Abstract
Thoracic aortic aneurysm is one of the manifestations of Marfan syndrome (MFS) that is known to affect men more severely than women. However, the incidence of MFS is similar between men and women. The aim of this study is to show that during pathological aortic dilation, sex-dependent severity of thoracic aortopathy in a mouse model of MFS translates into sex-dependent alterations in cells and matrix of the ascending aorta, consequently affecting aortic biomechanics. Fibrillin-1 C1041G/+ (Het) mice were used as a mouse model of MFS. Ultrasound measurements from 3 to 12 mo showed increased aortic diameter in Het aorta, with larger percentage increase in diameter for males compared with females. Immunohistochemistry showed decreased contractile smooth muscle cells in Het aortic wall compared with healthy aorta, which was accompanied by decreased contractility measured by wire myography. Elastin autofluorescence, second-harmonic generation microscopy of collagen fibers, and passive biomechanical assessments using myography showed more severe damage to elastin fibers, increased medial fibrosis, and increased stiffness of the aortic wall in MFS males but not females. Male and female Het mice showed increased expression of Sca-1-positive adventitial progenitor cells versus controls at young ages. In agreement with clinical data, Het mice demonstrate sex-dependent severity of thoracic aortopathy. It was also shown that aging exacerbates the disease state especially for males. Our findings suggest that female mice are protected from progression of aortic dilation at early ages, leading to a lag in aneurysm growth.
NEW & NOTEWORTHY Male Fbn1C1041G/+ mice show more severe thoracic aortic changes compared with females, especially at 12 mo of age. Up to 6 mo of age, Sca-1+ smooth muscle progenitor cells are more abundant in the adventitia of both male and female Fbn1 Het mice compared with wild types (WTs). Male and female Het mice show similar patterns of expression of Sca-1+ cells at early ages.
Keywords: Marfan syndrome, progenitor cells, sex dependency, thoracic aortic aneurysm, vascular remodeling
INTRODUCTION
Aneurysm is a local and irreversible chronic vascular disease, characterized by progressive enlargement of arterial diameter and weakening of the vessel wall, that ranks as the 13th leading cause of death in the United States because of rupture and hemorrhage (1). The main features of aneurysm are fragmentation of elastic fibers, loss of contractile smooth muscle cells (SMCs), and pathological remodeling of extracellular matrix (ECM) (2). Thoracic aortic aneurysm (TAA) is the second most common aortic aneurysm, with an incidence of 5 to 10 per 100,000 people per year. TAA is associated with a number of genetic mutations (3, 4). Marfan syndrome (MFS) is an autosomal dominant inherited disorder of connective tissue arising from mutation of the FBN1 gene (5), and it is the most common genetic disorder leading to TAA (1). With advances in clinical treatments, an increase in life expectancy of patients with MFS has resulted in more age-related complications (6). Clinical data show that incidence rates of MFS are the same for females and males; however, male patients suffer more severe aortic complications compared with females (7, 8). Currently, treatment of TAA in patients with MFS is limited to the use of angiotensin receptor blockers, such as losartan (9), to reduce the chance of dissection by limiting stresses acting on the vessel wall, and prophylactic surgery on the aortic root when the aortic diameter reaches 1.5 times normal. Aortic root dilatation, prophylactic aortic root replacement, and previous aortic dissection were reported more frequently in adult male compared with adult female patients with MFS (7). Interestingly, there is currently limited experimental evidence relating the dependency of TAA progression in MFS on age and sex.
Patients with MFS typically have a mutation in the FBN1 gene encoding fibrillin-1 glycoprotein, a structural macromolecule that acts as a scaffold for elastin fibers. Fibrillin-1 deficiency can cause elastic fiber defects in the aortic media that initiate a series of degenerative cellular and extracellular events leading to aneurysm progression (10). Chronic remodeling of ECM constituents in MFS shows progressive changes in elastin fragmentation and collagen deposition and degradation, concomitant with upregulation of matrix metalloproteinases (MMP)-2 and -9, causing alterations in the passive mechanical response of the arterial wall such as increased stiffness. Cellular changes also promote the progression of TAA by altering vascular tone, such as reducing SMC contractility, which prevents the artery from adapting to acute increases in pressure and flow. Several observations have suggested that elastic fiber breaks can cause detachment of contractile SMCs from elastic laminae, resulting in either apoptosis (11) or phenotypic switching (12). Synthetic SMCs are less contractile than contractile SMCs and do not contribute to active force generation or maintaining vascular tone. However, these cells are highly proliferative and secrete ECM components such as collagen that directly influence the passive biomechanics of the wall. Recently, some studies have focused on the role of progenitor cells, challenging the commonly accepted idea that phenotypic switching is the sole cellular phenomenon in TAA progression (13). A major group of arterial progenitor cells are adventitial SMC progenitor cells, which are mesenchymal-like cells distinguished as Sca-1+/CD45− by surface antigen expression (14). These cells can differentiate to SMCs, lose Sca-1 expression, and express SMC markers (15). Migration of these progenitor cells in vascular diseases such as atherosclerosis (16) and hypertension (17) has been studied before, but whether these cells also play a role in aneurysms is still unknown.
During progression of TAA, changes in cell and ECM components directly affect biomechanical properties of the aortic wall and potentially lead to rupture (18). Active and passive properties of the aneurysmal aortic wall have been investigated in different human (19) and mouse models (18, 20). Using wire myography, decreased active force and weakening of the wall were previously reported in the aortic arch and descending aorta of MFS mice (18). Conversely, increased contractility relative to wild-type (WT) mice was observed in the ascending aorta of male MFS mice but not in female mice by wire myography (21). Furthermore, biaxial mechanical testing of the ascending aorta of MFS mice showed increased circumferential but not axial stiffness of both ascending and descending aortas (22). Thus, the discrepancies between age, sex-related differences, and clinical outcomes in patients with MFS remain unclear. Understanding how temporal changes in thoracic aortopathy related to MFS are linked to age and sex can aid in the development of patient-specific medications or interventions to mitigate aortic dilation. To that end, we investigated the progression of thoracic aortopathy in male and female MFS mice (Fbn1C1041G/+) over a time course of 3–12 mo and showed differences in expression of contractile SMC markers, deposition of medial collagen, and aortic contractility to reveal sex-dependent differences in the MFS mouse model. Furthermore, we show that adventitial Sca-1+ progenitor cells in MFS mice increase at the early stages of thoracic aortopathy development.
MATERIALS AND METHODS
Mice
All animal studies were approved by the University of South Carolina Institutional Animal Care and Use Committee. A mouse model of MFS, Fbn1C1041G/+ (Het) (Jackson Laboratory Stock No. 012885) (23), on C57Bl/6 background was used to study the progression of MFS-related thoracic aortopathy over a time course of 3–12 mo in comparison with their wild-type (WT) littermates as the control group. Three mice for myography and five mice for all other experiments per sex and genotype were used at each timepoint of each experiment. Mice were euthanized by controlled-rate carbon dioxide asphyxiation at timepoints of 3, 6, 9, and 12 mo and perfused with heparinized normal saline at physiological pressure. The excised ascending aorta was processed as described in Immunohistochemistry and Biomechanical Assessments for each experiment.
Ultrasound
Small animal ultrasound (Vevo 3100, Fuji VisualSonics) with a MX400 transducer (15–30 MHz) was performed 1 wk before euthanasia to monitor aortic dilation. Mice were maintained under nominal 2% isoflurane anesthesia during imaging; isoflurane concentration was adjusted incrementally to keep the heart rate between 350 and 450 beats/min. Images were taken in the parasternal long-axis view in B mode at the aortic root (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.16832323.v2), proximal ascending aorta just after the aortic root, and distal ascending aorta just before the innominate artery. Aortic diameter was measured at each point perpendicular to the direction of mean blood flow using Vevo LAB 3.2.0 software.
Immunohistochemistry
Ten-micrometer frozen cross sections of the proximal ascending aorta were prepared and immunostained with specific antibodies. To ensure correct regional comparisons, all slides used for staining were collected immediately after no part of the aortic valve remained visible. Contractile SMCs were labeled with 2.7 µg/mL of α-smooth muscle actin (rabbit anti-α-SMA, Thermo Scientific) and 5 µg/mL of smooth muscle myosin heavy chain [rabbit anti-smooth muscle myosin heavy chain (SM-MHC), Abcam]. Stem cell antigen-1 (5 µg/mL; rat anti-Sca-1, StemCell Technologies, Inc.) was used as a marker for smooth muscle progenitor cells. Rhodamine Red X conjugated secondary antibodies (15 µg/mL; donkey anti-rabbit IgG) and goat anti-rat IgG (16 µg/mL; Jackson ImmunoResearch) were used to visualize proteins of interest by fluorescence microscopy with excitation/emission of 546/575–640 nm. Elastin autofluorescence was visualized using an FITC filter set with excitation/emission wavelengths of 450–490/515 nm. Cell nuclei were stained using Hoechst 33258 and imaged with excitation/emission filters at 365/445 nm.
Images were taken from entire aortic rings using a fluorescence microscope (Axio Imager.A1, Zeiss) attached to a camera (AxioCam MRc5, Zeiss) and analyzed using ImagePro Plus 6 software (Media Cybernetics, Inc.). Each aortic ring consisted of ∼15 single images that were stitched together using Autopano Pro 3.6.0 software (Kolor). Expression of each marker is reported as a fractional area of the aortic layer of interest (media for SM-MHC and α-SMA or adventitia for Sca-1). Internal and external elastic laminae were used as medial layer borders, and adventitia is defined as a ring outside the media with a fixed thickness of ∼20% of the medial thickness. Elastin breaks were counted over the entire area of each aortic ring using immunohistochemistry (IHC) images. Break count was done by two independent investigators blinded to the sample groups.
Multiphoton Second-Harmonic Generation Microscopy
A Leica SP8 multiphoton confocal microscope was used to measure medial collagen deposition by second-harmonic generation. The same sections were used for both IHC and second-harmonic generation (SHG) microscopy. Collagen fibers were imaged over the entire aortic area using a tunable Chameleon Ultra II laser at 800 nm with signal collection in the forward direction. Images were then processed using ImagePro Plus 6 software similar to IHC sections and reported as fractional area of the media.
Biomechanical Assessments
Active and passive mechanical response to uniaxial loading was measured using a wire myograph (Radnoti) for both the healthy and dilated aorta, using our published protocol (24). Mice were euthanized at each timepoint, and the ascending aorta was harvested and divided into proximal and distal segments. All biomechanical measurements were done within 4 h after tissue harvest. Each aortic segment was mounted on two 50-µm wires and incubated for 30 min in oxygenated Krebs buffer at 37°C to equilibrate. The segments were then preconditioned by stretching aortic segments three times to a stretch ratio of ∼1.7, which corresponds to normal stretch at physiological conditions. To measure the total force (FT = active and passive), the aortic rings were treated with 80 mM KCl in Krebs buffer to cause maximal contraction of SMCs. The initial distance (at F = 0) between two wires for the undeformed state of the aorta was considered L. The segments were then stretched 200 µm at a time and kept for 3 min at each stretch length until the force stabilized. Images were taken at each stretch point to measure the actual stretch which is denoted as l. To measure passive force (FP), the aorta was brought back to length L and treated with 10−5 M sodium nitroprusside (SNP) in Krebs buffer to cause complete relaxation of the SMCs and to inhibit active force generation. The rings were then stretched uniaxially in steps of 200 µm as described for total force measurement. To analyze the data, stretch ratio was measured from images as where d = 50 µm is the diameter of the wires. As schematically shown in Fig. 1C, unloaded aortic thickness (t; in mm) was measured from ring images taken after myography, and segment width at relaxed state (w; in mm) was measured from images to calculate stress as Collected data were then processed to calculate active stress (σA) from the total and passive stresses (σA = σT − σP).
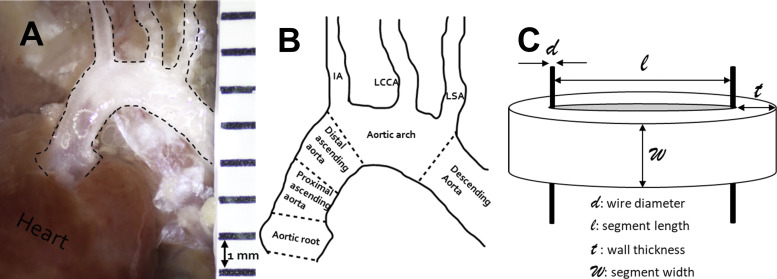
Figure 1.
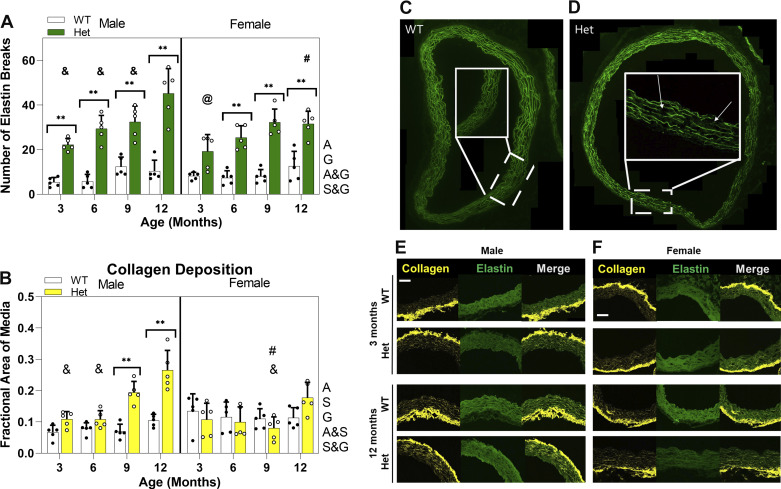
A: ascending aorta in situ showing aorta in dashed line. B: different regions of the aorta. C: schematic picture of aortic segment mounted for myography. IA, innominate artery; LCCA, left common carotid artery; LSA, left subclavian artery.
Furthermore, Fp and λ were used to plot stress-stretch curves, and slopes of low- and high-stretch regions of this plot were used as indicators of stiffness of the aortic wall. Low-stretch region is defined as the stretches below the stretch at which active stress was maximum (σA-max) and extends to lower stretches throughout the linear region (i.e., the part of the curve where the linear trend line has R2 > 0.9), and high-stretch region is defined as the stretches above σA-max.
Statistical Analysis
To determine the effect and interactions of age, sex, and genotype, three-way ANOVA was performed together with a post hoc Tukey test using Prism (GraphPad, La Jolla, CA), with statistical significance defined as P < 0.05. Correlation tests were also performed using the same software.
RESULTS
Aortic Dilation Is More Pronounced in Male Mice than in Females
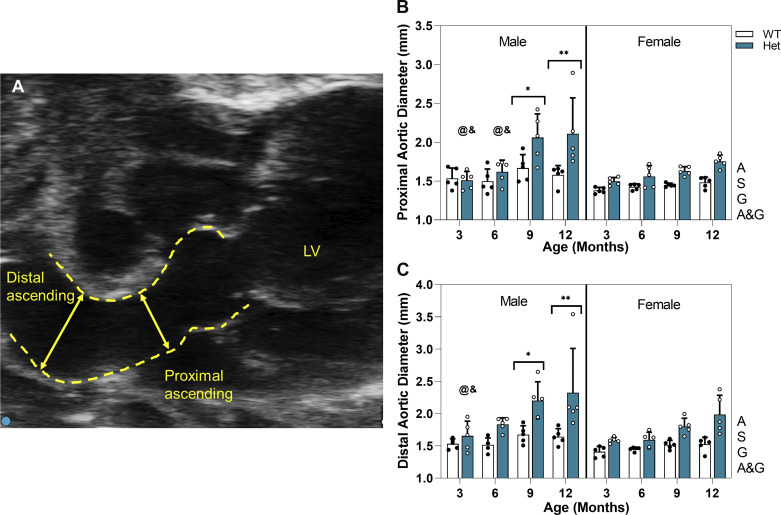
Aortic diameter was measured at the proximal and the distal ascending aorta as presented in Fig. 2, B and C. In both regions, male Hets show significantly greater diameter compared with WTs at 9 and 12 mo. Ascending aortic diameter for female Hets increases with age, but the difference from WTs is not significant. The extent of aortic dilation in Hets is highly dependent on age, sex, and region as evaluated using three-way ANOVA with age, genotype, and region (proximal and distal) as independent variables. Females show region dependency (P = 0.0006); however, that is not the case for males (P = 0.09). Table 1 lists the percentage increase of the aortic diameter at different regions. For both males and females, there were no significant changes in the aortic diameter of WT mice at any timepoint. For male mice at both proximal and distal regions, aortic diameter at 3 and 6 mo in Hets was comparable with that for WTs. It is evident in Fig. 2 that at both regions, the dilation grows very rapidly in Het male mice from 6 to 9 mo, leading to a significantly larger diameter at 9 and 12 mo compared with 3 and 6 mo in the proximal region and compared with 3 mo in the distal region. Diameter in male Hets stays almost constant from 9 to 12 mo. In both regions, aortic dilation is age, sex, and genotype dependent, and there is an interaction between age and genotype, which means aging worsens the dilation in Het mice.
Figure 2.
A: representative ultrasound image showing ascending aorta of 9-mo Het male mouse. Arrows show diameter measurement points at each region. Graphs show means and standard deviation for diameter of ascending aorta of WT and Het male and female mice at proximal (B) and distal ascending aorta (C) over a time course of 3–12 mo. White circles, Het mice; black circles, WT mice. Using three-way ANOVA for n = 80 (5 mice per study group), * and ** indicate significant differences between genotypes at P < 0.05 and P < 0.01, respectively. & and @ indicate significantly smaller diameter compared with 9 and 12 mo, respectively, for same sex and genotype. Letters next to each graph indicate dependency and interactions. A, age; A&G: age and genotype; G, genotype; LV, left ventricle; S, sex; WT, wild type.
Table 1.
Aortic diameter for male and female mice at proximal and distal region at different timepoints
| Proximal |
Distal |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Month 3 | ||||
| D, mm | ||||
| WT | 1.54 ± 0.13 | 1.38 ± 0.04 | 1.53 ± 0.07 | 1.41 ± 0.08 |
| Het | 1.51 ± 0.11 | 1.50 ± 0.05 | 1.65 ± 0.23 | 1.59 ± 0.04 |
| P value | >0.99 | 0.99 | >0.99 | 0.99 |
| %Change | −5.048 | 7.77 | 6.88 | 10.85 |
| Month 6 | ||||
| D, mm | ||||
| WT | 1.50 ± 0.16 | 1.43 ± 0.04 | 1.52 ± 0.11 | 1.45 ± 0.03 |
| Het | 1.62 ± 0.15 | 1.56 ± 0.14 | 1.83 ± 0.10 | 1.59 ± 0.11 |
| P value | 0.99 | 0.99 | 0.68 | 0.99 |
| %Change | 11.89 | 9.42 | 20.89 | 15.55 |
| Month 9 | ||||
| D, mm | ||||
| WT | 1.67 ± 0.17 | 1.45 ± 0.24 | 1.64 ± 0.14 | 1.51 ± 0.07 |
| Het | 2.06 ± 0.30 | 1.63 ± 0.06 | 2.20 ± 0.29 | 1.80 ± 0.13 |
| P value | 0.042 | 0.94 | 0.03 | 0.81 |
| %Change | 23.50 | 12.38 | 31.45 | 35.33 |
| Month 12 | ||||
| D, mm | ||||
| WT | 1.58 ± 0.12 | 1.48 ± 0.07 | 1.64 ± 0.12 | 1.52 ± 0.11 |
| Het | 2.11 ± 0.46 | 1.75 ± 0.08 | 2.32 ± 0.69 | 1.99 ± 0.30 |
| P value | 0.0007 | 0.46 | 0.001 | 0.11 |
| %Change | 26.28 | 16.51 | 35.33 | 31.29 |
Diameter values are means ± SE. Percentage change of aortic diameter calculated as [(DHet − DWT)/DWT]·100, where D is diameter, WT is wild type, and Het is Fbn1C1041G/+. With three-way ANOVA (n = 5 mice/study group), P value calculated between genotypes, with P < 0.05 considered significant.
Expression of Contractile SMC Markers Shows Different Patterns in Male and Female Het Mice
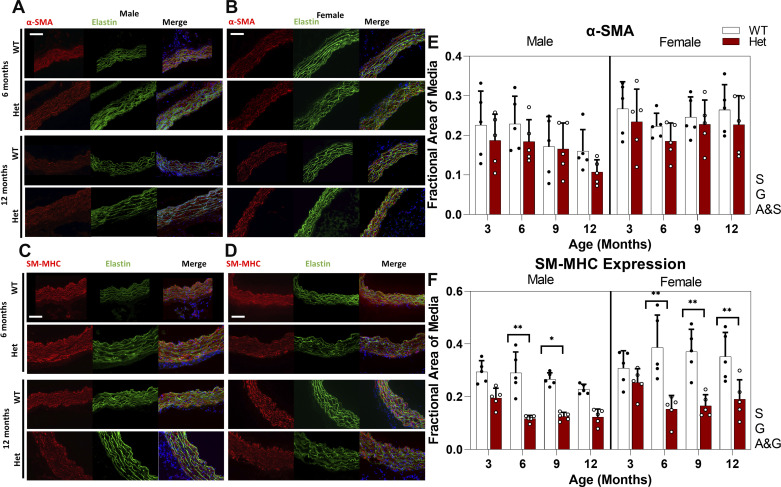
Loss of contractile SMC phenotype was confirmed by measuring the expression of specific markers for this cell type. Although there are no markers absolutely specific to contractile SMCs, smooth muscle myosin heavy chain (SM-MHC) is known to be one of the most specific (25). Quantification of IHC images, presented in Fig. 3, E and F, shows that the expression of SM-MHC in WTs remains nearly constant for female mice but gradually decreases with age in male mice. Decreased levels of SM-MHC expression in Het mice compared with WTs are significant at 6 and 9 mo for male mice and from 6 to 12 mo for female mice. Expression of SM-MHC is sex and genotype dependent and shows an interaction of age and genotype, which means there are different patterns of expression with aging for WT and Het mice.
Figure 3.
Representative IHC images showing expression of α-SMA at 6 and 12 mo (A, B) and SM-MHC at 6 and 12 mo (C, D) for male (A, C) and female (B, D) mice. Green shows elastin autofluorescence, blue shows nuclear staining, and red shows the marker of interest. Images were taken at ×200 magnification and the scale bar is 100 µm. E and F: bar graph showing means and standard deviation of fractional area of media with positive staining for each marker for n = 80 (5 mice per study group). White circles, Het mice; black circles, WT mice. Using three-way ANOVA, * and ** indicate significant differences between genotypes with P <0.05 and P < 0.01, respectively. Letters next to each graph indicates dependency and interactions. A&G, age and genotype; A&S, age and sex; α-SMA, α-smooth muscle actin; G, genotype; IHC, immunohistochemistry; S, sex; SM-MHC, smooth muscle myosin heavy chain; WT, wild type.
Smooth muscle α-actin (α-SMA), a less-specific marker for contractile SMCs, showed no significant pairwise differences in expression level between WT and Hets at any timepoint for both males and females. However, analysis of the data by three-way ANOVA shows that expression of this marker is sex and genotype dependent with an interaction between age and sex, meaning that aging affects the expression of α-SMA differently for males and females.
Adventitial Progenitor Cells Are More Abundant in Young Male and Female Hets
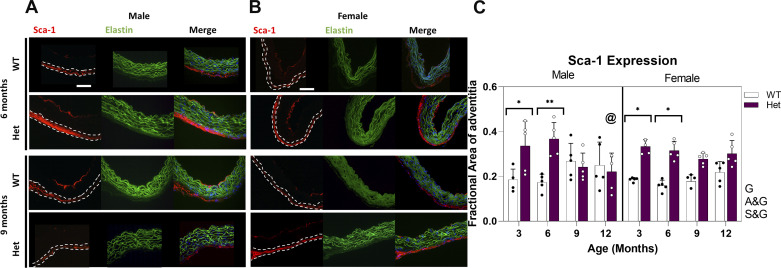
Involvement of multipotent mesenchymal progenitor cells in TAA was studied by measuring the expression of Sca-1 marker. These cells have been shown to differentiate to mature cell types including SMCs in response to injury and to repair the wall (17). As shown in Fig. 4C, expression of Sca-1 marker is significantly higher in Hets compared with WTs at 3 and 6 mo in both males and females. However, at 9 and 12 mo, expression of Sca-1 in WTs and Hets becomes similar. At 12 mo, male Hets show significantly lower Sca-1 expression compared with at 6 mo. Expression of adventitial Sca-1 is genotype dependent, and this factor shows interactions with age. An increase in adventitial Sca-1+ cells suggests the involvement of mesenchymal progenitor cells during progression of the aortic dilation.
Figure 4.
Representative IHC images showing expression of Sca-1 at 6 and 9 mo for male (A) and female (B) Het and WT mice. Green, blue, and red represent elastin autofluorescence, nuclear staining, and Sca-1, respectively. Dashed line delineates adventitia. Images were taken at ×200 magnification and the scale bar is 100 µm. C: bar graph showing means and standard deviation of fractional area of adventitia with positive staining (n = 80, 5 mice per study group). White circles, Het mice; black circles, WT mice. Using three-way ANOVA and with P < 0.05, @ indicates significantly lower expression compared with 6 mo for comparable sex and genotype, * and ** indicate significant differences between genotypes for P < 0.05 and P < 0.01, respectively. Letters next to the graph indicate dependency and interactions. A&G, age and genotype; G, genotype; IHC, immunohistochemistry; S&G, sex and genotype; WT, wild type.
Collagen Deposition and Elastin Breaks Show Significant ECM Remodeling during TAA Progression
Multiphoton second-harmonic generation (MP-SHG) microscopy was used to study collagen deposition in the aortic media. Figure 5B shows that, for both male and female WTs, there is a nearly constant level of medial collagen at all ages. Male Hets show significantly higher levels of medial collagen deposition compared with WTs at 9 and 12 mo. The gradual increase in medial collagen deposition for male Hets leads to significantly higher levels at 12 mo compared with at 3 and 6 mo. Collagen deposition is sex dependent, as increased levels of collagen deposition in the media of 9- and 12-mo Het males lead to significantly higher levels compared with females at 9 mo but not at 12 mo, mostly because of the slight increase of collagen deposition in female Hets at 12 mo. Furthermore, there is an interaction between age and sex for medial collagen deposition, meaning that males and females show different patterns of collagen deposition with aging.
Figure 5.
Left: bar graphs showing means and standard deviation for number of elastin breaks in complete aortic ring (n = 80, 5 mice per study group) (A) and quantification of fractional area of the media positive for collagen (n = 80, 5 mice per study group) (B). White circles, Het mice; black circles, WT mice. Using three-way ANOVA and with P < 0.05, # indicates significant difference between sexes for comparable age and genotype, @ and & indicate significant difference compared with 9 and 12 mo, respectively, for same sex and genotype. ** indicates significant difference between genotypes for P < 0.01. Right: representative images showing healthy (C) and aneurysmal (D) aortic rings at 12 mo for male mice. Insets show higher magnification of part of the ring and arrows point to elastin breaks. Representative SHG images of collagen showing parts of ascending aorta of WT and Het mice at 3 and 12 mo for male (E) and female (F). Green represents autofluorescence of elastin and yellow represents collagen. All images were taken at ×200 magnification and the scale bar is 100 µm. Letters next to each graph indicates dependency and interactions. A, age; G, genotype; A&G, age and genotype; A&S, age and sex; S&G, sex and genotype; WT, wild type.
With the IHC images, the number of elastin breaks over the entire aortic area was measured, as shown in Fig. 5A. There is a significantly higher number of elastin breaks in the media of Het mice compared with WT mice at all ages for males and starting from 6 mo for females. For both male and female Hets, the number of breaks gradually increases from 3 to 9 mo. The number of breaks continues to increase for male Hets, so that 12-mo males show significantly more elastin breaks compared with all earlier ages. For female Hets, the number of breaks is significantly higher at 9 mo than at 3 mo. This different temporal pattern makes 12 mo the only timepoint having significantly more elastin breaks in male Hets compared with females. The number of elastin breaks is age dependent. Furthermore, genotype shows an interaction with age and sex, which means WT and Het mice have different temporal patterns of elastin breaks and also different patterns between male and females.
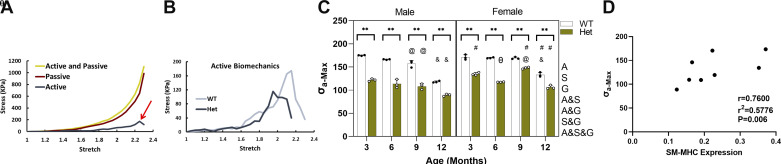
Maximum Active Stress Generation Is Significantly Reduced in Fbn1 Hets
Wire myography was used to measure the active and passive biomechanical responses of the aortic wall under circumferential uniaxial stretch. Although myography was performed independently for proximal and distal ascending aorta segments, the data from both segments have been averaged for statistical analysis since there was no significant difference between the maximum active stress generation (σA-max) in the two regions for Hets. Figure 6C shows that σA-max is significantly reduced in ascending aorta of Het mice compared with age-matched WT controls at all timepoints and for both sexes. Aging affects σA-max in a similar way in the WT mice for both sexes. There is a gradual decrease in σA-max from 3 to 9 mo followed by a more pronounced decrease from 9 to 12 mo, making σA-max at 12 mo significantly lower than at all other timepoints. However, there is a difference in the effect of aging on σA-max between male and female Hets. Although male Hets show a trend similar to WTs with reduced σA-max as a function of age, female Hets show an unexpected increase of σA-max at 9 mo, followed by a decrease at 12 mo. This pattern leads to σA-max in female Hets being significantly higher at 9 mo than at 3 and 12 mo. σA-max significantly depends on all variables examined and their interaction. Decreased contractility in Het mice compared with WTs is consistent with the loss of SM-MHC expression. To further investigate the relationship, a correlation test was performed between σA-max and SM-MHC expression, and it demonstrated a significant correlation of r = 0.76 as shown in Fig. 6D.
Figure 6.
A: schematic stress-stretch curve representing stresses from the two cycles of myography and calculated active stress. Red arrow points to location of maximum active stress. B: schematic stress-stretch curve representing only active curves for WT and Het groups. C: bar graph representing means and standard deviation of maximum active stress generation of WT and Het aortic rings measured at different timepoints for male and female mice (n = 48, 3 mice per study group). White circles, Het mice; black circles, WT mice. Using three-way ANOVA and with P < 0.05, # indicates significantly higher stress in the specified sex for comparable age and genotype, & indicates significantly lower stress compared with all other timepoints for the same sex and genotype. @ indicates significant difference compared with 3 mo and θ compared with 3 and 9 mo. ** indicates significant difference between genotypes for P < 0.01. D: correlation between the maximum active stress and smooth muscle-myosin heavy chain expression. Letters next to the graph indicate dependency and interactions. A, age; A&G; age and genotype; A&S: age and sex; A&S&G, age and sex and genotype; G, genotype; S, sex, S&G, sex and genotype; SM-MHC, smooth muscle myosin heavy chain; WT, wild type.
Altered Passive Mechanical Response of MFS Aortas Correlates with Increased Number of Elastin Breaks
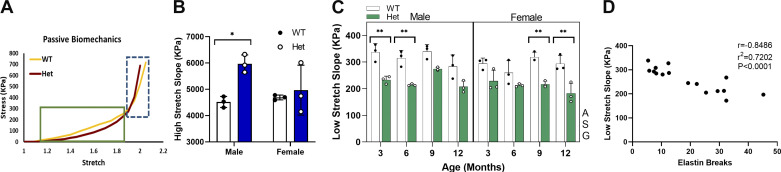
To evaluate passive mechanical properties of the aorta, the slope of the stress-stretch curve was calculated separately at two regions of low and high stretches, to study the involvement of elastin and collagen, respectively (26). Low- and high-stretch regions were defined as stretch values before and after the stretch corresponding to σa-max, respectively. Slopes calculated for proximal and distal ascending aortic segments were averaged for statistical analysis since the values in the two regions were not significantly different. It is well accepted that the mechanical response of the artery at low-stretch values mainly represents engagement of elastin fibers at small loads. As shown in Fig. 7C, low-stretch slope in the ascending aorta is age, sex, and genotype dependent; however, there is no interaction among the variables. Although at all ages there is a trend of lower slope for Hets compared with WT, this difference is only significant at 3 and 6 mo for males and at 9 and 12 mo for females. The small number of samples tested is a limitation of this study, and it is believed to be the reason that the trends are not showing significance.
Figure 7.
A: schematic stress-stretch curve representing regions defined as low stretch in green outlined box and high stretch in dashed blue box. Bar graph showing means and standard deviation for slope of high-stretch (B) (n = 12; 3 mice per study group) and low-stretch (C) (n = 48; 3 mice per study group) regions of the stress-stretch curve plotted for WT and Het mice as a function of age. White circles, Het mice; black circles, WT mice. Using three-way ANOVA, * and ** indicate significant differences between genotypes for P < 0.05 and P < 0.01, respectively. D: correlation between the slope of the low-stretch region and number of elastin breaks. Letters next to each graph indicate the dependency on that independent variable. A, age; G, genotype; S, sex; WT, wild type.
The measured decrease in low-stretch slope of the aortopathic samples can be attributed to elastin fragmentation and loss of elasticity of the vessel wall. A correlation test was performed between the number of elastin breaks and the slope of the low-stretch region in the proximal ascending aorta, demonstrating a significant inverse correlation with r = −0.85 as presented in Fig. 7D.
Aortic Wall Is Stiffer in MFS Mice Compared with WTs for Males at 12 Mo
Medial collagen deposition reflects a response to changes in cell phenotype in the aneurysmal aorta and constitutes part of the abnormal ECM remodeling response. Increased collagen deposition can lead to arterial stiffening. The slope of the passive stress-stretch curve in the high-stretch region can be used as an indicator of arterial stiffness, representing the strain-stiffening behavior typical of collagen fibers. At early timepoints in this study, forces in the high-stretch region exceeded the limits of the myograph; therefore, no comparisons between WT and Het mice were possible. Data collected for 12 mo are presented in Fig. 7B. The data collected from proximal and distal ascending aortic rings showed no significant difference; for that reason, data from the two regions in each mouse were averaged for statistical analysis. The data show a significant increase in slopes in the high-stretch region for aged male Hets compared with WT. These data are consistent with SHG microscopy observations of significantly higher medial collagen deposition in male Hets compared with WTs at 12 mo.
DISCUSSION
Although clinical studies show a clear sex dependency of aortic events in MFS (7), few animal studies have focused on understanding how these different outcomes in men and women appear at the molecular and cellular levels in the aortic wall. The findings of our study confirm a similar sex dependency of MFS-related aortopathy in mice as observed in human patients with MFS. Although Het mice show similar levels of elastin breaks, considered to be the initial matrix damage in the aneurysm, between males and females, patterns of aortic dilation, medial collagen deposition, contractile protein expression, and SMC contractility are different between males and females and suggest a protective response in females. Moreover, we showed for both sexes that at early stages of thoracic aortopathy, Sca-1+ progenitor cells are more abundant in the adventitia of the aneurysmal aorta, suggesting their involvement during progression of aortopathy.
Aneurysm in patients with MFS initiates at the sinus of Valsalva in the aortic root and can extend to the ascending and the descending aorta. Standard evaluation of TAA in these patients includes monitoring aortic dilatation using echocardiography, which can provide information such as aortic dilation, extent of the dilated segment, and rate of dilation. These parameters are directly proportional to the severity of aortic disease (27). During the past decade, many studies inside and outside the United States (8, 28–31) used national or institutional databases of patients with MFS to find correlations between age, sex, disease severity, and, more to our interest, aortic events. All nationwide studies were unanimous in reporting similar incidence in both sexes but more severe aortic events in men compared with women. One of the most comprehensive studies, performed in 2010 on 1,013 patients (53% men) from 38 countries (32), showed that men develop aortic dilatation significantly earlier in life, meaning that by the age of 30, more men show aortic dilation compared with women. However, by the age of 60, similar numbers of men and women show aortic dilation, suggesting a lag in the initiation of aortic dilation in women. However, when considering aortic events such as aortic dissection and need for prophylactic surgery, the occurrence is higher in men than in women even before the age of 60. More recently, Roman et al. (7) performed a similar study using data from the Genetically Triggered Thoracic Aortic Aneurysm and Cardiovascular Conditions (GenTAC) registry. Although their findings were similar to those of the earlier study, inclusion of children from the age of 7 in their study showed that aortic dilatation starts from very early ages.
To study age, sex, and regional dependency of TAA progression, we used a mouse model, heterozygous Fbn1C1041G/+ mice, which has a genetic mutation commonly seen in human MFS (23). Over a time course of 3–12 mo, we studied different manifestations of TAA on cells and ECM of the ascending aorta in male and female mice.
As mentioned earlier, monitoring the aortic diameter is one of the main prognostic indicators of TAA. Aortic diameter varies depending on age, sex, and body weight. The criteria for considering a dilation as aneurysm is when the diameter is 150% of a healthy aorta of the same group (33). Although over the time course of this study, the dilations never reached the clinical criterion for being considered true aneurysms, Het mice show dilations that were considerable and indicative of thoracic aortopathy. We used ultrasound to monitor the aortic dilation at different regions of the ascending aorta (Fig. 2) and aortic root (Supplemental Fig. S1) of male and female WT and Het mice. Aortic root dilation, which is the initial focus of dilation in MFS, begins to develop as early as 2 wk in these mice (9); however, here we focus on the ascending aorta to track the progression of aortopathies in MFS in different regions of the aorta that have been less well studied. Furthermore, previous studies have shown that SMCs in the proximal and distal ascending aorta have different embryonic origins (34), which raises the question whether this would lead to different behavior in aneurysm progression. We showed a different pattern of aortic dilation in male and female mice by 12 mo. Although the aortic diameter of male mice increases rapidly from 6 to 9 mo (proximal: 27.11% growth; distal: 20.13% growth) with no additional change up to 12 mo, females show a very gradual increase in the diameter, with the fastest growth occurring from 9 to 12 mo (proximal: 7.74%, distal: 11.48%), which is still less severe than aortic dilation in the males. The patterns of aortic dilation suggest a lag of dilation progression and an early protection from dilation growth in female mice compared with males. Furthermore, female Hets show greater dilations in the distal region compared with the proximal region, whereas in male Hets, this difference was not significant.
Elastin fragmentation caused by fibrillin-1 deficiency can lead to SMC detachment from elastic fibers. When cells lose their anchorage, vessel architecture disintegrates. Environmental factors such as hemodynamic stress can further promote the detachment of cells from ECM. When SMCs lose their anchorage to the ECM, they cannot generate force (35). The detached cells might undergo apoptosis (11) or phenotypic changes (12). Either way, contractility and elastic tension of the wall will be affected (36). We demonstrated that expression of SM-MHC during progression of TAA is sex and genotype dependent. Although both male and female Hets show decreased medial expression from 6 mo, male mice lose the significant difference between the genotypes at 12 mo, mostly due to decreased levels in older WTs. Loss of contractile SMCs agrees with our myography data showing decreased contractility of the dilated aortas compared with WTs. Previous studies on different human samples (12, 37) and animal models also reported decreased expression of specific markers for contractile SMCs. Our data showed no change in α-SMA expression between genotypes. However, genotype dependency of expression of this marker together with the age and sex interaction suggests a synergistic effect of age and genotype in male mice, whereas age had no effect on SMA expression in females. Previous investigations have provided conflicting reports on the expression of this marker. Studies on human tissue (37) as well as animal models of induced aneurysm (38) and a more severe MFS mouse model (Fbn1mgR/mgR) (36) reported decreased expression of α-SMA. However, our data are in agreement with a previous study that also used the Fbn1C1039G/+ mouse model (18). It has been shown that, with the possible exception of SM-MHC, all other known markers for contractile SMCs are at least transiently expressed in other cell types (25).
Tunica adventitia, the outermost layer of the aortic wall, has long been considered as merely a supportive tissue. Over the past decade, however, evidence has accumulated suggesting that the arterial adventitia is an active layer, signaling to other wall layers and playing a considerable role in remodeling all three layers of the wall during disease or repair (39). Previously, in a search for additional sources of progenitor cells, Hu et al. (16) showed that the highest number of progenitor cells in the adventitia are Sca-1+, accounting for 21% of total adventitial cells of the aortic root. Using a vein graft model, these investigators showed that Sca-1+ cells differentiate to SMCs and contribute to formation of neointimal lesions. Sca-1+ cells were also shown to be involved in vascular remodeling in hypertension, as one of the progenitors of collagen-producing cells in a mouse model (17). In the present study, we provide evidence that Sca-1+ cells are more abundant in the adventitia of both male and female Hets compared with WTs up to 6 mo of age. At 12 mo, male Hets show significantly lower expression of adventitial Sca-1 compared with at 6 mo. Although the decline in stem cell renewal ability with aging is very well accepted (40), sex differences in this process are not completely understood. However, there are some studies suggesting a potential role of estrogens in preventing stem cell senescence by reducing reactive oxygen species (ROS) (41).
The similarity in the time course of reduced expression of Sca-1 at late ages in Het males and more severe aortopathic outcomes in this group compared with females of the same age suggests a relation between the two factors. We speculate that protective behavior observed in female Hets may be due to persistent support of progenitor cells, which is lacking in male Hets at older ages. However, due to pluripotency of Sca-1+ cells, differentiation of these cells to specific cell types can potentially lead to either regenerative responses or further progression of aortopathies. Although in vivo, Sca-1+ cells can differentiate to adipocytes, osteoblasts, chondrocytes, fibroblasts, and SMCs, the path they will take depends on environmental cues. Studies on neointima formation showed that Sca-1+ cells can differentiate to SMC by a process dependent on collagen/integrin interactions (42). Conversely, some studies consider Sca-1+ cells to be a subset of fibroblasts (43) in adventitial (44) and cardiac (45) tissue that show plasticity and can differentiate to pathological fibroblasts. Furthermore, in hypertension, Sca-1+ cells showed their ability to differentiate to fibroblasts and produce collagen, thus taking part in increased fibrosis and stiffening of the vessel wall (17). Adventitial Sca-1+ cells have been shown to contain subpopulations of SMC progenitors (Sca-1+/CD45−) and macrophage progenitors (Sca-1+/CD45+) (46). On the one hand, differentiation of Sca-1+/CD45+ cells can increase the inflammatory response and further damage the aortic wall due to their association with increased MMP secretion (2), but on the other hand, macrophages provide chemokines necessary for migration of Sca-1+/CD45− cells into the media (42). However, the mechanism by which Sca-1+ cells contribute to either protection or destruction of the vessel wall in MFS needs further investigation. Lineage tracing of Sca-1+ cells and colocalization of Sca-1 marker with specific markers of possible progeny cells can shed light on the fate of these progenitor cells during aneurysm progression.
Fragmentation of elastin fibers, changes in medial resident cells, activation of signaling pathways leading to collagen deposition, and overactivation of elastolytic MMPs all lead to unbalanced remodeling of the ECM. Interestingly, it has been shown that estrogen levels have an inverse relationship with MMP-2 and -9 levels, matrix metalloproteases that are involved in the abnormal matrix remodeling in abdominal aortic aneurysm (47).
In this study, we measured elastin breaks and medial collagen deposition as indicators of ECM remodeling. We showed that initially, for both WT and Hets, male and female mice have comparable numbers of elastin breaks, which increase with age as expected. This finding can be regarded as evidence of similar susceptibility of both males and females to initiate thoracic aortopathy through damage to the vessel matrix. We measured the low-stretch slope of stress-stretch curves obtained by myography. These data showed an inverse correlation between number of elastin breaks and slope of the low-stretch region of the stress-stretch curve, which confirms that with increased elastin breaks, the vessel becomes less resistant to deformation. Chen et al. (48) used ultrasound to measure internal aortic diameters of 11-wk-old male and female Fbn1mgR/mgR mice at systole and diastole. They introduced Green–Lagrange strain as an indicator of compliance and showed decreased compliance of the aneurysmal aorta. Chen et al. (48) reported the diameter values for male mice at systole as 1.5 mm and 2.35 mm for WT and Fbn1mgR/mgR mice, respectively; however, they did not report the ex vivo zero load values for the aortic diameter. To be able to compare their stretch ratios with those used in our study, we used previously published zero load values (49) for the same strain of mice at 14–18 wk, which were 909 and 1,221 µm for WT and Fbn1mgR/mgR mice, respectively. With use of these values, the estimated stretch ratio at systole will be 1.6 and 1.9 for WT and Fbn1mgR/mgR, respectively, and for diastole will be 1.42 and 1.84 for WT and Fbn1mgR/mgR, respectively. When comparing these values with ours, for WTs, the circumferential stretch is within our definition of the low-stretch region, whereas for the Fbn1mgR/mgR mice, it is within the high-stretch region, so the smaller expansion for the Fbn1mgR/mgR aorta agrees with our data showing higher stiffness of the aorta in the high-stretch region. However, it is necessary to emphasize that in vivo stretch and ex vivo uniaxial stretch are not directly comparable. A more relevant comparison can be achieved by comparing in vivo data with biaxial mechanical test results.
Damaged elastic fibers can stretch more under smaller applied forces, making the aortic wall less resistant to deformation, but at the same time, increased deposition of collagen leads to a stiffer aortic wall, which lowers its distensibility and consequently limits elastic fiber stretch (22). Although there are no significant differences in the levels of medial collagen deposition in female Hets compared with WTs, the increased levels in males are significantly higher than in females at 9 mo but not at 12 mo. The increase in medial collagen in female Hets between 9 and 12 mo suggests that medial collagen accumulation does occur in these mice with a lag compared with males. Although we focused on medial collagen deposition, other reports show no changes in adventitial collagen from 3 to 9 mo (50) and a decrease at 12 mo (51) compared with WT in the same mouse model of MFS. However, there was no mention of the sex of the mice in these studies. These findings together with ours may suggest that the higher stiffness of the MFS aorta is due to increased medial collagen deposition. However, studies on human aortas of patients with MFS (26) that examined both quantity and organization of collagen fibers showed no differences in medial collagen organization between MFS and control groups but dramatically disturbed adventitial collagen organization in MFS compared with highly organized collagen fibers in healthy aortas. However, patient sex was not specified in this study.
Proximity to the heart subjects the ascending aorta to very high axial and cyclic-circumferential stretch due to heart motion and cyclical changes in blood pressure, respectively. The highly elastic behavior of the aorta accommodates these cycles of high stress and maintains the normal contraction of smooth muscle cells (52). However, loss of elasticity and increased stiffness of the aortic wall in patients with MFS can increase afterload on the heart, leading to increased cyclic loading on the aorta and eventually changing mechanical properties of the wall (22). Use of angiotensin receptor blockers or β-blockers helps to lower blood pressure and decrease this load, consequently preventing rupture. Interestingly, estrogen is known to enhance vasorelaxation via nitric oxide secretion from endothelial cells, whereas androgen plays a more complicated role and can also elevate blood pressure in vivo by causing vascular SMC contraction (47). The different effects of male and female sex hormones on vascular tone could be one mechanism by which young females show protection from aneurysm.
Although the majority of stress applied on the vessel wall is borne by the ECM (52), alterations in the biomechanical environment can disrupt normal cellular mechanosensing, potentially changing SMC contractility. Although passive biomechanics of healthy and aneurysmal aortic wall has been studied extensively, cell contractility has received substantially less attention. In a similar study on Fbn1C1041G/+ mice, Chung et al. (18) used wire myography to measure the contractility of aortic arch and descending aorta of MFS and WT mice. These authors reported decreased contractility in the MFS aorta from 3 to 9 mo, which is consistent with our findings. However, there was no mention of sex as a biological variable in their study. Later, Jimenes-Altayo et al. (21) used the same mouse model to study the contractility of ascending and descending aortas at 3 and 6 mo in MFS and WT mice, comparing male and female mice. Their data show an increase in the contractility of male MFS mice compared with WT and no difference in females. However, their measurement of contractility was based on changes in force and not stress, which does not take into account geometric changes of the wall, whereas it is known that the aortic wall is thicker in MFS mice than in WT mice due to medial thickening (9). In our work, we showed that from 3–12 mo, the σa-max is significantly lower at all timepoints for both male and female Hets than for WTs. Decreased σa-max at 12 mo for all the groups further shows that wall contractility is age dependent, independent of genotype, sex, and aortic region. Similar to most of our findings in this study, the largest differences between males and females are found at the later timepoints of 9 and 12 mo. As might be expected, the σa-max data show a significant correlation to SM-MHC expression for both males and females. Furthermore, similar biomechanical properties in the proximal and distal ascending aorta can suggest that, regardless of their embryonic origins, mature SMCs behave similarly in the diseased state in MFS.
In conclusion, this study showed that, in agreement with clinical data, the Fbn1C1041G/+ mouse model demonstrates sex dependency during progression of the thoracic aortopathy related to MFS. Studying the age dependency of the aortopathy revealed that events such as elastin breaks, decreased contractility, and increased adventitial Sca-1 expression start early on, followed by late events such as medial collagen deposition and aortic dilation. Studying sex dependency of the thoracic aortopathy showed that males and females display similarities in early events, whereas they differ in the late events. These findings emphasize that thoracic aortopathy in MFS starts similarly between males and females. With aging, males display a more severe disease phenotype, which suggests a degree of protection in females. Furthermore, male and female Hets show similarly high levels of expression of Sca-1+ multipotent progenitor cells in the adventitia at young ages. However, although the expression levels between WT and Hets become similar at older ages, for Het males at 12 mo, the expression level of Sca-1 is significantly lower compared with at 6 mo, suggesting that these cells may play a role in protecting the aortic wall from dilation. Determining whether the contribution of estrogen in maintaining vascular tone and/or stem cell renewal is the mechanism by which female mice build protection against MFS aortopathies can be the subject of future studies.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.16832323.v2.
GRANTS
This work was supported by National Institutes of Health Grants R01HL133662 and R03EB019663 (to S. M. L.) and by a SPARC graduate research grant (to N. Gharraee) from the Office of Vice President for Research at the University of South Carolina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.G. and S.M.L. conceived and designed research; N.G., Y.S., and J.A.S. performed experiments; N.G., Y.S., and J.A.S. analyzed data; N.G. and S.M.L. interpreted results of experiments; N.G. prepared figures; N.G. drafted manuscript; N.G. and S.M.L. edited and revised manuscript; N.G., Y.S., J.A.S., and S.M.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brooks A. Lane and Liya Du for assistance in myograph data collection and analysis and imaging.
REFERENCES
- 1.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: focus on restenosis and aneurysm. Cardiovasc Res 88: 395–405, 2010. doi: 10.1093/cvr/cvq224. [DOI] [PubMed] [Google Scholar]
- 2.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, Geng YJ, Milewicz DM. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg 131: P671–P678.E2, 2006. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Milewicz DM, Guo D-C, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet 9: 283–302, 2008. doi: 10.1146/annurev.genom.8.080706.092303. [DOI] [PubMed] [Google Scholar]
- 4.Milewicz DM, Urbán Z, Boyd C. Genetic disorders of the elastic fiber system. Matrix Biol 19: 471–480, 2000. doi: 10.1016/S0945-053X(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez F, Caescu C, Wondimu E, Galatioto J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFβ signaling and cell stemness. Matrix Biol 71–72: 82–89, 2018. doi: 10.1016/j.matbio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pyeritz RE. Marfan syndrome: 30 years of research equals 30 years of additional life expectancy. Heart 95: 173–175, 2009. doi: 10.1136/hrt.2008.160515. [DOI] [PubMed] [Google Scholar]
- 7.Roman MJ, Devereux RB, Preiss LR, Asch FM, Eagle KA, Holmes KW, LeMaire SA, Maslen CL, Milewicz DM, Morris SA, Prakash SK, Pyeritz RE, Ravekes WJ, Shohet RV, Song HK, Weinsaft JW, Dietz HC, Habashi J, Silberbach GM, Bavaria JE, Milewski K, McDonnell N, Tolunay HE, Desvigne-Nickens P, Tseng H, Kroner BL; GenTAC Investigators. Associations of age and sex with Marfan phenotype: The National Heart, Lung, and Blood Institute GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) Registry. Circ Cardiovasc Genet 10: e001647, 2017. doi: 10.1161/CIRCGENETICS.116.001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda N, Inuzuka R, Maemura S, Morita H, Nawata K, Fujita D, Taniguchi Y, Yamauchi H, Yagi H, Kato M, Nishimura H, Hirata Y, Ikeda Y, Kumagai H, Amiya E, Hara H, Fujiwara T, Akazawa H, Suzuki JI, Imai Y, Nagai R, Takamoto S, Hirata Y, Ono M, Komuro I. Impact of pathogenic FBN1 variant types on the progression of aortic disease in patients with Marfan syndrome. Circ Genomic Precis Med 11: e002058, 2018. doi: 10.1161/CIRCGEN.117.002058. [DOI] [PubMed] [Google Scholar]
- 9.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121, 2006. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Agarwal S. Marfan syndrome: an eyesight of syndrome. Meta Gene 2: 96–105, 2014. doi: 10.1016/j.mgene.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emrich F, Okamura H, Arakawa M, Dalal A, Penov K, Merk D, Raaz U, Hennigs J, Chin J, Miller M, Blankenberg F, Connolly A, Mohr F, Fischbein M. Apoptosis in Marfan syndrome aneurysms - it’s not only the loss of cells. Thorac Cardiovasc Surg 63: OP227, 2015. doi: 10.1055/s-0035-1544479. [DOI] [Google Scholar]
- 12.Crosas-Molist E, Meirelles T, López-Luque J, Serra-Peinado C, Selva J, Caja L, Gorbenko Del Blanco D, Uriarte JJ, Bertran E, Mendizábal Y, Hernández V, García-Calero C, Busnadiego O, Condom E, Toral D, Castellà M, Forteza A, Navajas D, Sarri E, Rodríguez-Pascual F, Dietz HC, Fabregat I, Egea G. Vascular smooth muscle cell phenotypic changes in patients with Marfan syndrome. Arterioscler Thromb Vasc Biol 35: 960–972, 2015. doi: 10.1161/ATVBAHA.114.304412. [DOI] [PubMed] [Google Scholar]
- 13.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun 3: 875, 2012. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res 116: 1392–1412, 2015. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 15.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: Building and repairing blood vessels. Circ Res . 108: 365–377, 2011. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atheroscleroses of vein grafts in ApoE-deficient mice. J Clin Invest 113: 1258–1265, 2004. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, Montaniel KRC, Saleh MA, Xiao L, Chen W, Owens GK, Humphrey JD, Majesky MW, Paik DT, Hatzopoulos AK, Madhur MS, Harrison DG. Origin of matrix-producing cells that contribute to aortic fibrosis in hypertension. Hypertension 67: 461–468, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung AWY, Au Yeung K, Sandor GGS, Judge DP, Dietz HC, Van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res 101: 512–522, 2007. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 19.Iliopoulos DC, Deveja RP, Kritharis EP, Perrea D, Sionis GD, Toutouzas K, Stefanadis C, Sokolis DP. Regional and directional variations in the mechanical properties of ascending thoracic aortic aneurysms. Med Eng Phys 31: 1–9, 2009. doi: 10.1016/j.medengphy.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Bellini C, Bersi MR, Caulk AW, Ferruzzi J, Milewicz DM, Ramirez F, Rifkin DB, Tellides G, Yanagisawa H, Humphrey JD. Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. J R Soc Interface 14: 20161036, 2017. doi: 10.1098/rsif.2016.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiménez-Altayó F, Siegert A-M, Bonorino F, Meirelles T, Barberà L, Dantas AP, Vila E, Egea G. Differences in the thoracic aorta by region and sex in a murine model of Marfan syndrome. Front Physiol 8: 933, 2017. doi: 10.3389/fphys.2017.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellini C, Korneva A, Zilberberg L, Ramirez F, Rifkin DB, Humphrey JD. Differential ascending and descending aortic mechanics parallel aneurysmal propensity in a mouse model of Marfan syndrome. J Biomech 49: 2383–2389, 2016. doi: 10.1016/j.jbiomech.2015.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114: 172–181, 2004. doi: 10.1172/jci20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson SR, Cooper KM, Liu P, Gharraee N, Du L, Han SM, Peña EA, Sutton MA, Eberth JF, Lessner SM. Diet alters age-related remodeling of aortic collagen in mice susceptible to atherosclerosis. Am J Physiol Heart Circ Physiol 320: H52–H65, 2021. doi: 10.1152/ajpheart.00420.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 26.Lindeman JHN, Ashcroft BA, Beenakker JWM, Van Es M, Koekkoek NBR, Prins FA, Tielemans JF, Abdul-Hussien H, Bank RA, Oosterkamp TH. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc Natl Acad Sci USA 107: 862–865, 2010. doi: 10.1073/pnas.0910312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation 111: e150–e157, 2005. doi: 10.1161/01.CIR.0000155243.70456.F4. [DOI] [PubMed] [Google Scholar]
- 28.Gray J, Bridges A, West R, McLeish L, Stuart A, Dean J, Porteous M, Boxer M, Davies S. Life expectancy in British Marfan syndrome populations. Clin Genet 54: 124–128, 1998. doi: 10.1111/j.1399-0004.1998.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 29.Groth KA, Stochholm K, Hove H, Andersen NH, Gravholt CH. Causes of mortality in the Marfan syndrome(from a Nationwide Register Study). Am J Cardiol 122: 1231–1235, 2018. doi: 10.1016/j.amjcard.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Meijboom LJ, Timmermans J, Zwinderman AH, Engelfriet PM, Mulder BJM. Aortic root growth in men and women with the Marfan’s syndrome. Am J Cardiol 96: 1441–1444, 2005. doi: 10.1016/j.amjcard.2005.06.094. [DOI] [PubMed] [Google Scholar]
- 31.Vanem TT, Geiran OR, Krohg-Sørensen K, Røe C, Paus B, Rand-Hendriksen S. Survival, causes of death, and cardiovascular events in patients with Marfan syndrome. Mol Genet Genomic Med 6: 1114–1123, 2018. doi: 10.1002/mgg3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Détaint D, Faivre L, Collod-Beroud G, Child AH, Loeys BL, Binquet C, Gautier E, Arbustini E, Mayer K, Arslan-Kirchner M, Stheneur C, Halliday D, Beroud C, Bonithon-Kopp C, Claustres M, Plauchu H, Robinson PN, Kiotsekoglou A, De Backer J, Ads L, Francke U, De Paepe A, Boileau C, Jondeau G. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur Heart J 31: 2223–2229, 2010. doi: 10.1093/eurheartj/ehq258. [DOI] [PubMed] [Google Scholar]
- 33.Goldfinger JZ, Halperin JL, Marin ML, Stewart AS, Eagle KA, Fuster V. Thoracic aortic aneurysm and dissection. J Am Coll Cardiol 64: 1725–1739, 2014. doi: 10.1016/j.jacc.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Sawada H, Rateri DL, Moorleghen JJ, Majesky MW, Daugherty A. Smooth muscle cells derived from second heart field and cardiac neural crest reside in spatially distinct domains in the media of the ascending aorta-brief report. Arterioscler Thromb Vasc Biol 37: 1722–1726, 2017. doi: 10.1161/ATVBAHA.117.309599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milewicz DM, Trybus KM, Guo DC, Sweeney HL, Regalado E, Kamm K, Stull JT. Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler Thromb Vasc Biol 37: 26–34, 2017. [Erratum in Arterioscler Thromb Vasc Biol 37: e12, 2017]. doi: 10.1161/ATVBAHA.116.303229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res 88: 37–43, 2001. doi: 10.1161/01.RES.88.1.37. [DOI] [PubMed] [Google Scholar]
- 37.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg 138: 1392–1399, 2009. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao N, Gu T, Shi E, Zhang G, Yu L, Wang C. Phenotypic switching of vascular smooth muscle cells in animal model of rat thoracic aortic aneurysm. Interact Cardiovasc Thorac Surg 21: 62–70, 2015. doi: 10.1093/icvts/ivv074. [DOI] [PubMed] [Google Scholar]
- 39.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: An underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol 31: 2391–2396, 2011. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 40.Oh J, Lee YD, Wagers AJ. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat Med 20: 870–880, 2014. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulken B, Brunet A. Stem cell aging and sex: are we missing something? Cell Stem Cell 16: 588–590, 2015. doi: 10.1016/j.stem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Wong MM, Campagnolo P, Simpson R, Winkler B, Margariti A, Hu Y, Xu Q. Adventitial stem cells in vein grafts display multilineage potential that contributes to neointimal formation. Arterioscler Thromb Vasc Biol 33: 1844–1851, 2013. doi: 10.1161/ATVBAHA.113.300902. [DOI] [PubMed] [Google Scholar]
- 43.Pedroza AJ, Tashima Y, Shad R, Cheng P, Wirka R, Churovich S, Nakamura K, Yokoyama N, Cui JZ, Iosef C, Hiesinger W, Quertermous T, Fischbein MP. Single-Cell transcriptomic profiling of vascular smooth muscle cell phenotype modulation in Marfan syndrome aortic aneurysm. Arterioscler Thromb Vasc Biol 40: 2195–2211, 2020. doi: 10.1161/ATVBAHA.120.314670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsukui T, Sun K-H, Wetter JB, Wilson-Kanamori JR, Hazelwood LA, Henderson NC, Adams TS, Schupp JC, Poli SD, Rosas IO, Kaminski N, Matthay MA, Wolters PJ, Sheppard D. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun 11: 1920, 2020. doi: 10.1038/S41467-020-15647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Bracamonte-Baran W, Diny NL, Hou X, Talor MV, Fu K, Liu Y, Davogustto G, Vasquez H, Taegtmeyer H, Frazier OH, Waisman A, Conway SJ, Wan F, Čiháková D. Sca-1+ cardiac fibroblasts promote development of heart failure. Eur J Immunol 48: 1522–1538, 2018. doi: 10.1002/EJI.201847583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A, Dong XR, Poczobutt J, Nemenoff RA, Weiser-Evans MCM. Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by Klf4. Circ Res 120: 296–311, 2017. doi: 10.1161/CIRCRESAHA.116.309322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boese AC, Kim SC, Yin KJ, Lee JP, Hamblin MH. Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol 313: H524–H545, 2017. doi: 10.1152/ajpheart.00217.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen JZ, Sawada H, Moorleghen JJ, Weiland M, Daugherty A, Sheppard MB. Aortic strain correlates with elastin fragmentation in fibrillin-1 hypomorphic mice. Circ Rep 1: 199–205, 2019. doi: 10.1253/circrep.cr-18-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwill S, Seppelt P, Grünhagen J, Ott CE, Jugold M, Ruhparwar A, Robinson PN, Karck M, Kallenbach K. The fibrillin-1 hypomorphic mgR/mgR murine model of Marfan syndrome shows severe elastolysis in all segments of the aorta. J Vasc Surg 57: 1628–1636.e1-3, 2013. doi: 10.1016/J.JVS.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Cui JZ, Tehrani AY, Jett KA, Bernatchez P, van Breemen C, Esfandiarei M. Quantification of aortic and cutaneous elastin and collagen morphology in Marfan syndrome by multiphoton microscopy. J Struct Biol 187: 242–253, 2014. doi: 10.1016/j.jsb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Tehrani AY, Cui JZ, Bucky Jones T, Hotova E, Castro M, Bernatchez P, van Breemen C, Esfandiarei M. Characterization of doxycycline-mediated inhibition of Marfan syndrome-associated aortic dilation by multiphoton microscopy. Sci Rep 10: 7154, 2020. doi: 10.1038/s41598-020-64071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell biology: dysfunctional mechanosensing in aneurysms. Science 344: 477–479, 2014. doi: 10.1126/science.1253026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.16832323.v2.