Abstract
Molecular detection of herpes simplex virus (HSV) DNA is recognized as the reference standard assay method for the sensitive and specific diagnosis of central nervous system infections caused by HSV. In this study, a molecular assay based on real-time PCR on the LightCycler (LC) instrument was evaluated and compared with a home-brew molecular assay. The detection limit of the LC assay was determined with 10-fold dilutions of plasmid pS4 with the SalI restriction fragment of the DNA polymerase gene and with the First European Union Concerted Action HSV Proficiency Panel. A total of 59 cerebrospinal fluid (CSF) specimens were investigated for the comparative study. With plasmid pS4, the detection limit of the LC assay was found to be 104 copies per ml, i.e., 12.5 copies per run. When samples of the First European Union Concerted Action HSV Proficiency Panel were tested, 2 × 103 to 5 × 103 HSV type 1 genome equivalents (GE) per ml, i.e., 2.5 to 6.3 GE per run, could consistently be detected. There was a correlation between the LC assay and the home-brew assay in 55 of 59 specimens. In conclusion, the LC assay allows very rapid detection of HSV DNA in CSF. It was found to be laborsaving and showed sufficient sensitivity.
Herpes simplex virus (HSV) causes a wide spectrum of clinical manifestations in the central nervous system (CNS). Neonatal HSV infection following exposure to the virus at delivery can produce severe disseminated infection and death. Presently, effective therapeutic management is possible; however, antiviral drugs must be administered early (28). Therefore, rapid laboratory diagnosis is essential for decreasing the lethality as well as the sequelae of HSV infection.
Today, PCR is recognized as the reference standard assay method for the sensitive and specific diagnosis of CNS infections caused by HSV (1, 4, 13, 17, 18). Studies on detection of HSV DNA are usually based on home-brew protocols. These assays commonly involve toxic and/or radioactive substances and a high number of manipulations (12, 22, 23). To replace these shortcomings, molecular assays including rapid extraction protocols with nontoxic substances and detection protocols based on nonradioactive and largely automated methods have been developed (10, 24).
Eight years ago, kinetic PCR analysis by real-time monitoring of DNA amplification reactions was described (7). The LightCycler (LC) instrument, which allows high-speed thermal cycling by using air instead of thermal blocks and online real-time fluorescence monitoring, has recently been introduced (29). Thirty to 40 PCR cycles can be completed in just 20 to 30 min. With regard to utilization of the LC technology for detection of pathogens, there have been very few studies published thus far (5, 9, 19).
In the present study, a qualitative molecular assay based on a rapid DNA extraction protocol and real-time PCR with the LC system was evaluated. After determination of the detection limit, clinical specimens were investigated. Results were compared with those of a qualitative home-brew PCR assay.
MATERIALS AND METHODS
Study design.
In the first step, a plasmid (pS4), kindly provided by K. W. Knopf, German Cancer Research Center, Heidelberg, Germany, containing a single copy of a SalI restriction fragment of the HSV polymerase gene from the HSV type 1 (HSV-1) strain Angelotti served as a standard for the determination of the detection limit. Tenfold dilutions of the plasmid were subjected to the molecular assay based on a rapid DNA extraction protocol and to real-time PCR on the LC instrument. Experiments were repeated five times on different days.
In the second step, the First European Union Concerted Action HSV Proficiency Panel, which contained different concentrations of HSV type 1 strain MacIntyre (American Type Culture Collection), HSV type 2 strain MS (American Type Culture Collection), varicella-zoster virus (VZV), and negative samples, was used (Table 1). Samples were tested five times on different days by the new molecular assay including real-time PCR on the LC instrument and, except for vial no. 5, two times on different days by the home-brew assay.
TABLE 1.
First European Union Concerted Action HSV Proficiency Panel
| Vial no. | Virus | No. of HSV GE/ml | No. of positive results/no. of testsa |
|---|---|---|---|
| 1 | None | 0/7 | |
| 2 | HSV-1 | 2 × 104–5 × 104 | 7/7 |
| 3 | HSV-2 | 2 × 103–5 × 103 | 1/7 |
| 4 | HSV-1 | 2 × 102–5 × 102 | 3/7 |
| 5 | HSV-1 | 2 × 105–5 × 105 | 7/7 |
| 6 | HSV-2 | 2 × 106–5 × 106 | 7/7 |
| 7 | HSV-1 | 2 × 103–5 × 103 | 7/7 |
| 8 | None | 0/7 | |
| 9 | HSV-2 | 2 × 105–5 × 105 | 6/7 |
| 10 | HSV-2 | 2 × 104–5 × 104 | 6/7 |
| 11 | VZV | 0/7 | |
| 12 | HSV-1 | 0.7 × 103–1.7 × 103 | 5/7 |
Panel tested by six reference laboratories using a total of seven different molecular methods before distribution.
In the third step, a total of 59 cerebrospinal fluid (CSF) specimens were investigated in a comparative study. All specimens had been collected from patients who were admitted to University Hospital, Frankfurt am Main, Germany, and had been obtained prior to the start of therapy. CSF samples were divided into aliquots. One aliquot was routinely investigated with a home-brew molecular assay at the Institute of Medical Virology, Frankfurt am Main, Germany. Each positive result was confirmed by a second PCR run. Another aliquot was stored frozen at −70°C and later sent to the Institute of Hygiene, Graz, Austria, for blind investigation with the new molecular assay. Each sample was run twice with real-time PCR on the LC instrument.
DNA extraction.
For the home-brew molecular assay, a DNA blood kit (Qiagen, Hilden, Germany) that required 200 μl of sample was used according to the manufacturer's instructions. For the real-time PCR assay, a rapid DNA extraction protocol was used. In a 1.5-ml tube, 100 μl of sample was added to 300 μl of a suspension consisting of 20% (wt/vol) Chelex 100 resin (Bio-Rad Laboratories, Richmond, Calif.) in 10 mM Tris-HCl (pH 8.0)–0.1 mM EDTA–0.1% sodium azide. After the tube sample was vortexed for 10 s, incubated at 100°C for 10 min, and vortexed for another 10 s, the tube was allowed to cool to room temperature. Following complete settlement of the resin, 5 μl of the supernatant was directly used for amplification.
Primer design.
For the home-brew molecular assay, oligonucleotides deduced from the published sequence of the glycoprotein D region of the HSV genome were used (16, 27). This set of primers has recently been used for diagnosis of herpes simplex virus encephalitis by nested PCR (20, 21). The primer and probe sequences and characteristics are shown in Table 2.
TABLE 2.
Oligonucleotides used for home-brew molecular assay
| Assay step and primer (sequence) or product | Virus and positions amplifieda | Length (nucleotides) | G+C content (%) | Melting temp (°C) |
|---|---|---|---|---|
| Initial PCR | ||||
| Primers | ||||
| Forward (5′-TGCTCCTACAACAAGTC) | HSV-1, 138845–138861; HSV-2, 141422–141438 | 17 | 47.1 | 43.8 |
| Reverse (5′-CGGTGCTCCAGGATAAA) | HSV-1, 139028–139044; HSV-2, 141625–141641 | 17 | 52.9 | 51.4 |
| Product | HSV-1, 138845–139044; HSV-2, 141422–141641 | 200 | ||
| Nested PCR | ||||
| Primers | ||||
| Forward (5′-ATCCGAACGCAGCCCCGCTG) | HSV-1, 138878–138897; HSV-2, 141475–141494 | 20 | 70.0 | 70.1 |
| Reverse (5′-TCTCCGTCCAGTCGTTTATCTTC) | HSV-1, 138997–139019; HSV-2, 141594–141616 | 23 | 47.8 | 57.3 |
| Product | HSV-1, 138878–139019; HSV-2, 141475–141616 | 142 |
For the real-time PCR assay, oligonucleotides deduced from the published sequence of the DNA polymerase gene-coding region from HSV were used (15, 25). This set of primers, which was chosen within a highly conserved region of the DNA polymerase gene from the herpesvirus group, allows amplification of a 92-bp fragment from each of the HSV-1 and HSV-2 DNA polymerase genes in clinical samples (2, 3). The TaqMan probe (TIB MOLBIOL, Berlin, Germany) was labeled with 6FAM at the 5′ end and with TAMRA at the 3′ end. The primer and probe sequences and characteristics are shown in Table 3.
TABLE 3.
Oligonucleotides used for real-time PCR assay
| Primer, product, or probe (sequence) | Virus and positions amplifieda | Length (nucleotides) | G+C content (%) | Melting temp (°C) |
|---|---|---|---|---|
| Primers | ||||
| Forward (5′-CATCACCGACCCGGAGAGGGAC) | HSV-1, 65866–65887; HSV-2; 66339–66360 | 22 | 68.2 | 66.9 |
| Reverse (5′-GGGCCAGGCGCTTGTTGGTGTA) | HSV-1, 65936–65957; HSV-2, 66409–66430 | 22 | 63.6 | 69.0 |
| Product | HSV-1, 65866–65957; HSV-2, 66430–66339 | 92 | ||
| TaqMan probe (5′-6FAM-CCGCCGAACTGAGCAGACACCCGCGC-TAMRA) | HSV-1, 65907–65932; HSV-2, 66380–66405 | 26 | 73.1 | 79.8 |
Home-brew DNA amplification and detection assay.
The PCR was carried out in a 50-μl reaction mixture containing 25 μl of Taq PCR master mix solution (Qiagen); 13 μl of double-distilled, DNase-free water; a 1 μM concentration of each primer; and 10 μl of the extracted sample. PCR was performed on a PE 9600 thermocycler (Perkin-Elmer Cetus, Branchburg, N.J.). Thirty-five cycles consisting of denaturation at 95°C for 30 s (5 min during cycle 1), annealing at 55°C for 30 s, and extension at 72°C for 30 s were run. After the final cycle, the tubes were incubated for an additional 5 min at 72°C and cooled down to 5°C. Nested amplification was done with a 5-μl aliquot from the first run; 25 μl of Taq PCR master mix solution; 18 μl of double-distilled, DNase-free water; and a 1 μM concentration of each nested primer by the same cycle protocol as described above. Each amplification run contained one negative and one positive control. The negative control consisted of blank reagent and water. For the positive control, total cellular nucleic acid extracted from virus stocks was used. After amplification, electrophoretic separation of PCR products (10 μl) was performed on 2% agarose gels in 0.5× Tris-borate-EDTA buffer, stained with ethidium bromide, and visualized by UV illumination.
Real-time PCR on the LC instrument.
The real-time PCR was performed on the specially designed LC instrument (Roche Diagnostics, Mannheim, Germany). Evaluation of the different assay formats has been described in detail elsewhere (11). For the present study, all samples were tested by the LC-DNA Master Hybridization Probes assay (Roche Diagnostics) using a TaqMan probe (Table 3). Additionally, the hot start technique was used. TaqStart antibody (Clontech, Palo Alto, California) was added directly to the 10× DNA Master solutions, and the mixtures were incubated at room temperature for 5 min. Then, MgCl2, primers, TaqMan probe, and water were added. Fifteen microliters of master mix and 5 μl of DNA template were added in each capillary. Sealed capillaries were centrifuged in a microcentrifuge and placed into the LC rotor. After denaturation for 2 min at 95°C, 55 PCR cycles were run.
RESULTS
When tenfold dilutions of plasmid pS4 were tested by real-time PCR on the LC instrument, the detection limit was found to be 104 copies per ml, i.e., 12.5 copies per LC PCR run. With the dilution containing 103 copies per ml, i.e., approx. 1 copy per LC PCR run, the assay employed produced inconsistent negative and positive results.
When samples of the First European Union Concerted Action HSV Proficiency Panel were tested with the real-time PCR assay, 2 × 103 to 5 × 103 HSV-1 genome equivalents (GE) per ml, i.e., 2.5 to 6.3 GE per LC PCR run, could consistently be detected. With the dilution containing 0.7 × 103 to 1.7 × 103 HSV-1 GE per ml (vial no. 12), i.e., 1 to 2 GE per LC-PCR run, the assay produced inconsistent (negative and positive) results. When HSV-2 samples from the same panel were tested, 2 × 104 to 5 × 104 GE per ml, i.e., 25 to 62.5 GE per LC PCR run, could consistently be detected, whereas 2 × 103 to 5 × 103 HSV-2 GE per ml (vial no. 3), i.e., 2.5 to 6.3 GE per LC PCR run, were not detected at all. With the home-brew assay, 2 × 102 to 5 × 102 and 2 × 103 to 5 × 103 HSV-2 GE per ml, i.e., all the positive samples of the First European Union Concerted Action HSV Proficiency Panel, could be detected.
From a total of 59 CSF samples, 20 were repeatedly found to be positive by real-time PCR on the LC instrument and by the home-brew PCR assay and 35 were found to be negative by both PCR assays (Fig. 1). Four samples yielded discrepant results: two of them were positive with the home-brew PCR assay and negative with the real-time PCR assay, and the other two were positive with the real-time PCR assay and negative with the home-brew PCR assay (Table 4). Upon repetition, one of the samples that had been positive with the real-time PCR assay and another one that had been positive with the home-brew PCR assay both yielded negative results. The remaining two samples were repeatedly positive or negative.
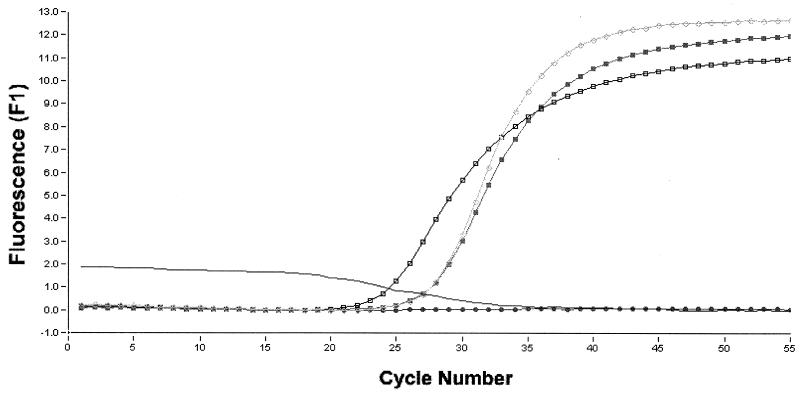
FIG. 1.
LC PCR of clinical samples. Fluorescence curves from TaqMan probe detection with hot start technique are shown. ●, sample 1 (negative); □, sample 2 (positive); ■, sample 3 (positive); ○, positive control; line without symbol, negative control.
TABLE 4.
Discrepant results
| Patient no. | Age (yr)/sexa | Result of PCR assay (R)b
|
HSV serologyc | Final diagnosisd | |
|---|---|---|---|---|---|
| Home-brew | Real-time | ||||
| 1 | 55/M | N | P (N) | N | Meningitis |
| 2 | 30/M | N | P (P) | IgG P, IgM N | Pericarditis |
| 3 | 36/F | P (N) | N (N) | ND | HIV encephalitis |
| 4 | 55/M | P (P) | N (N) | IgG P, IgM N | Meningitis |
F, female; M, male.
R, repeat result; N, negative; P, positive.
ND, not done; N, negative; P, positive; IgG, immunoglobulin G; IgM, immunoglobulin M.
HIV, human immunodeficiency virus.
The real-time PCR assay on the LC instrument proved to be very quick and laborsaving. The whole procedure could be finished within less than 1 h. The time required for DNA isolation was 15 min, followed by 10 min for pipetting and another 30 min for cycling and detection combined. In contrast, the home-brew PCR assay took about 5 h. Thirty minutes were required for DNA extraction, 4 h were required for nested amplification, and another 30 min were required for detection by gel electrophoresis.
DISCUSSION
In the present study, a real-time PCR assay on the LC instrument was evaluated and compared to a conventional home-brew PCR assay. Compared with the home-brew assay, the detection limit of the LC assay was found to be one log unit higher. For the LC assay, 100 μl of sample was used for DNA extraction and the theoretical detection limit of the LC assay could almost be achieved, i.e., 2 to 3 GE could be detected. For the home-brew PCR, a sample of double the above-mentioned volume was taken. Increasing the extract volume may improve the sensitivity of the LC assay. This is presently impossible, however, because of the LC capillary format, which allows only a small total reaction mixture volume (20 μl). For detection of pathogens, larger capillaries would be useful in the future.
Another reason for decreased sensitivity may be insufficient sample preparation. For molecular assays to be applicable in the routine diagnostic laboratory, sample preparation should be as simple as possible. In this study, the cation exchanger Chelex 100, which allows DNA extraction within less than half an hour, was employed. In comparison with classic nucleic acid extraction protocols, Chelex 100 protocols have been shown to increase sensitivity of molecular assays for detection of cytomegalovirus in cultures and clinical samples, for detection of human immunodeficiency virus type 1 proviral DNA in blood, and for detection of Legionella pneumophila in bronchoalveolar lavage fluids (6, 8, 30). A sample concentration step prior to the start of the Chelex 100 protocol may further increase the yield of DNA in the future.
The formation of primer dimers during PCR may also contribute to decreased sensitivity of a molecular assay. Especially in targets with low copy number, a relatively high primer dimer concentration has to be considered. To inhibit the formation of primer dimers, the LC assay included a hot start (11).
Because high viral titers should be present in CSF during herpes simplex virus encephalitis, lack of sensitivity appears to be only the minor problem. Contamination may be the major problem: despite special efforts to prevent contamination, all discrepant results turned out to be false-positives in this study. Because of the increased number of manipulations, it had originally been supposed that the home-brew assay would be more prone to contamination than the LC assay. In this study, however, contaminations may have occurred with both assays. The major weaknesses in assay design that could lead to false-positives include sample preparation and pipetting steps between the first-round PCR and the second-round PCR. To reduce the danger of contamination during sample preparation, techniques that required only a few manipulation steps were used in this study. Automation of sample preparation, however, is most desirable to minimize danger of contamination. The hot start technique, applied for the LC assay, requires an additional pipetting step after the addition of the extracted DNA. In the future, this step can be avoided by using the LC Fast Start assay, which will include a polymerase specially designed to remain inactive until heated during the PCR before thermal cycling and which will be brought on the market soon.
Detection formats of the LC technology include general detection of double-stranded DNA (SYBR Green technology), which corresponds to gel electrophoresis of home-brew assays, and specific detection of the target sequence by using a TaqMan probe or hybridization probes (14). Probes prevent false-positive results due to nonspecific amplification products and guarantee the specificity of results. Therefore, probes should always be employed for molecular assays to be applicable in the routine diagnostic laboratory.
False-negatives, which may result from PCR inhibitors, did not occur in this study. Removal of inhibitors, however, is crucial in molecular assays and may be better achieved by the use of classic extraction protocols. It has, however, been reported that even Chelex 100 may decrease false-negative results due to PCR inhibitors (26). Nevertheless, introduction of an internal control, which should be extracted and coamplified with the target DNA from the clinical specimen, would be helpful in the future.
In summary, the LC assay proved to be suitable for the routine diagnostic laboratory. Compared to a conventional home-brew assay, it was found to be very quick and very easy to use. Because of the significantly lower number of manipulations, there may be a lower probability of getting results that are due to false-positive contamination.
REFERENCES
- 1.Aslanzadeh J, Garner J G, Feder H M, Ryan R W. Use of polymerase chain reaction for laboratory diagnosis of herpes simplex virus encephalitis. Ann Clin Lab Sci. 1993;23:196–202. [PubMed] [Google Scholar]
- 2.Brice S L, Krzemien D, Weston W L, Huff J C. Detection of herpes simplex virus DNA in cutaneous lesions of erythema multiforme. J Investig Dermatol. 1989;93:183–187. doi: 10.1111/1523-1747.ep12277397. [DOI] [PubMed] [Google Scholar]
- 3.Cao M, Xiao X, Egbert T, Darragh T M, Yen T S B. Rapid detection of cutaneous herpes simplex virus infection with the polymerase chain reaction. J Investig Dermatol. 1989;92:391–392. doi: 10.1111/1523-1747.ep12277232. [DOI] [PubMed] [Google Scholar]
- 4.Cinque P, Vago L, Marenzi R, Giudici B, Weber T, Corradini R, Caresa D, Lazzarin A, Linde A. Herpes simplex virus infections of the central nervous system in human immunodeficiency virus infected patients: clinical management by polymerase chain reaction assay of cerebrospinal fluid. Clin Infect Dis. 1998;27:303–309. doi: 10.1086/514657. [DOI] [PubMed] [Google Scholar]
- 5.Espy M J, Uhl J R, Mitchell P S, Thorvilson J N, Svien K A, Wold A D, Smith T F. Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol. 2000;38:795–799. doi: 10.1128/jcm.38.2.795-799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essary L R, Kinard S J, Butcher A, Wang H, Laycock K A, Donegan E, McCreedy B, Connell S, Batchelor J, Harris J, Spadoro J, Pepose J S. Screening potential corneal donors for HIV-1 by polymerase chain reaction and a colorimetric microwell hybridization assay. Am J Ophthalmol. 1996;122:526–534. doi: 10.1016/s0002-9394(14)72113-0. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi R, Dollinger G, Walsh P S, Griffith R. Simultaneous amplification and detection of specific DNA sequences. Biotechnology. 1992;10:413–417. doi: 10.1038/nbt0492-413. [DOI] [PubMed] [Google Scholar]
- 8.Jaulhac B, Reyrolle M, Sodahlon Y K, Jarraud S, Kubina M, Monteil H, Piemont Y, Etienne J. Comparison of sample preparation methods for detection of Legionella pneumophila in culture-positive bronchoalveolar lavage fluids by PCR. J Clin Microbiol. 1998;36:2120–2122. doi: 10.1128/jcm.36.7.2120-2122.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke D, Menard C, Picard F J, Boissinot M, Ouellette M, Roy P H, Bergeron M G. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem. 2000;46:324–331. [PubMed] [Google Scholar]
- 10.Kessler H H, Pierer K, Weber B, Sakrauski A, Santner B, Stuenzner D, Gergely E, Marth E. Detection of herpes simplex virus DNA from cerebrospinal fluid by PCR and a rapid, nonradioactive hybridization technique. J Clin Microbiol. 1994;32:1881–1886. doi: 10.1128/jcm.32.8.1881-1886.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler H H. Qualitative detection of herpes simplex virus DNA on the LightCycler. In: Meuer S, Wittwer C T, Nakagawara K, editors. Rapid cycle real-time PCR—methods and applications, in press. Heidelberg, Germany: Springer-Verlag; 2000. [Google Scholar]
- 12.Klapper P E, Cleator G M, Dennett C, Lewis A G. Diagnosis of herpes encephalitis via Southern blotting of cerebrospinal fluid DNA amplified by polymerase chain reaction. J Med Virol. 1990;32:261–264. doi: 10.1002/jmv.1890320413. [DOI] [PubMed] [Google Scholar]
- 13.Koskiniemi M, Piiparinen H, Mannonen L, Rantalaiho T, Vaheri A. Herpes encephalitis is a disease of middle aged and elderly people: polymerase chain reaction for detection of herpes simplex virus in the CSF of 516 patients with encephalitis. J Neurol Neurosurg Psychiatry. 1996;60:174–178. doi: 10.1136/jnnp.60.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landt O. Selection of hybridization probe sequences for use with the LightCycler. In: Meuer S, Wittwer C T, Nakagawara K, editors. Rapid cycle real-time PCR—methods and applications, in press. Heidelberg, Germany: Springer-Verlag; 2000. [Google Scholar]
- 15.Larder B A, Kemp S D, Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987;6:169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasky L A, Dowbenko D J. DNA sequence analysis of the type-common glycoprotein-D genes of herpes simplex virus types 1 and 2. DNA. 1984;3:23–29. doi: 10.1089/dna.1.1984.3.23. [DOI] [PubMed] [Google Scholar]
- 17.Madhavan H N, Priya K, Anand A R, Therese K L. Detection of herpes simplex virus (HSV) genome using polymerase chain reaction (PCR) in clinical samples. Comparison of PCR with standard laboratory methods for the detection of HSV. J Clin Virol. 1999;14:145–151. doi: 10.1016/s1386-6532(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell P S, Espy M J, Smith T F, Toal D R, Rys P N, Berbari E F, Osmon D R, Persing D H. Laboratory diagnosis of central nervous system infections with herpes simplex virus by PCR performed with cerebrospinal fluid specimens. J Clin Microbiol. 1997;35:2873–2877. doi: 10.1128/jcm.35.11.2873-2877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitsche A, Steuer N, Schmidt C A, Landt O, Siegert W. Different real-time PCR formats compared for the quantitative detection of human cytomegalovirus DNA. Clin Chem. 1999;45:1932–1937. [PubMed] [Google Scholar]
- 20.Powell K F, Anderson N E, Frith R W, Croxson M C. Non-invasive diagnosis of herpes simplex encephalitis. Lancet. 1990;335:357–358. doi: 10.1016/0140-6736(90)90648-o. [DOI] [PubMed] [Google Scholar]
- 21.Read S J, Jeffery K J M, Bangham C R M. Aseptic meningitis and encephalitis: the role of PCR in the diagnostic laboratory. J Clin Microbiol. 1997;35:691–696. doi: 10.1128/jcm.35.3.691-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowley A H, Whitley R J, Lakeman F D, Wolinsky S M. Rapid detection of herpes simplex virus DNA in cerebrospinal fluid of patients with herpes simplex virus encephalitis. Lancet. 1990;335:440–441. doi: 10.1016/0140-6736(90)90667-t. [DOI] [PubMed] [Google Scholar]
- 23.Rozenberg F, Lebon P. Amplification and characterization of herpesvirus DNA in cerebrospinal fluid from patients with acute encephalitis. J Clin Microbiol. 1991;29:2412–2417. doi: 10.1128/jcm.29.11.2412-2417.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakrauski A, Weber B, Kessler H H, Pierer K, Doerr H W. Comparison of two hybridization assays for the rapid detection of PCR amplified HSV genome sequences from cerebrospinal fluid. J Virol Methods. 1994;50:175–184. doi: 10.1016/0166-0934(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 25.Tsurumi T, Maeno K, Nishiyama Y. Nucleotide sequence of the DNA polymerase gene of herpes simplex virus type 2 and comparison with the type 1 counterpart. Gene. 1987;52:129–137. doi: 10.1016/0378-1119(87)90039-4. [DOI] [PubMed] [Google Scholar]
- 26.Vignoli C, de Lamballerie X, Zandotti C, Tamalet C, de Micco P. Advantage of a rapid extraction method of HIV1 DNA suitable for polymerase chain reaction. Res Virol. 1995;146:159–162. doi: 10.1016/0923-2516(96)81085-5. [DOI] [PubMed] [Google Scholar]
- 27.Watson R J, Weis J H, Salstrom J S, Enquist L W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982;218:381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- 28.Whitley R J, Kimberlin D W, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541–555. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 29.Wittwer C T, Ririe K M, Andrew R V, David D A, Gundry R A, Balis U J. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]
- 30.Zandotti C, de Lamballerie X, Guignole-Vignoli C, Bollet C, de Micco P. A rapid DNA extraction method from culture and clinical samples. Suitable for the detection of human cytomegalovirus by the polymerase chain reaction. Acta Virol. 1993;37:106–108. [PubMed] [Google Scholar]