaves in R. roxburghii. Compared to polyoxin or control, allicin + chitosan, allicin and chitosan significantly (p < 0.01) increased the contents of proline, soluble sugar, and flavonoid of R. roxburghii leaves, and significantly (p < 0.01) enhanced their SOD and PPO activities, as well as effectively reducing leaf MDA content. The enhancing effect of allicin + chitosan on Pro, flavonoid, SOD activity and PPO activity of R. roxburghii leaves were significantly (p < 0.01) higher than that of allicin or chitosan alone. Soluble sugar of R. roxburghii leaves treated by allicin + chitosan was significantly higher than that of allicin (p < 0.05) or chitosan (p < 0.01) alone. MDA of R. roxburghii leaves treated by allicin + chitosan was significantly (p < 0.05) lower than that of allicin, but had no significant difference to that of chitosan. These results indicate that the co-application of allicin and chitosan effectively improved enhancing or inhibiting effects of allicin or chitosan on the proline, soluble sugar, MDA, flavonoid, SOD activity and PPO activity of leaves in R. roxburghii.
Figure 1.
The effects of allicin and chitosan on the Pro (a), soluble sugar (b), malonaldehyde (c), flavonoid (d), SOD activity (e), and PPO activity (f) of leaves in R. roxburghii. Values and error bars indicate the mean and SD of three replicates, respectively. Different small letters indicate significant differences at 5% level (p < 0.05), and different capital letters indicate significant differences at 1% level (p < 0.01).
3.4. Effects of Allicin and Chitosan on Photosynthetic Rate and Chlorophyll Content of R. roxburghii Leaves
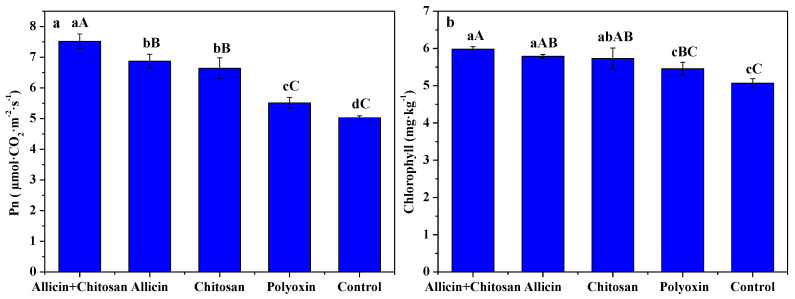
The effects of allicin + chitosan, allicin, chitosan and polyoxin on photosynthetic rate and chlorophyll content in R. roxburghii leaves are shown in Figure 2. Compared to polyoxin or control, allicin + chitosan, allicin and chitosan significantly (p < 0.01) enhanced the photosynthetic rate of R. roxburghii leaves. R. roxburghii leaves treated by allicin + chitosan exhibited an excellent photosynthetic rate with 7.52 μmol·CO2·m−2·s−1, which was significantly (p < 0.01) higher than 6.87 μmol·CO2·m−2·s−1 of allicin, and 6.64 μmol·CO2·m−2·s−1 of chitosan. Compared to control, allicin + chitosan, allicin and chitosan significantly (p < 0.01) increased the chlorophyll of R. roxburghii leaves, and there was no significant difference among the three treatments. Compared to polyoxin, allicin + chitosan could significantly (p < 0.01) increase the chlorophyll of R. roxburghii leaves, while allicin and chitosan could only significantly (p < 0.05) increase that of R. roxburghii leaves. The results presented here indicate that the co-application of allicin and chitosan effectively promoted leaf chlorophyll of R. roxburghii, thereby enhancing its photosynthesis.
Figure 2.
The effects of allicin and chitosan on the photosynthetic rate (a) and chlorophyll (b) of leaves in R. roxburghii. Values and error bars indicate the mean and SD of three replicates, respectively. Different small letters indicate significant differences at 5% level (p < 0.05), and different capital letters indicate significant differences at 1% level (p < 0.01).
3.5. Effects of Allicin and Chitosan on Yield and Quality of R. roxburghii
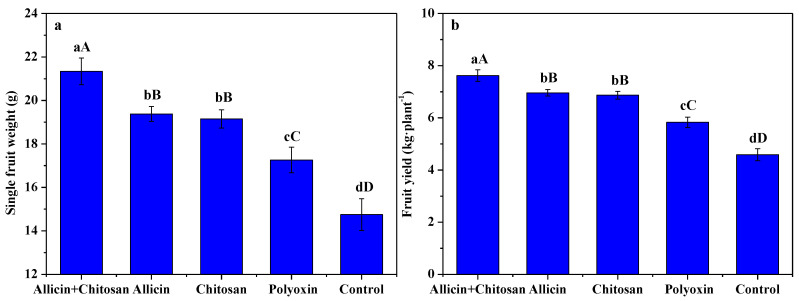
Figure 3 displays the effects of allicin + chitosan, allicin, chitosan and polyoxin on the single fruit weight and fruit yield per plant of R. roxburghii. Compared to polyoxin or control, allicin + chitosan, allicin and chitosan could significantly (p < 0.01) enhance the single fruit weight and fruit yield of R. roxburghii, and allicin + chitosan was the most efficient. The single fruit weight and fruit yield per plant of R. roxburghii treated by allicin + chitosan was 21.34 g and 7.62 kg, which significantly (p < 0.01) increased by 10.11% and 9.52%, 11.44% and 10.84%, 23.64% and 30.62%, and 44.68% and 66.11% compared to allicin, chitosan, polyoxin and control, respectively. The results of this work reveal that the co-application of allicin and chitosan effectively promoted fruit growth and yield formation of R. roxburghii.
Figure 3.
The effects of allicin and chitosan on single fruit weight (a) and fruit yield per plant (b) of R. roxburghii. Values and error bars indicate the mean and SD of three replicates, respectively. Different small letters indicate significant differences at 5% level (p < 0.05), and different capital letters indicate significant differences at 1% level (p < 0.01).
The effects of allicin + chitosan, allicin, chitosan and polyoxin on quality of R. roxburghii fruits are displayed in Table 4. Allicin + chitosan, allicin, and chitosan could significantly (p < 0.05) increase vitamin C, soluble solid, soluble sugar, total acidity, soluble protein, flavonoid, and SOD activity of R. roxburghii fruits compared to polyoxin or control. Vitamin C, soluble solid, soluble sugar, total acidity and SOD activity of R. roxburghii fruits treated by allicin + chitosan was significantly (p < 0.05) higher than that of allicin or chitosan. In addition, there were no significant (p < 0.05) differences between treatments of allicin and chitosan. These findings show that allicin used together with chitosan could effectively improve R. roxburghii fruit quality, and allicin and chitosan should have a notably synergistic effect in improving quality of R. roxburghii fruits.
Table 4.
The effects of allicin and chitosan on quality of R. roxburghii fruits.
| Treatments | Vitamin C (mg·g−1) | Soluble Solid (%) | Soluble Sugar(%) | Total Acidity (%) | Soluble Protein(%) | Flavonoid ggx(mg·g−1) | SOD Activity ggx(U·g−1 FW) |
|---|---|---|---|---|---|---|---|
| Allicin + Chitosan | 23.85 ± 0.16 a | 12.65 ± 0.08 a | 4.21 ± 0.10 a | 3.94 ± 0.06 a | 15.63 ± 0.47 a | 0.127 ± 0.006 a | 454.89 ± 2.05 a |
| Allicin | 22.78 ± 0.66 b | 12.18 ± 0.15 b | 3.92 ± 0.04 b | 3.62 ± 0.15 b | 14.87 ± 0.72 a | 0.119 ± 0.005 a | 444.45 ± 4.89 b |
| Chitosan | 22.56 ± 0.59 b | 12.12 ± 0.11 b | 3.87 ± 0.10 b | 3.53 ± 0.14 b | 14.59 ± 0.59 a | 0.117 ± 0.004 a | 441.12 ± 9.72 b |
| Polyoxin | 19.64 ± 0.52 c | 11.17 ± 0.13 c | 3.26 ± 0.03 c | 2.86 ± 0.09 c | 13.42 ± 0.61 b | 0.108 ± 0.008 b | 407.62 ± 5.04 c |
| Control | 17.88 ± 0.61 d | 10.35 ± 0.22 d | 3.14 ± 0.07 c | 2.51 ± 0.14 d | 12.65 ± 0.55 b | 0.096 ± 0.003 c | 376.95 ± 1.49 d |
Values indicate the mean ± SD of three replicates. Different small letters indicate significant differences at 5% level (p < 0.05).
4. Discussion
Previous findings have demonstrated that allicin could effectively inhibit the growth of Plectospherella cucumerina, Botrytis cinerea, Phytophthora infestans, Xanthomonas axonopodis, Magnaporthe grisea and Alternaria brassicicola, etc. [13,29], and chitosan had antifungal activity against various fungal pathogens [15,16,32,33,34,35]. The results here show that 5% allicin ME displayed outstanding toxicity against Sphaerotheca sp., with an EC50 value of 148.65 mg kg−1, which was 2.80-, 1.24- or 6.95-fold higher than chitosan, 3% polyoxin WP or 6% kasugamycin WP, respectively. This work extended the antimicrobial spectrum of allicin. Although chitosan exhibited a relatively inferior toxicity against Sphaerotheca sp., its EC50 value was still 2.48-fold higher than that of 6% kasugamycin WP. Moreover, the control effect of powdery mildew of R. roxburghii by allicin + chitosan was 85.97%, which was significantly (p < 0.01) higher than 76.70% of allicin, 70.93% of chitosan and 60.23% of polyoxin, respectively. Chitosan can trigger plant defense responses by inducing a variety of defense-related reactions [16,34,35,36,37,38]. Our previous results show that the inducing control effect of 1.0~1.5% chitosan against Sphaerotheca sp. was 69.30~72.87% [7]. In this study, the co-application of allicin and chitosan significantly (p < 0.01) enhanced the control effect of powdery mildew in R. roxburghii compared with allicin, chitosan or conventional antibiotic polyoxin alone. This suggests that allicin and chitosan had a notably synergetic effect in the control of powdery mildew of R. roxburghii. The effective control effect of allicin + chitosan was probably derived from the superior antimicrobial activity of allicin, as well as the excellent antimicrobial and induced resistance effect of chitosan.
The inducing of disease resistance is an effective agricultural practice for controlling plant diseases [39,40]. Pro and soluble sugar are important regulators of cell permeability, MDA is an important indicator of membrane lipid peroxidation and flavonoid is an important disease-resistant substance, as well as SOD and PPO being defense enzymes associated with plant disease resistance [38,40]. Many studies have also shown that chitosan could induce increases in sugar, Pro, flavonoid, polyphenolics and lignin in the plant and boost its defense enzyme activity, thereby enhancing its disease resistance [16,30,31,32,34,35,36,37,38,39,40]. Our previous results also indicate that the foliar application of 1.0~1.5% chitosan significantly (p < 0.01) increased Pro, soluble sugar and flavonoid contents, as well as SOD and POD activities of R. roxburghii leaves, and decreased their MDA [7]. The present results show that as compared with polyoxin or control, allicin + chitosan, allicin and chitosan could effectively increase Pro, soluble sugar, and flavonoid of R. roxburghii leaves, and enhance their SOD and PPO activities, as well as reduce their MDA, which is consistent with the above studies. Moreover, the enhancing or inhibiting effects of allicin + chitosan on Pro, soluble sugar, flavonoid and MDA contents, as well as SOD and PPO activities of R. roxburghii leaves were higher than those of allicin or chitosan alone. These results emphasize that the co-application of allicin and chitosan was more helpful in improving the disease resistance of R. roxburghii, and an obviously synergetic effect of allicin and chitosan was available.
Chlorophyll is an essential pigment for plant photosynthesis, and photosynthesis is the physiological basis of plant growth and development. Chitosan can promote plant growth and development by enhancing the photosynthetic rate by increasing chlorophyll content [16]. Our previous results show that foliar application of 0.5~1.5% chitosan effectively enhanced the photosynthetic rate, the content of chlorophyll a, chlorophyll b, and chlorophyll a + b of R. roxburghii leaves [7]. In this work, the co-application of allicin and chitosan more effectively promoted the chlorophyll and photosynthetic rate of R. roxburghii leaves compared with allicin, chitosan or polyoxin alone. This is closely related to the synergistic effect between allicin protecting plant leaf organs from pathogens and chitosan promoting plant growth. The growth and development of R. roxburghii determine its fruit yield and quality. Chitosan can also promote plant growth by activating the auxin and cytokinin signal transduction and gene expression, as well as increasing the nutrient intake [16,41]. Our previous results also indicate that the foliar application of 1.0~1.5% chitosan notably improved yield and quality of R. roxburghii fruits [7]. The present results indicate that the co-application of allicin and chitosan effectively enhance R. roxburghii fruit growth and yield formation. Moreover, vitamin C, soluble solid, soluble sugar, total acidity and SOD activity of R. roxburghii fruits treated by allicin + chitosan was significantly (p < 0.05) higher than that of treatments by allicin or chitosan alone. These notable effects were probably derived from their division of labor; allicin can protect R. roxburghii from pathogen infection and chitosan can induce the disease resistance of R. roxburghii, which guarantee the healthy growth of R. roxburghii plants.
At present, increasing attention has been focused on natural products as effective fungicides for controlling plant fungal disease, with high efficacy, nontoxicity and low food safety risks [24,42]. Therefore, natural products as an alternative to traditional antibiotics have been recognized by the public. Allicin, extracted from garlic, is used for daily consumption, and chitosan is a natural, nontoxic substance widely used in food, cosmetics and other fields. Moreover, the safe interval period (from April 29 to September 2, more than 120 days) of R. roxburghii was very long. Thus, the food safety risks caused by allicin or chitosan are almost nonexistent. This study highlights that the co-application of allicin and chitosan can be used as a green, cost-effective and environmentally friendly alternative approach to conventional antibiotics for controlling powdery mildew of R. roxburghii and enhancing its resistance, growth, yield and quality.
5. Conclusions
In conclusion, allicin displayed outstanding antifungal activity against Sphaerotheca sp. compared with conventional antibiotics including polyoxin and kasugamycin. The co-application of allicin and chitosan effectively controlled powdery mildew of R. roxburghii, and reliably enhanced Pro, soluble sugar and flavonoid contents, SOD and PPO activities in R. roxburghii leaves and reduced their MDA contents, as well as notably promoted the photosynthetic rate and chlorophyll contents of R. roxburghii. Moreover, the co-application of allicin and chitosan was more effective than allicin or chitosan alone in enhancing growth of R. roxburghii plants and improving quality of R. roxburghii fruits. This work highlights that the co-application of allicin and chitosan can be used as an ideal alternative to conventional antibiotics for controlling powdery mildew of R. roxburghii.
Author Contributions
X.W. constructed the project; X.W., H.A., J.L. and R.L. designed the experiments; J.L., R.L., C.Z. and Z.G. performed the experiments; J.L., R.L. and C.Z. analyzed the data; J.L., R.L., X.W. and H.A. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (no. 32160656), the Science-Technology Support Program of Guizhou Province (no. (2019)2407, (2020)1Y134, (2021) YB243), the “Hundred” Level Innovative Talent Foundation of Guizhou Province (no. 20164016), and the Cultivation Program of Guizhou University (no. (2019)09).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used or analyzed during the current study available from the corresponding author upon reasonable request.
Conflicts of Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang L.-T., Lv M.-J., An J.-Y., Fan X.-H., Dong M.-Z., Zhang S.-D., Wang J.-D., Wang Y.-Q., Cai Z.-H., Fu Y.-J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021;12:1432–1451. doi: 10.1039/D0FO02603D. [DOI] [PubMed] [Google Scholar]
- 2.Qi L.L., Zhou R.L. The Healthcare Function and Development Trend of Toxburgh Rose. Food Res. Dev. 2016;37:212–214. [Google Scholar]
- 3.Wang D., Lu M., Ludlow R.A., Zeng J., Ma W., An H. Comparative ultrastructure of trichomes on various organs of Rosa roxburghii. Microsc. Res. Tech. 2021;84:2095–2103. doi: 10.1002/jemt.23765. [DOI] [PubMed] [Google Scholar]
- 4.Liu X.Z., Zhao H.B., Li Y.F., Yu Z.H., Liu X.H., Huang M.Z. Identification and Oenological Properties Analysis of a Strain of Hanseniaspora uvarum from Rosa roxburghii. Food Ferment. Ind. 2020;46:97–104. [Google Scholar]
- 5.Huang X., Yan H., Zhai L., Yang Z., Yi Y. Characterization of the Rosa roxburghii Tratt transcriptome and analysis of MYB genes. PLoS ONE. 2019;14:e0203014. doi: 10.1371/journal.pone.0203014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan W.G., Pan X.J., Chen H., Yang H.R., Gong F.F., Guan J.Y., Wang M.L., Mu R. Effects of Oxalic Acid on the Nutrient of Calcareous Cultivated Soil and Leaf, Fruit Yield and Quality of Rosa roxburghii Tratt. J. Fruit Sci. 2021;38:1113–1122. doi: 10.13925/j.cnki.gsxb.20200495. [DOI] [Google Scholar]
- 7.Li J., Guo Z., Luo Y., Wu X., An H. Chitosan Can Induce Rosa roxburghii Tratt. against Sphaerotheca sp. and Enhance Its Resistance, Photosynthesis, Yield, and Quality. Horticulturae. 2021;7:289. doi: 10.3390/horticulturae7090289. [DOI] [Google Scholar]
- 8.Yan K., Wang J.L., Zhou Y., Fu D.P., Huang R.M. Efficacy of Five Fungicides in Rosa roxburghii Tratt against Sphaerotheca sp. Agrochemicals. 2018;57:609–610. [Google Scholar]
- 9.Xiang J., He B. Toxicity Determination of Several Bio-fungicides to Powdery Mildew in Laboratory. Sci. Technol. Modern Agric. 2013;19:147. [Google Scholar]
- 10.Meena R.S., Kumar S., Datta R., Lal R., Vijayakumar V., Brtnicky M., Sharma M.P., Yadav G.S., Jhariya M.K., Jangir C.K., et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land. 2020;9:34. doi: 10.3390/land9020034. [DOI] [Google Scholar]
- 11.Wang Q., Zhang C., Long Y., Wu X., Su Y., Lei Y., Ai Q. Bioactivity and Control Efficacy of the Novel Antibiotic Tetramycin against Various Kiwifruit Diseases. Antibiotics. 2021;10:289. doi: 10.3390/antibiotics10030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massi F., Torriani S.F.F., Borghi L., Toffolatti S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms. 2021;9:119. doi: 10.3390/microorganisms9010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slusarenko A.J., Patel A., Portz D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008;121:313–322. doi: 10.1007/s10658-007-9232-7. [DOI] [Google Scholar]
- 14.Borlinghaus J., Albrecht F., Gruhlke M.C.H., Nwachukwu I., Slusarenko A.J. Allicin: Chemistry and Biological Properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verlee A., Mincke S., Stevens C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017;164:268–283. doi: 10.1016/j.carbpol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty M., Hasanuzzaman M., Rahman M., Khan A.R., Bhowmik P., Mahmud N.U., Tanveer M., Islam T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture. 2020;10:624. doi: 10.3390/agriculture10120624. [DOI] [Google Scholar]
- 17.Torres-Rodriguez J.A., Reyes-Pérez J.J., Castellanos T., Angulo C., Quiñones-Aguilar E.E., Hernandez-Montiel L.G. A biopolymer with antimicrobial properties and plant resistance inducer against phytopathogens: Chitosan. Not. Bot. Horti Agrobot. Cluj-Napoca. 2021;49:12231. doi: 10.15835/nbha49112231. [DOI] [Google Scholar]
- 18.Rahman M., Mukta J.A., Sabir A.A., Gupta D.R., Mohi-Ud-Din M., Hasanuzzaman M., Miah M.G., Rahman M., Islam M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE. 2018;13:e0203769. doi: 10.1371/journal.pone.0203769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutinho T.C., Ferreira M.C., Rosa L.H., de Oliveira A.M., Júnior E.N.D.O. Penicillium citrinum and Penicillium mallochii: New phytopathogens of orange fruit and their control using chitosan. Carbohydr. Polym. 2020;234:115918. doi: 10.1016/j.carbpol.2020.115918. [DOI] [PubMed] [Google Scholar]
- 20.El Amerany F., Meddich A., Wahbi S., Porzel A., Taourirte M., Rhazi M., Hause B. Foliar Application of Chitosan Increases Tomato Growth and Influences Mycorrhization and Expression of Endochitinase-Encoding Genes. Int. J. Mol. Sci. 2020;21:535. doi: 10.3390/ijms21020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallito C.J., Bailey J.H. Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944;66:1950–1951. doi: 10.1021/ja01239a048. [DOI] [Google Scholar]
- 22.Auger J., Arnault I., Diwo-Allain S., Ravier N., Molia F., Pettiti M. Insecticidal and fungicidal potential of Allium substances as biofumigants. Agroindustria. 2004;3:5–8. [Google Scholar]
- 23.Khodavandi A., Alizadeh F., Harmal N.S., Sidik S.M., Othman F., Jahromi M.A.F., Sekawi Z., Ng K.-P., Chong P.P. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. FEMS Microbiol. Lett. 2011;315:87–93. doi: 10.1111/j.1574-6968.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- 24.Marchese A., Barbieri R., Sanches-Silva A., Daglia M., Nabavi S.F., Jafari N.J., Izadi M., Ajami M., Nabavi S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016;52:49–56. doi: 10.1016/j.tifs.2016.03.010. [DOI] [Google Scholar]
- 25.Choo S., Chin V.K., Wong E.H., Madhavan P., Tay S.T., Yong P.V.C., Chong P.P. Review: Antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020;65:451–465. doi: 10.1007/s12223-020-00786-5. [DOI] [PubMed] [Google Scholar]
- 26.Omar S., Al-Wabel N. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010;18:51–58. doi: 10.1016/j.jsps.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwivedi V.P., Bhattacharya D., Singh M., Bhaskar A., Kumar S., Fatima S., Sobia P., Van Kaer L., Das G. Allicin enhances antimicrobial activity of macrophages during Mycobacterium tuberculosis infection. J. Ethnopharmacol. 2019;243:111634. doi: 10.1016/j.jep.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Buendía A.S.A., González M.T., Reyes O.S., Arroyo F.E.G., García R.A., Tapia E., Lozada L.G.S., Alonso H.O. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2018;19:3107. doi: 10.3390/ijms19103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis H., Noll U., Störmann J., Slusarenko A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004;65:79–89. doi: 10.1016/j.pmpp.2004.11.006. [DOI] [Google Scholar]
- 30.Zhang C., Long Y.-H., Wang Q.-P., Li J.-H., Wu X.-M., Li M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit. Hortic. Sci. 2019;46:180–194. doi: 10.17221/84/2018-HORTSCI. [DOI] [Google Scholar]
- 31.Zhang C., Long Y., Li J., Li M., Xing D., An H., Wu X., Wu Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy. 2020;10:265. doi: 10.3390/agronomy10020265. [DOI] [Google Scholar]
- 32.Xing K., Zhu X., Peng X., Qin S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015;35:569–588. doi: 10.1007/s13593-014-0252-3. [DOI] [Google Scholar]
- 33.Berger L.R.R., Stamford N.P., Willadino L.G., Laranjeira D., de Lima M.A.B., Malheiros S.M.M., de Oliveira W.J., Stamford T.C.M. Cowpea resistance induced against Fusarium oxysporum f. sp. tracheiphilum by crustaceous chitosan and by biomass and chitosan obtained from Cunninghamella elegans. Biol. Control. 2016;92:45–54. doi: 10.1016/j.biocontrol.2015.09.006. [DOI] [Google Scholar]
- 34.Obianom C., Romanazzi G., Sivakumar D. Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol. Technol. 2019;147:214–221. doi: 10.1016/j.postharvbio.2018.10.004. [DOI] [Google Scholar]
- 35.El-Mohamedya R.S.R., Abd El-Aziz M.E., Kamel S. Antifungal Activity of Chitosan Nanoparticles against Some Plant Pathogenic Fungi In Vitro. Agric. Eng. Int. CIGR J. 2019;21:201–209. [Google Scholar]
- 36.Yan J., Cao J., Jiang W., Zhao Y. Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Sci. Hortic. 2012;142:196–204. doi: 10.1016/j.scienta.2012.05.025. [DOI] [Google Scholar]
- 37.Ma Z., Yang L., Yan H., Kennedy J.F., Meng X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013;94:272–277. doi: 10.1016/j.carbpol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q., Zhang C., Wu X., Long Y., Su Y. Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules. 2021;11:1257. doi: 10.3390/biom11091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vlot A.C., Sales J.H., Lenk M., Bauer K., Brambilla A., Sommer A., Chen Y., Wenig M., Nayem S. Systemic propagation of immunity in plants. New Phytol. 2021;229:1234–1250. doi: 10.1111/nph.16953. [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Moya F., Suarez-Fernandez M., Lopez-Llorca L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019;20:332. doi: 10.3390/ijms20020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzung N.A., Khanh V.T.P., Dzung T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011;84:751–755. doi: 10.1016/j.carbpol.2010.07.066. [DOI] [Google Scholar]
- 42.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study available from the corresponding author upon reasonable request.