Keywords: gut-brain axis, gut microbiota, nervous system, neuroactive steroids, steroids
Abstract
The intricate connection between central and enteric nervous systems is well established with emerging evidence linking gut microbiota function as a significant new contributor to gut-brain axis signaling. Several microbial signals contribute to altered gut-brain communications, with steroids representing an important biological class that impacts central and enteric nervous system function. Neuroactive steroids contribute pathologically to neurological disorders, including dementia and depression, by modulating the activity of neuroreceptors. However, limited information is available on the influence of neuroactive steroids on the enteric nervous system and gastrointestinal function. In this review, we outline how steroids can modulate enteric nervous system function by focusing on their influence on different receptors that are present in the intestine in health and disease. We also highlight the potential role of the gut microbiota in modulating neuroactive steroid signaling along the gut-brain axis.
INTRODUCTION
Evidence from various disciplines implicates bidirectional signals between the gut and central nervous systems (known as the gut-brain axis) in disease development. Considerable evidence over recent years also connects the gut microbiota to this signaling conduit, extending the commonly used terminology to the “microbiota-gut-brain axis” (1). Different signaling molecules, including steroids are involved in these complex communications (2). Steroids are lipid-derived molecules that generally contain four fused rings of carbon atoms with a chemical structure that plays an important role in a wide range of biological functions, including reproduction, metabolism, and immune responses. Some steroids also regulate neural activity independently of their origin. To highlight their importance in neuroscience the term “neuroactive steroids” was introduced to describe steroids that rapidly modulate neural activity through binding to membrane-bound receptors (3), whereas the term “neurosteroid” specifically refers to neuroactive steroids that are synthesized locally in the brain (4). Although the importance of neuroactive steroids in the central nervous system (CNS) has been extensively explored, limited information has linked this class of molecules to the enteric nervous system (ENS), which is often referred to as the “little brain” or “brain-in-the-gut.” This review summarizes how different steroids act on the CNS, and outlines how steroid signaling is involved in the microbiome-gut-brain axis in relation to potential effects on the ENS.
NEURO-MODULATORY EFFECTS OF STEROIDS
Steroids and steroid-like molecules include cholesterol, sex hormones, bile acids, corticosteroids, and vitamin D, all of which play important roles in maintaining human health. As previous reviews have extensively described neuroactive sex hormones and corticosteroids (5–9), here we consider how different steroid classes (Fig. 1) modulate central and peripheral nervous systems.
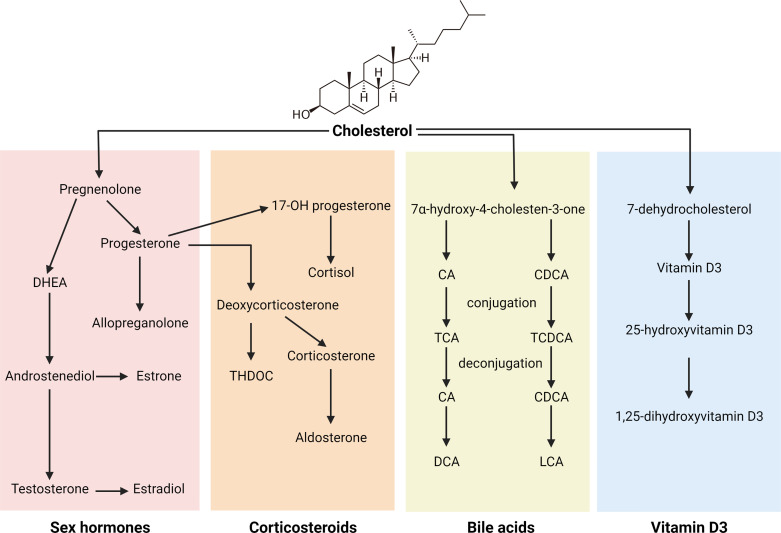
Figure 1.
Summary of synthesis pathway of the major steroids classes. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; DHEA, dehydroepiandrosterone; LCA, lithocholic acid.
Sex Steroids
Sex hormones including testosterone, estradiol, and progesterone are extensively studied steroids. Although they play a critical role in reproductive and endocrine functions, hormonal steroids and their precursors or metabolites also modulate neural activity. For example, testosterone regulates the hypothalamic-pituitary-adrenal (HPA) axis in men (10). Higher testosterone levels are also associated with elevated serotonergic tone in healthy males (11). Sex hormones and certain metabolite-derivatives exhibit genomic effects through classical hormonal receptors including estrogen receptors (ERs), androgen receptor (AR), and progesterone receptor (PR). These receptors are also involved in nervous system functions. For instance, ER activation promotes development and survival of dorsal root ganglion and serotonergic neurons in the dorsal raphe (12, 13). However, it is also well recognized that neuroactive steroids mediate rapid neuromodulatory signals through nongenomic pathways via interaction with ion channels and membrane receptors, including γ-aminobutyric acid type A (GABAA) and N-methyl-d-aspartate (NMDA) neuroreceptors (9). Sex hormones also exhibit nongenomic effects through binding and activation of G protein-coupled receptors (GPCRs), including G protein-coupled estrogen receptor (GPER) (14) and G protein-coupled receptor class C group 6 member A (GPRC6A) that are activated by androgens (15). Sex hormones also indirectly modulate GPCRs, for example serotonin receptors, which are neuroreceptors that are widely distributed throughout central and enteric nervous systems. Estrogens modulate the expression of serotonin 2A (5-HT2A) receptors (16), which is involved in cognition and intestinal smooth muscle contraction, as well as cognitive and mood changes in postmenopausal women (17). The precise mechanisms by which estrogens regulate serotonin receptors remain unclear but are suggested to act through ligand binding to estrogen receptors or GPER (17).
Accumulating evidence suggests that progesterone- and testosterone-related compounds are particularly potent in altering neural excitability and synaptic function. Studies have analyzed a spectrum of these neuroactive steroids in patients and found altered associated levels with neurological disorders such as depression, autism, multiple sclerosis, and schizophrenia (18–22). The neural impact of steroids may also be regulated by altered enzymatic activity modulating their synthesis. Several reviews have described how sex hormone-related steroidogenesis is carried out in the nervous system (23–25). In brief, cholesterol is transported across the mitochondrial membrane, a process regulated by steroidogenic acute regulatory protein (StAR) and translocator protein (TSPO). Cytochrome P450 side-chain cleavage (P450scc) then catalyzes the conversion of cholesterol into pregnenolone, which is further metabolized into other hormones including progesterone and testosterone. Downstream enzymes including 5α-reductase and 3α-hydroxysteroid dehydrogenase then catalyze the conversion of the hormones into other types of neuroactive steroids. Allopregnanolone, a 5α-reduced progesterone metabolite synthesized by the brain, was recently approved by the US Food and Drug Administration (FDA) as Zulresso for treatment of postpartum depression (26). It is a strong positive allosteric GABAA receptor modulator and is deficient in patients with depression (19, 27). In addition to its influence on mood fluctuations, it demonstrates analgesic properties in neuropathic pain models, possibly through T-type calcium channels and GABAA receptor (28, 29). A recent study also reported its inhibitory activity on Toll-like receptor 4 (TLR4), via MyD88-dependent signals that suppress neuroinflammation (30, 31). Pregnenolone sulfate, in contrast to allopregnanolone, inhibits GABAA receptor signaling by enhancing receptor desensitization (32). Pregnenolone sulfate also potentiates NMDA and activates the nociceptor transient receptor potential M3 (TRPM3) channel, which is involved in insulin secretion and noxious heat sensation (33–35). Dehydroepiandrosterone (DHEA) is an androgenic precursor of testosterone, and its sulfated form (DHEA-S) represents the most abundant steroid in the systemic circulation. Because these steroids are synthesized by the adrenal glands they represent important biomarkers of HPA axis function and the stress response. They are associated with antidepressive effects and their abundance is lower in the circulation of patients with depression (36, 37). They are also reported to be involved in various neurological functions including neurogenesis, catecholamine signaling, and neuronal survival (38). DHEA and DHEA-S antagonizes the GABAA receptor (39, 40) and positively modulate sigma-1 (41, 42) and NMDA (43) receptors. One study reported that DHEA-S potentiates NMDA-induced pain through activation of the sigma-1 receptor (44), whereas another study suggested that DHEAS enhances synaptic plasticity through chronic activation of sigma-1 receptor (45). The inhibitory effect of GABAA agonist muscimol on medial vestibular nucleus neurons is suppressed by DHEA-S. Furthermore, muscimol reduces DHEA-S-induced mechanical allodynia (46). This appears to be predominantly associated with sulfation as the impact of DHEA on GABAA receptor signaling is less potent (47, 48). Regarding their effect on gastrointestinal function, DHEA-S treatment alleviates visceral allodynia and reduces colonic permeability in stressed male rats through GABAA, nitric oxide (NO), opioid, central dopamine D2, and peripheral CRF2 signaling (49). Another androgenic neuroactive steroid (androstenediol) suppresses inflammation of microglia through Estrogen receptor β (ERβ) in the brain (50). It also reduces demyelination-induced axonopathy and promotes remyelination in rodents (51, 52), suggesting it may have protective properties against neurological disease, such as multiple sclerosis.
As some neurological disorders including irritable bowel syndrome (IBS) and autism demonstrate sex bias in incidence and severity (2, 53), sexually dimorphic effects of steroids (particularly sex hormones) have been suggested as a possible etiology. Neuroactive hormone abundance under normal physiological conditions is different in females and males. For instance, DHEA-S is more abundant in males (54, 55). Furthermore, fluctuations in the levels of neuroactive steroids under stress and/or pathological conditions also show sex-dependent patterns. Changes in neuroactive steroids following a forced swimming test in rats were more prominent in females (56). Pregnenolone and 3α-Androstanediol (3α-diol) measured in the sciatic nerve are decreased in diabetic males but not in female rats; whereas progesterone and isopregnanolone are reduced only in diabetic female rats (57). In addition to sex-bias, the activity of neuroactive steroids demonstrates significant regional specificity. For example, neuroactive steroids are abundant in the circulation but this does not always correlate with levels in cerebrospinal fluid (58). Diabetes-induced changes in neuroactive steroid levels are also markedly different in sciatic nerve versus the cerebral cortex (57). Therefore, although sex hormone-related steroids modulate the nervous system and activity of receptors, it is also important to consider potential confounding factors when studying their effects.
Corticosteroids
Corticosteroids are mainly synthesized by the adrenal cortex from progesterone. There are two main types of corticosteroids: glucocorticoids and mineralcorticoids. Glucocorticoids including cortisol and corticosterone play a primary role in the stress response, by activating the HPA axis and releasing cortisol. In the adrenal cortex, progesterone and 17-hydroxyprogesterone are converted into intermediate products 11-deoxycorticosterone and 11-deoxycortisol respectively, a process catalyzed by 21-hydroxylase (CYP21A2) (59). These intermediate compounds are then transformed into corticosterone and corticosterone by 11β-hydroxylase and represent potential enzymatic targets of corticosteroid activity. Cortisol activity is antagonized by DHEA/DHEA-S; thus its ratio against DHEA/DHEA-S is a measure of stress level and HPA dysfunction (60, 61). Furthermore, deoxycorticosterone is a precursor of corticosterone, whereas 3α,5α-tetrahydrodeoxycorticosterone (THDOC; a 3α-reduced metabolite of deoxycorticosterone) is more abundant in patients with depression (62). At the same time, hypercorticism is believed to damage brain structure and contribute to dementia (63–65). In female patients with IBS, the cortisol:DHEA ratio is higher compared with controls (66), which could be caused by altered adrenocorticotropic hormone (ACTH) responses to corticotropin-releasing hormone (CRH) (67). Through the action of aldosterone synthase, glucocorticoids are converted into mineralcorticoids, including aldosterone that regulates electrolyte and fluid homeostasis, for example through the renin-angiotensin-aldosterone system, which contributes to blood pressure regulation. Excess aldosterone is associated with obesity and visceral adiposity, possibly due to the aldosterone-releasing factors released by visceral fat (68). Regarding its effect on neurological health, aldosterone is positively associated with anxiety and depression (69, 70).
Corticosteroids exert their effects by genomic and nongenomic actions through cytosolic and membrane-associated glucocorticoid receptors (GR) and mineralcorticoid receptors (MR), which are expressed throughout the body including the brain (71–74). These receptors regulate neuronal survival and neurogenesis (75), as well as synaptic function, including memory, mood changes, and pain sensation (76–78). They are also co-localized in neurons and their regulated balance is critical in mediating an appropriate stress response (79, 80). Corticosteroids and their metabolites are important regulators of the HPA axis. Treatment with THDOC protects against behavioral and neuroendocrine consequences of early adverse life stress in rats (81). Although MR and GR are involved in regulating the HPA axis (80), GABAA receptor signaling also targets the HPA axis through suppressing the activity of corticotrophin-releasing hormone (CRH) neurons under steady-state (nonstressful) conditions. This inhibitory effect involves the K+/Cl− co-transporter KCC2 that maintains low extracellular Cl− concentrations. As a positive allosteric GABAA receptor modulator, THDOC therefore suppresses CRH neural signaling (82). Interestingly, these GABAA-mediated effects are reversed under stress conditions, possibly mediated by upregulation of the Na+-K+-2Cl− cotransporter NKCC1 in terminal endings of CRH neurons (83). THDOC also upregulates the HPA axis following stress through dephosphorylation of KCC2 residue-Ser940, resulting in a Cl− gradient shift (82). Corticosteroid also activates GPCRs, for example adhesion G protein-coupled receptor G3 (GPR97) that regulates B cell function and antimicrobial activity by human granulocytes (84, 85), whereas aldosterone activates the angiotensin II (AngII) type-1 receptor (AT1R) regulating blood pressure (86). In general, glucocorticoids regulate the stress response together with GABAA receptor differently under steady-state and stress conditions, whereas mineralcorticoids play a critical role in regulating blood pressure. The importance of balance between cortisol and DHEA-S level also indicate cross talk between steroids, indicating the importance of evaluating the role of a spectrum of steroids instead of a single compound.
Bile Acids
Bile acids are important mediators of microbiome-gut-brain axis signaling. Bile acids are steroid-like compounds that are synthesized by the liver from cholesterol, mainly through the classic pathway regulated by rate-limiting enzyme CYP7A1. The major human primary bile acids are cholic acid (CA) and chenodeoxycholic acid (CDCA), and their ratio is controlled by the activity of CYP8B1. After conversion into primary bile acids, they are conjugated with taurine or glycine in the liver before being secreted into the small intestine where they promote digestion and absorption of nutrients. In the intestine, bile acids are deconjugated and converted into secondary bile acids by the gut microbiota (discussed NEUROACTIVE STEROIDS IN MICROBIOTA-GUT-BRAIN AXIS SIGNALING). In addition to the extensive bile acid pool in the small intestine, bile acids are also present in the brain, where they are either transported from the systemic circulation and/or synthesized locally. In particular, CDCA is highly abundant in brain compared with systemic circulating levels in rodents (87).
In addition to their critical role in digestion, bile acids are recognized as important signaling molecules. Over the past few years, their impact on neural function has received much attention as altered bile acid levels are reported in several neuropathological conditions. For instance, brain bile acid levels are altered in patients with Alzheimer’s disease compared with cognitively normal individuals (88). Similarly, bile acid-related disorders are also associated with changes in neural activity. Cholestasis is associated with suppression of HPA axis activity, possibly through the interactions between bile acids and the glucocorticoid receptor in the brain (89). Also, cerebrotendinous xanthomatosis (CTX), a genetic disorder associated with decreased bile acid synthesis contributes to neural dysfunction including dementia, a finding that is supported by CDCA supplementation alleviating dementia symptoms (90).
Bile acids activate farnesoid X receptor (FXR) and G protein-coupled bile acid receptor (TGR5) that serve multiple functions and are expressed in various tissues, including in central and peripheral neurons throughout the intestine. Importance of the nuclear receptor FXR in nervous system function is demonstrated by altered neurotransmitter levels, and impaired cognitive function and motor coordination after deletion of FXR (91). It is also reported that FXR mediates bile acid-induced visceral hypersensitivity, with the involvement of mucosal mast cells, nerve growth factor (NGF), and transient receptor potential (TRP) vanilloid family 1 channel (TRPV1) (92). Meanwhile, the G protein-coupled bile acid receptor TGR5 is expressed in brain and peripheral neurons, and functions as a neurosteroid receptor after activation by allopregnanolone (93). Animal studies also suggest that TGR5 mediates bile acid-induced stimulation of sensory nerves and contributes to symptoms, including itching and analgesia in cholestatic disease (94). Furthermore, TGR5 is expressed by enteric neurons and mediates region-specific bile acid regulation of gastrointestinal motility. In mouse stomach and small intestine, bile acids activating TGR5 suppress motility and promote efficient absorption (95). Conversely, TGR5 overexpression enhances colonic motility, whereas ablation of TGR5 delays colonic transit (96). Certain bile acid species also activate pregnane X receptor (PXR), vitamin D receptor (VDR), and glucocorticoid receptor (GR), which independently regulate nervous system activity (89, 97–99). In addition to these receptors that show promiscuous activation by bile acids, CDCA in particular is highly abundant in brain and is reported to be a potential antagonist of NMDA and GABAA receptors (100). Furthermore, ursodeoxycholic acid (UDCA) that shows neuroprotective effects against dementia enhances alertness via suppression of GABAA receptor signaling (101). These findings support that bile acids are important mediators in the gut-brain axis and in regulating gastrointestinal function.
Vitamin D3
Vitamin D3 is a fat-soluble vitamin as well as a steroid. It is consumed as part of the diet or is synthesized from cholesterol after exposure to sunlight. In its active form, it has to be hydroxylated to 25-hydroxyvitamin D3 [25(OH)D3] by 25-hydroxylase (CYP27A1) and is further hydroxylated to 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] by 1α-hydroxylase (1α-OHase, also known as CYP27B1). It plays an important role in calcium absorption, immunity, and metabolic health. In recent years, vitamin D3 is also recognized as a neurosteroid (102). 1α-OHase, the enzyme that transforms vitamin D into its active form is expressed by the human brain (103). Endothelial cells and neurons are capable of converting Vitamin D3 into 25(OH)D3, which is further metabolized into 1,25(OH)2D3 by neurons and microglia (104). Evidence from epidemiological studies and animal experiments shows that vitamin D3 deficiency is associated with pain sensitization and neurological dysfunction, including Alzheimer’s disease, depression, and multiple sclerosis (105–109). This could be explained by the importance of vitamin D3 in various neurological functions, including neuroinflammation, neurogenesis, synaptic function, and neuronal survival in the CNS (110–112). Furthermore, vitamin D3 maintains blood-brain barrier integrity in in vitro and in vivo models (113). It has also been reported that vitamin D3 deficiency leads to elevated basal GABA levels in rodents (110, 114). However, confounding factors, particularly sunlight exposure and lifestyle confounders, should be taken into account when studying the effects of vitamin D3 in humans (108).
Similar to other steroids, vitamin D3 acts through genomic or nongenomic pathways. Genomic actions are mainly mediated through the nuclear vitamin D receptor (VDR), which is present in various organs including the brain (103). In humans, VDR polymorphisms are associated with susceptibility and worsening symptoms in Parkinson’s disease and dementia (115, 116). VDR knockout animals also demonstrate altered locomotor behavior (117). Nongenomic actions also involve the VDR, as well as the membrane receptor protein disulfide isomerase family member (PDIA3) (118, 119). Although VDR is mostly expressed by astrocytes, PDIA3 is highly expressed by various cell types in the brain and is therefore suggested to be the major nongenomic vitamin D receptor type in the CNS (104). VDR and PDIA3 may also interact to mediate nongenomic effects (120). In addition, vitamin D3 modulates signaling via other receptors. For instance, vitamin D3 downregulates the expression of epidermal growth factor receptor (EGFR), a transmembrane protein involved in pain processing (121). Vitamin D3 also targets opioid receptor signaling, as vitamin D3 supplementation modulates the expression of genes involved in the signaling pathway and reduces neuropathic pain (122). Vitamin D3 deficiency is recently reported to increase the risk for opioid dependence, possibly due to deficiencies in VDR signaling (123), suggesting interactions between VDR with other receptors that regulate nervous system function. Furthermore, vitamin D3 is recently reported as an agonist of the calcium-selective channel TRPV1 that mediates pain sensation (124). In general, vitamin D3 plays an important role in controlling neuroinflammation and neuropathic pain, which likely is mediated through VDR and different membrane receptors.
In summary, a plethora of evidence supports the neuromodulatory effect of steroids, capable of regulating various receptors associated with neural signaling pathways. In particular, several steroids modulate GABAA and NMDA receptors, implicating a potential involvement of steroids in clinical disorders involving synaptic dysfunction. Although many membrane receptors are influenced by steroids, some studies also suggest cross-activation between receptors upon steroid activation that deserves further investigation. It is also demonstrated that steroids regulate gastrointestinal function, which has led to intense interest in understanding how steroids signal along the gut-brain axis.
NEUROACTIVE STEROIDS IN MICROBIOTA-GUT-BRAIN AXIS SIGNALING
The gut is innervated by the ENS, which is the largest division of the peripheral nervous system and the third division of the autonomic nervous system. It plays an important role in regulating gastrointestinal function, including gastric secretion, gut motility, and intestinal epithelial cell activity. ENS dysfunction is associated with a number of clinical disorders, including autism spectrum disorder and IBS (125, 126). The ENS usually communicates with the CNS, but is also capable of local function independently of the brain. Many of the structural and neurochemical components of the ENS resemble that of the CNS. For instance, neurons (and enteroendocrine) cells secrete serotonin or gastrointestinal hormones that activate signals transmitted through the vagal nerve to the brain (127). Also, neurons of the ENS are surrounded by enteric glia that are structurally similar to CNS glia and support neural networks (128). Furthermore, membrane channels or receptors including NMDA and TGR5 that are modulated by steroids are expressed in the gut (129). These features suggest that molecules including steroids that act on the CNS could also influence the enteric nervous counterpart.
The cross talk between the gut and CNS is well recognized, and in the last decade the gut microbiota has been included as a significant contributor to this signaling network (130). The term “psychobiome” was introduced to describe gut bacteria that influence how we think, feel, and act (131). Growing clinical evidence indicates that shifts in gut microbiome composition are associated with changes in central and enteric nervous system function and behavior. For example, specific gut microbiome features are associated with quality of life and depression in the Flemish Gut Flora Project (132). Also, probiotic use is reported to alleviate anxiety and depression (133, 134). The significance of the microbiome-gut-brain axis is also demonstrated by dysregulated hippocampus neurogenesis, serotonergic signaling, and microglial responses to antibiotics or by inducing a germ-free state in animal models (135–137). Regarding the ENS, germ-free rodents show abnormal myenteric plexus structure and altered intrinsic primary afferent neuron excitability (138, 139). In addition, the gut microbiota can mediate dietary effects on the nervous system, for instance the antiseizure properties of ketogenic diets (140).
Mechanisms by which gut microbiota modulate the nervous system remain incompletely understood, but considerable evidence suggests that microbial metabolites are involved. Gut microbiota release and/or regulate the synthesis of neurotransmitters and neuromodulators that act locally on enteric neurons or transmit signals to the brain via for example the vagus nerve (141). At the same time, they convert steroids and/or other compounds into microbial metabolites that may exhibit different biological effects on the nervous system compared with their parent forms. Finasteride is a drug for benign prostate hyperplasia and androgenetic alopecia, as well as an irreversible inhibitor of 5α-reductase, an enzyme involved in the synthesis of some neuroactive steroids. Patients with postfinasteride often have altered neuroactive steroid profiles and demonstrate depressive symptoms (142, 143). Functional magnetic resonance imaging has confirmed abnormal function in brain areas related to sexual arousal and depression in these patients (144). Recently, an altered gut microbiota composition was reported in patients with postfinasteride (145). Although microbiota community shifts associated with steroid levels were not investigated, these results implicate a potential connection between the gut microbiota and neuroactive steroids. This section summarizes how different types of steroids interact with the gut microbiota and subsequently how this interaction may regulate gut-brain axis signaling (Table 1 and Fig. 2).
Table 1.
Examples of how different steroids interact with gut microbiota
| Steroids | Modulation of Steroids by Gut Microbiota | Effect of Steroids on Gut Microbiota |
|---|---|---|
| Sex hormones | Deconjugation by enzymes including sulfatase and glucuronidases Biotransformation of unconjugated hormones |
Enhances intestinal alkaline phosphatase (IAP) activity Facilitates intraluminal transport of sIgA which neutralizes pathogenic bacteria Regulates plasma immunoglobulin levels and B cell function |
| Corticosteroids | Regulate intestinal corticosteroid production in response to stress Biotransform into 21-dehydroxylated products or androgens Influence GR activity |
Mediate the effects of stress through modulation of the microbiome Modulate the oral microbiome in a metatranscriptomic study Modulate infant intestinal microbiota composition with altered maternal cortisol level |
| Bile acids | Deconjugates by bile salt hydrolase (BSH) Conversion of primary into secondary bile acids by 7-dehydroxylation, 12α-dehydrogenation and desulfation |
Exhibit antimicrobial and cytotoxic properties by inducing membrane damage or through FXR Regulate gut microbiota composition through FXR |
| Vitamin D3 | Increase 25-hydroxyvitamin D and biosynthesis of 7-dehydrocholesterol by probiotics and prebiotics, respectively Upregulates VDR expression by probiotics Hydroxylate and activate vitamin D3 by cytochrome enzymes |
Increase gut microbial diversity and Akkermansia abundance Elevate tight junction protein expression and improves intestinal barrier function Enhance the production of antimicrobial peptides |
FXR, farnesoid X receptor; VDR, vitamin D receptor.
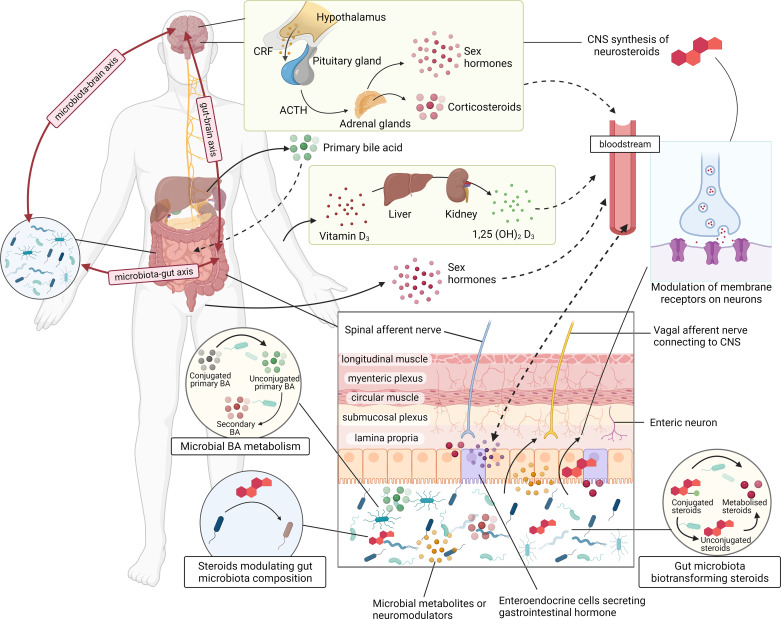
Figure 2.
Schematic diagram of how steroids are involved in microbiota-gut-brain axis signaling. CNS, central nervous system.
Sex Hormones
Gut microbiota composition is different in males and females, for instance, Akkermansia is reportedly more abundant in females than in males (146). As gut microbiota composition appears to be sex-biased, systemic sex hormone levels are also associated with altered gut microbiota profiles (147, 148). Furthermore, alteration of serotonin metabolism and microglial responses in the absence of gut microbiota are sex-biased, demonstrating the importance of sex and consideration of estrus cycle when studying the microbiota-gut-brain axis (135, 137).
Gut microbiota and sex steroids interact in reciprocal ways. On the one hand, the gut microbiota regulate the levels of sex steroids, with animal studies showing that shifts in gut microbiota composition result in abundant changes of neuroactive sex steroids. For instance, prebiotic supplementation reduces the elevation of neuroactive pregnanolone-type compounds induced by stress (149). In a study of type 1 diabetes, transfer of gut microbiota from male to female mice induced an elevation in testosterone levels and conferred protection from autoimmune disease (150). These effects are also reported to be dependent on AR activity. The gut microbiota express enzymes such as sulfatase and glucuronidases that deconjugate steroids for intestinal reabsorption. Therefore, conjugated steroid levels are higher in germ-free compared with conventional mice (151). The deconjugation process may also influence the potency of sex steroids on the nervous system. It is reported that DHEA induces neural AR expression and activity whereas DHEA-S fails to do so (152). At the same time, unconjugated sex steroids can be further converted into other metabolic products by specific bacteria. For instance, Steroidobacter denitrificans biotransforms testosterone through the 9,10-seco-pathway (153). Microbial metabolism of estrogens may also modify their estrogenic potential through producing different estrogenic metabolites (147, 154).
On the other hand, sex hormones regulate gut microbiome structure and function, which in part could explain differences in gut microbiome profiles between the sexes. Ovariectomy changes gut microbiota composition in rodents as demonstrated by an increase in the relative abundance of Firmicutes and Proteobacteria, with decreases in Bactoidetes (155, 156). Neuroactive DHEA treatment modulates gut microbiota composition and alleviates intestinal inflammation in experimental dextran sulfate sodium (DSS)-induced colitis (157). In a study of metabolic syndrome, 17β-estradiol reduces Proteobacteria and increases Akkermansia in male mice, and protected the mice against metabolic syndrome (155). Furthermore, fecal DHEA-S levels are elevated and gut microbiota composition is altered in polycystic ovary syndrome (PCOS), which is characterized by hyperandrogenism (157, 158). The mechanisms by which steroids regulate gut microbiota likely involve regulation of host intestinal function, such as motility and immune responses. Estrogens are reported to upregulate intestinal alkaline phosphatase activity that dephosphorylates LPS and antimicrobial peptides, which prevents Escherichia coli overgrowth (155). In an in vitro study, estrogens also facilitated the transport of secretory immunoglobulin A (IgA), which binds to and neutralizes pathogenic bacteria (159). Furthermore, an increase in estrogen to androgen ratio in male mice is associated with elevated plasma immunoglobulin levels and altered B cell function (160). In general, microbiota metabolize sex hormone-related steroids and modulate their activity, while steroids also play important roles in shaping the gut microbiome and host immunity. Sex-dependent effects of steroids are also reported in disease models. It is therefore important to investigate how their cross talk differs between sexes and contributes to disease pathogenesis. There is also inadequate direct evidence on how desulfation of steroids by the gut microbiota modulates their potency. Given that the inhibition of GABAA receptor by DHEA-S is associated with its sulfation, it is possible that the microbial desulfation will reduce the inhibitory effect.
Corticosteroids
Glucocorticoids are the primary hormones driving the stress response, whereas stress is also a major factor that alters gut microbiota composition. The gut microbiome is associated with HPA axis activity, which is often measured based on glucocorticoid levels (161). For example, in infants, gut microbiota diversity is associated with stress-induced saliva cortisol responses (162).
Emerging evidence supports a role for gut microbiota regulation of glucocorticoid synthesis and metabolism. Germ-free male mice shows higher plasma corticosterone levels after restraint stress compared with SPF mice, and this increase was alleviated by Bifidobacterium infantis (161). Another potential psychobiotic, Bifidobacterium longum 1714, reduces reported stress and attenuates increases in stress-induced salivary cortisol in healthy volunteers (163). Furthermore, intestinal corticosterone production in response to stress is reduced in germ-free mice (164). Anaerobic bacteria including Eggerthella lenta metabolize glucocorticoids into 21-dehydroxylated products, 11β-OH-progesterone or 11β-OH-(allo)-5α-preganolones, which are associated with hypertension (165, 166). Clostridium scindens has also been reported to transform glucocorticoids into androgens (167). Gut microbiota also influence depression and anxiety through downstream actions on the glucocorticoid receptor in germ-free animals (168). Glucocorticoids are suggested to mediate the effects of stress through modulation of the microbiome. For instance, cortisol treatment modulated the oral microbiome in a metatranscriptomic study (169). Furthermore, maternal prenatal and postpartum stress that are measured by saliva cortisol levels are associated with infant intestinal microbiota composition (170, 171).
In addition to glucocorticoids, mineralcorticoids are also influenced by gut microbiota activity. In an early study, antibiotics reduced urinary 21-dehydroxylated aldosterone products in healthy volunteers and in patients with liver cirrhosis, suggesting a role for the gut microbiota in aldosterone metabolism (172). As the renin-angiotensin-aldosterone system is closely associated with hypertension, this could possibly reflect how the gut microbiota may regulate blood pressure. Taken together, the interaction between corticosteroids and gut microbiota is likely to mediate their effect on stress response and blood pressure regulation. While stress and glucocorticoids induce various changes to host phenotype, including inflammation and oxidative stress, gut microbiota are also closely influenced by these factors. At the same time, gut microbiota play important roles in regulating host immune responses and steroid metabolism. These form a multifactorial link between gut microbiota, steroids, and stress.
Bile Acids
Primary bile acids are synthesized by the liver and are secreted as conjugated molecules into the small intestine as bile. Over 95% of bile acids are reabsorbed, primarily as conjugated species in the distal ileum, and recirculate back to the liver as part of the enterohepatic circulation. The remaining bile acids reach the large intestine and are excreted in feces while interacting with the microbiota in the colonic lumen.
Together with the enterohepatic circulation, the gut microbiota play an important role in regulating bile acid compositional profiles. Germ-free mice have a larger bile acid pool, and less fecal bile acids are excreted compared with conventional mice (173). Gene expression profiles involved in bile acid metabolism are also different in the absence of gut microbiota, including elevated mRNA accumulation of hepatic bile acid synthesis genes (173). Significant alterations in bile acid species in the brain of Alzheimer’s disease patients suggest an association with the microbiota-gut-brain axis (88). Indeed, the gut microbiota metabolize bile acids and increase the diversity of the bile acid pool. Numerous strains of gut bacteria possess bile salt hydrolase (BSH) activity (174–176) and deconjugate bile acids, which is a requirement for microbial conversion into secondary bile acids in the colon. In the small intestine, this action prevents the reabsorption, as conjugated bile acids are transported more effectively through active transport (177). Unconjugated bile acids enter the colon where they are metabolized into secondary bile acids by a much more limited repertoire of bacteria that are capable of 7-dehydroxylation including some Clostridium or Eubacterium species (178). Generally, cholic acid (CA) is transformed into deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA) is transformed into lithocholic acid (LCA). Certain strains including Clostridium scindens and Eggerthella lenta have been a focus because of their potential to produce secondary bile acids (179, 180), and alterations in bile acid profiles and circulating secondary bile acid levels influence central and peripheral nervous systems, including the ENS. Changes in bile acid profiles are potentially important as bile acid receptor signaling is preferentially activated by specific bile acid ligands with distinct signaling potency. For instance, CDCA is more potent in activating FXR compared with DCA and LCA (181), whereas the murine-specific tauro-β-muricholic acid is recognized as an FXR antagonist (173). Also, unconjugated bile acids, particularly LCA and DCA promote colonic transit, with more potent effects on male compared with female mice (96, 182). Mechanistically, the effect could be mediated through TGR5 activation and/or the serotonergic system. Furthermore, DCA excites colonic afferent nerves, implicating its potential role in visceral hypersensitivity (183). Apart from 7-dehydroxylation, other microbial modifications including 12α-dehydrogenation and desulfation occur producing different bile acid species with unknown signaling potential (184).
Although the gut microbiota influences bile acid profiles, they also serve as important host factors in shaping the gut microbiome. A recent study reported bile acids as signals for gut microbiota maturation in newborn mice (185). Also, neonatal cholestasis is associated with gut dysbiosis in infants (186). In in vivo studies, bile acid treatment and bile duct ligation alter gut microbiota composition (187–189). Certain unconjugated bile acids including CDCA and DCA exhibit antimicrobial and cytotoxic properties and inhibit growth of commensal bacteria by inducing membrane damage (190). This could possibly explain the bacterial overgrowth and translocation when there is a blockage of bile flow (191, 192). Furthermore, bile acid receptors may mediate the effect of bile acids on gut microbiota. For instance, FXR-deficient mice have reduced Firmicutes:Bacteroidetes ratio and Proteobacteria, as well as higher abundance of Desulfovibrionaceae, Deferribacteraceae, and Helicobacteraceae (193). FXR is also associated with antibacterial activity and protects against bacterial overgrowth and mucosal injury (194). However, future studies that characterize microbiota changes in the absence of specific intestinal bile acid species are still required to evaluate their modulatory role on gut microbiota community structure and function. For example, CYP8B1-deficient mice lacking cholic acid and its secondary bile acid derivatives did not demonstrate marked shifts in microbiota composition or function (195). In this same bile-acid deficient model rederived under germ-free conditions, microbiota protection (both human and murine) against neurogenic inflammation of the colon was demonstrated to occur independently of bile acid signaling. Given the considerable amount of evidence demonstrating a close relationship between bile acids and gut microbiota, as well as studies reporting the effect of secondary bile acids on visceral sensitivity and gut motility, there is solid ground to continue exploring how their interactions contribute to central and enteric nervous system function and underlying disease mechanisms. However, there is a need to test such findings in animal models that rigorously address causation.
Vitamin D3
Gut microbiota are associated with vitamin D3 metabolism and VDR signaling. Probiotics studies have suggested that vitamin D3 is involved in their protective effects on intestinal disease, such as colitis (196). Oral probiotic supplementation of patients with hypercholesterolemia showed increased systemic 25-hydroxyvitamin D levels, indicating an improvement in vitamin D status (197). It is also proposed that prebiotics could enhance biosynthesis of provitamin D3 7-dehydrocholesterol (198). Gut microbiota may also regulate vitamin D3 signaling by modulating VDR expression and activity, with animal models demonstrating that probiotics elevate VDR expression (199). In addition, gut microbiota species such as Streptomyces griseolus and Amycolata autotrophica express cytochrome enzymes that hydroxylate and activate vitamin D3 (200, 201).
Accumulating evidence suggests that vitamin D3 modulates gut microbiota composition, although changes reported have been inconsistent (202). In a recent study of healthy women with vitamin D3 deficiency, supplementation increased gut microbial diversity and abundance of Akkermansia and Bifidobacterium (203). One consistent finding with other studies is that higher vitamin D3 baseline or vitamin D3 supplementation is positively associated with Akkermansia abundance in healthy individuals, as well as in patients with multiple sclerosis (204, 205). A genome-wide association study (GWAS) identified a significant association between VDR genetic variants and human gut microbiota composition, while Vdr-knockout mice support this finding by showing a different gut microbiota composition compared with wild-type mice (206). As vitamin D3 plays important roles in immune system function, it may regulate the gut microbiome through regulation of intestinal immunity. Mice with VDR deletion or vitamin D3 deficiency are more prone to intestinal inflammation, pathogen infection, and bacterial overgrowth (207–209). In terms of adaptive immunity, vitamin D3 supplementation elevates tight junction protein expression and improves intestinal barrier function in in vitro and in vivo studies (210, 211). For innate immunity, vitamin D3 enhances the production of antimicrobial peptides, which exhibit antimicrobial activity and protects the host against pathogen colonization (212, 213). As gut microbiota modulates vitamin D3 metabolism, these protective effects of vitamin D3 signaling may also mediate some of the beneficial effects of probiotics. Also, while altered Akkermansia abundance is found in multiple diseases (214, 215), the clinical significance of the consistent positive association between vitamin D3 and Akkermansia warrants further investigation.
Overall, steroids and gut microbiota closely interact in a bidirectional fashion that may influence signaling to either gut or brain. Evidence also suggests that steroids interact with the gut microbiota and these signals may serve as mediators of beneficial probiotic effects. Nevertheless, we still lack a detailed understanding of how these complex interrelationships regulate the gut-brain axis, as direct evidence is limited and requires further investigation.
ALTERED STEROID-GUT MICROBIOTA INTERACTIONS IN DISEASE STATES
Considerable evidence has independently shown that steroids and gut microbiota play critical roles in various diseases; however, how they interact under pathological conditions remains unclear. Alterations in secondary bile acid profiles that originate from gut microbiota activity are found in different types of disease, suggesting dysregulated steroid-microbiota interactions. Understanding these interactions in the context of disease development may assist in advancing future diagnostic tools and therapeutic interventions.
Alzheimer’s Disease
Alzheimer’s disease is a major neurodegenerative disease characterized by the progressive damage and/or dysfunction of neurons, with symptoms including cognitive impairment and mood changes. As steroids are important regulators of neuronal survival and differentiation, altered neurosteroid levels are often found in patients with dementia. For example, DHEA-S and allopregnanolone are reduced in patients with Alzheimer’s disease (216, 217). At the same time, allopregnanolone supplementation reverses neurogenic and cognitive deficits in a translational mouse model (218). Furthermore, steroidogenesis regulators including StAR are altered in Alzheimer’s tissue (219). Mechanisms underlying neuronal death in Alzheimer’s disease include dysregulation of neuroinflammation, protein aggregation, oxidative stress, and DNA damage. Evidence has shown that various neurosteroids modulate these mechanisms. Regarding protein aggregation, pregnenolone sulfate and DHEA-S are negatively associated with β-amyloid peptide detection and phosphorylated tau proteins in brain, respectively (220). Furthermore, androstenediol, progesterone, and allopregnanolone promote myelin regeneration, suggesting that myelin represents a target for protection against neurodegenerative disease (52, 221). Meanwhile, the human gut microbiome structure shifts during aging and could contribute to neurodegenerative disease (222). β-Amyloid is also reported to induce gut dysbiosis (223). Various studies have reported gut dysbiosis in patients and animal models with neurodegenerative diseases (224–227) and these studies form the foundation for probiotic therapy (228). Studies also found positive associations between cognition and secondary bile acids in Alzheimer’s brain tissues and serum, indicating potential involvement of steroid-microbiota interactions in cognition (88, 229). This is possibly because secondary bile acid DCA induces oxidative stress and DNA damage, which are risk factors for neurodegeneration (230, 231). Furthermore, Lactobacillus helveticus NS8 treatment alleviated cognitive symptoms and restored brain serotonin levels in a chronic stress model while lowering plasma corticosterone and ACTH (232), suggesting stress-associated steroids are involved in the beneficial effects of probiotics against dementia.
Although the mechanisms underlying the effects of steroids and gut microbiota are not fully known, various membrane receptors are likely to be involved. As described in previous sections, both gut microbiota and steroids modulates synthesis and receptor activity of neurotrophic factors that are also important factors in Alzheimer’s disease. The balance between inhibitory GABA and excitatory glutamate transmission is important for proper neuronal function. GABAA receptor dysfunction is reported to be associated with Alzheimer’s disease (233). Although an earlier study suggested GABAergic neurons are resistant to β-amyloid toxicity (234), later studies reported that β-amyloid impairs GABA inhibitory interneuron function (235, 236). At the same time, GABA treatment and activation of GABAA receptor during early life protects against β-amyloid-induced cognitive impairment in mice (237), which may explain reduced GABA levels in patients with Alzheimer’s disease (238). As neurosteroids are modulators of the GABAA receptor, allopregnanolone and DHEA protect neurons from apoptosis while upregulating α1 and β2 mRNAs of GABAA receptor (239). The promotion of myelin formation by allopregnanolone is abolished by the selective GABAA receptor antagonist bicuculline, suggesting the neuroprotective effect is GABAA-dependent (221). DHEA-S also demonstrates neuroprotective effects that are abolished by bicuculline, despite DHEAS commonly being regarded as a GABAA antagonist (240). In addition to the GABAA receptor, NMDA receptors play important roles in cognitive function and neuronal survival. Although glutamate is important for neuronal survival and synaptic transmission, excitotoxicity resulting from excessive glutamate and glutamatergic activity leads to neuronal dysfunction and death. Synaptic dysfunction caused by Ca2+ influx into neurons through the NMDA receptor has also been suggested as a mechanism underlying β-amyloid toxicity in Alzheimer’s disease (241). In this regard, steroids including allopregnanolone, DHEA(S) and vitamin D protect against NMDA-induced toxicity (242–244). Furthermore, pregnenolone sulfate that positively modulates the NMDA receptor enhances memory and reduces competitive NMDA receptor antagonist-induced retention deficits (245–247). These findings implicate neurosteroids as modulators of pathological components of Alzheimer’s disease through GABAA and NMDA receptors. At the same time, the gut microbiota regulates neurotransmitter synthesis and neuroreceptor activity in Alzheimer’s disease. Using the Mendelian randomization approach, a study reported that GABA and Blautia, a potential GABA producing genus, are protective against Alzheimer’s disease (248). NMDA receptor expression is also influenced by the presence of gut microbiota (249). Taken together, the interaction between steroids and gut microbiota could be involved in Alzheimer’s disease through regulating GABAA and NMDA receptor activity.
In addition to neurotrophic factors and neuroreceptors, altered bile acid profiles in Alzheimer’s disease suggest that bile acid receptors could be involved. FXR activation is reported to enhance β-amyloid-induced neural apoptosis, whereas the TGR5 agonist has an opposite effect (250, 251). As the subtypes and conjugation extent of bile acids affect their potency as ligands activating receptors, microbiota-regulation of bile acid profiles is likely to modulate the balance of the receptors. Furthermore, cortisol increases the risk of Alzheimer’s disease, indicating the potential involvement of stress response and GR (64). Also, the GR antagonist mifepristone is protective against cognitive deficit and reduces both β-amyloid and tau protein in brain (252, 253). As cortisol level changes in response to gut microbiota alterations, it could mediate the impact of dysbiosis on Alzheimer’s disease through activating GR. Nevertheless, the evidence linking Alzheimer’s disease and steroid-microbiota interactions is still tentative and largely correlative, there is still a lack of consensus on the underlying mechanisms and this merits further investigation.
Irritable Bowel Syndrome
Although patients with IBS often experience gastrointestinal discomfort, IBS is considered a disorder caused by ENS dysfunction and gut-brain axis dysregulation. At the same time, the gut microbiota is closely associated with ENS function and IBS symptoms. For instance, fecal transplant from patients with IBS to germ-free animals induced increases in visceral sensitivity (254). The female predominance of IBS suggests that sex hormones are involved in its pathogenesis (2). Indeed, steroids are associated with IBS symptoms including visceral hypersensitivity, gastrointestinal dysmotility, and depression. For instance, estrogens increase visceral sensitivity in animals, which may explain the higher sensitivity to pain in females (255). This could be due to estrogen potentiating TRPV1 channel activity (256), which contributes to pain sensation and is upregulated in IBS (125). Furthermore, sex steroids regulate the serotonergic system (2), which could also explain why sex dependence is evident in alterations of the serotonergic system in IBS, which is another signal associated with pain (257). Despite the lack of consistency in IBS-associated microbiome signatures, the gut microbiota could mediate the effect of steroids in IBS pathogenesis. For example, Akkermansia muciniphila is positively associated with estradiol and vitamin D, as well as increased colonic serotonin concentration (258) in patients with IBS-C (259). At the same time, the microbial metabolite butyrate is a TRPV1 channel agonist and modulates neuropathic pain (260), indicating that gut microbiota activity may modulate visceral sensitivity through producing metabolites that modulate ion channels.
As IBS often manifests clinical symptoms at prepubertal age when gonadal hormone production is still low, other steroids including adrenal hormones could be preferentially involved in the sex bias of functional gastrointestinal (GI) disorders in children. A study reported that DHEA-S and DHEA-S/DHEA is lower in patients with irritable bowel syndrome (IBS) (predominantly males) (261), whereas another study reported higher DHEA levels in IBS females under stress compared with controls (66). These studies also reported an altered cortisol:DHEA(S) ratio in IBS, implicating HPA-axis dysfunction. How these steroids influence IBS symptoms is still unclear but could possibly involve modulation of neuroreceptors. As described in previous sections, DHEA-S antagonizes the GABAA receptor and potentiates sigma-1 and NMDA receptors. At the same time, these signaling pathways are reported as altered in IBS. Activation of the colonic NMDA receptor contributes to visceral hypersensitivity (262). In contrast, GABAergic compounds, including pregabalin and gabapentin alleviate pain in patients with IBS (263, 264), while reduced GABAergic signals are found in patients with IBS-D (265). Bifidobacterium dentium produces GABA and desensitizes sensory neuron activity in a rat model of visceral hypersensitivity (266), demonstrating the role of gut microbiota in regulating GABAergic signals. However, before attributing neurotransmitter signaling by specific members of the microbiota community there is a need to establish that neurotransmission is maintained in vivo under physiological conditions, and that the impacted signaling pathway is validated using chemical and/or genetic intervention in the host. Taken together, these findings implicate that steroid-microbiota interaction may alter neurotransmitter synthesis and neuroreceptor activity, and subsequently modulate IBS symptoms. It should also be noted that changes in DHEA(S) appears to be sex-dependent in IBS, and therefore warrants study results to be stratified by sex when evaluating underlying mechanisms.
In addition to sex-related steroids, altered bile acid and related microbiome features are reported in some patients with IBS where they associate with gut dysmotility. Patients with IBS-C have lower fecal bile acids and DCA, whereas patients with IBS-D have excess fecal bile acids and a Clostridia-rich microbiome (267, 268). Secondary bile acids including DCA activate TGR5 that promotes colonic motility. At the same time, Coriobacteriaceae including its genus Eggerthella that regulates steroid and bile acid metabolism (269) are also increased in IBS (270, 271). Taken together, steroids and gut microbiota overlap in modulating many potential mechanisms underlying IBS pathology, including neuroreceptor activity, serotonergic tone, TRP channels, and bile acid signaling. Given that all of these factors are important in regulating gastrointestinal function, further investigations of how their cross talk modulates ENS function may provide further insight into IBS pathogenesis.
PHYTOSTEROL IMPACT ON THE MICROBIOTA-GUT-BRAIN AXIS
In addition to endogenous steroids, humans are exposed to plant steroids contained in the diet. These include phytosterols, steroid saponins, brassinosteroids, etc., which can influence microbiota-gut-brain axis signaling. Phytosterols including sitosterol, campesterol, stigmasterol, and cycloartenol are structurally similar to cholesterol. Dietary phytosterols are negatively associated with serum cholesterol levels (272). As cholesterol is important for the nervous system, the cholesterol-lowering property of phytosterols is possibly associated with neurological function. It is reported in animal studies that phytosterols can pass through the blood-brain barrier and accumulate in the brain, although no associated phenotypic alterations has been identified (273, 274). Some studies propose phytosterol intake is protective against cognitive and mood disorders. A study reported that stigmasterol inhibits the formation of amyloid-β and may protect against Alzheimer’s disease (275). Another study found that protective cognitive properties of stigmasterol are mediated by estrogen or NMDA receptors (276). Meanwhile, β-sitosterol exhibits antidepressant and anxiolytic effects (277, 278), possibly mediated through serotonergic, GABAergic, and dopaminergic mechanisms. Furthermore, phytosterols modulate the activity of endogenous steroid receptors. For instance, stigmasterol is reported to antagonize the bile acid receptor FXR (279). However, contrasting evidence show phytosterols do not impact neurocognitive function or mood changes, thus further confirmation is needed (280). In terms of gastrointestinal function, phytosterol alleviates symptoms of DSS-induced colitis in rodents, accompanied by changes to intestinal bile acid profiles and bile acid-deconjugating bacteria (281).
A reciprocal relationship between microbiota and phytosterols is also reported, connecting phytosterols to the microbiota-gut-brain axis. Gut microbiota biotransform phytosterols into phytostanone and phytostenone, and subsequently into phytostanols (282). At the same time, phytosterols modulate gut microbiota composition. In vitro fermentation of phytosterol increased Eubacterium hallii and reduced Erysipelotrichaceae abundance, which is associated with metabolic disorders (283). Consistent with this finding, plant sterol esters reduced the abundance of Erysipelotrichaceae in hamsters (269). A study with high-fat diet fed rats also reported changes in gut microbiome by phytosterol esters, with partial restoration of a healthy microbiota (284). Based on the current evidence, phytosterols are potential candidates for use as therapeutic interventions in disorders of the gut-brain axis. Future studies are needed to investigate how they could be applied to reverse alterations in steroids-microbiota interactions.
CONCLUSIONS
Steroids are well recognized for their role in regulating the gut-brain axis through modulating membrane receptors activities. Considerable evidence also demonstrates that the gut microbiota influences this network. Furthermore, both steroids and the microbiota-gut-brain axis are implicated in multiple disease etiologies. Multiple studies indicate steroids-microbiota interaction could influence disease development through modulating GABAA and NMDA receptors. However, there is limited direct evidence of detailed mechanisms underlying their disease associations. There is also inadequate information on the effect of neurosteroids on the ENS. Further effort is required to unravel this complex interplay involving multiple factors and signals. This review highlights how various steroids are involved in the microbiota-gut-brain axis via different potential mechanisms, and provides a fundamental basis to explore the many interconnected facets that comprise this network.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) P30-DK56338 and R01DK130517, NIAID U01-AI24290 and P01-AI152999, and National Institute of Nursing Research (NINR) R01-NR013497.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and T.C.S. conceived and designed research; S.S. prepared figures; S.S. and T.C.S. drafted manuscript; S.S. and T.C.S. edited and revised manuscript; S.S. and T.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Figures were created using Biorender.com.
REFERENCES
- 1.Luna RA, Savidge TC, Williams KC. The brain-gut-microbiome axis: what role does it play in autism spectrum disorder? Curr Dev Disord Rep 3: 75–81, 2016. doi: 10.1007/s40474-016-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.So SY, Savidge TC. Sex-bias in irritable bowel syndrome: linking steroids to the gut-brain axis. Front Endocrinol (Lausanne) 12: 684096, 2021. doi: 10.3389/fendo.2021.684096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul SM, Purdy RH. Neuroactive steroids. FASEB J 6: 2311–2322, 1992. doi: 10.1096/fasebj.6.6.1347506. [DOI] [PubMed] [Google Scholar]
- 4.Baulieu E-E, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol 37: 395–403, 1990. doi: 10.1016/0960-0760(90)90490-C. [DOI] [PubMed] [Google Scholar]
- 5.Carta MG, Bhat KM, Preti A. GABAergic neuroactive steroids: a new frontier in bipolar disorders? Behav Brain Funct 8: 61, 2012. doi: 10.1186/1744-9081-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giatti S, Garcia-Segura LM, Barreto GE, Melcangi RC. Neuroactive steroids, neurosteroidogenesis and sex. Prog Neurobiol 176: 1–17, 2019. doi: 10.1016/j.pneurobio.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Giatti S, Romano S, Pesaresi M, Cermenati G, Mitro N, Caruso D, Tetel MJ, Garcia-Segura LM, Melcangi RC. Neuroactive steroids and the peripheral nervous system: an update. Steroids 103: 23–30, 2015. doi: 10.1016/j.steroids.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 28: 139–168, 2003. doi: 10.1016/S0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 9.Tuem KB, Atey TM. Neuroactive steroids: receptor interactions and responses. Front Neurol 8: 442, 2017. doi: 10.3389/fneur.2017.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, Nieman L. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology 30: 1906–1912, 2005. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perfalk E, Cunha-Bang SD, Holst KK, Keller S, Svarer C, Knudsen GM, Frokjaer VG. Testosterone levels in healthy men correlate negatively with serotonin 4 receptor binding. Psychoneuroendocrinology 81: 22–28, 2017. doi: 10.1016/j.psyneuen.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Patrone C, Andersson S, Korhonen L, Lindholm D. Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci USA 96: 10905 –10910, 1999. doi: 10.1073/pnas.96.19.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Barros RPA, Sugiyama N, Krishnan V, Yaden BC, Kim H-J, Warner M, Gustafsson J-Å. Involvement of estrogen receptor β in maintenance of serotonergic neurons of the dorsal raphe. Mol Psychiatry 18: 674–680, 2013. doi: 10.1038/mp.2012.62. [DOI] [PubMed] [Google Scholar]
- 14.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7: 715–726, 2011. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalyvianaki K, Panagiotopoulos AA, Malamos P, Moustou E, Tzardi M, Stathopoulos EN, Ioannidis GS, Marias K, Notas G, Theodoropoulos PA, Castanas E, Kampa M. Membrane androgen receptors (OXER1, GPRC6A AND ZIP9) in prostate and breast cancer: a comparative study of their expression. Steroids 142: 100–108, 2019. doi: 10.1016/j.steroids.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Cyr M, Landry M, Di Paolo T. Modulation by estrogen-receptor directed drugs of 5-hydroxytryptamine-2A receptors in rat brain. Neuropsychopharmacology 23: 69–78, 2000. doi: 10.1016/S0893-133X(00)00085-3. [DOI] [PubMed] [Google Scholar]
- 17.Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry 160: 1522–1524, 2003. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 18.Caruso D, Melis M, Fenu G, Giatti S, Romano S, Grimoldi M, Crippa D, Marrosu MG, Cavaletti G, Melcangi RC. Neuroactive steroid levels in plasma and cerebrospinal fluid of male multiple sclerosis patients. J Neurochem 130: 591–597, 2014. doi: 10.1111/jnc.12745. [DOI] [PubMed] [Google Scholar]
- 19.Dichtel LE, Lawson EA, Schorr M, Meenaghan E, Paskal ML, Eddy KT, Pinna G, Nelson M, Rasmusson AM, Klibanski A, Miller KK. Neuroactive steroids and affective symptoms in women across the weight spectrum. Neuropsychopharmacology 43: 1436–1444, 2018. doi: 10.1038/npp.2017.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology 37: 543–553, 2012. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology 31: 1249–1263, 2006. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- 22.Needham BD, Adame MD, Serena G, Rose DR, Preston GM, Conrad MC, Campbell AS, Donabedian DH, Fasano A, Ashwood P, Mazmanian SK. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol Psychiatry 89: 451–462, 2021. doi: 10.1016/j.biopsych.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon M-C, Pelletier G, Vaudry H. Neurosteroid biosynthesis: enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front Neuroendocrinol 30: 259–301, 2009. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Liang JJ, Rasmusson AM. Overview of the molecular steps in steroidogenesis of the GABAergic neurosteroids allopregnanolone and pregnanolone. Chronic Stress (Thousand Oaks) 2: 2470547018818555, 2018. doi: 10.1177/2470547018818555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd-Evans E, Waller-Evans H. Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease. Essays Biochem 64: 591–606, 2020. doi: 10.1042/EBC20200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell JG, Garland S, Preston K, Piszczatoski C. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother 54: 157–163, 2020. doi: 10.1177/1060028019873320. [DOI] [PubMed] [Google Scholar]
- 27.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA 95: 3239–3244, 1998. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kavaliers M, Wiebe JP. Analgesic effects of the progesterone metabolite, 3α-hydroxy-5α-pregnan-20-one, and possible modes of action in mice. Brain Res 415: 393–398, 1987. doi: 10.1016/0006-8993(87)90228-9. [DOI] [PubMed] [Google Scholar]
- 29.Pathirathna S, Todorovic SM, Covey DF, Jevtovic-Todorovic V. 5α-reduced neuroactive steroids alleviate thermal and mechanical hyperalgesia in rats with neuropathic pain. Pain 117: 326–339, 2005. doi: 10.1016/j.pain.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Balan I, Aurelian L, Schleicher R, Boero G, O'Buckley T, Morrow AL. Neurosteroid allopregnanolone (3α,5α-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl Psychiatry 11: 145, 2021. doi: 10.1038/s41398-021-01266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balan I, Beattie MC, O'Buckley TK, Aurelian L, Morrow AL. Endogenous neurosteroid (3α,5α)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci Rep 9: 1220, 2019. doi: 10.1038/s41598-018-37409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. J Neurosci 20: 3571–3579, 2000. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciurtin C, Majeed Y, Naylor J, Sukumar P, English AA, Emery P, Beech DJ. TRPM3 channel stimulated by pregnenolone sulphate in synovial fibroblasts and negatively coupled to hyaluronan. BMC Musculoskelet Disord 11: 111, 2010. doi: 10.1186/1471-2474-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70: 482–494, 2011. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Wagner TFJ, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nat Cell Biol 10: 1421–1430, 2008. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- 36.Barrett-Connor E, von Mühlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc 47: 685–691, 1999. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, Simpson St. Clair L, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry 62: 154–162, 2005. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- 38.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30: 65–91, 2009. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demirgören S, Majewska MD, Spivak CE, London ED. Receptor binding and electrophysiological effects of Dehydroepiandrosterone sulfate, an antagonist of the GABAA receptor. Neuroscience 45: 127–135, 1991. doi: 10.1016/0306-4522(91)90109-2. [DOI] [PubMed] [Google Scholar]
- 40.Gartside SE, Griffith NC, Kaura V, Ingram CD. The neurosteroid dehydroepiandrosterone (DHEA) and its metabolites alter 5-HT neuronal activity via modulation of GABAA receptors. J Psychopharmacol 24: 1717–1724, 2010. doi: 10.1177/0269881109105836. [DOI] [PubMed] [Google Scholar]
- 41.Moriguchi S, Shinoda Y, Yamamoto Y, Sasaki Y, Miyajima K, Tagashira H, Fukunaga K. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One 8: e60863, 2013. [Erratum in PLoS One 9, 2014]. doi: 10.1371/journal.pone.0060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noda Y, Kamei H, Kamei Y, Nagai T, Nishida M, Nabeshima T. Neurosteroids ameliorate conditioned fear stress: an association with sigma1 receptors. Neuropsychopharmacology 23: 276–284, 2000. doi: 10.1016/S0893-133X(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci USA 101: 3202–3207, 2004. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoon S-Y, Roh D-H, Seo H-S, Kang S-Y, Moon J-Y, Song S, Beitz AJ, Lee J-H. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology 59: 460–467, 2010. doi: 10.1016/j.neuropharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Dai X-N, Sokabe M. Chronic administration of dehydroepiandrosterone sulfate (DHEAS) primes for facilitated induction of long-term potentiation via sigma 1 (σ1) receptor: optical imaging study in rat hippocampal slices. Neuropharmacology 50: 380–392, 2006. doi: 10.1016/j.neuropharm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Yoon S-Y, Roh D-H, Seo H-S, Kang S-Y, Han H-J, Beitz AJ, Lee J-H. Intrathecal injection of the neurosteroid, DHEAS, produces mechanical allodynia in mice: involvement of spinal sigma-1 and GABAA receptors. Br J Pharmacol 157: 666–673, 2009. doi: 10.1111/j.1476-5381.2009.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res 830: 72–87, 1999. doi: 10.1016/S0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- 48.Sousa A, Ticku MK. Interactions of the neurosteroid dehydroepiandrosterone sulfate with the GABAA receptor complex reveals that it may act via the picrotoxin site. J Pharmacol Exp Ther 282: 827–833, 1997. [PubMed] [Google Scholar]
- 49.Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Dehydroepiandrosterone sulfate improves visceral sensation and gut barrier in a rat model of irritable bowel syndrome. Eur J Pharmacol 852: 198–206, 2019. doi: 10.1016/j.ejphar.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 50.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145: 584–595, 2011. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalakh S, Mouihate A. The promyelinating properties of androstenediol in gliotoxin-induced demyelination in rat corpus callosum. Neuropathol Appl Neurobiol 41: 964–982, 2015. doi: 10.1111/nan.12237. [DOI] [PubMed] [Google Scholar]
- 52.Kalakh S, Mouihate A. Androstenediol reduces demyelination-induced axonopathy in the rat corpus callosum: impact on microglial polarization. Front Cell Neurosci 11: 49, 2017. doi: 10.3389/fncel.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol 26: 146–153, 2013. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glei DA, Goldman N, Weinstein M, Liu I-W. Dehydroepiandrosterone sulfate (DHEAS) and health: does the relationship differ by sex? Exp Gerontol 39: 321–331, 2004. [Erratum in Exp Gerontol 41: 337, 2006]. doi: 10.1016/j.exger.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Goldman N, Glei DA. Sex differences in the relationship between DHEAS and health. Exp Gerontol 42: 979–987, 2007. doi: 10.1016/j.exger.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sze Y, Gill AC, Brunton PJ. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J Neuroendocrinol 30: e12644, 2018. doi: 10.1111/jne.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav 57: 46–55, 2010. doi: 10.1016/j.yhbeh.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Kancheva R, Hill M, Novák Z, Chrastina J, Kancheva L, Stárka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience 191: 22–27, 2011. doi: 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 59.Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 94: 3–21, 2006. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- 60.Goodyer IM, Herbert J, Altham PME. Adrenal steroid secretion and major depression in 8- to 16-year-olds. III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol Med 28: 265–273, 1998. doi: 10.1017/S0033291797006314. [DOI] [PubMed] [Google Scholar]
- 61.Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem 131: 99–104, 1994. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- 62.Ströhle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3α-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol Psychiatry 45: 274–277, 1999. doi: 10.1016/S0006-3223(98)00328-X. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Zhang J, Tan H, Li J, Yu Y. Detrimental effects of hypercortisolism on brain structure and related risk factors. Sci Rep 10: 12708, 2020. doi: 10.1038/s41598-020-68166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ouanes S, Popp J. High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature. Front Aging Neurosci 11: 43, 2019. doi: 10.3389/fnagi.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pires P, Santos A, Vives-Gilabert Y, Webb SM, Sainz-Ruiz A, Resmini E, Crespo I, de Juan-Delago M, Gómez-Anson B. White matter alterations in the brains of patients with active, remitted, and cured cushing syndrome: a DTI study. AJNR Am J Neuroradiol 36: 1043–1048, 2015. doi: 10.3174/ajnr.A4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugaya N, Izawa S, Saito K, Shirotsuki K, Nomura S, Shimada H. Effect of prolonged stress on the adrenal hormones of individuals with irritable bowel syndrome. Biopsychosoc Med 9: 4, 2015. doi: 10.1186/s13030-015-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kano M, Muratsubaki T, Van Oudenhove L, Morishita J, Yoshizawa M, Kohno K, Yagihashi M, Tanaka Y, Mugikura S, Dupont P, Ly HG, Takase K, Kanazawa M, Fukudo S. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep 7: 12425, 2017. doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA 100: 14211–14216, 2003. doi: 10.1073/pnas.2336140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Emanuele E, Geroldi D, Minoretti P, Coen E, Politi P. Increased plasma aldosterone in patients with clinical depression. Arch Med Res 36: 544–548, 2005. doi: 10.1016/j.arcmed.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 70.Murck H, Held K, Ziegenbein M, Künzel H, Koch K, Steiger A. The renin-angiotensin-aldosterone system in patients with depression compared to controls—a sleep endocrine study. BMC Psychiatry 3: 15, 2003. doi: 10.1186/1471-244X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102: 19204–19207, 2005. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krug AW, Pojoga LH, Williams GH, Adler GK. Cell membrane—associated mineralocorticoid receptors. Hypertension 57: 1019–1025, 2011. doi: 10.1161/HYPERTENSIONAHA.110.159459. [DOI] [PubMed] [Google Scholar]
- 73.Olijslagers JE, De Kloet ER, Elgersma Y, Van Woerden GM, Joëls M, Karst H. Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci 27: 2542–2550, 2008. doi: 10.1111/j.1460-9568.2008.06220.x. [DOI] [PubMed] [Google Scholar]
- 74.Vernocchi S, Battello N, Schmitz S, Revets D, Billing AM, Turner JD, Muller CP. Membrane glucocorticoid receptor activation induces proteomic changes aligning with classical glucocorticoid effects. Mol Cell Proteomics 12: 1764–1779, 2013. doi: 10.1074/mcp.M112.022947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 38: 872–883, 2013. doi: 10.1038/npp.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rozeboom AM, Akil H, Seasholtz AF. Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA 104: 4688–4693, 2007. doi: 10.1073/pnas.0606067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwabe L, Tegenthoff M, Höffken O, Wolf OT. Mineralocorticoid receptor blockade prevents stress-induced modulation of multiple memory systems in the human brain. Biol Psychiatry 74: 801–808, 2013. doi: 10.1016/j.biopsych.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 78.Ye L, Xie W, Strong JA, Zhang J-M. Blocking the mineralocorticoid receptor improves effectiveness of steroid treatment for low back pain in rats. Anesthesiology 121: 632–643, 2014. doi: 10.1097/ALN.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res 51: 371–381, 2005. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 80.Harris AP, Holmes MC, de Kloet ER, Chapman KE, Seckl JR. Mineralocorticoid and glucocorticoid receptor balance in control of HPA axis and behaviour. Psychoneuroendocrinology 38: 648–658, 2013. doi: 10.1016/j.psyneuen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 81.Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OF. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest 99: 962–966, 1997. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis Is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci 31: 18198–18210, 2011. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kakizawa K, Watanabe M, Mutoh H, Okawa Y, Yamashita M, Yanagawa Y, Itoi K, Suda T, Oki Y, Fukuda A. A novel GABA-mediated corticotropin-releasing hormone secretory mechanism in the median eminence. Sci Adv 2: e1501723, 2016. doi: 10.1126/sciadv.1501723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hsiao C-C, Chu T-Y, Wu C-J, Biggelaar M, Pabst C, Hébert J, Kuijpers T, Scicluna B, K-Y I, Chen T-C, Liebscher I, Hamann J, Lin H-H. The adhesion g protein-coupled receptor GPR97/ADGRG3 is expressed in human granulocytes and triggers antimicrobial effector functions. Front Immunol 9: 2830, 2018. doi: 10.3389/fimmu.2018.02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ping Y-Q, Mao C, Xiao P, Zhao R-J, Jiang Y, Yang Z, An W-T, Shen D-D, Yang F, Zhang H, Qu C, Shen Q, Tian C, Li Z, Li S, Wang G-Y, Tao X, Wen X, Zhong Y-N, Yang J, Yi F, Yu X, Xu HE, Zhang Y, Sun J-P. Structures of the glucocorticoid-bound adhesion receptor GPR97–Go complex. Nature 589: 620–626, 2021. doi: 10.1038/s41586-020-03083-w. [DOI] [PubMed] [Google Scholar]
- 86.Lemarié CA, Simeone SMC, Nikonova A, Ebrahimian T, Deschênes M-E, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-induced activation of signaling pathways requires activity of angiotensin type 1a receptors. Circ Res 105: 852–859, 2009. doi: 10.1161/CIRCRESAHA.109.196576. [DOI] [PubMed] [Google Scholar]
- 87.Mano N, Goto T, Uchida M, Nishimura K, Ando M, Kobayashi N, Goto J. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J Lipid Res 45: 295–300, 2004. doi: 10.1194/jlr.M300369-JLR200. [DOI] [PubMed] [Google Scholar]
- 88.Baloni P, Funk CC, Yan J, Yurkovich JT, Kueider-Paisley A, Nho K, Heinken A, Jia W, Mahmoudiandehkordi S, Louie G, Saykin AJ, Arnold M, Kastenmüller G, Griffiths WJ, Thiele I; Alzheimer’s Disease Metabolomics Consortium; Kaddurah-Daouk R, Price ND. Metabolic network analysis reveals altered bile acid synthesis and metabolism in Alzheimer’s disease. Cell Rep Med 1: 100138, 2020. doi: 10.1016/j.xcrm.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McMillin M, Frampton G, Quinn M, Divan A, Grant S, Patel N, Newell-Rogers K, DeMorrow S. Suppression of the HPA axis during cholestasis can be attributed to hypothalamic bile acid signaling. Mol Endocrinol 29: 1720–1730, 2015. doi: 10.1210/me.2015-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med 311: 1649–1652, 1984. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- 91.Huang F, Wang T, Lan Y, Yang L, Pan W, Zhu Y, Lv B, Wei Y, Shi H, Wu H, Zhang B, Wang J, Duan X, Hu Z, Wu X. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front Behav Neurosci 9: 70, 2015. doi: 10.3389/fnbeh.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li W-T, Luo Q-Q, Wang B, Chen X, Yan X-J, Qiu H-Y, Chen S-L. Bile acids induce visceral hypersensitivity via mucosal mast cell–to–nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. FASEB J 33: 2435–2450, 2019. doi: 10.1096/fj.201800935RR. [DOI] [PubMed] [Google Scholar]
- 93.Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 58: 1794–1805, 2010. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 94.Alemi F, Kwon E, Poole DP, Lieu T, Lyo V, Cattaruzza F, Cevikbas F, Steinhoff M, Nassini R, Materazzi S, Guerrero-Alba R, Valdez-Morales E, Cottrell GS, Schoonjans K, Geppetti P, Vanner SJ, Bunnett NW, Corvera CU. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest 123: 1513–1530, 2013. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 22: 814–825, 2010. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 144: 145–154, 2013. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han S, Li T, Ellis E, Strom S, Chiang JYL. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol 24: 1151–1164, 2010. doi: 10.1210/me.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann–Pick C disease. Proc Natl Acad Sci USA 103: 13807–13812, 2006. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, Willson TM, Koller BH, Kliewer SA. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA 98: 3369–3374, 2001. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schubring SR, Fleischer W, Lin JS, Haas HL, Sergeeva OA. The bile steroid chenodeoxycholate is a potent antagonist at NMDA and GABAA receptors. Neurosci Lett 506: 322–326, 2012. doi: 10.1016/j.neulet.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 101.Yanovsky Y, Schubring SR, Yao Q, Zhao Y, Li S, May A, Haas HL, Lin J-S, Sergeeva OA. Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block. PLoS One 7: e42512, 2012. doi: 10.1371/journal.pone.0042512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Groves NJ, McGrath JJ, Burne THJ. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr 34: 117–141, 2014. doi: 10.1146/annurev-nutr-071813-105557. [DOI] [PubMed] [Google Scholar]
- 103.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat 29: 21–30, 2005. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 104.Landel V, Stephan D, Cui X, Eyles D, Feron F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J Steroid Biochem Mol Biol 177: 129–134, 2018. doi: 10.1016/j.jsbmb.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 105.Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, Freedman MS, Hartung H-P, Miller DH, Montalbán X, Edan G, Barkhof F, Pleimes D, Radü E-W, Sandbrink R, Kappos L, Pohl C. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol 71: 306–314, 2014. doi: 10.1001/jamaneurol.2013.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chai B, Gao F, Wu R, Dong T, Gu C, Lin Q, Zhang Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: an updated meta-analysis. BMC Neurol 19: 284, 2019. doi: 10.1186/s12883-019-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lucas RM, Ponsonby A-L, Dear K, Valery PC, Pender MP, Taylor BV, Kilpatrick TJ, Dwyer T, Coulthard A, Chapman C, van der Mei I, Williams D, McMichael AJ. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 76: 540–548, 2011. doi: 10.1212/WNL.0b013e31820af93d. [DOI] [PubMed] [Google Scholar]
- 108.Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, Beekman ATF, Smit JH, Penninx BWJH. The association between low vitamin D and depressive disorders. Mol Psychiatry 19: 444–451, 2014. doi: 10.1038/mp.2013.36. [DOI] [PubMed] [Google Scholar]
- 109.von Känel R, Müller-Hartmannsgruber V, Kokinogenis G, Egloff N. Vitamin D and central hypersensitivity in patients with chronic pain. Pain Med 15: 1609–1618, 2014. doi: 10.1111/pme.12454. [DOI] [PubMed] [Google Scholar]
- 110.Kasatkina LA, Tarasenko AS, Krupko OO, Kuchmerovska TM, Lisakovska OO, Trikash IO. Vitamin D deficiency induces the excitation/inhibition brain imbalance and the proinflammatory shift. Int J Biochem Cell Biol 119: 105665, 2020. doi: 10.1016/j.biocel.2019.105665. [DOI] [PubMed] [Google Scholar]
- 111.Latimer CS, Brewer LD, Searcy JL, Chen K-C, Popović J, Kraner SD, Thibault O, Blalock EM, Landfield PW, Porter NM. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci USA 111: E4359–E4366, 2014. doi: 10.1073/pnas.1404477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morello M, Landel V, Lacassagne E, Baranger K, Annweiler C, Féron F, Millet P. Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer’s disease. Mol Neurobiol 55: 6463–6479, 2018. doi: 10.1007/s12035-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takahashi S, Maeda T, Sano Y, Nishihara H, Takeshita Y, Shimizu F, Kanda T. Active form of vitamin D directly protects the blood–brain barrier in multiple sclerosis. Clin Exp Neuroimmunol 8: 244–254, 2017. doi: 10.1111/cen3.12398. [DOI] [Google Scholar]
- 114.Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne THJ. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res 241: 120–131, 2013. doi: 10.1016/j.bbr.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 115.Gatto NM, Paul KC, Sinsheimer JS, Bronstein JM, Bordelon Y, Rausch R, Ritz B. Vitamin D receptor gene polymorphisms and cognitive decline in Parkinson’s disease. J Neurol Sci 370: 100–106, 2016. doi: 10.1016/j.jns.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee YH, Kim J-H, Song GG. Vitamin D receptor polymorphisms and susceptibility to Parkinson’s disease and Alzheimer’s disease: a meta-analysis. Neurol Sci 35: 1947–1953, 2014. doi: 10.1007/s10072-014-1868-4. [DOI] [PubMed] [Google Scholar]
- 117.Burne THJ, McGrath JJ, Eyles DW, Mackay-Sim A. Behavioural characterization of Vitamin D receptor knockout mice. Behav Brain Res 157: 299–308, 2005. doi: 10.1016/j.bbr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 118.Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem 285: 37041–37050, 2010. doi: 10.1074/jbc.M110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zanello LP, Norman AW. Rapid modulation of osteoblast ion channel responses by 1 α,25(OH)2-vitamin D3 requires the presence of a functional vitamin D nuclear receptor. Proc Natl Acad Sci USA 101: 1589–1594, 2004. doi: 10.1073/pnas.0305802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen J, Doroudi M, Cheung J, Grozier AL, Schwartz Z, Boyan BD. Plasma membrane Pdia3 and VDR interact to elicit rapid responses to 1α,25(OH)2D3. Cell Signal 25: 2362–2373, 2013. doi: 10.1016/j.cellsig.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 121.Shen Z, Zhang X, Tang J, Kasiappan R, Jinwal U, Li P, Hann S, Nicosia SV, Wu J, Zhang X, Bai W. The coupling of epidermal growth factor receptor down regulation by 1alpha,25-dihydroxyvitamin D3 to the hormone-induced cell cycle arrest at the G1-S checkpoint in ovarian cancer cells. Mol Cell Endocrinol 338: 58–67, 2011. doi: 10.1016/j.mce.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poisbeau P, Aouad M, Gazzo G, Lacaud A, Kemmel V, Landel V, Lelievre V, Feron F. Cholecalciferol (vitamin D3) reduces rat neuropathic pain by modulating opioid signaling. Mol Neurobiol 56: 7208–7221, 2019. doi: 10.1007/s12035-019-1582-6. [DOI] [PubMed] [Google Scholar]
- 123.Kemény LV, Robinson KC, Hermann AL, Walker DM, Regan S, Yew YW, Lai YC, Theodosakis N, Rivera PD, Ding W, Yang L, Beyer T, Loh Y-HE, Lo JA, van der Sande AAJ, Sarnie W, Kotler D, Hsiao JJ, Su MY, Kato S, Kotler J, Bilbo SD, Chopra V, Salomon MP, Shen S, Hoon DSB, Asgari MM, Wakeman SE, Nestler EJ, Fisher DE. Vitamin D deficiency exacerbates UV/endorphin and opioid addiction. Sci Adv 7: eabe4577, 2021. doi: 10.1126/sciadv.abe4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Long W, Fatehi M, Soni S, Panigrahi R, Philippaert K, Yu Y, Kelly R, Boonen B, Barr A, Golec D, Campbell SA, Ondrusova K, Hubert M, Baldwin T, Lemieux MJ, Light PE. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J Physiol 598: 4321–4338, 2020. doi: 10.1113/JP279961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Akbar A, Yiangou Y, Facer P, Walters JRF, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57: 923–929, 2008. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, Witherspoon K, Gerdts J, Baker C, Vulto-van Silfhout AT, Schuurs-Hoeijmakers JH, Fichera M, Bosco P, Buono S, Alberti A, Failla P, Peeters H, Steyaert J, Vissers LELM, Francescatto L, Mefford HC, Rosenfeld JA, Bakken T, O'Roak BJ, Pawlus M, Moon R, Shendure J, Amaral DG, Lein E, Rankin J, Romano C, de Vries BBA, Katsanis N, Eichler EE. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 158: 263–276, 2014. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dockray GJ. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol 13: 954–958, 2013. doi: 10.1016/j.coph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 128.Savidge TC, Sofroniew MV, Neunlist M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab Invest 87: 731–736, 2007. doi: 10.1038/labinvest.3700600. [DOI] [PubMed] [Google Scholar]
- 129.Savidge T. S-nitrosothiol signals in the enteric nervous system: lessons learnt from big brother. Front Neurosci 5: 31, 2011. doi: 10.3389/fnins.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 146: 1500–1512, 2014. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pennisi E. Meet the psychobiome. Science 368: 570–573, 2020. doi: 10.1126/science.368.6491.570. [DOI] [PubMed] [Google Scholar]
- 132.Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 4: 623–632, 2019. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 133.Chao L, Liu C, Sutthawongwadee S, Li Y, Lv W, Chen W, Yu L, Zhou J, Guo A, Li Z, Guo S. Effects of probiotics on depressive or anxiety variables in healthy participants under stress conditions or with a depressive or anxiety diagnosis: a meta-analysis of randomized controlled trials. Front Neurol 11: 421, 2020. doi: 10.3389/fneur.2020.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noonan S, Zaveri M, Macaninch E, Martyn K. Food & mood: a review of supplementary prebiotic and probiotic interventions in the treatment of anxiety and depression in adults. BMJ Nutr Prev Health 3: 351–362, 2020. doi: 10.1136/bmjnph-2019-000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18: 666–673, 2013. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 136.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep 15: 1945–1956, 2016. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 137.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo P-M, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell 172: 500–516.e16, 2018. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dupont J-R, Jervis HR, Sprinz H. Auerbach’s plexus of the rat cecum in relation to the germfree state. J Comp Neurol 125: 11–18, 1965. doi: 10.1002/cne.901250103. [DOI] [PubMed] [Google Scholar]
- 139.McVey Neufeld KA, Mao YK, Bienenstock J, Foster JA, Kunze WA. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil 25: 183–e88, 2013. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 140.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173: 1728–1741.e13, 2018. [Erratum in Cell 174: 497, 2018]. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O'Donnell MP, Fox BW, Chao P-H, Schroeder FC, Sengupta P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 583: 415–420, 2020. doi: 10.1038/s41586-020-2395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dusková M, Hill M, Hanus M, Matousková M, Stárka L. Finasteride treatment and neuroactive steroid formation. Prague Med Rep 110: 222–230, 2009. [PubMed] [Google Scholar]
- 143.Melcangi RC, Caruso D, Abbiati F, Giatti S, Calabrese D, Piazza F, Cavaletti G. Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of post-finasteride patients showing persistent sexual side effects and anxious/depressive symptomatology. J Sex Med 10: 2598–2603, 2013. doi: 10.1111/jsm.12269. [DOI] [PubMed] [Google Scholar]
- 144.Basaria S, Jasuja R, Huang G, Wharton W, Pan H, Pencina K, Li Z, Travison TG, Bhawan J, Gonthier R, Labrie F, Dury AY, Serra C, Papazian A, O’Leary M, Amr S, Storer TW, Stern E, Bhasin S. Characteristics of men who report persistent sexual symptoms after finasteride use for hair loss. J Clin Endocrinol Metab 101: 4669–4680, 2016. doi: 10.1210/jc.2016-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Borgo F, Macandog AD, Diviccaro S, Falvo E, Giatti S, Cavaletti G, Melcangi RC. Alterations of gut microbiota composition in post-finasteride patients: a pilot study. J Endocrinol Invest 44: 1263–1273, 2021. doi: 10.1007/s40618-020-01424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takagi T, Naito Y, Inoue R, Kashiwagi S, Uchiyama K, Mizushima K, Tsuchiya S, Dohi O, Yoshida N, Kamada K, Ishikawa T, Handa O, Konishi H, Okuda K, Tsujimoto Y, Ohnogi H, Itoh Y. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol 54: 53–63, 2019. [Erratum in J Gastroenterol 54: 96–98, 2019]. doi: 10.1007/s00535-018-1488-5. [DOI] [PubMed] [Google Scholar]
- 147.Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J, Goedert JJ. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med 10: 253, 2012. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shin J-H, Park Y-H, Sim M, Kim S-A, Joung H, Shin D-M. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol 170: 192–201, 2019. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 149.Thompson RS, Vargas F, Dorrestein PC, Chichlowski M, Berg BM, Fleshner M. Dietary prebiotics alter novel microbial dependent fecal metabolites that improve sleep. Sci Rep 10: 3848, 2020. doi: 10.1038/s41598-020-60679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339: 1084–1088, 2013. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 151.Colldén H, Landin A, Wallenius V, Elebring E, Fändriks L, Nilsson ME, Ryberg H, Poutanen M, Sjögren K, Vandenput L, Ohlsson C. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Physiol 317: E1182–E1192, 2019. doi: 10.1152/ajpendo.00338.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mo Q, Lu S-F, Hu S, Simon NG. DHEA and DHEA sulfate differentially regulate neural androgen receptor and its transcriptional activity. Brain Res Mol Brain Res 126: 165–172, 2004. doi: 10.1016/j.molbrainres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 153.Wang P-H, Leu Y-L, Ismail W, Tang S-L, Tsai C-Y, Chen H-J, Kao A-T, Chiang Y-R. Anaerobic and aerobic cleavage of the steroid core ring structure by Steroidobacter denitrificans. J Lipid Res 54: 1493–1504, 2013. doi: 10.1194/jlr.M034223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tamura M, Hori S, Nakagawa H. Intestinal bacterium TM-30: an S-equol-producing bacterium isolated from human feces is involved in estrogen metabolism in vitro. Food Sci Technol Res 20: 309–316, 2014. doi: 10.3136/fstr.20.309. [DOI] [Google Scholar]
- 155.Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, Wang B, Hao L, Bhan AK, Kang JX. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6: 205, 2018. doi: 10.1186/s40168-018-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Z, Chen Y, Xiang L, Wang Z, Xiao GG, Hu J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 9: 1146, 2017. doi: 10.3390/nu9101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Wang L, Gonzalez FJ, Patterson AD, Liu H, Mu L, Zhou Z, Zhao Y, Li R, Liu P, Zhong C, Pang Y, Jiang C, Qiao J. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 25: 1225–1233, 2019. doi: 10.1038/s41591-019-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou L, Ni Z, Yu J, Cheng W, Cai Z, Yu C. Correlation between fecal metabolomics and gut microbiota in obesity and polycystic ovary syndrome. Front Endocrinol (Lausanne) 11: 628, 2020. doi: 10.3389/fendo.2020.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Diebel ME, Diebel LN, Liberati DM. Gender dimorphism in the gut: mucosal protection by estrogen stimulation of IgA transcytosis. J Trauma 71: 474–479, 2011. doi: 10.1097/ta.0b013e318228239d. [DOI] [PubMed] [Google Scholar]
- 160.Aguilar-Pimentel JA, Cho Y-L, Gerlini R, Calzada-Wack J, Wimmer M, Mayer-Kuckuk P, Adler T, Schmidt-Weber CB, Busch DH, Fuchs H, Gailus-Durner V, Ollert M, Hrabě de Angelis M, Ohlsson C, Poutanen M, Teperino R, Strauss L. Increased estrogen to androgen ratio enhances immunoglobulin levels and impairs B cell function in male mice. Sci Rep 10: 18334, 2020. doi: 10.1038/s41598-020-75059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558: 263–275, 2004. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Keskitalo A, Aatsinki A-K, Kortesluoma S, Pelto J, Korhonen L, Lahti L, Lukkarinen M, Munukka E, Karlsson H, Karlsson L. Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress 24: 551–560, 2021. doi: 10.1080/10253890.2021.1895110. [DOI] [PubMed] [Google Scholar]
- 163.Allen AP, Hutch W, Borre YE, Kennedy PJ, Temko A, Boylan G, Murphy E, Cryan JF, Dinan TG, Clarke G. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl Psychiatry 6: e939, 2016. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ergang P, Vagnerová K, Hermanová P, Vodička M, Jágr M, Šrůtková D, Dvořáček V, Hudcovic T, Pácha J. The gut microbiota affects corticosterone production in the murine small intestine. Int J Mol Sci 22: 4229, 2021. doi: 10.3390/ijms22084229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Feighner SD, Hylemon PB. Characterization of a corticosteroid 21-dehydroxylase from the intestinal anaerobic bacterium, Eubacterium lentum. J Lipid Res 21: 585–593, 1980. [PubMed] [Google Scholar]
- 166.Winter J, Bokkenheuser VD, Ponticorvo L. Bacterial metabolism of corticoids with particular reference to the 21-dehydroxylation. J Biol Chem 254: 2626–2629, 1979. doi: 10.1016/S0021-9258(17)30117-5. [DOI] [PubMed] [Google Scholar]
- 167.Ridlon J, Ikegawa S, Alves J, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, Guzman A, Cooper P, Buck G, Hylemon P. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res 54: 2437–2449, 2013. doi: 10.1194/jlr.M038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Luo Y, Zeng B, Zeng L, Du X, Li B, Huo R, Liu L, Wang H, Dong M, Pan J, Zheng P, Zhou C, Wei H, Xie P. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry 8: 187, 2018. doi: 10.1038/s41398-018-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duran-Pinedo AE, Solbiati J, Frias-Lopez J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. NPJ Biofilms Microbiomes 4: 25, 2018. doi: 10.1038/s41522-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jahnke JR, Roach J, Azcarate-Peril MA, Thompson AL. Maternal precarity and HPA axis functioning shape infant gut microbiota and HPA axis development in humans. PLoS One 16: e0251782, 2021. doi: 10.1371/journal.pone.0251782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zijlmans MAC, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology 53: 233–245, 2015. doi: 10.1016/j.psyneuen.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 172.Miyamori I, Koshida H, Matsubara T, Ikeda M, Takeda Y, Takeda R, Vecsei P. Role of intestinal bacteria in the metabolism of aldosterone in man. Horm Res 29: 147–150, 1988. doi: 10.1159/000180992. [DOI] [PubMed] [Google Scholar]
- 173.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall H-U, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17: 225–235, 2013. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 174.Grill J, Schneider F, Crociani J, Ballongue J. Purification and characterization of conjugated bile salt hydrolase from Bifidobacterium longum BB536. Appl Environ Microbiol 61: 2577–2582, 1995. doi: 10.1128/aem.61.7.2577-2582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hernández-Gómez JG, López-Bonilla A, Trejo-Tapia G, Ávila-Reyes SV, Jiménez-Aparicio AR, Hernández-Sánchez H. In vitro bile salt hydrolase (BSH) activity screening of different probiotic microorganisms. Foods 10: 674, 2021. doi: 10.3390/foods10030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tanaka H, Doesburg K, Iwasaki T, Mierau I. Screening of lactic acid bacteria for bile salt hydrolase activity. J Dairy Sci 82: 2530–2535, 1999. doi: 10.3168/jds.S0022-0302(99)75506-2. [DOI] [PubMed] [Google Scholar]
- 177.Al-Dury S, Marschall H-U. Ileal bile acid transporter inhibition for the treatment of chronic constipation, cholestatic pruritus, and NASH. Front Pharmacol 9: 931, 2018. doi: 10.3389/fphar.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Takamine F, Imamura T. Isolation and characterization of bile acid 7-dehydroxylating bacteria from human feces. Microbiol Immunol 39: 11–18, 1995. doi: 10.1111/j.1348-0421.1995.tb02162.x. [DOI] [PubMed] [Google Scholar]
- 179.Harris SC, Devendran S, Méndez-García C, Mythen SM, Wright CL, Fields CJ, Hernandez AG, Cann I, Hylemon PB, Ridlon JM. Bile acid oxidation by Eggerthella lenta strains C592 and DSM 2243(T). Gut Microbes 9: 523–539, 2018. doi: 10.1080/19490976.2018.1458180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marion S, Studer N, Desharnais L, Menin L, Escrig S, Meibom A, Hapfelmeier S, Bernier-Latmani R. In vitro and in vivo characterization of Clostridium scindens bile acid transformations. Gut Microbes 10: 481–503, 2019. doi: 10.1080/19490976.2018.1549420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 284: 1362–1365, 1999. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 182.Li N, Koester ST, Lachance DM, Dutta M, Cui JY, Dey N. Microbiome-encoded bile acid metabolism modulates colonic transit times. iScience 24: 102508, 2021. doi: 10.1016/j.isci.2021.102508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu Y, Villalobos-Hernandez EC, Pradhananga S, Baker CC, Keating C, Grundy D, Lomax AE, Reed DE. Deoxycholic acid activates colonic afferent nerves via 5-HT3 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 317: G275–G284, 2019. doi: 10.1152/ajpgi.00016.2019. [DOI] [PubMed] [Google Scholar]
- 184.Robben J, Caenepeel P, Van Eldere J, Eyssen H. Effects of intestinal microbial bile salt sulfatase activity on bile salt kinetics in gnotobiotic rats. Gastroenterology 94: 494–502, 1988. doi: 10.1016/0016-5085(88)90443-x. [DOI] [PubMed] [Google Scholar]
- 185.van Best N, Rolle-Kampczyk U, Schaap FG, Basic M, Olde Damink SWM, Bleich A, Savelkoul PHM, von Bergen M, Penders J, Hornef MW. Bile acids drive the newborn’s gut microbiota maturation. Nat Commun 11: 3692, 2020. doi: 10.1038/s41467-020-17183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li M, Liu S, Wang M, Hu H, Yin J, Liu C, Huang Y. Gut microbiota dysbiosis associated with bile acid metabolism in neonatal cholestasis disease. Sci Rep 10: 7686, 2020. doi: 10.1038/s41598-020-64728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cabrera-Rubio R, Patterson AM, Cotter PD, Beraza N. Cholestasis induced by bile duct ligation promotes changes in the intestinal microbiome in mice. Sci Rep 9: 12324, 2019. doi: 10.1038/s41598-019-48784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Islam KBMS, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773–1781, 2011. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 189.Van den Bossche L, Hindryckx P, Devisscher L, Devriese S, Van Welden S, Holvoet T, Vilchez-Vargas R, Vital M, Pieper DH, Vanden Bussche J, Vanhaecke L, Van de Wiele T, De Vos M, Laukens D. Ursodeoxycholic acid and its taurine- or glycine-conjugated species reduce colitogenic dysbiosis and equally suppress experimental colitis in mice. Appl Environ Microbiol 83: e02766-16, 2017. doi: 10.1128/AEM.02766-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol 188: 1979–1986, 2006. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Deitch EA, Sittig K, Li M, Berg R, Specian RD. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg 159: 79–84, 1990. doi: 10.1016/S0002-9610(05)80610-5. [DOI] [PubMed] [Google Scholar]
- 192.Parks RW, Clements WD, Pope C, Halliday MI, Rowlands BJ, Diamond T. Bacterial translocation and gut microflora in obstructive jaundice. J Anat 189: 561–565, 1996. [PMC free article] [PubMed] [Google Scholar]
- 193.Sheng L, Jena PK, Liu H-X, Kalanetra KM, Gonzalez FJ, French SW, Krishnan VV, Mills DA, Wan Y-JY. Gender differences in bile acids and microbiota in relationship with gender dissimilarity in steatosis induced by diet and FXR inactivation. Sci Rep 7: 1748, 2017. doi: 10.1038/s41598-017-01576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Inagaki T, Moschetta A, Lee Y-K, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 103: 3920–3925, 2006. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aguirre AM, Yalcinkaya N, Wu Q, Swennes A, Tessier ME, Roberts P, Miyajima F, Savidge T, Sorg JA. Bile acid-independent protection against Clostridioides difficile infection. PLoS Pathog 17: e1010015, 2021. doi: 10.1371/journal.ppat.1010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wu S, Yoon S, Zhang Y-G, Lu R, Xia Y, Wan J, Petrof EO, Claud EC, Chen D, Sun J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol 309: G341–G349, 2015. doi: 10.1152/ajpgi.00105.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab 98: 2944–2951, 2013. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]
- 198.Gokhale S, Bhaduri A. Provitamin D3 modulation through prebiotics supplementation: simulation based assessment. Sci Rep 9: 19267, 2019. doi: 10.1038/s41598-019-55699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Appleyard CB, Cruz ML, Isidro AA, Arthur JC, Jobin C, De Simone C. Pretreatment with the probiotic VSL#3 delays transition from inflammation to dysplasia in a rat model of colitis-associated cancer. Am J Physiol Gastrointest Liver Physiol 301: G1004–G1013, 2011. doi: 10.1152/ajpgi.00167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kawauchi H, Sasaki J, Adachi T, Hanada K, Beppu T, Horinouchi S. Cloning and nucleotide sequence of a bacterial cytochrome P-450VD25 gene encoding vitamin D-3 25-hydroxylase. Biochim Biophys Acta 1219: 179–183, 1994. doi: 10.1016/0167-4781(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 201.Sawada N, Sakaki T, Yoneda S, Kusudo T, Shinkyo R, Ohta M, Inouye K. Conversion of vitamin D3 to 1α,25-dihydroxyvitamin D3 by Streptomyces griseolus cytochrome P450SU-1. Biochem Biophys Res Commun 320: 156–164, 2004. doi: 10.1016/j.bbrc.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 202.Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, Neale RE. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr 58: 2895–2910, 2019. doi: 10.1007/s00394-018-1842-7. [DOI] [PubMed] [Google Scholar]
- 203.Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep 10: 21641, 2020. doi: 10.1038/s41598-020-77806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, Venkatesan A, Fraser CM, Mowry EM. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med 63: 729–734, 2015. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Charoenngam N, Shirvani A, Kalajian TA, Song A, Holick MF. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: a randomized, double-blinded, dose-response study. Anticancer Res 40: 551–556, 2020. doi: 10.21873/anticanres.13984. [DOI] [PubMed] [Google Scholar]
- 206.Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet 48: 1396–1406, 2016. doi: 10.1038/ng.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Assa A, Vong L, Pinnell LJ, Rautava J, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency predisposes to adherent-invasive Escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflamm Bowel Dis 21: 297–306, 2015. doi: 10.1097/MIB.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 208.He L, Zhou M, Li YC. Vitamin D/vitamin D receptor signaling is required for normal development and function of group 3 innate lymphoid cells in the gut. iScience 17: 119–131, 2019. doi: 10.1016/j.isci.2019.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu S, Zhang Y-G, Lu R, Xia Y, Zhou D, Petrof EO, Claud EC, Chen D, Chang EB, Carmeliet G, Sun J. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 64: 1082–1094, 2015. doi: 10.1136/gutjnl-2014-307436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 211.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 12: 57, 2012. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, Meddings J, O’Sullivan M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J 3: 294–302, 2015. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH, Hanrahan JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173: 2909–2912, 2004. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 214.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L; MICRO-Obes Consortium; Dumas M-E, Rizkalla SW, Doré J, Cani PD, Clément K. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436, 2016. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 215.Earley H, Lennon G, Balfe Á, Coffey JC, Winter DC, O'Connell PR. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep 9: 15683, 2019. doi: 10.1038/s41598-019-51878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer’s disease. Biol Psychiatry 60: 1287–1294, 2006. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 217.Pan X, Wu X, Kaminga AC, Wen SW, Liu A. Dehydroepiandrosterone and dehydroepiandrosterone sulfate in Alzheimer’s disease: a systematic review and meta-analysis. Front Aging Neurosci 11: 61, 2019. doi: 10.3389/fnagi.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang JM, Singh C, Liu L, Irwin RW, Chen S, Chung EJ, Thompson RF, Brinton RD. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 107: 6498–6503, 2010. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Webber KM, Stocco DM, Casadesus G, Bowen RL, Atwood CS, Previll LA, Harris PLR, Zhu X, Perry G, Smith MA. Steroidogenic acute regulatory protein (StAR): evidence of gonadotropin-induced steroidogenesis in Alzheimer disease. Mol Neurodegener 1: 14, 2006. doi: 10.1186/1750-1326-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Weill-Engerer S, David J-P, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu E-E, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab 87: 5138–5143, 2002. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- 221.Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem 86: 848–859, 2003. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 222.Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, Earls JC, Zimmer A, Glusman G, Robinson M, Yurkovich JT, Kado DM, Cauley JA, Zmuda J, Lane NE, Magis AT, Lovejoy JC, Hood L, Gibbons SM, Orwoll ES, Price ND. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab 3: 274–286, 2021. [Erratum in Nat Metab 3: 586, 2021]. doi: 10.1038/s42255-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dos Santos Guilherme M, Todorov H, Osterhof C, Möllerke A, Cub K, Hankeln T, Gerber S, Endres K. Impact of acute and chronic amyloid-β peptide exposure on gut microbial commensals in the mouse. Front Microbiol 11: 1008, 2020. doi: 10.3389/fmicb.2020.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Guo M, Peng J, Huang X, Xiao L, Huang F, Zuo Z. Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis 80: 299–310, 2021. doi: 10.3233/JAD-201040. [DOI] [PubMed] [Google Scholar]
- 225.Kundu P, Torres ERS, Stagaman K, Kasschau K, Okhovat M, Holden S, Ward S, Nevonen KA, Davis BA, Saito T, Saido TC, Carbone L, Sharpton TJ, Raber J. Integrated analysis of behavioral, epigenetic, and gut microbiome analyses in AppNL-G-F, AppNL-F, and wild type mice. Sci Rep 11: 4678, 2021. doi: 10.1038/s41598-021-83851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, Zhang L, Jia L, Yue S, Zhou K, Li L, Luo B, Wang B. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 80: 633–643, 2019. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 227.Zhuang Z-Q, Shen L-L, Li W-W, Fu X, Zeng F, Gui L, Lü Y, Cai M, Zhu C, Tan Y-L, Zheng P, Li H-Y, Zhu J, Zhou H-D, Bu X-L, Wang Y-J. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimers Dis 63: 1337–1346, 2018. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 228.Arora K, Green M, Prakash S. The microbiome and Alzheimer’s disease: potential and limitations of prebiotic, synbiotic, and probiotic formulations. Front Bioeng Biotechnol 8: 537847, 2020. doi: 10.3389/fbioe.2020.537847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—an emerging role for gut microbiome. Alzheimers Dement 15: 76–92, 2019. [Erratum in Alzheimers Dement 15: 604, 2019]. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, Wang J-Y, Cheng E, Meyer F, Wang DH, Zhang Q, Spechler SJ, Souza RF. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κB activation in benign Barrett’s epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G278–G286, 2011. doi: 10.1152/ajpgi.00092.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ignacio Barrasa J, Olmo N, Pérez-Ramos P, Santiago-Gómez A, Lecona E, Turnay J, Antonia Lizarbe M. Deoxycholic and chenodeoxycholic bile acids induce apoptosis via oxidative stress in human colon adenocarcinoma cells. Apoptosis 16: 1054–1067, 2011. doi: 10.1007/s10495-011-0633-x. [DOI] [PubMed] [Google Scholar]
- 232.Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310: 561–577, 2015. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 233.Limon A, Reyes-Ruiz JM, Miledi R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc Natl Acad Sci USA 109: 10071–10076, 2012. doi: 10.1073/pnas.1204606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pike CJ, Cotman CW. Cultured GABA-immunoreactive neurons are resistant to toxicity induced by β-amyloid. Neuroscience 56: 269–274, 1993. doi: 10.1016/0306-4522(93)90331-9. [DOI] [PubMed] [Google Scholar]
- 235.Ulrich D. Amyloid-β impairs synaptic inhibition via GABAA receptor endocytosis. J Neurosci 35: 9205–9210, 2015. doi: 10.1523/JNEUROSCI.0950-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149: 708–721, 2012. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sun X, Meng X, Zhang J, Li Y, Wang L, Qin X, Sui N, Zhang Y. GABA attenuates amyloid toxicity by downregulating its endocytosis and improves cognitive impairment. J Alzheimers Dis 31: 635–649, 2012. doi: 10.3233/JAD-2012-120535. [DOI] [PubMed] [Google Scholar]
- 238.Bai X, Edden RAE, Gao F, Wang G, Wu L, Zhao B, Wang M, Chan Q, Chen W, Barker PB. Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J Magn Reson Imaging 41: 1326–1331, 2015. doi: 10.1002/jmri.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xilouri M, Papazafiri P. Anti-apoptotic effects of allopregnanolone on P19 neurons. Eur J Neurosci 23: 43–54, 2006. doi: 10.1111/j.1460-9568.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- 240.Lapchak PA, Chapman DF, Nunez SY, Zivin JA. Dehydroepiandrosterone sulfate is neuroprotective in a reversible spinal cord ischemia model. Stroke 31: 1953–1957, 2000. doi: 10.1161/01.STR.31.8.1953. [DOI] [PubMed] [Google Scholar]
- 241.Kodis EJ, Choi S, Swanson E, Ferreira G, Bloom GS. N-methyl-D-aspartate receptor-mediated calcium influx connects amyloid-β oligomers to ectopic neuronal cell cycle reentry in Alzheimer’s disease. Alzheimers Dement 14: 1302–1312, 2018. doi: 10.1016/j.jalz.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. Protective effects of 1 α,25-(OH)2D3 against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology 40: 761–771, 2001. doi: 10.1016/s0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 243.Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA 95: 1852–1857, 1998. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany R-M. Allopregnanolone attenuates N-methyl-d-aspartate-induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci Lett 328: 33–36, 2002. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- 245.Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA 89: 1567–1571, 1992. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci USA 92: 10806–10810, 1995. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mathis C, Vogel E, Cagniard B, Criscuolo F, Ungerer A. The neurosteroid pregnenolone sulfate blocks deficits induced by a competitive NMDA antagonist in active avoidance and lever-press learning tasks in mice. Neuropharmacology 35: 1057–1064, 1996. doi: 10.1016/S0028-3908(96)00041-X. [DOI] [PubMed] [Google Scholar]
- 248.Zhuang Z, Yang R, Wang W, Qi L, Huang T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J Neuroinflammation 17: 288, 2020. doi: 10.1186/s12974-020-01961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23: 255–264, e119, 2011. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 250.Chen Q, Ma H, Guo X, Liu J, Gui T, Gai Z. Farnesoid X receptor (FXR) aggravates amyloid-β-triggered apoptosis by modulating the cAMP-response element-binding protein (CREB)/brain-derived neurotrophic factor (BDNF) pathway in vitro. Med Sci Monit 25: 9335–9345, 2019. doi: 10.12659/MSM.920065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wu X, Lv Y-G, Du Y-F, Chen F, Reed MN, Hu M, Suppiramaniam V, Tang S-S, Hong H. Neuroprotective effects of INT-777 against Aβ1-42-induced cognitive impairment, neuroinflammation, apoptosis, and synaptic dysfunction in mice. Brain Behav Immun 73: 533–545, 2018. doi: 10.1016/j.bbi.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 252.Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry 74: 357–366, 2013. doi: 10.1016/j.biopsych.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lesuis SL, Weggen S, Baches S, Lucassen PJ, Krugers HJ. Targeting glucocorticoid receptors prevents the effects of early life stress on amyloid pathology and cognitive performance in APP/PS1 mice. Transl Psychiatry 8: 53, 2018. doi: 10.1038/s41398-018-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Crouzet L, Gaultier E, Del'Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil 25: e272–e282, 2013. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 255.Hubbard CS, Karpowicz JM, Furman AJ, da Silva JT, Seminowicz DA, Traub RJ. Estrogen-dependent visceral hypersensitivity following stress in rats: an fMRI study. Mol Pain 12: 174480691665414, 2016. doi: 10.1177/1744806916654145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yamagata K, Sugimura M, Yoshida M, Sekine S, Kawano A, Oyamaguchi A, Maegawa H, Niwa H. Estrogens exacerbate nociceptive pain via up-regulation of TRPV1 and ANO1 in trigeminal primary neurons of female rats. Endocrinology 157: 4309–4317, 2016. doi: 10.1210/en.2016-1218. [DOI] [PubMed] [Google Scholar]
- 257.Nakai A, Kumakura Y, Boivin M, Rosa P, Diksic M, D’Souza D, Kersey K. Sex differences of brain serotonin synthesis in patients with irritable bowel syndrome using α-[11C]methyl-l-tryptophan, positron emission tomography and statistical parametric mapping. Can J Gastroenterol 17: 572127, 2003. doi: 10.1155/2003/572127. [DOI] [PubMed] [Google Scholar]
- 258.Yaghoubfar R, Behrouzi A, Ashrafian F, Shahryari A, Moradi HR, Choopani S, Hadifar S, Vaziri F, Nojoumi SA, Fateh A, Khatami S, Siadat SD. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci Rep 10: 22119, 2020. doi: 10.1038/s41598-020-79171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion 63: 188–194, 2001. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 260.Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, Zima AV, Brown IA, Dugas LR, Zakharian E, Aubert G, Alonzo F, Calcutt NA, Mansuy-Aubert V. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci USA 117: 26482–26493, 2020. doi: 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sugaya N, Izawa S, Kimura K, Ogawa N, Yamada KC, Shirotsuki K, Mikami I, Hirata K, Nagano Y, Nomura S, Shimada H. Adrenal hormone response and psychophysiological correlates under psychosocial stress in individuals with irritable bowel syndrome. Int J Psychophysiol 84: 39–44, 2012. doi: 10.1016/j.ijpsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 262.Qi Q, Chen F, Zhang W, Wang P, Li Y, Zuo X. Colonic N-methyl-d-aspartate receptor contributes to visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol 32: 828–836, 2017. doi: 10.1111/jgh.13588. [DOI] [PubMed] [Google Scholar]
- 263.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation α2δ ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut 56: 1218–1225, 2007. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 22: 981–988, 2005. doi: 10.1111/j.1365-2036.2005.02685.x. [DOI] [PubMed] [Google Scholar]
- 265.Aggarwal S, Ahuja V, Paul J. Dysregulation of GABAergic signalling contributes in the pathogenesis of diarrhea-predominant irritable bowel syndrome. J Neurogastroenterol Motil 24: 422–430, 2018. doi: 10.5056/jnm17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Matsunami RK, Lugo M, Major A, Mori-Akiyama Y, Hollister EB, Dann SM, Shi XZ, Engler DA, Savidge T, Versalovic J. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 29: e12904, 2017. doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin Gastroenterol Hepatol 16: 522–527, 2018. doi: 10.1016/j.cgh.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhao L, Yang W, Chen Y, Huang F, Lu L, Lin C, Huang T, Ning Z, Zhai L, Zhong LLD, Lam W, Yang Z, Zhang X, Cheng C, Han L, Qiu Q, Shang X, Huang R, Xiao H, Ren Z, Chen D, Sun S, El-Nezami H, Cai Z, Lu A, Fang X, Jia W, Bian Z. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J Clin Invest 130: 438–450, 2020. doi: 10.1172/JCI130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Martínez I, Perdicaro DJ, Brown AW, Hammons S, Carden TJ, Carr TP, Eskridge KM, Walter J. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl Environ Microbiol 79: 516–524, 2013. doi: 10.1128/AEM.03046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Barandouzi ZA, Lee J, Maas K, Starkweather AR, Cong XS. Altered gut microbiota in irritable bowel syndrome and its association with food components. J Pers Med 11: 35, 2021. doi: 10.3390/jpm11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Hollister EB, Oezguen N, Chumpitazi BP, Luna RA, Weidler EM, Rubio-Gonzales M, Dahdouli M, Cope JL, Mistretta T-A, Raza S, Metcalf GA, Muzny DM, Gibbs RA, Petrosino JF, Heitkemper M, Savidge TC, Shulman RJ, Versalovic J. Leveraging human microbiome features to diagnose and stratify children with irritable bowel syndrome. J Mol Diagn 21: 449–461, 2019. doi: 10.1016/j.jmoldx.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Klingberg S, Ellegård L, Johansson I, Hallmans G, Weinehall L, Andersson H, Winkvist A. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am J Clin Nutr 87: 993–1001, 2008. doi: 10.1093/ajcn/87.4.993. [DOI] [PubMed] [Google Scholar]
- 273.Jansen PJ, Lütjohann D, Abildayeva K, Vanmierlo T, Plösch T, Plat J, von Bergmann K, Groen AK, Ramaekers FCS, Kuipers F, Mulder M. Dietary plant sterols accumulate in the brain. Biochim Biophys Acta 1761: 445–453, 2006. doi: 10.1016/j.bbalip.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 274.Vanmierlo T, Rutten K, van Vark-van der Zee LC, Friedrichs S, Bloks VW, Blokland A, Ramaekers FC, Sijbrands E, Steinbusch H, Prickaerts J, Kuipers F, Lütjohann D, Mulder M. Cerebral accumulation of dietary derivable plant sterols does not interfere with memory and anxiety related behavior in Abcg5−/− mice. Plant Foods Hum Nutr 66: 149–156, 2011. doi: 10.1007/s11130-011-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Burg VK, Grimm HS, Rothhaar TL, Grösgen S, Hundsdörfer B, Haupenthal VJ, Zimmer VC, Mett J, Weingärtner O, Laufs U, Broersen LM, Tanila H, Vanmierlo T, Lütjohann D, Hartmann T, Grimm MOW. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J Neurosci 33: 16072–16087, 2013. doi: 10.1523/JNEUROSCI.1506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Park SJ, Kim DH, Jung JM, Kim JM, Cai M, Liu X, Hong JG, Lee CH, Lee KR, Ryu JH. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur J Pharmacol 676: 64–70, 2012. doi: 10.1016/j.ejphar.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 277.Panayotis N, Freund PA, Marvaldi L, Shalit T, Brandis A, Mehlman T, Tsoory MM, Fainzilber M. β-Sitosterol reduces anxiety and synergizes with established anxiolytic drugs in mice. Cell Rep Med 2: 100281, 2021. doi: 10.1016/j.xcrm.2021.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yin Y, Liu X, Liu J, Cai E, Zhao Y, Li H, Zhang L, Li P, Gao Y. The effect of beta-sitosterol and its derivatives on depression by the modification of 5-HT, DA and GABA-ergic systems in mice. RSC Adv 8: 671–680, 2018. doi: 10.1039/C7RA11364A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Carter BA, Taylor OA, Prendergast DR, Zimmerman TL, Von Furstenberg R, Moore DD, Karpen SJ. Stigmasterol, a soy lipid–derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res 62: 301–306, 2007. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 280.Schiepers OJG, de Groot RHM, van Boxtel MPJ, Jolles J, de Jong A, Lütjohann D, Plat J, Mensink RP. Consuming functional foods enriched with plant sterol or stanol esters for 85 weeks does not affect neurocognitive functioning or mood in statin-treated hypercholesterolemic individuals. J Nutr 139: 1368–1373, 2009. doi: 10.3945/jn.108.103721. [DOI] [PubMed] [Google Scholar]
- 281.Aldini R, Micucci M, Cevenini M, Fato R, Bergamini C, Nanni C, Cont M, Camborata C, Spinozzi S, Montagnani M, Roda G, D’Errico-Grigioni A, Rosini F, Roda A, Mazzella G, Chiarini A, Budriesi R. Antiinflammatory effect of phytosterols in experimental murine colitis model: prevention, induction, remission study. PLoS One 9: e108112, 2014. doi: 10.1371/journal.pone.0108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wong A. Chemical and microbiological considerations of phytosterols and their relative efficacies in functional foods for the lowering of serum cholesterol levels in humans: a review. J Funct Foods 6: 60–72, 2014. doi: 10.1016/j.jff.2013.10.023. [DOI] [Google Scholar]
- 283.Cuevas-Tena M, Gómez del Pulgar EM, Benítez-Páez A, Sanz Y, Alegría A, Lagarda MJ. Plant sterols and human gut microbiota relationship: an in vitro colonic fermentation study. J Funct Foods 44: 322–329, 2018. doi: 10.1016/j.jff.2018.03.023. [DOI] [Google Scholar]
- 284.Song L, Li Y, Qu D, Ouyang P, Ding X, Wu P, Guan Q, Yang L. The regulatory effects of phytosterol esters (PSEs) on gut flora and faecal metabolites in rats with NAFLD. Food Funct 11: 977–991, 2020. doi: 10.1039/C9FO01570A. [DOI] [PubMed] [Google Scholar]