Keywords: MIST1, trafficking, vesicle, zymogenic cells
Abstract
A single transcription factor, MIST1 (BHLHA15), maximizes secretory function in diverse secretory cells (like pancreatic acinar cells) by transcriptionally upregulating genes that elaborate secretory architecture. Here, we show that the scantly studied MIST1 target, ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1), is an evolutionarily conserved, novel mannose-6-phosphate receptor (M6PR) domain-containing protein. ELAPOR1 expression was specific to zymogenic cells (ZCs, the MIST1-expressing population in the stomach). ELAPOR1 expression was lost as tissue injury caused ZCs to undergo paligenosis (i.e., to become metaplastic and reenter the cell cycle). In cultured cells, ELAPOR1 trafficked with cis-Golgi resident proteins and with the trans-Golgi and late endosome protein: cation-independent M6PR. Secretory vesicle trafficking was disrupted by expression of ELAPOR1 truncation mutants. Mass spectrometric analysis of co-immunoprecipitated proteins showed ELAPOR1 and CI-M6PR shared many binding partners. However, CI-M6PR and ELAPOR1 must function differently, as CI-M6PR co-immunoprecipitated more lysosomal proteins and was not decreased during paligenosis in vivo. We generated Elapor1−/− mice to determine ELAPOR1 function in vivo. Consistent with in vitro findings, secretory granule maturation was defective in Elapor1−/− ZCs. Our results identify a role for ELAPOR1 in secretory granule maturation and help clarify how a single transcription factor maintains mature exocrine cell architecture in homeostasis and helps dismantle it during paligenosis.
NEW & NOTEWORTHY Here, we find the MIST1 (BHLHA15) transcriptional target ELAPOR1 is an evolutionarily conserved, trans-Golgi/late endosome M6PR domain-containing protein that is specific to gastric zymogenic cells and required for normal secretory granule maturation in human cell lines and in mouse stomach.
INTRODUCTION
Exocrine secretory cells throughout the body use a simple two-step transcription factor sequence to induce and maintain a long-lived, protein-secreting phenotype. The bZIP domain transcription factor, XBP1, massively upregulates rough endoplasmic reticulum (rER), which increases protein production. XBP1 also directly binds and induces expression of MIST1 (a.k.a. BHLHA15), a highly conserved basic helix-loop-helix (bHLH) transcription factor that scales up and maintains the apical secretory cellular architecture, in particular, the large secretory granules harboring cargo like digestive enzymes (1–8). Constitutive loss of MIST1 leads to defective, deficient zymogenic cell (ZC) granules and loss of normal basal nuclear localization (9). In addition, deleting MIST1 in already mature ZCs recapitulates the Mist1 knockout phenotype, and the ectopic expression of the protein is sufficient to scale up the secretory apparatus in parietal cells or hepatocytes which normally lack MIST1, proving MIST1 is a bona fide “scaling factor” that is both necessary and sufficient for maintaining secretory cell architecture (1, 10).
MIST1 exerts its function through transcriptional activation of multiple downstream targets, many of whose function has been elucidated in the past decade. For example, MIST1 induces the E3-ubiquitin ligase Mindbomb 1 (MIB1), which is critical for endocytic trafficking (9). Without MIB1, MIST1-expressing cells mislocalize apical death-associated protein kinase-1 (DAPK1), apical microtubules, secretory granules, and basal nuclei (9). Another downstream target, a small protein GTPase RAB26, the expression of which is largely confined to MIST1-expressing secretory tissues, coordinates redistribution of lysosomes and mitochondria to aid in proper secretory cell function (11). The MIST1 target, CCPG1, is critical in regulating targeted autophagy of the rER, in turn maintaining production and maintenance of secreted cargo and secretory granules (12).
In a recent screen, one of the most conserved targets of MIST1 (across tissues and species) was a scantly characterized gene previously designated EIG121/KIAA1324 in humans and 5330417C22Rik in mice (13). Recently redesignated ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator) gene encodes a protein whose molecular characterization has been limited, with one previous report being the basis for its name: that is, showing the protein localizing to endosomes and lysosomes with effects upon gain or loss of function on autophagy (14). There have been no prior reports on mouse ELAPOR1 nor on mice null for Elapor1. As ELAPOR1 is a MIST1 target, and MIST1 is lost early in progression to gastric cancer (15), it is not surprising that the only other manuscript concerning ELAPOR1 specifically showed it was expressed in normal human stomach and lost in gastric cancer (16).
The loss of MIST1 in carcinogenesis is because MIST1 is rapidly downregulated after an injury that induces paligenosis, the conserved cellular program for reprogramming mature cells into regenerative progenitors (17, 18). This rapid loss of MIST1 has been demonstrated in paligenosis of ZCs in stomach (9, 15, 19, 20), pancreas (21, 22), and salivary gland (23). MIST1 is likely shut off to allow cells to downscale their secretory apparatus to retool as wound-healing cells, because large-scale secretion of digestive enzymes would be counterproductive to repair. More specifically, loss of MIST1 targets like ELAPOR1 must help permit or facilitate the massive cellular reorganization, and thus, understanding MIST1 targets helps us understand this key process that is critical for metaplasia and tumorigenesis (24, 25).
Here, we confirm the initial functional characterization of this MIST1 target and detail for the first time its role in normal exocrine secretory cells in vitro and in vivo within cells in tissue. We confirm ELAPOR1 is expressed in normal stomach corpus and show that its expression is specifically within the MIST1-regulated cell lineage: digestive-enzyme-secreting ZCs. We analyze truncation mutants of ELAPOR1 in vitro, showing that ELAPOR1 largely localizes to trans-Golgi and late endosomes. We also demonstrate it is critical for proper trafficking of vesicles containing transfected secretory cargo. Moreover, for the first time, we characterize Elapor1–/– mice. Consistent with in vitro results and with its role as a MIST1 target, we find that ELAPOR1 is necessary for proper maturation of zymogenic granules in mouse stomach. Our results characterize a scantly understood new gene and reveal another important molecular mechanism underlying how the universal secretory cell architect MIST1 orchestrates secretory function during homeostasis and cell dismantling in paligenosis.
MATERIALS AND METHODS
Mice
All experiments involving animals were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Germline Elapor1−/− mice were generated as part of a workflow pipeline that generated deletion mutants of 472 genes that has been described previously (26). Briefly, targeted or gene trap mutations in this workflow are generated in strain 129S5/SvEvBrd-derived embryonic stem (ES) cells with chimeric mice bred to C57BL/6-Tyrc-Brd albino mice to generate F1 heterozygotes. Those are intercrossed to generate F2 wild-type, heterozygous, and homozygous mutant progeny. In some cases of few F1 mice being obtained from the chimera, F1 heterozygous mice are crossed to 129S5/SvEvBrd × C57BL/6 hybrid mice to yield additional heterozygotes intercross to generate F2 mice. Tamoxifen (250 mg/kg body wt; Toronto Research Chemicals, Inc., Toronto, Canada) was prepared in a 10% ethanol and 90% sunflower oil solution by sonication and administered intraperitoneally once every day for 3 days to induce paligenosis in the stomach as previously described (27). All mice used were between 8 and 12 wk old, and both sexes were used. There were no differences in results observed between male and female mice. When possible, mice of equivalent age and sex (littermate controls) were used for experimental and control groups; otherwise, mice were randomly chosen and sorted into experimental groups. Primer sequences used for genotyping are shown in Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.5281/zenodo.5598174).
Immunoblot and Immunoprecipitation
Approximately 35 to 70 μg mouse corpus stomach tissues were lysed in T-PER Tissue Protein Extraction Reagent buffer (Thermo) with protease inhibitors (Thermo). Protein was separated using a 4%–12% gradient SDS-PAGE gel and transferred to a PVDF membrane, blocked with 3% bovine serum albumin (BSA) in Tris-buffered saline with 0.05% Tween-20 (TBS-T), and then incubated for 16 h at 4°C with primary antibodies (see Supplemental Table S2 for the list of antibodies). The membranes were washed three times in TBS-T, incubated for 1 h at room temperature in horseradish peroxidase (HRP)-conjugated secondary antibody in 5% nonfat dairy milk or 3% BSA in TBS-T. Secondary antibody signals were imaged and detected by SuperSignal West Pico PLUS Chemiluminescent Substrate Kit (Thermo). When the LI-COR system was used, fluorescent intensity values were determined and quantified using Image Studio Lite Ver 5.2 software (LI-COR Biosciences). Protein signal intensities were normalized against a β-actin loading control for each sample.
For immunoprecipitation, cell lysates were prepared using IP lysis buffer (Thermo) with protease inhibitor. Lysate was separated from cell debris via centrifugation at 14,000 rpm for 10 min at 4°C. Then, 50 μL of protein A beads were washed with phosphate-buffered saline (PBS) with 0.02% Tween-20 (PBS-T) and incubated with either CI-M6PR antibody or normal rabbit IgG (negative control; Cell Signaling) for 10 min at room temperature, washed three times in PBS-T, and incubated overnight at 4°C with rotation. To immunoprecipitate green fluorescent protein (GFP) tagged ELAPOR1, GFP-Trap Dynabeads (Chromotek) were used. After overnight incubation, the beads were washed three times in PBS-T followed by PBS and were used for mass spectrometry or boiled with sample buffer and reducing agent (Thermo) for immunoblotting.
Plasmid Constructs
Mouse Elapor1 cDNA was purchased from GenScript and cloned into pcDNA3.1 (Flag). To construct enhanced green fluorescent protein (EGFP) fusion, full-length cDNA (NheI/SacII) fragments were subcloned into pEGFP-N1 vector (Clontech) and confirmed by Sanger sequencing. Two deleted forms (Δ1-4 in which codons encoding residues 89–368 were deleted; Δ5-6 in which residues 659–856 were deleted) were constructed by the site-directed mutagenesis.
Cell Culture and Transfection
AGS and AR42J cells were cultured in RPMI-1640 medium with 10% fetal bovine serum and Primocin. AR42J cells were additionally supplemented with 2 mM Glutamax (Gibco). LS174T cells were cultured in DMEM medium with 10% fetal bovine serum and Primocin. All cells were maintained at 37°C in a 5% CO2 humidified atmosphere. To induce differentiation of AR42J cells (which increases the endogenous MIST1 expression and secretory apparatus), the cells were incubated for 72 h in culture medium supplemented with 200 nM dexamethasone (Sigma-Aldrich). The plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Lysotracker was incubated for 1 h in the cell culture media at concentration of 50 nM per instruction from the supplier.
Imaging and Tissue Analysis
Mouse tissue was fixed for 16 h at 4°C in 10% formalin, washed in PBS followed by 70% ethanol. All stomach samples were immobilized in 3% agar, embedded in paraffin, and sectioned at 5 to 7 µm thickness. For IHC, sections were deparaffinized in Histo-Clear (National Diagnostics) and rehydrated in a gradient of ethanol, quenched for endogenous peroxidase for 15 min. Heat-induced epitope retrieval was conducted in sodium citrate buffer (pH 6.0). Samples were subsequently blocked with 5% normal serum in 0.15% Triton X-100 for 1 h at room temperature and incubated for 16 h at 4°C with primary antibodies. After washing in PBS three times, samples were incubated for 1 h with biotin-conjugated secondary antibodies followed by additional 1 h with avidin/biotin complex (ABC, Vector Laboratories) at room temperature. Sections were developed with DAB Substrate Kit then counterstained in hematoxylin and eosin before mounting. For immunofluorescence (IF) staining, sections were deparaffinized in xylene and rehydrated using isopropanol and underwent heat-induced epitope retrieval in sodium citrate buffer (pH 6.0). Samples were then blocked with 1% BSA in 0.15% Triton X-100 for 1 h at room temperature and incubated overnight with primary antibodies. Primary antibodies were detected with Alexa-Fluor (Invitrogen) secondary antibodies and mounted in ProLong Gold antifade mountant with 4′,6-diamidino-2-phenylindole (DAPI) added to detect nuclei in IF images. IF images were taken either on a Zeiss Apotome or LSM880 Laser Scanning Confocal Microscope (Zeiss), keeping constant exposure times across samples to be compared.
IF of cells was performed on the cultures grown on coverslips in 12-well culture plates. Cells were fixed with 10% formalin, washed three times in PBS, blocked with 1% BSA in 0.15% Triton X-100 for 1 h at room temperature and incubated overnight with primary antibodies. After washing in PBS three times, samples were incubated for 1 h with Alexa-Fluor (Invitrogen) secondary antibodies followed by mounting onto slides with ProLong Gold antifade mountant with DAPI.
qRT-PCR
RNA was isolated using RNeasy and treated with DNase (Qiagen) per the manufacturer’s protocol. The quality of the mRNA was verified with a Nanodrop spectrophotometer (Thermo). Then, 500 ng of RNA was reverse transcribed with PrimeScript RT Reagent kit (Takara) following the manufacture’s protocol. Measurements of cDNA abundance were performed by qRT-PCR using Stratagene MX3000P detection system. Power SYBR Green master mix (Thermo) fluorescence was used to quantify the relative amplicon amounts of each gene. Primer sequences are shown in Supplemental Table S1.
Phylogenetic Analysis of Evolutionary Conserved Elapor1
ELAPOR1 protein sequences were obtained from Uniprot (http://www.uniprot.org). We selected sequences of human (Homo sapiens, Q6UXG2), house mouse (Mus musculus, A2AFS3), zebrafish (Danio rerio, A0A2R8Q613), and sea anemone (Nematostalla vectensis, A7RIP3), thereby spanning several examples of metazoans. We used Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) to align multiple sequences with default parameters. Jalview Version 2.11.1.3 was used to visualize the data. Similar pan-metazoan conservation was confirmed using the OMA (“Orthologous MAtrix”) project algorithm (28).
Electron Microscopy
Stomach corpus preparation and imaging for transmission electron microscopy (TEM) were as previously described (29). Briefly, whole stomach was collected, fixed overnight at 4°C in modified Karnovsky’s fixative. Next day, a single slice of stomach corpus was sectioned and submitted. Tissue was processed by the Washington University in St. Louis Department of Pathology and Immunology Electron Microscopy Facility. Electro-density and vesicle size of the granules were measured by ImageJ using the “Analyze-Measure” function to obtain area and mean gray value (30). Electro-density of each zymogenic granule was normalized to adjacent mitochondrial density in the same image. The pixel-based area measurements for each vesicle were converted into actual area by calibrating against standards.
Statistical Analyses
Statistical analyses were performed in GraphPad Prism 8 (San Diego, CA). In general, datapoints were approximately normally distributed and t test (for 2-sample assessments of error) was used to quantify likelihood of true differences in means. Distributions of vesicle electro-density and area were obviously nonnormal, so the nonparametric Mann–Whitney U test was performed to assess the likelihood of differences between samples. For qRT-PCR, when more than two samples were being assessed in the same experiment, one-way ANOVA with post hoc correction (Tukey) was used to assess statistical significance.
RESULTS
Evolutionarily Conserved ELAPOR1 is Expressed in Pre- and Mature Zymogenic Cells
Previous work on ELAPOR1 function had been limited. We analyzed curated databases of protein structure to generate clues about ELAPOR1 function. We observed that it 1) harbors a transmembrane domain sandwiched by four Ephrin receptor-like domains in its N-terminus and two mannose-6-phosphate receptor-like domains in its COOH-terminus and 2) a signal peptide, suggestive of its being a transmembrane protein. Based on that information and our previous finding that ELAPOR1 is a MIST1 target, we predicted ELAPOR1 would work to promote granule homeostasis, consistent with other MIST1 targets like MIB1, which traffics endosomes, and RAB26, which traffics lysosomes.
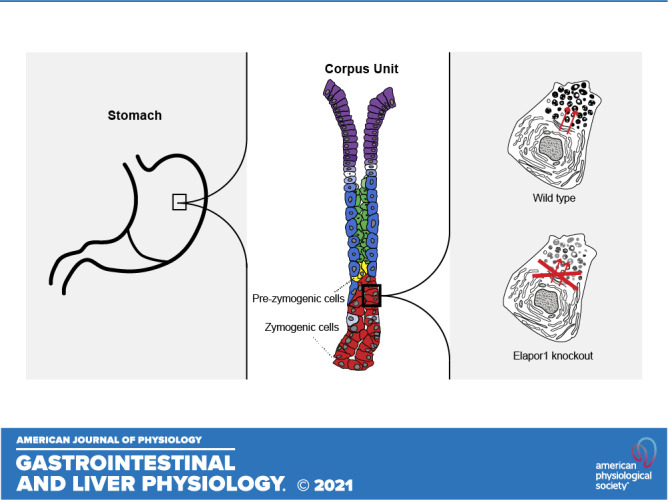
We first sought to determine ELAPOR1 expression in vivo in the stomach, as ELAPOR1 expression in specific cell lineages has not been studied, and the organization of the gastric corpus allows for easy lineage analysis (i.e., allows easy determination of which cell type expresses which protein). We observe that ELAPOR1 is expressed in the lower third of the stomach, in the cytoplasm of ZCs, the MIST1-expressing cells in the stomach (Fig. 1A). Expression was often stronger in ZCs higher in the gastric unit, on the border with the progenitor zone in the neck. Such cells express MIST1 but have a secretory architecture phenotype hybrid between that of mucous neck cells (a progenitor population) and mature ZCs (29, 31), consistent with their being early or pre-ZCs, as has been proposed (32). These pre-ZCs harbor many immature secretory granules and are likely highly active in delivering digestive enzymes to granules and packaging and maturing the granules. Pre-ZCs are also characterized by marked expression of the trans-Golgi and late endosome protein cation-independent mannose-6-phosphate receptor (CI-M6PR; Supplemental Fig. S1A). CI-M6PR is critical for lysosome development, and although it is not a direct MIST1 transcriptional target, its cellular localization is highly aberrant in Mist1–/– mice (9).
Figure 1.
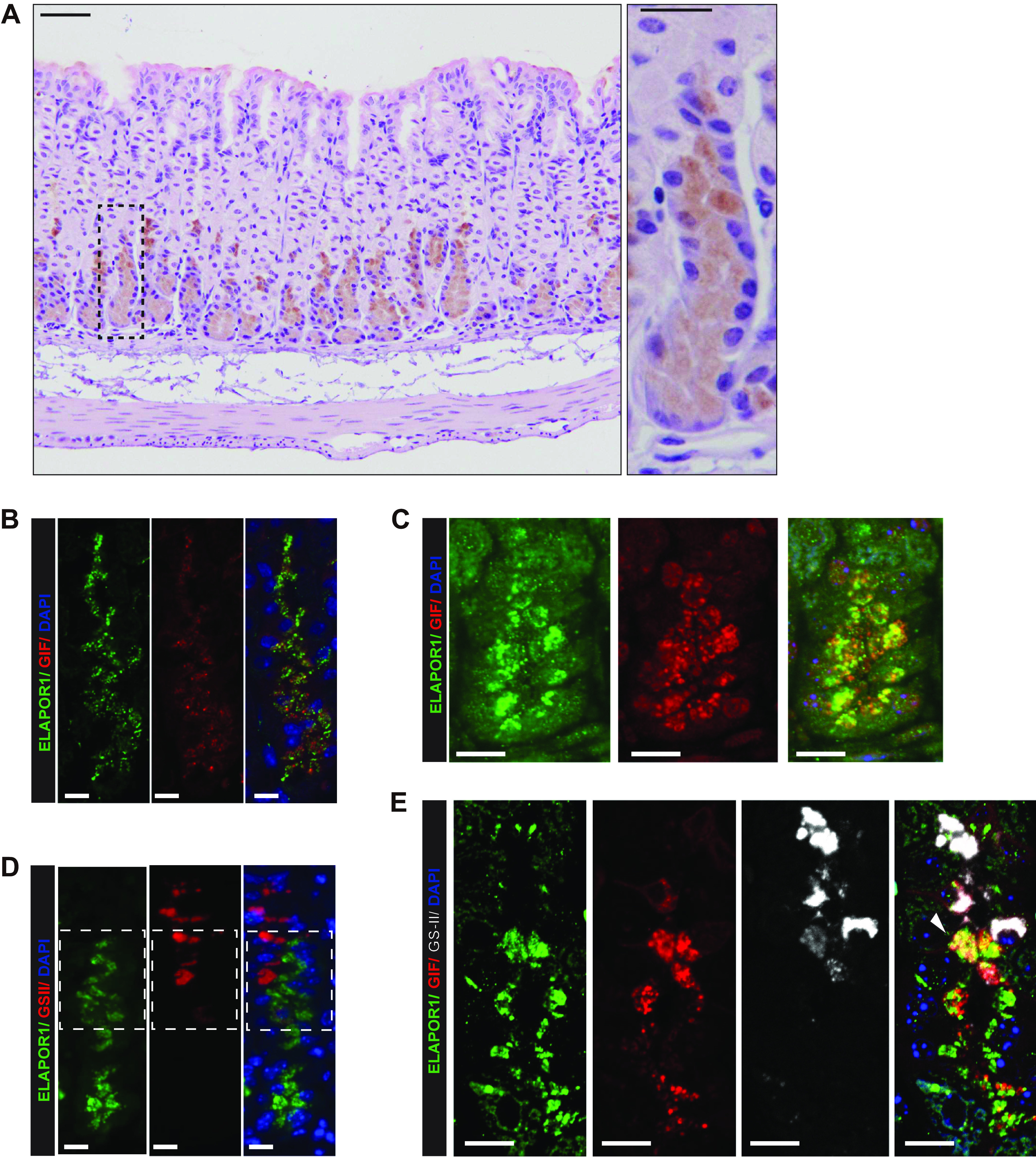
ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1) is expressed predominantly in zymogenic chief cells (ZC) of the stomach. A: representative immunohistochemistry staining of ELAPOR1 in gastric units in wild-type mouse. Hematoxylin and Eosin counterstain. ELAPOR1 is expressed in cells in the base of the stomach corpus; cells have the morphology of ZCs. Box is shown in higher magnification to the right. Scale bars: 50 μm (left image), 20 μm (right image). B: base and lower neck zone of a gastric unit; anti-ELAPOR1 (green), anti-gastric intrinsic factor (GIF, red), and nuclei [4′,6-diamidino-2-phenylindole (DAPI), blue]. Scale bars 10 µm. C: base and lower neck zone of a different gastric unit; ELAPOR1 (green), GIF (red), and nuclei (DAPI, blue). Scale bars 10 µm. D: base and neck zone of a gastric unit; ELAPOR1 (green), Griffonia simplicifolia-II lectin (GS-II, red; mucous neck cell marker), and nuclei (DAPI, blue); box highlights the transition between neck and base zones. Scale bars 10 µm. E: base and neck zone of a gastric unit; ELAPOR1 (green), GIF (red), GS-II (white), and nuclei (DAPI, blue). Scale bars 10 µm. (White arrowhead: ELAPOR1, GIF, GS-II copositive pre-ZC, or transitional cell.) Scale bars 10 µm.
ELAPOR1 was co-expressed in cells positive for gastric intrinsic factor (GIF), which is a key cargo of secretory granules of mouse ZCs (Fig. 1, B and C). The ELAPOR1-positive cells closest to the gastric lumen were found in the transition zones between the lower neck and the base. In that region, it has been reported that there are occasional cells that co-express the epitope for GSII, a lectin that marks mucous neck cells, and GIF (Fig. 1D). We also observed such GSII/GIF-positive cells, which are thought to be transitional between mucous neck and chief cells and often express MIST1 (29). Fig. 1E shows that some of these transitional cells are copositive for ELAPOR1, confirming that ELAPOR1 is in pre-ZCs and neck/ZC transitional cells. Intracellularly, ELAPOR1 localized in vesicles that were in and among GIF-containing secretory granules without substantial direct localizing of ELAPOR1 to granules.
Figure 2.
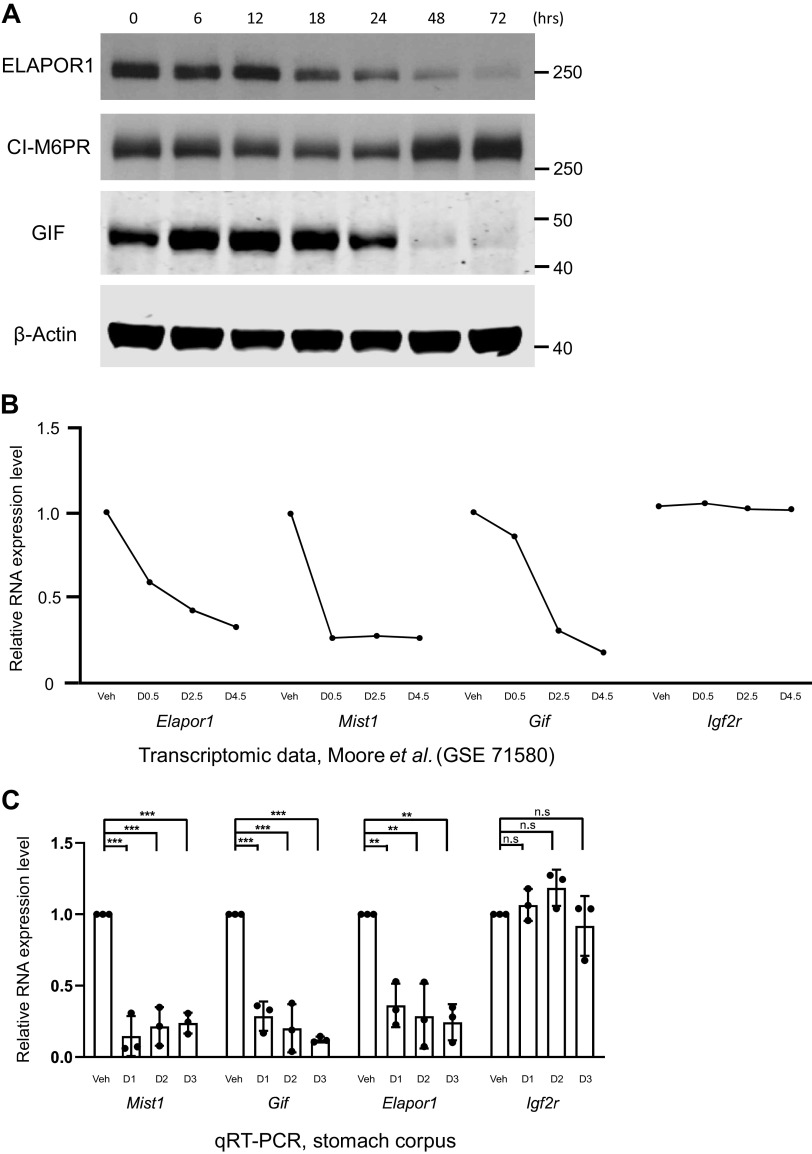
ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1) expression decreases along with expression of other secretory granule-associated genes during paligenosis. A: representative Western blots from gastric corpus for indicated proteins and β-actin loading control at the designated timepoints following high-dose tamoxifen induction of paligenosis. B: mRNA expression of indicated genes at designated days following tamoxifen induction of paligenosis (data from previously published microarray, n = 1). C: as for B but qRT-PCR. Significance by one-way ANOVA and post hoc analysis by Tukey’s test. n = 3 mice per timepoint. ***P < 0.001, **P < 0.01; n.s., not significant. CI-M6PR, cation-independent mannose-6-phosphate receptor; GIF, gastric intrinsic factor.
Our earlier bioinformatics studies as well as public databases showed that Elapor1 transcript is present only in tissues (like stomach) with substantial exocrine secretory role and abundant Mist1 expression (1). Despite the cell type and tissue specificity, which suggests ELAPOR1 does not have some sort of generalized housekeeping function, we found ELAPOR1 is highly evolutionarily conserved. ELAPOR1 paralogs were identified in animals as distant from humans as Nematostella vectensis (sea anemone), a Cnidarian which is among the most primitive of metazoans (Supplemental Fig. S1, B and C). Summarizing findings so far, ELAPOR1 is a highly conserved protein whose expression overlaps in mammals with MIST1-expressing professional secretory cells.
ELAPOR1 Expression Decreases along with Other Secretory Granule Components during Chief Cell Plasticity (Paligenosis)
Previously, we showed Elapor1 transcript was reduced in Mist1–/– mice, and we used ChIP-Seq to show that MIST1 occupied a canonical first intronic site in the Elapor1 locus, inducing gene expression (1). We next sought to determine whether ELAPOR1 was lost during the stereotyped cellular injury response program known as paligenosis. In stomach, ZCs can change identity within 3 days following gastric damage by toxins, like high doses of tamoxifen, to a more embryonic/progenitor-like proliferating cell that help fuel repair (17, 33). One of the first changes in paligenosis is loss of MIST1 and dismantling of the secretory cell architecture by autophagic and lysosomal activation that targets rER and secretory granules (4, 15, 17, 34, 35). As ELAPOR1 is a known downstream target of MIST1, and as high-dose tamoxifen injury of the stomach induces paligenosis in ZCs and accompanying decrease in MIST1 expression, we followed ELAPOR1 expression after high-dose tamoxifen injury (17). As expected, ELAPOR1 protein decreased over the paligenosis time course (Fig. 2A). The ZC cargo protein GIF was also decreased, although the gene encoding the GIF protein is not a direct MIST1 target (MIST1 regulates secretory apparatus in diverse secretory cells without affecting the transcription of specific cargo genes within each cell). We have shown that secretory granule diminution via the autophagy/lysosome pathway trails loss of MIST1 and its targets (9), so the results are consistent with ELAPOR1’s specific dependence on MIST1.
CI-M6PR had a biphasic pattern. It decreased until 24 h post-high-dose tamoxifen injury but then showed abrupt increase after 48 h, suggesting it may have a yet unknown role in the final stage of paligenosis, when cells are progenitor-like and proliferative (Fig. 2A). Abundance of Igf2r mRNA, which encodes CI-M6PR, did not differ significantly during paligenosis (Fig. 2, B and C). Finally, we show that Elapor1 mRNA, consistent with its direct transcriptional activation by MIST1, decreased both in our previous transcriptomic study (36) and in new qRT-PCR performed in the current study to confirm those results (Fig. 2, B and C). The data collectively point to the fact that Elapor1 is a direct MIST1 target not just in homeostasis but also, due to loss of transcriptional maintenance by MIST1, during paligenosis. Finally, we confirmed that ELAPOR1 returns after paligenosis when metaplastic cells reprogram back to mature ZCs again 7 and 14 days after tamoxifen injury (Supplemental Fig. S2, A and B).
ELAPOR1 Localizes to Golgi and Late Endosomes; Truncation Mutants Traffic Abnormally and Disrupt Secretory Vesicles
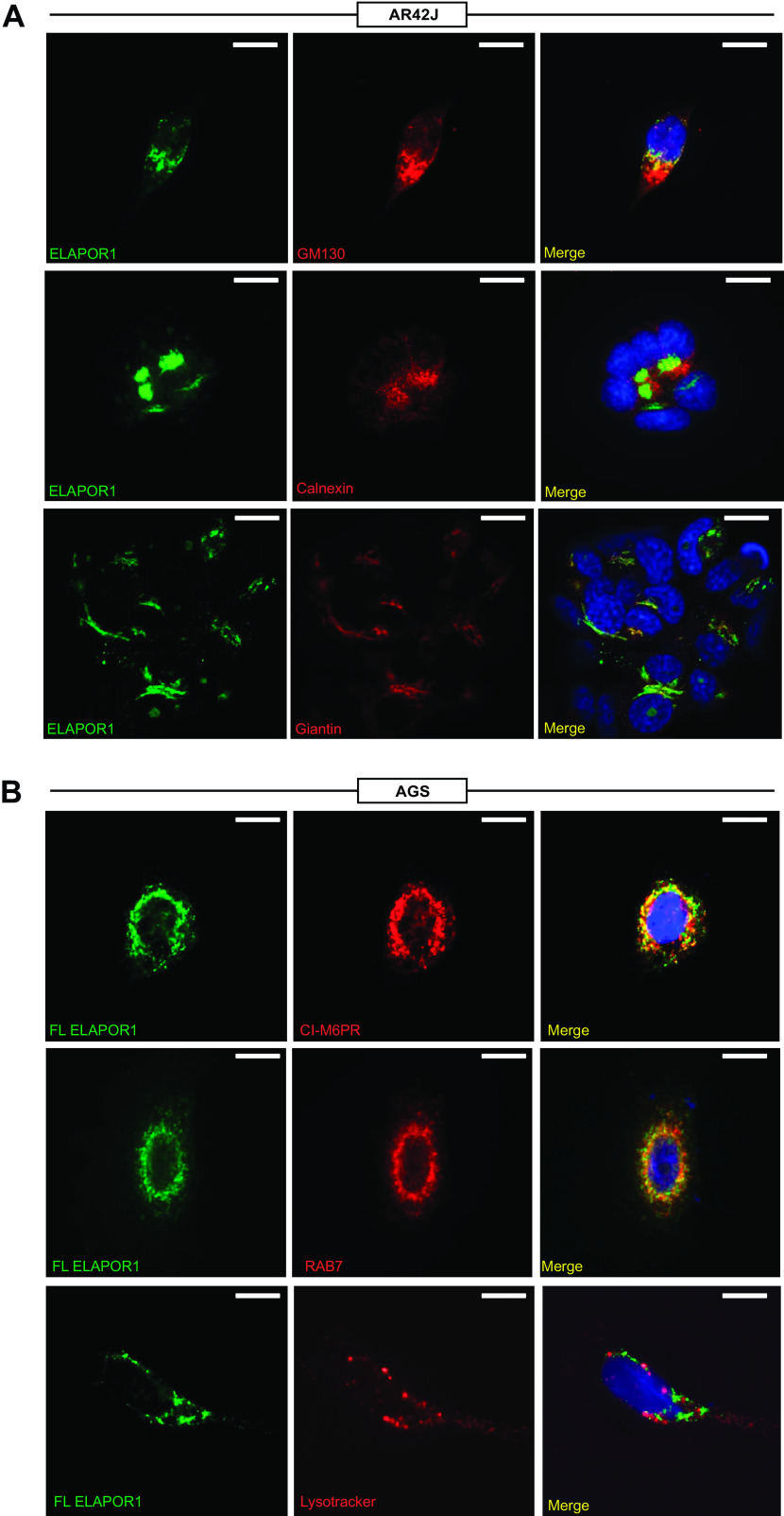
To determine subcellular localization and to mechanistically dissect the function of ELAPOR1, we examined its expression and trafficking in two cell lines. One, AR42J, is from a tumor of pancreatic acinar cells (a MIST1-expressing population) and is commonly used to model secretory cell function by us and others (11); the other, AGS, is a commonly used gastric cell line. Endogenous ELAPOR1 overlapped substantially with Golgi markers, Giantin and GM130 (Fig. 3A). ELAPOR1 showed minimal overlap with the ER marker Calnexin (Fig. 3A). The previous study of in vitro ELAPOR1 implicating it in autophagy, along with its overlap with Golgi and its harboring of mannose-6-phosphate receptor domains, suggested ELAPOR1 might traffic to trans-Golgi and late endosomes and lysosomes, the membrane trafficking pathway that delivers enzymes to lysosomes and is critical for ZC granule maturation. As expected, transfected, full-length (Supplemental Fig. S3A) ELAPOR1 overlapped substantially with CI-M6PR (Fig. 3B, top). Moreover, it partially overlapped with RAB7, a late endosome and lysosome marker. (Fig. 3B, middle).
Figure 3.
ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1) traffics with Golgi network and late endosomes. A: staining patterns in the pancreatic acinar AR42J secretory cell line of endogenous ELAPOR1 (green) and DAPI (blue, nuclei) with various subcellular compartment markers: GM130 (red, cis-Golgi), calnexin (red, rough endoplasmic reticulum), and Giantin (red, cis-golgi). Scale bars 10 µm. B: staining patterns in the AGS gastric cell line of DAPI (blue, nuclei) and transfected, full-length ELAPOR1 (green via GFP tag) with various subcellular compartment markers: CI-M6PR (red, trans-Golgi and late endosomes), RAB7 (red, late endosomes and lysosomes), lysotracker (red, acidified, mature lysosomes). Scale bars 10 µm. CI-M6PR, cation-independent mannose-6-phosphate receptor; FL, full length; GFP, green fluorescent protein.
In contrast, ELAPOR1 did not overlap with vesicles fluorescent from Lysotracker labeling, which stains acidic, mature lysosomes, corroborating the idea that ELAPOR1 is trafficked in trans-Golgi and late endosomes but does not accumulate in a mature lysosome compartment (Fig. 3B, bottom). We next immunoprecipitated CI-M6PR and ELAPOR1 to determine their binding partners by mass spectrometry (MS). For this study, we used a human gastrointestinal cell line, LS174T, that we work with extensively because the cells grow rapidly, are easily transfected, and maintain a fully functioning secretory apparatus. We used the FL ELAPOR1-GFP construct and immunoprecipitated with anti-GFP antibody, and we used endogenous anti-CI-M6PR as its abundance was sufficient without transfection (Supplemental Fig. S3, B and C). Interestingly, in these experiments, we were never able to co-immunoprecipitate CI-M6PR and ELAPOR1, suggesting that despite their overlapping expression patterns in certain cellular compartments, they do not directly interact with each other (Supplemental Fig. S3C), a result further corroborated by the MS data showing neither protein co-immunoprecipitated the other (Table 1; original data for the proteomics experiment is available at https://www.ebi.ac.uk/pride/archive/projects/PXD027491). However, similar to the cell localization studies, both proteins shared a substantially overlapping pool of interacting proteins including, as expected, Golgi-resident proteins, as well as various vesicular trafficking proteins like RABs (Table 1). There were also some differences in cohorts of interacting proteins. Proteins exclusively expressed in lysosomes tended to bind CI-M6PR rather than ELAPOR1, suggesting that ELAPOR1 may be less directly involved in dictating homeostasis of mature lysosomes and may work to shape other intracellular membrane compartments. That result also correlates with the earlier observation that ELAPOR1 did not traffic with lysotracker-positive, mature lysosomes. Indeed, ELAPOR1 associated more with various mucin proteins, which are the key endogenous cargo of this cell line, supporting a specific role for ELAPOR1 in secretory granule trafficking or homeostasis.
Table 1.
Comparison of mass spectrometry data from human LS174T cell line
| Protein (Gene) | Identifier | CI-M6PR | Negative Control | ELAPOR1-GFP |
|---|---|---|---|---|
| ER targeting and translocation | ||||
| CI-M6PR (IGF2R) | P11717 | 549 | 0 | 0 |
| ELAP1 (ELAPOR1) | Q6UXG2 | 0 | 0 | 136 |
| S61A1 (SEC61A1) | P61619 | 4 | 0 | 8 |
| SC61B | P60468 | 3 | 0 | 2 |
| SSRD (SSR4) | P51571 | 3 | 0 | 2 |
| SRPRA (SRPRA) | P08240 | 2 | 0 | 9 |
| SRPRB (SRPRB) | Q9Y5M8 | 4 | 2 | 4 |
| SC11A (SEC11A) | P67812 | 0 | 0 | 2 |
| CALR (CALR) | P27797 | 5 | 0 | 0 |
| Sorting nexin and Golgi-associated | ||||
| SNX9 (SNX9) | Q9Y5X1 | 0 | 0 | 10 |
| GOGA4 (GOLGA4) | Q13439 | 0 | 0 | 67 |
| GSLG1 (GLG1) | Q92896 | 2 | 0 | 7 |
| N-linked glycosylation | ||||
| RPN1 (RPN1) | P04843 | 9 | 6 | 15 |
| RPN2 (RPN2) | P04844 | 4 | 0 | 8 |
| DAD1 (DAD1) | P61803 | 2 | 0 | 3 |
| OST48 (DDOST) | P39656 | 8 | 3 | 6 |
| MOGS (MOGS) | Q13724 | 2 | 4 | 14 |
| Protein folding | ||||
| DJB11 (DNAJB11) | Q9UBS4 | 5 | 0 | 11 |
| DJB12 (DNAJB12) | Q9NXW2 | 11 | 3 | 11 |
| DJC10 (DNAJC10) | Q8IXB1 | 0 | 0 | 16 |
| PPIB (PPIB) | P23284 | 0 | 0 | 6 |
| PDIA6 (PDIA6) | Q15084 | 0 | 0 | 3 |
| Vesicular trafficking | ||||
| COPB2 (COPB2) | P35606 | 2 | 0 | 2 |
| COPE (COPE) | O14579 | 0 | 0 | 2 |
| LMAN2 (LMAN2) | Q12907 | 4 | 0 | 2 |
| COPA (COPA) | P53621 | 4 | 2 | 12 |
| RAB-associated | ||||
| RAB1B (RAB1B) | Q9H0U4 | 4 | 2 | 8 |
| RAB7A (RAB7A) | P51149 | 3 | 0 | 6 |
| RAB5C (RAB5C) | P51148 | 4 | 0 | 3 |
| RAB10 (RAB10) | P61026 | 0 | 4 | 5 |
| RB11A (RAB11A) | P62491 | 3 | 0 | 3 |
| RAB2A (RAB2A) | P61019 | 2 | 0 | 0 |
| RAB32 (RAB32) | Q13637 | 2 | 0 | 0 |
| RAB18 (RAB18) | Q9NP72 | 0 | 0 | 2 |
| RELCH (RELCH) | Q9P260 | 2 | 0 | 0 |
| RFIP1 (RAB11FIP1) | Q6WKZ4 | 0 | 0 | 4 |
| Lysosome-associated | ||||
| MA2B1 (MAN2B1) | O00754 | 25 | 0 | 0 |
| LYAG (GAA) | P10253 | 6 | 0 | 0 |
| PCP (PRCP) | P42785 | 4 | 0 | 0 |
| LICH (LIPA) | P38571 | 2 | 0 | 0 |
| ARSA (ARSA) | P15289 | 5 | 0 | 0 |
| GNS (GNS) | P15586 | 12 | 0 | 0 |
| Mucins | ||||
| MUC5A (MUC5AC) | P98088 | 0 | 0 | 37 |
| MUC5B (MUC5B) | Q9HC84 | 0 | 0 | 14 |
| MUC2 (MUC2) | Q02817 | 5 | 0 | 7 |
| Cathepsin | ||||
| CATD (CTSD) | P07339 | 14 | 0 | 3 |
| CATZ (CTSZ) | Q9UBR2 | 5 | 0 | 0 |
| CATB (CTSB) | P07858 | 3 | 0 | 0 |
| PPGB (CTSA) | P10619 | 15 | 0 | 0 |
CI-M6PR, cation-independent mannose-6-phosphate receptor; ELAPOR1, endosome/lysosome-associated apoptosis and autophagy regulator 1; GFP, green fluorescent protein.
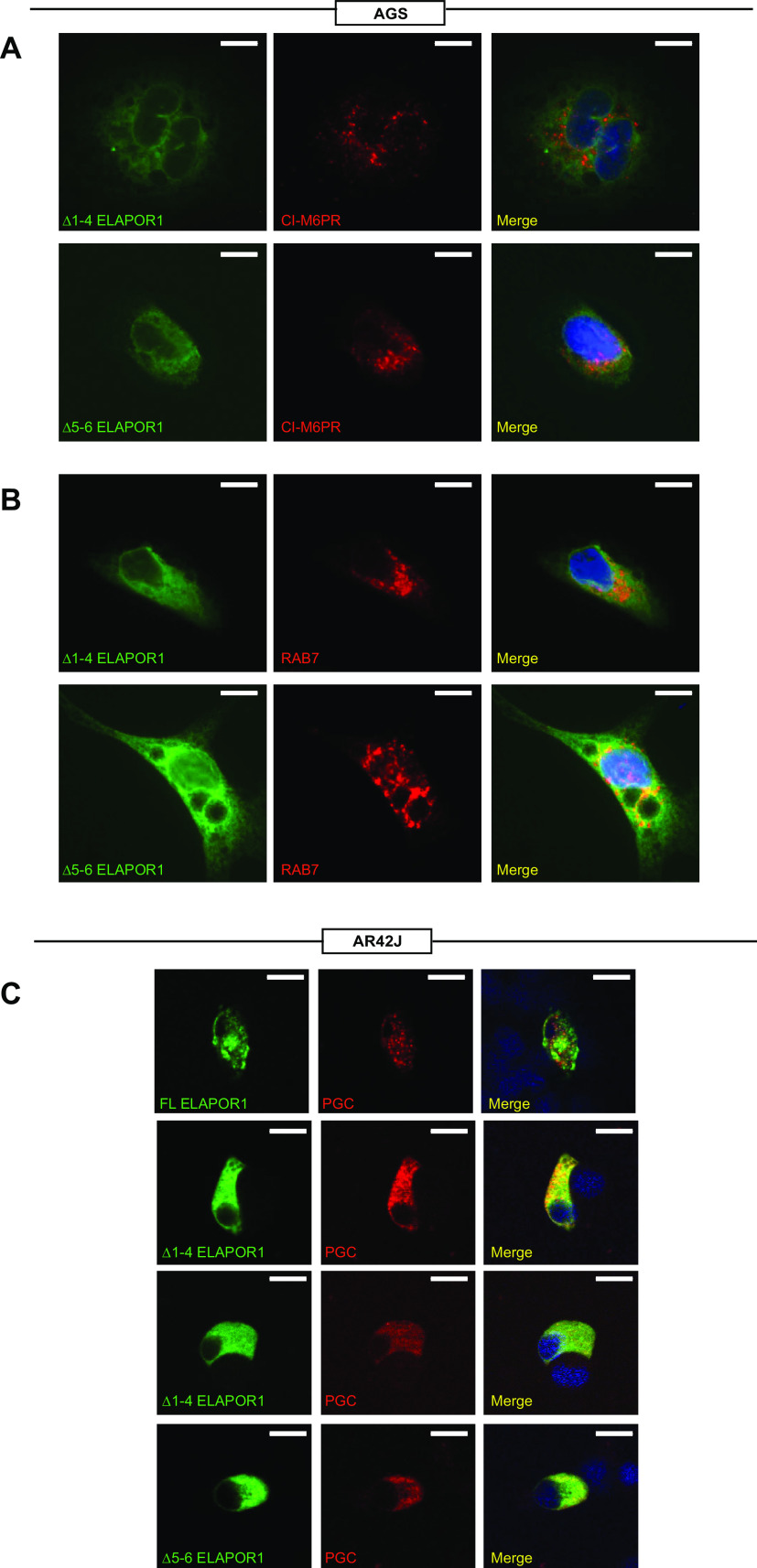
To further clarify ELAPOR1’s function, we examined the importance of ELAPOR1 subcellular domains in directing cellular vesicular trafficking. We transfected constructs encoding the truncated form of ELAPOR1 lacking the Ephrin A/B receptor-like region (Δ1-4) or lacking mannose-6-phosphate receptor (M6PR) domain at the COOH-terminus (Δ5-6) (Supplemental Fig. S3A). In contrast to the normal speckled, vesicular expression of FL ELAPOR1 (Fig. 3), both Δ1-4 and Δ5-6 mutants dispersed throughout the cytoplasm (Fig. 4, A and B). Neither mutants overlapped substantially with CI-M6PR or RAB7 (Fig. 4, A and B). To determine whether ELAPOR1 was required for proper secretory vesicular trafficking, we transfected a bona fide cargo protein secreted by ZCs in AR42J cells (which express MIST1 endogenously and traffic digestive enzyme secretory cargo; 11). Specifically, we cotransfected GFP-tagged FL or each mutant ELAPOR1 with plasmid expressing the full-length ZC digestive enzyme cargo pepsinogen C (PGC) tagged with red fluorescent protein (37). Prior work with cells endogenously expressing MIST1 (like AR42J cells) has shown that PGC traffics to discrete cytoplasmic granules, and FL ELAPOR1 encouraged or permitted that trafficking (Fig. 4C, top row, Supplemental Fig. S3E, and see Ref. 37). Note that the ELAPOR1 vesicles were distributed near and among the secretory granules as might be expected if ELAPOR1 Golgi-endolysosomal trafficking helps traffic proteins to and from granules. The distribution of ELAPOR1 relative to secretory granules was thus similar to the in vivo pattern (Fig. 1, B, C, and E). Both truncation mutants disrupted trafficking of PGC into discrete compartments (Fig. 4C, bottom three rows). Overall, the in vitro experiments show that ELAPOR1 has a similar cellular distribution to the canonical M6PR domain containing protein CI-M6PR in Golgi and lysosomes but may have distinct functions and binding partners that govern ELAPOR1’s requirement for trafficking, generation, or maintenance of secretory granules.
Figure 4.
Localization of ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1) to Golgi network and late endosomes are lost upon truncation. A: truncated (Δ1-4 and Δ5-6) ELAPOR1-GFP mutants transiently transfected into AGS cells were colabeled with CI-M6PR (red, trans-Golgi, late endosomes); DAPI (blue, nuclei). Scale bars 10 µm. B: as for A but colabeled with RAB7 (red, late endosomes and lysosomes). Scale bars 10 µm. C: as for A but with full-length (FL) ELAPOR1 also transfected along with secretory cargo marker PGC-RFP (red) into secretory AR42J cells. Note that PGC localizes to punctate granules when full-length ELAPOR1 is cotransfected but is more diffused with mutants. Scale bars 10 µm. CI-M6PR, cation-independent mannose-6-phosphate receptor; GFP, green fluorescent protein; PGC, pepsinogen C; RFP, red fluorescent protein.
Loss of ELAPOR1 Causes Defective Secretory Granules in Vivo in Zymogenic Cells
We next sought to investigate the phenotype induced by global deletion of the Elapor1 gene in mice. We acquired an Elapor1–/– mouse pedigree that had been generated as part of a large-scale insertional mutagenesis program but never characterized beyond the initial analysis by program staff. These mice have disruption of the Elapor1 gene due to insertion of a lacZ cassette in exon 2 (26). Elapor1–/– mice were born at Mendelian ratio, but the homozygous males were infertile, confirming the observation of the mouse repository where the mice were generated.
As expected, ELAPOR1 protein in null mice was undetectable (Fig. 5A), and primers for Elapor1 transcript showed no signal above background (Supplemental Fig. S3D). Furthermore, IHC using ELAPOR1 antibody showed no staining in the Elapor1–/– mice (Supplemental Fig. S3F). Interestingly, ELAPOR1 protein produced a single band that runs in our Western blots at 250 kDa, which is more than double the expected molecular mass of 110.7 kDa, suggesting either extensive modification and/or dimerization that resisted our attempts at reducing intermolecular associations. We, and others, have previously shown that maturation of zymogenic secretory granules involves modification and packing of secretory cargo that depends on endolysosomal delivery of enzymes like furin (9, 38–41). ELAPOR1’s co-expression in cells with abundant, MIST1-dependent secretory granules, its requirement for secretory granule formation and maintenance in vitro, and its endolysosomal distribution adjacent to secretory granules in vitro and in vivo all led us to determine if ELAPOR1 was required for secretory granule homeostasis in vivo. Defects in granule maturation manifest as failure to acquire electron density and full size in TEM (9). Accordingly, EM analysis of 1,637 vesicles across two wild-type (WT) mice and 1,792 vesicles in chief cells of two different Elapor1–/– mice revealed that zymogenic granules in Elapor1–/– mice were significantly lighter (more electron-lucent) than that of WT mice (0.96 ± 0.34 vs. 1.63 ± 0.71, arbitrary units, P < 0.0001; Fig. 5, B and C, Fig. 5E for higher magnification). In addition, examination of 1,880 WT and 1,774 vesicles in chief cells of two WT and two Elapor1–/– mice showed that median vesicle size was significantly smaller in Elapor1–/– mice [0.390 µm2, interquartile range (IQR), 0.266–0.564 vs. 0.297 µm2, IQR, 0.215–0.410, P < 0.0001; Fig. 5D, Fig. 5E for higher magnification]. Mist1–/– mice similarly have smaller secretory granule size (29). The data distribution indicated that a cohort of Elapor1–/– granules achieved the same electron density as WT granules, but the distribution as a whole was much broader and skewed toward electron lucency. On the other hand, Elapor1–/– granule size had a distribution that was similar to WT in shape but shifted downward. The results are consistent with a marked slowing of Elapor1–/– granule maturation such that some granules eventually mature, whereas granule size in the null mice is reduced at all stages of maturation.
Figure 5.
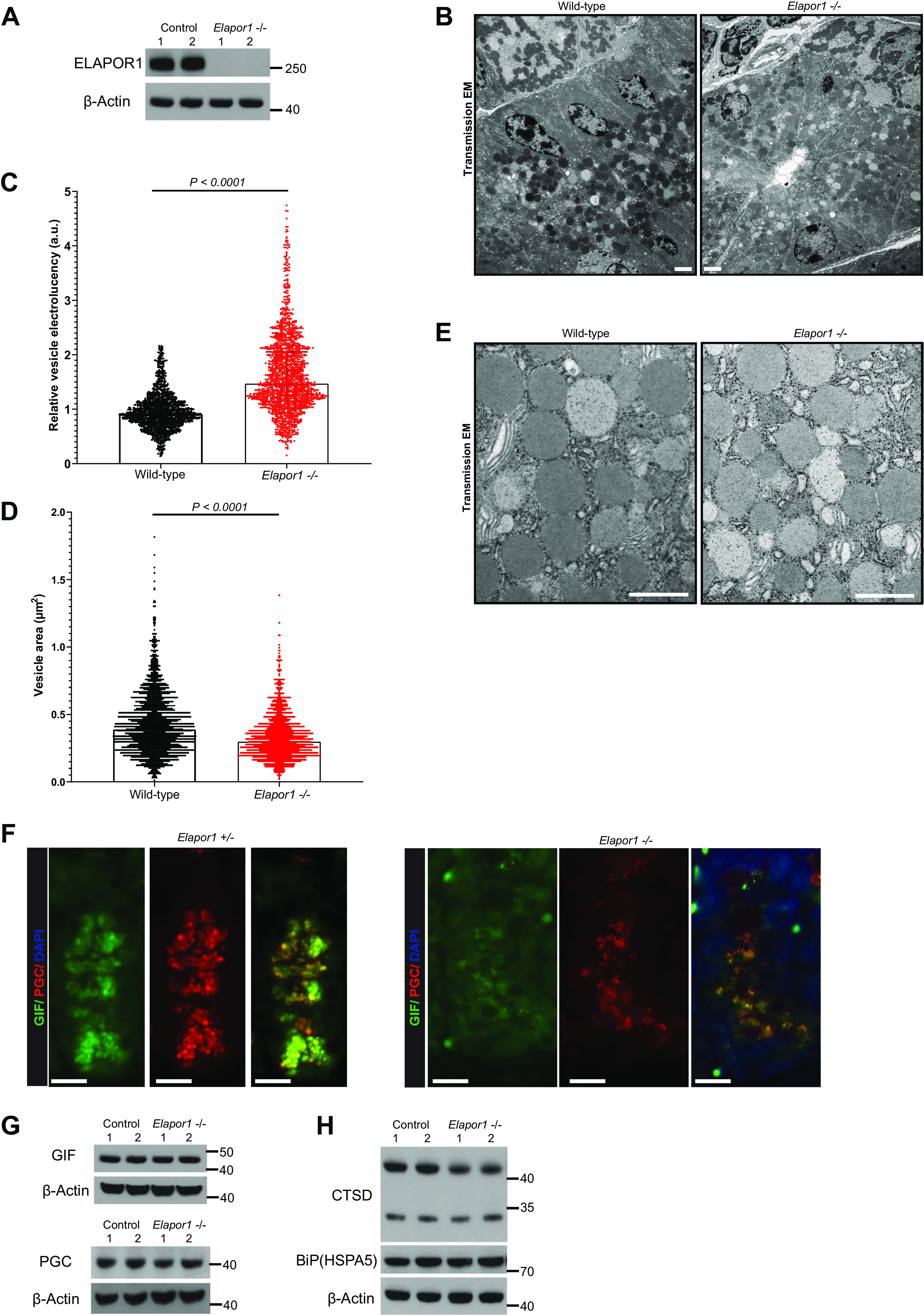
Loss of ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1) causes defective secretory granules in vivo in zymogenic cells (ZCs). A: Western blot of ELAPOR1 from two wild-type and two Elapor1–/– mice, β-actin as a loading control. B: representative transmission electron microscopy (TEM) images of ZCs in the bases of representative wild-type and Elapor1–/– mice (scale bar, 2 μm). C: relative secretory granule electro-lucency of ZC granules in wild-type vs. Elapor1–/– mice. Significance by Mann–Whitney U test. Granules were scored in multiple thin sections, n = 2 mice per genotype. D: as for C but secretory granule area was calculated. E: representative TEM image of granules in a wild-type and Elapor1−/− mature ZC at the base of a gastric unit. Null ZCs have granules that rarely reach the maximal electro-density of typical wild-type granules, and they have many more electro-lucent granules. Scale bar, 600 nm. F: a gastric unit base in control (Elapor1+/–) or Elapor1–/– mice with anti-GIF (green, ZC secretory cargo), PGC (red, ZC secretory cargo), and DAPI (blue, nuclei). Note intracellular distribution of PGC and GIF is more diffuse in null ZCs. Scale bar, 10 μm. G: Western blot from two wild-type and two Elapor1–/– mice, β-actin as loading control. H: Western blot showing pro- (higher-mass band, >40 kDa) and mature- (lower-mass, <35 kDa) Cathepsin D and BiP from two wild-type and two Elapor1–/– mice, β-actin as loading control. a.u., arbitrary unit; GIF, gastric intrinsic factor; FL, full length; PGC, pepsinogen C.
In addition, in Elapor1–/– mice, there was an even more striking defect in the granules in the cells in the transition zone between the neck of the gland and the base where mature ZCs reside (Fig. 6A, Fig. 6B for higher magnification images). Because these transitional cells are normally characterized by hybrid-type granules mottled with both electron-lucent and electron-dense materials, and many such cells express MIST1, they have been assumed to represent cells at an earlier stage of ZC development (i.e., pre-ZCs; see Refs. 29, 32, and 42; Fig. 6C). In WT mice, there are 1–2 such cells per gastric unit (Fig. 6E); in addition to their location between neck and base, and their mottled granules, they can be identified by intermediate contact with the basement membrane that is more extensive than that of mucous neck cells (Fig. 6D), but usually less than that of mature ZCs (29, 31, 32). Finally, the granules in these transitional cells are more discrete versus the saccular, interconnected organization of mucin granules in mucous neck cells (29). Consistent with published literature, we observed on TEM of control transitional ZCs, 88.4% (137/155; WT mouse 1) and 77.2% (51/66; WT mouse 2) of the granules were mottled (defined as electron-dense mottling occupying >50% of the overall granules), as expected. In stark contrast, we observed only 6.4% (11/174; knockout mouse 1) or 19.3% (21/109; knockout mouse 2) of granules were mottled in the equivalent Elapor1–/– cells in the transition zone (Fig. 6A, Fig. 6B for higher magnification images). The cells could still be identified as transitional cells by the morphology of their granules and the substantial extent of their basement membrane contact (cf. WT transitional cell in Fig. 6E). The transitional cells in heterozygous (Elapor1+/−) mice were indistinguishable from that in WT (Fig. 6F).
Figure 6.
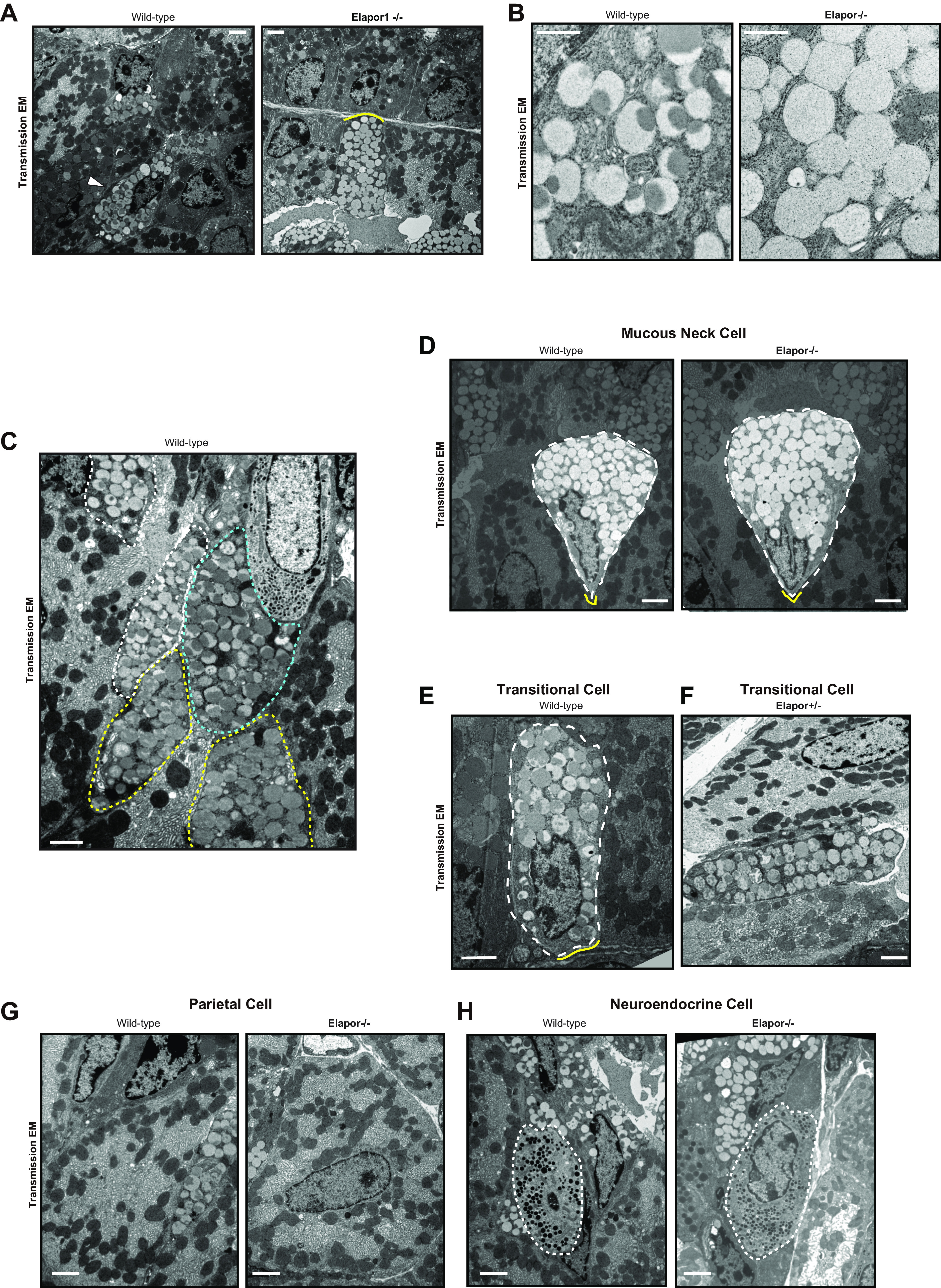
Loss of ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1) causes defective secretory granules in vivo in pre-zymogenic/transitional cells. A: representative transmission electron microscopy (TEM) images of pre-ZCs from wild-type and Elapor1–/– mice (scale bar, 2 μm). Note that null mice harbor frequent cells that are clearly of the pre-ZC/transitional phenotype because they reside in the transition zone between neck and base, have discrete and globoid granules (vs. interloculated ones that characterize neck cells), and have extensive basement membrane contact (right, yellow bracket). However, the granules in these cells are uniformly electro-lucent, without the characteristic mottling of wild-type transitional cells (left, arrowhead). B: representative TEM image of granules in pre-ZC/transitional cells from a wild-type and Elapor1−/− mouse. Note the uniform electro-lucency that typifies the abnormal pattern in null mice. Scale bar, 600 nm. C: representative TEM image showing multiple pre-ZCs at various degree of maturation in a neck-base transition zone of a wild-type mouse. White dotted outline depicts the most neck-like or least mature ZC (“pre-ZC”, <25% of granules mottled); cyan outline shows moderately mature pre-ZC (∼50% mottling), yellow outline shows nearly fully mature ZC with >75% mottling. Scale bar, 2 µm. D: representative TEM image of mucous neck cell in wild-type and Elapor1−/− mouse. Cell of interest was outlined with dotted lines and adjacent cells were darkened for better recognition. Neck cells (which do not express MIST1 or ELAPOR1) appear similar in the two genotypes, with globally electro-lucent, loosely interloculated network of granules. Yellow brackets depict basement membrane contact. Scale bar, 2 µm. E: representative TEM image of a pre-ZC in wild-type mouse. Cell of interest was outlined with dotted lines with adjacent cells shaded. Yellow bracket depicts basement membrane contact. Scale bar, 2 µm. F: representative TEM image of pre-ZC in mouse heterozygous for ELAPOR1. Scale bar, 2 µm. G: parietal cells in wild-type (left) and Elapor1−/−(right) mice show no obvious differences. Scale bar, 2 µm. H: endocrine cells (outlined in white) in wild-type (left) and Elapor1−/− (right) mice show no obvious differences. Scale bar, 2 µm; ZC, zymogenic cell.
Lack of ELAPOR1 did not affect mucous neck cells above the transition zone (Fig. 6D) nor were abnormalities in other cell types—parietal cells (Fig. 6G) and neuroendocrine cells (Fig. 6H), for example—noted. In line with the EM data, immunofluorescence (IF) consistently revealed scanter, smaller GIF and PGC-positive granules in Elapor1–/– ZCs (Fig. 5F). However, the total amount of both proteins did not decrease significantly on Western blots of whole stomach (Fig. 5G). Thus, loss of ELAPOR1 does not cause decreased overall levels of secretory cargo. Rather, cargo may be dispersed more diffusely across the cytoplasm (as happens with disrupted ELAPOR1 function in vitro) and/or the granule maturation defect means cargo proteins within secretory granules do not get modified and packaged appropriately. In contrast, pro-cathepsin D (the precursor form of the mature lysosomal protein Cathepsin D) was modestly but consistently decreased in Elapor1–/– mice relative to the mature form (Fig. 5H, right top). This observation further corroborates the idea that loss of ELAPOR1 disrupts the trafficking/maturation process between the trans-Golgi complex and late endosome/zymogenic granules, because cathepsin D is produced by the partial cleavage of pro-cathepsin D that occurs in lysosomes (43, 44). Pro-cathepsin D is the form of the protein in Golgi and endosomes; thus, a decrease in this form of the protein would be consistent with decreased trans-Golgi and endosomal vesicles in the absence of ELAPOR1 (45). HSPA5 protein abundance, which was previously reported to be affected by ELAPOR1, did not differ in the stomach corpus (Fig. 5H, right bottom). Overall, the results are consistent with ELAPOR1 governing trafficking of components via the trans-Golgi/late endosome vesicular network to facilitate packing, growth, and maturation of secretory granules without changing expression or abundance of secretory cargo itself.
DISCUSSION
Here, we substantially expand in vitro and in vivo work on the function of ELAPOR1, a direct transcriptional target of MIST1, the master regulator of exocrine secretory cell architecture. We show that ELAPOR1 is highly evolutionarily conserved and, as expected, that its expression is confined specifically to ZCs, the MIST1-expressing lineage in the stomach. Loss of MIST1 is one of the first steps during paligenosis, the injury-induced program during which ZCs scale down their secretory apparatus, then retool their gene expression, and finally reenter the cell cycle (9, 15, 17, 19, 20). We show here that ELAPOR1, like other MIST1 targets, also decreases during the first, downscaling stage of paligenosis. Finally, loss or disruption of ELAPOR1 in vitro in cells and in vivo in mouse stomachs causes aberrant maturation of secretory granules.
Published data on ELAPOR1 have been scarce, especially with regard to in vivo analysis of cell-specific expression in tissue. In addition, the homeostatic role of the protein in noncancerous, noninjured tissue has not previously been reported. Using in vitro and in vivo studies with truncation mutants and after injury, we both refine and expand the potential functional roles for ELAPOR1. It was previously reported that ELAPOR1 might act as a tumor suppressor in gastric cancer (16). The authors found ELAPOR1 expression was high in normal tissues and lost in cancer. That pattern would be expected from our current study, too, as ELAPOR1 was expressed only in ZCs and lost during the paligenosis that occurs during atrophy and metaplasia. The vast majority of human cancers arise in atrophic, metaplastic tissue that lacks mature ZCs and the expression of MIST1 and its targets is lost from the epithelium (9, 15, 19, 20).
It was previously reported that ELAPOR1 binds GRP78 (a.k.a. HSPA5 or BiP), which in turn inhibits HSPA5 interaction with Caspase-7, thereafter hampering AKT activation and inducing apoptosis. However, in our study, knockout of ELAPOR1 did not affect HSPA5 protein abundance in homeostatic mouse stomach tissue. In our cell line, MS study, ELAPOR1 did co-immunoprecipitate HSPA5, but HSPA5 is a chaperone that binds with hundreds of proteins. Indeed, it was immunoprecipitated in our study at equivalent levels with anti-CI-M6PR antibody as well as nonspecific IgG control antibodies. Furthermore, HSPA5 is an ER-resident protein, and ELAPOR1 does not, in our experiments, localize to or affect ER.
Another group reported ELAPOR1 regulates autophagy and survival in a transformed cell line (14). The first stage of paligenosis involves loss of MIST1 and massive macroautophagy (stage 1). Like other MIST1 targets we have studied (e.g., MIB1 and RAB26), ELAPOR1 decreases more slowly than MIST1. Functionally, that means ELAPOR1 is still present in the first 6–24 h of paligenosis, which is when stage 1 occurs in ZCs after tamoxifen injury, so ELAPOR1 could potentially still participate in paligenosis in this early stage of the process (17, 18), even though it was lost rapidly after this phase. However, we did not notice any defects in Elapor1−/− mice during paligenosis, which argues ELAPOR1 is not required for the massive autophagic/lysosomal activity (17).
It should be noted that, although ELAPOR1 is a MIST1 target, it is not likely entirely MIST1 dependent, which is consistent with other MIST1 targets. MIST1 acts as a scaling factor, a rheostat as opposed to an on-off switch (1, 11). Accordingly, ELAPOR1’s expression pattern is not strictly limited to those cells and tissues known to express MIST1. For example, the Protein Atlas expression database indicates expression in humans in brain, whereas MIST1 is not prominent there (http://www.proteinatlas.org; see expression of “KIAA1324” in basal ganglial nerve bodies, for example). Note Protein Atlas immunolabeling for ELAPOR1 shows a punctate, ZC-specific pattern similar to what we observe in mouse stomach (46). It is not clear why the null mice have male infertility, although secretory cells in the male reproductive tract in humans have ELAPOR1, particularly prostatic acinar cells (26). In addition, in terms of the MIST1-ELAPOR1 relationship, we report here that ELAPOR1 is exceptionally conserved across metazoans, and it is not clear if MIST1 would have an orthologous relationship across all these species.
Just as ELAPOR1 is not dependent on MIST1 in every tissue, MIST1 clearly has critical targets besides ELAPOR1 that collaborate to enact MIST1’s master control of secretion. Thus, it would be expected that in the absence of ELAPOR1, maturation of zymogenic vesicles is incompletely blocked, and the loss of ELAPOR1 does not wholly recreate the massive disruption of secretion in Mist1−/− ZCs. We and others have characterized function of several other MIST1 targets. As mentioned earlier, MIB1 is a ubiquitin ligase that regulates early to late endocytic trafficking which helps localize DAPK, which in turn regulates microtubule localization and stability (9). In the absence of MIST1, the apical cytoplasmic space (i.e., the space between the nucleus and the gland lumen) is foreshortened. That space, where maturing secretory granules reside, is in part determined by the microtubular network, which in turn helps transports granules. Mib1−/− ZCs have blunted apical microtubular networks with nuclei closer to the cell apex. Similar to ZCs lacking ELAPOR1, they also have a pronounced granule maturation defect. MIST1 also induces CCPG1, which resides in the rER; its knockout results in greatly increased ER stress, even at baseline, in secretory cells, so CCPG1 likely plays a role in the ER stress exhibited by Mist1−/− cells (3, 12, 47). RAB3D is another well-studied target of MIST1 whose function seems obvious, as RAB3D is a well-characterized regulator of exocrine secretory granule trafficking (2, 37). Another downstream target, the small protein GTPase RAB26, whose expression is largely confined to MIST1-expressing secretory tissues, coordinates cellular trafficking of lysosomes and mitochondria (11, 48–50). Thus, loss of RAB26 in the absence of MIST1 may be why Mist1−/− cells accumulate abnormally large lysosomes (1).
The molecular network governing secretory granule maturation is incompletely understood but must involve substantial potential redundancy. There is evidence that granule maturation may involve or overlap lysosomal trafficking pathways, especially as immature granules first bud from the Golgi before definitive sorting occurs (51, 52). Our MS data hints that perhaps sorting nexins are involved in the sorting of secretory cargo from lysosomal hydrolases as secretory granules mature. For example, SNX9 was bound to ELAPOR1 and not to CI-M6PR or negative control. It has also been shown that SNX10 is abundantly present in the ZCs in the stomach and involved in gastric maturation (53).
CI-M6PR, although not a MIST1 target, overlaps ELAPOR1 expression with substantial enrichment in ZCs. Subcellularly, we have observed that the cloud of CI-M6PR+ vesicles that normally surround ZC secretory granules is disrupted in the absence of MIST1 (9). Most hydrolases and lysosomal proteins (e.g., cathepsins) exhibit mannose bound to N-acetylglucosamine (GlcNAc) as part of a glycan ultimately attached to an asparagine residue (54). The mannose is subsequently phosphorylated on its C6 hydroxyl group to form M-6-P in the Golgi. The final step in the maturation of this key targeting glycan is removal of the terminal GlcNAC so that M6P can be unmasked and recognized by the lectin protein CI-M6PR. CI-M6PR shuttles enzymes between trans-Golgi and late endosome, delivering acid hydrolases and cathepsins to the late endosome (55). It leaves lysosomes as they mature by acidification. How MIST1 might affect CI-M6PR localization is unclear.
It has long been thought that ZCs develop in adult gastric units from progenitor cells higher up in the unit. These progenitors, known as mucous neck cells, in turn differentiate from the gastric epithelial stem cell. However, this concept—at least at homeostasis—has recently been challenged by multiple observations (42, 56), and the revised model now suggests that most of the chief cells maintain their census by autoduplication and only a small proportion of chief cells might derive from pre-ZCs. Here, we observe distinct morphological differences caused by the loss of ELAPOR1 in these transitional cells. Currently, the implications of these differences are unclear; however, mice null for Elapor1 may be useful in the future for better understanding of how these cells behave in homeostasis and after injury. At this point, it seems safe to speculate that if these transition cells are indeed maturing ZCs, then loss of ELAPOR1, which our data suggest is required for granule maturation, might particularly affect them. Likewise, ELAPOR1 seems particularly concentrated in these cells in WT mice, whereas it is entirely undetectable in mucous neck cells.
In summary, our results broaden our knowledge of how MIST1 exerts its scaling function via its downstream mediators in zymogenic cells of the stomach. Furthermore, ELAPOR1 provides some additional clues about how secretory granules are generated and maintained and about the nature of cells that are transitional between mucous neck and zymogenic phenotypes in the stomach. Finally, our deep understanding of how this highly conserved protein that is markedly downregulated in the conserved regeneration program, paligenosis, works might also shed light on conserved cellular processes that scale differentiated cell physiology up and down in development and disease.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.5281/zenodo.5598174.
GRANTS
C.J.C. was funded by T32-CA009547(NIH NCI) and J.C.M. was funded by P30DK056338, R01s AR064793, DK094989, DK105129, and DK110406; by the Alvin J. Siteman Cancer Center–Barnes Jewish Hospital Foundation Cancer Frontier Fund; and NIH National Cancer Institute P30 CA091842 and R01 CA246208. The proteomic experiments were performed at the Washington University Proteomics Shared Resource (WU-PSR, Dr. R. Reid Townsend, Director, and Dr. Robert Sprung, Co-Director). The WU-PSR is supported in part by the WU Institute of Clinical and Translational Sciences (NCATS UL1 TR000448), the Mass Spectrometry Research Resource (NIGMS P41 GM103422; R24GM136766), and the Siteman Comprehensive Cancer Center Support Grant (NCI P30 CA091842). Microscopy and tissue sectioning were performed by the Advanced Imaging and Tissue Analysis Core (AITAC) of the Washington University Digestive Disease Research Core Center (DDRCC: P30 DK052574).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.C., D.P., and J.C.M. conceived and designed research; C.J.C., D.P., and J.C.M. performed experiments; C.J.C., D.P., and J.C.M. analyzed data; C.J.C., D.P., and J.C.M. interpreted results of experiments; C.J.C., D.P., and J.C.M. prepared figures; C.J.C., D.P., and J.C.M. drafted manuscript; C.J.C., D.P., and J.C.M. edited and revised manuscript; C.J.C., D.P., and J.C.M. approved final version of manuscript.
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at https://www.ebi.ac.uk/pride/archive/projects/PXD027491. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
ACKNOWLEDGMENTS
The expert technical assistance of Petra Erdmann-Gilmore, Dr. Yiling Mi, and Rose Connors is gratefully acknowledged.
REFERENCES
- 1.Lo H-YG, Jin RU, Sibbel G, Liu D, Karki A, Joens MS, Madison BB, Zhang B, Blanc V, Fitzpatrick JAJ, Davidson NO, Konieczny SF, Mills JC. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev 31: 154–171, 2017. doi: 10.1101/gad.285684.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson CL, Kowalik AS, Rajakumar N, Pin CL. Mist1 is necessary for the establishment of granule organization in serous exocrine cells of the gastrointestinal tract. Mech Dev 121: 261–272, 2004. doi: 10.1016/j.mod.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Hess DA, Strelau KM, Karki A, Jiang M, Azevedo-Pouly AC, Lee AH, Deering TG, Hoang CQ, MacDonald RJ, Konieczny SF. MIST1 links secretion and stress as both target and regulator of the unfolded protein response. Mol Cell Biol 36: 2931–2944, 2016. doi: 10.1128/MCB.00366-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore BD, Khurana SS, Huh WJ, Mills JC. Hepatocyte nuclear factor 4α is required for cell differentiation and homeostasis in the adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol 311: G267–G275, 2016. doi: 10.1152/ajpgi.00195.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 139: 2038–2049, 2010. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Lemercier C, To RQ, Swanson BJ, Lyons GE, Konieczny SF. Mist1: a novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Dev Biol 182: 101–113, 1997. doi: 10.1006/dbio.1996.8454. [DOI] [PubMed] [Google Scholar]
- 8.Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec 259: 157–167, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Capoccia BJ, Jin RU, Kong YY, Peek RM Jr, Fassan M, Rugge M, Mills JC. The ubiquitin ligase Mindbomb 1 coordinates gastrointestinal secretory cell maturation. J Clin Invest 123: 1475–1491, 2013. doi: 10.1172/JCI65703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills JC, Taghert PH. Scaling factors: transcription factors regulating subcellular domains. BioEssays 34: 10–16, 2012.doi: 10.1002/bies.201100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin RU, Mills JC. RAB26 coordinates lysosome traffic and mitochondrial localization. J Cell Sci 127: 1018–1032, 2014. doi: 10.1242/jcs.138776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MD, Harley ME, Kemp AJ, Wills J, Lee M, Arends M, von Kriegsheim A, Behrends C, Wilkinson S. CCPG1 is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis. Dev Cell 44: 217–232.e11, 2018. doi: 10.1016/j.devcel.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng L, Broaddus RR, McCampbell A, Shipley GL, Loose DS, Stancel GM, Pickar JH, Davies PJ. Identification of a novel estrogen-regulated gene, EIG121, induced by hormone replacement therapy and differentially expressed in type I and type II endometrial cancer. Clin Cancer Res 11: 8258–8264, 2005. doi: 10.1158/1078-0432.CCR-05-1189. [DOI] [PubMed] [Google Scholar]
- 14.Deng L, Feng J, Broaddus RR. The novel estrogen-induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Dis 1: e32, 2010. doi: 10.1038/cddis.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol 177: 1514–1533, 2010. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JM, Park S, Kim SJ, Kim H, Lee B, Kim J, Park J, Kim ST, Yang HK, Kim WH, Kim SJ. KIAA1324 suppresses gastric cancer progression by inhibiting the oncoprotein GRP78. Cancer Res 75: 3087–3097, 2015. doi: 10.1158/0008-5472.CAN-14-3751. [DOI] [PubMed] [Google Scholar]
- 17.Willet SG, Lewis MA, Miao ZF, Liu D, Radyk MD, Cunningham RL, Burclaff J, Sibbel G, Lo HG, Blanc V, Davidson NO, Wang ZN, Mills JC. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J 37: e98311, 2018. doi: 10.15252/embj.201798311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao ZF, Lewis MA, Cho CJ, Adkins-Threats M, Park D, Brown JW, Sun JX, Burclaff JR, Kennedy S, Lu J, Mahar M, Vietor I, Huber LA, Davidson NO, Cavalli V, Rubin DC, Wang ZN, Mills JC. A dedicated evolutionarily conserved molecular network licenses differentiated cells to return to the cell cycle. Dev Cell 55: 178–194.e7, 2020. doi: 10.1016/j.devcel.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weis VG, Petersen CP, Weis JA, Meyer AR, Choi E, Mills JC, Goldenring JR. Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 312: G67–G76, 2017. doi: 10.1152/ajpgi.00326.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu T, Sohn Y, Choi E, Petersen CP, Prasad N, Goldenring JR. Decrease in MiR-148a expression during initiation of chief cell transdifferentiation. Cell Mol Gastroenterol Hepatol 9: 61–78, 2020. doi: 10.1016/j.jcmgh.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins MA, Yan W, Sebolt-Leopold JS, Pasca di Magliano M. MAPK signaling is required for dedifferentiation of acinar cells and development of pancreatic intraepithelial neoplasia in mice. Gastroenterology 146: 822–834.e7, 2014. doi: 10.1053/j.gastro.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Lahmy R, Riha C, Yang C, Jakubison BL, van Niekerk J, Staub C, Wu Y, Gates K, Dong DS, Konieczny SF, Itkin-Ansari P. The basic helix-loop-helix transcription factor E47 reprograms human pancreatic cancer cells to a quiescent acinar state with reduced tumorigenic potential. Pancreas 44: 718–727, 2015. doi: 10.1097/MPA.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shubin AD, Sharipol A, Felong TJ, Weng PL, Schutrum BE, Joe DS, Aure MH, Benoit DSW, Ovitt CE. Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res 380: 487–497, 2020. doi: 10.1007/s00441-019-03157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao ZF, Sun JX, Adkins-Threats M, Pang MJ, Zhao JH, Wang X, Tang KW, Wang ZN, Mills JC. DDIT4 licenses only healthy cells to proliferate during injury-induced metaplasia. Gastroenterology 160: 260–271.e10, 2021. doi: 10.1053/j.gastro.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin RU, Mills JC. The cyclical hit model: how paligenosis might establish the mutational landscape in Barrett’s esophagus and esophageal adenocarcinoma. Curr Opin Gastroenterol 35: 363–370, 2019. doi: 10.1097/MOG.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, Forrest W, Ghilardi N, Oravecz T, Platt KA, Rice DS, Hansen GM, Abuin A, Eberhart DE, Godowski P, Holt KH, Peterson A, Zambrowicz BP, de Sauvage FJ. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 28: 749–755, 2010. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 27.Saenz JB, Burclaff J, Mills JC. Modeling murine gastric metaplasia through tamoxifen-induced acute parietal cell loss. Methods Mol Biol (Clifton, NJ) 1422: 329–339, 2016. doi: 10.1007/978-1-4939-3603-8_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altenhoff AM, Train CM, Gilbert KJ, Mediratta I, Mendes de Farias T, Moi D, Nevers Y, Radoykova HS, Rossier V, Warwick Vesztrocy A, Glover NM, Dessimoz C. OMA orthology in 2021: website overhaul, conserved isoforms, ancestral gene order and more. Nucleic Acids Res 49: D373–D379, 2021. doi: 10.1093/nar/gkaa1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 33.Radyk MD, Burclaff J, Willet SG, Mills JC. Metaplastic cells in the stomach arise, independently of stem cells, via dedifferentiation or transdifferentiation of chief cells. Gastroenterology 154: 839–843.e2, 2018. doi: 10.1053/j.gastro.2017.11.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134: 511–522, 2008. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radyk MD, Spatz LB, Peña BL, Brown JW, Burclaff J, Cho CJ, Kefalov Y, Shih CC, Fitzpatrick JA, Mills JC. ATF3 induces RAB7 to govern autodegradation in paligenosis, a conserved cell plasticity program. EMBO Rep 22: e51806, 2021.doi: 10.15252/embr.202051806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore BD, Jin RU, Osaki L, Romero-Gallo J, Noto J, Peek RM Jr, Mills JC. Identification of alanyl aminopeptidase (CD13) as a surface marker for isolation of mature gastric zymogenic chief cells. Am J Physiol Gastrointest Liver Physiol 309: G955–G964, 2015. doi: 10.1152/ajpgi.00261.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Błazewska KM, McKenna CE, Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol Cell Biol 30: 1269–1284, 2010. doi: 10.1128/MCB.01328-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills JC, Andersson N, Stappenbeck TS, Chen CC, Gordon JI. Molecular characterization of mouse gastric zymogenic cells. J Biol Chem 278: 46138–46145, 2003. doi: 10.1074/jbc.M308385200. [DOI] [PubMed] [Google Scholar]
- 39.Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J 13: 18–33, 1994. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol 146: 345–359, 1999. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol 3: 753–766, 2002. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burclaff J, Willet SG, Sáenz JB, Mills JC. Proliferation and differentiation of gastric mucous neck and chief cells during homeostasis and injury-induced metaplasia. Gastroenterology 158: 598–609.e5, 2020. doi: 10.1053/j.gastro.2019.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boonen M, Staudt C, Gilis F, Oorschot V, Klumperman J, Jadot M. Cathepsin D and its newly identified transport receptor SEZ6L2 can modulate neurite outgrowth. J Cell Sci 129: 557–568, 2016. doi: 10.1242/jcs.179374. [DOI] [PubMed] [Google Scholar]
- 44.Benes P, Vetvicka V, Fusek M. Cathepsin D—many functions of one aspartic protease. Crit Rev Oncol Hematol 68: 12–28, 2008. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laurent-Matha V, Derocq D, Prebois C, Katunuma N, Liaudet-Coopman E. Processing of human cathepsin D is independent of its catalytic function and auto-activation: involvement of cathepsins L and B. J Biochem 139: 363–371, 2006.doi: 10.1093/jb/mvj037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science 347: 1260419, 2015. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 47.Kowalik AS, Johnson CL, Chadi SA, Weston JY, Fazio EN, Pin CL. Mice lacking the transcription factor Mist1 exhibit an altered stress response and increased sensitivity to caerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol 292: G1123–G1132, 2007. doi: 10.1152/ajpgi.00512.2006. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Zhou Y, Qiu H, Zhuang R, Han Y, Liu X, Qiu X, Wang Z, Xu L, Tan R, Hong W, Wang T. Rab26 suppresses migration and invasion of breast cancer cells through mediating autophagic degradation of phosphorylated Src. Cell Death Dis 12: 284, 2021. doi: 10.1038/s41419-021-03561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong W, He B, Qian H, Liu Q, Wang D, Li J, Wei Z, Wang Z, Xu Z, Wu G, Qian G, Wang G. RAB26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy 14: 1677–1692, 2018. doi: 10.1080/15548627.2018.1476811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binotti B, Pavlos NJ, Riedel D, Wenzel D, Vorbrüggen G, Schalk AM, Kühnel K, Boyken J, Erck C, Martens H, Chua JJ, Jahn R. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. eLife 4: e05597, 2015.doi: 10.7554/eLife.05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic beta-cells. J Cell Biol 126: 77–86, 1994. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J Cell Biol 137: 595–608, 1997. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye L, Morse LR, Zhang L, Sasaki H, Mills JC, Odgren PR, Sibbel G, Stanley JRL, Wong G, Zamarioli A, Battaglino RA. Osteopetrorickets due to Snx10 deficiency in mice results from both failed osteoclast activity and loss of gastric acid-dependent calcium absorption. PLoS Genet 11: e1005057, 2015. doi: 10.1371/journal.pgen.1005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem 61: 307–330, 1992. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 55.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell 52: 329–341, 1988. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 56.Han S, Fink J, Jörg DJ, Lee E, Yum MK, Chatzeli L, Merker SR, Josserand M, Trendafilova T, Andersson-Rolf A, Dabrowska C, Kim H, Naumann R, Lee JH, Sasaki N, Mort RL, Basak O, Clevers H, Stange DE, Philpott A, Kim JK, Simons BD, Koo BK. Defining the identity and dynamics of adult gastric isthmus stem cells. Cell Stem Cell 25: 342–356.e7, 2019. doi: 10.1016/j.stem.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S2 and Supplemental Figs. S1–S3: https://doi.org/10.5281/zenodo.5598174.