Abstract
Mice are routinely used to investigate molecular mechanisms underlying the atrial fibrillation (AF) substrate. We sought to optimize transesophageal rapid atrial pacing (RAP) protocols for the detection of AF susceptibility in mouse models. Hypertensive and control C57Bl/6J mice were subjected to burst RAP at a fixed stimulus amplitude. The role of parasympathetic involvement in pacing-related atrioventricular (AV) block and AF was examined using an intraperitoneal injection of atropine. In a crossover study, burst and decremental RAP at twice diastolic threshold were compared for induction of AV block during pacing. The efficacy of burst and decremental RAP to elicit an AF phenotype was subsequently investigated in mice deficient in the lymphocyte adaptor protein (Lnk−/−) resulting in systemic inflammation, or the paired-like homeodomain-2 transcription factor (Pitx2+/−) as a positive control. When pacing at a fixed stimulus intensity, pacing-induced AV block with AF induction occurred frequently, so that there was no difference in AF burden between hypertensive and control mice. These effects were prevented by atropine administration, implicating parasympathetic activation due to ganglionic stimulation as the etiology. When mice with AV block during pacing were eliminated from the analysis, male Lnk−/− mice displayed an AF phenotype only during burst RAP compared with controls, whereas male Pitx2+/− mice showed AF susceptibility during burst and decremental RAP. Notably, Lnk−/− and Pitx2+/− females exhibited no AF phenotype. Our data support the conclusion that multiple parameters should be used to ascertain AF inducibility and facilitate reproducibility across models and studies.
NEW & NOTEWORTHY Methods were developed to optimize transesophageal rapid atrial pacing (RAP) to detect AF susceptibility in new and established mouse models. High stimulus intensity and pacing rates caused parasympathetic stimulation, with pacing-induced AV block and excessive AF induction in normal mice. For a given model, pacing at twice TH enabled improved phenotype discrimination in a pacing mode and sex-specific manner. Transesophageal RAP should be individually optimized when developing a mouse model of AF.
Keywords: atrial fibrillation, mouse models, rapid atrial pacing, transesophageal atrial pacing, transesophageal pacing
INTRODUCTION
Animal models are routinely used to assess the molecular determinants of atrial fibrillation (AF) susceptibility. Among small animals, mouse models have largely demonstrated excellent correlation with genetic (1) and acquired (2, 3) risk factors that promote clinical AF. As spontaneous AF rarely occurs in mice (4), rapid atrial pacing (RAP) is used to assess AF susceptibility. RAP can be performed using either intracardiac (5) or transesophageal (6) pacing dependent upon the study design. Although both have benefits, transesophageal RAP is particularly advantageous as a survival procedure.
Regardless of the method used, the utility of RAP in mice is complicated by the diverse array of proposed protocols that differ for multiple parameters, including the basic mode of stimulus delivery (Table 1) (7, 9). Although some investigators use decremental pacing (13), others employ burst pacing using a range of constant pacing cycle lengths (8). In addition, there is considerable variability for other parameters, including the number of pacing trains, pacing duration, and stimulus amplitude, as well as the definition of a positive study. Despite these major differences, there are limited comparisons of mouse RAP protocols (6) with no systematic attempt at protocol optimization. Thus, experimental time and resources are often spent developing protocols with limited insight into strategies that could improve sensitivity and specificity for a specific model.
Table 1.
Representative murine pacing protocols for AF induction
| Reference | Type | Cycle Length, ms |
Mode | Trials | Amplitude, mA | Pulse Width, ms | AF Definition, s | |
|---|---|---|---|---|---|---|---|---|
| Start | Stop | |||||||
| Verheule et al. (7) | TE | 40 | 20 | ↓2 ms q 2 s | 2 | 1.5× TH | 1 | ≥2 |
| Fukui et al. (2) | TE | 40, 20 | N/A | 2 s burst | 1 | NR | 5, 10 | ≥1 |
| Faggioni et al. (8) | TE | 50, 40, 30, 25, 20, 15 | N/A | 15 s burst | 1 | 2× TH; 3 mA | 1, 2 | ≥1 |
| Sood et al. (9) | IC | 40 | 20 | ↓2 ms q 2 s | 2 | 1.5× TH | 1 | ≥2 |
| Voight et al. (10) | IC | 40 | 20 | ↓2 ms q 2 s | 3 | 1.5× TH | 1 | ≥1 |
| Purhoit et al. (11) | IC | 40 | 20 | ↓2 ms q 2 s | 5 | NR | 2 | ≥1 |
| Egom et al. (12) | IC | 40, 20 | N/A | 2 s burst | 1 | 0.4 | 2 | ≥1 |
| Li et al. (4) | IC | 40 | 20 | ↓2 ms q 2 s | 3 | 1.5× TH | 2 | ≥1 |
| Yao et al. (13) | IC | 40 | 10 | ↓2 ms q 2 s | 3 | 1.5× TH | 1 | ≥1 |
AF, atrial fibrillation; IC, intracardiac; N/A, not applicable; NR, not reported; TE, transesophageal; TH, diastolic threshold.
Murine RAP has been used to assess AF susceptibility across a range of clinical risk factors such as hypertension (14), obesity (15), and genetic variants (16). Although increasing experimental evidence links inflammation to AF (13), only two mouse models of inflammation-mediated AF have been reported, both of which also demonstrate evidence of structural heart disease (17). Recently, genome-wide association studies (GWAS) have linked variants of the lymphocyte adaptor protein (LNK) or SH2B3, a negative regulator of cytokine signaling, to cardiovascular disease (18). Notably, loss of LNK in mice (Lnk−/−) has been shown to cause systemic inflammation and oxidative stress making Lnk−/− mice a potential tool to investigate inflammation-mediated AF (19, 20).
While developing mouse models of AF, we observed significant variability in AF induction for both control and AF susceptible mice, depending upon the protocol used. In addition, we found that transesophageal atrial pacing intermittently caused exaggerated pacing-related atrioventricular (AV) block that was frequently associated with AF induction, a phenomenon not seen with intracardiac pacing (Li N and Mesubi O, personal communication). Given that ganglionic plexi are clustered in the posterior left atrium, we tested the hypothesis that transesophageal pacing in this region could cause parasympathetic stimulation leading to excessive AV block during pacing and also AF, potentially contaminating experimental results. In addition, we investigated the role of stimulus intensity and pacing mode to generate this phenomenon. After optimizing for these considerations, we hypothesized that decremental and burst pacing modes would be equally effective to detect AF susceptibility in a specific model, i.e., Lnk−/− mice, as well as mice deficient in the paired-like homeobox transcription factor 2, or Pitx2. GWAS has identified chromosome 4q25 variants near the Pitx2 gene as the strongest genetic risk factor for AF (21) and reduced Pitx2 expression (Pitx2+/−) in mice increases AF susceptibility (16), enabling these mice to serve as a positive control. Finally, we analyzed the role of individual pacing trains to optimize sensitivity and specificity for both pacing protocols. Our results provide an experimental strategy to optimize transesophageal RAP to assess AF susceptibility in mouse models.
METHODS
Animal Use
Male C57Bl/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 9 wk of age and acclimated for 2 wk. To induce hypertension, mice were fed a high-salt diet (8% NaCl; Research Diets; New Brunswick, NJ) for 1 wk starting at 11 wk of age before subcutaneous minipump (model 2002; Alzet; Cupertino, CA) implantation under isoflurane anesthesia (3% for induction, 1% for maintenance) at 12 wk of age for 14 days of angiotensin II (140 ng/kg/min; Sigma; St. Louis, MO) infusion. A subcutaneous injection of ketoprofen (5 mg/kg; Zoetis; Parsippany-Troy Hills, NJ) was administered before and 24 h after the surgery. Meanwhile, high-salt-diet feeding continued until euthanizing the mice at 14 wk of age. Control mice were fed with a normal salt diet (0.259% NaCl; Research Diets; New Brunswick, NJ) and implanted with minipumps filled with vehicle (sodium chloride/acetic acid solution). Following acclimation, blood pressure was obtained by tail-cuff measurements at baseline, day 7, and day 14 of angiotensin II infusion (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.16926037.v1). Based on previous data (14), animals were assessed for AF susceptibility at 14 wk of age.
To determine parasympathetic involvement in AV block during pacing, baseline AF susceptibility was measured by burst RAP in male C57Bl/6J mice after which mice were given an intraperitoneal administration of atropine (0.5 mg/kg; Med-Pharmex; Pomona, CA) with AF susceptibility reassessed 15 min after injection. To compare decremental and burst pacing, mice were subjected to a crossover study with each RAP procedure separated by 24 h. Male and female LNK knockout (Lnk−/−) and paired-like homeodomain 2 transcription factor deficient (Pitx2+/−) mice were generated as described previously (22, 23) and maintained on a C57Bl/6J background. AF susceptibility was assessed at 14–16 and 16–18 wk of age for Lnk−/− and Pitx2+/− mice, respectively. Mice were euthanized ∼1–2 wk after each study for tissue procurement. Briefly, mice were anesthetized with isoflurane (3%–4%) until deep anesthesia was achieved. Upon loss of the pedal withdrawal reflex, mice were euthanized by cervical dislocation, which was subsequently confirmed by excision of the heart.
Transesophageal Electrophysiological Studies
All electrophysiological studies were conducted between 9:00 AM and 12:00 PM. Mice were anesthetized with isoflurane (3% for induction, 1% for maintenance) and a surface electrocardiogram (ECG) obtained by subcutaneous insertion of 27-G needles into the forelimbs. For all studies, a 2-Fr octapolar electrode catheter (NuMED; Hopkington, NY) was inserted into the esophagus with proper placement confirmed by atrial capture, followed by measurement of the atrial diastolic capture threshold (TH) when pacing at a stimulus amplitude of twice TH. For hypertension and atropine studies, pacing was performed using a fixed stimulus amplitude of 3 mA with a pulse width of 1 ms as described previously (6). To reduce parasympathetic activation, all other studies were conducted using a stimulus amplitude twice TH with a pulse width of 2 ms in the absence of atropine (8). For burst RAP, mice were subjected to six pacing trains of 15 s each at cycle lengths of 50, 40, 30, 25, 20, and 15 ms as described previously (8). For decremental RAP, an established protocol was used in which mice were subjected to three pacing trains having an initial cycle length of 40 ms that decreased by 2 ms every 2 s to 20 ms, followed by termination (7). Between all pacing trains, there was a recovery time of 30 s. AF was considered present if rapid atrial activity and an irregularly irregular ventricular response occurred lasting for 1 s or more. If an AF episode lasted for 10 min, the study was terminated. Total AF duration, or AF burden, was defined as the sum of all AF episodes induced during a complete protocol.
AV Block Analysis
During RAP, all pacing was initiated at a rate faster than the mouse Wenckebach cycle length (∼70 ms), resulting typically in 2:1 AV conduction. Because we occasionally observed periods of excessive pacing-related AV block during atrial capture causing ventricular bradycardia, methods were developed to detect and quantify this (i.e., AV block) as illustrated in Fig. 1. The average baseline RR interval was determined by analyzing 10 s of the ECG recording during sinus rhythm before pacing. The onset of AV block was defined as either the initial R wave of three consecutively prolonged RR intervals or the initial R wave of an RR interval ≥ 3 baseline RR intervals. AV block was considered to have terminated with the initial R wave of three consecutive RR intervals ≤ the baseline RR interval. If the total time spent in AV block was ≥ 10% of a pacing train duration (e.g., 1.5 s for burst protocol or 2.2 s for decremental) the pacing response was deemed AV block. This definition was compared with the interpretation of two clinical cardiac electrophysiologists to assess accuracy and reproducibility. Each physician reviewed the ECG recordings from 20 mice receiving RAP for AV block in a blinded manner with ambiguous cases reviewed by both clinical electrophysiologists together, and our definition detected 93% of physician-identified AV block with an 8% false-positive rate.
Figure 1.
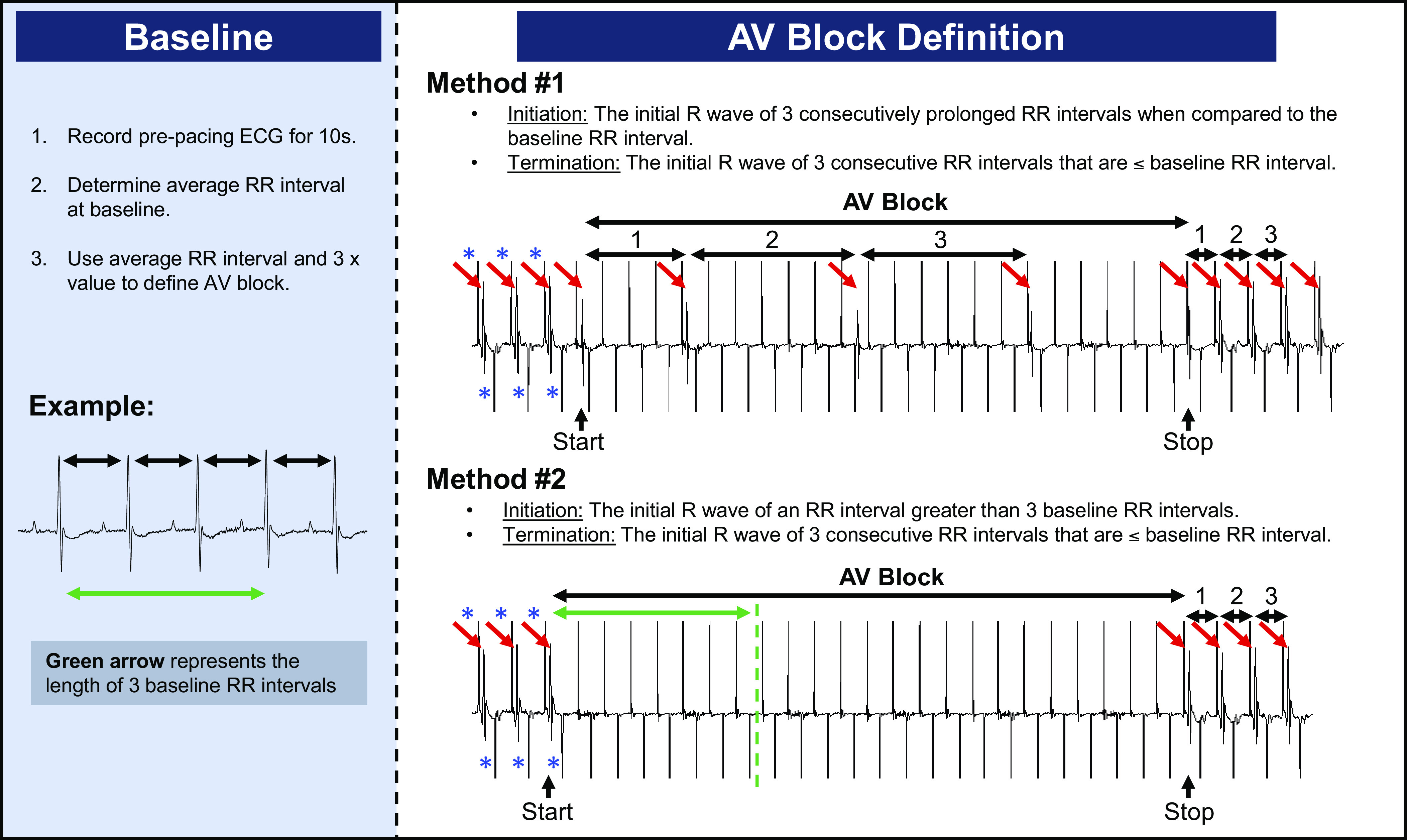
Definition of pacing-induced AV block. ECG recording demonstrating initial 2:1 AV conduction during RAP (rate exceeding Wenckebach cycle length) followed by further loss of AV conduction causing ventricular bradycardia, defined as AV block, and the two methods used to identify this. An unexpectedly prolonged AV block response was defined as total pacing-induced AV block ≥ 10% of the pacing train duration. Red arrows denote QRS complexes and blue asterisks denote atrial pacing spikes. AV, atrioventricular; RAP, rapid atrial pacing.
Statistical Analysis
Data are displayed as means ± SE. Normally distributed data were compared with Student’s t test and nonparametric data by Mann–Whitney U test. Paired categorical data were analyzed by McNemar’s test and unpaired data by Fisher’s exact test. Differences in systolic blood pressure were examined using a two-way ANOVA for repeated measures followed by Sidak’s multiple-comparisons test. GraphPad Prism software version 8.2.1 was used for the analysis. A two-tailed P value < 0.05 was considered statistically significant.
Study Approval
All animal procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee and adhered to the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services.
RESULTS
Fixed Stimulus Amplitude Promotes AV Block and AF Induction in Normotensive Mice during Transesophageal Pacing
In previously published pacing protocols (7, 8, 12) and our own studies, a range of stimulus amplitudes have been employed. To evaluate the utility of a fixed stimulus that would obviate the need for TH testing, we subjected hypertensive and normotensive mice to AF inducibility studies using a burst pacing protocol with a stimulus amplitude of 3 mA that resulted in consistent capture. However, during pacing, obvious and profound AV block was intermittently observed (Fig. 2, A and B; Supplemental Fig. S2) that was not due to the loss of atrial capture (Supplemental Fig. S3). This occurred in at least 80% of mice studied irrespective of phenotype and was most frequent with pacing at shorter cycle lengths (CLs; Supplemental Table S1).
Figure 2.
Fixed stimulus intensity induces AV block and AF in normotensive mice. A: representative tracings of atrial paced rhythm with 2:1 AV conduction (upper) followed by excessive pacing-induced AV block (lower). Red arrows denote QRS complexes. B: incidence of AV block during any pacing train was similar between normotensive (sham; 80%) and hypertensive (ANG II; 93.3%) mice. C: total AF duration was similar between hypertensive and normotensive mice during burst RAP at 3 mA [n = 10, 15 mice; nonsignificant (ns), P = 0.76 by Mann–Whitney U test]. AF, atrial fibrillation; ANG II, angiotensin II; AV, atrioventricular; RAP, rapid atrial pacing.
Atrial pacing-related AV block was often associated with AF induction. Notably, the total AF duration was 184.9 ± 59.3 s in the normotensive sham-treated mice, and there was no difference in burden observed between these and the hypertensive mice (246.0 ± 77.8 s; Fig. 2C). As with AV block, the induction of any AF (i.e., 1 s in duration) and total AF duration were increased as pacing CL was shortened (Supplemental Table S1). These data demonstrate that a fixed stimulus amplitude results in not only exaggerated AV block during pacing but also substantial AF induction in control mice.
Pacing-Induced Parasympathetic Stimulation Mediates AV Block and Promotes AF Susceptibility
To test the hypothesis that these effects were parasympathetically driven, we subjected 10 control mice to burst RAP before and immediately after injection of the muscarinic receptor antagonist atropine. At baseline, six out of 10 mice experienced AV block during pacing, and this was completely prevented by atropine administration (Fig. 3A). Notably, mice had a total AF duration at baseline of 90.3 s, and this was reduced to 33.2 s following atropine administration (Fig. 3B). These data indicate that parasympathetic stimulation is responsible for both pacing-induced AV block and the elevated AF burden in normal mice during transesophageal pacing.
Figure 3.
Pacing-induced AV block and AF induction are mediated by parasympathetic stimulation. A: atropine significantly reduced the occurrence of pacing-induced AV block (n = 10 mice each; P = 0.0001 by McNemar’s test). B: after assessing baseline AF burden, atropine administration reduced AF susceptibility in wild-type mice during burst RAP at 3 mA (n = 10 each). AF, atrial fibrillation; AV, atrioventricular; RAP, rapid atrial pacing.
Incidence of AV Block Is Similar between Burst and Decremental Pacing
To determine whether stimulus amplitude was critical to parasympathetic activation, experiments were performed using an amplitude of twice TH. In addition, a cohort of control mice underwent both burst and decremental RAP in a crossover study to determine if exaggerated pacing-induced AV block was protocol-specific. The stimulus amplitudes were comparable between protocols averaging 1.430 A ± 0.022 mA for burst and 1.425 ± 0.028 mA for decremental (Fig. 4A). Notably, the incidence of excessive AV block during pacing was similar between burst (30%) and decremental (25%) protocols (Fig. 4B) and substantially reduced compared with pacing at 3 mA. These findings indicate that pacing at twice TH decreases the incidence of parasympathetic-mediated AV block regardless of the protocol used and that this response is not pacing mode-specific.
Figure 4.
Incidence of AV block is similar between burst and decremental protocols. A: there was no difference in pacing amplitude during comparison of protocols at twice diastolic threshold (n = 20 mice each). B: incidence of pacing-mediated AV block was similar in wild-type C57Bl/6J mice paced with burst (30%) and decremental (25%) protocols (n = 20 each). AV, atrioventricular.
AF Phenotypes Are Restored at Twice Diastolic Threshold Stimulus Amplitude and Vary by Sex
Based on the reduced incidence of AV block and associated AF, we sought to determine if RAP at twice TH would restore AF phenotypes in two separate mouse models. As noted previously, mice deficient in the lymphocyte adapter protein (Lnk−/−) demonstrate evidence of systemic inflammation with no evidence of structural heart disease (18, 19, 24). Using burst RAP, male Lnk−/− mice displayed a significant increase in total AF duration compared with wild-type littermate controls (Fig. 5A; 109.9 ± 36.0 vs. 19.5 ± 6.5 s, respectively; n = 34, 24; P = 0.017). Notably, decremental pacing failed to provoke an AF phenotype in these mice (Fig. 5B). Moreover, excluding mice with AV block from analysis (Fig. 5, C and D) reduces variability within wild-type controls by eliminating the contribution of vagally mediated AF induction. Based on these results, Lnk−/− mice that experienced AV block associated with AF were excluded from analysis in subsequent experiments.
Figure 5.
Lnk−/− mice display a protocol-specific and sex-specific AF phenotype during pacing at twice diastolic threshold. A: male Lnk−/− mice demonstrated a significant increase in total AF duration compared with wild-type littermate (WT) controls during burst RAP (109.9 ± 36.0 vs. 19.5 ± 6.5 s, respectively; n = 34, 24 mice; P value by Mann–Whitney U test). B: this was not observed between WT controls and male Lnk−/− mice during decremental RAP (52.0 ± 30.9 vs. 78.4 ± 23.1 s, respectively; n = 42, 22). C: removal of male mice with AV block from the analysis reduced variability and excessive AF induction in WT controls for both burst (7.9 ± 2.8 vs. 124.8 ± 43.3 s, WT vs. Lnk−/−; n = 16, 28) and D: decremental RAP (9.9 ± 4.4 vs. 67.5 ± 27.9 s, WT vs. Lnk−/−; n = 15, 30). E: Lnk−/− female mice showed no difference in total AF duration compared with WT controls during burst (57.1 ± 51.0 vs. 11.8 ± 4.3 s, respectively; n = 12, 11) or F: decremental RAP (18.0 ± 5.3 vs. 61.4 ± 52.7 s, respectively; n = 17, 12). AF, atrial fibrillation; AV, atrioventricular; RAP, rapid atrial pacing.
To determine if the AF phenotype was seen in male Lnk−/− mice was dependent upon sex, a cohort of female mice underwent RAP. Compared with wild-type littermate controls, total AF duration was not increased in Lnk−/− females during burst pacing (Fig. 5E; 57.4 ± 51.0 vs. 11.8 ± 4.3 s, respectively; n = 12, 11; P = 0.5). Similarly, decremental pacing failed to elicit an AF phenotype in Lnk−/− females (Fig. 5F).
As male Lnk−/− mice appear to represent a novel mouse model of AF, we investigated the accuracy of the burst and decremental protocols at twice TH using the established PITX2-deficiency mouse model as a positive control (16). Compared with wild-type littermate controls, Pitx2+/− male mice displayed a significant increase in total AF duration during both burst and decremental RAP (Fig. 6, A and B). However, when excluding mice with AV block from analysis only decremental RAP provoked an AF phenotype (Fig. 6, C and D). Importantly, removal of these mice reduced excessive AF induction in control mice. Analogous to Lnk−/− mice, Pitx2+/− females without AV block displayed no difference in AF duration compared with littermate controls during burst (Fig. 6E) or decremental (Fig. 6F) pacing.
Figure 6.
Decremental and burst pacing elicits an AF phenotype in male Pitx2+/− mice that are not seen in females. A: male Pitx2+/− mice showed a significant increase in total AF duration compared with wild-type littermate (WT) controls during burst RAP (145.1 ± 42.1 vs. 52.9 ± 22.6 s, respectively; n = 26, 47 mice; P value by Mann–Whitney U test) and B: decremental RAP (162.3 ± 45.3 vs. 44.2 ± 10.7 s, respectively; n = 25, 46). C: removal of male mice with AV block from the analysis reduced excessive AF induction in WT controls for both burst (9.0 ± 2.8 vs. 76.3 ± 53.0 s, WT vs. Pitx2+/−; n = 11, 28) and D: decremental RAP (34.2 ± 15.3 vs. 169.4 ± 61.2 s, WT vs. Pitx2+/−; n = 16, 27). E: no difference in total AF duration was seen in Pitx2+/− female mice compared with WT littermate controls during burst (67.5 ± 54.2 vs. 8.8 ± 4.1 s, respectively; n = 13, 8) or F: decremental RAP (19.4 ± 7.1 vs. 3.4 ± 2.5 s, respectively; n = 11, 9). AF, atrial fibrillation; AV, atrioventricular; RAP, rapid atrial pacing.
Pacing Protocol Analysis
As the number of pacing trials varies among RAP protocols, we conducted a post hoc analysis to determine the effect of pacing train number on AF burden in Lnk−/− and Pitx2+/− male mice. Notably, during burst pacing, male Lnk−/− mice displayed an increase in total AF duration using pacing trains from 50 ms to 20 ms compared with controls, indicating that a 15 ms train may not be necessary (Supplemental Fig. S4; Supplemental Table S1), whereas Pitx2+/− male mice exhibited no difference. Furthermore, during decremental pacing Pitx2+/− male mice showed a significant increase in AF duration after both the first and second trains were compared with controls, whereas Lnk−/− mice showed no difference (Supplemental Fig. S5; Supplemental Table S2). However, inclusion of a third pacing train further increased statistical significance for both models.
Using both protocols, we also examined the binary endpoint of AF incidence. There were no statistically significant differences between male Lnk−/− mice and WT controls during both burst and decremental pacing (Supplemental Tables S1 and S2). However, Pitx2+/− male mice displayed a trend of increased AF incidence during decremental pacing compared with WT controls (Supplemental Table S2; 82% vs. 48%; n = 16, 27, P = 0.053). Nevertheless, it should be noted that continuous variables such as total AF duration have greater statistical power than categorical data (i.e., incidence of AF).
DISCUSSION
RAP is routinely used to assess AF susceptibility in mice, as mouse models that spontaneously develop AF are rare and typically have structural heart disease (17). Although RAP can be performed with intracardiac or transesophageal pacing, transesophageal RAP is advantageous for being a survival procedure enabling serial testing in an individual animal. However, the use of transesophageal RAP is complicated by numerous protocols reported in the literature (2, 7, 8) and the lack of systematic attempts at protocol comparison and optimization. Due to the occurrence of excessive pacing-induced AV block and variability in total AF duration in control mice, we sought to optimize and compare transesophageal RAP protocols to assess AF susceptibility in both a novel and established mouse model.
Although a previous study compared fixed amplitude with TH-determined pacing in mice (6), only WT C57Bl/6J mice were studied, and AV block during pacing was not reported. Using fixed stimulus amplitude pacing in hypertensive mice, we encountered a major limitation of this method of pacing: the development of AF due to parasympathetic stimulation which obscured the distinction between control and AF susceptible mice. Fortunately, we found that this limitation could be minimized by pacing at twice TH, enabling the detection of AF phenotypes in Lnk−/− and Pitx2+/− mice. Given the increased susceptibility to AF in Lnk−/− mice, additional studies are underway using this model to investigate mechanisms of inflammation-mediated AF in the absence of structural heart disease (24). Taken together, these findings support the use of TH-guided stimulus delivery during transesophageal atrial pacing and exclusion of mice with parasympathetically mediated AF that confounds results.
In addition, we found that it is also critical to individually optimize the pacing mode for each AF model. Our results showed that male Pitx2+/− mice demonstrated an AF phenotype compared with controls during both burst and decremental RAP, whereas only burst RAP elicited AF susceptibility in male Lnk−/− mice. Notably, removal of mice with AV block from analysis reduced excessive AF induction in controls but also prevented burst RAP from detecting an AF phenotype in Pitx2+/− males. However, since the effect size is nearly identical between data showing all Pitx2+/− males (Fig. 6, A and B) and data excluding those with AV block (Fig. 6, C and D), we speculate that this loss of significance seen when excluding mice with AV block is most likely due to the reduced sample size. Importantly, to our knowledge, this is the first report to compare the effect of different pacing modes on AF burden and corroborates the findings of Wang et al. (16) that decremental pacing promotes AF susceptibility in Pitx2+/− mice. Furthermore, the effects of biological sex on AF phenotypes should be assessed during the development of AF models. Murine AF induction studies routinely use males or fail to report the effect of biological sex on AF susceptibility; however, our studies show that Lnk−/− and Pitx2+/− males, and not females, display AF susceptibility. Although the molecular basis of this sex difference remains unknown, multiple studies have demonstrated sex differences in multiple animal models and humans (25, 26).
There are several limitations of our study. Despite being commonly used in murine RAP (8, 11), isoflurane anesthesia can suppress autonomic function (27) and our results may have been different if a general anesthetic with negligible autonomic effects was used. Although our definition of AV block is both sensitive (93% accurate) and specific (8% false-positive rate), it is possible that episodes of AV block were not identified or misclassified. Although the differential response of Lnk−/− and Pitx2+/− mice to burst and decremental pacing modes is intriguing, experiments to investigate mechanisms responsible for this, as well as sex-based differences in AF susceptibility, are beyond the scope of the present study. Finally, although multiple definitions of AF inducibility exist (7, 28), we defined AF inducibility as ≥ 1 s due to its well-established use (10–13).
In summary, our results support the conclusion that multiple parameters should be used to ascertain AF inducibility and facilitate reproducibility in a specific murine model using transesophageal pacing, including the following approach: 1) atrial pacing should be performed at stimulus amplitude no greater than twice TH, 2) mice that exhibit excessive AV block during pacing in conjunction with AF induction should be eliminated from analysis to increase specificity, and 3) the utility of both decremental and burst pacing modes should be examined.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S5: https://doi.org/10.6084/m9.figshare.16926037.v1.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL096844 and HL133127; American Heart Association, Southeast Affiliate Grant 2160035 and National Center Grant 18SFRN34230125 (to K.T.M., basic project principal investigator); and the National Center for Advancing Translational Sciences of NIH Grant UL1 TR000445.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.B.M., K.K., P.J.K., J.V.B., and K.T.M. conceived and designed research; M.B.M., K.K., and P.J.K. performed experiments; M.B.M., K.K., and P.J.K. analyzed data; M.B.M., P.J.K., J.V.B., and K.T.M. interpreted results of experiments; M.B.M. and K.T.M. prepared figures; M.B.M. and K.T.M. drafted manuscript; M.B.M., K.K., P.J.K., T.S., J.C.V.A., J.V.B., and K.T.M. edited and revised manuscript; M.B.M., K.K., P.J.K., T.S., J.C.V.A., J.V.B., and K.T.M. approved final version of manuscript.
REFERENCES
- 1.Sumitomo N, Sakurada H, Taniguchi K, Matsumura M, Abe O, Miyashita M, Kanamaru H, Karasawa K, Ayusawa M, Fukamizu S, Nagaoka I, Horie M, Harada K, Hiraoka M. Association of atrial arrhythmia and sinus node dysfunction in patients with catecholaminergic polymorphic ventricular tachycardia. Circ J 71: 1606–1609, 2007. doi: 10.1253/circj.71.1606. [DOI] [PubMed] [Google Scholar]
- 2.Fukui A, Takahashi N, Nakada C, Masaki T, Kume O, Shinohara T, Teshima Y, Hara M, Saikawa T. Role of leptin signaling in the pathogenesis of angiotensin II-mediated atrial fibrosis and fibrillation. Circ Arrhythm Electrophysiol 6: 402–409, 2013. doi: 10.1161/CIRCEP.111.000104. [DOI] [PubMed] [Google Scholar]
- 3.Savio-Galimberti E, Kannankeril PJ, Wasserman D, Darbar D. Weight loss reduces atrial fibrillation inducibility and burden in severe obesity induced by either high-fat diet or genetic hyperphagia in mice (Abstract). Circulation 132, 2015. doi: 10.1161/circ.132.suppl_3.14729. [DOI] [Google Scholar]
- 4.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Müller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 129: 1276–1285, 2014. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakimoto H, Maguire CT, Kovoor P, Hammer PE, Gehrmann J, Triedman JK, Berul CI. Induction of atrial tachycardia and fibrillation in the mouse heart. Cardiovasc Res 3: 463–473, 2001. doi: 10.1016/s0008-6363(01)00264-4. [DOI] [PubMed] [Google Scholar]
- 6.Schrickel JW, Bielik H, Yang A, Schimpf R, Shlevkov N, Burkhardt D, Meyer R, Grohé C, Fink K, Tiemann K, Lüderitz B, Lewalter T. Induction of atrial fibrillation in mice by rapid transesophageal atrial pacing. Basic Res Cardiol 97: 452–460, 2002. doi: 10.1007/s003950200052. [DOI] [PubMed] [Google Scholar]
- 7.Verheule S, Sato T, Everett T 4th, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-β1. Circ Res 94: 1458–1465, 2004. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faggioni M, Savio-Galimberti E, Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D, Knollmann BC. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2-null hearts. Circ Arrhythm Electrophysiol 7: 313–320, 2014. doi: 10.1161/CIRCEP.113.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XHT. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm 5: 1047–1054, 2008. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circ 125: 2059–2070, 2012. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XHT, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 128: 1748–1757, 2013. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egom EE, Vella K, Hua R, Jansen HJ, Moghtadaei M, Polina I, Bogachev O, Hurnik R, Mackasey M, Rafferty S, Ray G, Rose RA. Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C. J Physiol 593: 1127–1146, 2015. doi: 10.1113/jphysiol.2014.283135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsima KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMarie SA, Schmitz W, Müller FU, El-Armouche A, Eissa NT, Beeton C, Nattel S, Wehrens XHT, Dobrev D, Li N. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circ 138: 2227–2242, 2018. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prinsen JK, Kannankeril PJ, Sidorova TN, Yermalitskaya LV, Boutaud O, Zagol-Ikapitte I, Barnett JV, Murphy MB, Subati T, Stark JM, Christopher IL, Jafarian-Kerman SR, Saleh MA, Norlander AE, Loperena R, Atkinson JB, Fogo AB, Luther JM, Amarnath V, Davies SS, Kirabo A, Madhur MS, Harrison DG, Murray KT. Highly reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC Basic Transl Sci 5: 602–615, 2020. doi: 10.1016/j.jacbts.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCauley MD, Hong L, Sridhar A, Menon A, Perike S, Zhang M, da Silva IB, Yan J, Bonini MG, Ai X, Rehman J, Darbar D. Ion channel and structural remodeling in obesity-mediated atrial fibrillation. Circ Arrhythm Electrophysiol 13: 755–767, 2020. doi: 10.1161/CIRCEP.120.008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XHT, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci USA 107: 9753–9758, 2010. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schüttler D, Bapat A, Kääb S, Lee K, Tomsits P, Clauss S, Hucker WJ. Animal models of atrial fibrillation. Circ Res 127: 91–110, 2020. doi: 10.1161/CIRCRESAHA.120.316366. [DOI] [PubMed] [Google Scholar]
- 18.Devallière J, Charreau B. The adaptor Lnk (SH2B3): an emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem Pharmacol 82: 1391–1402, 2011. doi: 10.1016/j.bcp.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Mori T, Suzuki-Yamazaki N, Takaki S. Lnk/Sh2b3 regulates adipose inflammation and glucose tolerance through group 1 ILCs. Cell Rep 24: 1830–1841, 2018. doi: 10.1016/j.celrep.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 20.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, et al. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 448: 353–357, 2007. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 22.Takaki S, Sauer K, Iritani BM, Chien S, Ebihara Y, Tsuji K, Takatsu K, Perlmutter RM. Control of B cell production by the adaptor protein lnk. Definition of a conserved family of signal-modulating proteins. Immunity. 13: 599–609, 2000. doi: 10.1016/s1074-7613(00)00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisúa-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401: 279–282, 1999. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- 24.Murphy MB, Subati T, Van Amburg JC, Smart CD, Pitzer AL, Kannankeril PJ, Zhenjiang Y, Stark JM, Christopher IL, Kirabo A, Madhur MS, Barnett JV, Roden DM, Murray KT. Abstract 14785: the reactive lipid mediators isolevuglandins promote atrial fibrillation mediated by inflammation. Circulation 142: A14785, 2020. doi: 10.1161/circ.142.suppl_3.14785. [DOI] [Google Scholar]
- 25.Tadros R, Ton A, Fiset C, Nattel S. Sex differences in cardiac electrophysiology and clinical arrhythmias: epidemiology, therapeutics, and mechanisms. Can J Cardiol 30: 783–792, 2014. [Erratum in Can J Cardiol 30: 1244, 2014]. doi: 10.1016/j.cjca.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Du X. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc Res 63: 510–519, 2004. doi: 10.1016/j.cardiores.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Komatsu T, Kimura T, Sugiyama F, Nakashima K, Shimada Y. Spectral analysis of heart rate variability during isoflurane anesthesia. Anesthesiology 7: 669–674, 1992. doi: 10.1097/00000542-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, Wang Q, Farman GP, Yang F, Yang W, Dorian D, Simpson JA, Tuomi JM, Jones DL, Nanthakumar K, Cox B, Wehrens XHT, Dorian P, Backx PH. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat Commun 6: 6018, 2015. doi: 10.1038/ncomms7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S5: https://doi.org/10.6084/m9.figshare.16926037.v1.







