Abstract
This review reports on the effects of fermentation on the chemical constituents and antioxidant activity of plant-based food materials. Fermentation involves a series of reactions that modify the chemical components of the substrate. It could be considered a tool to increase the bioactive compounds and functional properties of food plant materials. Oxidative damage is key to the progression of many human diseases, and the production of antioxidant compounds by fermentation will be helpful to reduce the risk of these diseases. Fermentation also can improve antioxidant activity given its association with increased phytochemicals, antioxidant polysaccharides, and antioxidant peptides produced by microbial hydrolysis or biotransformation. Additionally, fermentation can encourage the breakdown of plant cell walls, which helps to liberate or produce various antioxidant compounds. Overall, results indicated that fermentation in many cases contributed to enhancing antioxidants’ content and antioxidant capacity, supporting the fermentation use in the production of value-added functional food. This review provides an overview of the factors that impact the effects of fermentation on bioactive compound composition and antioxidant activity. The impacts of fermentation are summarized as a reference to its effects on food plant material.
Keywords: plant-based food, fermentation, antioxidant activity, chemical composition, phytochemicals
1. Introduction
Today’s increased awareness of functional foods has resulted in attempts to modify available food to have higher health benefits. Plants, especially medicinal and edible plants that have high phenolic content and other bioactive compounds, can be used for the production of functional foods mainly because of the antioxidant capacity of its phytochemicals [1]. Natural antioxidants in foods are getting more and more attention due to their health and functional properties, and the expectation is that the development of natural antioxidants, such as proteins, flavonoids, and phenolic compounds inside plants, will enhance safety. For instance, phenolic compounds are naturally occurring compounds that consist of several groups, including phenols, flavonoids, tannins, and phenolic acids [2]. Much past research has focused on different methods to increase phenolic compounds in an effort to decrease or eliminate oxidative damage in the body. It has been recommended that some treatments induce levels of antioxidant activity and biologically active compounds [3]. The phenolics in food products could be affected by processing techniques, such as fermentation [4,5], enzymatic pretreatment [6], autoclaving pretreatment [7], pulsed electric fields (PEF) treatment [8], ultrasound-assisted extraction (UAE) [9], and sun-drying [10,11]. Fermentation is a biotechnological process that can improve the nutritional value and organoleptic characteristics of food by converting conjugated phenolic forms to free phenolic forms using enzymes produced by microorganisms [4]. It can also alter composition by changing protein fractions [12]. Thus, fermentation deserves attention due to its potential health benefits, such as lowering the risk of chronic diseases and cardiovascular diseases. This work provides an overview of the influence of fermentation on the antioxidant activity of different plant-based food items.
2. Impact of Fermentation Strain on Antioxidant Activity of Plant-Based Food Material
2.1. Single Strain Fermentation
Single-strain fermentation has typically been used to explore how microorganisms affect food materials. A functional and effective single strain would be more suitable for industry fermentation. Meanwhile, the properties of different strains could be screened and compared during single-strain fermentation. Hur et al. noticed that fermentation could increase the phenolic content and antioxidant activity of different plants in a manner that depended on the starting microorganisms [13]. Moreover, Ricci et al. concluded that the metabolism of phenolics depends on strain features and species [14]. For example, fermentation with cerevisiae had no significant effect on the inhibitory influence of 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) in some cereal species, while fermentation with L. rhamnosus had a positive impact on the DPPH inhibitory effect in the same cereal species [15].
2.1.1. Yeast Fermentation
Yeast has been one of the most widely-used microorganisms in food fermentation. The use of yeast is integral to processes such as dough fermentation and beer brewing, showing that antioxidant activity is positively correlated with yeast fermentation. For example, flavonoids, a subclass of phenolic compounds, were found to increase during the first 12 h of tea fermentation with yeast, followed by a gradual decrease. The total phenolic content increased during the first 24 h of fermentation, followed by a slight decrease after 48 h [16]. Further, yeast fermentation improved the radical scavenging activity of black tea, increasing metal chelating activities, DPPH, and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate diammonium) during the 24 h of fermentation. In addition, Ooi et al. investigated the DPPH free radical scavenging activity, total flavonoid content, and total polyphenols content after fermentation of cocoa beans with Hanseniaspora thailandica and Pichia kudriavzevii. The result showed that yeast fermentation increased antioxidant potential [17]. However, Laaksonen et al. [18] found that fermentation with commercial yeast had only a minor impact on the phenolic product contents. Authors used the commercial yeast strains Saccharomyces bayanus, Saccharomyces cerevisiae, and Torulaspora delbrueckii, and the results showed that fermentation with S. bayanus produced freer hydroxycinnamic acids (phenolic compounds) than fermentation with S. cerevisiae, indicating higher enzyme production for breaking the ester bonds between quinic acid and hydroxycinnamic acids. In summary, according to previous research, selected yeast strains impact fermentation results by increasing antioxidant activity, flavonoids, and metal chelating activity.
There are several different yeast strains for food fermentation, such as Saccharomyces cerevisiae, Saccharomyces bayanus, Torulaspora delbrueckii, Hanseniaspora thailandica, and Pichia kudriavzevii [19]. Kim et al. demonstrated that antioxidant activity has a positive correlation with the yeast fermentation time [20]. Polyphenols are the major natural antioxidants in food, as illustrated by Verni et al. [21]. Additionally, Reis et al. showed that fermentation of goji berries with Saccharomyces cerevisiae increased the total phenolic content [22]. Anson et al. fermented wheat bran with Saccharomyces cerevisiae and noticed an increase of p-coumaric, sinapic, and ferulic acids content three-fold higher than unfermented wheat bran [23].
2.1.2. Fungal Fermentation
Similar to yeasts, fungi have been frequently used in the food, condiment, and brewing industry, as a starter to convert the starch in raw materials into fermentable sugars. Ajila et al. [24] found that carbohydrate-degrading enzymes produced by fungi during fermentation convert conjugated phenolic compounds to free form, which improves their ability to work as good antioxidants. They showed that an increase in phenolic amounts during fermentation might be attributed to the fungi β-glucosidases which can hydrolyze β-glucosidic linkages, then mobilize phenolic compounds to react with the Folin–Ciocalteau reagent. Accordingly, Aspergillus niger is the most used microorganism in solid-state fermentation because of its ability to produce a large number of enzymes [25]. Hifney et al., who used various fungal species to ferment the seaweed Cystoseira trinodis before extracting alginate and fucoidan, showed that all the fungal species chosen produced alginate lyase and fucoidanase, decreasing the molecular weight of alginate and fucoidan. This process led to the enhancement of the antioxidants’ ferric-reducing power (FRAP), hydroxyl radical scavenging activity (HRSA), and total antioxidant capacity (TAC) of fucoidan and alginate [26]. However, some studies indicated that not all fermentations play a positive role in antioxidation activity. For example, it was found that Aspergillus carbonarius fermentation increased the antioxidant activity of Cabernet Sauvignon grapes but did not affect the antioxidant activity of Moscato Italico grapes, demonstrating that fruit variety has an impact on fermentation results [27]. Additionally, Monascus purpureus fermentation of defatted soybean flour increased isoflavone content but caused almost no change in total phenolic content or antioxidant activity. Thus, the increase in isoflavone content could be attributed to the selective action of β-glucosidase, which may result in polyphenol aglycones having a lower capacity for reducing the Folin–Ciocalteu reagent, or alternatively, to the synthesis of β-glucosidases having low activity on other soybean flour phenolic glucosides [28]. On the other hand, the results from Lee et al. showed that the total isoflavone content of diseased soybeans decreased significantly due to Phomopsis longicolla and Cercospora kikuchii fungi [29], which suggests that the effects of different fungi on antioxidant compounds in plants may be selective.
2.1.3. Bacterial Fermentation
It is well known that lactic acid bacteria have been applied in fermented dairy, fermented vegetables, and fermented meat products in the food industry. The potential of lactic acid bacteria for enhancing the antioxidant activity of plant materials may be due to a decrease in pH and the enzymolysis effect during fermentation. Tkace et al. studied the effect of fermentation by using the Oenococcus oeni, Lactobacillus plantarum, and Lactobacillus plantarum subsp. argentoratensis strains on 100% Sea buckthorn (Hippophaë rhamnoides L.) berries mixed with apple (1:1) juices [30]. It was found that Lactobacillus plantarum strains were effective in increasing flavonols and enhancing the antioxidant activity of sea buckthorn–apple juices. Additionally, fermentation of quinoa flour by lactic acid bacteria achieved higher antioxidant activity than spontaneous fermentation, especially with Lactobacillus plantarum T0A10, through proteolysis of native quinoa proteins during fermentation [31]. Additionally, fermentation by Lactobacillus casei increased the isoflavone aglycone and phenolic content of whole soybeans [32]. All the results above indicate that bacterial fermentation plays an important role in the improvement of antioxidant activity.
2.2. Mixed Strain Fermentation
Some past research has suggested that fermentation using a complex of strains was better than a single strain [15]. It was found that mixed cultures enhanced the growth of L. plantarum strains, which may relate to the production of nutrients, such as vitamins, by other strains [33]. The pure and mixed cultures of L. plantarum LP3, L. plantarum AF1, and L. plantarum subsp. plantarum PTCC 1896 were used to ferment bergamot juice. Mixed ternary cultures had the highest radical-scavenging effect and DPPH scavenging ability. Wang et al. fermented guava leaf tea with Monascus anka and Saccharomyces cerevisiae and then hydrolyzed the mixture with complex enzymes. Fermentation resulted in an increase in the reducing power of soluble phenolics, an increase in antioxidant activity, and inhibitory efficacy towards α-glucosidase, and the hydrolyzing enzymes increased the positive effect of fermentation [34]. They reported that antioxidant activity increased due to an increase in the solubility of phenolic compounds in the guava leaf tea extract and due to the production of more phenolic compounds with higher bioactivities, such as kaempferol and quercetin.
3. Impact of Fermentation on Antioxidant Activity of Various Plant-Based Food Materials
3.1. Cereals
Cereals exhibit good antioxidant capacity due to the phenolic compounds distributed in the outer layer of the grain, and since most phenolics exist in the bound state [35]. Fermented cereal samples (including buckwheat, wheat germ, barley, and rye) with L. rhamnosus had higher antioxidant activity than those fermented with S. cerevisiae [15]. This indicated that fermentation with S. cerevisiae did not significantly influence lipid peroxidation inhibition in cereals, while fermentation with L. rhamnosus did have such influence. Therefore, the increase in antioxidant activity depended on the selected microorganism species. Additionally, fermentation of rice bran with lactic acid bacteria increased antioxidant activity due to an increase of free and soluble conjugated phenolic compounds and the bioavailability of free hydroxyl groups [36]. Fermenting Radix Puerariae combined with red yeast rice led to an increase in total phenolic content and pigment intensity and to enhanced antioxidant activity [37]. By contrast, fermentation with L. rhamnosus and S. cerevisiae did not show any significant influence on the ferric-reducing antioxidant power of buckwheat, wheat germ, barley, and rye [15]. Zhao et al. fermented barley with Lactobacillus plantarum for one day, and it was found that saccharides, amino acids, nucleosides, and some organic acids decreased, while lipids and bioactive molecules in barley were released and metabolites were accumulated during fermentation. Moreover, characteristic components in fermented barley were revealed, including functional molecules such as indole-3-lactic acid, phenyllactic acid, homovanillic acid, cafestol, among others. [38].
3.2. Legumes
The functional properties of legumes and their products have been the focus of research in recent years, and their antioxidant activity has received increased attention. In this regard, Limón et al. concluded that the fermentation technology encouraged obtaining bioactive compounds from kidney beans, which led to their useful utilization in added-value functional foods [39]. Dueñas et al. reported that Lactobacillus plantarum for fermentation of cowpeas could enhance the antioxidant activity [40]. Starzyńska–Janiszewska et al. suggested that fermentation of legumes decreases their allergenicity and lowes the non-nutritional factors [41], and Crujeiras et al. indicated that fermented legumes exhibited protective effects against cardiovascular disease [42]. Xiao et al. found that the solid-state fermentation of chickpeas with Cordyceps militaris SN-18 possessed higher total phenolic content and antioxidant activity compared with unfermented samples [43]. During fermentation, phenolic compounds such as chlorogenic acid, shikimic acid, biochanin A, daidzein, and rutin. They used 80% methanol, 80% ethanol, and water for extraction and demonstrated that methanolic extracts exhibited the highest scavenging DPPH radical ability compared with other solvent extracts. Thus, solvent selection may lead to variation in DPPH radical scavenging ability. Therefore, a strong correlation has been found between specific phenolic compounds and the antioxidant activity of some fermented legumes [39]. Marazza et al. reported that the isoflavone aglycones produced by fermentation are more active antiradical compounds [44]. In addition, higher reducing power was obtained from soybean fermented with Streptomyces sp., Acetobacter sp., Saccharomyces sp., and Lactobacillus sp., which might be related to the hydrogen-donating ability of the contained reductones [45,46].
3.3. Vegetables
Vegetables are considered to be rich in antioxidants. Sawicki et al. investigated the effect of boiling and spontaneous fermentation on the betalains of red beetroot. It was found that matrix softening and microbial activity increased the release of betalains during fermentation [47]. Firstly, an increase in betalains content by 99% within the first seven days of the fermentation process was observed, followed by a decrease by 17% during days 8–12. Afterwards, the fermentation process of the inner tissue of red beet slices could not overcome the degradation processes. Degradation continued during the next or 13th day of fermentation, and finally, the degradation processes began to prevail in the fermented juice. The authors reported that 14-day fermented red beets had the lowest value in antioxidant activity. Although prior research has shown that storage affects the antioxidant activity of fermented products, the opposite was observed by Wiczkowski et al., who studied the effect of fermentation on the antioxidant capacity of red cabbage. Lower antioxidant capacity was found in fermented red cabbage than fresh red cabbage due to decreased anthocyanin content, contributing to antioxidant capacity [48]. However, they noted that fermented red cabbage is still rich in anthocyanin content. Olennikov et al. pointed that novel melanoidin is an essential antioxidant found in the fermented leaves of willowherb with a content of 145.31 ± 4.35 mg per gram dry plant weight, and it is worth further investigation as potential food and medical antioxidant [49].
3.4. Fruits
Fruits are a great source of natural antioxidants in the human diet. Dulf et al. investigated the effect of solid-state fermentation (SSF) by Rhizopus oligosporus and Aspergillus niger on the antioxidant activity of plum pomaces and noticed greater free-radical scavenging activities leading to an increase in the antioxidant activity of plum pomaces [25]., Dachery et al. also evaluated the effect of Aspergillus carbonarius on the antioxidant activity of Cabernet Sauvignon (CS, red) grapes and Moscato Italico (MI, white) grapes. It was found that an increase in antioxidant activity for red grapes, but a decrease in antioxidant activity for white grapes [27]. However, Jiménez-López et al. noticed only a slight increase in antioxidant activity during the fermentation of caper berries (Capparis spinosa L.) [1]. They mentioned that glucosinolate glucocapparin was fully degraded after the fermentation process of berries, producing methyl isothiocyanate as the main degradation product. They also observed that most phenolic compounds remained unaltered during the fermentation of caper berries except in the case of hydrolysis of quercetin glycosides and degradation of glucosinolates, which was probably due to pH changes during the fermentation process. Besides this, a decrease in epicatechin concentration was also observed.
4. Changes of Antioxidant Constituents in Plant-Based Food Material during Fermentation
4.1. Phytochemicals
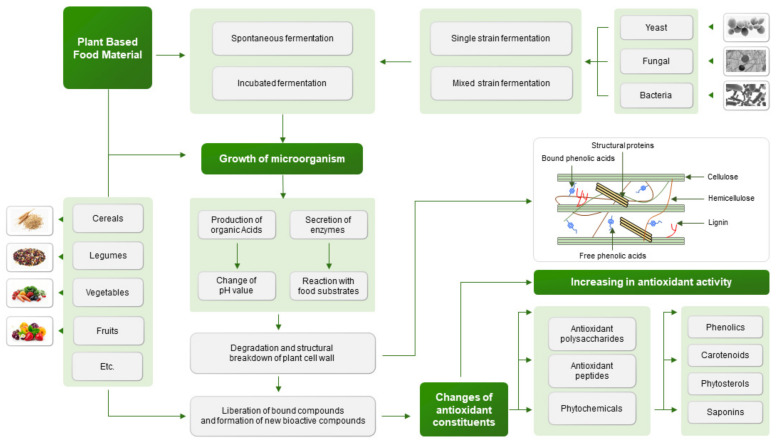
Microorganisms can modify antioxidant constituents during the fermentation process. Fermentation can lead to the structural breakdown of plant cell walls, leading to the synthesis and liberation of various bioactive compounds, as shown in Table 1.
Table 1.
Effect of fermentation on antioxidant activity of plant-based food material.
| Food Material | The Used Microorganism | Effect on Bioactive Compounds of Plant-Based Food Material | Fermentation Type | Literature |
|---|---|---|---|---|
| Wheat | Lactobacillus fermentum (MR13), L. rhamnosus (C249, C1272), L. plantarum (LB102, LB124, LB126, LB245, 29DAN, 83DAN, 6BHI, 98A) and L. brevis (3BHI) |
|
Liquid and solid | [50] |
| Oats | Monascus anka GIM 3.592 |
|
Liquid and solid | [51] |
| Goat milk | Lactobacillus plantarum 60 |
|
Liquid | [52] |
| Buckwheat, wheat germ, barley, and rye | Lactobacillus rhamnosus, and Saccharomyces cerevisiae |
|
Liquid and solid | [15] |
| Defatted soybean flour | Monascus purpureus or Aspergillus oryzae |
|
Solid | [28] |
| Bergamot juice | Pure and mixed cultures of L. plantarum subsp. plantarum PTCC 1896, L. plantarum AF1 and L. plantarum LP3 |
|
Liquid | [33] |
| Whole soybean flour | Lactobacillus casei |
|
Liquid and solid | [32] |
| Kidney beans | Solid-state fermentation was carried out by Bacillus subtilis, whilst liquid state fermentation was performed either by natural fermentation (NF) or by Lactobacillus plantarum strain (LPF) |
|
Liquid and solid | [39] |
| Rice bran | It steamed with α-amylase, fermented with lactic acid bacteria, and hydrolyzed with complex enzymes |
|
Liquid and solid | [36] |
| Cocoa beans | 13 naturally existing yeast strains |
|
Liquid and solid | [17] |
| Elderberry juices | Ten strains of Lactobacillus |
|
Liquid | [14] |
| Sea buckthornberries | Lactobacillus plantarum, Lactobacillus plantarum subsp. argentoratensis and Oenococcus oeni strains |
|
Liquid | [30] |
| Guava leaves tea | It first fermented with Monascus anka and Saccharomyces cerevisiae and then hydrolyzed with complex enzymes |
|
Liquid and solid | [34] |
| Chickpeas | Cordyceps militaris SN-18 |
|
Solid | [43] |
| Buckwheat flours | Selected lactic acid bacteria (LAB) and Rhizopus oligosporus fungi |
|
Liquid | [53] |
4.1.1. Phenolic Compounds
Phenolic compounds are important phytochemicals found widely in plants, and they are well-known antioxidants because of the high reactivity of hydroxyl substitution and their ability to scavenge free radicals. Đorđević et al. used the yeast Saccharomyces cerevisiae and bacteria Lactobacillus rhamnosus to ferment four kinds of cereal, including wheat germ, buckwheat, rye, and barley. It was found that total phenolic content (TPC) was enhanced after fermentation. Moreover, rye had the lowest total phenolic content with 13.3 mg of gallic acid equivalents (GAE) per gram of dried extract (d.e.) in the unfermented sample, 16.2 mg GAE/g d.e. in S. cerevisiae-fermented extract, and 18.4 mg GAE/g d.e. in L. rhamnosus-fermented extract [15]. Chung et al. showed that the total phenolic contents in 12-h and 24-h fermented ginseng extracts with Lactococcus lactis KC24 increased by 6.1% and 4.1%, respectively, and were greater than in nonfermented extracts [54]. Moreover, Antognoni et al. used different lactic acid bacteria strains to ferment wheat (Triticum aestivum L.) and detected ferulic, p-coumaric, cinnamic, caffeic, sinapic, p-hydroxybenzoic, and gallic acids in the fermented dough [50]. Liu et al. also detected 12 phenolic compounds after fermentation of rice bran: ferulic acid, protocatechuic acid, isoferulic acid, gallic acid, caffeic acid, chlorogenic acid, coumaric acid, syringic acid, kaempferol, vanillic acid, catechin, and (−)-epicatechin [36]. Ricci et al. noticed that fermentation was able to increase flavanol glycosides and anthocyanins, produce phenyllactic acids, and modify hydroxycinnamic acids [14]. It was also found that catechol and dihydrocaffeic acid were produced during the fermentation of elderberry juice, but protocatechuic acid and caffeic acid were consumed by lactic acid bacteria.
The fermented elderberry juice was characterized by flavonols, flavonol glycosides, anthocyanins, hydroxybenzoic acid, phenyllactic acids, and hydroxycinnamic acids [14]. The increase in hydroxycinnamic acids could be attributed to the increase of 5-O-caffeoylquinic acid in Lactobacillus plantarum 285, Lactobacillus rhamnosus 1019, 1473, 2360, and Lactobacillus casei 2107, which was used to ferment the juice [14]. Phenyllactic acids can derive from amino acid metabolism, such as converting phenylalanine to phenylpyruvic acid by a transamination reaction, followed by metabolizing into phenyllactic acid by hydroxyl acid dehydrogenase [14]. Additionally, solid-state fermentation of kidney beans exhibited high soluble phenolic compound content and antioxidant activity due to the production of β-glucosidases by Bacillus subtilis [39]. Liu et al. noted that antioxidant activity increased due to a release of antioxidative phenolics and an increased bioavailability of free hydroxyl groups during fermentation [36]. However, Dulf et al. reported a slight decrease in the free phenolic content of plum by-products after fermentation, perhaps attributable to oxidative enzymes that polymerize released phenolics [25]. Further, most phenolic compounds remained without alteration during the fermentation of caper berries (Capparis spinosa L.), although there were some changes, such as a decrease in epicatechin concentration and the hydrolysis of quercetin glycosides, which may be due to pH changes during the fermentation process [1]. Even though glucosinolates were fully degraded upon fermentation, antioxidant activity slightly increased upon the fermentation process.
4.1.2. Carotenoids
Carotenoids are vital compounds because of their antioxidant mechanisms [55]. Fermentation can increase the release of carotenoids [55]. For example, lactic acid fermentation can preserve β-carotene more than other processing techniques, such as deep-frying, blanching, and drying [56]. In addition, Lactobacillus plantarum (29DAN, 83DAN) was able to increase the carotenoid content in wheat dough due to the higher solubility of these antioxidant compounds [50]. The effect of fermentation on carotenoid concentration depends on the plant material, the carotenoid involved, the fermentation conditions, and the enzyme activity of strains because some enzymes favour carotenoid extractions [55].
4.1.3. Phytosterols
Phytosterols are naturally occurring potential antioxidants that contribute to the antioxidant capacity of soy germ [57]. Stigmasterol, D-7-avenasterol, campesterol, β-sitosterol, and 5-avenasterol are examples of phytosterols [57,58]. Adeyeye compared the changes of phytosterol content in African locust bean seeds by fermentation, and the result showed a higher value in the fermented samples than the unfermented samples [58]. However, Hubert et al. used lactic acid bacteria to ferment soy germ and studied the effect of fermentation on its phytochemical composition. A reduction in phytosterol content in soy germ was observed upon the fermentation for 48 h. This could be attributed to dehydration or oxidation that occurs in an oxygen environment when substrates are exposed to heat or ultraviolet light. Additionally, it was found that phytosterols were poorly active in scavenging the superoxide anion radical in soy germ. However, it was noted that the relative distribution of the individual phytosterols remained constant during all the fermentation stages. A 6-h incubation period is the best time to obtain conserved amounts of phytosterols in fermented soy germ, perhaps due to the low pH value. Thus, controlling incubation time can help preserve the phytosterol levels [57].
4.1.4. Saponins
Saponins are chemical compounds composed of a triterpenoid or steroid sapogenin nucleus with one or more carbohydrate branches [59]. The triterpenoid saponins could be classified into major groups A and B, each containing a glycone fraction attached to one or more oligosaccharide chains [57]. Saponins are natural glycosides that contribute to the antioxidant capacity of soy germ [57]. However, the soyasaponins were poorly active in scavenging the superoxide anion radical . It was found that considerable changes in the conjugation profile of soyasaponins during the fermentation of soy germ by lactic acid bacteria. Moreover, it was reported that during the incubation period, the conjugation profile of soyasaponins B was modified, and 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP)-conjugated saponin significantly decreased after the fermentation of soy germ with lactic acid bacteria for 10 h. This could be attributed to the loss of their terminal sugar through enzymatic hydrolysis, followed by conversion into other unidentified structures. Hubert et al. discussed that (DDMP)-conjugated soyasaponins were converted into their DDMP-moieties with the release of maltol molecules during fermentation. After a 10-h to 48-h incubation, soyasaponins B remained unchanged [57].
4.2. Antioxidant Peptides
Antioxidant peptides are the peptides with the function of inhibiting peroxidation and scavenging free radicals. Generally, the antioxidant activity of peptides is related to the molecular weight, amino acid sequence, and amino acid side chain group. It was reported that the addition of purified peptides to goat milk powder increased antioxidant capacities [52]. Rapeseed and bitter bean peptides exhibited high scavenging free radical activities but low ferrous ion-chelating activity [60,61]. Additionally, peptides that chelate metal ions could be used as an indirect antioxidant agent [62]. Concerning the fermentation process, Muhialdin et al. noticed that the fermentation process of bitter beans produced higher peptides content with lower molecular weight than the boiling process. Furthermore, they concluded that the increased antioxidant activity in Lactobacillus fermentum ATCC9338 fermented bitter beans was due to an increase in low-molecular-weight peptides [60]. Additionally, fermentation can decrease glucosinolate content, increasing the nutritional value of the hydrolyzed peptide products, while the fermentation of different food items increases peptides via protein hydrolysis through microbial proteolytic enzymes [62]. Chen et al. mentioned that the release of antioxidant peptides was affected by selected lactic acid bacteria species. Three antioxidative peptides were obtained after fermentation of goat milk with Lactobacillus plantarum 60 [52]. They explained that the radical scavenging activity of the antioxidant peptides depends on certain proteolytic enzymes inside bacterial strains rather than the high proteolytic state of fermented products.
4.3. Antioxidant Polysaccharides
Polysaccharides are important compounds that exhibit many biological activities, such as antioxidants related to the structure of polysaccharides and their molecular mass [36]. It was reported that fermentation is a good way to improve the antioxidant activities of Auricularia auricula polysaccharides [61]. Fermentation degraded the pyran type polysaccharide to furan type polysaccharide with improved antioxidant activity. In addition, Liu et al. noticed that the antioxidant activity of rice bran polysaccharides increased by fermentation with the Grifola frondosa fungus [36]. After nine days of fermentation, it was noticed that a decrease in molecular weight of the polysaccharides from the range of approximately 103–104 Da to the range of about 102 and 103 Da, with a 2.9% increase between 103 and 104 Da. The changes in molecular weight distribution may be due to extracellular enzymes secreted from Grifola frondose [36]. In other research, Liu et al. prepared a polysaccharide from Inonotus hispidus by fermentation and isolated two types of polysaccharide fractions and studied the characteristics of the fractions that had the higher antioxidant activity [63]. They described how fungal polysaccharides produced by solid-state fermentation were characterized by a shorter production cycle and high conductivity to biological circulation. They also showed that the polysaccharides produced by fermentation had higher antioxidant activity than unfermented polysaccharides from the fruiting body of Inonotus hispidus. Fermented polysaccharides reduced H2O2-induced cellular damage and improved the cell survival rate.
4.4. Conversion Effect of Fermentation on Antioxidant Components
As already mentioned, the antioxidant peptides and polysaccharides could be degraded or synthesized by microorganisms, usually accompanied by the conversion from large to smaller molecules. For the phytochemicals, there are not only changes in content but also to their metabolism and other biotransformation effect during fermentation. Cho et al. investigated phytochemical contents in soybean paste fermented with Bacillus subtilis CS90. It was found that the levels of isoflavone aglycones, flavanols, and gallic acid increased, while the β-glucosidase and esterase activities, isoflavone glycosides and flavanol gallates contents decreased [64]. Koistinen et al. processed rye bran with the combination of enzymatic processing and yeast fermentation. Different from sourdough fermentation, a significant increase of free phenolic acids in bran was observed, some of the hexose moieties were released from benzoxazinoids, and alkylresorcinols experienced moderate degradation [65]. It indicated that the conversion of phytochemicals was variance attributed to the bioprocessing type and conditions. Sheih et al. considered that the novel antioxidants could be produced from the soy germ materials fermented with A. niger M46. Moreover, the fermentation altered the composition of isoflavones, and the isoflavones were further converted into other microbial-induced metabolites [66]. Rodríguez et al. pointed that L. plantarum was able to degrade some food phenolic compounds and metabolize some of the hydroxycinnamic and hydroxybenzoic acids [67,68]. Thus, further studies are needed to investigate the fermentation characteristics of strains, the role of enzymes, and their relationship with antioxidants’ release, synthesis, and metabolism.
5. Effects of Various Factors on Changes to Antioxidant Components and Activities during Fermentation
5.1. Effect of Enzyme on Fermentation Results
An enzyme is one of the key factors to promote the release and production of bioactive components in the fermentation process of microbial growth and metabolism. There are some enzymes, such as cellulase, amylase, esterase, tannase, and glucosidase, that can break down plant cell walls, liberating or producing bioactive compounds such as flavonoids, phenolic acids, etc. [13]. Aspergillus niger can produce more than 19 types of enzymes, including cellulase and pectinase. As reported, increases in phenolic content in the early stages of solid-state fermentation were attributable to glycosidases and lignin-degrading enzymes, which can help release cell wall-bound phenolic compounds. However, after nine days of fermentation, a slight decrease in free phenolics of plum by-products was noticed. This may be attributed to oxidative enzymes that polymerize released phenolics [25]. Hur et al. also reported that grain constituents exposed to bacterial and endogenous enzymes caused the solubility of some components and the production of new nutritionally active molecules [13]. Phospho-β-glucosidases plays a significant role during the fermentation of plant-based foods related to the release of phenolic compounds. Acin-Albiac et al. characterized the metabolism of Lactiplantibacillus plantarum and Leuconostoc pseudomesenteroides during their fermentation with brewers’ spent grain and noted an increased metabolic activity for gentiobiose, cellobiose and β-glucoside conjugates of phenolic compounds during fermentation [69]. In addition, de Araújo et al. investigated the hydrolytic potential of the enzymes of filamentous fungi isolated from the natural cocoa fermentation. Nineteen different species of fungi were isolated, and most of them exhibited the ability to secrete enzymes, including amylase, pectinase, cellulase, and xylanase [70].
Therefore, the addition of hydrolases helped convert bound bioactive compounds into a free state, whether exogenous or endogenous. Hydrolysis can enhance the liberation of phytochemicals in food items. Solid-state fermentation was employed by Costa et al. to produce feruloyl esterase and xylanase, and the addition of fermented wheat bran and brewer’s spent grain led to an increase in soluble ferulic acid of 159% and 198% in the bioprocessing bread, respectively [71]. Moreover, Bei et al. pointed that additional time added to cellulase resulted in significant variance in phenolic content during the solid-state fermentation of oats, but there was no significant difference if cellulase was added during inoculation [51]. In summary, the addition of specific enzymes could enhance the effect of fermentation by increasing the content of bioactive substances.
5.2. Effect of Fermentation Conditions on Fermentation Results
As mentioned, fermentation does not always play a positive role in the release and production of bioactive components. In addition to the selection of strains, fermentation conditions are significant factors in this process. Hur et al. reported that changes in temperature during the fermentation of plant-based foods influence the resulting antioxidant activity, including, for instance, those of phenolic compounds [13]. Optimum temperature led to enhanced microbial growth and enzymatic activity that resulted in improved fermentation. Extremely low or high temperatures negatively affected antioxidant activity during fermentation. Yao et al. evaluated the antioxidant activities of Eurotium cristatum-fermented loose tea at different fermentation temperatures, and it was found that higher temperature increased antioxidant activity in the loose tea [72]. The effects of different fermentation temperatures were evaluated, and 37 °C was considered the optimum temperature to produce better ferric reducing ability, which was also favourable for increasing DPPH scavenging ability in fermented tea. However, they noticed that the increase in fermentation temperature decreased the hydroxyl radical scavenging ability in fermented tea, for which 28 °C was the optimum temperature. Thus, increasing fermentation temperature had varying effects on ferric reducing, hydroxyl radical scavenging, and DPPH scavenging in fermented tea. It is important to monitor how quickly the process will reach a pH value lower than 4.0 since this can inhibit the growth of most food-borne pathogens [73]. Some microorganisms can increase or decrease pH value. For example, at the beginning of the fermentation of caper berries, lactic acid bacteria growth rapidly lowers the pH value of the medium, displacing other microbial groups such as enterobacteria [1]. Meanwhile, the release and production of some compounds can also increase or decrease pH value.
5.3. Selection of Liquid Fermentation and Solid-State Fermentation
The fermentation type was also important for the fermentation effect. As is known, the vital change between solid-state fermentation (SSF) and liquid fermentation (LF) is the water content, which would affect the interactions between microorganisms, enzymes, liquids, air, and fermentation matrix. Slightly different from traditional SSF, the plant-based food substrates should supply enough nutrients such as carbon and nitrogen for microbial utilization, which put forward higher requirements for the fermentation features of strains and the control of fermentation conditions [74]. While the LF needs a larger scale fermentation vessel to provide controlled conditions such as optimum temperature, pH value, and others. It occurs in a liquid media with high water content, and all nutrients are present in the liquid medium for microbial growth, which might be a challenge for the enrichment of products. It is shown in their advantages and limitations in Table 2 for microorganism growing [75].
Table 2.
Comparison between solid-state fermentation and liquid fermentation.
| Factor for Comparison | Solid-State Fermentation | Liquid Fermentation |
|---|---|---|
| Advantages |
|
|
| Limitations |
|
|
6. Conclusions
In this work, we described microbial bioconversion in the chemical composition of various food items. Fermented food is a good source of bioactive compounds compared to most nonfermented food. Fermentation can increase amino acids and isoflavone content, and it helps to improve the ratio of nutritive to antinutritive components in plants, which encourages the production of new functional foods (Figure 1). Microorganisms such as L. rhamnosus, L. plantarum, Rhizopus oligosporus, Aspergillus niger, Saccharomyces cerevisiae, etc., lead to a series of reactions that result in higher antioxidant activity. This activity is linked to increases in antioxidant compounds, such as phenolic compounds, which have been converted from conjugated form to free form by enzymes produced by microorganisms. With the continuous development of fermentation technology, more studies are needed to discuss this topic. Fermentation’s ability to increase bioactive components, especially antioxidants in plant-based food materials, could be used to design new functional food.
Figure 1.
The process by which antioxidants are released and produced by fermentation.
Several factors influence the effect of fermentation on the liberation and production of antioxidants from plant-based food materials, including starting microorganisms, pH value, fermentation time, fermentation type, and enzymes. More studies are needed on the activities of relevant enzymes and their interactions with food during fermentation. Research has suggested that the type of food and food composition influence fermentation results. For example, it has been found that gelatinization of cereals affects the behaviour of microorganisms. Overall, fermentation can enhance the functional properties of food. This review has provided helpful information on the effect of the fermentation process on the bioactive substances and antioxidant activity of different plant-based food items.
Author Contributions
Conceptualization, Y.-S.Z.; writing—original draft preparation, A.S.E. and Y.-S.Z.; writing—review and editing, Y.-S.Z., J.-Y.Z., Y.Z., J.B., H.-B.Z., O.M.D., and X.X.; supervision, X.X.; funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the Natural Science Foundation of China (Grant No. 32072200), Jiangsu Agriculture Science and Technology Innovation Fund (Grant No. CX(20)2036) and the Key Research and Development Project of Yangzhou (Grant No. YZ2020043). The funders played no roles in the study design; data collection and analysis; or the decision to publish the study.
Data Availability Statement
The data presented in this study are available are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jiménez-López J., Ruiz-Medina A., Ortega-Barrales P., Llorent-Martínez E.J. Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process. Food Chem. 2018;250:54–59. doi: 10.1016/j.foodchem.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Chung I.-M., Seo S.-H., Ahn J.-K., Kim S.-H. Effect of processing, fermentation, and aging treatment to content and profile of phenolic compounds in soybean seed, soy curd and soy paste. Food Chem. 2011;127:960–967. doi: 10.1016/j.foodchem.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 3.Ravichandran K., Saw N.M.M.T., Mohdaly A.A.A., Gabr A.M.M., Kastell A., Riedel H., Cai Z., Knorr D., Smetanska I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013;50:670–675. doi: 10.1016/j.foodres.2011.07.002. [DOI] [Google Scholar]
- 4.Frias J., Miranda M.L., Doblado R., Vidal-Valverde C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005;92:211–220. doi: 10.1016/j.foodchem.2004.06.049. [DOI] [Google Scholar]
- 5.Septembre-Malaterre A., Remize F., Poucheret P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018;104:86–99. doi: 10.1016/j.foodres.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Alrahmany R., Avis T.J., Tsopmo A. Treatment of oat bran with carbohydrases increases soluble phenolic acid content and influences antioxidant and antimicrobial activities. Food Res. Int. 2013;52:568–574. doi: 10.1016/j.foodres.2013.03.037. [DOI] [Google Scholar]
- 7.Budaraju S., Mallikarjunan K., Annor G., Schoenfuss T., Raun R. Effect of pre-treatments on the antioxidant potential of phenolic extracts from barley malt rootlets. Food Chem. 2018;266:31–37. doi: 10.1016/j.foodchem.2018.05.110. [DOI] [PubMed] [Google Scholar]
- 8.López-Gámez G., Elez-Martínez P., Martín-Belloso O., Soliva-Fortuny R. Enhancing phenolic content in carrots by pulsed electric fields during post-treatment time: Effects on cell viability and quality attributes. Innov. Food Sci. Emerg. Technol. 2020;59:102252. doi: 10.1016/j.ifset.2019.102252. [DOI] [Google Scholar]
- 9.Goldsmith C.D., Vuong Q.V., Stathopoulos C.E., Roach P.D., Scarlett C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT Food Sci. Technol. 2018;89:284–290. doi: 10.1016/j.lwt.2017.10.065. [DOI] [Google Scholar]
- 10.Peinado J., López de Lerma N., Peralbo-Molina A., Priego-Capote F., de Castro C., McDonagh B. Sunlight exposure increases the phenolic content in postharvested white grapes. An evaluation of their antioxidant activity in Saccharomyces cerevisiae. J. Func. Foods. 2013;5:1566–1575. doi: 10.1016/j.jff.2013.06.007. [DOI] [Google Scholar]
- 11.Maseko I., Mabhaudhi T., Ncube B., Tesfay S., Araya H.T., Fessehazion M.K., Chimonyo V.G.P., Ndhlala A.R., Du Plooy C.P. Postharvest drying maintains phenolic, flavonoid and gallotannin content of some cultivated African leafy vegetables. Sci. Hortic. 2019;255:70–76. doi: 10.1016/j.scienta.2019.05.019. [DOI] [Google Scholar]
- 12.El Khalifa A.O., El Tinay A.H. Effect of fermentation on protein fractions and tannin content of low- and high-tannin cultivars of sorghum. Food Chem. 1994;49:265–269. doi: 10.1016/0308-8146(94)90171-6. [DOI] [Google Scholar]
- 13.Hur S.J., Lee S.Y., Kim Y.-C., Choi I., Kim G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- 14.Ricci A., Cirlini M., Calani L., Bernini V., Neviani E., Del Rio D., Galaverna G., Lazzi C. In vitro metabolism of elderberry juice polyphenols by lactic acid bacteria. Food Chem. 2019;276:692–699. doi: 10.1016/j.foodchem.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Đorđević T.M., Šiler-Marinković S.S., Dimitrijević-Branković S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- 16.Maria John K.M., Thiruvengadam M., Enkhtaivan G., Kim D.H. Variation in major phenolic compounds and quality potential of CTC black tea elicited by Saccharomyces cercevisiae and its correlation with antioxidant potential. Ind. Crop. Prod. 2014;55:289–294. doi: 10.1016/j.indcrop.2014.02.006. [DOI] [Google Scholar]
- 17.Ooi T.S., Ting A.S.Y., Siow L.F. Influence of selected native yeast starter cultures on the antioxidant activities, fermentation index and total soluble solids of Malaysia cocoa beans: A simulation study. LWT Food Sci. Technol. 2020;122:108977. doi: 10.1016/j.lwt.2019.108977. [DOI] [Google Scholar]
- 18.Laaksonen O., Kuldjärv R., Paalme T., Virkki M., Yang B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017;233:29–37. doi: 10.1016/j.foodchem.2017.04.067. [DOI] [PubMed] [Google Scholar]
- 19.Puškaš V.S., Miljić U.D., Djuran J.J., Vučurović V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Sci. Nutr. 2020;8:1489–1498. doi: 10.1002/fsn3.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M.J., John K.M.M., Choi J.N., Lee S., Kim A.J., Kim Y.M., Lee C.H. Changes in secondary metabolites of green tea during fermentation by Aspergillus oryzae and its effect on antioxidant potential. Food Res. Int. 2013;53:670–677. doi: 10.1016/j.foodres.2012.12.053. [DOI] [Google Scholar]
- 21.Verni M., Verardo V., Rizzello C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods. 2019;8:362. doi: 10.3390/foods8090362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis B., Gnazzo A.K.-C., Schmitt R., Andlauer W. Fermentation of plant material—Effect on sugar content and stability of bioactive compounds. Pol. J. Food Nutr. Sci. 2014;64:235–241. doi: 10.2478/pjfns-2013-0017. [DOI] [Google Scholar]
- 23.Anson N.M., Selinheimo E., Havenaar R., Aura A.M., Mattila I., Lehtinen P., Bast A., Poutanen K., Haenen G.R. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009;57:6148–6155. doi: 10.1021/jf900492h. [DOI] [PubMed] [Google Scholar]
- 24.Ajila C.M., Gassara F., Brar S.K., Verma M., Tyagi R.D., Valéro J.R. Polyphenolic antioxidant mobilization in apple pomace by different methods of solid-state fermentation and evaluation of its antioxidant activity. Food Bioprocess Tech. 2012;5:2697–2707. doi: 10.1007/s11947-011-0582-y. [DOI] [Google Scholar]
- 25.Dulf F.V., Vodnar D.C., Socaciu C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016;209:27–36. doi: 10.1016/j.foodchem.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Hifney A.F., Fawzy M.A., Abdel-Gawad K.M., Gomaa M. Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018;269:387–395. doi: 10.1016/j.foodchem.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Dachery B., Hernandes K.C., Veras F.F., Schmidt L., Augusti P.R., Manfroi V., Zini C.A., Welke J.E. Effect of Aspergillus carbonarius on ochratoxin a levels, volatile profile and antioxidant activity of the grapes and respective wines. Food Res. Int. 2019;126:108687. doi: 10.1016/j.foodres.2019.108687. [DOI] [PubMed] [Google Scholar]
- 28.Handa C.L., de Lima F.S., Guelfi M.F.G., Fernandes M.d.S., Georgetti S.R., Ida E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019;271:274–283. doi: 10.1016/j.foodchem.2018.07.188. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.H., Hwang S.-R., Lee Y.-H., Kim K., Cho K.M., Lee Y.B. Changes occurring in compositions and antioxidant properties of healthy soybean seeds [Glycine max (L.) Merr.] and soybean seeds diseased by Phomopsis longicolla and Cercospora kikuchii fungal pathogens. Food Chem. 2015;185:205–211. doi: 10.1016/j.foodchem.2015.03.139. [DOI] [PubMed] [Google Scholar]
- 30.Tkacz K., Chmielewska J., Turkiewicz I.P., Nowicka P., Wojdyło A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020;332:127382. doi: 10.1016/j.foodchem.2020.127382. [DOI] [PubMed] [Google Scholar]
- 31.Rizzello C.G., Lorusso A., Russo V., Pinto D., Marzani B., Gobbetti M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017;241:252–261. doi: 10.1016/j.ijfoodmicro.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Li S., Jin Z., Hu D., Yang W., Yan Y., Nie X., Lin J., Zhang Q., Gai D., Ji Y., et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT Food Sci. Technol. 2020;125:109264. doi: 10.1016/j.lwt.2020.109264. [DOI] [Google Scholar]
- 33.Hashemi S.M.B., Jafarpour D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT Food Sci. Technol. 2020;131:109803. doi: 10.1016/j.lwt.2020.109803. [DOI] [Google Scholar]
- 34.Wang L., Luo Y., Wu Y., Liu Y., Wu Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem. 2018;264:189–198. doi: 10.1016/j.foodchem.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Deng H., Bai J., Zhou X., Zhao Y., Zhu Y., McClements D.J., Xiao X., Sun Q. Health-promoting properties of barley: A review of nutrient and nutraceutical composition, functionality, bioprocessing, and health benefits. Crit. Rev. Food Sci. 2021 doi: 10.1080/10408398.2021.1972926. Early Access. [DOI] [PubMed] [Google Scholar]
- 36.Liu L., Zhang R., Deng Y., Zhang Y., Xiao J., Huang F., Wen W., Zhang M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with α-amylase. Food Chem. 2017;221:636–643. doi: 10.1016/j.foodchem.2016.11.126. [DOI] [PubMed] [Google Scholar]
- 37.Huang Q., Zhang H., Xue D. Enhancement of antioxidant activity of Radix Puerariae and red yeast rice by mixed fermentation with Monascus purpureus. Food Chem. 2017;226:89–94. doi: 10.1016/j.foodchem.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Wu C., Zhu Y., Zhou C., Xiong Z., Samy Eweys A., Zhou H., Dong Y., Xiao X. Metabolomics strategy for revealing the components in fermented barley extracts with Lactobacillus plantarum dy-1. Food Res. Int. 2021;139:109808. doi: 10.1016/j.foodres.2020.109808. [DOI] [PubMed] [Google Scholar]
- 39.Limón R.I., Peñas E., Torino M.I., Martínez-Villaluenga C., Dueñas M., Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–352. doi: 10.1016/j.foodchem.2014.09.084. [DOI] [PubMed] [Google Scholar]
- 40.Dueñas M., Fernández D., Hernández T., Estrella I., Muñoz R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agr. 2005;85:297–304. doi: 10.1002/jsfa.1924. [DOI] [Google Scholar]
- 41.Starzyńska-Janiszewska A., Stodolak B., Mickowska B. Effect of controlled lactic acid fermentation on selected bioactive and nutritional parameters of tempeh obtained from unhulled common bean (Phaseolus vulgaris) seeds. J. Sci. Food Agr. 2014;94:3068. doi: 10.1002/jsfa.6851. [DOI] [PubMed] [Google Scholar]
- 42.Crujeiras A.B., Parra D., Abete I., Martínez J.A. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic. Res. 2007;41:498–506. doi: 10.1080/10715760601131935. [DOI] [PubMed] [Google Scholar]
- 43.Xiao Y., Xing G., Rui X., Li W., Chen X., Jiang M., Dong M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods. 2014;10:210–222. doi: 10.1016/j.jff.2014.06.008. [DOI] [Google Scholar]
- 44.Marazza J.A., Nazareno M.A., de Giori G.S., Garro M.S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods. 2012;4:594–601. doi: 10.1016/j.jff.2012.03.005. [DOI] [Google Scholar]
- 45.Yang J.-H., Mau J.-L., Ko P.-T., Huang L.-C. Antioxidant properties of fermented soybean broth. Food Chem. 2000;71:249–254. doi: 10.1016/S0308-8146(00)00165-5. [DOI] [Google Scholar]
- 46.Lee I.H., Hung Y.-H., Chou C.-C. Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int. J. Food Microbiol. 2008;121:150–156. doi: 10.1016/j.ijfoodmicro.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 47.Sawicki T., Wiczkowski W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018;259:292–303. doi: 10.1016/j.foodchem.2018.03.143. [DOI] [PubMed] [Google Scholar]
- 48.Wiczkowski W., Szawara-Nowak D., Topolska J. Changes in the content and composition of anthocyanins in red cabbage and its antioxidant capacity during fermentation, storage and stewing. Food Chem. 2015;167:115–123. doi: 10.1016/j.foodchem.2014.06.087. [DOI] [PubMed] [Google Scholar]
- 49.Olennikov D.N., Kirillina C.S., Chirikova N.K. Water-soluble melanoidin pigment as a new antioxidant component of fermented willowherb leaves (Epilobium angustifolium) Antioxidants. 2021;10:1300. doi: 10.3390/antiox10081300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antognoni F., Mandrioli R., Potente G., Taneyo Saa D.L., Gianotti A. Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem. 2019;292:211–216. doi: 10.1016/j.foodchem.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 51.Bei Q., Wu Z., Chen G. Dynamic changes in the phenolic composition and antioxidant activity of oats during simultaneous hydrolysis and fermentation. Food Chem. 2020;305:125269. doi: 10.1016/j.foodchem.2019.125269. [DOI] [PubMed] [Google Scholar]
- 52.Makkar H.P.S., Sidhuraju P., Becker K. Plant Secondary Metabolites. Humana Press; Totowa, NJ, USA: 2007. [DOI] [PubMed] [Google Scholar]
- 53.Zieliński H., Szawara-Nowak D., Bączek N., Wronkowska M. Effect of liquid-state fermentation on the antioxidant and functional properties of raw and roasted buck wheat flours. Food Chem. 2019;271:291–297. doi: 10.1016/j.foodchem.2018.07.182. [DOI] [PubMed] [Google Scholar]
- 54.Chung Y., Park J.-Y., Lee J.-E., Kim K.-T., Paik H.-D. Antioxidant Activity and Inhibitory Effect on Nitric Oxide Production of Hydroponic Ginseng Fermented with Lactococcus lactis KC24. Antioxidants. 2021;10:1614. doi: 10.3390/antiox10101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mapelli-Brahm P., Barba F.J., Remize F., Garcia C., Fessard A., Mousavi Khaneghah A., Sant’Ana A.S., Lorenzo J.M., Montesano D., Meléndez-Martínez A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Tech. 2020;99:389–401. doi: 10.1016/j.tifs.2020.03.013. [DOI] [Google Scholar]
- 56.Oloo B.O., Shitandi A.A., Mahungu S., Malinga J., Ogata R. Effects of lactic acid fermentation on the retention of β-carotene content in orange fleshed sweet potatoes. Int. J. Food Stud. 2014;3:13–33. doi: 10.7455/ijfs/3.1.2014.a2. [DOI] [Google Scholar]
- 57.Hubert J., Berger M., Nepveu F., Paul F., Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–721. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- 58.Adeyeye E. The effect of fermentation on the dietary quality of lipids from African locust bean (Parkia biglobosa) seeds. Elixir Food Sci. 2013;58:14912–14922. [Google Scholar]
- 59.Chen L., Hui Y., Gao T., Shu G., Chen H. Function and characterization of novel antioxidant peptides by fermentation with a wild Lactobacillus plantarum 60. LWT Food Sci. Technol. 2021;135:110162. doi: 10.1016/j.lwt.2020.110162. [DOI] [Google Scholar]
- 60.Muhialdin B.J., Abdul Rani N.F., Meor Hussin A.S. Identification of antioxidant and antibacterial activities for the bioactive peptides generated from bitter beans (Parkia speciosa) via boiling and fermentation processes. LWT Food Sci. Technol. 2020;131:109776. doi: 10.1016/j.lwt.2020.109776. [DOI] [Google Scholar]
- 61.Miao J., Shi W., Zhang J., Zhang X., Zhang H., Wang Z., Qiu J. Response surface methodology for the fermentation of polysaccharides from Auricularia auricula using Trichoderma viride and their antioxidant activities. Int. J. Biol. Macromol. 2020;155:393–402. doi: 10.1016/j.ijbiomac.2020.03.183. [DOI] [PubMed] [Google Scholar]
- 62.He R., Ju X., Yuan J., Wang L., Girgih A.T., Aluko R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res. Int. 2012;49:432–438. doi: 10.1016/j.foodres.2012.08.023. [DOI] [Google Scholar]
- 63.Liu X., Hou R., Xu K., Chen L., Wu X., Lin W., Zheng M., Fu J. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 2019;123:468–476. doi: 10.1016/j.ijbiomac.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 64.Cho K.M., Lee J.H., Yun H.D., Ahn B.Y., Kim H., Seo W.T. Changes of phytochemical constituents (isoflavones, flavanols, and phenolic acids) during cheonggukjang soybeans fermentation using potential probiotics Bacillus subtilis CS90. J. Food Compos. Anal. 2011;24:402–410. doi: 10.1016/j.jfca.2010.12.015. [DOI] [Google Scholar]
- 65.Koistinen V.M., Katina K., Nordlund E., Poutanen K., Hanhineva K. Changes in the phytochemical profile of rye bran induced by enzymatic bioprocessing and sourdough fermentation. Food Res. Int. 2016;89:1106–1115. doi: 10.1016/j.foodres.2016.06.027. [DOI] [Google Scholar]
- 66.Sheih I.C., Fang T.J., Wu T.-K., Chen R.-Y. Effects of fermentation on antioxidant properties and phytochemical composition of soy germ. J. Sci. Food Agr. 2014;94:3163–3170. doi: 10.1002/jsfa.6666. [DOI] [PubMed] [Google Scholar]
- 67.Rodríguez H., Landete J.M., de las Rivas B., Muñoz R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008;107:1393–1398. doi: 10.1016/j.foodchem.2007.09.067. [DOI] [Google Scholar]
- 68.Rodríguez H., Curiel J.A., Landete J.M., de las Rivas B., de Felipe F.L., Gómez-Cordovés C., Mancheño J.M., Muñoz R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009;132:79–90. doi: 10.1016/j.ijfoodmicro.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 69.Acin-Albiac M., Filannino P., Arora K., Da Ros A., Gobbetti M., Di Cagno R. Role of lactic acid bacteria phospho-β-glucosidases during the fermentation of cereal by-products. Foods. 2021;10:97. doi: 10.3390/foods10010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Araújo J.A., Ferreira N.R., da Silva S.H.M., Oliveira G., Monteiro R.C., Alves Y.F.M., Lopes A.S. Filamentous fungi diversity in the natural fermentation of Amazonian cocoa beans and the microbial enzyme activities. Ann. Microbiol. 2019;69:975–987. doi: 10.1007/s13213-019-01488-1. [DOI] [Google Scholar]
- 71.Costa R.d.S., de Almeida S.S., Cavalcanti E.d.A.C., Freire D.M.G., Moura-Nunes N., Monteiro M., Perrone D. Enzymes produced by solid state fermentation of agro-industrial by-products release ferulic acid in bioprocessed whole-wheat breads. Food Res. Int. 2021;140:109843. doi: 10.1016/j.foodres.2020.109843. [DOI] [PubMed] [Google Scholar]
- 72.Yao Y., Wu M., Huang Y., Li C., Pan X., Zhu W., Huang Y. Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum-fermented loose tea. LWT Food Sci. Technol. 2017;82:248–254. doi: 10.1016/j.lwt.2017.04.032. [DOI] [Google Scholar]
- 73.Nguyen T.T.T., Loiseau G., Icard-Vernière C., Rochette I., Trèche S., Guyot J.-P. Effect of fermentation by amylolytic lactic acid bacteria, in process combinations, on characteristics of rice/soybean slurries: A new method for preparing high energy density complementary foods for young children. Food Chem. 2007;100:623–631. doi: 10.1016/j.foodchem.2005.09.080. [DOI] [Google Scholar]
- 74.Darwesh O.M., El-Latief A.H.A., Abuarab M.E., Kasem M.A. Enhancing the efficiency of some agricultural wastes as low-cost absorbents to remove textile dyes from their contaminated solutions. Biomass Convers. Biorefinery. 2021:1–10. doi: 10.1007/s13399-020-01142-w. [DOI] [Google Scholar]
- 75.Manan M., Webb C. Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017;4:1–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available are included in the article.