Abstract
The emergence of nosocomial multidrug-resistant Klebsiella pneumoniae is an escalating public health threat worldwide. The prevalence of nosocomial infections due to K. pneumoniae was recorded up to 10%. In this systematic review and meta-analysis, which were conducted according to the guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis, 1092 articles were screened from four databases of which 47 studies fulfilled the selected criteria. By performing a random-effect model, the pooled prevalence of nosocomial multidrug-resistant K. pneumoniae was estimated at 32.8% (95% CI, 23.6–43.6), with high heterogeneity (I2 98.29%, p-value < 0.001). The estimated prevalence of this pathogen and a few related studies were discussed, raising awareness of the spread of multidrug-resistant K. pneumoniae in the healthcare setting. The emergence of nosocomial multidrug-resistant K. pneumoniae is expected to increase globally in the future, and the best treatments for treating and preventing this pathogen should be acknowledged by healthcare staff.
Keywords: worldwide, prevalence, nosocomial, multidrug-resistant, Klebsiella pneumoniae, antibiotic resistance, systematic review, meta-analysis
1. Introduction
The rise of antibiotic resistance among infectious disease-causing bacteria, such as Klebsiella pneumoniae (K. pneumoniae), is an escalating public health threat around the world. It does not only increase the morbidity and mortality rates in patients, but also prolongs hospital stays and increases the treatment costs [1]. K. pneumoniae is a gram-negative, encapsulated, rod-shaped, non-motile bacterium [2], and an important opportunistic pathogen from the Enterobacteriaceae family that causes a large number of nosocomial infections, particularly in developing countries [3]. As stated by Ghashghaee et al. [4], the global prevalence of hospitalized patients exposed to nosocomial infections was 8.7% and the effects could be more burdensome for patients suffering from cancer, organ transplants, and surgery. Additionally, up to 10% of the nosocomial infections are caused by K. pneumoniae [5].
K. pneumoniae can be found almost anywhere in the body and the most frequent infections that occur in humans are urinary tract infections (UTIs), meningitis, respiratory tract infections (RTIs), pneumonia, bloodstream infections (BSIs), and surgical site infections (SSIs). Neonates, the elderly, and immunocompromised patients are the most vulnerable groups to K. pneumoniae infection [6]. Neonates are at risk for K. pneumoniae infections due to the undeveloped body physiology [7]. As recorded, 1.6 million neonatal death occurred every year due to sepsis, predominantly in middle and low income countries [8]. Immunocompromised patients who are hospitalized and suffer from underlying chronic illnesses as well as the elderly are prone to be infected with K. pneumoniae as their immune system’s defences are low [9].
Antibiotics have previously been acknowledged to have saved a large amount of lives, but the emergence of resistance towards antibiotics has threatened healthcare systems worldwide. Antibiotic resistance can be classified into three groups: Multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR). These three types of antibiotic resistance are classified based on their susceptibility to several classes of antibiotics. MDR K. pneumoniae developed resistance to at least one agent from three or more antibiotic classes. Meanwhile, XDR K. pneumoniae strains are resistant to at least one agent in all of the antibiotic classes except two or fewer classes. Lastly, K. pneumoniae which is resistant to all of the agents in all antibiotic classes is classified as PDR, and this type of antibiotic resistance is the worst infection scenario in the healthcare setting [10]. The emergence of MDR K. pneumoniae arose due to the extensive use of antibiotics when treating hospitalized patients [6], making it a challenge to treat and prevent the spread of the infection in the hospital setting.
Extended spectrum beta lactamase-producing K. pneumoniae (ESBL-KP) and carbapenem-resistant K. pneumoniae (CRKP) strains have been reported to cause severe infections in humans. Most of the ESBL-KP and CRKP have contributed to the emergence of multidrug-resistance strains, diminishing treatment options for patients [6,11]. Furthermore, both resistance (blaTEM, blaSHV, blaKPC, blaOXA, qnrB1, oqxA, dfrA12, sul2, fosA, and mgrB) [12], and virulence genes (iro, kpn, fimH, mrkD, entB, traT, rmpA, fyuA, magA, and hlyA) [13] encoded in the bacterium genome played significant factors in the emergence of MDR K. pneumoniae.
Nosocomial infections often arise during the process of receiving a health treatment when an infection is absent upon hospital admission, but it can appear up to 48 h after admission [14]. The infections may happen in various settings of healthcare delivery, including hospitals, long-term care facilities, and ambulatory settings. In addition, it may show up following a discharge from the hospital. Nosocomial infections not only affect patients, but can also affect the healthcare staff [15].
The aim of this systematic review and meta-analysis is to estimate the prevalence of nosocomial infections due to multidrug-resistant K. pneumoniae worldwide and portray the reality of the emergence of these fatal strains.
2. Results
2.1. Search and Screening Results
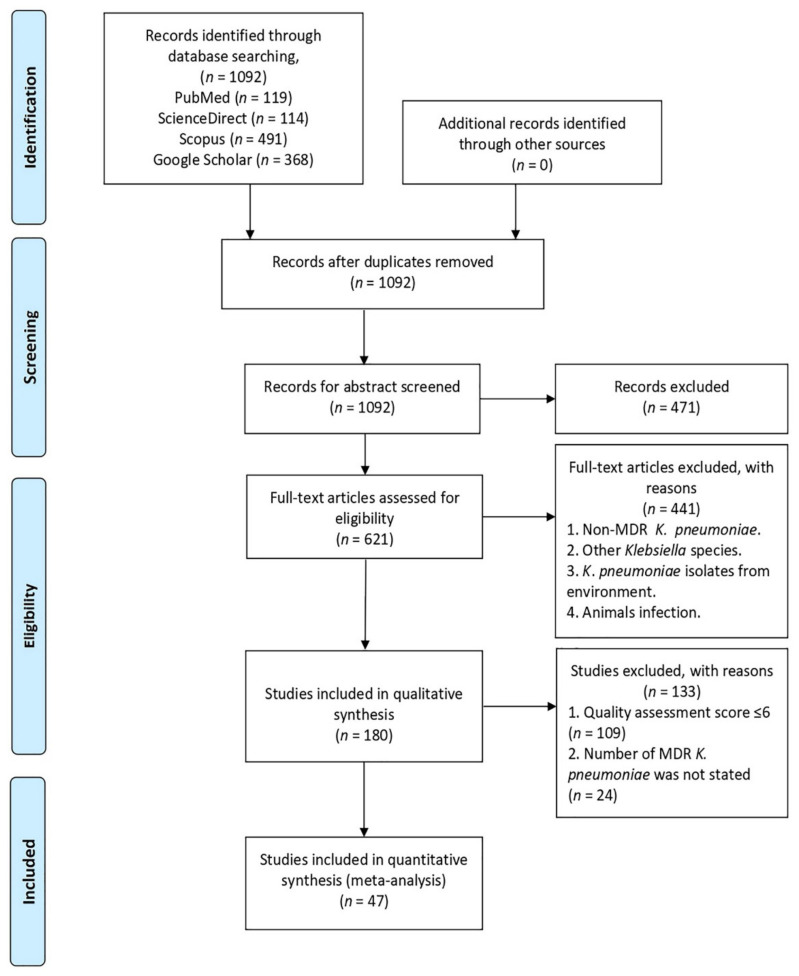
A total of 1092 studies were identified from four electronic databases (PubMed, ScienceDirect, Google Scholar, Scopus). The screening of abstracts was done on 1092 articles based on the inclusion and exclusion criteria. As a result, 621 articles were included in full text-screening. Then, 441 articles were excluded after full-article assessment based on the same inclusion and exclusion criteria. In total, 180 articles were selected for data extraction prior to removing 109 articles from this review, due to the high and moderate risk bias based on the quality assessment score (≤6 score) (Appendix A; Table A1). Thereafter, from the 71 articles selected, only 47 studies portrayed all of the selected criteria, and they were included in this analysis (Figure 1).
Figure 1.
PRISMA flow diagram illustrating the selection process of the studies in this analysis.
2.2. Characteristics of the Included Studies
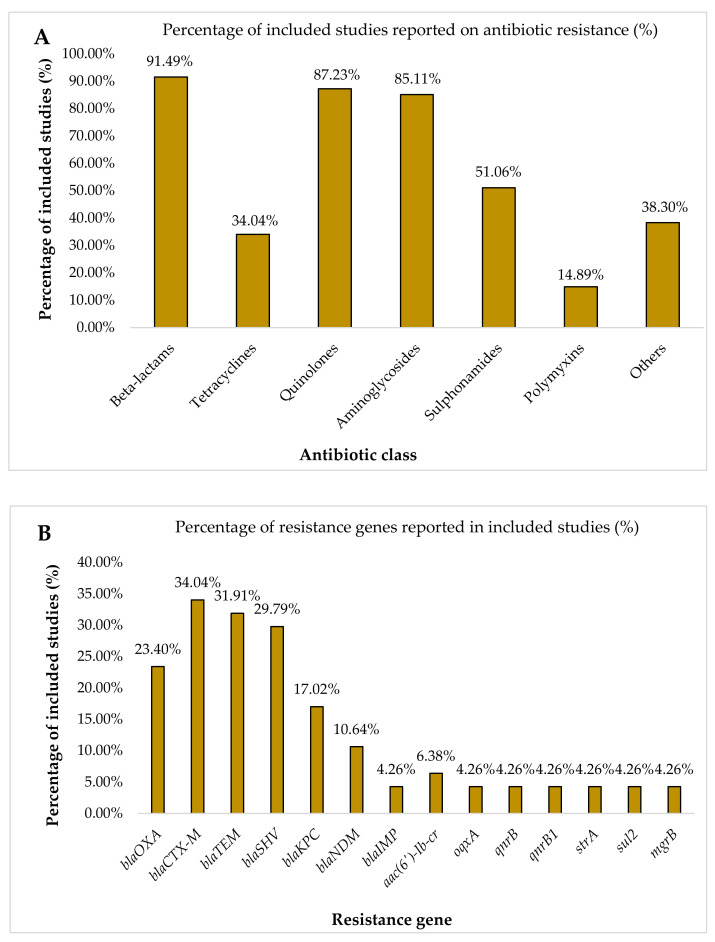
The recorded antibiotic resistance profiles from the included studies varied between one another. Based on the 47 studies, 43 (91.49%), 41 (87.23%), 40 (85.11%), 24 (51.06%), 18 (38.30%), 16 (34.04%), and 7 (14.89%) of the included studies showed consistent resistance to beta-lactams, quinolones, aminoglycosides, sulphonamides, other classes of antibiotics, tetracyclines, and polymyxins, respectively (Figure 2A). Beta-lactams can be divided into six classes which are penicillins, penicillins/beta-lactamase inhibitor, cephalosporins, cephalosporins/beta-lactamase inhibitor, carbapenems, and monobactams (Appendix A; Table A2). In these studies, the examples of the other classes of antibiotics were chloramphenicol, trimethoprim, nitrofurantoin, fosfomycin, and rifampin, while four (8.51%) studies did not record the antibiotic resistance profile as they only stated the number of nosocomial MDR K. pneumoniae cases. Information on the characteristics of the included studies is presented in Table 1. Genes encoded for antibiotic resistance were reported in 24 (51.06%) of the included studies. The majority of the reported antibiotic resistance genes were genes encoded for β-lactamases enzymes, which account from 22 studies (46.81%). According to Figure 2B, the percentages of the included studies that recorded the resistance genes for the class of beta-lactams were blaOXA (23.40%), blaCTX-M (34.04%), blaTEM (31.91%), blaSHV (29.79%), blaKPC (17.02%), blaNDM (10.64%), and blaIMP (4.26%), while these three genes, blaDHA, blaVIM, and blaCMY, were recorded once in three different studies (2.13%). Furthermore, the other resistance genes were also recorded in the included studies, such as aac(6′)-Ib-cr (6.38%), and each of these resistance genes, oqxA, qnrB, qnrB1, strA, sul2, and mgrB, have the same percentage which was 4.26%.
Figure 2.
Percentage of resistance according to (A) antibiotic classes and (B) resistance genes in the included studies.
Table 1.
Characteristics of the 47 included studies in this analysis.
| Author ID | Country | Number of Isolates | Number of K. pneumoniae | Number of MDR K. pneumoniae |
Resistance Profile to Antibiotic Class | Genes Encoded for Antibiotic Resistance |
|---|---|---|---|---|---|---|
| Abdul et al. 2020 [20] | Iraq | 30 | 14 | 9 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Abdul Momin et al. 2017 [16] | Brunei | 5 | 5 | 5 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaOXA-232, blaCTX-M-15, blaTEM-1b, blaSHV-11 |
| Alcántar-Curiel et al. 2018 [3] | Mexico | 168 | 168 | 28 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides. | NR |
| Aljanaby and Alhasani 2016 [21] | Iraq | 439 | 32 | 27 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Chloramphenicol, Nitrofurantoin. | NR |
| Amani et al. 2020 [2] | Iran | 193 | 36 | 13 | Beta-lactams, Aminoglycosides, Chloramphenicol, Nitrofurantoin. | o qxA |
| Anes et al. 2017 [6] | United Kingdom | 11 | 11 | 11 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | blaCTX-M-15, blaSHV-12, blaTEM-1B, oqxAB, qnrB |
| Ashayeri-Panah et al. 2014 [5] | Iran | 35 | 35 | 32 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Nitrofurantoin. | blaSHV |
| Badamchi et al. 2018 [22] | Iran | 93 | 93 | 84 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Rifampin. | blaKPC |
| Bandic-Pavlovic et al. 2020 [23] | Croatia | 97 | 8 | 4 | Beta-lactams, Quinolones, Aminoglycosides. | blaCTX-M-15, blaOXA-48 |
| Bidell et al. 2017 [24] | United States of America | 6093 | 1039 | 105 | Beta-lactams, Quinolones. | NR |
| Caneiras et al. 2019 [11] | Portugal | 31 | 31 | 12 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Fosfomycin. | blaTEM-10, blaTEM-24, blaCTX-M-15, blaKPC-3, blaSHV-11 |
| Chakraborty et al. 2016 [25] | Bangladesh | 500 | 108 | 60 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Das and Debnath 2015 [26] | India | 2273 | 671 | 151 | NR | NR |
| Dolejska et al. 2012 [9] | Czech Republic | 50 | 36 | 36 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | blaCTX-M-15, blaTEM-1, blaOXA-1, aac(6’)-Ib-cr, qnrB1, strA, sul2, tet(A), aac(3’)-II |
| Durdu et al. 2019 [27] | Turkey | 208 | 208 | 84 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin. | NR |
| Eghbalpoor et al. 2019 [13] | Iran | 60 | 60 | 29 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaCTX-M, blaTEM, blaSHV |
| Eid et al. 2020 [1] | Egypt | 95 | 22 | 13 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Chloramphenicol. | NR |
| Folgori et al. 2014 [28] | Italy | 136 | 37 | 23 | NR | blaKPC, blaOXA-48 |
| Giufre et al. 2018 [29] | Italy | 569 | 52 | 22 | Beta-lactams, Quinolones, Sulphonamides. | blaCTX-M-14, blaCTX-M-15, blaTEM-24, blaTEM-52, blaSHV-12, blaKPC-3 |
| Glasser et al. 2010 [30] | United States of America | 82 | 22 | 19 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Imtiaz et al. 2021 [31] | Pakistan | 200 | 200 | 125 | Beta-lactams, Quinolones, Aminoglycosides, Polymyxin. | blaTEM, blaSHV, blaCTX-M-14, blaCTX-M-15, blaOXA, blaNDM-1, blaKPC, blaOXA-48 type, mcr-1, mcr-2 |
| Jin et al. 2017 [7] | China | 16 | 16 | 12 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Fosfomycin. | blaCTX-M-14, blaCTX-M-15, blaDHA-1, blaIMP-4, blaIMP-8, blaNDM-1, blaTEM-1 |
| John et al. 1983 [32] | United States of America | 60 | 60 | 60 | NR | NR |
| Keen et al. 2010 [19] | United States of America | 2647 | 695 | 25 | NR | NR |
| Kim et al. 2020 [12] | South Korea | 26 | 26 | 26 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Chloramphenicol, Fosfomycin, Nitrofurans. | aph(3’)-VIa, armA, aac(6’)-Ib-cr, aadA2, aadA1, aac(3)-IId, strA, strB, blaOXA-1, blaTEM-1A, blaOXA-9, blaCTX-M-15, blaSHV-28, blaNDM-1, blaOXA-232, catB3, catA1, cmlA1, mph(E), msr(E), ere(A), qnrB1, oqxA, oqxB, dfrA12, dfrA1, sul1, sul2, ARR-3, fosA, mgrB, phoP |
| Kocsis et al. 2014 [17] | Italy | 5 | 3 | 3 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides. | blaKPC-3, blaTEM-1, blaOXA-9, blaSHV-11, aac(6’)Ib |
| Kolpa et al. 2018 [33] | Poland | 291 | 44 | 10 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Kooti et al. 2019 [18] | Iran | 150 | 150 | 150 | Beta-lactams, Quinolones, Aminoglycosides. | blaIMP, blaVIM |
| Lee et al. 2020 [34] | Malaysia | 39 | 36 | 36 | Beta-lactams, Quinolones, Aminoglycosides. | blaTEM, blaSHV, blaOXA-1, blaCTX-M-1, blaCTX-M-9 |
| Lima et al. 2014 [35] | Brazil | 29 | 29 | 21 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | NR |
| Mahmoudi et al. 2017 [36] | Iran | 2325 | 263 | 200 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Mansour et al. 2017 [37] | Tunisia | 940 | 220 | 29 | Beta-lactams, Tetracyline, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Trimethoprim. | mgrB, blaOXA-48, blaOXA-204, blaCMY-4, blaNDM-1, blaCMY-16, blaCTX-M-15 |
| Moges et al. 2019 [38] | Ethiopia | 185 | 97 | 85 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Chloramphenicol. | NR |
| Nirwati et al. 2019 [39] | Indonesia | 962 | 167 | 91 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Okomo et al. 2020 [40] | Gambia | 94 | 6 | 6 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides. | NR |
| Oli et al. 2017 [41] | Nigeria | 34 | 5 | 5 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
| Petro et al. 2014 [42] | Tanzania | 172 | 113 | 113 | Beta-lactams. | NR |
| Saeed et al. 2010 [10] | Kingdom of Saudi Arabia | 710 | 96 | 62 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Polymyxin, Chloramphenicol. | NR |
| Shahi et al. 2019 [43] | Iran | 104 | 104 | 24 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaKPC-2 |
| Sharahi et al. 2021 [44] | Iran | 165 | 52 | 5 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Sulphonamides, Fosfomycin. | blaTEM, blaSHV, blaCTX-M, blaNDM-1, blaNDM-6 |
| Traub et al. 2000 [45] | Germany | 14 | 14 | 14 | Beta-lactams, Quinolones, Aminoglycosides, Polymyxin, Chloramphenicol, Fosfomycin + Glucose-6-phosphate, Nitrofurantoin, Rifampin | NR |
| Vaziri et al. 2020 [46] | Iran | 126 | 126 | 69 | Beta-lactams, Quinolones, Aminoglycosides. | qnrB, qnrS, aac(6′)-Ib-cr |
| Yazdansetad et al. 2019 [47] | Iran | 100 | 100 | 100 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides, Nitrofurantoin. | blaTEM, blaCTX-M, blaSHV |
| Yin et al. 2020 [48] | China | 2930 | 452 | 134 | Beta-lactams, Tetracyclines, Quinolones, Aminoglycosides, Rifamycins. | |
| Zaman et al. 2014 [49] | Kingdom of Saudi Arabia | 23 | 23 | 23 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaOXA-48, blaOXA-D, blaTEM-1, blaSHV-1, blaSHV-11, blaSHV-12, blaCTX-M-14, blaCTX-M-15, aadB, dfrA7 |
| Zeng et al. 2020 [50] | China | 37 | 37 | 37 | Beta-lactams, Quinolones, Aminoglycosides, Sulphonamides. | blaKPC-2, blaSHV, blaTEM, blaOXA-1, blaCTX-M-15, blaCTX-M-177, blaCTX-M-3, blaCTX-M-14 |
| Zhong et al. 2012 [51] | China | 124 | NR | 13 | Beta-lactams, Quinolones, Aminoglycosides. | NR |
NR: Not reported.
2.3. Prevalence of Nosocomial MDR K. pneumoniae
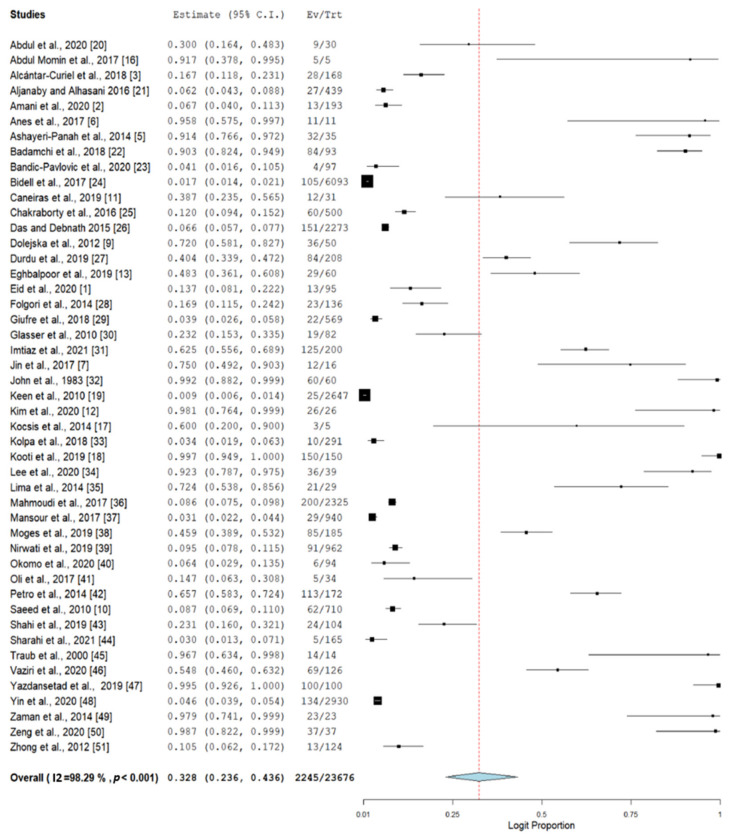
The 47 studies included in this meta-analysis comprised a total of 23,676 isolates from nosocomial infections. From these studies, 5822 isolates were positively identified as an infection due to K. pneumoniae. Using a random-effect model, the pooled prevalence of nosocomial MDR K. pneumoniae was estimated at 32.8% (95% CI, 23.6–43.6), but with high heterogeneity (I2 98.29%, p-value < 0.001) (Figure 3).
Figure 3.
Forest plot of the prevalence of nosocomial MDR K. pneumoniae. The prevalence of reported nosocomial MDR K. pneumoniae cases was estimated by a random-effect model using the DerSimonian-Laird method of meta-analysis.
To investigate the potential sources of heterogeneity, a subgroup analysis from different countries and regions was performed (Table 2). Data recorded from the included articles showed that the studies from Iran (n = 10) accounted for the majority of the studies. The highest estimate of 98.1% (95% CI, 76.4–99.9) was observed from South Korea (n = 1) and the lowest pooled prevalence was estimated at 3.1% (95% CI, 2.2–4.4) from Tunisia (n = 1). Most of the estimates from different countries observed the same heterogeneity as the pooled prevalence (I2 94.38–98.14%). According to regions, studies from the North America region (n =5) observed the lowest estimate at 12.9% (95% CI, 3.1–40.3) and studies from the South America region (n = 1) gave the highest estimate at 72.4% (95% CI, 53.8–85.6). The studies from the North America region showed a significant estimated prevalence for nosocomial MDR K. pneumoniae.
Table 2.
Subgroup analysis of the prevalence of nosocomial MDR K. pneumoniae according to countries and regions.
| Subgroup | No. of Studies | Prevalence | 95% CI | p-Value | I2 (%) | Q | Heterogeneity Test | |
|---|---|---|---|---|---|---|---|---|
| DF | p-Value | |||||||
| Location | ||||||||
| Iraq | 2 | 14.0 | 2.5–50.5 | 0.053 | 94.38 | 17.793 | 1 | <0.001 |
| Mexico | 1 | 16.7 | 11.8–23.1 | - | - | - | - | - |
| Iran | 10 | 55.0 | 27.5–79.8 | 0.736 | 98.14 | 484.982 | 9 | <0.001 |
| United Kingdom | 1 | 95.8 | 57.5–99.7 | - | - | - | - | - |
| Portugal | 1 | 38.7 | 23.5–56.5 | - | - | - | - | - |
| Italy | 3 | 15.5 | 3.9–45.5 | 0.028 | 94.42 | 35.86 | 2 | <0.001 |
| Bangladesh | 1 | 12.0 | 9.4–15.2 | - | - | - | - | - |
| India | 1 | 6.6 | 5.7–7.7 | - | - | - | - | - |
| Czech republic | 1 | 72.0 | 58.1–82.7 | - | - | - | - | - |
| Egypt | 1 | 13.7 | 8.1–22.2 | - | - | - | - | - |
| USA | 4 | 11.6 | 2.5–40.6 | 0.016 | 98.12 | 159.439 | 3 | <0.001 |
| Pakistan | 1 | 62.5 | 55.6–68.9 | - | - | - | - | - |
| China | 4 | 38.6 | 9.5–79.0 | 0.612 | 96.36 | 82.437 | 3 | <0.001 |
| South Korea | 1 | 98.1 | 76.4–99.9 | - | - | - | - | - |
| Turkey | 1 | 40.4 | 33.9–47.2 | - | - | - | - | - |
| Malaysia | 1 | 92.3 | 78.7–97.5 | - | - | - | - | - |
| Brazil | 1 | 72.4 | 53.8–85.6 | - | - | - | - | - |
| Tunisia | 1 | 3.1 | 2.2–4.4 | - | - | - | - | - |
| Brunei | 1 | 91.7 | 37.8–99.5 | - | - | - | - | - |
| Indonesia | 1 | 9.5 | 7.8–11.5 | - | - | - | - | - |
| Gambia | 1 | 6.4 | 2.9–13.5 | - | - | - | - | - |
| Nigeria | 1 | 14.7 | 6.3–30.8 | - | - | - | - | - |
| Tanzania | 1 | 65.7 | 58.3–72.4 | - | - | - | - | - |
| Saudi Arabia | 2 | 64.3 | 0.4–99.9 | 0.849 | 94.64 | 18.640 | 1 | <0.001 |
| Germany | 1 | 96.7 | 63.4–99.8 | - | - | - | - | - |
| Croatia | 1 | 4.1 | 1.6–10.5 | - | - | - | - | - |
| Ethiopia | 1 | 45.9 | 38.9–53.2 | - | - | - | - | - |
| Poland | 1 | 3.4 | 1.9–6.3 | - | - | - | - | - |
| Region | ||||||||
| Asia | 11 | 39.6 | 22.1–60.3 | 0.324 | 98.35 | 607.235 | 10 | <0.001 |
| South America | 1 | 72.4 | 53.8–85.6 | - | - | - | - | - |
| North America | 5 | 12.9 | 3.1– 40.3 | 0.014 | 98.45 | 258.186 | 4 | <0.001 |
| Europe | 9 | 31.2 | 11.5–61.2 | 0.213 | 95.84 | 192.35 | 8 | <0.001 |
| Africa | 4 | 28.7 | 11.5–55.5 | 0.114 | 95.78 | 71.023 | 3 | <0.001 |
| Middle East | 17 | 35.4 | 21.2–52.7 | 0.097 | 97.79 | 723.876 | 16 | <0.001 |
Bold font indicates significant p-values. I1 represents the heterogeneity in meta-analysis for 47 studies.
Another approach that provides a partial explanation of heterogeneity is the analysis of sensitivity and publication bias. In this meta-analysis, sensitivity is assessed by evaluating the impact of small sample size and leave-one-out analysis. Two studies [16,17] with a sample size of 10 and below were excluded and the re-estimated prevalence was 31.5% (95% CI, 22.4–42.2), indicating a slight decline from the original prevalence of 32.8%. Furthermore, the leave-one-out analysis was performed by removing one study at a time, using a random-effects model. The lowest estimate of 30.9%, which was observed following the analysis, was obtained when the study from Kooti et al. [18] was removed. Meanwhile, the highest prevalence of 34.7% was observed when the study from Keen et al. [19] was removed. Overall, the prevalence estimate of nosocomial MDR K. pneumoniae was stable.
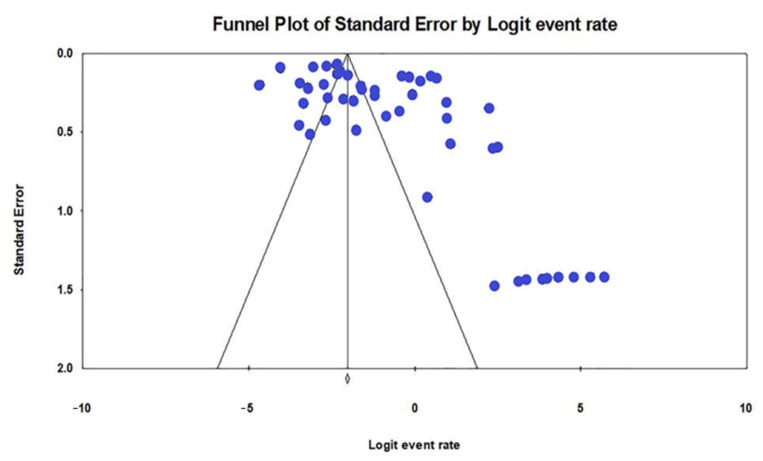
The selected studies in this review had a good methodological quality according to the JBI Quality Assessment Tool for Prevalence Studies (Appendix A; Table A1). A visual observation of the funnel plot, which was generated for all of the included studies, showed a relatively asymmetrical plot with evidence of publication bias (Figure 4). Furthermore, Egger’s test for the asymmetrical funnel plot revealed a significant publication bias (p-value < 0.001).
Figure 4.
Funnel plot showing the evidence of publication bias with Egger’s test (p-value < 0.001).
3. Discussion
This study is the first meta-analysis that estimates the prevalence of antibiotic resistance in nosocomial MDR K. pneumoniae worldwide. Here, 47 studies were strictly systematized to be included in this analysis. Based on the included studies, there were 2245 nosocomial MDR K. pneumoniae isolates recorded from 28 countries in six regions. A random-effect model was used to analyze the data. As a result, the pooled prevalence of nosocomial MDR K. pneumoniae was estimated to be 32.8% (95% CI, 23.6–43.6). A study in Iran showed that the prevalence of nosocomial infections due to K. pneumoniae was found at 6.6% (95% CI, 2.1–19.6) [4], which was lower compared to the pooled prevalence of nosocomial MDR K. pneumoniae in this study and the prevalence estimate for nosocomial MDR K. pneumoniae in Iran, 55.0% (95% CI, 27.5–79.8). While in Ethiopia, the pooled proportional estimates of ESBL-producing K. pneumoniae were 61.8% (95% CI, 48.7–73.4) and the pooled proportion estimates of MDR isolates for both K. pneumoniae and E. coli were 82.7% (95% CI, 72.6–89.6), which was higher when compared with the current study, 45.9% (95% CI, 38.9–53.2). Ethiopia is a resource-limited country where it can be a considerable reason for the high prevalence of K. pneumoniae [52].
Other meta-analysis studies under the family of K. pneumoniae, Enterobacteriaceae, were reported from a few countries and regions. The overall pooled estimate of ESBL-producing Enterobacteriaceae was 40% (95% CI, 34.0–47.0) in Pakistan [53] and 42% (95% CI, 34.0–50.0) in East Africa [54], which included five countries. The estimated prevalence of Enterobacteriaceae in these two studies was slightly higher than the estimated prevalence of nosocomial MDR K. pneumoniae in this analysis. Another study by Mansouri et al. [55] showed a pooled prevalence of ESBL-producing Enterobacteriaceae at 25% (95% CI, 18.0–32.0) globally. In addition, the recorded estimated prevalence in Africa, Asia, Europe, South America, and North America was 45% (95% CI, 22.0–67.0), 15% (95% CI, 6.0–24.0), 5% (95% CI, 2.0–8.0), 4% (95% CI, 1.0–11.0), and 3% (95% CI, 1.0–5.0), respectively. This study is likely to share a quite similar result with Cantón et al. [56], where the prevalence of K. pneumoniae in the United States of America showed a declining pattern from 7.2% to 4.4%. This result can be proven by Ghashghaee et al. [4], where they estimated that hospital infection rates were between 3.5–12% for the developed countries and between 5.7–19.1% for the developing countries. However, these studies were contradicted with the current study, as the prevalence of nosocomial MDR K. pneumoniae in Europe, South America, and North America regions was estimated at 31.2% (95% CI, 11.5–61.2), 72.4% (95% CI, 53.8–85.6), and 12.9% (95% CI, 3.1–40.3), respectively, which was higher than the two studies [55,56]. In this case, the reason that can be proposed was that the transmission of nosocomial MDR K. pneumoniae strains was higher in these regions. Eastern and South-Western Europe, together with the Mediterranean countries, are endemic to MDR K. pneumoniae, due to the ESBL strain. In these countries, the resistance rates were more than 50–60% for the third generation of cephalosporins, fluoroquinolones, and aminoglycosides antibiotic classes [57].
Based on the included studies, the information on antibiotic resistance profiles and the responsible genes encoded for antibiotic resistance were tabulated in Table 1 and Figure 2. Based on Figure 2A, 43 (91.49%), 41 (87.23%), 40 (85.11%), 24 (51.06%), 18 (38.30%), 16 (34.04%), and 7 (14.89%) of the included studies showed consistent resistance to beta-lactams, quinolones, aminoglycosides, sulphonamides, other classes of antibiotics, tetracyclines, and polymyxins, respectively. All of the MDR K. pneumoniae isolates from nosocomial infections were completely resistant to aminopenicillins, penicillins with inhibitors of β-lactamases (except piperacillin/tazobactam), cephalosporins, quinolones, tigecycline, and tobramycin [58]. While a study by Hou et al. [59] stated the high prevalence in resistance to aminoglycosides, macrolides, quinolones, and beta-lactams MDR K. pneumoniae isolates. Furthermore, a majority of the included studies described the resistant genes encoded for β-lactamases enzymes as contributing to the high resistance to the class of beta-lactams.
Since the treatment for patients infected with MDR K. pneumoniae is quite challenging and costly, this high prevalence estimate is alarming enough. There were some efficient treatments used in treating the patients with MDR K. pneumoniae in hospitals, such as colistin [60], fosfomycin [58], and double carbapenem therapy [61]. Furthermore, risk factors that contribute to nosocomial infections, such as poor hygienic conditions in healthcare settings, immunosuppression in patients, extended days in intensive care units (ICU), prolonged consumption of antibiotics, inappropriate use of injection techniques and invasive devices (catheters), a lack of knowledge of basic infection control measures, and inadequacy of control policies, must be addressed and managed in order to control this emergence [62].
There were several limitations in this study. First, this analysis does not cover all of the countries in order to understand the complete overview of prevalence in nosocomial MDR K. pneumoniae, which is due to the lack of resources for some countries. In addition, the analysis on antibiotic resistance profiles could not be conducted due to the varied antibiotics that were used, although they were the same classes of antibiotics in the included studies. Therefore, the highest prevalence of antibiotic classes that contribute to the nosocomial MDR K. pneumoniae could not be identified in this study.
4. Materials and Methods
4.1. Selection Criteria
All of the articles related to the isolates of MDR K. pneumoniae from clinical samples of patients with nosocomial infections were considered for inclusion. The articles with isolates of MDR K. pneumoniae from patients with community-acquired infection, MDR K. pneumoniae from the environment and animals, non-MDR K. pneumoniae, other Klebsiella spp. isolated from patients, as well as unrecoverable full texts and unrelated results were excluded from this study.
4.2. Literature Search
A combination of specific keywords in the title or abstract, such as “Klebsiella pneumoniae” AND (“antibiotic resistance” OR “antibiotic susceptibility”) were used during the search in four electronic databases: PubMed, ScienceDirect, Google Scholar, and Scopus.
The analysis was conducted on those studies that had enough information to answer the objective. In the initial screening, the abstracts were screened by three authors (NAMA, NMH, NFMZ) based on the inclusion and exclusion criteria. The articles that fulfilled the selection criteria were included and proceeded for full-text screening. Disagreement was resolved by discussion and was further verified by two authors (NYY, SA).
This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO), with a registered ID of CRD42021262133.
4.3. Data Extraction and Quality Assessment
Data extraction was conducted based on the objective. In this study, the following data were extracted from the included studies: URL, authors, publication year, study design, study period, country, number, gender and age of patients, types of diseases, area of infection, types of samples, total number of isolates, total number of K. pneumoniae isolates, total number of MDR K. pneumoniae isolates, total number of antibiotics tested, percentage of resistance and susceptibility to antibiotic classes (Beta-lactams, tetracyclines, quinolones, aminoglycosides, sulphonamides, polymyxins, and others), percentage of MDR K. pneumoniae, type of treatment to MDR K. pneumoniae, and genes encoded for antibiotic resistance.
The quality of eligible studies was assessed with the Joanna Brigs Institute (JBI) Quality Assessment Tool for prevalence studies. The quality scores (proportion) were computed for all of the articles. The risk of bias was considered low when more than 70% of the answers were “yes”, moderate when 50–69% of the answers were “yes”, and high when less than 50% of the answers were “yes”. The articles with a low risk of bias only were included in the studies, while the articles that showed a high and moderate risk of bias were excluded from the review.
4.4. Data Synthesis and Data Analysis
Data analysis was performed using OpenMeta Analyst. The pooled prevalence of nosocomial MDR K. pneumoniae was measured and the subgroup analysis was performed according to the location and geographical region. A random-effect model using the DerSimonian-Laird method of meta-analysis was used to create the pooled estimates of the reported nosocomial MDR K. pneumoniae cases. The potential publication bias was examined by creating a funnel plot with the comprehensive meta-analysis and the asymmetry of the plot was further assessed using Egger’s regression test. The heterogeneity of the study-level estimates was evaluated using Cochran’s Q test and I2 statistic as outlined: 0 to 40% might not be important; 30 to 60% may represent moderate heterogeneity; 50 to 90% may represent substantial heterogeneity; and 75 to 100% would be considerable heterogeneity [63]. A sensitivity test using the leave-one-out analysis was conducted. For all of the tests, a p-value of < 0.05 was considered to be statistically significant.
5. Conclusions
In conclusion, based on the 28 countries in six regions from the included studies, this meta-analysis showed that the pooled prevalence of nosocomial MDR K. pneumoniae was estimated at 32.8% (95% CI, 23.6–43.6), which was considerably moderate, but the emergence of this pathogen was accelerated. Based on the 47 studies, all of the studies with an antibiotic resistance profile showed high resistance to the beta-lactams class (n = 43), followed by quinolones (n = 41), and aminoglycosides class (n = 40). Most of the studies recorded for resistance genes showed the resistance genes that were encoded for beta-lactams and they likely contributed to the antibiotic resistance in the class of beta-lactams. Immediately, the proper protocol needs to be emphasized in the healthcare setting to ensure that the emergence and spread of the nosocomial MDR K. pneumoniae can be prevented.
Appendix A
Table A1.
Quality of the included studies by the JBI critical appraisal checklist for studies reporting prevalence data.
| No | Author ID | Checklist 1 | Overall Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||
| 1. | Abdul et al. 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 2. | Abdul Momin et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 3. | Alcántar-Curiel et al. 2018 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 4. | Aljanaby and Alhasani 2016 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 5. | Amani et al. 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 6. | Anes et al. 2017 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 7. | Ashayeri-Panah et al. 2014 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 8. | Badamchi et al. 2018 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| 9. | Bandic-Pavlovic et al. 2020 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| 10 | Bidell et al. 2017 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| 11. | Caneiras et al. 2019 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 12. | Chakraborty et al. 2016 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 13. | Das and Debnath 2015 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 14. | Dolejska et al. 2012 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 15. | Durdu et al. 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 16. | Eghbalpoor et al. 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 17. | Eid et al. 2020 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 18. | Folgori et al. 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 7 |
| 19. | Giufre et al. 2018 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 20. | Glasser et al. 2010 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 21. | Imtiaz et al. 2021 | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 22. | Jin et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 23. | John et al. 1983 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 24. | Keen et al. 2010 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 25. | Kim et al. 2020 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 26. | Kocsis et al. 2014 | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| 27. | Kolpa et al. 2018 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 28. | Kooti et al. 2019 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 29. | Lee et al. 2020 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 7 |
| 30. | Lima et al. 2014 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 7 |
| 31. | Mahmoudi et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 32. | Mansour et al. 2017 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 33. | Moges et al. 2019 | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| 34. | Nirwati et al. 2019 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 35. | Okomo et al. 2020 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 36. | Oli et al. 2017 | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| 37. | Petro et al. 2014 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| 38. | Saeed et al. 2010 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 39. | Shahi et al. 2019 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 40. | Sharahi et al. 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 41. | Traub et al. 2000 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 42. | Vaziri et al. 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 43. | Yazdansetad et al. 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 44. | Yin et al. 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| 45. | Zaman et al. 2014 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| 46. | Zeng et al. 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 8 |
| 47. | Zhong et al. 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
1 The checklist questions to determine the risk of bias for the included studies: 1. Was the sample frame appropriate to address the target population? 2. Were the study participants sampled in an appropriate way? 3. Was the sample size adequate? 4. Were the study subjects and the setting described in detail? 5. Was a sample size justification, power description or variance and effect estimates provided? 6. Were valid methods used for the identification of the condition? 7. Was the condition measured in a standard, reliable way for all participants? 8. Was there an appropriate statistical analysis? 9. Was the response rate adequate, and if not, was the low response rate managed appropriately?
Table A2.
The list of antibiotic classes under Beta-lactams with resistance to MDR K. pneumoniae in 47 studies.
| Author ID | Antibiotic Resistance to the Beta-Lactams Class |
|---|---|
| Abdul et al. 2020 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Abdul Momin et al. 2017 | Beta-lactams; Cephalosporins, Carbapenems. |
| Alcántar-Curiel et al. 2018 | Beta-lactams; Penicillins, Cephalosporins. |
| Aljanaby and Alhasani 2016 | Beta-lactams; Penicillins, Cephalosporins, Carbapenems. |
| Amani et al. 2020 | Beta-lactams; Penicillins, Cephalosporins, Carbapenems. |
| Anes et al. 2017 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Ashayeri-Panah et al. 2014 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Monobactams. |
| Badamchi et al. 2018 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Bandic-Pavlovic et al. 2020 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Bidell et al. 2017 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Caneiras et al. 2019 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins. |
| Chakraborty et al. 2016 | Beta-lactams; Penicillins, Cephalosporins. |
| Das and Debnath 2015 | Beta-lactams; NR |
| Dolejska et al. 2012 | Beta-lactams; Carbapenems. |
| Durdu et al. 2019 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Cephalosporins/Beta-lactamase inhibitor, Carbapenems. |
| Eghbalpoor et al. 2019 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Eid et al. 2020 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Folgori et al. 2014 | Beta-lactams; NR |
| Giufre et al. 2018 | Beta-lactams; Penicillins, Cephalosporins. |
| Glasser et al. 2010 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins. |
| Imtiaz et al. 2021 | Beta-lactams; Penicillins, Cephalosporins, Carbapenems, Monobactams. |
| Jin et al. 2017 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| John et al. 1983 | Beta-lactams; NR |
| Keen et al. 2010 | Beta-lactams; NR |
| Kim et al. 2020 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Kocsis et al. 2014 | Beta-lactams; Cephalosporins, Carbapenems, Monobactams. |
| Kolpa et al. 2018 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Cephalosporins/Beta-lactamase inhibitor, Carbapenems. |
| Kooti et al. 2019 | Beta-lactams; Cephalosporins, Carbapenems, Monobactams. |
| Lee et al. 2020 | Beta-lactams; Penicillins, Cephalosporins. |
| Lima et al. 2014 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Mahmoudi et al. 2017 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Mansour et al. 2017 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Moges et al. 2019 | Beta-lactams; Penicillins, Cephalosporins. |
| Nirwati et al. 2019 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Okomo et al. 2020 | Beta-lactams; Penicillins, Cephalosporins. |
| Oli et al. 2017 | Beta-lactams; Penicillins, Cephalosporins. |
| Petro et al. 2014 | Beta-lactams; Penicillins/Beta-lactamase inhibitor. |
| Saeed et al. 2010 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Shahi et al. 2019 | Beta-lactams; Cephalosporins, Carbapenems. |
| Sharahi et al. 2021 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Traub et al. 2000 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems, Monobactams. |
| Vaziri et al. 2020 | Beta-lactams; Cephalosporins, Monobactams. |
| Yazdansetad et al. 2019 | Beta-lactams; Cephalosporins, Carbapenems. |
| Yin et al. 2020 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Cephalosporins/Beta-lactamase inhibitor, Carbapenems. |
| Zaman et al. 2014 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems. |
| Zeng et al. 2020 | Beta-lactams; Penicillins/Beta-lactamase inhibitor, Cephalosporins, Carbapenems |
| Zhong et al. 2012 | Beta-lactams; Penicillins, Penicillins/Beta-lactamase inhibitor, Cephalosporins. |
NR is not recorded.
Author Contributions
Conceptualization and methodology, N.Y.Y., S.A., N.A.M.A., and N.M.H.; data extraction, synthesis, and interpretation, N.A.M.A., N.M.H., N.F.M.Z., S.A., and N.Y.Y.; formal analysis, N.A.M.A.; validation, S.A. and A.A.I.; writing (original draft preparation), N.A.M.A.; writing (review and editing), S.A., R.M., R.H.S., L.C.Y., C.Y.Y., F.H.M., N.M.N., and N.Y.Y.; supervision, N.Y.Y.; funding acquisition, N.Y.Y. and C.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was sponsored by the Universiti Sains Malaysia (USM) Short Term Grant (grant number 304.CIPPM.6315337) and School of Medical Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eid D., Sayed O.M., Hozayen W.G., Azmy A.F. Battling biofilm forming nosocomial pathogens using chitosan and pluronic F127. J. Pure Appl. Microbiol. 2020;14:1893–1903. doi: 10.22207/JPAM.14.3.28. [DOI] [Google Scholar]
- 2.Amani I., Salehi M.B., Mansour F.N. Frequency of prevalence of Klebsiella pneumoniae in clinical samples and the evaluation of the role of efflux pump in determining antibiotic resistance. Khazar J. Sci. Technol. 2020;4:41–64. [Google Scholar]
- 3.Alcántar-Curiel M.D., Ledezma-Escalante C.A., Jarillo-Quijada M.D., Gayosso-Vázquez C., Morfín-Otero R., Rodríguez-Noriega E., Cedillo-Ramírez M.L., Santos-Preciado J.I., Girón J.A. Association of antibiotic resistance, cell adherence, and biofilm production with the endemicity of nosocomial Klebsiella pneumoniae. BioMed. Res. Int. 2018;2018:7012958. doi: 10.1155/2018/7012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghashghaee A., Behzadifar M., Azari S., Farhadi Z., Bragazzi N.L., Behzadifar M., Shahri S.S.S., Ghaemmohamadi M.S., Ebadi F., Mohammadibakhsh R., et al. Prevalence of nosocomial infections in Iran: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran. 2018;32:273–282. doi: 10.14196/mjiri.32.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashayeri-Panah M., Feizabadi M.M., Eftekhar F. Correlation of multi-drug resistance, integron and blaESBL gene carriage with genetic fingerprints of extended-spectrum β-lactamase producing Klebsiella pneumoniae. Jundishapur J. Microbiol. 2014;7:e8747. doi: 10.5812/jjm.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anes J., Hurley D., Martins M., Fanning S. Exploring the genome and phenotype of multi-drug resistant Klebsiella pneumoniae of clinical origin. Front. Microbiol. 2017;8:1913. doi: 10.3389/fmicb.2017.01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin Y., Dong C., Shao C., Wang Y., Liu Y. Molecular epidemiology of clonally related metallo-ß-lactamase-producing Klebsiella pneumoniae isolated from newborns in a hospital in Shandong, China. Jundishapur J. Microbiol. 2017;10:e14046. doi: 10.5812/jjm.14046. [DOI] [Google Scholar]
- 8.Marwah P., Chawla D., Chander J., Guglani V., Marwah A. Bacteriological profile of neonatal sepsis in a tertiary-care hospital of Northern India. Indian Pediatr. 2015;52:158–159. [PubMed] [Google Scholar]
- 9.Dolejska M., Brhelova E., Dobiasova H., Krivdova J., Jurankova J., Sevcikova A., Dubska L., Literak I., Cizek A., Vavrina M., et al. Dissemination of IncFIIK-type plasmids in multiresistant CTX-M-15-producing Enterobacteriaceae isolates from children in hospital paediatric oncology wards. Int. J. Antimicrob. Agents. 2012;40:510–515. doi: 10.1016/j.ijantimicag.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Saeed N.K., Kambal A.M., El-Khizzi N.A. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med. J. 2010;31:179–187. [PubMed] [Google Scholar]
- 11.Caneiras C., Lito L., Melo-Cristino J., Duarte A. Community-and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: Virulence and antibiotic resistance. Microorganisms. 2019;7:138. doi: 10.3390/microorganisms7050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y.J., Kim S., Kim J., Bae S. Tracking short-term changes in the genetic diversity and antimicrobial resistance of OXA-232-producing Klebsiella pneumoniae ST14 in clinical settings. Clin. Microbiol. Infect. 2020;26:78–86. doi: 10.1016/j.cmi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Eghbalpoor F., Habibi M., Azizi O., Karam M.R.A., Bouzari S. Antibiotic resistance, virulence and genetic diversity of Klebsiella pneumoniae in community- and hospital-acquired urinary tract infections in Iran. Acta Microbiol. Immunol. Hung. 2019;66:349–366. doi: 10.1556/030.66.2019.006. [DOI] [PubMed] [Google Scholar]
- 14.Le T., Wang L., Zeng C., Fu L., Liu Z., Hu J. Clinical and microbiological characteristics of nosocomial, healthcare-associated, and community-acquired Klebsiella pneumoniae infections in Guangzhou, China. Antimicrob. Resist. Infect. Control. 2021;10:1–11. doi: 10.1186/s13756-021-00910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Biotechnology Information. [(accessed on 10 August 2021)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK559312/
- 16.Abdul Momin M.H.F., Liakopoulos A., Phee L.M., Wareham D.W. Emergence and nosocomial spread of carbapenem-resistant OXA-232-producing Klebsiella pneumoniae in Brunei Darussalam. J. Glob. Antimicrob. Resist. 2017;9:96–99. doi: 10.1016/j.jgar.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Kocsis E., Cascio G.L., Piccoli M., Cornaglia G. KPC-3 carbapenemase harbored in FIIk plasmid from Klebsiella pneumoniae ST512 and Escherichia coli ST43 in the same patient. Microb. Drug Resist. 2014;20:377–382. doi: 10.1089/mdr.2013.0152. [DOI] [PubMed] [Google Scholar]
- 18.Kooti S., Zamani K., Sisakht M.T., Mansury D., Motamedifar M. Phenotypic and genotypic detection of antibiotic resistance among metallo-beta-lactamases producing Klebsiella pneumoniae strains isolated from patients in intensive care units in Shiraz, Iran. Gene Rep. 2019;17:100522. doi: 10.1016/j.genrep.2019.100522. [DOI] [Google Scholar]
- 19.Keen E.F., Robinson B.J., Hospenthal D.R., Aldous W.K., Wolf S.E., Chung K.K., Murray C.K. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns. 2010;36:819–825. doi: 10.1016/j.burns.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Jabar H.H., Abd A.K.H., Abdulamir A.S. Efficacy of combinations of piperacilline/tazobactam, ceftazidime, amikacin and bacteriophage against Enterobacteriaceae sepsis in neonates: In vitro study. Sys. Rev. Pharm. 2020;11:165–170. [Google Scholar]
- 21.Aljanaby A.A.J., Alhasani A.H.A. Virulence factors and antibiotic susceptibility patterns of multidrug resistance Klebsiella pneumoniae isolated from different clinical infections. Afr. J. Microbiol. Res. 2016;10:829–843. [Google Scholar]
- 22.Badamchi A., Farahani R.K., Naghadalipoor M., Etemadi M.R., Tabatabaie A. Phenotypic and genotypic characterization of antibiotic resistance in Klebsiella pneumoniae isolated from patients admitted to a third-level hospital in Tehran, Iran. Curr. Pediatr. Rev. 2018;22:258–262. [Google Scholar]
- 23.Bandić-pavlović D., Zah-bogović T., Žižek M., Bielen L., Bratić V., Hrabač P., Slačanac D., Mihaljević S., Bedenić B. Gram-negative bacteria as causative agents of ventilator-associated pneumonia and their respective resistance mechanisms. J. Chemother. 2020;32:344–358. doi: 10.1080/1120009X.2020.1793594. [DOI] [PubMed] [Google Scholar]
- 24.Bidell M.R., Opraseuth M.P., Yoon M., Mohr J., Lodise T.P. Effect of prior receipt of antibiotics on the pathogen distribution and antibiotic resistance profile of key Gram-negative pathogens among patients with hospital-onset urinary tract infections. BMC Infect. Dis. 2017;17:176. doi: 10.1186/s12879-017-2270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakraborty S., Mohsina K., Sarker P.K., Alam M.Z., Abdul Karim M.I., Abu Sayem S.M. Prevalence, antibiotic susceptibility profiles and ESBL production in Klebsiella pneumoniae and Klebsiella oxytoca among hospitalized patients. Period. Biol. 2016;118:53–58. doi: 10.18054/pb.2016.118.1.3160. [DOI] [Google Scholar]
- 26.Das P.K., Debnath J. Prevalence and antibiotic susceptibility pattern of Klebsiella pneumoniae isolated from various clinical specimen in a tertiary care hospital of Tripura. Scholars Acad. J. Biosci. 2015;3:931–935. [Google Scholar]
- 27.Durdu B., Koc M.M., Hakyemez I.N., Akkoyunlu Y., Daskaya H., Gultepe B.S., Aslan T. Risk factors affecting patterns of antibiotic resistance and treatment efficacy in extreme drug resistance in intensive care unit-acquired Klebsiella pneumoniae infections: A 5-year analysis. Med. Sci. Monit. 2019;25:174–183. doi: 10.12659/MSM.911338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folgori L., Livadiotti S., Carletti M., Bielicki J., Pontrelli G., Ciofi Degli Atti M.L., Bertaina C., Lucignano B., Ranno S., Carretto E., et al. Epidemiology and Clinical Outcomes of Multidrug-resistant, Gram-negative Bloodstream Infections in a European Tertiary Pediatric Hospital During a 12-month Period. J. Pediatr. Infect. Dis. 2014;33:929–932. doi: 10.1097/INF.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 29.Giufrè M., Accogli M., Ricchizzi E., Barbanti F., Farina C., Fazii P., Mattei R., Sarti M., Barozzi A., Buttazzi R., et al. Multidrug-resistant infections in long-term care facilities: Extended-spectrum β-lactamase–producing Enterobacteriaceae and hypervirulent antibiotic resistant Clostridium difficile. Diagn. Microbiol. Infect. Dis. 2018;91:275–281. doi: 10.1016/j.diagmicrobio.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Glasser J.S., Guymon C.H., Mende K., Wolf S.E., Hospenthal D.R., Murray C.K. Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns. 2010;36:1172–1184. doi: 10.1016/j.burns.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Imtiaz W., Syed Z., Rafaque Z., Andrews S.C., Dasti J.I. Analysis of antibiotic resistance and virulence traits (genetic and phenotypic) in Klebsiella pneumoniae clinical isolates from Pakistan: Identification of significant levels of carbapenem and colistin resistance. Infect. Drug Resist. 2021;14:227–236. doi: 10.2147/IDR.S293290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John J.F., McKee K.T., Twitty J.A., Schaffner W. Molecular epidemiology of sequential nursery epidemics caused by multiresistant Klebsiella pneumoniae. J. Pediatr. 1983;102:825–830. doi: 10.1016/S0022-3476(83)80006-7. [DOI] [PubMed] [Google Scholar]
- 33.Kołpa M., Wałaszek M., Gniadek A., Wolak Z., Dobroś W. Incidence, Microbiological Profile and Risk Factors of Healthcare-Associated Infections in Intensive Care Units: A 10 Year Observation in a Provincial Hospital in Southern Poland. Int. J. Environ. Res. Public Health. 2018;15:112. doi: 10.3390/ijerph15010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y.Q., Ahmad Kamar A., Velayuthan R.D., Chong C.W., The C.S.J. Clonal relatedness in the acquisition of intestinal carriage and transmission of multidrug resistant (MDR) Klebsiella pneumoniae and Escherichia coli and its risk factors among preterm infants admitted to the neonatal intensive care unit (NICU) Pediatr. Neonatol. 2021;62:129–137. doi: 10.1016/j.pedneo.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Lima A.M.S., de Melo M.E.S., Alves L.C., Brayner F.A., Lopes A.C.S. Investigation of class 1 integrons in Klebsiella pneumoniae clinical and microbiota isolates belonging to different phylogenetic groups in Recife, State of Pernambuco. Rev. Soc. Bras. Med. Trop. 2014;47:165–169. doi: 10.1590/0037-8682-0021-2014. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoudi S., Mahzari M., Banar M., Pourakbari B., Haghi Ashtiani M.T., Mohammadi M., Valian S.K., Mamishi S. Antimicrobial resistance patterns of gram-negative bacteria isolated from bloodstream infections in an Iranian referral paediatric hospital: A 5.5-year study. J. Glob. Antimicrob. Resist. 2017;11:17–22. doi: 10.1016/j.jgar.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Mansour W., Haenni M., Saras E., Grami R., Mani Y., Khalifa A.B.H., el Atrouss S., Kheder M., Hassen M.F., Boujâafar N., et al. Outbreak of colistin-resistant carbapenemase-producing Klebsiella pneumoniae in Tunisia. J. Glob. Antimicrob. Resist. 2017;10:88–94. doi: 10.1016/j.jgar.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Moges F., Eshetie S., Abebe W., Mekonnen F., Dagnew M., Endale A., Amare A., Feleke T., Gizachew M., Tiruneh M. High prevalence of extended-spectrum beta-lactamase-producing Gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS ONE. 2019;14:e0215177. doi: 10.1371/journal.pone.0215177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nirwati H., Sinanjung K., Fahrunissa F., Wijaya F., Napitupulu S., Hati V.P., Hakim M.S., Meliala A., Aman A.T., Nuryastuti T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019;13:20. doi: 10.1186/s12919-019-0176-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okomo U., Senghore M., Darboe S., Bojang E., Zaman S.M.A., Hossain M.J., Nwakanma D., Le Doare K., E Holt K., Hos N.J., et al. Investigation of sequential outbreaks of Burkholderia cepacia and multidrug-resistant extended spectrum β-lactamase producing Klebsiella species in a West African tertiary hospital neonatal unit: A retrospective genomic analysis. Lancet Microbe. 2020;1:e119–e129. doi: 10.1016/S2666-5247(20)30061-6. [DOI] [PubMed] [Google Scholar]
- 41.Oli A.N., Eze D.E., Gugu T.H., Ezeobi I., Maduagwu U.N., Ihekwereme C.P. Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections. Pan Afr. Med. J. 2017;27:66. doi: 10.11604/pamj.2017.27.66.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petro D., Mushi M.F., Moremi N., Iddi S., Mirambo M., Seni J., Mshana S.E. In vitro susceptibility of multi-drug resistant Pseudomonas aeruginosa and extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from clinical specimens at Bugando Medical Centre, Tanzania to piperacillin-Tazobactam. Tanzan. J. Health Res. 2014;16:54–57. doi: 10.4314/thrb.v16i1.8. [DOI] [PubMed] [Google Scholar]
- 43.Shahi A., Hasani A., Rezaee M.A., Jafarabadi M.A., Hasani A., Kafil H.S., Memar M.Y., Soltani E. Klebsiella pneumoniae carbapenemase production among K. pneumoniae isolates and its concern on antibiotic susceptibility. Microbiol. Res. 2019;10:8–11. doi: 10.4081/mr.2019.7587. [DOI] [Google Scholar]
- 44.Sharahi J.Y., Hashemi A., Ardebili A., Davoudabadi S. Molecular characteristics of antibiotic-resistant Escherichia coli and Klebsiella pneumoniae strains isolated from hospitalized patients in Tehran, Iran. Ann. Clin. Microbiol. Antimicrob. 2021;20:32. doi: 10.1186/s12941-021-00437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traub W.H., Schwarze I., Bauer D. Nosocomial outbreak of cross-infection due to multiple-antibiotic-resistant Klebsiella pneumoniae: Characterization of the strain and antibiotic susceptibility studies. Chemotherapy. 2000;46:1–14. doi: 10.1159/000007250. [DOI] [PubMed] [Google Scholar]
- 46.Vaziri S., Afsharian M., Mansouri F., Azizi M., Nouri F., Madadi-Goli N., Afshar Z.M., Zamanian M.H., Alvandi A., Ahmadi K. Frequency of qnr and aac(6′)Ib-cr genes among ESBL-producing Klebsiella pneumoniae strains isolated from burn patients in Kermanshah, Iran. Jundishapur J. Microbiol. 2020;13:e100348. doi: 10.5812/jjm.100348. [DOI] [Google Scholar]
- 47.Yazdansetad S., Alkhudhairy M.K., Najafpour R., Farajtabrizi E., Al-Mosawi R.M., Saki M., Jafarzadeh E., Izadpour F., Ameri A. Preliminary survey of extended-spectrum β-lactamases (ESBLs) in nosocomial uropathogen Klebsiella pneumoniae in north-central Iran. Heliyon. 2019;5:e02349. doi: 10.1016/j.heliyon.2019.e02349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin Y., Zhao C., Li H., Jin L., Wang Q., Wang R., Zhang Y., Zhang J., Wang H., Yang C., et al. Clinical and microbiological characteristics of adults with hospital-acquired pneumonia: A 10-year prospective observational study in China. Eur. J. Clin. Microbiol. Infect. Dis. 2020;40:683–690. doi: 10.1007/s10096-020-04046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uz Zaman T., Aldrees M., Al Johani S.M., Alrodayyan M., Aldughashem F.A., Balkhy H.H. Multi-drug carbapenem-resistant Klebsiella pneumoniae infection carrying the OXA-48 gene and showing variations in outer membrane protein 36 causing an outbreak in a tertiary care hospital in Riyadh, Saudi Arabia. Int. J. Infect. Dis. 2014;28:186–192. doi: 10.1016/j.ijid.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Zeng L., Zhang J., Li C., Fu Y., Zhao Y., Wang Y., Zhao J., Guo Y., Zhang X. The determination of gyrA and parC mutations and the prevalence of plasmid-mediated quinolone resistance genes in carbapenem resistant Klebsiella pneumonia ST11 and ST76 strains isolated from patients in Heilongjiang Province, China. Infect. Genet. Evol. 2020;82:104319. doi: 10.1016/j.meegid.2020.104319. [DOI] [PubMed] [Google Scholar]
- 51.Zhong L., Men T.Y., Li H., Peng Z.H., Gu Y., Ding X., Xing T.-H., Fan J.-W. Multidrug-resistant gram-negative bacterial infections after liver transplantation-spectrum and risk factors. J. Infect. 2012;64:299–310. doi: 10.1016/j.jinf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Abayneh M., Worku T. Prevalence of multidrug-resistant and extended-spectrum beta-lactamase (ESBL)-producing gram-negative bacilli: A meta-analysis report in Ethiopia. Drug Target Insights. 2020;14:16–25. doi: 10.33393/dti.2020.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrar S., Hussain S., Khan R.A., Ain N.U., Haider H., Riaz S. Prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae: First systematic meta-analysis report from Pakistan. Antimicrob. Resist. Infect. Control. 2018;7:26. doi: 10.1186/s13756-018-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonda T., Kumburu H., van Zwetselaar M., Alifrangis M., Lund O., Kibiki G., Aarestrup F.M. Meta-analysis of proportion estimates of extended-spectrum-beta-lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob. Resist. Infect. Control. 2016;5:18. doi: 10.1186/s13756-016-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansouri F., Sheibani H., Masroor M.J., Afsharian M. Extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae and urinary tract infections in pregnant/postpartum women: A systematic review and meta-analysis. Int. J. Clin. Pract. 2019;73:e13422. doi: 10.1111/ijcp.13422. [DOI] [PubMed] [Google Scholar]
- 56.Cantón R., Novais A., Valverde A., Machado E., Peixe L., Baquero F., Coque T. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2008;14:144–153. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 57.Navon-Venezia S., Kondratyeva K., Carattoli A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017;41:252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 58.Mączyńska B., Paleczny J., Oleksy-Wawrzyniak M., Choroszy-Król I., Bartoszewicz M. In vitro susceptibility of multi-drug resistant Klebsiella pneumoniae strains causing nosocomial infections to fosfomycin. A comparison of determination methods. Pathogens. 2021;10:512. doi: 10.3390/pathogens10050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou X.H., Song X.Y., Ma X.B., Zhang S.Y., Zhang J.Q. Molecular characterization of multidrug-resistant Klebsiella pneumoniae isolates. Braz. J. Microbiol. 2015;46:759–768. doi: 10.1590/S1517-838246320140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou S.Y., Wu D., Feng X.H. Polymyxin monotherapy versus polymyxin-based combination therapy against carbapenem-resistant Klebsiella pneumoniae: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2020;23:197–202. doi: 10.1016/j.jgar.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 61.Li Y.Y., Wang J., Wang R., Cai Y. Double-carbapenem therapy in the treatment of multidrug resistant gram-negative bacterial infections: A systematic review and meta-analysis. BMC Infect. Dis. 2020;20:408. doi: 10.1186/s12879-020-05133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan H.A., Baig F.K., Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017;7:478–482. doi: 10.1016/j.apjtb.2017.01.019. [DOI] [Google Scholar]
- 63.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions Cochrane. 4th ed. John Wiley & Sons; Hoboken, NJ, USA: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are included in the manuscript.