Abstract
Central adiposity is associated with greater sympathetic support of blood pressure. β-adrenergic receptors (β-AR) buffer sympathetically mediated vasoconstriction and β-AR-mediated vasodilation is attenuated in preclinical models of obesity. With this information, we hypothesized β-AR vasodilation would be lower in obese compared with normal weight adults. Because β-AR vasodilation in normal weight adults is limited by cyclooxygenase (COX) restraint of nitric oxide synthase (NOS), we further explored the contributions of COX and NOS to β-AR vasodilation in this cohort. Forearm blood flow (FBF, Doppler ultrasound) and mean arterial blood pressure (MAP, brachial arterial catheter) were measured and forearm vascular conductance (FVC) was calculated (FVC = FBF/MAP). The rise in FVC from baseline (ΔFVC) was quantified during graded brachial artery infusion of isoproterenol (Iso, 1–12 ng/100 g/min) in normal weight (n = 36) and adults with obesity (n = 22) (18–40 yr old). In a subset of participants, Iso-mediated vasodilation was examined before and during inhibition of NOS [NG-monomethyl-l-arginine (l-NMMA)], COX (ketorolac), and NOS + COX (l-NMMA + ketorolac). Iso-mediated increases in FVC did not differ between groups (P = 0.57). l-NMMA attenuated Iso-mediated ΔFVC in normal weight (P = 0.03) but not adults with obesity (P = 0.27). In normal weight adults, ketorolac increased Iso-mediated ΔFVC (P < 0.01) and this response was lost with concurrent l-NMMA (P = 0.67). In contrast, neither ketorolac (P = 0.81) nor ketorolac + l-NMMA (P = 0.40) altered Iso-mediated ΔFVC in adults with obesity. Despite shifts in COX and NOS, β-AR vasodilation is preserved in young adults with obesity. These data highlight the presence of a compensatory shift in microvascular control mechanisms in younger humans with obesity.
NEW & NOTEWORTHY We examined β-adrenergic receptor-mediated vasodilation in skeletal muscle of humans with obesity and normal weight. Results show that despite shifts in the contribution of cyclooxygenase and nitric oxide synthase, β-adrenergic-mediated vasodilation is relatively preserved in young, otherwise healthy adults with obesity. These data highlight the presence of subclinical changes in microvascular control mechanisms early in the obesity process and suggest duration of obesity and/or the addition of primary aging may be necessary for overt dysfunction.
Keywords: blood flow, cyclooxygenase, isoproterenol, nitric oxide, obesity
INTRODUCTION
The incidence of adult obesity has more than doubled in the past 30 years, with over 60% of the US population deemed overweight or obese (1). Obesity is an independent risk factor for the development of a number of cardiovascular disease conditions, including but not limited to hypertension, metabolic syndrome, and coronary artery disease (2). Unfortunately, mechanisms contributing to the development of cardiovascular disease in human obesity are unclear and the majority of available data are confounded by aging and/or age-associated comorbidities such as diabetes and/or hypertension. With this, gaps in understanding remain as to the role of obesity per se in the progression toward overt cardiovascular disease and contributing mechanisms.
The presence of central obesity puts individuals at increased risk for the development of neurogenic hypertension (3–5). Recent work in humans indicates β-adrenergic receptors play an important role in mitigating sympathetically mediated vasoconstriction, and thus sympathetic support of blood pressure (6). Given central adiposity is associated with greater autonomic support of blood pressure (7), it is reasonable to speculate that humans with obesity may display attenuated vascular responsiveness to β-adrenergic receptor stimulation. In support of this idea, data from preclinical rodent models show attenuated β-adrenergic-mediated vasodilation with obesity (8, 9), although controversy exists (10). Similarly, there is disagreement in the human literature regarding the impact of obesity and related conditions on β-adrenergic-mediated vasodilation (11–14). Part of this discrepancy may be due to differences in age and/or progressive disease state of the individuals studied (e.g., obesity vs. overt diabetes). Thus, it remains unclear whether humans with obesity display attenuated vasodilatory responses to vascular β-adrenergic receptor stimulation. Such questions are of major importance given impairments in β-adrenergic receptor-mediated vasodilation with obesity may augment total peripheral resistance, contributing to impaired blood pressure recovery following acute stress (8) and ultimately increased cardiovascular disease risk (15–17).
β-Adrenergic-mediated dilation is thought to be primarily endothelium-dependent with data supporting endothelial nitric oxide synthase (NOS) accounts for anywhere from 0% to 60% of the vasodilatory response to β-adrenoceptor stimulation (18–24). The reason behind this wide range (0–60%) may include a variable role of cyclooxygenase (COX) in β-adrenergic-mediated dilation. Recently, our group (22) and others (25) have shown COX restrains NOS-mediated vasodilation in response to β-adrenergic receptor stimulation in healthy humans. With this, we speculate the level of COX restraint of NOS following β-adrenergic receptor stimulation could contribute to the wide-ranging role of NOS in the literature. This may be especially true in the context of obesity, where COX-dependent vasoconstriction has been shown to contribute to endothelial dysfunction in adipose and renal circulations (26, 27).
With this information in mind, we aimed to determine the effect of obesity on the skeletal muscle microvascular response to β-adrenergic receptor stimulation in otherwise healthy adults. We hypothesized that young adults with obesity would exhibit blunted vasodilator responses to β-adrenoceptor stimulation compared with young normal weight adults. Because β-mediated vasodilation in normal weight adults is limited by COX restraint of NOS (22, 25), we further explored these mechanisms in the present cohort.
MATERIALS AND METHODS
Fifty-eight young (18–40 yr) normal weight adults (n = 36) and adults with obesity (n = 22) participated in the current study (Table 1, Fig. 1). Isoproterenol (Iso) data from a subset of normal weight participants were previously published (22, 28). All participants reported being healthy, nonsmokers, and were taking no medications known to alter cardiovascular physiology. Normal weight individuals were required to have a body mass index (BMI) <25 kg/m2 (range 18.0–24.7 kg/m2) and individuals with obesity exhibited a BMI > 30 kg/m2 (range 30.4–47.7 kg/m2).
Table 1.
Participant demographics
| Normal Weight | Obese | P Value | |
|---|---|---|---|
| n | 36 | 22 | |
| %Female (male/female) | 53 (17/19) | 68 (7/15) | 0.248 |
| Age, yr | 26 ± 6 | 29 ± 6 | 0.115 |
| Height, cm | 171 ± 8 | 170 ± 10 | 0.606 |
| Weight, kg | 64 ± 8 | 106 ± 19 | <0.001 |
| Waist circumference, cm | 77 ± 7 | 111 ± 14 | <0.001 |
| Body mass index, kg/m2 | 22 ± 2 | 36 ± 5 | <0.001 |
| Body fat, % | 26 ± 9 | 47 ± 7 | <0.001 |
| Lean forearm mass, g | 760 ± 208 | 877 ± 272 | 0.128 |
| Glucose, mg/dL | 68 ± 8 | 71 ± 7 | 0.068 |
| Insulin, μIU/mL | 8.7 ± 2.9 | 16.0 ± 4.2 | <0.001 |
| HOMA-IR | 1.5 ± 0.5 | 2.9 ± 0.6 | <0.001 |
| Total cholesterol, mg/dL | 150 ± 23 | 156 ± 22 | 0.314 |
| Systolic blood pressure, mmHg | 110 ± 6 | 123 ± 6 | <0.001 |
| Diastolic blood pressure, mmHg | 66 ± 7 | 73 ± 7 | <0.001 |
| Forearm blood flow, mL/min/100 g | 6.2 ± 2.8 | 8.1 ± 4.5 | 0.078 |
| Forearm vascular conductance, mL/min/100 g/100 mmHg | 7.5 ± 3.2 | 9.2 ± 5.6 | 0.461 |
Values are means ± SD. Body fat and waist circumference (n = 35 normal weight). Insulin and homeostatic model assessment for insulin resistance (HOMA-IR; n = 19 normal weight; n = 11 obese). %Female compared using χ2 test. All other variables compared using one-way analysis of variance or Kruskal–Wallis one-way analysis of variance on ranks (normality, Shapiro–Wilk; equal variance, Brown–Forsythe).
Figure 1.
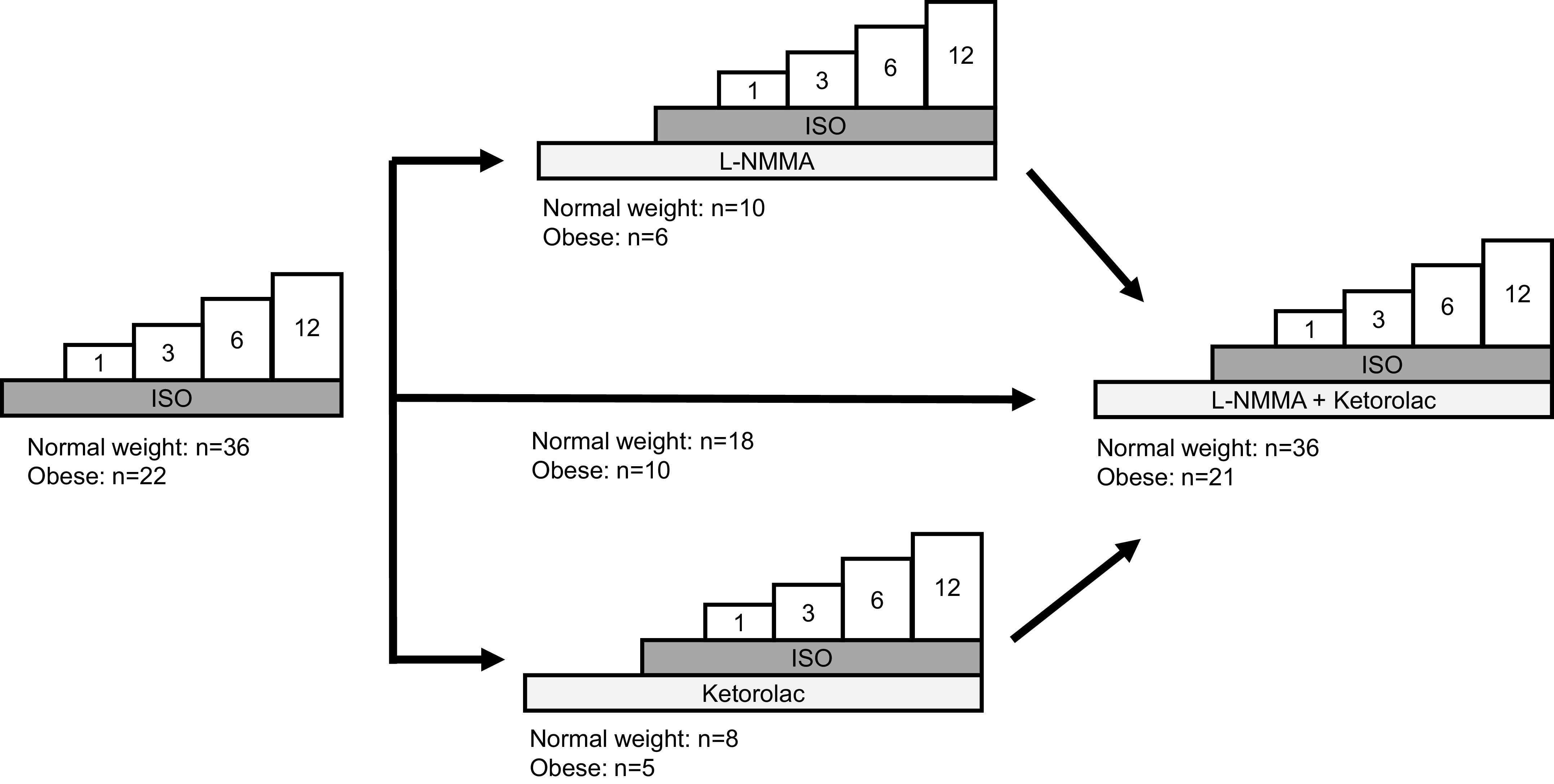
Study protocol. All participants completed an isoproterenol (Iso) dose-response test (1, 3, 6, and 12 ng/100 g/min). Following a washout period, a subset of participants received a loading dose of NG-monomethyl-l-arginine (l-NMMA) or ketorolac, followed by a continuous maintenance dose. Iso infusions were then repeated. After final washout period, a loading dose of the second inhibitor (ketorolac or l-NMMA) was administered and Iso infusions were repeated a final time.
Women were not pregnant (confirmed via negative urine test on study day) and were studied during the early follicular phase (days 1–5) of the menstrual cycle or placebo phase of oral contraceptive use as determined by self-report (oral contraceptive use: normal weight, n = 4/19, 21%; obese, n = 5/15, 33%). Study participants were asked to undergo a minimum 10-h fast, and refrain from exercise, nonsteroidal anti-inflammatory drugs, alcohol, and caffeine for 24 h before the study visit. Written informed consent was obtained from all participants. All procedures were approved by the Institutional Review Board at the University of Wisconsin-Madison and conformed to the standards set by the Declaration of Helsinki except for registration in a database.
Following informed consent, participants completed a fasted blood draw for measures of glucose, insulin, and total cholesterol (CardioChek; PTS Panels, Indianapolis, IN). Participant height, weight, and waist circumference were measured, and BMI (kg/m2) was calculated. Body fat and lean forearm mass were determined via dual energy X-ray absorptiometry (DEXA; GE Lunar Prodigy, GE Healthcare, Milwaukee, WI). Resting blood pressure was measured in the supine position using an automated upper arm cuff. To ensure both normal weight and participants with obesity were otherwise healthy, individuals exhibiting overt metabolic (e.g., fasting glucose > 100 mg/dL, total cholesterol > 200 mg/dL, triglycerides > 150 mg/dL) and/or cardiovascular (e.g., blood pressure: normal weight adults > 120/80 mmHg, adults with obesity > 140/90 mmHg) abnormalities were excluded.
Forearm blood flow (artery diameter, blood velocity) was measured using Doppler ultrasound (Vivid 7, General Electric; Milwaukee, WI) with the participant supine using methods previously published (22, 28). The Doppler audio information was converted into a real-time digital flow velocity signal using fast-Fourier transform (29), and all data were recorded continuously on a computer and analyzed offline (PowerLab Chart5, ADInstruments, Colorado Springs, CO). Beat-to-beat brachial artery blood velocity, heart rate (ECG, Datex-Ohmeda; Helsinki, Finland), and blood pressure (Arterial catheter; Transpac IV Monitoring Kit; ICU medical; San Clemente, CA) were obtained throughout each trial. Brachial artery diameters were measured at the end of each measurement period and assessed offline from B-mode images, and the median of five measurements in late diastole was used to calculate blood flow. Blood flow was calculated as the product of mean blood velocity (cm/s) and vessel cross-sectional area (radius in cm2). Blood flow values were normalized for lean forearm mass (mL/min/100 g). To account for potential changes in blood pressure and assess vasodilation, vascular conductance (mL/min/100 g/100 mmHg) was calculated (blood flow ÷ mean arterial blood pressure × 100).
All pharmacological agents were infused via a brachial artery catheter placed in the antecubital fossa. Isoproterenol (Iso; isoproterenol hydrochloride injection, Marathon Pharmaceuticals, LLC) was infused at four separate doses (1, 3, 6, and 12 ng/100 g/min), similar to those previously published (21, 22, 28, 30, 31). NG-monomethyl-l-arginine (l-NMMA acetate, Clinalfa BaChem) was infused at a rate of 10 mg/min over 5 min, followed by a 1 mg/min maintenance dose (22, 32). Ketorolac (ketorolac tromethamine, Wockhardt, Limited) was infused at a rate of 1.2 mg/min for 5 min, followed by a 0.1 mg/min maintenance dose (22, 32). The doses of l-NMMA and ketorolac were selected based on previous work, which has shown successful blockade (33–35).
Three study protocols were conducted (Fig. 1). Two minutes of resting data were acquired at the start of each trial. Each dose of Iso was infused for 3 min, for a total infusion length of 12 min (protocol 1). After a minimum of 10 min of quiet rest, participants received a loading dose of l-NMMA (protocol 2) or ketorolac (protocol 3) administered over 5 min, followed by a continuous maintenance dose, an approach shown to reduce endothelium dependent dilation (36, 37). The maintenance dose was given for a minimum of 20 min before repeated infusion of Iso. After a minimum 10-min Iso washout, a loading dose of the second inhibitor (ketorolac or l-NMMA) was administered over 5 min, followed by a continuous maintenance dose (given for a minimum of 20 min before additional testing). Iso infusions were then repeated (double blockade). Data from the Iso alone (protocol 1) and Iso + l-NMMA + ketorolac (double blockade) trials were pooled across protocols for analysis (see Fig. 1). In the instance where participants completed multiple study visits/protocols, Iso alone and double blockade trials from each visit were averaged within individuals before data were pooled. Importantly, vasodilatory responses to Iso in these individuals were comparable between visits [forearm vascular conductance (FVC), area under the curve, visit 1 vs. visit 2: R = 0.84, P < 0.01] and serve as an important time control.
To account for any group differences in resting vascular conductance, the main dependent variable was a change in lean forearm vascular conductance (FVC) from baseline levels (ΔFVC; FVCinfusion – FVCbaseline). The primary analysis was to examine differences in Iso-mediated vasodilation (ΔFVC) between normal weight and participants with obesity (protocol 1). Area under the curve (AUC) from ΔFVC data was calculated and compared between conditions. Sex differences in ΔFVCAUC were not detected in either group for any of the study interventions (P value = 0.132–0.975); thus data from both sexes were pooled. Sample size was determined a priori by a power test equation with α = 0.05 and power = 0.80, using group differences from previously published data (19, 31, 38, 39). Protocol 1 (primary analysis) required a minimum of 13 participants per group, which was exceeded (31).
Statistical analysis was completed using SigmaPlot 14.0 (Systat Software, Inc.). Participant characteristics were compared across groups using a one-way analysis of variance (ANOVA) or Kruskal–Wallis one-way ANOVA on ranks (normality: Shapiro–Wilk; equal variance: Brown–Forsythe; multiple comparisons: Dunn’s method). ΔFVC data were analyzed using a two-way repeated-measures ANOVA to determine the significance of the fixed effect of group, dose, and/or blockade on parameters of interest, with supplemental pairwise comparisons performed using the Holm–Sidak method. Effect size analysis (Cohen’s d) was conducted on area-under-the-curve data. Changes in heart rate, mean arterial blood pressure, forearm blood flow, and brachial artery diameter from baseline (Δ) were analyzed using a two-way repeated-measures ANOVA to determine the significance of the fixed effect of dose and/or blockade. All data are presented as means ± SD and significance was determined a priori at P < 0.05.
RESULTS
Participant demographics are reported in Table 1. Groups were not different in regard to age, height, fasting glucose, or total cholesterol (P > 0.05). By design, individuals with obesity exhibited significantly greater BMI and waist circumference, as well as weight and percent body fat when compared with normal weight controls (P < 0.05). Fasting insulin, homeostatic model assessment for insulin resistance (HOMA-IR), and resting systolic and diastolic blood pressures were elevated in individuals with obesity compared with normal weight controls (Table 1).
Iso infusion resulted in a dose-dependent increase in forearm blood flow (main effect of Iso, P = 0.014) and vascular conductance (main effect of Iso, P < 0.001; Fig. 2). The Iso-mediated increases in blood flow and vascular conductance were not different between groups (interaction of group and Iso, P = 0.194 and P = 0.569, respectively) and, if anything, vascular conductance was greater in obese compared with normal weight adults (main effect of group, P = 0.040). ΔFVC area-under-the-curve data support higher vascular conductance in obese (139 ± 62 AU) compared with normal weight adults (108 ± 54 AU; P = 0.044; Cohen’s d = 0.533).
Figure 2.
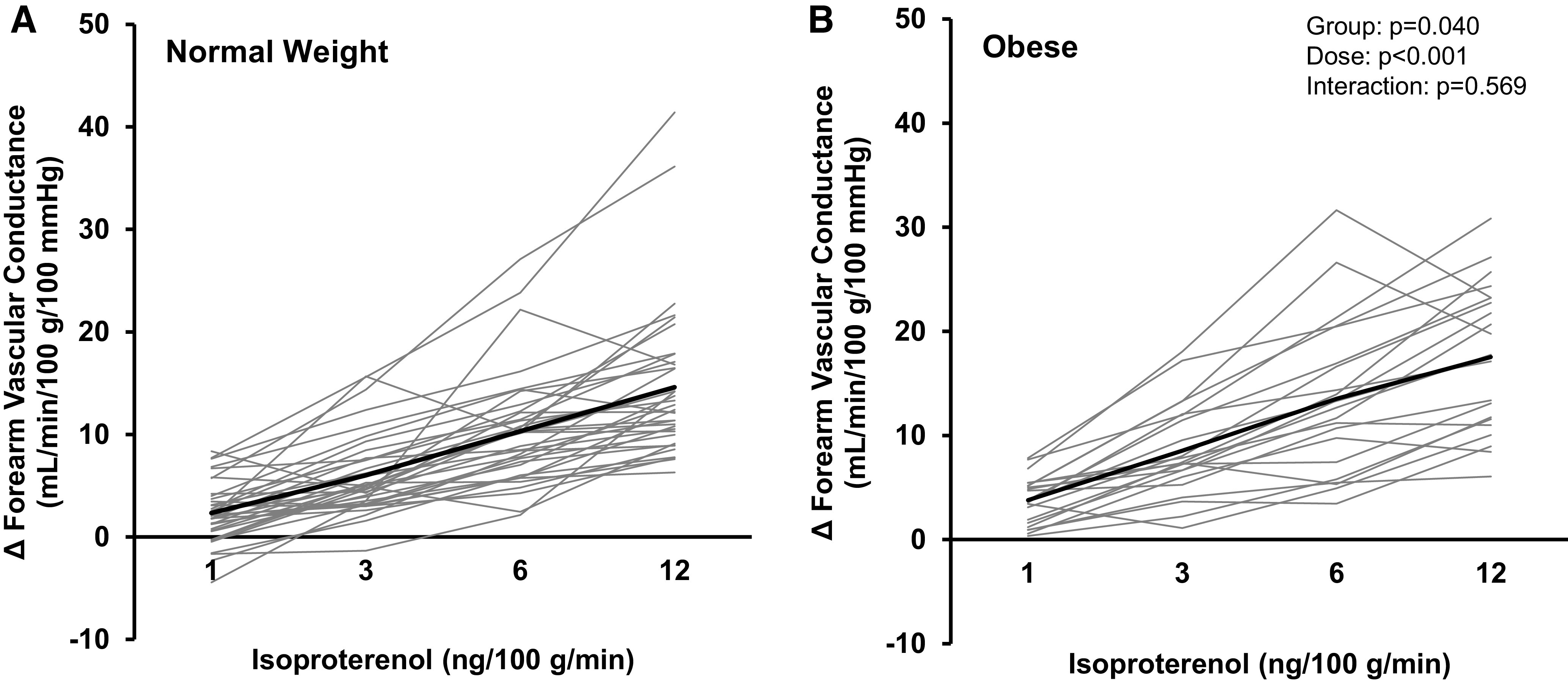
Forearm vascular response to β-adrenergic receptor stimulation. All participants (A: normal weight, n = 36, 17 males/19 females; B: obese, n = 22, 7 males/15 females) completed an isoproterenol (Iso) dose-response test (1, 3, 6, and 12 ng/100 g/min). Both groups (normal weight, obese) exhibited an increase in forearm vascular conductance during Iso infusion (main effect of Iso, P < 0.001) and the magnitude of increase did not differ between groups (interaction of group and Iso, P = 0.569). Solid black lines = group average. Light gray lines = individual data. Two-way repeated-measures analysis of variance.
In a subset of participants (normal weight n = 10, obese n = 6), coinfusion of l-NMMA resulted in a reduction in Iso-mediated blood flow (interaction of Iso and l-NMMA, P = 0.027; Table 2) and vascular conductance (main effect of l-NMMA, P = 0.028) in normal weight adults (Fig. 3A). In contrast, there was no effect of l-NMMA on Iso-mediated blood flow (main effect of l-NMMA, P = 0.704; Table 2) or vascular conductance (main effect of l-NMMA, P = 0.347) in adults with obesity (Fig. 3B). ΔFVC area-under-the-curve data confirm group differences in the effect of l-NMMA on Iso-mediated vasodilation to be moderate-to-high (normal weight: 140 ± 71 to 99 ± 64 AU, P = 0.002; obese: 110 ± 44 to 89 ± 30 AU, P = 0.320; AUC % control: normal weight 70 ± 20%, obese 93 ± 55%; Cohen’s d = 0.558).
Table 2.
Contribution of NOS to the hemodynamic response to Iso
| 1 ng/100 g/min | 3 ng/100 g/min | 6 ng/100 g/min | 12 ng/100 g/min | Main Effect of Iso | Main Effect of l-NMMA | Interaction | |
|---|---|---|---|---|---|---|---|
| Brachial artery diameter, cm | |||||||
| Normal weight | |||||||
| Control | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.001 | 0.026 | |
| l-NMMA | 0.00 ± 0.01 | 0.00 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.02 | |||
| Obese | |||||||
| Control | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.010 | 0.738 | |
| l-NMMA | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | |||
| Forearm blood flow, mL/min/100 g | |||||||
| Normal weight | |||||||
| Control | 1.5 ± 3.0 | 6.8 ± 4.8 | 11.6 ± 5.7 | 17.1 ± 10.8 | <0.001 | 0.075 | 0.027 |
| l-NMMA | 2.1 ± 3.4 | 5.9 ± 6.3 | 7.9 ± 5.1* | 12.2 ± 7.0* | |||
| Obese | |||||||
| Control | 2.6 ± 1.6 | 5.4 ± 3.8 | 9.4 ± 4.4 | 14.4 ± 5.4 | <0.001 | 0.704 | |
| l-NMMA | 2.8 ± 3.1 | 5.4 ± 1.5 | 8.7 ± 3.5 | 12.4 ± 5.1 | |||
| Mean arterial blood pressure, mmHg | |||||||
| Normal weight | |||||||
| Control | 1 ± 2 | 1 ± 4 | 0 ± 6 | 1 ± 9 | 0.831 | 0.554 | |
| l-NMMA | −1 ± 2 | 0 ± 5 | 0 ± 3 | 1 ± 5 | |||
| Obese | |||||||
| Control | −1 ± 3 | 0 ± 2 | −1 ± 2 | 2 ± 4 | 0.171 | 0.266 | |
| l-NMMA | 0 ± 4 | 1 ± 3 | 2 ± 5 | 3 ± 7 | |||
| Heart rate, beats/min | |||||||
| Normal weight | |||||||
| Control | 2 ± 9 | 1 ± 7 | 1 ± 6 | 6 ± 6 | 0.177 | 0.214 | |
| l-NMMA | −6 ± 12 | −3 ± 13 | −1 ± 13 | −2 ± 13 | |||
| Obese | |||||||
| Control | −1 ± 8 | −2 ± 8 | −1 ± 5 | 3 ± 8 | 0.032 | 0.863 | |
| l-NMMA | 0 ± 8 | 0 ± 4 | 1 ± 7 | 2 ± 6 |
Values are changes from baseline and are means ± SD from a subset of participants: normal weight (n = 10; 3 males/7 females) and obese (n = 6; 2 males/4 females). Two-way repeated-measures ANOVA. From the initial model that included the isoproterenol (Iso)-by-NG-monomethyl-l-arginine (L-NMMA) interaction effect, no significant interaction was detected (P > 0.05). *P < 0.05 vs. control. NOS, nitric oxide synthase.
Figure 3.
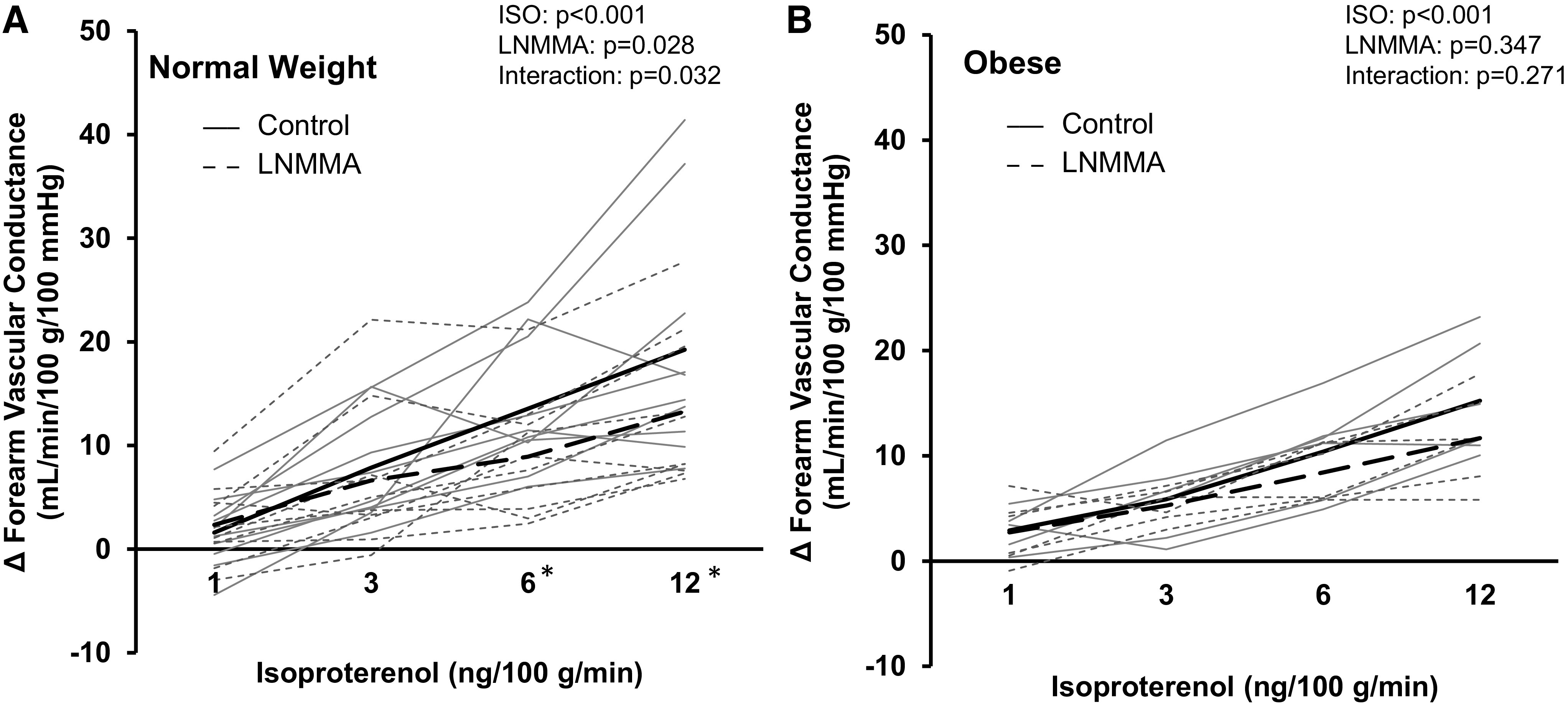
Contribution of nitric oxide synthase (NOS) to the forearm vascular response to β-adrenergic receptor stimulation. Data are reported from a subset of participants (A: normal weight, n = 10, 3 males/7 females; B: obese, n = 6, 2 males/4 females). Both groups (normal weight, obese) exhibited an increase in forearm vascular conductance during Iso infusion (main effect of Iso, P < 0.001). Coinfusion of NG-monomethyl-l-arginine (l-NMMA; NOS blockade) elicited a reduction in forearm vascular conductance in normal weight (main effect of l-NMMA, P = 0.028) but not adults with obesity (P = 0.347). Solid black lines = group average control. Dashed black lines = group average l-NMMA. Solid gray lines = individual data control. Dashed gray lines = individual data l-NMMA. Two-way repeated-measures analysis of variance. *P < 0.05 control vs. l-NMMA within Iso dose. Iso, isoproterenol.
In a second subset of participants (normal weight n = 8, obese n = 5), normal weight adults exhibited a significant increase in Iso-mediated blood flow and vascular conductance with ketorolac (main effect of ketorolac, P = 0.015 and P = 0.009, respectively; Table 3, Fig. 4A). Any effect of ketorolac on blood flow or vascular conductance in normal weight adults was lost with concurrent l-NMMA infusion (P = 0.133 and P = 0.027, respectively; Table 4, Fig. 5A). In contrast, no effect of ketorolac on Iso-mediated blood flow or vascular conductance was observed in adults with obesity (main effect of ketorolac, P = 0.079 and P = 0.120; Table 3, Fig. 4B) and double blockade (l-NMMA + ketorolac) did not significantly alter Iso-mediated changes in forearm blood flow or vascular conductance (P = 0.139 and P = 0.063; Table 4, Fig. 5B). ΔFVC area-under-the-curve data confirm group differences in the effect of ketorolac on Iso-mediated vasodilation to be moderate to high (normal weight: 107 ± 81 to 211 ± 139 AU, P = 0.008; obese: 125 ± 69 to 161 ± 60 AU, P = 0.073; AUC % control: normal weight 214 ± 96%, obese 146 ± 53%; Cohen’s d = 0.877).
Table 3.
Contribution of COX to the hemodynamic response to Iso
| 1 ng/100 g/min | 3 ng/100 g/min | 6 ng/100 g/min | 12 ng/100 g/min | Main Effect of Iso | Main Effect of Ketorolac | Interaction | |
|---|---|---|---|---|---|---|---|
| Brachial artery diameter, cm | |||||||
| Normal weight | |||||||
| Control | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.062 | 0.258 | |
| Ketorolac | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.03 ± 0.02 | |||
| Obese | |||||||
| Control | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | <0.001 | 0.962 | |
| Ketorolac | 0.01 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | |||
| Forearm blood flow, mL/min/100 g | |||||||
| Normal weight | |||||||
| Control | 1.6 ± 2.1 | 4.5 ± 3.0 | 8.4 ± 6.9 | 11.0 ± 6.2 | <0.001 | 0.015 | 0.003 |
| Ketorolac | 3.2 ± 3.6 | 9.8 ± 6.9* | 15.5 ± 12.9* | 20.9 ± 13.3* | |||
| Obese | |||||||
| Control | 4.0 ± 1.9 | 6.4 ± 2.2 | 9.6 ± 7.0 | 13.5 ± 5.4 | <0.001 | 0.079 | |
| Ketorolac | 4.5 ± 3.5 | 8.5 ± 2.1 | 12.0 ± 2.1 | 18.1 ± 7.7 | |||
| Mean arterial blood pressure, mmHg | |||||||
| Normal weight | |||||||
| Control | 1 ± 8 | 2 ± 8 | 0 ± 8 | -1 ± 8 | 0.005 | 0.280 | |
| Ketorolac | −1 ± 1 | −2 ± 2 | −3 ± 2 | −3 ± 2 | |||
| Obese | |||||||
| Control | −1 ± 1 | −2 ± 1 | −1 ± 5 | −3 ± 5 | 0.440 | 0.400 | |
| Ketorolac | −5 ± 6 | −5 ± 6 | −6 ± 10 | −6 ± 10 | |||
| Heart rate, beats/min | |||||||
| Normal weight | |||||||
| Control | −3 ± 15 | −4 ± 13 | −3 ± 15 | −3 ± 15 | 0.224 | 0.530 | |
| Ketorolac | −2 ± 8 | 0 ± 2 | 0 ± 5 | 3 ± 7 | |||
| Obese | |||||||
| Control | −4 ± 3 | −4 ± 2 | −4 ± 3 | −3 ± 3 | 0.855 | 0.801 | |
| Ketorolac | −3 ± 6 | −4 ± 4 | −4 ± 12 | −7 ± 6 |
Values are changes from baseline and are means ± SD from a subset of participants: normal weight (n = 8; 3 males/5 females) and obese (n = 5; 3 males/2 females). Two-way repeated-measures ANOVA. From the initial model that included the isoproterenol (Iso)-by-ketorolac interaction effect, no significant interaction was detected (P > 0.05). *P < 0.05 vs. control. COX, cyclooxygenase.
Figure 4.
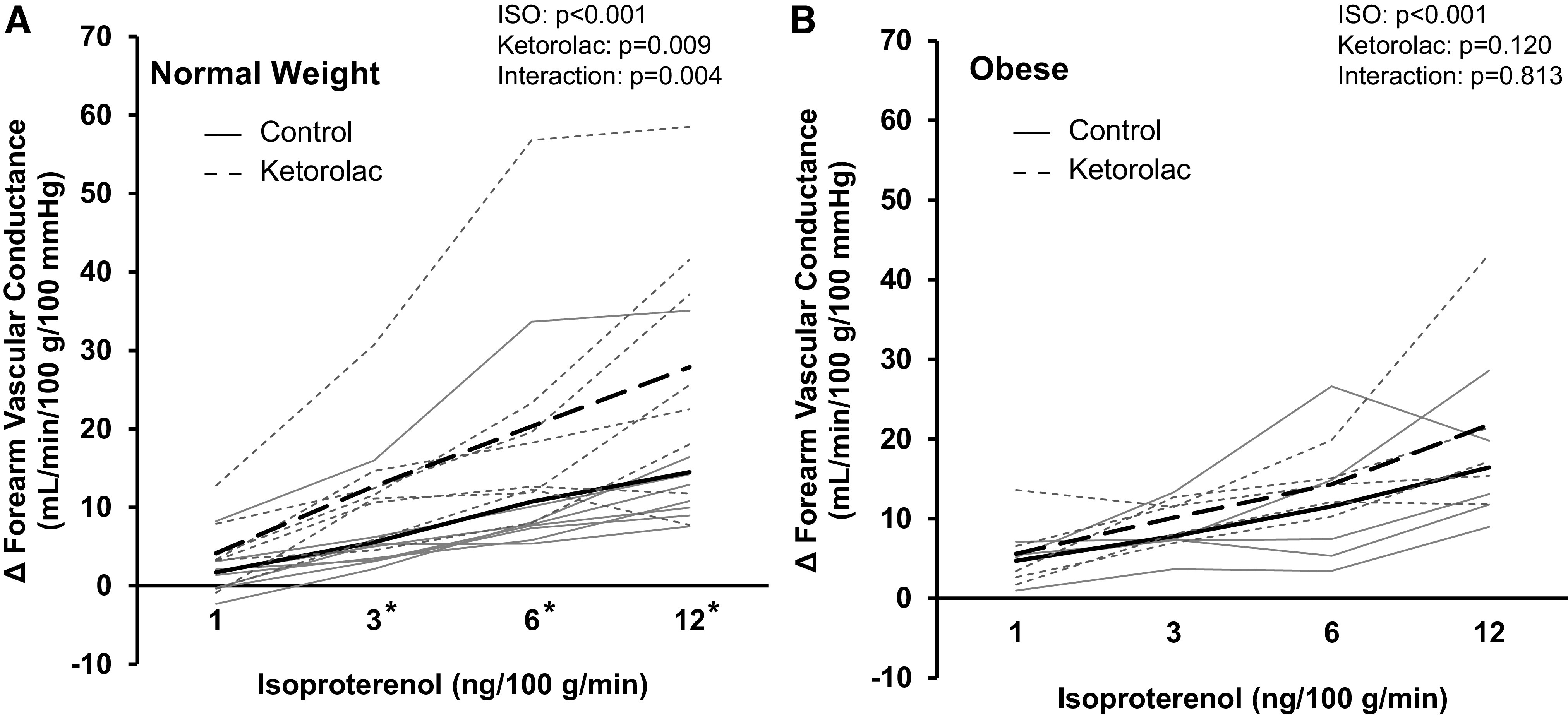
Contribution of cyclooxygenase (COX) to the forearm vascular response to β-adrenergic receptor stimulation. Data are reported from a subset of participants (A: normal weight, n = 8, 3 males/5 females; B: obese, n = 5, 3 males/2 females). Both groups (normal weight, obese) exhibited an increase in forearm vascular conductance during Iso infusion (main effect of Iso, P < 0.001). Coinfusion of ketorolac (COX blockade) elicited an increase in forearm vascular conductance in normal weight (main effect of ketorolac, P = 0.009) but not adults with obesity (P = 0.120). Solid black lines = group average control. Dashed black lines = group average ketorolac. Solid gray lines = individual data control. Dashed gray lines = individual data ketorolac. Two-way repeated-measures analysis of variance. *P < 0.05 control vs. ketorolac within dose. Iso, isoproterenol.
Table 4.
Contribution of NOS and COX to the hemodynamic response to Iso
| 1 ng/100 g/min | 3 ng/100 g/min | 6 ng/100 g/min | 12 ng/100 g/min | Main Effect of Iso | Main Effect of DB | |
|---|---|---|---|---|---|---|
| Brachial artery diameter, cm | ||||||
| Normal weight | ||||||
| Control | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | <0.001 | 0.450 |
| DB | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.01 ± 0.02 | 0.02 ± 0.02 | ||
| Obese | ||||||
| Control | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.02 | <0.001 | 0.050 |
| DB | 0.00 ± 0.01 | 0.00 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | ||
| Forearm blood flow, mL/min/100 g | ||||||
| Normal weight | ||||||
| Control | 2.0 ± 2.4 | 5.0 ± 3.3 | 8.5 ± 4.5 | 12.1 ± 6.5 | <0.001 | 0.133 |
| DB | 1.3 ± 2.0 | 4.3 ± 3.1 | 7.5 ± 5.1 | 11.8 ± 6.7 | ||
| Obese | ||||||
| Control | 3.4 ± 2.1 | 7.5 ± 3.9 | 11.9 ± 6.2 | 15.4 ± 6.0 | <0.001 | 0.139 |
| DB | 1.5 ± 3.5 | 5.4 ± 4.9 | 9.3 ± 6.3 | 15.2 ± 8.2 | ||
| Mean arterial blood pressure, mmHg | ||||||
| Normal weight | ||||||
| Control | 0 ± 2 | −1 ± 3 | −1 ± 4 | −1 ± 5 | 0.422 | 0.336 |
| DB | 0 ± 3 | −1 ± 4 | 0 ± 4 | 1 ± 4 | ||
| Obese | ||||||
| Control | −1 ± 3 | −1 ± 2 | −1 ± 3 | −1 ± 5 | 0.756 | 0.900 |
| DB | −1 ± 3 | −2 ± 3 | −1 ± 4 | −1 ± 4 | ||
| Heart rate, beats/min | ||||||
| Normal weight | ||||||
| Control | −2 ± 12 | −3 ± 11 | −2 ± 11 | 0 ± 12 | 0.855 | 0.649 |
| DB | −1 ± 7 | 0 ± 8 | 1 ± 11 | −2 ± 17 | ||
| Obese | ||||||
| Control | −2 ± 5 | 0 ± 6 | 1 ± 6 | 1 ± 6 | 0.035 | 0.828 |
| DB | 0 ± 6 | −2 ± 5 | 0 ± 5 | 1 ± 4 |
Values are changes from baseline and are means ± SD from 36 (17 males/19 females) normal weight and 21 (7 males/14 females) participants with obesity. Two-way repeated-measures ANOVA. From the initial model that included the isoproterenol (iso)-by-double blockade (DB) interaction effect, no significant interaction was detected (P > 0.05). COX, cyclooxygenase; NOS, nitric oxide synthase.
Figure 5.
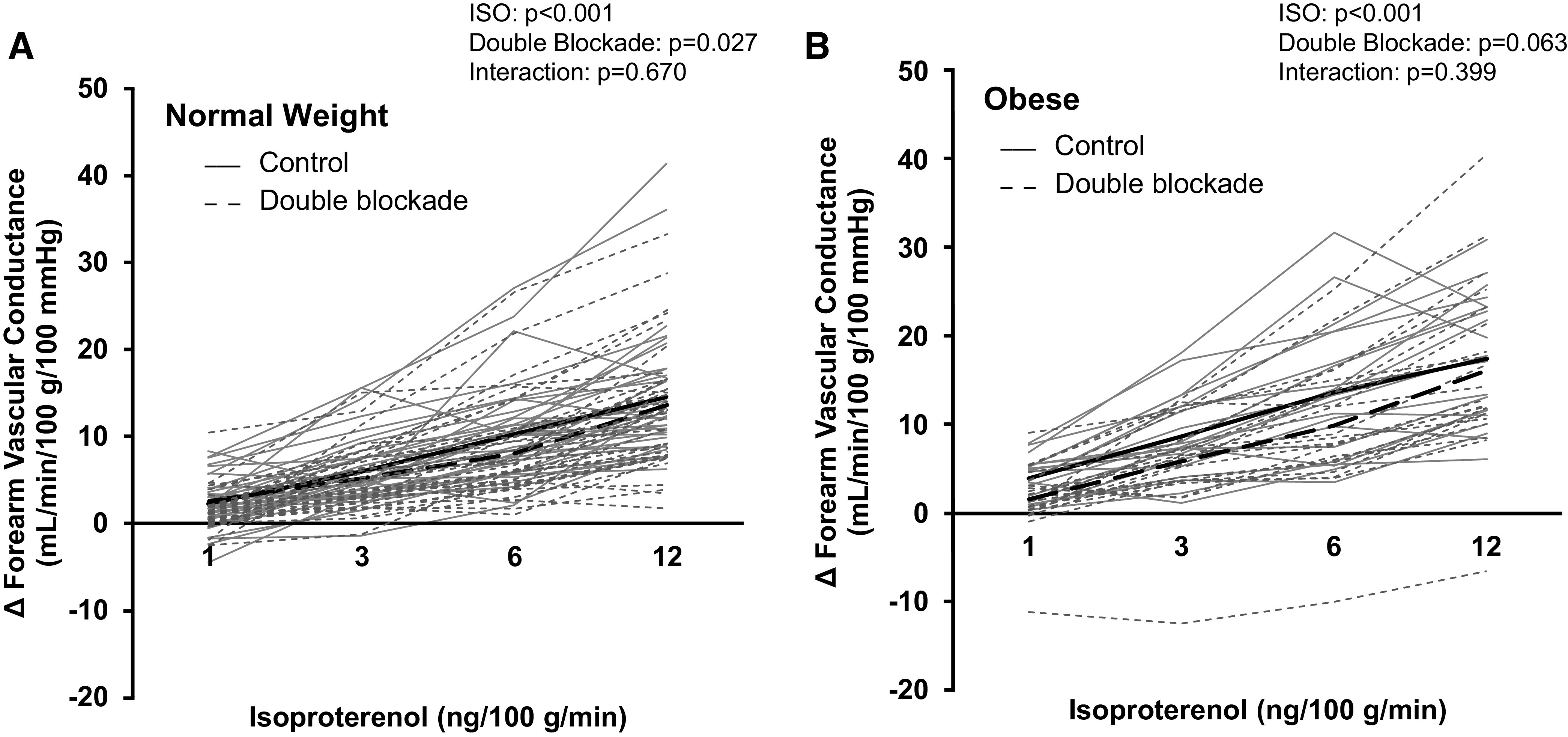
Contribution of NOS and COX to the forearm vascular response to β-adrenergic receptor stimulation. Data are reported from 36 (17 males/19 females) normal weight (A) and 21 (7 males/14 females) participants with obesity (B). Both groups (normal weight, obese) exhibited an increase in forearm vascular conductance during Iso infusion (main effect of Iso, P < 0.001). Double blockade [NG-monomethyl-l-arginine (l-NMMA) + ketorolac] did not significantly alter Iso-mediated changes in forearm vascular conductance in normal weight (interaction of Iso and blockade, P = 0.670) or adults with obesity (P = 0.399). Solid black lines = group average control. Dashed black lines = group average double blockade. Solid gray lines = individual data control. Dashed gray lines = individual data double blockade. Two-way repeated-measures analysis of variance. COX, cyclooxygenase; Iso, isoproterenol; NOS, nitric oxide synthase.
Iso-infusion resulted in an increase in brachial artery diameter in both normal weight and adults with obesity (main effect of Iso, P > 0.05; Tables 2–4). Any Iso-mediated change in brachial artery diameter was attenuated in normal weight adults during l-NMMA infusion (main effect of l-NMMA, P = 0.026), with no effect in adults with obesity (main effect of l-NMMA, P = 0.738). Neither ketorolac nor double blockade (l-NMMA + ketorolac) had any effect on brachial artery diameter in either group (P > 0.05, Tables 2–4). Mean arterial blood pressure was unaffected by intraarterial Iso infusion and/or NOS, COX, or double blockade in the individuals with obesity (P > 0.05, Tables 2, 3 and 4). In normal weight adults, mean arterial blood pressure was similarly preserved across conditions, except in protocol 2 where a small (0–3 mmHg) reduction in blood pressure across doses was observed (main effect of Iso, P = 0.005; Table 3). Heart rate was unaffected by intra-arterial Iso infusion and/or NOS, COX, or double blockade in the normal weight individuals (P > 0.05, Tables 2–4). In adults with obesity, a small (−2 to +3 beats/min) change in heart rate was observed with Iso infusion across trials (main effect of Iso, P = 0.032, Table 2; P = 0.035, Table 4).
DISCUSSION
Contrary to our hypothesis, primary findings indicate β-adrenergic-mediated vasodilation is maintained, or even enhanced, in young, otherwise healthy adults with obesity. Interestingly, mechanistic probing further suggests 1) humans with obesity do not achieve β-adrenergic-mediated vasodilation via NOS signaling, 2) β-adrenergic-mediated vasodilation is not suppressed by COX signals in adults with obesity, and 3) COX does not suppress NOS signaling in response to β-adrenergic receptor stimulation in human obesity. These responses are clearly divergent from what is observed in normal weight adults and highlight the presence of a compensatory shift in microvascular control mechanisms in younger humans with obesity.
In the present investigation, the vasodilatory responses to β-adrenoceptor stimulation are preserved in individuals with obesity under 40 yr of age (Fig. 2). Our findings are in contrast with Van Guilder et al. (13) who described impaired β-adrenergic-mediated vasodilation in older (54 yr) overweight and obese humans. On the other hand, our data agree with findings from Agapitov et al. (11) who found Iso-mediated vasodilation was not impaired in younger (40 yr) normotensive obese (BMI, 35 kg/m2) compared with normal weight (BMI, 22 kg/m2) adults. Importantly, Agapitov et al. (11) had an overrepresentation of women in their protocol (80% female) and phase of the menstrual cycle was not controlled for. Sex hormones (i.e., estradiol) may modulate both β-adrenergic receptor number and sensitivity (28, 39, 40). With this, present new data confirm findings by Agapitov in a group of equal men and women of which women were studied during the early follicular phase of the menstrual cycle, when circulating estradiol is low. Together these data increase the confidence in the primary conclusion that in younger otherwise healthy adults with obesity, β-adrenergic receptor-mediated vasodilation is preserved. In contrast, dysfunction likely occurs with disease progression (i.e., duration of obesity, aging, addition of comorbidities).
We speculate β-adrenergic receptor-mediated vasodilation may be preserved, or even enhanced, in early obesity because of an upregulation of alternative/compensatory vascular control mechanisms that (due to redundancy in vascular signaling) obscure early pathological changes (41, 42). Consistent with this, our group and others have shown the presence of early, subclinical vascular changes in the obesity process (43, 44). Given such changes may be strong predictors of skeletal muscle microvascular pathology, we further explored potential changes in vascular control mechanisms (NOS, COX) mediating β-adrenergic receptor vasodilation in the present cohort.
In normal weight adults, we observed a measurable contribution of NOS to β-adrenergic-mediated vasodilation. Specifically, we saw β-adrenergic-mediated vasodilation was reduced ∼30% during coinfusion of l-NMMA in the present cohort (Fig. 3A). These findings are consistent with prior work from others which show NOS may contribute to up to 60% of β-adrenergic-mediated vasodilation in young normal weight individuals (19, 20, 39, 45). Notably, present findings are in contrast to previous work from our group, where we observed very little (1%–12%) contribution of NOS to the vasodilatory response to β-adrenergic receptor stimulation in young men and women (22). Discrepancies between past and present findings may be due to the more selective inclusion criteria applied to the current study cohort to ensure all participants (both normal weight and obese) were otherwise healthy and free of overt metabolic and/or cardiovascular disease.
Despite relatively normal vasodilatory responses to β-adrenergic receptor stimulation in the individuals with obesity studied, we uncovered a distinct shift in vascular control mechanisms. We observed no change in Iso-mediated vasodilation with coinfusion of l-NMMA (Fig. 3B), as well as a lack of increase in β-adrenergic-mediated vasodilation with COX suppression (ketorolac) in the individuals with obesity (Fig. 4B). These findings were surprising, given previous data suggesting COX-dependent vasoconstriction contributes to endothelial dysfunction in other models of obesity (26, 27). We propose two likely explanations for a lack of observable COX restraint of NOS in human obesity. First, we speculate NOS is not activated during β-receptor stimulation in individuals with obesity, and this loss of NOS activation limits any increase in Iso-mediated vasodilation with ketorolac infusion. These ideas are consistent with recent work from our group showing a limited contribution of NOS to endothelial-dependent vasodilation (acetylcholine) in younger otherwise healthy humans with obesity (32), which may be attributed to higher levels of inflammation, oxidative stress, leptin, and/or sedentary lifestyles commonly observed in this cohort (43, 44). Second, it is possible the COX pathway is not activated by β-adrenoceptor stimulation in individuals with obesity, and/or the ability of COX to suppress NOS is lost in human obesity. Given an independent effect of l-NMMA on Iso-mediated vasodilation was not observed in adults with obesity, we feel this conclusion is unlikely.
Despite a clear change in β-adrenergic activation of NOS and COX signaling, β-adrenergic-mediated vasodilation is maintained in adults with obesity. These results suggest young humans with obesity achieve β-adrenergic vasodilation via alternative vascular control mechanisms and/or upregulation of vasodilator signals, which are not active in normal weight controls. There are data to support an increased contribution of endothelium-derived hyperpolarizing factors (EDHF) following a loss of NOS in disease states (41). Nitric oxide can inhibit EDHF-induced coronary dilation (46) and following a reduction in functional nitric oxide in obesity, EDHF’s involvement in β-adrenergic vasodilation may be upregulated. Furthermore, EDHFs play a more important role in Iso-mediated vasodilation in coronary artery disease (41) when compared with healthy control conditions (42). Hydrogen peroxide has also been shown to be an important compensatory vasodilator following β-adrenergic receptor stimulation in disease states (41, 47, 48), and we speculate such mechanisms may play a role in preserving Iso-mediated vasodilation in the obese individuals studied.
Experimental Considerations
There are some important limitations to consider. First, the present study included both men and women. Although there are data to suggest sex differences may modulate β-adrenergic-mediated vasodilation (49), we have shown previously that Iso-mediated vasodilation does not differ between men and women (22) and therefore data from both sexes were pooled. Notably, a post hoc analysis supports a lack of sex differences in Iso-mediated vasodilation in either group (P value = 0.132–0.975); however, based on small samples sizes, these results should be interpreted with caution and should be replicated in larger study cohorts. Second, ketorolac is a nonselective inhibitor of COX. Thus, it is possible that shifts in the balance between COX-1 and COX-2 occur with obesity that were not examined presently. Furthermore, although doses of both l-NMMA and ketorolac were based on prior publications that show effective blockade (33–35), it is challenging to verify complete inhibition of NOS and/or COX in humans. Incomplete inhibition may lead to an underestimation of the role of NOS and/or COX in β-adrenergic-mediated vasodilation, independent of group. Third, the action of isoproterenol is not specific to the β2-adrenergic receptors. However, prior work has shown metoprolol does not impair isoproterenol-mediated vasodilation, whereas vasodilatory responses are abolished with propranolol (50). These data indicate vasodilation via isoproterenol is primarily β2-adrenoceptor mediated. Furthermore, group differences in vasodilator responses to isoproterenol (31) can be replicated using the β2-adrenergic receptor agonist, terbutaline (45), supporting the likelihood that present results are consistent with action of isoproterenol at the level of the β2-adrenoceptor. Last, despite the large number (n = 58) of individuals in the present study, protocols 2 (NOS) and 3 (COX) had smaller cohorts due to the secondary nature of the experiment (22, 28, 32). For transparency, we provide individual data points. In addition, we conducted an effect size analysis on area-under-the-curve data that confirmed group differences in the effect of l-NMMA (Cohen’s d = 0.558) and ketorolac (Cohen’s d = 0.877) on Iso-mediated vasodilation are moderate to high. With this in mind, we believe cohort size does not limit our conclusions; however, additional studies in larger study cohorts are warranted.
Conclusions
β-Adrenergic-mediated vasodilation is not impaired in young adults with obesity. In the context of previous studies, these new data support the concept that aging and/or greater disease duration is necessary to uncover overt impairments in β-adrenergic-mediated vasodilation (11, 13). Although β-adrenoceptor activation of COX suppresses NOS in normal weight adults, this is not observed in individuals with obesity. These results suggest humans with obesity maintain vascular β-adrenoceptor vasodilation via alternate mechanisms and highlight the presence of early subclinical changes in vascular control in human obesity before the development of overt dysfunction. Present findings enhance our understanding of the time course and mechanisms associated with the pathophysiology of cardiovascular disease development in obesity-related conditions.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL105820 (to W.G.S.) and HL153523 (to J.K.L.). The project described was supported by the Clinical and Translational Science Award program, through NIH Grant UL1TR000427.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K.L., R.E.J., M.W.E., and W.G.S. conceived and designed research; R.E.J., G.L.P., J.W.H., J.M.K., M.W.E., J.J.S., B.J.W., and W.G.S. performed experiments; J.K.L., R.E.J., and K.J.C. analyzed data; J.K.L., R.E.J., K.J.C., G.L.P., J.W.H., J.M.K., M.W.E., and W.G.S. interpreted results of experiments; J.K.L. prepared figures; J.K.L., R.E.J., and W.G.S. drafted manuscript; J.K.L., R.E.J., K.J.C., G.L.P., J.W.H., J.M.K., M.W.E., J.J.S., B.J.W., and W.G.S. edited and revised manuscript; J.K.L., R.E.J., K.J.C., G.L.P., J.W.H., J.M.K., M.W.E., J.J.S., B.J.W., and W.G.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Our deepest appreciation and thanks to our research participants and Meghan Crain, Josh Trierweiler, Cameron Rousseau, Brady Ernst, Mariah Marino, and Trent Evans for technical assistance.
REFERENCES
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 53: 1925–1932, 2009. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 3.Carnagarin R, Gregory C, Azzam O, Hillis GS, Schultz C, Watts GF, Bell D, Matthews V, Schlaich MP. The role of sympatho-inhibition in combination treatment of obesity-related hypertension. Curr Hypertens Rep 19: 99, 2017. doi: 10.1007/s11906-017-0795-1. [DOI] [PubMed] [Google Scholar]
- 4.Head GA, Lim K, Barzel B, Burke SL, Davern PJ. Central nervous system dysfunction in obesity-induced hypertension. Curr Hypertens Rep 16: 466, 2014. doi: 10.1007/s11906-014-0466-4. [DOI] [PubMed] [Google Scholar]
- 5.Stocker SD, Kinsman BJ, Sved AF. Recent advances in neurogenic hypertension: dietary salt, obesity and inflammation. Hypertension 70: 474–478, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briant LJ, Burchell AE, Ratcliffe LE, Charkoudian N, Nightingale AK, Paton JF, Joyner MJ, Hart EC. Quantifying sympathetic neuro-haemodynamic transduction at rest in humans: insights into sex, ageing and blood pressure control. J Physiol 594: 4753–4768, 2016. doi: 10.1113/JP272167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christou DD, Jones PP, Pimentel AE, Seals DR. Increased abdominal-to-peripheral fat distribution contributes to altered autonomic-circulatory control with human aging. Am J Physiol Heart Circ Physiol 287: H1530–H1537, 2004. doi: 10.1152/ajpheart.00322.2004. [DOI] [PubMed] [Google Scholar]
- 8.D’Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressor recovery in obese Zucker rats. Hypertension 48: 1109–1115, 2006. doi: 10.1161/01.HYP.0000247306.53547.d4. [DOI] [PubMed] [Google Scholar]
- 9.Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol 294: H1840–H1850, 2008. doi: 10.1152/ajpheart.00692.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 282: H816–H820, 2002. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- 11.Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically mediated vascular tone in normotensive human obesity. Hypertension 52: 687–695, 2008. doi: 10.1161/HYPERTENSIONAHA.107.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh HS, Park YW, Kang JH, Lee SH, Lee HS, Shim KW. Vascular endothelial dysfunction tested by blunted response to endothelium-dependent vasodilation by salbutamol and its related factors in uncomplicated pre-menopausal obese women. Int J Obes (Lond) 29: 217–222, 2005. doi: 10.1038/sj.ijo.0802642. [DOI] [PubMed] [Google Scholar]
- 13.Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol 294: H1685–H1692, 2008. doi: 10.1152/ajpheart.01281.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webber J, Taylor J, Greathead H, Dawson J, Buttery PJ, Macdonald IA. A comparison of the thermogenic, metabolic and haemodynamic responses to infused adrenaline in lean and obese subjects. Int J Obes Relat Metab Disord 18: 717–724, 1994. [PubMed] [Google Scholar]
- 15.Gerin W, Pickering TG. Association between delayed recovery of blood pressure after acute mental stress and parental history of hypertension. J Hypertens 13: 603–610, 1995. doi: 10.1097/00004872-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Schuler JL, O'Brien WH. Cardiovascular recovery from stress and hypertension risk factors: a meta-analytic review. Psychophysiology 34: 649–659, 1997. doi: 10.1111/j.1469-8986.1997.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 17.Sung BH, Wilson MF, Izzo JL Jr, Ramirez L, Dandona P. Moderately obese, insulin-resistant women exhibit abnormal vascular reactivity to stress. Hypertension 30: 848–853, 1997. doi: 10.1161/01.HYP.30.4.848. [DOI] [PubMed] [Google Scholar]
- 18.Béa ML, Ghaleh B, Giudicelli JF, Berdeaux A. Lack of importance of NO in beta-adrenoceptor-mediated relaxation of large epicardial canine coronary arteries. Br J Pharmacol 111: 981–982, 1994. doi: 10.1111/j.1476-5381.1994.tb14839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO 3rd, Panza JA. Decreased vasodilator response to isoproterenol during nitric oxide inhibition in humans. Hypertension 30: 918–921, 1997. doi: 10.1161/01.HYP.30.4.918. [DOI] [PubMed] [Google Scholar]
- 20.Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation 95: 2293–2297, 1997. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- 21.Eisenach JH, Schroeder DR, Pike TL, Johnson CP, Schrage WG, Snyder EM, Johnson BD, Garovic VD, Turner ST, Joyner MJ. Dietary sodium restriction and beta2-adrenergic receptor polymorphism modulate cardiovascular function in humans. J Physiol 574: 955–965, 2006. doi: 10.1113/jphysiol.2006.112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limberg JK, Johansson R, Peltonen GL, Harrell JW, Kellawan JM, Eldridge MW, Sebranek JJ, Schrage WG. β-adrenergic mediated vasodilation in young men and women: cyclooxygenase restrains nitric oxide synthase. Am J Physiol Heart Circ Physiol 310: H756–H764, 2016. doi: 10.1152/ajpheart.00886.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majmudar NG, Anumba D, Robson SC, Ford GA. Contribution of nitric oxide to beta2-adrenoceptor mediated vasodilatation in human forearm arterial vasculature. Br J Clin Pharmacol 47: 173–177, 1999. doi: 10.1046/j.1365-2125.1999.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Queen LR, Ferro A. Beta-adrenergic receptors and nitric oxide generation in the cardiovascular system. Cell Mol Life Sci 63: 1070–1083, 2006. doi: 10.1007/s00018-005-5451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii N, McNeely BD, Kenny GP. Nitric oxide synthase and cyclooxygenase modulate β-adrenergic cutaneous vasodilatation and sweating in young men. J Physiol 595: 1173–1184, 2017. doi: 10.1113/JP273502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farb MG, Tiwari S, Karki S, Ngo DT, Carmine B, Hess DT, Zuriaga MA, Walsh K, Fetterman JL, Hamburg NM, Vita JA, Apovian CM, Gokce N. Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity (Silver Spring) 22: 349–355, 2014. doi: 10.1002/oby.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz M, Sánchez A, Pilar Martínez M, Benedito S, López-Oliva ME, García-Sacristán A, Hernández M, Prieto D. COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic Biol Med 84: 77–90, 2015. doi: 10.1016/j.freeradbiomed.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Limberg JK, Peltonen GL, Johansson RE, Harrell JW, Kellawan JM, Eldridge MW, Sebranek JJ, Walker BJ, Schrage WG. Greater beta-adrenergic receptor mediated vasodilation in women using oral contraceptives. Front Physiol 7: 215, 2016. doi: 10.3389/fphys.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010. doi: 10.1152/ajpheart.00713.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenach JH, Schroeder DR, Pavey ES, Penheiter AR, Knutson JN, Turner ST, Joyner MJ. Interactions between beta-2 adrenoceptor gene variation, cardiovascular control and dietary sodium in healthy young adults. J Physiol 592: 5221–5233, 2014. doi: 10.1113/jphysiol.2014.276469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey RE, Barnes JN, Charkoudian N, Curry TB, Eisenach JH, Hart EC, Joyner MJ. Forearm vasodilator responses to a β-adrenergic receptor agonist in premenopausal and postmenopausal women. Physiol Rep 2: e12032, 2014. doi: 10.14814/phy2.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrell JW, Johansson RE, Evans TD, Sebranek JJ, Walker BJ, Eldridge MW, Serlin RC, Schrage WG. Preserved microvascular endothelial function in young, obese adults with functional loss of nitric oxide signaling. Front Physiol 6: 387, 2015. doi: 10.3389/fphys.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF, Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol 589: 1979–1990, 2011. doi: 10.1113/jphysiol.2011.205013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol (1985) 98: 1251–1257, 2005. doi: 10.1152/japplphysiol.00966.2004. [DOI] [PubMed] [Google Scholar]
- 35.Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB, Joyner MJ. Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low-intensity exercise in untrained humans. J Appl Physiol (1985) 109: 768–777, 2010. doi: 10.1152/japplphysiol.00326.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011. doi: 10.1113/jphysiol.2010.203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000. doi: 10.1016/S0735-1097(00)00849-4. [DOI] [PubMed] [Google Scholar]
- 40.Riedel K, Deussen AJ, Tolkmitt J, Weber S, Schlinkert P, Zatschler B, Friebel C, Müller B, El-Armouche A, Morawietz H, Matschke K, Kopaliani I. Estrogen determines sex differences in adrenergic vessel tone by regulation of endothelial β-adrenoceptor expression. Am J Physiol Heart Circ Physiol 317: H243–H254, 2019. doi: 10.1152/ajpheart.00456.2018. [DOI] [PubMed] [Google Scholar]
- 41.Durand MJ, Gutterman DD. Diversity in mechanisms of endothelium-dependent vasodilation in health and disease. Microcirculation 20: 239–247, 2013. doi: 10.1111/micc.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satake N, Shibata M, Shibata S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br J Pharmacol 119: 505–510, 1996. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisbee JC, Butcher JT, Frisbee SJ, Olfert IM, Chantler PD, Tabone LE, d'Audiffret AC, Shrader CD, Goodwill AG, Stapleton PA, Brooks SD, Brock RW, Lombard JH. Increased peripheral vascular disease risk progressively constrains perfusion adaptability in the skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 310: H488–H504, 2016. doi: 10.1152/ajpheart.00790.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limberg JK, Morgan BJ, Schrage WG. Peripheral blood flow regulation in human obesity and metabolic syndrome. Exerc Sport Sci Rev 44: 116–122, 2016. doi: 10.1249/JES.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey RE, Ranadive SM, Limberg JK, Baker SE, Nicholson WT, Curry TB, Barnes JN, Joyner MJ. Forearm vasodilatation to a β2 -adrenergic receptor agonist in premenopausal and postmenopausal women. Exp Physiol 105: 886–892, 2020. doi: 10.1113/EP088452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol Heart Circ Physiol 279: H459–H465, 2000. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol 285: H1213–H1219, 2003. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 48.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 49.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol 589: 5285–5297, 2011. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao F, de Beer VJ, Hoekstra M, Xiao C, Duncker DJ, Merkus D. Both beta1- and beta2-adrenoceptors contribute to feedforward coronary resistance vessel dilation during exercise. Am J Physiol Heart Circ Physiol 298: H921–H929, 2010. doi: 10.1152/ajpheart.00135.2009. [DOI] [PubMed] [Google Scholar]


