Abstract
Simple Summary
This study was conducted to explore the antibacterial ability of sheep β-defensin 2 (SBD-2) against E. coli F17 infection in ovine intestinal epithelial cells (OIECs). Our data revealed that E. coli F17 induces SBD-2 expression in OIECs in vitro, which appears to be mediated through the activation of the signaling pathways NF-κB and MAPK. Our results provide a novel insight for the functionality of SBD-2, which could be useful for developing anti-infective drugs and/or breeding for E. coli diarrhea disease-resistant sheep.
Abstract
Escherichia coli (E. coli) F17 is a member of enterotoxigenic Escherichia coli, which can cause massive diarrhea and high mortality in newborn lambs. β-defensin is mainly produced by the epithelial tissue of the gastrointestinal tract in response to microbial infection. However, the molecular mechanism of sheep β-defensin 2 (SBD-2) against E. coli F17 remains unclear. This study aims to reveal the antibacterial ability of SBD-2 against E. coli F17 infection in sheep. Firstly, we established the culture system of ovine intestinal epithelial cells (OIECs) in vitro, treated with different concentrations of E. coli F17 for an indicated time. Secondly, we performed RNA interference and overexpression to investigate the effect of SBD-2 expression on E. coli F17 adhesion to OIECs. Finally, inhibitors of NF-κB and MAPK pathways were pre-treated to explore the possible relationship involving in E. coli F17 infection regulating SBD-2 expression. The results showed that E. coli F17 markedly (p < 0.01) upregulated the expression levels of SBD-2 mRNA and protein in a concentration- and time-dependent manner. Overexpression of SBD-2 contributed to enhancing E. coli F17 resistance in OIECs, while silencing SBD-2 dramatically improved the adhesion of E. coli F17 to OIECs (p < 0.05 or p < 0.01). Furthermore, E. coli F17 stimulated SBD-2 expression was obviously decreased by pre-treatment with NF-κB inhibitor PDTC, p38 MAPK inhibitor SB202190 and ERK1/2 MAPK inhibitor PD98095 (p < 0.05 or p < 0.01). Interestingly, adhesion of E. coli F17 to OIECs were highly enhanced by pre-treated with PDTC, SB202190 and PD98095. Our data suggested that SBD-2 could inhibit E. coli F17 infection in OIECs, possibly through NF-κB and MAPK signaling pathways. Our results provide useful theoretical basis on developing anti-infective drug and breeding for E. coli diarrhea disease-resistant sheep.
Keywords: sheep, SBD-2, Escherichia coli F17, inflammation, NF-κB pathway, MAPK pathway
1. Introduction
Enterotoxigenic Escherichia coli (ETEC) F17 is one of the most common pathogens causing E. coli diarrhea in lambs. Its fatality rate is very high, which seriously hinders the production and profit of large-scale sheep farms [1,2]. ETEC is characterized by its ability to produce two types of virulence factor: adhesin and enterotoxin [3]. Once colonized by adhesin in the intestines of neonatal animals, ETEC will produce two major classes of enterotoxins, namely, heat-labile toxin (LT) and heat-stable toxin (ST). According to their antigenic properties, the main adhesins can be classified into different sub-types, such as F4 (also designated K88), F5 (K99), F6 (987p), F17 (Fy), F18 and F41 [4]. F17 fimbriae were first found in diarrhea calves, which are typically colonized in small intestinal mucosa to produce STa enterotoxin, leading to diarrhea in lambs [5].
Defensins, as the first line of defense against pathogens, have broad-spectrum bactericidal effect, especially in killing Gram-negative and Gram-positive bacteria [6]. Due to the side effects of traditional antibiotics and the emergence of drug-resistant strains [7], the use of defensin as a new drug or functional target gene to breed disease-resistant animals may be helpful to control bacterial infection. β-defensins are mainly distributed in the epithelial tissues of the gastrointestinal tract, which had been found in human, pigs, cattle, sheep, etc. [8]. In sheep, two types of β-defensins, sheep β-defensin 1 (SBD-1) and sheep β-defensin 2 (SBD-2), have been described [9]. However, the function of SBD-2 involved with E. coli F17 infection in sheep has not been reported. In other animals, accumulated evidence showed that β-defensins not only have strong antibacterial activity, but also participate in regulating inflammatory response. It has been previously reported that human β-defensin 118 could reduce the inflammation and intestinal injury in mice induced by E.coli F4 [10]. For the inflammatory response of E. coli, it is mainly induced by lipopolysaccharide (LPS), which can be recognized by Toll-like receptors (TLRs) family, mainly resulting in the activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPK) signaling [11]. In a study related to HBD-3, the researchers found that HBD-3 could down-regulate the expression of cytokines in macrophages through inhibiting the activation of NF-κB and MAPK pathways induced by LPS [12]. Another report revealed that PBD-2 could improve inflammatory response by affecting the activation of NF-κB signal pathway [13]. Based on the above research progress, we hypothesized that SBD-2 could play a potential role in regulating E. coli F17 infection in sheep and two inflammatory pathways were involved in the process of E. coli F17 infection.
Thus, in this study, we constructed the model of ovine intestinal epithelial cells (OIECs) infected by E. coli F17, and verified the relationship between the expression of SBD-2 and E. coli F17 infection in sheep using RT-PCR and ELISA analysis. Furthermore, we performed RNA interference and overexpression to inquiry the effect of SBD-2 expression on E. coli F17 adhesion to OIECs. Further, we used RT-PCR analysis to detect whether NF-κB and MAPK pathways were activated after E. coli F17 stimulation. Then, we conducted pathway inhibition experiment to study the effect on SBD-2 expression. Finally, colony count analysis was conducted to explore the influence of pathway inhibition on the adhesion of E. coli F17 to OIECs. This study not only comprehensively explored the essential role of SBD-2 regulating E. coli F17 infection in OIECs, but also deeply studied the status of the NF-κB and MAPK pathways in this process. Our results could provide feasible treatment direction for E. coli F17 resistance in lambs and theoretical basis on breeding for E. coli diarrhea disease-resistant sheep.
2. Materials and Methods
2.1. Experimental Sample and Ethical Statement
OIECs used in this experiment were isolated from two healthy lambs of Hu sheep at the age of 3 to 5 days (Jiangsu Xilaiyuan Ecological Agriculture Co., Ltd., Taizhou, China). The detailed isolation method was documented in Figure S1. Purification and identification work had been finished in the laboratory of our research group. E. coli F17 strain (DN1502) was offered by Prof. Dr. Dongfang Shi, Northeast Agricultural University (Harbin, China).
The Institutional Animal Care and Use Committee (IACUC) of the government of Jiangsu Province (Permit Number 45) and the Ministry of Agriculture of China (Permit Number 39) approved the animal study proposal. All experimental procedures were conducted in strict compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals of Jiangsu Province and of the Animal Care and Use Committee of the Chinese Ministry of Agriculture.
2.2. Cell Culture and E. coli F17 Stimulation in OIECs
OIECs were cultured with a DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (All from Sigma-Aldrich, St. Louis, MO, USA) in 5% CO2 atmosphere at 37 °C. The growth of cells was observed under an inverted fluorescence microscope. E. coli F17 strain, which was preserved with glycerin, was inoculated into a Luria-Bertani (LB) agar Petri dish and incubated for 16–18 h at 37 °C. Then, a single colony was picked up with the end of a sterilization inoculation ring and inoculated it into LB liquid medium, and then they were incubated overnight on a shaker with 150 rpm (more details about E. coli F17 were shown in Table S1).
In order to determine the best infection condition related to SBD-2 gene, here we designed different infection concentrations and times. Cells were inoculated to 6-well plates, cultured in DMEM/F12 medium without serum and antibiotics, and then performed E. coli F17 stimulation. Firstly, OIECs were treated with five different concentrations of E. coli F17, namely, 0 CFU/mL, 105 CFU/mL, 106 CFU/mL, 107 CFU/mL and 108 CFU/mL, respectively. Then, based on the best concentration obtained from the previous experiment, OIECs were treated with six different infection times, namely, 0 h, 2 h, 4 h, 6 h, 8 h and 10 h. Cells collection was used for RT-PCR analysis and Enzyme-linked Immunosorbent Assay (ELISA) to detect mRNA and protein expression levels of SBD-2, respectively. Specific primers for RT-PCR were shown in Table S2.
2.3. Plasmid Construction and RNA Oligonucleotides
According to the coding sequence (CDS) of SBD-2 in NCBI (https://www.ncbi.nlm.nih.gov/ (accessed on 23 November 2021)), primers were designed by premier primer 5.0 software (Premier Biosoft International, Palo Alto, CA, USA) and were documented in Table S3. Full-length DNA encoding SBD-2 were amplified using PrimeSTAR Max DNA Polymerase reagent (Takara, Kusatsu, Shiga, Japan). The obtained PCR product was purified and recovered by SanPrep column PCR product purification kit (Sangon Biotech, Shanghai, China) and then was sequenced by Beijing Tsingke Biotechnology Co., Ltd. (Nanjing, China). The pGH plasmid (Generay, Shanghai, China) and SBD-2 target fragment were digested with restriction enzyme QuickCut EcoR V (Takara), then connected the target fragment into the linear vector according to the instruction of DNA Ligation Kit Ver.2.1 kit (Takara). After enzyme digestion identification and sequencing verification, the successfully constructed cloning plasmid was named pGH-SBD-2. A schematic diagram for the construction of SBD-2 overexpression vector was shown in Figure S2. Small-interfering ribonucleic acids (siRNAs) of SBD-2 together with negative control (NC) were synthesized by GenePharma Pharmaceutical Technology Co., Ltd. (Shanghai, China). All sequences were shown in Table S4.
2.4. Cell Transfection
When the cell confluence reached 50–60%, pGH-SBD-2 plasmid and siRNAs of SBD-2 were transfected into cells with jetPRIME transfection reagent (Polyplus transfection, Illkirch, France) following manufacturer’s instructions. Each transfection had been treated with at least three replications. After 24 h, cells were collected for RT-PCR analysis to verify transfection efficiency.
2.5. Pathway Inhibitors Blocking Signaling Pathway Test
To investigate the function of NF-κB and MAPK signaling pathway in the process of SBD-2 regulating E. coli F17 infection in OIECs, pathway inhibitors (Beyotime, Shanghai, China) were added into four groups of well-growing OIECs, namely, blank control group, positive control group, negative control group and experimental group. The detailed treatment of each group was shown in Table 1. Then, cells were collected to quantify the mRNA expression levels of p65, p50, p38, ERK1 and JNK. Using these cells, the mRNA and protein expression levels of SBD-2 were been quantified as well. The primers used for RT-PCR were documented in Table S2.
Table 1.
Different treatment of grouping.
| A Blank Control Group |
B Positive Control Group |
C Negative Control Group |
D Experimental Group |
|---|---|---|---|
| No treatment | E. coli F17 only | NF-κB pathway inhibitor PDTC (25 μM), | NF-κB pathway inhibitor PDTC (25 μM) + E. coli F17, |
| p38 pathway inhibitor SB202190 (25 μM), | p38 pathway inhibitor SB202190 (25 μM) + E. coli F17, | ||
| ERK1/2 pathway inhibitor PD98059 (50 μM), | ERK1/2 pathway inhibitor PD98059 (50 μM) + E. coli F17, | ||
| (Add separately) | (Add separately) |
2.6. Total RNA Extraction and Real-Time PCR (RT-PCR)
Total RNA was extracted from cells using the TRIzol reagent (TIANGEN, Beijing, China). The purity and concentration were detected by 1% agarose gel electrophoresis and NanoReady spectrophotometer (Life Real, Hangzhou, China). All RNA samples were stored at −80 °C. Reverse transcription was implemented using the FastKing gDNA Dispelling RT Super Mix (TIANGEN). The reverse transcription reaction system and reaction condition were summed up in Table S5. RT-PCR was conducted using the 2× TSINGKE Master qPCR Mix (SYBR Green I) (TSE201, Tsingke, Beijing, China). The detailed amplification program was shown in Table S5. The GAPDH was used as an internal reference gene. RT-PCR was implemented by using CFX96 Connect™ Real-Time System (BIO-RAD, CA, USA). All RT-PCR results were analyzed using 2−∆∆Ct method [14].
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
To assess SBD-2 mass concentration, the cell culture medium was collected to implemented ELISA after plasmid transfection and E. coli F17 infection, referring to the sheep β-Defensin 2 enzyme-linked immunosorbent assay detection kit instructions (mlbio, Shanghai, China), following specific steps. Firstly, the above cell medium was centrifuged at 4 °C, 2000–3000 rpm for 20 min and supernatant was saved as samples. Secondly, 50 µL standard substance of different concentrations was added into the standard wells; meanwhile, 50 µL sample was added into the sample wells except the blank wells. Thirdly, 100 µL enzyme-labeled reagent was added into standard wells and sample wells except the blank wells, and they were incubated at 37 °C for 1 h. Then, 350 µL washing liquid was added into each well for 1 min and absorbent paper was used to pat dry, 5 times repeatedly. Finally, 50 µL substrate A and B were added into each well, and they were incubated at 37 °C for 15 min in the dark, then 50 µL stop solution was added into each well. OD value of each well was measured at 450 nm wavelength in the microplate reader at 26 °C within 15 min (Tecan, Shanghai, China). Additionally, the OD value of the measured standard substance was used as the abscissa and the concentration value was used as the ordinate to draw the standard curve.
2.8. Plate Counting Method for Bacteria Enumeration
To assess adhesion of E. coli F17 to OIECs, plate count was performed according to Jouve et al. [15] after plasmid transfection and E. coli F17 infection. First of all, cells were washed gently three times with PBS buffer (Sigma-Aldrich). Then, 300 µL 0.5% Triton X-100 (Solarbio, Beijing, China) were added into each well to lyse the cells for 30 min, and the cell lysate was collected. Next, 200 µL PBS buffer were added to wash twice and a total of 700 µL liquid were collected into a 1.5 mL sterile centrifuge tube, and they were mixed uniformly. Last, liquid was diluted by multiple times, then the diluent was spread on LB Petri dish, and they were inverted in a constant temperature incubator at 37 °C overnight. The number of colonies were counted.
2.9. Statistical Analysis
Before statistical analysis, the normality of data were been tested by Kolmogorov–Smirnov Test using SPSS 25.0. An analysis of variance (ANOVA) was applied to analyze the differences of SBD-2 expression level in OIECs between the E. coli F17 stimulation group and non-treated group with different bacteria concentration and infection times by using SPSS 25.0 software (SPSS, Inc., Chicago, IL, USA). Independent t test was performed to analyze the differences of SBD-2 expression level in OIECs between pGH group and pGH-SBD-2 group, the differences of SBD-2 expression level in OIECs between negative control group and siRNA-SBD-2 group, the differences of number of E. coli F17 colonies between pGH group and pGH-SBD-2 group, and the differences of number of E. coli F17 colonies between negative control group and siRNA-SBD-2 group. Independent t test was performed to analyze the differences of genes (p65, p50, p38, ERK1/2, JNK) mRNA expression levels in OIECs between non-treated group and E. coli F17 infection group, the differences of p50, p38, ERK1/2 and SBD-2 expression levels in OIECs between non-treated group and positive control group, negative control group and experimental group, respectively. Independent t test was performed to analyze the differences of number of E. coli F17 colonies between non-treated group and pathway inhibitors treatment group. Results were represented as mean ± SD (* denotes p < 0.05, significant difference; ** denotes p < 0.01, extremely significant difference).
3. Results
3.1. Effect of E. coli F17 Different Infection Concentration and Time on SBD-2 Expression
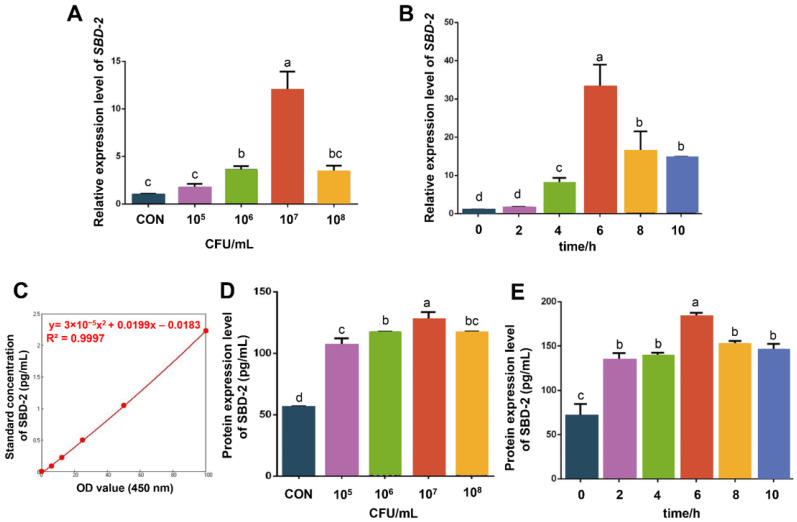
The resuscitated OIECs had consistent normal morphology and good activity, growing adherently and closely connected (Figure S3). They could be used in the later experiment. Using 1% agarose gel electrophoresis to check the quality of the total RNA extracted from cells, clear 28 S and 18 S bands can be observed, indicating that the integrity of the extracted RNA is of high quality (Figure S4). As clearly reflected in Figure 1A, mRNA expression levels of SBD-2 with 107 CFU/mL infection concentration reached maximum, which were also extremely significantly higher than those of the control group (p < 0.01) (The extremely significant level was shown in Figure S5, the same below).
Figure 1.
E. coli F17 stimulates mRNA and protein expression levels of SBD-2. (A) SBD-2 mRNA expression levels detected by RT-PCR in OIECs treated with the indicated concentrations of E. coli F17 compared with non-treated controls. (B) SBD-2 mRNA expression levels detected by RT-PCR in OIECs treated with E. coli F17 (107 CFU/mL) for various time intervals compared with non-treated controls. (C) Standard curve drawn by SBD-2 protein concentration value (y axis) and OD value at 450 nm (x axis). (D) SBD-2 protein expression levels detected by ELISA in OIECs treated with the indicated concentrations of E. coli F17 compared with non-treated controls. (E) SBD-2 protein expression levels detected by ELISA in OIECs treated with E. coli F17 (107 CFU/mL) for various time intervals compared with non-treated controls. Mean values with different letters in the same row are significantly different (p < 0.05) according to Duncan’s multiple range test; data were shown as mean ± SD, n = 3 biological replicates.
Hence, we used 107 CFU/mL infection concentration to optimize the infection time. The result of relative quantification (Figure 1B) showed that mRNA expression levels of SBD-2 achieved maximum at 6 h, which were also extremely significantly higher than those of the control group (p < 0.01). We also detected SBD-2 protein level using ELISA. As demonstrated in Figure 1C, this standard curve could be used to calculate SBD-2 protein level in samples. Results obviously showed that changes in SBD-2 protein levels shared the same trend with SBD-2 mRNA levels that it reached the peak when we used 107 CFU/mL infection concentration (Figure 1D) with 6 h of infection (Figure 1E), which were both extremely significantly higher than those of the control group (p < 0.01). Therefore, we took 107 CFU/mL of E. coli F17 concentration and 6 h of infection as optimal conditions in subsequent experiments.
3.2. Expression Level of SBD-2 Regulates E. coli F17 Resistance in OIECs
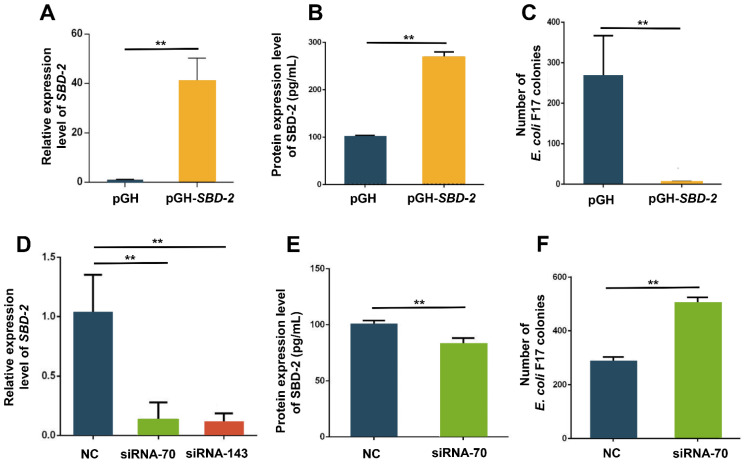
To probe into whether SBD-2 expression has the function of resisting E. coli F17 infection in OIECs, we performed SBD-2 overexpression and RNA interference (Figure 2). The results of relative quantification (Figure 2A) and ELISA (Figure 2B) showed mRNA and protein expression levels of SBD-2 in OIECs treated with pGH-SBD-2 were extremely significantly higher than those of pGH treated cells (p < 0.01). The result of bacteria enumeration (Figure 2C) showed the remarkably decreased adhesion of E. coli F17 to OIECs treated with pGH-SBD-2 compared with that of pGH treated cells (p < 0.01). Besides, we used RNA interference to knockdown SBD-2 expression. As shown in Figure 2D, the interference efficiency of SBD-2 reached more than 50%, and we chose siRNA-70 for next functional verification experiment. The result from ELISA (Figure 2E) showed that SBD-2 protein expression level was significantly reduced in OIECs treated with siRNA-SBD-2 compared with that of non-treated cells (p < 0.05). The result from bacteria enumeration (Figure 2F) showed SBD-2 knockdown could dramatically improve the adhesion of E. coli F17 to OIECs. All these results indicated that overexpression of SBD-2 contributed to enhancing E. coli F17 resistance in OIECs, while SBD-2 knockdown improved adhesion of E. coli F17 to OIECs.
Figure 2.
The effect of SBD-2 expression on the adhesion of E. coli F17 to OIECs. (A) Overexpression efficiency of SBD-2 mRNA expression level detected by RT-PCR in pGH (Control) cells and pGH-SBD-2 cells. (B) Overexpression efficiency of SBD-2 protein expression level detected by ELISA in pGH (Control) cells and pGH-SBD-2 cells. (C) Adhesion of the F17 fimbria to OIECs transfected with pGH-SBD-2 compared with pGH (Control) analyzed by bacteria enumeration. (D) Interference efficiency of SBD-2 mRNA expression level detected by RT-PCR in OIECs transfected with siRNA-SBD-2 compared with negative control. (E) Interference efficiency of SBD-2 protein expression level detected by ELISA in OIECs transfected with siRNA-SBD-2 compared with negative control. (F) Adhesion of the F17 fimbria to OIECs transfected with siRNA-SBD-2 compared with negative control analyzed by bacteria enumeration. ** p < 0.01, extremely significant difference; NS = no difference. Data were shown as mean ± SD, n = 3 biological replicates.
3.3. Effect of E. coli F17 Stimulation in OIECs on NF-κB and MAPK Pathways
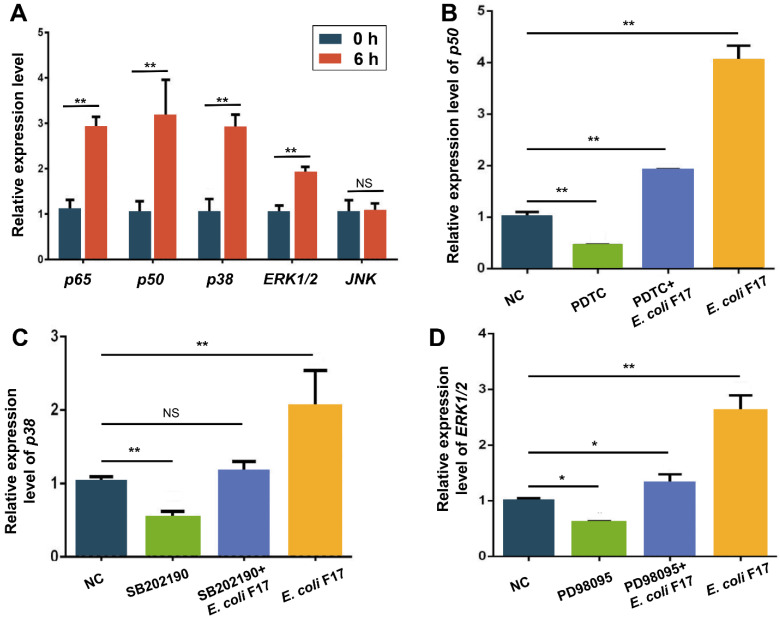
To confirm whether NF-κB and MAPK pathways participating in the process of E. coli F17 infection in OIECs, we used 107 CFU/mL E. coli F17 to infect OIECs for 6 h. Then, RT-PCR was to detect mRNA expression levels of p50, p65, p38, ERK1 and JNK. The result of relative quantification (Figure 3A) showed that expression levels of p50, p65, p38, ERK1 and JNK were highly up-regulated in infection group compared with non-treated group (p < 0.01), which indicated NF-κB and MAPK pathways were activated after E. coli F17 stimulating OIECs. Then, we conducted experiments on co-treatment of OIECs with pathway inhibitors and E. coli F17. The result of relative quantification (Figure 3B–D) reflected obviously down-regulated mRNA expression levels of p50 (Figure 3B), p38 (Figure 3C) and ERK1/2 (Figure 3D) in inhibitor addition groups in comparison with the non-treated groups (p < 0.05 or p < 0.01), while mRNA expression levels of p50 and ERK1/2 were markedly increased after co-treatment with E. coli F17 (p < 0.05 or p < 0.01). The above results indicated that pathway inhibitors could be used for the subsequent experiments.
Figure 3.
The effect of E. coli F17 stimulation in OIECs on NF-κB and MAPK pathways. (A) p65, p50, p38, ERK1/2 and JNK expression level determined by RT-PCR in OIECs treated with E. coli F17 (107 CFU/mL) for 6 h compared with non-treated control. (B) p50 expression level determined by RT-PCR in OIECs treated with NF-κB inhibitor PDTC, PDTC+E. coli F17 and E. coli F17 compared with non-treated control. (C) p38 expression level determined by RT-PCR in OIECs treated with p38 MAPK inhibitor SB202190, SB202190+E. coli F17 and E. coli F17 compared with non-treated control. (D) ERK1/2 expression level determined by RT-PCR in OIECs treated with ERK1/2 MAPK inhibitor PD98095, PD98095+E. coli F17 and E. coli F17 compared with non-treated control. * p < 0.05, significant difference; ** p < 0.01, extremely significant difference; NS = no difference. Data were shown as mean ± SD, n = 3 biological replicates.
3.4. NF-κB and MAPK Pathways Influence SBD-2 Expression at the mRNA and Protein Levels
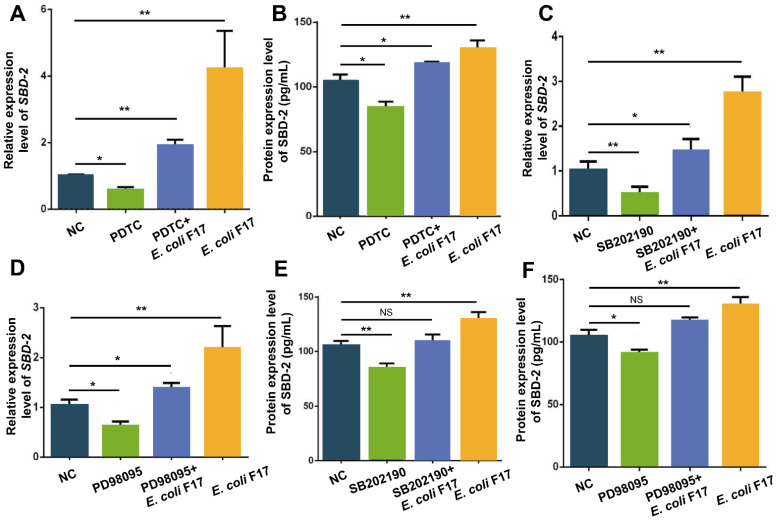
Since we have confirmed that NF-κB and MAPK pathways could be activated after E. coli F17 infecting OIECs, the effects of pathways on SBD-2 expression is unknown. First, we added NF-κB pathway inhibitor PDTC and E. coli F17 infection treatment to OIECs. The relative quantitative results revealed that the mRNA expression level of SBD-2 was significantly down-regulated after the addition of PDTC inhibitors compared with non-treated group (p < 0.05), then very highly up-regulated after co-treatment with E. coli F17 (p < 0.01) (Figure 4A). The result of ELISA (Figure 4B) showed the changes of SBD-2 protein level were consistent with the mRNA level. Next, we added p38 pathway inhibitor SB202190 and ERK1/2 pathway inhibitor PD98095, co-treatment with E. coli F17. The result of relative quantification (Figure 4C,D) showed an obvious decrease with SBD-2 mRNA expression levels in OIECs treated with pathway inhibitors compared with those in non-treated cells (p < 0.05 or p < 0.01), then highly up-regulated after co-treatment with E. coli F17 (p < 0.01). The result of ELISA (Figure 4E,F) confirmed that protein level of SBD-2 shared the same trend of mRNA level. Therefore, NF-κB and MAPK pathways could be involved in regulating the SBD-2 expression in OIECs infected by E. coli F17.
Figure 4.
The effect of NF-κB and MAPK pathways on SBD-2 expression. (A) SBD-2 relative expression determined by RT-PCR in OIECs treated with PDTC, PDTC+E. coli F17 and E. coli F17 compared with non-treated control. (B) SBD-2 protein expression determined by ELISA in OIECs treated with NF-κB inhibitor PDTC, PDTC+E. coli F17 and E. coli F17 compared with non-treated control. (C) SBD-2 relative expression determined by RT-PCR in OIECs treated with p38 MAPK inhibitor SB202190, SB202190+E. coli F17 and E. coli F17 compared with non-treated control. (D) SBD-2 relative expression determined by RT-PCR in OIECs treated with ERK1/2 MAPK inhibitor PD98095, PD98095+E. coli F17 and E. coli F17 compared with non-treated control. (E) SBD-2 protein expression determined by ELISA in OIECs treated with p38 MAPK inhibitor SB202190, SB202190+E. coli F17 and E. coli F17 compared with non-treated control. (F) SBD-2 protein expression determined by ELISA in OIECs treated with ERK1/2 MAPK inhibitor PD98095, PD98095+E. coli F17and E. coli F17 compared with non-treated control. * p < 0.05, significant difference; ** p < 0.01, extremely significant difference; NS = no difference. Data were shown as mean ± SD, n = 3 biological replicates.
3.5. NF-κB and MAPK Pathways Regulate Adhesion of E. coli F17 to OIECs
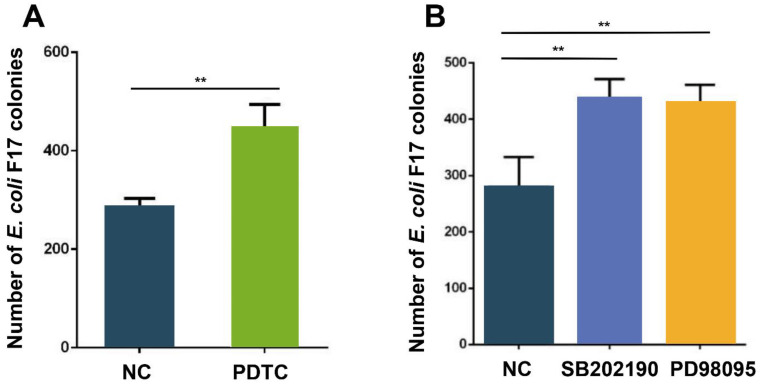
In order to further determine the role of the NF-κB and MAPK pathways in the process of E. coli F17 infecting OIECs, NF-κB pathway inhibitor PDTC, p38 pathway inhibitor SB202190 and ERK1/2 pathway inhibitor PD98095 were added to OIECs, respectively, and then collected cells for colony count analysis. The result of bacteria enumeration (Figure 5A) showed that distinctly increased adhesion of E. coli F17 to OIECs treated with PDTC compared with that of non-treated cells (p < 0.01). Similarly, adhesion of E. coli F17 to OIECs were both highly enhanced in OIECs treated with SB202190 and PD98095 contrast with those of non-treated cells (p < 0.01) (Figure 5B). These results suggested that NF-κB and MAPK pathways could affect the adhesion of E. coli F17 to OIECs.
Figure 5.
The effect of NF-κB and MAPK pathways on the adhesion of E. coli F17 to OIECs. (A) Adhesion of the F17 fimbria to OIECs treated with NF-κB inhibitor PDTC compared with negative control analyzed by bacteria enumeration. (B) Adhesion of the F17 fimbria to OIECs treated with p38 MAPK inhibitor SB202190 and ERK1/2 MAPK inhibitor PD98095 compared with negative control analyzed by bacteria enumeration. ** p < 0.01, extremely significant difference; data were shown as mean ± SD, n = 3 biological replicates.
4. Discussion
4.1. Effect of SBD-2 Expression on E. coli F17 Adhesion to OIECs
Defensin is a kind of antimicrobial peptide, which exists widely in mammals and has an extensive killing effect on bacteria, fungi, viruses and parasites that invade the body. β-defensin was first found in bovine neutrophils, which is considered to be an important component of the anti-microbial barrier on the mucosal surface [16]. Thus far, SBD-1 and SBD-2 have been annotated in sheep and are located on chromosome 26. SBD-2 is mainly distributed in the epithelial cells of the gastrointestinal tract and digestive tract [17]. In recent years, studies focused on sheep β-defensin seem to be more inclined to study SBD-1, because the strong expression of SBD-1 in rumen epithelium makes researchers interested in exploring the mechanism of SBD-1 expression in the process of sheep rumen innate immunity [18,19,20,21,22]. However, we cannot ignore that sheep colibacillosis is one of the important factors restricting the development of sheep breeding industry in China, which is caused by E. coli F17. Previous studies have found that SBD-2 expression level is the strongest in the intestinal tract, and the tissue distribution is higher in fetal sheep and newborn lambs, which plays a vital role in developmental regulation before and after birth [23,24]. However, there is no detailed report related to the relationship between SBD-2 and E. coli F17 infection in OIECs.
In this study, we successfully established a model of E. coli F17 infection in OIECs in vitro. To explore the expression changes of SBD-2 in OIECs infected by E. coli F17, cells were infected with different concentrations of E. coli F17. We found that different concentrations of E. coli F17 could cause significant changes in SBD-2 expression in OIECs, and the expression level of SBD-2 in OIECs reached the maximum when the infection concentration was 107 CFU/mL. Further, cells were infected with E. coli F17 at the concentration of 107 CFU/mL for different times, and we found that the expression level of SBD-2 in OIECs reached the maximum at 6 h. Interestingly, E. coli F17 infection markedly upregulated SBD-2 mRNA and protein expression levels in a concentration- and time-dependent manner. Based on the above results, we figured out that the best condition for infection test was E. coli F17 infecting OIECs with 107 CFU/mL for 6 h.
Recently, scholars in various fields tend to use gene overexpression technology to explore the mechanism of β-defensin regulating cancer diseases, species including human [25,26], pig [27,28], mice [29,30], etc. Similarly, RNAi technology is also increasingly used in gene functional analysis [31]. However, to comprehensively explore the mechanism of gene action, we are supposed to combine gene overexpression with interference to explore the molecular mechanism of β-defensin [32]. In this study, we carried out SBD-2 overexpression and knockdown to explore the ability of E. coli F17 adhering to OIECs. The results showed that overexpression of SBD-2 could dramatically enhance E. coli F17 resistance in OIECs, while interfering with SBD-2 could obviously improve the adhesion of E. coli F17 to OIECs. Thus, SBD-2 is likely to play an essential role in the resistance of OIECs to E. coli F17 infection in lambs. It is well established that the distribution of β-defensins differ in different species [33], resulting in their different anti-infection ability to bacteria. In human, Lin et al. reported that Human β-Defensin 118 (DEFB118) showed antimicrobial activities against E. coli K88 and E. coli DH5α with a minimum inhibitory concentration (MIC) of 4 μg/mL [34]. In pig, Su et al. showed that porcine β-defensin 114 (PBD114) inhibited the activities of E. coli DH5α and K88 with MIC of 64 and 128 μg/mL, respectively [35]. In sheep, we have confirmed the molecular mechanism by which SBD-2 regulated the adhesion of E. coli F17 to OIECs. Therefore, we believe that SBD-2 has antibacterial activity against E. coli F17 and has the potential to be used as a novel antibiotic against diarrhea caused by E. coli F17 in lambs. It is worth noting that the in vitro cell model cannot completely reflect the subtle changes of the immune environment in vivo. Thus, the function of SBD-2 should be further verified in vivo.
4.2. Effect of NF-κB and MAPK Pathways on SBD-2 Expression and E. coli F17 Adhesion to OIECs
NF-κB and MAPK signaling pathways are two key pathways activated by E. coli induced inflammatory response [36]. The NF-κB family consists of five related transcription factors: p50, p52, REL (also known as cREL), REL-A (p65) and REL-B, which is involved in the differentiation, proliferation and survival of almost all multicellular organisms [37]. The MAPK pathway including ERK1/2, p38 and JNK has been found in mammals. ERK1/2 signaling is mainly involved in the regulation of gene expression, protein translation, cell growth and differentiation. JNK and p38 signaling mainly play a significant role in inflammation, apoptosis and immune system response [38]. A wealth of studies has shown that the expression of induced β-defensin is usually mediated by NF-κB and MAPK pathways [20,39,40,41,42,43]. Among the reports related to Gram-negative bacteria, p38, ERK1/2 and JNK pathways are the most common MAPK pathways that cause inflammatory and immune responses [44,45]. In this study, detected mRNA expression levels of p50, p65, p38, ERK1/2 and JNK were significantly up-regulated after OIECs infected by E. coli F17, indicating that MAPK and NF-κB pathways were activated. Furthermore, we added p50 pathway inhibitor PDTC, p38 pathway inhibitor SB202190 and ERK1/2 pathway inhibitor PD98095 to OIECs, respectively. RT-PCR and ELISA analysis showed that the mRNA and protein expression levels of SBD-2 were significantly down-regulated after adding NF-κB and MAPK pathway inhibitors, indicating that SBD-2 expression induced by E. coli F17 is possibly mediated by NF-κB and MAPK signaling pathways. Finally, in order to more directly clarify the effects of NF-κB and MAPK pathways in OIECs infected by E. coli F17, we found that the addition of pathway inhibitors enhanced the adherence of E. coli F17 to OIECs. A similar study reported that LPS-induced SBD-1 expression is mainly mediated by the TLR4-P38 MAPK pathway [46]. Recently, Su et al. introduced a vital inflammation of NF-κB-dependent that induces porcine β-defensin 114 by using an infected porcine model [47]. Another report showed the novel anti-inflammatory effect of DEFB118 on ETEC-infected mice [48]. Hence, an effective animal model aims to uncover the novel anti-inflammatory function of SBD-2 needs to be further excavated, associated with the receptors recognized and the most vital pathway involved in the E. coli F17 infection.
5. Conclusions
In conclusion, the findings of the present study demonstrate that E. coli F17 induces SBD-2 expression in OIECs in vitro, which appears to be mediated through the activation of the signaling pathways, NF-κB and MAPK. The regulation of SBD-2 expression and the elucidation of the host signaling pathways that contribute to the induction of SBD-2 expression are conducive to enhance the innate immune response of the host against bacterial invasion. Our results provide a novel insight for the functionality of SBD-2, which could be useful for selecting sheep resisting E. coli F17.
Acknowledgments
The authors thank all the members of the Wei Sun’s group for their contribution to take care of the animals and sample determinations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10121356/s1, Figure S1: Specific isolation method of ovine intestinal epithelial cells, Figure S2: Illustration of pGH-SBD-2 plasmid construction, Figure S3: Ovine intestinal epithelial cells of Hu sheep under inverted microscope, ×40, Figure S4: Agarose gel electrophoresis of cell total RNA, Figure S5: E. coli F17 stimulates mRNA and protein expression levels of SBD-2, Table S1: A detailed description of the clinical symptoms, laboratory characteristics and identification of E. coli F17, Table S2: Specific primers used for RT-PCR, Table S3: Primers used in plasmid construction, Table S4: Sequence information of RNA oligonucleotides, Table S5: Reaction systems and procedures for PCR.
Author Contributions
Conceptualization, W.S., Z.Y., S.W., X.C. and X.L.; methodology, L.G., S.Z. and W.C.; data curation, L.G.; writing—original draft preparation, L.G.; writing—review and editing, W.S., Z.Y., S.W., X.C., X.L., T.G., J.M.M. and A.H.; project administration, W.S.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China-CGIAR (32061143036), National Natural Science Foundation of China (31872333, 32172689), Key Research and Development Plan (modern agriculture) in Jiangsu Province (BE2018354), Major New Varieties of Agricultural Projects in Jiangsu Province (PZCZ201739), Jiangsu Agricultural Science and Technology Innovation Fund (CX(18)2003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagy B., Fekete P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005;295:443–454. doi: 10.1016/j.ijmm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Shabana I.I., Bouqellah N.A., Zaraket H. Investigation of viral and bacterial enteropathogens of diarrheic sheep and goats in Medina, Saudi Arabia. Trop. Biomed. 2017;34:944–955. [PubMed] [Google Scholar]
- 3.Kopic S., Geibel J.P. Toxin mediated diarrhea in the 21 century: The pathophysiology of intestinal ion transport in the course of ETEC, V. cholerae and rotavirus infection. Toxins. 2010;2:2132–2157. doi: 10.3390/toxins2082132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubreuil J.D., Isaacson R.E., Schifferli D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus. 2016;7 doi: 10.1128/ecosalplus.ESP-0006-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lintermans P.F., Pohl P., Bertels A., Charlier G., Vandekerckhove J., Van Damme J., Schoup J., Schlicker C., Korhonen T., De Greve H., et al. Characterization and purification of the F17 adhesin on the surface of bovine enteropathogenic and septicemic Escherichia coli. Am. J. Vet. Res. 1988;49:1794–1799. [PubMed] [Google Scholar]
- 6.Holly M.K., Diaz K., Smith J.G. Defensins in Viral Infection and Pathogenesis. Annu. Rev. Virol. 2017;4:369–391. doi: 10.1146/annurev-virology-101416-041734. [DOI] [PubMed] [Google Scholar]
- 7.Brogden K.A., Ackermann M., McCray P.B., Jr., Tack B.F. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents. 2003;22:465–478. doi: 10.1016/S0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T., Lehrer R.I. Antimicrobial peptides of vertebrates. Curr. Opin. Immunol. 1998;10:41–44. doi: 10.1016/S0952-7915(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 9.Huttner K.M., Lambeth M.R., Burkin H.R., Burkin D.J., Broad T.E. Localization and genomic organization of sheep antimicrobial peptide genes. Gene. 1998;206:85–91. doi: 10.1016/S0378-1119(97)00569-6. [DOI] [PubMed] [Google Scholar]
- 10.Hou J., Liu H.Y., Diao H., Yu H. The truncated human beta-defensin 118 can modulate lipopolysaccharide mediated inflammatory response in RAW264.7 macrophages. Peptides. 2021;136:170438. doi: 10.1016/j.peptides.2020.170438. [DOI] [PubMed] [Google Scholar]
- 11.Ostuni R., Zanoni I., Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell. Mol. Life Sci. CMLS. 2010;67:4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L., Bian T., Lyu J., Cui D., Lei L., Yan F. Human β-defensin-3 alleviates the progression of atherosclerosis accelerated by Porphyromonas gingivalis lipopolysaccharide. Int. Immunopharmacol. 2016;38:204–213. doi: 10.1016/j.intimp.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Han F., Zhang H., Xia X., Xiong H., Song D., Zong X., Wang Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015;194:1882–1893. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- 14.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Jouve M., Garcia M.I., Courcoux P., Labigne A., Gounon P., Le Bouguénec C. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect. Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell R.M., Taganov K.D., Boldin M.P., Cheng G., Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza B.B., Barbosa E.M., Azevedo J.S., Campelo J.E., Rodrigues L.F., Pinheiro L.M., Silva S.C., Schierholt A.S., Souza P.H., Gonçalves E.C., et al. Genetic polymorphisms in β-defensin II gene in Amazon sheep from Brazil. Genet. Mol. Res. GMR. 2015;14:12805–12810. doi: 10.4238/2015.October.19.24. [DOI] [PubMed] [Google Scholar]
- 18.Jin X., Zhang M., Zhu X.M., Fan Y.R., Du C.G., Bao H.E., Xu S.G., Tian Q.Z., Wang Y.H., Yang Y.F. Modulation of ovine SBD-1 expression by Saccharomyces cerevisiae in ovine ruminal epithelial cells. BMC Vet. Res. 2018;14:134. doi: 10.1186/s12917-018-1445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M., Jin X., Yang Y.F. β-Glucan from Saccharomyces cerevisiae induces SBD-1 production in ovine ruminal epithelial cells via the Dectin-1-Syk-NF-κB signaling pathway. Cell. Signal. 2019;53:304–315. doi: 10.1016/j.cellsig.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Jin X., Zhang M., Yang Y.F. Saccharomyces cerevisiae β-glucan-induced SBD-1 expression in ovine ruminal epithelial cells is mediated through the TLR-2-MyD88-NF-κB/MAPK pathway. Vet. Res. Commun. 2019;43:77–89. doi: 10.1007/s11259-019-09747-x. [DOI] [PubMed] [Google Scholar]
- 21.Jin X., Zhang M., Cao G.F., Yang Y.F. Saccharomyces cerevisiae mannan induces sheep beta-defensin-1 expression via Dectin-2-Syk-p38 pathways in ovine ruminal epithelial cells. Vet. Res. 2019;50:8. doi: 10.1186/s13567-019-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin X., Tian Q.Z., Zhang M., Cao G.F., Yang Y.F. Modulation of SBD-1 expression by Saccharomyces cerevisiae cell wall components in ovine ruminal epithelial cells. Res. Vet. Sci. 2020;129:28–38. doi: 10.1016/j.rvsc.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Huttner K.M., Brezinski-Caliguri D.J., Mahoney M.M., Diamond G. Antimicrobial peptide expression is developmentally regulated in the ovine gastrointestinal tract. J. Nutr. 1998;128:297s–299s. doi: 10.1093/jn/128.2.297S. [DOI] [PubMed] [Google Scholar]
- 24.Meyerholz D.K., Gallup J.M., Grubor B.M., Evans R.B., Tack B.F., McCray P.B., Jr., Ackermann M.R. Developmental expression and distribution of sheep beta-defensin-2. Dev. Comp. Immunol. 2004;28:171–178. doi: 10.1016/S0145-305X(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 25.Shi N., Jin F., Zhang X., Clinton S.K., Pan Z., Chen T. Overexpression of human β-defensin 2 promotes growth and invasion during esophageal carcinogenesis. Oncotarget. 2014;5:11333–11344. doi: 10.18632/oncotarget.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y., Chen C., Di T., Yang J., Wang K., Zhu Y., Zhu R., Zhou A., Qian Y. Human β-Defensin-2 Improves Hyperoxia-Induced Lung Structural and Functional Injury in Neonatal Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:6074–6084. doi: 10.12659/MSM.915814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Cheng Y.T., Tan M.F., Zhang H.W., Liu W.Q., Zou G., Zhang L.S., Zhang C.Y., Deng S.M., Yu L., et al. Overexpression of Porcine Beta-Defensin 2 Enhances Resistance to Actinobacillus pleuropneumoniae Infection in Pigs. Infect. Immun. 2015;83:2836–2843. doi: 10.1128/IAI.03101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J., Yang X., Wang A., Huang C., Tang H., Zhang Q., Fang Q., Yu Z., Liu X., Huang Q., et al. Pigs Overexpressing Porcine β-Defensin 2 Display Increased Resilience to Glaesserella parasuis Infection. Antibiotics. 2020;9:903. doi: 10.3390/antibiotics9120903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi Y., Nagase T., Tomita T., Nakamura K., Fukuhara S., Amano T., Yamamoto H., Ide Y., Suzuki M., Teramoto S., et al. Beta-defensin overexpression induces progressive muscle degeneration in mice. Am. J. Physiol. Cell Physiol. 2007;292:C2141–C2149. doi: 10.1152/ajpcell.00295.2006. [DOI] [PubMed] [Google Scholar]
- 30.Röhrl J., Huber B., Koehl G.E., Geissler E.K., Hehlgans T. Mouse β-defensin 14 (Defb14) promotes tumor growth by inducing angiogenesis in a CCR6-dependent manner. J. Immunol. 2012;188:4931–4939. doi: 10.4049/jimmunol.1102442. [DOI] [PubMed] [Google Scholar]
- 31.Ziv C., Yarden O. Gene silencing for functional analysis: Assessing RNAi as a tool for manipulation of gene expression. Methods Mol. Biol. 2010;638:77–100. doi: 10.1007/978-1-60761-611-5_6. [DOI] [PubMed] [Google Scholar]
- 32.Sang Y., Ramanathan B., Ross C.R., Blecha F. Gene silencing and overexpression of porcine peptidoglycan recognition protein long isoforms: Involvement in beta-defensin-1 expression. Infect. Immun. 2005;73:7133–7141. doi: 10.1128/IAI.73.11.7133-7141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neil D.A., Cole S.P., Martin-Porter E., Housley M.P., Liu L., Ganz T., Kagnoff M.F. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect. Immun. 2000;68:5412–5415. doi: 10.1128/IAI.68.9.5412-5415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Q., Xie K., Chen D., Yu B., Mao X., Yu J., Luo J., Zheng P., Luo Y., Yan H., et al. Expression and Functional Characterization of a Novel Antimicrobial Peptide: Human Beta-Defensin 118. BioMed Res. Int. 2020;2020:1395304. doi: 10.1155/2020/1395304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su G., Xie K., Chen D., Yu B., Huang Z., Luo Y., Mao X., Zheng P., Yu J., Luo J., et al. Differential expression, molecular cloning, and characterization of porcine beta defensin 114. J. Anim. Sci. Biotechnol. 2019;10:60. doi: 10.1186/s40104-019-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen H., Karino M., Tobe T. Modulation of the Inflammasome Signaling Pathway by Enteropathogenic and Enterohemorrhagic Escherichia coli. Front. Cell. Infect. Microbiol. 2016;6:89. doi: 10.3389/fcimb.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y.J., Pan W.W., Liu S.B., Shen Z.F., Xu Y., Hu L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dou X., Han J., Ma Q., Cheng B., Shan A., Gao N., Yang Y. TLR2/4-mediated NF-κB pathway combined with the histone modification regulates β-defensins and interleukins expression by sodium phenyl butyrate in porcine intestinal epithelial cells. Food Nutr. Res. 2018;62:1493. doi: 10.29219/fnr.v62.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan J., Dou X., Li J., Yang Y., Xue C., Wang C., Gao N., Shan A. l-Arginine Ameliorates Lipopolysaccharide-Induced Intestinal Inflammation through Inhibiting the TLR4/NF-κB and MAPK Pathways and Stimulating β-Defensin Expression in Vivo and in Vitro. J. Agric. Food Chem. 2020;68:2648–2663. doi: 10.1021/acs.jafc.9b07611. [DOI] [PubMed] [Google Scholar]
- 41.Liu H., Yu H., Gu Y., Xin A., Zhang Y., Diao H., Lin D. Human beta-defensin DEFB126 is capable of inhibiting LPS-mediated inflammation. Appl. Microbiol. Biotechnol. 2013;97:3395–3408. doi: 10.1007/s00253-012-4588-9. [DOI] [PubMed] [Google Scholar]
- 42.Cui L., Wang H., Lin J., Wang Y., Dong J., Li J., Li J. Progesterone inhibits inflammatory response in E.coli- or LPS-Stimulated bovine endometrial epithelial cells by NF-κB and MAPK pathways. Dev. Comp. Immunol. 2020;105:103568. doi: 10.1016/j.dci.2019.103568. [DOI] [PubMed] [Google Scholar]
- 43.Cui L., Zheng Y., Wang H., Dong J., Li J., Song Q., Qian C., Li J. Cortisol inhibits the Escherichia coli-induced endometrial inflammatory response through NF-κB and MAPK pathways in postpartum goats. Anim. Reprod. Sci. 2020;215:106333. doi: 10.1016/j.anireprosci.2020.106333. [DOI] [PubMed] [Google Scholar]
- 44.Wang S., Cao Y., Deng S., Jiang X., Wang J., Zhang X., Zhang J., Liu G., Lian Z. Overexpression of Toll-like Receptor 4-linked Mitogen-activated Protein Kinase Signaling Contributes to Internalization of Escherichia coli in Sheep. Int. J. Biol. Sci. 2018;14:1022–1032. doi: 10.7150/ijbs.25275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae C.H., Choi Y.S., Song S.Y., Kim Y.K., Kim Y.D. Escherichia coli-derived and Staphylococcus aureus-derived extracellular vesicles induce MUC5AC expression via extracellular signal related kinase 1/2 and p38 mitogen-activated protein kinase in human airway epithelial cells. Int. Forum Allergy Rhinol. 2017;7:91–98. doi: 10.1002/alr.21844. [DOI] [PubMed] [Google Scholar]
- 46.Li Q., Bao F., Zhi D., Liu M., Yan Q., Zheng X., Ren L., Cong S., Li Y., Cao G. Lipopolysaccharide induces SBD-1 expression via the P38 MAPK signaling pathway in ovine oviduct epithelial cells. Lipids Health Dis. 2016;15:127. doi: 10.1186/s12944-016-0294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su G., Luo Y., Chen D., Yu B., He J. NF-κB-dependent induction of porcine β-defensin 114 regulates intestinal epithelium homeostasis. Int. J. Biol. Macromol. 2021;192:241–249. doi: 10.1016/j.ijbiomac.2021.09.163. [DOI] [PubMed] [Google Scholar]
- 48.Lin Q., Fu Q., Li X., Luo Y., Luo J., Chen D., Mao X., Yu B., Zheng P., Huang Z., et al. Human β-Defensin 118 Attenuates Escherichia coli K88-Induced Inflammation and Intestinal Injury in Mice. Probiotics Antimicrob. Proteins. 2021;13:586–597. doi: 10.1007/s12602-020-09725-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.