Abstract
Cardiovascular disease risk increases with age regardless of sex. Some of this risk is attributable to alterations in natural hormones throughout the life span. The quintessential example of this being the dramatic increase in cardiovascular disease following the transition to menopause. Plasma levels of adiponectin, a “cardioprotective” adipokine released primarily by adipose tissue and regulated by hormones, also fluctuate throughout one’s life. Plasma adiponectin levels increase with age in both men and women, with higher levels in both pre- and postmenopausal women compared with men. Younger cohorts seem to confer cardioprotective benefits from increased adiponectin levels yet elevated levels in the elderly and those with existing heart disease are associated with poor cardiovascular outcomes. Here, we review the most recent data regarding adiponectin signaling in the vasculature, highlight the differences observed between the sexes, and shed light on the apparent paradox regarding increased cardiovascular disease risk despite rising plasma adiponectin levels over time.
Keywords: adiponectin, age, sex, vascular
INTRODUCTION
Adiponectin, a substance released primarily from adipose tissue in healthy adult humans, has emerged as a critical signaling molecule within the systemic vasculature. Its deficiency in plasma has been strongly linked to obesity, diabetes, and cardiovascular disease (CVD) (1). Hypoadiponectinemia has also been associated with endothelial dysfunction (2–4), the precursor to many CVDs. Elevated levels of adiponectin in adults are linked to improvement in brachial artery flow-mediated dilation (FMD) (5); however, a U-shaped association is observed with CVD risk and the adipokine. In older adults without CVD, increases in adiponectin levels up to 20 mg/L appear to be cardioprotective. Beyond this concentration, further increases correlate with higher incidence of CVD (6). Aside from concentration, the age of the individual also contributes to the response to adiponectin. This is illustrated by studies showing that elevated levels appear to promote vascular health in younger adults (40+ yr of age) (7, 8), whereas studies that focused on adults age 60 yr and older had an increased risk for CVD (8–10).
Regardless of one’s sex, levels of natural hormones also fluctuate over time and can promote or prevent CVD depending on the hormone and age of the individual. Both testosterone and estradiol have opposing effects on adiponectin levels (11), which may account for differences in CVD risk observed between the sexes and over time (Fig. 1). Adiponectin regulates the vascular endothelium by promoting a quiescent environment, however, significant knowledge gaps remain regarding how this relationship varies with age. Although much is known about the effect of adipose and adiponectin on vascular function (12–18), very little has focused on age- and sex-specific differences. The goal of this mini-review is to briefly summarize up-to-date knowledge regarding adiponectin and its known protective role in the endothelium. Insight into sex differences and changes throughout the life span will be discussed. Finally, we will focus on potential targets within the adiponectin signaling pathway that may allow for prevention of vascular inflammation, endothelial dysfunction, and associated disease regardless of age, sex, or gender.
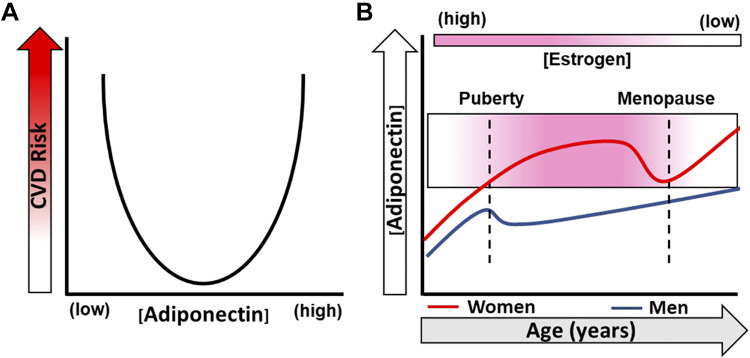
Figure 1.
Plasma adiponectin and cardiovascular (CVD) risk over time. A: both low and high levels of plasma adiponectin are associated with CVD risk and mortality. B: time course of plasma adiponectin levels in both women and men over the life span. Adiponectin levels increase in women at puberty and decrease during the transition to menopause before rising again postmenopause. Men experience a decrease in plasma adiponectin at puberty that recovers over time; however, levels are lower compared with women.
ADIPONECTIN AND RECEPTORS
Adiponectin
Adiponectin, a 30-kDa protein discovered in 1995 (19), is mainly released by adipocytes found in white, beige, and brown fat depots; however, other reports have shown that other cells such as cardiomyocytes (20), osteoblasts (21), skeletal muscle cells (22), and possibly endothelial cells (23) are capable of releasing it as well. Its 244 amino acid sequence contains G-X-Y repeats, which facilitates adiponectin’s ability to oligomerize (19). Three oligomeric forms have been identified in vivo: a 12–18mer known as the high-molecular weight (HWM) form, a hexamer or middle molecular weight (MMW), and a trimeric or low-molecular weight (LMW) structure (24–27). A fourth form of adiponectin, globular adiponectin, is a COOH-terminus cleavage product of full-length adiponectin. Of all the structural forms of adiponectin, evidence suggests that decreased HMW adiponectin is most closely associated with poor cardiovascular outcomes (28, 29).
Adiponectin Receptors, AdipoR1 and AdipoR2
The effects of adiponectin are primarily regulated by two surface membrane receptors, adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2). Although structurally similar to G protein-coupled receptors (GPCRs), AdipoRs have an intracellular NH2-terminus and an extracellular COOH-terminus (30). Both receptors have been identified in human aortic endothelial cells; however, data support preferential expression of AdipoR1 (31). It is currently unknown whether AdipoR1 or R2 exist in the human microvascular endothelium; however, data from our group would suggest that this to be true as chronic exogenous administration of adiponectin (∼16 h) improves endothelial function in microvessels collected from patients with known coronary artery disease (CAD) (32). In this setting, the addition of adiponectin had a positive effect on the vascular endothelium; however, it remains unknown whether the aging process affects receptor expression and/or function as well as whether it differs among men and women.
T-Cadherin
T-cadherin, a glycophosphatidylinositol (GPI)-linked plasma membrane protein, has been implicated as a third receptor for adiponectin, specifically HMW adiponectin (33, 34). Pascolutti and colleagues (35) discovered that T-cadherin preferentially binds to the globular domain of HWM adiponectin. The fact that T-cadherin is highly expressed in the cardiovascular system begs the question of whether it has a critical role in adiponectin’s cardioprotective effects. Wang and colleagues (36) recently demonstrated for the first time that loss of T-cadherin is associated with endothelial dysfunction. Acetylcholine (ACh)-induced vasodilation of aortic rings from T-cadherin knockout mice was significantly impaired compared with wild-type littermates. Furthermore, levels of NOx and phosphorylated Akt in the T-cadherin−/− mice were decreased compared with control animals (36). Other studies have shown that decreased expression of T-cadherin impairs adiponectin localization to the vascular endothelium (37, 38). Despite an absent intracellular structure, the presence of T-cadherin appears to be critical for adiponectin signaling and a functioning endothelium; however, details regarding its exact role are lacking.
Although little is known regarding how age or sex affects the expression level of adiponectin receptors, some evidence points to estrogen as being a key regulator of T-cadherin. Using a quantitative real-time RT-PCR assay, Bromhead and colleagues (39) demonstrated that β-estradiol (E2) regulates both transcriptional and posttranscriptional expression of T-cadherin. This suggests the possibility that loss of T-cadherin expression due to declining estrogen levels in menopause may result in decreased uptake or physiological action of plasma adiponectin. Therefore, despite having elevated plasma levels of adiponectin during the postmenopause period (40, 41), this may not truly reflect the bioavailability or action of this adipokine in the endothelium.
SEX AND AGE DIFFERENCES
In healthy, nonobese adult humans, plasma adiponectin concentrations are within the range of 2–10 µg/mL (42). Adiponectin levels have been shown to increase with age in healthy men and women (43), with greater levels observed in women (44). Interestingly, approximately half of the adiponectin pool in females consists of HMW compared with males where adiponectin is evenly divided among the three major forms. The significance of this is underscored by data from Pischon et al. (45) showing that elevated levels of HMW adiponectin and/or an increased HMW/total adiponectin ratio are associated with a lower risk of ischemic heart disease in women. Increases in both HMW adiponectin and the ratio were inversely related to body mass index (BMI), triglycerides, C-reactive protein, hemoglobin A1C, and low-density lipoprotein-C. Importantly, in postmenopausal women, the HMW/total adiponectin ratio is significantly lower when compared with early-menopausal women (46), potentially contributing to the increased risk of CVD postmenopause.
Plasma adiponectin levels in women are dynamic, with levels declining to their lowest during the menopausal transition and increasing again after menopause. The decrease in adiponectin during this time is also associated with increased androgens (40, 41). Although adiponectin levels rise in postmenopausal women, low plasma levels during this time in life strongly correlate with risk for metabolic syndrome in those with increased adiposity (47). In men, testosterone levels are inversely associated with adiponectin levels (48), and in transmales, adiponectin levels decrease following testosterone therapy (49). A decrease in adiponectin is also seen in pubertal boys, as their androgen levels increase, and by the end of puberty, males have lower adiponectin levels compared with females (50). Combs et al. (51) further tried to elucidate hormonal effects on adiponectin showing that female mice have higher levels of adiponectin in fat depots compared with male following sexual maturation, as seen in humans. Castration of neonatal male mice, but not adult mice, led to comparable adiponectin levels to the female mice. Interestingly, neonatal ovariectomy did not impact adiponectin levels, but adult ovariectomy further increased levels, similar to alterations seen in postmenopausal women. The researchers propose that neonatal testosterone levels might establish adiponectin set-point levels while estrogen dominates as the regulator of adiponectin later in life. It’s clear that the hormone changes experienced over time influence concentration of adiponectin and may account for differences in CVD risk we observe as individuals age. These preclinical studies have yet to be translated to humans and may have tremendous implications for hormone-replacement therapy for both postmenopausal women and the transgender population.
ADIPONECTIN AND VASCULAR CELL SIGNALING
Endothelial Cells
Adiponectin is a key player in promoting vascular homeostasis and is mainly categorized as an anti-inflammatory compound by increasing levels of nitric oxide (NO). Adiponectin binds both AdipoR1 and AdipoR2 on the extracellular (C) terminus (30), triggers calcium influx (52), and activates multiple downstream targets including AMPK (53), PPARα (54), and PI3K/Akt (55), which has been recently reviewed extensively by da Silva Rosa and colleagues (56). The link between AdipoR1/R2 and downstream pathway activation relies on an adapter protein, APPL1. APPL1 activates AMPK resulting in endothelial eNOS phosphorylation (57). Adiponectin-induced APPL1 binding also activates PI3K to phosphorylate eNOS (58, 59). In addition to direct increases in NO, studies performed in bovine aortic endothelial cells have shown that globular adiponectin stimulates binding of Hsp90 to eNOS, a critical process for proper eNOS homodimerization and function (60). Ex vivo experiments on human internal mammary arteries have shown that adiponectin levels increase tetrahydrobiopterin (BH4) bioavailability, thus promoting eNOS coupling and NO-producing efficiency (61). Experiments using siRNA to decrease expression of APPL1 in human umbilical vein endothelial cells (HUVECs) have demonstrated markedly reduced adiponectin-dependent NO production primarily as a result of decreased AMPK phosphorylation (62).
Studies in both animals and humans have shown a strong correlation between hypoadiponectinemia and impaired endothelium-dependent dilation (2–4), suggesting that lack of adiponectin promotes endothelial dysfunction. In aortic rings from adiponectin knockout mice, Cao et al. (63) demonstrated that NO bioavailability was significantly reduced because of decreased phosphorylated eNOS. This was reversed upon administration of exogenous globular adiponectin. Similarly, both globular and full-length adiponectin have been shown to induce NO-dependent vasodilation in nondiabetic male and female Zucker lean rats. However, in the diabetic fatty rats, administration of adiponectin failed to restore endothelial function, which was attributed to a decrease in APPL1, suggesting resistance to adiponectin signaling (64). In patients with heart failure (8 men and 5 women), adiponectin levels are fivefold higher compared with healthy subjects, but the expression of PPARa and AMPK genes and AdipoR1 mRNA are reduced in the skeletal muscle. This further supports a potential functional resistance to adiponectin in diseased states (65). In the vasculature specifically, a high-fat diet in male rats has been shown to decrease AdipoR1 and R2 expression, with an initial increase in adiponectin levels. The same study also showed a reduction in AMPK/eNOS signaling in response to recombinant adiponectin (66). Alterations in downstream signaling may explain the paradoxical scenario of elevated risk in older individuals, postmenopausal women, and those with heart failure despite increased plasma adiponectin levels.
Smooth Muscle Cells
Although this review focuses on the vascular endothelium, one would be remiss to not comment on how adiponectin affects smooth muscle cells (SMCs) within the medial layer. Both AdipoR1 and R2, as well as T-cadherin, have been identified in human coronary artery SMCs (67). Data thus far indicate that adiponectin is involved in phenotypic modulation of SMCs. Adiponectin promotes the contractile phenotype or elongated SMCs that primarily consist of contractile filaments. This is in opposition to the synthetic phenotype where SMCs contain organelles involved in protein synthesis allowing to create machinery necessary for cell migration and proliferation. Cerosimo and colleagues (67) demonstrated that the presence of adiponectin prevented human coronary SMC migration and proliferation induced by high glucose/palmitate. Although one would expect a similar situation with T-cadherin with its presence being protective, this does not appear to be the case. Studies done by Frismantiene et al. (68) have concluded that knock down of T-cadherin in human aortic SMCs promotes the contractile phenotype, whereas overexpression results in the dedifferentiated synthetic/secretory state. It is possible that the migration of T-cadherin from the endothelium to SMCs damages the vascular wall in two ways: 1) by decreasing the uptake of adiponectin in endothelial cells and 2) by triggering the SMC to enter the synthetic state to promote proliferation.
ADIPONECTIN AND THE SPHINGOLIPID RHEOSTAT
Sphingolipids, critical lipid messengers within the endothelium that regulate the balance between NO and reactive oxygen species (ROS) production, are strongly influenced by adiponectin. In healthy adults, ceramide levels are inversely correlated with adiponectin levels (69). Ceramide accumulation in endothelial cells leads to increased ROS production through both activation of NADPH oxidases (70) and mitochondria (71). Our group has shown that chronic exposure to exogenous ceramide induces human microvascular endothelial dysfunction (72) and more recently that blocking metabolism of ceramide thus causing accumulation, results in similar damage to human arterioles (32). Furthermore, ceramide is a potent stimulus for the formation of extracellular vesicles and we have shown that intraluminal exposure to these vesicles in a microvessel from a healthy patient also induced endothelial dysfunction (73).
The effects of ceramide can be mitigated if hydrolyzed to sphingosine by ceramidase enzymes. Once converted to sphingosine, it can further be phosphorylated to form sphingosine-1-phosphate (S1P), a known activator of eNOS (74). Both AdipoR1 and AdipoR2 possess intrinsic ceramidase activity and binding of adiponectin increases hydrolysis of ceramide by more than 20-fold (75). The inhibition of adiponectin production has been shown to decrease sphingosine and S1P levels and blocking ceramide production leads to an increase in HMW adiponectin (69), thereby highlighting a strong interplay between adiponectin signaling and the sphingolipid rheostat. Obata et al. (76) also found that through the binding of T-cadherin, adiponectin triggered the formation and release of ceramide containing exosomes, thus lowering endothelial cell ceramide content. Adiponectin-induced release of ceramide-filled vesicles did not occur in endothelial cells from T-cadherin-knockout mice. As previously mentioned, T-cadherin lacks intracellular signaling domains therefore studies such as this add important insight into its role and relationship with adiponectin. Taken together, adiponectin may be critical in limiting the potential damaging effects of elevated ceramides in the endothelium.
Elevated plasma ceramide levels are highly associated with many cardiovascular diseases thought to arise from a dysfunctional endothelium including both atherosclerosis (77) and heart failure with preserved ejection fraction (HFpEF) (78, 79). Interestingly, elevated levels of adiponectin are typically observed in individuals with HFpEF (80). Knowing that adiponectin is extremely efficient at decreasing cellular ceramides creates a bit of a quagmire. A similar scenario is observed in women of advanced age. After accounting for potential confounders, circulating ceramide levels have been shown to increase postmenopause (81) despite rising adiponectin levels (40, 41). The increased ceramide levels observed in aging women are strongly associated with the loss of estrogen (81) and yet the increased plasma levels of adiponectin appear to have little effect in mitigating ceramide accumulation. The elevated adiponectin levels associated with menopause may suggest the possibility that decreased and/or dysfunctional T-cadherin or adiponectin signaling may contribute to this confusing scenario (Fig. 2). In addition, sex differences also exist in the prevalence of HFpEF, a disease that tends to favor women over men by 2:1 (82) and is associated with elevated levels of both adiponectin and ceramide. This begs the question of whether dysfunctional T-cadherin/adiponectin signaling that occurs in aged females places them at higher risk for development of this particular form of heart failure.
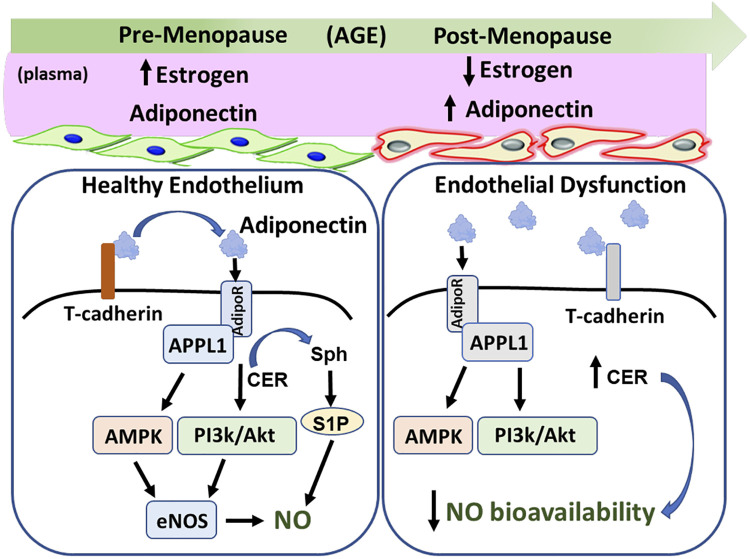
Figure 2.
The estrogen-adiponectin paradox in aging females. Plasma estrogen levels decline while adiponectin levels increase during the transition to menopause. T-cadherin is critical for the binding of adiponectin to adiponectin receptor (AdipoR) 1/2. Once receptors are activated, the adapter protein AAPL1 is required to activate various downstream pathways, including AMPK and PI3K/Akt, which results in an increase in endothelial nitric oxide (NO) synthase (eNOS)-generated NO. Activation of AdipoR1/2 dramatically increases hydrolysis of ceramide (Cer), a sphingolipid that induces endothelial dysfunction. The NO-generating pathways combined with shifting the sphingolipid rheostat toward sphingosine (Sph)-1-phosphate (S1P) promotes a healthy endothelium. Despite the rise in adiponectin during menopause, women have an elevated risk of cardiovascular disease, which may be due to dysfunctional T-cadherin, AdipoR1/2, and/or APPL1 signaling (gray). Both the loss of NO-stimulating pathways and decrease in AdipoR1/2 ceramidase activity contributes to decreased NO bioavailability and endothelial dysfunction.
TRANSLATIONAL STUDIES
Translational studies in humans have also highlighted the positive influence of adiponectin within the vascular endothelium. Perivascular adipose tissue (PVAT), an important source of adiponectin, has been shown to elicit NO-mediated vasodilation. In an elegant bioassay experiment, Greenstein and colleagues (83) demonstrated that medium collected from a human microvessel surrounded by PVAT-induced vasodilation in an acceptor vessel minus the surrounding adipose. This dilation was inhibited in the presence of either the nitric oxide synthase inhibitor NG-monomethyl-l-arginine or an adiponectin blocking peptide suggesting that adiponectin was responsible for the rise in NO and dilation. Unfortunately, women were underrepresented in this study and comprised only 20%–30% of the subjects studied. Using an in vitro approach, our group has recently demonstrated that chronic exposure (16 h) to exogenous adiponectin is able to restore NO-mediated flow-induced dilation in human arterioles collected from patients with CAD (32); however, over 80% microvessels were collected from male patients.
There is a paucity of in vivo work linking adiponectin to vascular function. Although Torigoe and colleagues (84) were able to demonstrate that NO-mediated brachial artery FMD correlates with HMW adiponectin levels, the study was done entirely in young men (∼30-yr old). Yoo et al. (5) recently showed that endothelial levels of adiponectin, but not circulating levels, were associated with greater brachial artery FMD in older men and women. A regression analysis performed by Okui et al. (85) concluded that plasma adiponectin levels positively correlate with ACh-induced increases in coronary blood flow. Interestingly, compared with body mass index (BMI), triglycerides, and the insulin resistance index, only adiponectin concentration could independently predict ACh-induced fluctuations in coronary flow (85). Although women were included in the Okui study, females represented only one-third of the total participants. There is no doubt that alterations in hormones complicate measured outcomes in clinical studies; however, it is critical that effort is given to include women in these in vivo studies to better elucidate adiponectin signaling in both sexes and within different age groups.
CLINICAL PERSPECTIVES
For clinical scenarios and possibly appropriate age groups in which low adiponectin levels are associated with cardiovascular risk, adiponectin replacement therapy may offer benefit to patients. Because of the complexity of the intact adiponectin protein, the majority of pharmacological advances have been made with small molecule agonists of the adiponectin receptor, primarily peptides that are structurally similar to the active site of globular adiponectin (86). One of the first to be discovered was ADP355, a peptidomimetic that although was shown to increase phosphorylation of both eNOS and AMPK and these effects were mainly observed in the liver (87). AdipoRon, a nonpeptide small molecule discovered in 2013, is the first orally active agonist for both AdipoR1 and R2. Our group has shown that as with globular adiponectin, chronic treatment with AdipoRon is capable of restoring NO-mediated FID in human arterioles from patients with known CAD (32). Very recently, Caldwell et al. (88) showed the importance of adiponectin in coronary microvascular function in response to exercise, and Iwabu and colleagues (89) demonstrated in AdipoR-humanized mice (mice that express human AdipoR1 in muscle of AdipoR1/R2 double knockout mice) that orally administered AdipoRon increased insulin sensitivity and exercise endurance. Aside from these mentioned studies, little is known regarding how small molecule agonists or peptide mimetics that target AdipoR1/R2 affect the cardiovascular system and improve endothelial function, much less in age- or sex-specific groups.
Recently, a member of the plant protein family, osmotin, was discovered as an AdipoR1/R2 agonist (90). AdipoR1/R2 do share homology with the receptor for osmotin, known as PHO36 (90). Osmotin has been shown to induce AMPK phosphorylation through adiponectin receptors in C2C12 myocytes (90). This stress-activated phytopeptide is found fairly ubiquitously in plants, but particularly in tomatoes, potatoes, and peppers (91, 92). Early evidence of osmotin as a potential therapeutic in the prevention of cardiovascular disease has been promising, as it was recently shown that osmotin infusion significantly decreased the amount of atherosclerotic plaque development in ApoE−/− mice (91) fed a high-fat diet. Endothelial effects of osmotin, including its potential to promote NO signaling similar to that of adiponectin, have yet to be determined but may serve as a potential therapy for hypoadiponectinemia or better yet, adiponectin resistance during advanced age or disease.
SUMMARY AND CONCLUSIONS
We experience a multitude of physiological changes as we age. All organ systems undergo transformation with time including the systemic vasculature. Age alone is a risk factor for CVD; however, this risk varies depending on sex and stage of life (93) (e.g., pre- vs. postmenopause). Low levels of adiponectin, an anti-inflammatory compound typically released from adipose tissue, have historically been associated with increased CVD risk despite being elevated in older individuals. It is becoming increasingly clear that the effect of adiponectin within the vasculature is strongly influenced by both age and sex. Increasing adiponectin to enhance vascular function has proven benefit in younger adult populations yet appears to be futile in older adults. Adiponectin signaling within the endothelium seems to confer resistance in disease states such as diabetes, obesity, and heart failure, where higher adiponectin levels correlate with worse outcomes. A similar resistance may occur in women, as adiponectin levels are elevated in postmenopause yet risk of CVD dramatically increases during this time, which may be due to altered downstream signaling of AMPK/eNOS. Adiponectin is highly efficient at reducing vascular-damaging ceramides, yet plasma ceramide levels also increase following menopause. Male and female sex hormones modulate the production of adiponectin, which may partly explain the difference in CVD risk in men compared with women as well as over time. Studies focused on human vascular adiponectin signaling across the life span are needed for males, females, and the transgender population. Insight into these mechanistic transformations may provide more precise therapeutic targets to mitigate cardiovascular disease for all.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants K08HL141562 (to J. K. Freed) and R38HL143561 (to K. E. Cohen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.E.C. and J.K.F. drafted manuscript; K.E.C., B.K., G.S., J.J.M., and J.K.F. edited and revised manuscript; K.E.C., B.K., G.S., J.J.M., and J.K.F. approved final version of manuscript.
REFERENCES
- 1.Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol 8: 93–100, 2016. doi: 10.1093/jmcb/mjw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42: 231–234, 2003. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 3.Tan KCB, Xu A, Chow WS, Lam MCW, Ai VHG, Tam SCF, Lam KSL. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab 89: 765–769, 2004. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 4.Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, Ueda S, Shimomura I, Funahashi T, Matsuzawa Y. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab 88: 3236–3240, 2003. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 5.Yoo J-K, Hwang M-H, Luttrell MJ, Kim H-K, Meade TH, English M, Segal MS, Christou DD. Higher levels of adiponectin in vascular endothelial cells are associated with greater brachial artery flow-mediated dilation in older adults. Exp Gerontol 63: 1–7, 2015. doi: 10.1016/j.exger.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kizer JR, Benkeser D, Arnold AM, Djousse L, Zieman SJ, Mukamal KJ, Tracy RP, Mantzoros CS, Siscovick DS, Gottdiener JS, Ix JH. Total and high-molecular-weight adiponectin and risk of coronary heart disease and ischemic stroke in older adults. J Clin Endocrinol Metab 98: 255–263, 2013. doi: 10.1210/jc.2012-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothenbacher D, Brenner H, März W, Koenig W. Adiponectin, risk of coronary heart disease and correlations with cardiovascular risk markers. Eur Heart J 26: 1640–1646, 2005. doi: 10.1093/eurheartj/ehi340. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med 167: 1510–1517, 2007. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 9.Poehls J, Wassel CL, Harris TB, Havel PJ, Swarbrick MM, Cummings SR, Newman AB, Satterfield S, Kanaya AM; Health ABC Study. Association of adiponectin with mortality in older adults: the health, aging, and body composition study. Diabetologia 52: 591–595, 2009. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab 93: 3357–3364, 2008. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capllonch-Amer G, Lladó I, Proenza AM, García-Palmer FJ, Gianotti M. Opposite effects of 17-β estradiol and testosterone on mitochondrial biogenesis and adiponectin synthesis in white adipocytes. J Mol Endocrinol 52: 203–214, 2014. doi: 10.1530/JME-13-0201. [DOI] [PubMed] [Google Scholar]
- 12.Meijer RI, Hoevenaars FPM, Serné EH, Yudkin JS, Kokhuis TJA, Weijers EM, van Hinsbergh VWM, Smulders YM, Eringa EC. JNK2 in myeloid cells impairs insulin's vasodilator effects in muscle during early obesity development through perivascular adipose tissue dysfunction. Am J Physiol Heart Circ Physiol 317: H364–H374, 2019. doi: 10.1152/ajpheart.00663.2018. [DOI] [PubMed] [Google Scholar]
- 13.Saxton SN, Whitley AS, Potter RJ, Withers SB, Grencis R, Heagerty AM. Interleukin-33 rescues perivascular adipose tissue anticontractile function in obesity. Am J Physiol Heart Circ Physiol 319: H1387–H1397, 2020. doi: 10.1152/ajpheart.00491.2020. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Jin D, Takai S, Hayakawa T, Ogata J, Yamanishi K, Okamura H. Impaired function of aorta and perivascular adipose tissue in IL-18-deficient mice. Am J Physiol Heart Circ Physiol 317: H1142–H1156, 2019. doi: 10.1152/ajpheart.00813.2018. [DOI] [PubMed] [Google Scholar]
- 15.Hazra S, Henson GD, Bramwell RC, Donato AJ, Lesniewski LA. Impact of high-fat diet on vasoconstrictor reactivity of white and brown adipose tissue resistance arteries. Am J Physiol Heart Circ Physiol 316: H485–H494, 2019. doi: 10.1152/ajpheart.00278.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatineau E, Cohn DM, Poglitsch M, Loria AS, Gong M, Yiannikouris F. Losartan prevents the elevation of blood pressure in adipose-PRR deficient female mice while elevated circulating sPRR activates the renin-angiotensin system. Am J Physiol Heart Circ Physiol 316: H506–H515, 2019. doi: 10.1152/ajpheart.00473.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones-Muhammad M, Warrington JP. When high-fat diet plus hypertension does not equal vascular dysfunction. Am J Physiol Heart Circ Physiol 321: H128–H130, 2021. doi: 10.1152/ajpheart.00284.2021. [DOI] [PubMed] [Google Scholar]
- 18.Yoon N, Dadson K, Dang T, Chu T, Noskovicova N, Hinz B, Raignault A, Thorin E, Kim S, Jeon JS, Jonkman J, McKee TD, Grant J, Peterson JD, Kelly SP, Sweeney G. Tracking adiponectin biodistribution via fluorescence molecular tomography indicates increased vascular permeability after streptozotocin-induced diabetes. Am J Physiol Endocrinol Physiol 317: E760–E772, 2019. doi: 10.1152/ajpendo.00564.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 20.Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, Ricks E, Yang Q. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol 43: 73–84, 2007. doi: 10.1016/j.yjmcc.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone 35: 842–849, 2004. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol 295: C203–C212, 2008. doi: 10.1152/ajpcell.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu C, Keen HL, Lu K-T, Liu X, Wu J, Davis DR, Ibeawuchi S-RC, Vogel S, Quelle FW, Sigmund CD. Retinol-binding protein 7 is an endothelium-specific PPARγ cofactor mediating an antioxidant response through adiponectin. JCI Insight 2: e91738, 2017. doi: 10.1172/jci.insight.91738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 278: 9073–9085, 2003. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 25.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 26.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278: 40352–40363, 2003. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 27.Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149: 2270–2282, 2008. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes 56: 2174–2177, 2007. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 29.Koenen TB, van Tits LJH, Holewijn S, Lemmers HLM, den Heijer M, Stalenhoef AFH, de Graaf J. Adiponectin multimer distribution in patients with familial combined hyperlipidemia. Biochem Biophys Res Commun 376: 164–168, 2008. doi: 10.1016/j.bbrc.2008.08.111. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, Motoyama K, Ikeda M, Wakiyama M, Terada T, Ohsawa N, Hato M, Ogasawara S, Hino T, Murata T, Iwata S, Hirata K, Kawano Y, Yamamoto M, Kimura-Someya T, Shirouzu M, Yamauchi T, Kadowaki T, Yokoyama S. Crystal structures of the human adiponectin receptors. Nature 520: 312–316, 2015. doi: 10.1038/nature14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Addabbo F, Nacci C, De Benedictis L, Leo V, Tarquinio M, Quon MJ, Montagnani M. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF- κB/COX-2 signaling in human aortic endothelial cells. Am J Physiol Endocrinol Physiol 301: E1143–E1154, 2011. doi: 10.1152/ajpendo.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz ME, Katunaric B, Hockenberry JC, Gutterman DD, Freed JK. Manipulation of the sphingolipid rheostat influences the mediator of flow-induced dilation in the human microvasculature. J Am Heart Assoc 8: e013153, 2019. doi: 10.1161/JAHA.119.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao T-S, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101: 10308–10313, 2004. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranscht B, Dours-Zimmermann MT. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron 7: 391–402, 1991. doi: 10.1016/0896-6273(91)90291-7. [DOI] [PubMed] [Google Scholar]
- 35.Pascolutti R, Erlandson SC, Burri DJ, Zheng S, Kruse AC. Mapping and engineering the interaction between adiponectin and T-cadherin. J Biol Chem 295: 2749–2759, 2020. doi: 10.1074/jbc.RA119.010970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Tao L, Ambrosio A, Yan W, Summer R, Lau WB, Wang Y, Ma X. T-cadherin deficiency increases vascular vulnerability in T2DM through impaired NO bioactivity. Cardiovasc Diabetol 16: 12, 2017. doi: 10.1186/s12933-016-0488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker-Duffen JL, Nakamura K, Silver M, Kikuchi R, Tigges U, Yoshida S, Denzel MS, Ranscht B, Walsh K. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem 288: 24886–24897, 2013. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda K, Fujishima Y, Maeda N, Mori T, Hirata A, Sekimoto R, Tsushima Y, Masuda S, Yamaoka M, Inoue K, Nishizawa H, Kita S, Ranscht B, Funahashi T, Shimomura I. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology 156: 934–946, 2015. doi: 10.1210/en.2014-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bromhead C, Miller JH, McDonald FJ. Regulation of T-cadherin by hormones, glucocorticoid and EGF. Gene 374: 58–67, 2006. doi: 10.1016/j.gene.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Zheng C, Chen D, Xie M. [Investigation of the change of adiponectin level with menopause status in middle aged women and its relationship with androgen]. Zhonghua Fu Chan Ke Za Zhi 50: 356–360, 2015. [PubMed] [Google Scholar]
- 41.Matsui S, Yasui T, Tani A, Kato T, Kunimi K, Uemura H, Kuwahara A, Matsuzaki T, Irahara M. Association of circulating adiponectin with testosterone in women during the menopausal transition. Maturitas 73: 255–260, 2012. doi: 10.1016/j.maturitas.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi K, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 43.Obata Y, Yamada Y, Takahi Y, Baden MY, Saisho K, Tamba S, Yamamoto K, Umeda M, Furubayashi A, Matsuzawa Y. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol (Oxf) 79: 204–210, 2013. doi: 10.1111/cen.12041. [DOI] [PubMed] [Google Scholar]
- 44.Song HJ, Oh S, Quan S, Ryu O-H, Jeong J-Y, Hong K-S, Kim D-H. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatr 14: 8, 2014. doi: 10.1186/1471-2318-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pischon T, Hu FB, Girman CJ, Rifai N, Manson JE, Rexrode KM, Rimm EB. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis 219: 322–329, 2011. doi: 10.1016/j.atherosclerosis.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsui S, Yasui T, Tani A, Kato T, Kunimi K, Uemura H, Kuwahara A, Matsuzaki T, Irahara M. Difference in the ratio of high-molecular weight (HMW) to total adiponectin and HMW adiponectin in late post-menopausal women. J Endocrinol Invest 36: 982–985, 2013. doi: 10.3275/9001. [DOI] [PubMed] [Google Scholar]
- 47.Henneman P, Janssens ACJW, Zillikens MC, Frolich M, Frants RR, Oostra BA, van Duijn CM, van Dijk KW. Menopause impacts the relation of plasma adiponectin levels with the metabolic syndrome. J Intern Med 267: 402–409, 2010. doi: 10.1111/j.1365-2796.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 48.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. J Androl 26: 85–92, 2005. [PubMed] [Google Scholar]
- 49.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Physiol 281: E1172–E1181, 2001. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 50.Bottner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, Blüher M, Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab 89: 4053–4061, 2004. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 51.Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52: 268–276, 2003. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- 52.Grossini E, Farruggio S, Qoqaiche F, Raina G, Camillo L, Sigaudo L, Mary D, Surico N, Surico D. Monomeric adiponectin modulates nitric oxide release and calcium movements in porcine aortic endothelial cells in normal/high glucose conditions. Life Sci 161: 1–9, 2016. doi: 10.1016/j.lfs.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304–1309, 2004. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278: 2461–2468, 2003. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 55.Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 64: 2207–2219, 2015. doi: 10.2337/db14-1011. [DOI] [PubMed] [Google Scholar]
- 56.da Silva Rosa SC, Liu M, Sweeney G. Adiponectin synthesis, secretion and extravasation from circulation to interstitial space. Physiology (Bethesda) 36: 134–149, 2021. doi: 10.1152/physiol.00031.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102: 1296–1301, 2000. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 58.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 59.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [Erratum in Nature 400: 792, 1999]. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3–Akt pathway contribute to globular adiponectin-induced NO production: Vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun 332: 200–205, 2005. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- 61.Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127: 2209–2221, 2013. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- 62.Cheng KKY, Lam KSL, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394, 2007. [Erratum in Diabetes 65: 3218, 2016]. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 63.Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B, Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol 46: 413–419, 2009. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmid PM, Resch M, Steege A, Fredersdorf-Hahn S, Stoelcker B, Birner C, Schach C, Buechler C, Riegger GAJ, Luchner A, Endemann DH. Globular and full-length adiponectin induce NO-dependent vasodilation in resistance arteries of Zucker lean but not Zucker diabetic fatty rats. Am J Hypertens 24: 270–277, 2011. doi: 10.1038/ajh.2010.239. [DOI] [PubMed] [Google Scholar]
- 65.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail 3: 185–194, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 66.Li R, Xu M, Wang X, Wang Y, Lau WB, Yuan Y, Yi W, Wei X, Lopez BL, Christopher TA, Wang X-M, Ma X-L. Reduced vascular responsiveness to adiponectin in hyperlipidemic rats–mechanisms and significance. J Mol Cell Cardiol 49: 508–515, 2010. doi: 10.1016/j.yjmcc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cersosimo E, Xu X, Terasawa T, Dong LQ. Anti-inflammatory and anti-proliferative action of adiponectin mediated by insulin signaling cascade in human vascular smooth muscle cells. Mol Biol Rep 47: 6561–6572, 2020. doi: 10.1007/s11033-020-05707-w. [DOI] [PubMed] [Google Scholar]
- 68.Frismantiene A, Kyriakakis E, Dasen B, Erne P, Resink TJ, Philippova M. Actin cytoskeleton regulates functional anchorage-migration switch during T-cadherin-induced phenotype modulation of vascular smooth muscle cells. Cell Adh Migr 12: 69–85, 2018. doi: 10.1080/19336918.2017.1319545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Field BC, Gordillo R, Scherer PE. The role of ceramides in diabetes and cardiovascular disease regulation of ceramides by adipokines. Front Endocrinol (Lausanne) 11: 569250, 2020. doi: 10.3389/fendo.2020.569250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, Förstermann U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 106: 2250–2256, 2002. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 71.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol 24: 762–768, 2001. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 72.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115: 525–532, 2014. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freed JK, Durand MJ, Hoffmann BR, Densmore JC, Greene AS, Gutterman DD. Mitochondria-regulated formation of endothelium-derived extracellular vesicles shifts the mediator of flow-induced vasodilation. Am J Physiol Heart Circ Physiol 312: H1096–H1104, 2017. doi: 10.1152/ajpheart.00680.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tolle M, Klöckl L, Wiedon A, Zidek W, van der Giet M, Schuchardt M. Regulation of endothelial nitric oxide synthase activation in endothelial cells by S1P1 and S1P3. Biochem Biophys Res Commun 476: 627–634, 2016. doi: 10.1016/j.bbrc.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 75.Vasiliauskaite-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, Hoh F, De Colibus L, Bechara C, Saied EM, Arenz C, Leyrat C, Granier S. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544: 120–123, 2017. doi: 10.1038/nature21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Obata Y, Kita S, Koyama Y, Fukuda S, Takeda H, Takahashi M, Fujishima Y, Nagao H, Masuda S, Tanaka Y, Nakamura Y, Nishizawa H, Funahashi T, Ranscht B, Izumi Y, Bamba T, Fukusaki E, Hanayama R, Shimada S, Maeda N, Shimomura I. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 3, 2018. doi: 10.1172/jci.insight.99680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation 109: III27–32, 2004. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 78.Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 18: 701–711, 2021. doi: 10.1038/s41569-021-00536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lam CS, Brutsaert DL. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J Am Coll Cardiol 60: 1787–1789, 2012. doi: 10.1016/j.jacc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Faxen UL, Hage C, Andreasson A, Donal E, Daubert J-C, Linde C, Brismar K, Lund LH. HFpEF and HFrEF exhibit different phenotypes as assessed by leptin and adiponectin. Int J Cardiol 228: 709–716, 2017. doi: 10.1016/j.ijcard.2016.11.194. [DOI] [PubMed] [Google Scholar]
- 81.Vozella V, Basit A, Piras F, Realini N, Armirotti A, Bossù P, Assogna F, Sensi SL, Spalletta G, Piomelli D. Elevated plasma ceramide levels in post-menopausal women: a cross-sectional study. Aging (Albany NY ) 11: 73–88, 2019. doi: 10.18632/aging.101719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355: 260–269, 2006. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 83.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119: 1661–1670, 2009. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 84.Torigoe M, Matsui H, Ogawa Y, Murakami H, Murakami R, Cheng XW, Numaguchi Y, Murohara T, Okumura K. Impact of the high-molecular-weight form of adiponectin on endothelial function in healthy young men. Clin Endocrinol (Oxf) 67: 276–281, 2007. doi: 10.1111/j.1365-2265.2007.02876.x. [DOI] [PubMed] [Google Scholar]
- 85.Okui H, Hamasaki S, Ishida S, Kataoka T, Orihara K, Fukudome T, Ogawa M, Oketani N, Saihara K, Shinsato T, Shirasawa T, Mizoguchi E, Kubozono T, Ichiki H, Ninomiya Y, Matsushita T, Nakasaki M, Tei C. Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA-R, body mass index, immunoreactive insulin, or triglycerides. Int J Cardiol 126: 53–61, 2008. doi: 10.1016/j.ijcard.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 86.Otvos L Jr. Potential adiponectin receptor response modifier therapeutics. Front Endocrinol (Lausanne) 10: 539, 2019. doi: 10.3389/fendo.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumar P, Smith T, Rahman K, Thorn NE, Anania FA. Adiponectin agonist ADP355 attenuates CCl4-induced liver fibrosis in mice. PLoS One 9: e110405, 2014. doi: 10.1371/journal.pone.0110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caldwell JT, Jones KMD, Park H, Pinto JR, Ghosh P, Reid-Foley EC, Ulrich B, Delp MD, Behnke BJ, Muller-Delp JM. Aerobic exercise training reduces cardiac function and coronary flow-induced vasodilation in mice lacking adiponectin. Am J Physiol Heart Circ Physiol 321: H1–H14, 2021. doi: 10.1152/ajpheart.00885.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwabu M, Okada-Iwabu M, Tanabe H, Ohuchi N, Miyata K, Kobori T, Odawara S, Kadowaki Y, Yokoyama S, Yamauchi T, Kadowaki T. AdipoR agonist increases insulin sensitivity and exercise endurance in AdipoR-humanized mice. Commun Biol 4: 45, 2021. doi: 10.1038/s42003-020-01579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Narasimhan ML, Coca MA, Jin J, Yamauchi T, Ito Y, Kadowaki T, Kim KK, Pardo JM, Damsz B, Hasegawa PM, Yun D-J, Bressan RA. Osmotin is a homolog of mammalian adiponectin and controls apoptosis in yeast through a homolog of mammalian adiponectin receptor. Mol Cell 17: 171–180, 2005. [Erratum in Mol Cell 17: 611, 2005]. doi: 10.1016/j.molcel.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi Y, Watanabe R, Sato Y, Ozawa N, Kojima M, Watanabe-Kominato K, Shirai R, Sato K, Hirano T, Watanabe T. Novel phytopeptide osmotin mimics preventive effects of adiponectin on vascular inflammation and atherosclerosis. Metabolism 83: 128–138, 2018. doi: 10.1016/j.metabol.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Anil Kumar S, Hima Kumari P, Shravan Kumar G, Mohanalatha C, Kavi Kishor PB. Osmotin: a plant sentinel and a possible agonist of mammalian adiponectin. Front Plant Sci 6: 163, 2015. doi: 10.3389/fpls.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, Proctor DN. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Heart Circ Physiol 317: H395–H404, 2019. doi: 10.1152/ajpheart.00430.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]