Abstract
Simple Summary
Chitin is a polysaccharide that forms the outer layer of many organisms, and it is widely used in industry. Chitinases are enzymes that can break down chitin into monomeric molecules and are used in the agro-industrial sectors. Because chitin is the key structural component of marine (mollusks, crustaceans, and marine invertebrates) and other species (algae, fungi, and insects), chitinases can be employed in the marine waste management and biocontrol of pathogenic fungi and harmful insects. Chitinase also has uses in the food industry, cosmetics, medicine, waste management, crop protection, and the production of single-cell proteins, among others. This study includes detailed information on the characterization, sources, and uses of chitinases in several areas.
Abstract
Chitinases are a large and diversified category of enzymes that break down chitin, the world’s second most prevalent polymer after cellulose. GH18 is the most studied family of chitinases, even though chitinolytic enzymes come from a variety of glycosyl hydrolase (GH) families. Most of the distinct GH families, as well as the unique structural and catalytic features of various chitinolytic enzymes, have been thoroughly explored to demonstrate their use in the development of tailor-made chitinases by protein engineering. Although chitin-degrading enzymes may be found in plants and other organisms, such as arthropods, mollusks, protozoans, and nematodes, microbial chitinases are a promising and sustainable option for industrial production. Despite this, the inducible nature, low titer, high production expenses, and susceptibility to severe environments are barriers to upscaling microbial chitinase production. The goal of this study is to address all of the elements that influence microbial fermentation for chitinase production, as well as the purifying procedures for attaining high-quality yield and purity.
Keywords: chitinolytic enzymes, microbial chitinases, chitooligosaccharides, fermentation, biocontrol
1. Introduction
Chitin, an insoluble polysaccharide, is the structural component of many organisms, such as mollusks, crustaceans, algae, fungi, and marine invertebrates [1]. Chitin, along with its derivatives, is of high industrial importance and possesses applications in medicine, dermatology, cosmetics, food, and agricultural sectors. Chitinases are enzymes that hydrolyze chitin, and mainly belong to four glycoside hydrolase (GH) families, 18, 19, 23, and 48 [2,3]. In chitin and chitodextrins, chitinases randomly cause the endo-hydrolysis of N-acetyl-β-D-glucosaminide (1→4)-β-linkages [4].
Chitinases convert the chitin polymer into chitooligosaccharides (COS), which are then converted to N-acetyl-D-glucosamine units by the action of chitobiases [5,6]. COS have several important biological activities, such as antimicrobial, antioxidant, anti-inflammatory, angiotensin I-converting enzyme (ACE) inhibiting, immunostimulating, antitumor, and hypocholesterolemic activities, as well as the ability to enhance the absorption of iron and calcium [7]. Further, since chitinous waste is generated in abundance, its sustainable valorization is essential to prevent environmental pollution. In this context, chitin-active enzymes are critical toolboxes for chitin waste management, with simultaneous generation of value-added products [8]. Apart from their myriad of industrial applications, chitinases, as biocontrol agents, are gaining popularity in the agricultural sector, for increasing crop productivity. They offer a safe alternative to toxic pesticides for the protection of crops, due to their unique ability to inhibit pathogenic fungi and insects without disturbing plants, vertebrates, and other components of an ecosystem.
Various groups of organisms, including plants, insects, bacteria, actinomycetes, and fungi, secrete chitinases. Though researchers report a few extremophilic chitinases secreted by fungi and plants, bacteria and archaea are the primary sources of extremophilic chitinases. Extremophilic chitinases can withstand extreme conditions, such as high salt concentrations, and extreme pH and temperature, making them suitable candidates for various industrial processes [9]. Since fermentation conditions and medium composition critically affect chitinase production from extremophilic, as well as mesophilic organisms, the review comprehensively covers the diverse factors impacting the microbial production of chitinase.
In order to grasp an understanding of the catalytic potential of distinct chitin-degrading enzymes, the review also provides vital insights into their widely varied categorization and structural organization. The current study focuses on the microbiological potential for producing cost-effective and robust chitinases, as well as the in depth exploration of their applications in various industries. The possibility of using a recombinant biotechnological tool or immobilization to boost the native catalytic efficacy has also been considered.
2. Classification of Chitin-Degrading Enzymes
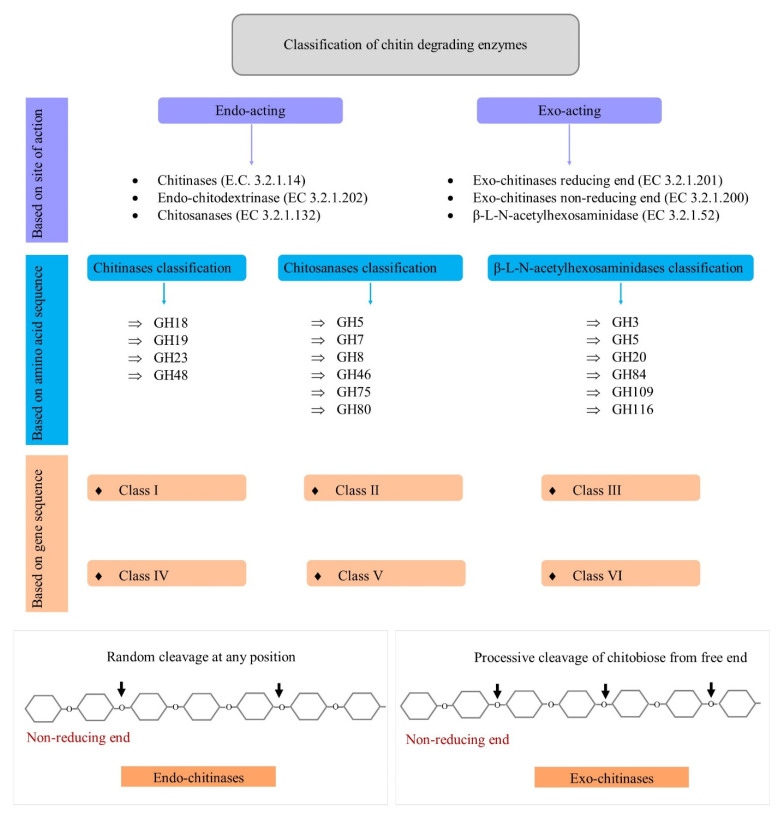
The enzyme commission has classified chitinolytic enzymes into different types, namely, chitinases (EC 3.2.1.14), exo-chitinase (reducing end) (EC 3.2.1.201), exo-chitinase (non-reducing end) (3.2.1.200), β-L-N-acetylhexosaminidases (EC 3.2.1.52), endo-chitodextrinases (EC 3.2.1.202), and chitosanases (EC 3.2.1.132) [4,5]. Various schemes of classification, based on different criteria, are subsequently discussed, and are schematically represented in Figure 1.
Figure 1.
Classification of chitin-degrading enzymes.
2.1. Site of Action-Based Classification
Chitin-degrading enzymes are categorized into endo- and exo-acting enzymes, based on their site of action. Chitinases (E.C 3.2.1.14), endo-chitodextrinases (EC 3.2.1.202), and chitosanases (EC 3.2.1.132) are endo-acting enzymes. Chitin is cleaved at random positions along the internal chain by chitinases (EC 3.2.1.14), to generate soluble oligomers of N-acetylglucosamine. Another endo-acting enzyme, endo-chitodextrinase (EC 3.2.1.202), is encoded by the endo I gene, and it hydrolyzes chitodextrins to release N, N′-diacetylchitobiose, and small amounts of N, N′, N′′-triacetylchitotriose. An endo-cleaving chitodextrinase (EC 3.2.1.202), characterized from the bacterium Vibrio furnissii, participates in the chitin catabolic pathway found in members of Vibrionaceae, and, dissimilarly to chitinase (EC 3.2.1.14), it has no activity on chitin [4]. Chitosanases (EC 3.2.1.132) are also endo-acting enzymes that cause endohydrolysis of the β-(1→4)-linkages between D-glucosamine residues in a partly acetylated chitosan [5,10].
Exo-chitinase (reducing end) (EC 3.2.1.201), exo-chitinase (non-reducing end) (3.2.1.200), and β-L-N-acetylhexosaminidases (EC 3.2.1.52) are exo-acting enzymes. Exo-chitinase (reducing end) (EC 3.2.1.201), encoded by the chiA gene, acts on the reducing end of chitin and chitodextrins, and causes the hydrolysis of N, N′-diacetylchitobiose. It liberates N, N′-diacetylchitobiose disaccharides by hydrolyzing the second glycosidic (1→4) linkage on the reducing end of chitin and chitodextrin molecules. The chiB gene encodes exo-chitinase (non-reducing end) (EC 3.2.1.200), and it generates N′-diacetylchitobiose from the non-reducing end of chitin and chitodextrins. Initially, exochitinases were subcategorized into chitobiosidases (EC 3.2.1.29) and 1,4-β-glucosamidases (EC 3.2.1.30) [4]. However, according to NC-IUBMB (the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology), EC 3.2.1.29 (chitobiosidases) and EC 3.2.1.30 (1,4-β-glucosamidases) have now been deleted and included in EC 3.2.1.52 (β-L-N-acetylhexosaminidase). EC 3.2.1.52 (β-L-N-acetylhexosaminidase) was created in 1972. In contrast, EC 3.2.1.30 (1,4-β-glucosamidases) was created in 1961 and incorporated into EC 3.2.1.52 (β-L-N-acetylhexosaminidase) in 1992, and EC 3.2.1.29 (chitobiosidases) was created in 1961 and incorporated into EC 3.2.1.52 (β-L-N-acetylhexosaminidase) in 1972 [4].
2.2. Gene Sequence-Based Classification
Chitinases are classified into six different classes, based on their gene sequence [11,12]. The class of chitinases is determined by various characteristics, such as their isoelectric pH, signal peptide, the sequence at the N-terminal, inducers, and localization of the enzyme.
The chitinases in class I have a cysteine-rich N-terminal. These undergo vacuolar localization, and possess valine or a leucine-rich signal peptide. These are further subdivided into two different classes, based on their acidic or basic nature. Plant chitinases, mostly endo-chitinases, are presented in class I.
Class II chitinases have a sequence similar to class I chitinases, but the cysteine-rich N-terminal is not present in class II chitinases. This class consists of only exo-chitinases, and contains chitinases from plants, fungi, and bacteria.
The sequence of class III chitinases is different from class I or class II chitinases. Class III chitinases have a GH18 catalytic domain with (β/α)8-barrel folding. These possess three conserved disulfide bonds, and the folding is somewhat similar to that of bacterial and fungal chitinases, despite the low sequence similarity.
As compared to class I chitinases, the size of class IV chitinases is significantly smaller, but they have similar characteristics, including immunological properties.
The chitinases in class V and class VI are not characterized well enough. Based on the gene sequence, it is reported that the cysteine-rich N-terminal appeared to be lost during evolution, as observed in one example of a class V chitinase, due to less selection pressure. There are two chitin-binding domains in tandem in class V chitinases [11,12].
2.3. Amino Acid Sequence-Based Classification
Based on their amino acid sequence, chitin-degrading enzymes are classified into four different GH families in the CAZy database [13], whereas β-L-N-acetylhexosaminidases and chitosanases are classified into six other GH families. Overall, Table 1 enlists the characteristics of chitin-degrading enzymes belonging to different GH families.
Table 1.
Characteristics of chitin-degrading enzymes belonging to different GH families.
| GH Family | Clan | Mechanism | Catalytic Domain 3D Structure | Catalytic Nucleophile/Base | Catalytic Proton Donor | Enzyme Name |
|---|---|---|---|---|---|---|
| GH3 | - | Retaining | - | Aspartate | Glutamate (for hydrolases)/Histidine (for phosphorylases) | β-L-N-acetylhexosaminidase |
| GH5 | GH-A | Retaining | (β/α)8-barrel | Glutamate | Glutamate | Chitosanase, β-L-N-acetylhexosaminidase |
| GH7 | GH-B | Retaining | β-jelly roll | Glutamate | Glutamate | Chitosanase |
| GH8 | GH-M | Inverting | (α/α)6 | Aspartate | Glutamate | Chitosanase |
| GH18 | GH-K | Retaining | (β/α)8-barrel | Carbonyl oxygen of C-2 acetamido group of the substrate | Glutamate | Chitinase |
| GH19 | - | Inverting | - | - | - | Chitinase |
| GH20 | GH-K | Retaining | (β/α)8 | Carbonyl oxygen of C-2 acetamido group of the substrate | Glutamate | β-L-N-acetylhexosaminidase |
| GH23 | - | - | - | - | Glutamate | Chitinase |
| GH46 | - | Inverting | - | Probably aspartate | Probably glutamate | Chitosanase |
| GH48 | GH-M | Inverting | (α/α)6-barrel | - | Glutamate | Chitinase |
| GH75 | - | Inverting | - | Probably aspartate | Probably glutamate | Chitosanase |
| GH80 | GH-I | Inverting | α+β | - | - | Chitosanase |
| GH84 | - | Retaining | (β/α)8-barrel | Carbonyl oxygen of C-2 acetamido group of the substrate | Aspartate | β-L-N-acetylhexosaminidase |
| GH109 | - | Retaining | - | Not applicable | None | β-L-N-acetylhexosaminidase |
| GH116 | GH-O | Retaining | (α/α)6-barrel | Glutamate | Aspartate | β-L-N-acetylhexosaminidase |
2.3.1. GH Family of Chitinases
According to the CAZy database [3,13], chitinases are mainly grouped into four different GH families, i.e., GH18, GH19, GH23, and GH48. GH18 family chitinases belong to the GH-K clan. The glycosidic bonds of chitin are cleaved by these enzymes with the retaining mechanism of configuration, and their catalytic domain has a (β/α)8-barrel 3D structure. Glutamate acts as a proton donor, and the carbonyl oxygen of the C-2 acetamido group of the substrate acts as a nucleophile. This family contains chitinases of class III and V. Chitinases belonging to the GH19 family cleave the glycosidic bonds with an inverting mechanism of configuration, and this family contains chitinases of classes I, II, and IV. The information about proton donors and nucleophiles in the reaction catalyzed by GH19 family chitinases is not known. Chitinases belonging to the GH23 family possess glutamate as a catalytic proton donor. GH48 chitinases cleave the glycosidic bonds with an inverting mechanism of configuration. Their catalytic domain has an (α/α)6-barrel 3D structure and belongs to the GH-M domain. Glutamate acts as a proton donor, and the information about catalytic nucleophiles is not known [3,13].
2.3.2. GH Family of β-L-N-acetylhexosaminidases
β-L-N-acetylhexosaminidase (EC 3.2.1.52) enzymes cause the hydrolysis of terminal non-reducing N-acetyl-D-hexosamine residues in N-acetyl-β-D-hexosaminides. In the CAZy database, they are grouped into six different GH families (GH3, GH5, GH20, GH84, GH109, and GH116) [3,13].
2.3.3. GH Family of Chitosanases
Chitosanases (EC 3.2.1.132), produced by some bacteria, deacetylate chitin to chitosan, which is finally converted to glucosamine residues. In the CAZy database, these are grouped into six different GH families (GH5, GH7, GH8, GH46, GH75, and GH80) [3,13].
3. Structural Organization of Chitinases
Various researchers have demonstrated the 3D structure of different chitinases belonging to the GH18 family. An eight-stranded α/β barrel represents the enzyme core of GH18 family chitinases, where eight strands of parallel β sheets are laid down with an α helix. The eight strands of β sheets bend into a barrel structure, with the helices forming a ring towards the outside. Most of the bacterial chitinases belonging to the GH18 family are characterized by a TIM-barrel (triosephosphate isomerase) fold in the catalytic subunit-containing conserved sequence Tyr-10, Ser-93, Asp-140 [14]. These enzymes degrade chitin with a retention mechanism of configuration. The 3D structure of the chitinase ChiA74 from Bacillus thuringiensis was elaborated by Juárez-Hernández et al. [15]. The crystal structure had the following four domains: a chitin-binding domain, fibronectin type III, chitinase insertion domain for barrel insertion, and the catalytic region with an (α/β)8-TIM barrel as a core structure. ChiA74 contains 676 amino acids that possess an N-terminal His-tag. The 442 residues form the catalytic region, which has a substrate-binding cleft and a semi-closed tunnel-shaped active site, with both an (α/β) 8-TIM barrel and CID domain. There are three disulfide bonds in the TIM barrel between the cysteine residues, at C78–C100, C140–C146, and C453–C461, and one in the CID domain at C348–C359. The FnIII linker loop (residues 476–484 at the opposite site of the catalytic region) links the FnIII-like domain to the TIM barrel. A calcium ion is present in a highly coordinated form in ChiA74. The CID domain containing 82 residues is present between the seventh beta-strand and the seventh alpha-helix of the (α/β)8-TIM barrel.
A similar structure was reported by Liu et al. [16] for the insect chitinase OfChi-h. Two domains, the fibronectin III domain and the catalytic domain, are connected via a linker of 25 amino acids. These domains were found to interact with each other via a motif consisting of two antiparallel β-strands and one short α-helix. The fibronectin III domain is an immunoglobulin-like β-sandwich domain that comprises eight β-strands, and the catalytic domain is a (β/α)8-barrel fold composed of eight β-strands and eight α-helices. A chitinase insertion domain, consisting of five antiparallel β-strands flanked by two α-helices, was also observed in the catalytic domain.
In GH19 family chitinases, 10 α-helical segments and one three-stranded β sheet are present in the secondary structure [17]. GH18 family chitinases catalyze the hydrolytic reaction by substrate-assisted mechanisms, whereas GH19 family chitinases have a high percentage of α-helices and adopt the single-displacement catalytic mechanism [18].
4. Sources of Chitinases
4.1. Plant Chitinases
Chitinases are expressed during various developmental stages of the plant, especially in response to biotic stress, to protect the plant against phytopathogens. Chitinase genes have been reported in plants such as Arabidopsis, Euphorbia characias, Solanum lycopersicum, and Oryza sativa. In Arabidopsis, 25 chitinase family genes have been identified, while, in rice, 49 chitinase family genes have been identified so far. Based on the available database, 43 chitinases have been identified in tomatoes [19]. Moreover, the plant genome contains many genes that code for catalytically inactive chitinases, also referred to as chitinase-like proteins (CLPs). These CLPs lack catalytic activity/binding ability due to substitutions in the chitin-binding domain (CBD) [20].
The expression of some proteins increases significantly in plants when the plants are exposed to different pathogens or stresses. These proteins are defined as PR (pathogenesis-related) proteins. These are a component of the plant’s systemic acquired resistance (SAR), and the expression of these PR proteins enhances the plant’s resistance to biotic (pathogens) and abiotic stresses (salinity and heavy metals). In the apple plant, the PR4 group is responsible for chitin recognition and the resistance response. In many plant species, the cell polarity of pathogenic fungi is destroyed by PR4 proteins, by binding at the tip of fungal hyphae [21]. The PR proteins are classified into 17 families, based on molecular mass, isoelectric point, localization, and biological activity [22,23]. These include β-1,3-glucanases, chitinases, thaumatin-like proteins, proteinase inhibitors, endoproteinase, peroxidases, ribonuclease-like proteins, thionins, nonspecific lipid transfer proteins, oxalate oxidase and oxalate oxidase-like proteins, and defensin. Among these 17 families, only four PR families, namely, PR3, PR4, PR8, and PR11, contain chitinases [23].
4.2. Chitinases from Insects and Other Organisms
Chitinases have also been reported in arthropods, mollusks, protozoans, nematodes, and coelenterates. Various insects, such as Drosophila melanogaster, Tribolium castaneum, Anopheles gambiae, Bombyx mori, Hyphantria cunea, Chironomus tentans, Spodoptera litura, Choristoneura fumiferana, Helicoverpa armigera, Aedes aegypti, Culex quinquefasciatus, Lacanobia oleracea, Spodoptera exigua, Mamestra brassicae, Ostrinia furnacalis, and Acyrthosiphon pisum, are known to produce chitinases [24,25]. In three mosquito species (Anopheles gambiae, Aedes aegypti, and Culex quinquefasciatus), multiple chitinase genes have been identified [24]. The first full-length cDNA of an insect chitinase gene was cloned from Manduca sexta [26]. Chitinase genes have also been isolated from different crustaceans, such as the Pacific white shrimp (Litopenaeus vannamei), Chinese mitten crab (Eriocheir sinensis), black tiger shrimp (Penaeus monodon), ridge tail white prawn (Exopalaemon carinicauda), and juvenile kuruma shrimp (Marsupenaeus japonicus) [27]. Acidic mammalian chitinase (AMCase) and chitotriosidase (Chit1) are two different types of chitinases that are produced by mammals. Pigs have also been reported to produce chitinases. The expression of chitinases in pig’s stomachs can be induced by the presence of chitin in their diet (insect powder). Kawasaki et al. [28] reported the production of acidic mammalian chitinase in piglets.
4.3. Microbial Chitinases
Chitinases are secreted by various microorganisms, such as bacteria, actinomycetes, fungi, and other organisms, as well as plants. Several bacteria (Bacillus, Streptomyces, Brevibacillus, Serratia, and Chromobacterium) [29] and fungi (Aspergillus, Trichoderma, Neurospora, Mucor, Lycoperdon, Metarhizium, Beauveria, and Lecanicillium) [30] are known for their chitinase production abilities. Table 2 summarizes the chitinases produced by these microbes, and their major characteristics.
Table 2.
Chitinases from different microbial sources and their characteristics.
| S.No. | Organism Name | Class of Enzyme | Molecular Weight (kDa) | Optimum pH/Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| 1. | Pseudoalteromonas sp. DL-6 | GH18 | 113.5 | 8.0/20 | Wang et al. [31] |
| 2. | Pseudoalteromonas sp. DC14, | - | 65 | 9.0/40 | Makhdoumi et al. [32] |
| 3. | Chitinibacter sp. GC72 | - | 65 | 6.8/40 | Gao et al. [33] |
| 4. | Bacillus licheniformis LHH100 | - | 65 | 4.0/75 | Laribi-Habchi et al. [34] |
| 5. | Paenibacillus barengoltzii | GH18 | 70 | 5.5/55 | Yang et al. [35] |
| 6. | Paenibacillus elgii HOA73 | - | 68 | 7.0/50 | Kim et al. [36] |
| 7. | Hydrogenophilus hirschii | - | 59 | 5.0/85 | Bouacem et al. [37] |
| 8. | Microbulbifer thermotolerans DAU221 | GH18 | 60 | 4.6/55 | Lee et al. [38] |
| 9. | Bacillus subtilis WB600 | GH18 | 62 | 5.0/60 | Pan et al. [39] |
| 10. | Corallococcus sp. EGB | GH18 | 52.9 | 6.0/50 | Li et al. [40] |
| 11. | Pseudomonas | - | - | 4.5/35 | Liu et al. [41] |
| 12. | Paenibacillus timonensis LK-DZ15 | GH18 | 70 | 4.5/80 | Yahiaoui et al. [42] |
| 13. | Chromobacterium violaceum | GH18 | 46 | 5.0/60 | Sousa et al. [43] |
| 14. | Paenibacillus pasadenensis CS0611 | GH18 | 69 | 5.0/50 | Guo et al. [44] and Xu et al. [45] |
| 15. | Chitiniphilus shinanonensis | GH18 | 45.3 | 6.0/50 | Bhuvanachandra and Podile [46] |
| 16. | Xenorhabdus nematophila | GH18 | 76 | - | Mahmood et al. [47] |
| 17. | Chitiniphilus shinanonensis | GH18 | 58.87 | 7.0/50 | Rani et al. [48] |
| 18. | Serratia marcescens | - | 55.6 | 6.0/55 | Li et al. [49] |
| 19. | Bacillus licheniformis J24 | GH18 | - | 7.0/70 | Essghaier et al. [50] |
| 20. | Paenibacillus sp. | - | 30 | 4.5/50 | Du et al. [51] |
| Actinomycetes | |||||
| 1. | Streptomyces sp. | - | 40 | 2&6/50 | Karthik et al. [52] |
| 2. | Microbispora sp. V2 | - | 35 | 3.0/60 | Nawani et al. [53] |
| 3. | Thermobifida fusca | GH18 | 46.3 | 6.0–8.0/40–45 | Gaber et al. [54] |
| 4. | Saccharothrix yanglingensis Hhs.015 | - | 77.9 | 7.0/49 | Lu et al. [55] |
| 5. | Streptomyces albolongus ATCC 27414 | GH18 | 47 | 5.0/55 | Gao et al. [56] |
| 6. | Streptomyces chilikensis RC1830 | - | 10.5 | 7.0/60 | Ray et al. [57] |
| 7. | Streptomyces alfalfae | GH19 | 29 | 8.0/45 | Lv et al. [58] |
| Fungi | |||||
| 1. | Aspergillus terreus | - | 60 | 5.6/50 | Farag et al. [59] |
| 2. | Humicola grisea | - | 50 | 3.0/70 | Kumar et al. [60] |
| 3. | Myceliophthora thermophila C1 | - | 43 | 6.0/55 | Krolicka et al. [61] |
| 4. | Aspergillus griseoaurantiacus KX010988 | - | 130 | 4.5/40 | Shehata et al. [62] |
| 5. | Trichoderma harzianum GIM 3.442 | GH18 | 45 | 6.0/45 | Deng et al. [63] |
4.3.1. Bacteria
Chitinolytic bacteria can decompose chitin under both aerobic and anaerobic conditions, and have been isolated from soil, hot springs, compost, and shellfish waste. Several bacteria, including Alteromonas, Aeromonas, Bacillus, Escherichia, Paenibacillus, Pseudomonas, Serratia, Streptomyces, and Vibrio, have been popularly reported for their chitinase activity [5,51,64]. Marine bacteria are also an important source of chitinases, and play an important role in chitin recycling in the aquatic environment; these include, in particular, Bacillus, Vibrio, Paenibacillus, Pseudoalteromonas, Aeromonas, Micrococcus, Streptomyces, Alteromonas, Actinomyces, Chitiniphilus, and Achromobacter [65]. Fu et al. [66] reported a chitinase of 67 kDa from the marine thermophilic bacterium Paenicibacillus barengoltzii CAU904, with an optimum pH and temperature of 3.5 and 60 ℃, respectively. Chitinases from extremophilic bacteria are important, due to their resistance to multiple factors, such as salt, temperature, heavy metals, etc. Thermostable chitinases are ideally suited for industrial purposes, as they can withstand the high-temperature operating conditions, which enable enhanced solubility of hydrophobic compounds and prevent microbial contamination [10]. A hyperthermostable chitinase from Brevibacillus formosus BISR-1, with a half-life of more than 5 h at 100 ℃, was isolated by Meena et al. [67]. Li et al. [68] characterized a thermophilic chitinase from the marine bacterium Microbulbifer sp. BN3, and the enzyme was stable at a high temperature, with maximum activity at 60 ℃. The thermostability of the chitinase enzyme can also be increased by site-directed mutagenesis and other molecular tools. Engineering of the Paenibacillus pasadenensis-derived chitinase PpChi1, by combinational mutagenesis and semi-rational design, improved the optimal temperature to 52.5 ℃, from 45 ℃ [45].
4.3.2. Actinomycetes
Actinomycetes are known for their antagonistic activities against various pathogens, due to their ability to produce a wide array of antibiotics. Besides antibiotics, actinomycetes are prolific producers of chitinases. Both mesophilic genera, such as Streptomyces, Saccharothrix, and Microbispora, and thermophilic genera, such as Thermobifida and Streptosporangium, are reported to produce chitinases [8,10]. A chitinase-producing actinomycete, Saccharothrix yanglingensis Hhs.015, was isolated from the roots of cucumber, which produced eight different chitinases [55].
4.3.3. Fungi
Fungal chitinases have been reported from various genera, such as Mucor, Aspergillus, Penicillium, Trichoderma, Beauveria, Coprinopsis, Metarhizium, Pistacia, and Lactarius, etc. The fungal cell wall is made of chitin and, therefore, has to protect itself from self-lysis. Due to cell wall protection in healthy hyphae vs. deprotection during a mycoparasitic attack, hyphal aging, and autolysis, it has been hypothesized that the degradation of self and non-self is regulated by substrate accessibility, rather than the speciation of individual chitinases. Fungi guard their cell walls against their hydrolytic enzymes by producing hydrophobic cell wall proteins, such as QID74 and carbohydrate-binding proteins. Carbohydrate-binding proteins bind to short oligosaccharides and chitin, and, therefore, mask them from degradation [69]. Rosado et al. [70] reported the role of the cell wall protein QID74, produced from Trichoderma harzianum, in cell protection and adherence to hydrophobic surfaces. The cell wall becomes more sensitive towards lytic enzymes in the absence of the QID74 protein. Repetitive peptide motifs in hydrophobic cell wall proteins also increase the molecular rigidity and hydrophobicity of cell walls, which helps in cell wall protection.
The molecular weight of fungal chitinases (35–50 kDa) is generally comparable to bacteria (20–60 kDa). In general, the optimum pH and temperature of fungal chitinases range from 4.0 to 8.0 and 40 to 50 °C, respectively [5,30]. Fungal chitinases exhibit anti-fungal activities against many plant pathogenic fungi, such as Aspergillus flavus, Botrytis cinerea, Rhizoctonia solani, Aspergillus niger, Aspergillus oryzae, Penicillum oxysporium, Candida albicans, Fusarium solani, and Sclerotinia sclerotiorum [59,63,71].
Generally, the inherent yield potential of wild organisms is low, which can be improved by the expression of the chitinase gene in a heterologous host, for the production of this enzyme in abundance. Chitinase genes from bacteria, actinobacteria, and fungi have been cloned and expressed in different expression hosts, such as E. coli BL21, Pichia pastoris, etc. [56,63]. Chitinase yields in heterologous hosts generally increase to a much higher titer (~22.6-fold) as compared to wild organisms. Nonetheless, chitinases from both wild and recombinant microorganisms are commercially available. Many chitinase formulations from wild strains of Aspergillus niger, Streptomyces griseus, and Trichoderma viride are commercially available. Recombinant chitinases are also commercially available, and include chitinase 18A from Clostridium thermocellum (activity—25 U/mg), chitinase 18A from Bacillus cereus (concentration—1 mg/mL), chitinase 18A from Bacillus licheniformis (concentration—1 mg/mL), and chitinase 18A from Clostridium thermocellum (activity—25 U/mg). These recombinant chitinases are produced at a large scale using E. coli (https://www.creative-enzymes.com/). Merck (Sigma-Aldrich), Megazyme, and Prospec Protein Specialists are the major commercial producers of chitinase. Prospec Protein Specialists manufacture a recombinant chitinase under the product name Chitinase Protein from Clostridium paraputrificum, expressed in E. coli (https://www.prospecbio.com/, 10 April 2021). Table 3 enlists the commercially available microbial chitinases.
Table 3.
Commercially available microbial chitinases.
| Trade Name and Producing Firm | Producing Organism | Formulation Type (Solid, Liquid) | Activity (U/g) |
|---|---|---|---|
| Chitinase from Aspergillus niger (food grade)/Creative Enzymes® | Aspergillus niger | Light-brown powdered form | 200 * |
| Native Streptomyces griseus Chitinase/Creative Enzymes® | Streptomyces griseus | Lyophilized powder (essentially salt-free) | >200 * |
| Native Trichoderma viride Chitinase/Creative Enzymes® | Trichoderma viride | Lyophilized powder form | >600 * |
| Chitinase (Clostridium thermocellum)/Megazyme | Clostridium thermocellum | Solid | 300 ** |
| Chitinase from Streptomyces griseus/Merck (Sigma-Aldrich) | Streptomyces griseus | Solid | ≥200 *** |
| Chitinase from Streptomyces griseus/Merck (Sigma-Aldrich) | Streptomyces griseus | Solid | ≥1000 *** |
| Chitinase from Trichoderma viride/Merck (Sigma-Aldrich) | Trichoderma viride | Lyophilized powder form | ≥600 *** |
Information adopted from the following three different websites: * https://www.creative-enzymes.com/, ** https://www.megazyme.com/, *** https://www.sigmaaldrich.com/india.html.
5. Microbial Fermentation for Chitinase Production
Both submerged fermentation (SmF) and solid-state fermentation (SSF) have been effectively used to produce chitinase from bacteria or fungi [72]. Bacteria are preferred for chitinase production, as compared to fungi, because of their high volumetric productivity [73]. For large-scale chitinase production, the substrate chitin is pretreated to increase its accessibility to chitinase, which helps to significantly improve the yields. Different techniques, such as ultra-sonication, acid treatment, and steam explosion, are used for the pretreatment of chitin. Lactic acid is also used to process chitin, which is a more environmentally favorable approach than hydrolysis using hydrochloric acid. Chitin processed using lactic acid has a higher potential to induce chitinase production when compared to colloidal chitin.
5.1. Submerged Fermentation (SmF) for Chitinase Production
SmF is the most widely used method for chitinase production from different microbes, such as Streptomyces pratensis strain KLSL55 [74], Trichoderma viride [75], Aspergillus niveus [76], Beauveria bassiana [77], and Bacillus cereus GS02 [78]. The fermentation may be operated in batch, fed-batch, or continuous mode, depending on the type of substrate and microorganism being used. SmF has several advantages, including enhanced oxygen delivery, increased mass transfer, and better process control. Different bioreactors, such as an airlift bioreactor, an airlift with a net draft tube reactor, a stirred-tank bioreactor, and a bubble column reactor, have been used for chitinase production [72].
5.2. Solid-State Fermentation (SSF) for Chitinase Production
Nutrient-rich waste materials, such as bagasse, paper pulp, and bran, can be used as substrates in SSF, for producing different metabolites, including chitinases. Chitin, being a solid material, can be directly used as the substrate for SSF, which potentially reduces the cost of the enzyme [72]. Chitinase production using SSF has been studied in various microorganisms, such as Trichoderma koningiopsis UFSMQ40 [79], Chromobacterium violaceum [80], Metarhizium anisopliae [81], and Bacillus thuringiensis R 176 [82]. The use of SSF for large-scale chitinase production is generally limited to laboratory scale, despite the low cost and high titer [83].
5.3. Factors Affecting Chitinase Production by Fermentation
Numerous physico-chemical factors, including the substrate, pH, agitation speed, fermentation period, inoculum size, carbon source, nitrogen source, metal ions, and temperature, affect the growth of chitinase-producing microorganisms and the subsequent titer of chitinase. These factors vary with the type of microbe used, and also with the method used for chitinase production. The effect of these factors on chitinase production is discussed below.
5.3.1. Substrate Used
Chitinase production is induced by the presence of (1,4)-glycosidic bonds in the substrate. Though various substrates, such as powdery chitin, colloidal chitin, chitosan, crab shell, shrimp powder, demineralized crab, wheat bran, sugarcane bagasse, and cellobiose, are used for the production of chitinase [64,79,84,85], colloidal chitin is the substrate of choice at the commercial scale, due to its higher productivity [64,86]. Colloidal chitin, chitin flakes, and other chitinous substrates, such as dimeric N-acetylglucosamine, are good inducers of chitinase. Glucose and xylose are also known to induce chitinase production in Streptomyces sp. K5 [84], and chitobiose was reported to be a strong inducer of chitinase in Trichoderma asperellum T4 [87].
5.3.2. Temperature and pH
Both temperature and pH are important factors that influence chitinase production. The fermentation temperature changes with the microorganism used. Most of the commercial strains used are mesophilic (Bacillus sp., Streptomyces sp., Aspergillus sp., and Trichoderma sp.), but a few thermophiles are also used at the commercial scale. The optimum pH for maximum chitinase production by various microorganisms generally varies between 6.0 and 8.0 [2,74,86,88].
5.3.3. Agitation Speed
As compared to static conditions, a good titer of chitinase is obtained at a higher rpm (revolutions per minute). However, when agitation is increased beyond a certain value, it causes cell sufferance and, consequently, low enzyme production, due to shear stress. Shear stress was also reported to partially inactivate the enzyme [89]. An airlift reactor with a net draft tube fermenter is generally used to improve agitation at the commercial scale.
5.3.4. Fermentation Period
For the production of chitinase, the fermentation period varies with the organism, substrate used, and other process parameters. The incubation time varies from 24 to 72 h in bacteria [73,74,86] and 48 to 96 h in fungi under SmF [79,90]. It was also reported that Bacillus and Paenibacillus require a shorter cultivation period (2–3 days) for chitinase production than Streptomyces thermocarboxydus TKU045, which takes 5 days [91].
5.3.5. Inoculum Size
Microbial growth and growth-related parameters are improved by increasing the inoculum size up to a certain level (1–5%, v/v), and a reduction in microbial activity is observed when the inoculum size is further increased, due to nutritional limitation. Gupta, Kumar, Laksh, and Rana [73] reported a maximum enzyme production of 0.53 IU with 1% of a 24 h starter culture of Bacillus sp. For Streptomyces pratensis strain KLSL55, production increased with inoculum size, and the highest titer of 141.20 IU was obtained with 1.25% inoculum (1 × 108 spores/mL) [74].
5.3.6. Carbon and Nitrogen Source
Yeast extract, malt extract, casein, ammonium sulfate, colloidal chitin, and chitin flakes are commonly used nitrogen sources for higher production [73]. The maximum chitinase production by Bacillus sp. R2 was observed with yeast extract as a nitrogen source [85], while, for Streptomyces macrosporus M1, the chitinase production was the highest with KNO3 [92]. Swollen chitin, sucrose, and cellulose improved the chitinase production from Chitinophaga sp. S167 [2], whereas starch increased the chitinase production from Paenibacillus sp. [51], when provided as a carbon source.
5.3.7. Metal Ions
The addition of Mn2+, Ca2+, and Co2+ improved the yield of chitinase, while Cu2+, Zn2+, Mg2+, and Fe2+ decreased the yield in bacterial fermentation [49,74,92]. Stimulation of the enzymatic activity in the presence of metal ions may be due to a change in electrostatic bonding. These metal ions may act as a binding link between the enzyme and the substrate. Ca2+ helps extracellular enzymes to withstand high temperatures, and is a well-known stimulator. The residues of aspartic and glutamic acid in chitinases bind to certain divalent cations, such as Zn2+ and Hg2+, which can inhibit chitinases [93].
5.4. Purification of Chitinases
Enzymes are needed in purified form to obtain information about their amino acid sequence, biochemical functions, and evolutionary relationships between proteins in diverse organisms [94]. For the purification of chitinase, the culture filtrate is generally precipitated using the ammonium sulfate precipitation method, followed by the use of various chromatography techniques for further purification (Table 4). Different researchers have obtained 4.3–14.4-fold purification with a recovery yield of 18.4–2.6%. The molecular weight of the purified chitinases varies from 20 to 120 kDa, with a pI range from 4.5 to 8.5.
Table 4.
Different techniques used for the purification of chitinases and their characteristics.
| Organism Name | Purification Technique | Molecular Weight (kDa) | Specific Activity (U/mg) | Optimum pH/Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Paenicibacillus barengoltzii | Ammonium sulfate precipitation (20–40%), ion-exchange chromatography | 67 | 12.4 | 3.5/60 | Fu, Yan, Wang, Yang and Jiang [66] |
| Aspergillus niveus | Ammonium sulfate precipitation, Sephadex G-100 gel filtration chromatography | 44 | 13.3 | 5.0/65 | Alves, de Oliveira Ornela, de Oliveira, Jorge and Guimarães [76] |
| Aspergillus griseoaurantiacus | Ammonium sulfate precipitation, DEAE-cellulose column chromatography, Sephacryl S-300 column chromatography | 130 | 93.75 | 4.5/40 | Shehata, Abd El Aty, Darwish, Abdel Wahab and Mostafa [62] |
| Bacillus altitudinis KA15 | Ammonium sulfate precipitation (30 and 60%), Sephacryl S-200 high-resolution size exclusion chromatography, high-performance ion-exchange chromatography (IEX) | 43 | 120,000 | 4.0/85 | Asmani et al. [95] |
| Myxococcus fulvus UM01 | Ni-NTA affinity chromatography | 26.99 | - | 7.0/35 | Shahbaz and Yu [64] |
| Paenibacillus sp. | Ammonium sulfate precipitation (60–80%), DEAE-IEC, and gel chromatography | 30 | 0.85 | 4.5/50 | Du, Duan, Miao, Zhai and Cao [51] |
| Shewanella inventionis HE3 | Ammonium sulfate precipitation (20–80%), gel filtration chromatography | 40 | 41,000 | 4.0/70 | Laribi-Habchi et al. [96] |
Further, the efficiency and stability of purified free chitinases are generally less, which can be improved by immobilization [97]. Enzyme immobilization is used to achieve stable, reusable, and more active enzymes by the simple act of fixing an enzyme on a support surface [98]. Various types of supports are used for immobilization, and, amidst them, magnetic nanoparticles (MNPs) of 10 to 20 nm are highly favored, due to their non-toxicity, large surface area and surface: volume ratio, and the simplicity in separating the biocatalyst after use. A chitinase immobilized on MNPs can be recovered easily, and can be used multiple times without losing much activity [99]. The immobilized enzyme is reported to be more active for COS production as compared to the free enzyme [97]. Different activators and cross-linking agents, such as 3-aminopropyl triethoxylsilane (APTES), cyanogen bromide, etc., are popularly used for the preparation of immobilized enzymes. Glutaraldehyde cross-linked chitosan beads were used to immobilize a recombinant, thermostable fungal chitinase from Thermomyces lanuginosus, which further increased the stability of the immobilized chitinase, as compared to the free enzyme [100]. Similarly, the stability and catalytic activity of the purified recombinant Chit36 enzyme were improved by immobilization on Ca2+cross-linked alginate beads and Ca2+ cross-linked alginate–sepiolite nanocomposite beads [101]. Though the immobilization of chitinases looks promising, more research is needed on non-toxic cross-linkers, especially for food-related applications.
5.5. Applications of Chitinases
Chitinases have a wide array of applications in various fields, including medical, industrial, and agricultural, which are elaborately discussed below.
5.5.1. Biocontrol Agent
Several pathogens, such as Fusarium sp., Botrytis cinerea, Sclerotinia sclerotiorum, and Rhizoctonia solani, possess chitin as the main cell wall constituent. Insects also contain chitin in their exoskeleton, which helps them to survive in adverse conditions. Chitinases can be used against both phytopathogenic fungi and insect pests. Chitinase from Penicillium ochrochloron has shown biocontrol activity against H. armigera, by increasing larval and pupal mortality, and reducing pupation [102]. The chitinase gene from Xenorhabdus nematophila was cloned in E. coli (DH5α) using a pGEMT easy vector, and expressed in E. coli BL-21 (DE3) using a pET-28b vector. This heterologous recombinant chitinase showed biocontrol activity against the larvae of H. armigera. The LD50 value was 20 µg/g, which caused 51.5% larval mortality on day 3 [47]. Further, chitinase production is also induced by the attack of phytopathogens in plant seeds, stems, tuber, and flowers. Some elicitors (such as COS) or growth regulators (ethylene) induce the production of chitinases, which act as PR proteins in plant self-defense [103].
5.5.2. Single-Cell Protein (SCP) Production
Single-cell proteins are used as food supplements, and are generated from single-celled microorganisms. Chitin-containing waste can be used as a substrate (shellfish chitin, shrimp cell waste, prawn cell waste, etc.) by chitin-utilizing microorganisms to produce SCP. Chitinases hydrolyze the chitin present in the waste products and release simpler products, which can be further used to grow yeast and other microbes to generate SCP. Various researchers have demonstrated the use of chitinase to produce SCP [104,105]. Patil and Jadhav [104] hydrolyzed shellfish chitin using chitinase from Penicillium ochrochloron to generate N-acetyl-D-glucosamine, which was used as the substrate for SCP production using Yarrowia lipolytica NCIM 3450.
5.5.3. Protoplast Isolation
Protoplasts are crucial for physiological and genetic studies on fungi [106]. Fungal protoplasts have been extensively used for the preparation of cell-free extracts and fungal organelles, for studying enzyme synthesis and secretion, cell wall synthesis and characterization, and genetic recombination and transformation. Chitinases are widely used to prepare fungal protoplasts of many fungi, including T. reesei, P. florida, A. bisporus, and A. niger [107].
5.5.4. Biomedical Applications
Chitinases also play important roles in the regulation of humoral and cellular immune responses. Allergens and helminths are known to induce the production of acidic mammalian chitinase (AMCase), which functions as a critical initiator of protective type 2 responses against intestinal nematodes. In an experiment conducted on AMCase-deficient mice, it was observed that when the mice were infected with the chitin-containing gastrointestinal nematodes Nippostrongylus brasiliensis and Heligmosomoides polygyrus bakeri, there was no type 2 immune response present [108]. Similarly, the chitinase produced by Lactobacillus rhamnosus GG inhibited the morphogenesis of the opportunist pathogen Candida albicans [109].
5.5.5. Chitooligosaccharide (COS) Production
Chitinases produce COS, which have immune-stimulant, anti-inflammatory, antibacterial, antioxidant, and prebiotic properties [91]. Chitin and COS are gaining interest due to their biocompatibility, non-toxicity, and biodegradability, which make them suitable for various applications. COS produced by the chitinase CsChiE from Chitiniphilus shinanonensis SAY3 efficiently hydrolyzed unmilled crab shell chitin waste (chitin flakes), and crystalline α-, β- and colloidal chitin. The predominant hydrolysis product was chitobiose, which is used as a cosmetic ingredient, dietary supplement, and in osteoarthritis therapeutics [48]. The chitinase PbChi70, from the thermophilic marine bacterium Paenibacillus barengoltzii, can efficiently produce N-acetyl chitobiose (GlcNAc)2 [35]. The hydrolysis of colloidal chitin by Streptomyces thermocarboxydus chitinase produced chitin oligomers with multiple degrees of polymerization (DP). The chitin oligomers with low DP exhibited enhanced 2, 2-diphenyl-1-picrylhydrazyl radical scavenging capability and promoted the growth of probiotic Lactobacillus lactis [91]. Pan, Li, Lv, Du, and Liu [39] increased the chitinase production from Bacillus sp. DAU101 for the enzymatic production of COS using molecular engineering. The expression level of chitinase increased from 35.54 U/mL to 51.67 U/mL by optimizing the ribosome binding sites (RBSs) with spacer sequences, and combining molecular docking technology with site-directed mutagenesis.
5.5.6. Waste Management
Waste from the sea, containing crab, lobster, and shrimp shells, is a challenging problem that requires massive landfills for disposal. Globally, approximately six to eight million tons of chitinous waste is produced annually. Chitinous waste can be degraded using physical and chemical methods, but these methods are expensive and not environmentally friendly. Chitinases can be an alternative eco-friendly approach for chitinous waste management. The application of chitinases is known to enhance the degradation rate of waste, and is being utilized for the management of chitinous waste globally [110]. An overexpressed chitinase from Bacillus subtilis degraded pretreated crystalline chitin substrates (such as α-chitin, β-chitin, and crab shells) more efficiently than SmChiA chitinase from Serratia marcescens and the commercial chitinase preparation from Streptomyces griseus [111].
6. Conclusions and Future Prospects
Chitinase, a commercially significant enzyme produced by bacteria, fungi, and insects, has a wide range of applications, including waste management, the production of SCP and COS, protoplast generation, biocontrol, etc. Its use as a biocontrol agent in agriculture has significant ramifications as a non-toxic alternative to chemical pesticides and insecticides. At the industrial scale, SmF is the technique of choice for chitinase production, but greater productivities can be obtained using SSF, which requires additional research, especially on heat transfer and downstream processing aspects, before commercial economic acceptability. Although immobilized chitinases offer the potential to reduce the cost of products such as COS, finding non-toxic cross-linkers and activators remains a major difficulty in developing immobilized enzyme formulations for food applications. Because of the numerous applications of chitinases, their demand is expected to rise in the near future.
Acknowledgments
The first author thanks Central University of Haryana, Mahendergarh (India) for providing fellowship during Ph.D.
Author Contributions
Writing—original draft, V.P., A.R., and A.K.; writing—review and editing, J.G., and K.P.; conceptualization, supervision, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the grant “The Fly ash as the precursors of functionalized materials for applications in environmental engineering, civil engineering and agriculture” no. POIR.04.04.00-00-14E6/18-00 project, carried out within the TEAM-NET programme of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel S., Goyal A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. Int. J. Biol. Macromol. 2017;97:331–338. doi: 10.1016/j.ijbiomac.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S., Kumar S., Khajuria A., Ohri P., Kaur R., Kaur R. Biocontrol potential of chitinases produced by newly isolated Chitinophaga sp. S167. World J. Microbiol. Biotechnol. 2020;36:90. doi: 10.1007/s11274-020-02864-9. [DOI] [PubMed] [Google Scholar]
- 3.Henrissat B., Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997;7:637–644. doi: 10.1016/S0959-440X(97)80072-3. [DOI] [PubMed] [Google Scholar]
- 4.McDonald A.G., Boyce S., Tipton K.F. ExplorEnz: The primary source of the IUBMB enzyme list. Nucleic Acids Res. 2009;37:D593–D597. doi: 10.1093/nar/gkn582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid R., Khan M., Ahmad M., Ahmad M.M., Abdin M., Musarrat J., Javed S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013;5:21–29. doi: 10.4103/0975-7406.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur N., Nath A.K., Chauhan A., Parmar S.C., Pandey H., Thakur K. Chitinases from microbial sources, their role as biocontrol agents and other potential applications. J. Entomol. Zool. Stud. 2019;7:837–843. [Google Scholar]
- 7.Bosquez-Molina E., Zavaleta-Avejar L. Chapter 2—New Bioactive Biomaterials Based on Chitosan. In: Bautista-Baños S., Romanazzi G., Jiménez-Aparicio A., editors. Chitosan in the Preservation of Agricultural Commodities. Academic Press; San Diego, CA, USA: 2016. pp. 33–64. [DOI] [Google Scholar]
- 8.Dhole N.P., Dar M.A., Pandit R.S. Recent advances in the bioprospection and applications of chitinolytic bacteria for valorization of waste chitin. Arch. Microbiol. 2021;203:1953–1969. doi: 10.1007/s00203-021-02234-5. [DOI] [PubMed] [Google Scholar]
- 9.Bibra M., Krishnaraj R.N., Sani R.K. An Overview on Extremophilic Chitinases. In: Sani R.K., Krishnaraj R.N., editors. Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy. Springer International Publishing; Cham, Switzerland: 2017. pp. 225–247. [DOI] [Google Scholar]
- 10.Mathew G.M., Madhavan A., Arun K.B., Sindhu R., Binod P., Singhania R.R., Sukumaran R.K., Pandey A. Thermophilic Chitinases: Structural, Functional and Engineering Attributes for Industrial Applications. Appl. Biochem. Biotechnol. 2021;193:142–164. doi: 10.1007/s12010-020-03416-5. [DOI] [PubMed] [Google Scholar]
- 11.Patil R.S., Ghormade V., Deshpande M.V. Chitinolytic enzymes: An exploration. Enzym. Microb. Technol. 2000;26:473–483. doi: 10.1016/S0141-0229(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 12.Passarinho P.A., de Vries S.C. Arabidopsis Chitinases: A Genomic Survey. Arab. Book. 2002;1:e0023. doi: 10.1199/tab.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Aalten D.M., Komander D., Synstad B., Gåseidnes S., Peter M.G., Eijsink V.G. Structural insights into the catalytic mechanism of a family 18 exo-chitinase. Proc. Natl. Acad. Sci. USA. 2001;98:8979–8984. doi: 10.1073/pnas.151103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juárez-Hernández E.O., Casados-Vázquez L.E., Brieba L.G., Torres-Larios A., Jimenez-Sandoval P., Barboza-Corona J.E. The crystal structure of the chitinase ChiA74 of Bacillus thuringiensis has a multidomain assembly. Sci. Rep. 2019;9:2591. doi: 10.1038/s41598-019-39464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu T., Chen L., Zhou Y., Jiang X., Duan Y., Yang Q. Structure, Catalysis, and Inhibition of OfChi-h, the Lepidoptera-exclusive Insect Chitinase*. J. Biol. Chem. 2017;292:2080–2088. doi: 10.1074/jbc.M116.755330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertus J.D., Monzingo A.F. The structure and action of chitinases. EXS. 1999;87:125–135. doi: 10.1007/978-3-0348-8757-1_9. [DOI] [PubMed] [Google Scholar]
- 18.Li H., Greene L.H. Sequence and Structural Analysis of the Chitinase Insertion Domain Reveals Two Conserved Motifs Involved in Chitin-Binding. PLoS ONE. 2010;5:e8654. doi: 10.1371/journal.pone.0008654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao J., Tan X. Comprehensive Analysis of the Chitinase Family Genes in Tomato (Solanum lycopersicum) Plants. 2019;8:52. doi: 10.3390/plants8030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kesari P., Patil D.N., Kumar P., Tomar S., Sharma A.K., Kumar P. Structural and functional evolution of chitinase-like proteins from plants. Proteomics. 2015;15:1693–1705. doi: 10.1002/pmic.201400421. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z., Zhu Y., Tian Y., Yao J.-L., Bian S., Zhang H., Zhang R., Gao Q., Yan Z. MdPR4, a pathogenesis-related protein in apple, is involved in chitin recognition and resistance response to apple replant disease pathogens. J. Plant Physiol. 2021;260:153390. doi: 10.1016/j.jplph.2021.153390. [DOI] [PubMed] [Google Scholar]
- 22.Morris J.S., Caldo K.M.P., Liang S., Facchini P.J. PR10/Bet v1-like Proteins as Novel Contributors to Plant Biochemical Diversity. ChemBioChem. 2021;22:264–287. doi: 10.1002/cbic.202000354. [DOI] [PubMed] [Google Scholar]
- 23.Jain D., Khurana J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In: Singh A., Singh I.K., editors. Molecular Aspects of Plant-Pathogen Interaction. Springer; Singapore: 2018. pp. 265–281. [Google Scholar]
- 24.Li D., Zhang J., Wang Y., Liu X., Ma E., Sun Y., Li S., Zhu K.Y., Zhang J. Two chitinase 5 genes from Locusta migratoria: Molecular characteristics and functional differentiation. Insect Biochem. Mol. Biol. 2015;58:46–54. doi: 10.1016/j.ibmb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Xi Y., Pan P.L., Ye Y.X., Yu B., Xu H.J., Zhang C.X. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2015;24:29–40. doi: 10.1111/imb.12133. [DOI] [PubMed] [Google Scholar]
- 26.Kramer K.J., Corpuz L., Choi H.K., Muthukrishnan S. Sequence of a cDNA and expression of the gene encoding epidermal and gut chitinases of Manduca sexta. Insect Biochem. Mol. Biol. 1993;23:691–701. doi: 10.1016/0965-1748(93)90043-R. [DOI] [PubMed] [Google Scholar]
- 27.Niu S., Yang L., Zuo H., Zheng J., Weng S., He J., Xu X. A chitinase from pacific white shrimp Litopenaeus vannamei involved in immune regulation. Dev. Comp. Immunol. 2018;85:161–169. doi: 10.1016/j.dci.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki K., Osafune T., Tamehira S., Yano K. Piglets can secrete acidic mammalian chitinase from the pre weaning stage. Sci. Rep. 2021;11:1297. doi: 10.1038/s41598-020-80368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veliz E.A., Martínez-Hidalgo P., Hirsch A.M. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017;3:689–705. doi: 10.3934/microbiol.2017.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karthik N., Akanksha K., Binod P., Pandey A. Production, purification and properties of fungal chitinases—A review. Indian J. Exp. Biol. 2014;52:1025–1035. [PubMed] [Google Scholar]
- 31.Wang X., Zhao Y., Tan H., Chi N., Zhang Q., Du Y., Yin H. Characterisation of a chitinase from Pseudoalteromonas sp. DL-6, a marine psychrophilic bacterium. Int. J. Biol. Macromol. 2014;70:455–462. doi: 10.1016/j.ijbiomac.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Makhdoumi A., Dehghani-Joybari Z., Mashreghi M., Jamialahmadi K., Asoodeh A. A novel halo-alkali-tolerant and thermo-tolerant chitinase from Pseudoalteromonas sp. DC14 isolated from the Caspian Sea. Int. J. Environ. Sci. Technol. 2015;12:3895–3904. doi: 10.1007/s13762-015-0848-4. [DOI] [Google Scholar]
- 33.Gao C., Zhang A., Chen K., Hao Z., Tong J., Ouyang P. Characterization of extracellular chitinase from Chitinibacter sp. GC72 and its application in GlcNAc production from crayfish shell enzymatic degradation. Biochem. Eng. J. 2015;97:59–64. doi: 10.1016/j.bej.2015.02.010. [DOI] [Google Scholar]
- 34.Laribi-Habchi H., Bouanane-Darenfed A., Drouiche N., Pauss A., Mameri N. Purification, Characterization, and molecular cloning of an extracellular chitinase from Bacillus licheniformis stain LHH100 isolated from wastewater samples in Algeria. Int. J. Biol. Macromol. 2015;72:1117–1128. doi: 10.1016/j.ijbiomac.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 35.Yang S., Fu X., Yan Q., Guo Y., Liu Z., Jiang Z. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii. Food Chem. 2016;192:1041–1048. doi: 10.1016/j.foodchem.2015.07.092. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y.H., Park S.K., Hur J.Y., Kim Y.C. Purification and Characterization of a Major Extracellular Chitinase from a Biocontrol Bacterium, Paenibacillus elgii HOA73. Plant Pathol. J. 2017;33:318–328. doi: 10.5423/PPJ.FT.01.2017.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouacem K., Laribi-Habchi H., Mechri S., Hacene H., Jaouadi B., Bouanane-Darenfed A. Biochemical Characterization of a novel thermostable chitinase from Hydrogenophilus hirschii strain KB-DZ44. Int. J. Biol. Macromol. 2018;106:338–350. doi: 10.1016/j.ijbiomac.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Lee H.-J., Lee Y.-S., Choi Y.-L. Cloning, purification, and Characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnol. Biofuels. 2018;11:303. doi: 10.1186/s13068-018-1299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan M., Li J., Lv X., Du G., Liu L. Molecular engineering of chitinase from Bacillus sp. DAU101 for enzymatic production of chitooligosaccharides. Enzym. Microb. Technol. 2019;124:54–62. doi: 10.1016/j.enzmictec.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Li Z., Xia C., Wang Y., Li X., Qiao Y., Li C., Zhou J., Zhang L., Ye X., Huang Y., et al. Identification of an endo-chitinase from Corallococcus sp. EGB and evaluation of its antifungal properties. Int. J. Biol. Macromol. 2019;132:1235–1243. doi: 10.1016/j.ijbiomac.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 41.Liu K., Ding H., Yu Y., Chen B. A Cold-Adapted Chitinase-Producing Bacterium from Antarctica and Its Potential in Biocontrol of Plant Pathogenic Fungi. Mar. Drugs. 2019;17:695. doi: 10.3390/md17120695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yahiaoui M., Laribi-Habchi H., Bouacem K., Asmani K.-L., Mechri S., Harir M., Bendif H., Aïssani-El Fertas R., Jaouadi B. Purification and biochemical Characterization of a new organic solvent-tolerant chitinase from Paenibacillus timonensis strain LK-DZ15 isolated from the Djurdjura Mountains in Kabylia, Algeria. Carbohydr. Res. 2019;483:107747. doi: 10.1016/j.carres.2019.107747. [DOI] [PubMed] [Google Scholar]
- 43.Sousa A.J.S., Silva C.F.B., Sousa J.S., Monteiro J.E., Freire J.E.C., Sousa B.L., Lobo M.D.P., Monteiro-Moreira A.C.O., Grangeiro T.B. A thermostable chitinase from the antagonistic Chromobacterium violaceum that inhibits the development of phytopathogenic fungi. Enzyme Microb. Technol. 2019;126:50–61. doi: 10.1016/j.enzmictec.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Guo X., Xu P., Zong M., Lou W. Purification and Characterization of alkaline chitinase from Paenibacillus pasadenensis CS0611. Chin. J. Catal. 2017;38:665–672. doi: 10.1016/S1872-2067(17)62787-6. [DOI] [Google Scholar]
- 45.Xu P., Ni Z.-F., Zong M.-H., Ou X.-Y., Yang J.-G., Lou W.-Y. Improving the thermostability and activity of Paenibacillus pasadenensis chitinase through semi-rational design. Int. J. Biol. Macromol. 2020;150:9–15. doi: 10.1016/j.ijbiomac.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 46.Bhuvanachandra B., Podile A.R. A transglycosylating chitinase from Chitiniphilus shinanonensis (CsChiL) hydrolyzes chitin in a processive manner. Int. J. Biol. Macromol. 2020;145:1–10. doi: 10.1016/j.ijbiomac.2019.12.134. [DOI] [PubMed] [Google Scholar]
- 47.Mahmood S., Kumar M., Kumari P., Mahapatro G.K., Banerjee N., Sarin N.B. Novel insecticidal chitinase from the insect pathogen Xenorhabdus nematophila. Int. J. Biol. Macromol. 2020;159:394–401. doi: 10.1016/j.ijbiomac.2020.05.078. [DOI] [PubMed] [Google Scholar]
- 48.Rani T.S., Madhuprakash J., Podile A.R. Chitinase-E from Chitiniphilus shinanonensis generates chitobiose from chitin flakes. Int. J. Biol. Macromol. 2020;163:1037–1043. doi: 10.1016/j.ijbiomac.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 49.Li J., Zheng J., Liang Y., Yan R., Xu X., Lin J. Expression and Characterization of a chitinase from Serratia marcescens. Protein Expr. Purif. 2020;171:105613. doi: 10.1016/j.pep.2020.105613. [DOI] [PubMed] [Google Scholar]
- 50.Essghaier B., Zouaoui M., Najjari A., Sadfi N. Potentialities and Characterization of an Antifungal Chitinase Produced by a Halotolerant Bacillus licheniformis. Curr. Microbiol. 2021;78:513–521. doi: 10.1007/s00284-020-02329-0. [DOI] [PubMed] [Google Scholar]
- 51.Du J., Duan S., Miao J., Zhai M., Cao Y. Purification and characterization of chitinase from Paenibacillus sp. Biotechnol. Appl. Biochem. 2021;68:30–40. doi: 10.1002/bab.1889. [DOI] [PubMed] [Google Scholar]
- 52.Karthik N., Binod P., Pandey A. Purification and Characterisation of an acidic and antifungal chitinase produced by a Streptomyces sp. Bioresour. Technol. 2015;188:195–201. doi: 10.1016/j.biortech.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Nawani N.N., Kapadnis B.P., Das A.D., Rao A.S., Mahajan S.K. Purification and characterization of a thermophilic and acidophilic chitinase from Microbispora sp. V2. J. Appl. Microbiol. 2002;93:965–975. doi: 10.1046/j.1365-2672.2002.01766.x. [DOI] [PubMed] [Google Scholar]
- 54.Gaber Y., Mekasha S., Vaaje-Kolstad G., Eijsink V.G.H., Fraaije M.W. Characterization of a chitinase from the cellulolytic actinomycete Thermobifida fusca. Biochim. Biophys. Acta. 2016;1864:1253–1259. doi: 10.1016/j.bbapap.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Lu Y., Wang N., He J., Li Y., Gao X., Huang L., Yan X. Expression and Characterization of a novel chitinase with antifungal activity from a rare actinomycete, Saccharothrix yanglingensis Hhs.015. Protein Expr. Purif. 2018;143:45–51. doi: 10.1016/j.pep.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 56.Gao L., Sun J., Secundo F., Gao X., Xue C., Mao X. Cloning, Characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414. Food Chem. 2018;261:329–336. doi: 10.1016/j.foodchem.2018.04.068. [DOI] [PubMed] [Google Scholar]
- 57.Ray L., Panda A.N., Mishra S.R., Pattanaik A.K., Adhya T.K., Suar M., Raina V. Purification and Characterization of an extracellular thermo-alkali stable, metal tolerant chitinase from Streptomyces chilikensis RC1830 isolated from a brackish water lake sediment. Biotechnol. Rep. 2019;21:e00311. doi: 10.1016/j.btre.2019.e00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lv C., Gu T., Ma R., Yao W., Huang Y., Gu J., Zhao G. Biochemical Characterization of a GH19 chitinase from Streptomyces alfalfae and its applications in crystalline chitin conversion and biocontrol. Int. J. Biol. Macromol. 2021;167:193–201. doi: 10.1016/j.ijbiomac.2020.11.178. [DOI] [PubMed] [Google Scholar]
- 59.Farag A.M., Abd-Elnabey H.M., Ibrahim H.A.H., El-Shenawy M. Purification, Characterization and antimicrobial activity of chitinase from marine-derived Aspergillus terreus. Egypt. J. Aquat. Res. 2016;42:185–192. doi: 10.1016/j.ejar.2016.04.004. [DOI] [Google Scholar]
- 60.Kumar M., Brar A., Vivekanand V., Pareek N. Process optimization, purification and Characterization of a novel acidic, thermostable chitinase from Humicola grisea. Int. J. Biol. Macromol. 2018;116:931–938. doi: 10.1016/j.ijbiomac.2018.05.125. [DOI] [PubMed] [Google Scholar]
- 61.Krolicka M., Hinz S.W.A., Koetsier M.J., Joosten R., Eggink G., van den Broek L.A.M., Boeriu C.G. Chitinase Chi1 from Myceliophthora thermophila C1, a Thermostable Enzyme for Chitin and Chitosan Depolymerization. J. Agric. Food Chem. 2018;66:1658–1669. doi: 10.1021/acs.jafc.7b04032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shehata A.N., Abd El Aty A.A., Darwish D.A., Abdel Wahab W.A., Mostafa F.A. Purification, physicochemical and thermodynamic studies of antifungal chitinase with production of bioactive chitosan-oligosaccharide from newly isolated Aspergillus griseoaurantiacus KX010988. Int. J. Biol. Macromol. 2018;107:990–999. doi: 10.1016/j.ijbiomac.2017.09.071. [DOI] [PubMed] [Google Scholar]
- 63.Deng J.-J., Shi D., Mao H.-H., Li Z.-W., Liang S., Ke Y., Luo X.-C. Heterologous expression and Characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion. Int. J. Biol. Macromol. 2019;134:113–121. doi: 10.1016/j.ijbiomac.2019.04.177. [DOI] [PubMed] [Google Scholar]
- 64.Shahbaz U., Yu X. Cloning, isolation, and Characterization of novel chitinase-producing bacterial strain UM01 (Myxococcus fulvus) J. Genet. Eng. Biotechnol. 2020;18:45. doi: 10.1186/s43141-020-00059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beygmoradi A., Homaei A., Hemmati R., Santos-Moriano P., Hormigo D., Fernández-Lucas J. Marine chitinolytic enzymes, a biotechnological treasure hidden in the ocean? Appl. Microbiol. Biotechnol. 2018;102:9937–9948. doi: 10.1007/s00253-018-9385-7. [DOI] [PubMed] [Google Scholar]
- 66.Fu X., Yan Q., Wang J., Yang S., Jiang Z. Purification and biochemical Characterization of novel acidic chitinase from Paenicibacillus barengoltzii. Int. J. Biol. Macromol. 2016;91:973–979. doi: 10.1016/j.ijbiomac.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 67.Meena S., Gothwal R.K., Krishna Mohan M., Ghosh P. Production and purification of a hyperthermostable chitinase from Brevibacillus formosus BISR-1 isolated from the Great Indian Desert soils. Extremophiles. 2014;18:451–462. doi: 10.1007/s00792-014-0630-4. [DOI] [PubMed] [Google Scholar]
- 68.Li R.K., Hu Y.J., He Y.J., Ng T.B., Zhou Z.M., Ye X.Y. A thermophilic chitinase 1602 from the marine bacterium Microbulbifer sp. BN3 and its high-level expression in Pichia pastoris. Biotechnol. Appl. Biochem. 2020;68:1076–1085. doi: 10.1002/bab.2027. [DOI] [PubMed] [Google Scholar]
- 69.Gruber S., Seidl-Seiboth V. Self versus non-self: Fungal cell wall degradation in Trichoderma. Microbiology. 2012;158:26–34. doi: 10.1099/mic.0.052613-0. [DOI] [PubMed] [Google Scholar]
- 70.Rosado I.V., Rey M., Codón A.C., Govantes J., Moreno-Mateos M.A., Benítez T. QID74 Cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fungal Genet. Biol. 2007;44:950–964. doi: 10.1016/j.fgb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Dolatabad H.K., Javan-Nikkhah M., Shier W.T. Evaluation of antifungal, phosphate solubilisation, and siderophore and chitinase release activities of endophytic fungi from Pistacia vera. Mycol. Prog. 2017;16:777–790. doi: 10.1007/s11557-017-1315-z. [DOI] [Google Scholar]
- 72.Stoykov Y.M., Pavlov A.I., Krastanov A.I. Chitinase biotechnology: Production, purification, and application. Eng. Life Sci. 2015;15:30–38. doi: 10.1002/elsc.201400173. [DOI] [Google Scholar]
- 73.Gupta N., Kumar A., Laksh S.A., Rana M. Process optimization of extracellular chitinase production from Bacillus sp. Isolated from fish waste Dumping site. Eur. J. Pharm. Med. Res. 2017;4:474–480. [Google Scholar]
- 74.Shivalee A., Lingappa K., Mahesh D. Influence of bioprocess variables on the production of extracellular chitinase under submerged fermentation by Streptomyces pratensis strain KLSL55. J. Genet. Eng. Biotechnol. 2018;16:421–426. doi: 10.1016/j.jgeb.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abu-Tahon M.A., Isaac G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020;66:32–40. doi: 10.2323/jgam.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 76.Alves T.B., de Oliveira Ornela P.H., de Oliveira A.H.C., Jorge J.A., Guimarães L.H.S. Production and Characterization of a thermostable antifungal chitinase secreted by the filamentous fungus Aspergillus niveus under submerged fermentation. 3 Biotech. 2018;8:369. doi: 10.1007/s13205-018-1397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elawati N.E., Pujiyanto S., Kusdiyantini E. Production of extracellular chitinase Beauveria bassiana under submerged fermentation conditions. J. Phys. Conf. Ser. 2018;1025:012074. doi: 10.1088/1742-6596/1025/1/012074. [DOI] [Google Scholar]
- 78.Dukariya G., Kumar A. Statistical optimization of chitinase production by Box–Behnken design in submerged fermentation using Bacillus cereus GS02. J. Appl. Biol. Biotechnol. 2021;9:60–66. doi: 10.7324/JABB.2021.9205. [DOI] [Google Scholar]
- 79.Baldoni D.B., Antoniolli Z.I., Mazutti M.A., Jacques R.J.S., Dotto A.C., de Oliveira Silveira A., Ferraz R.C., Soares V.B., de Souza A.R.C. Chitinase production by Trichoderma koningiopsis UFSMQ40 using solid state fermentation. Braz. J. Microbiol. 2020;51:1897–1908. doi: 10.1007/s42770-020-00334-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Michael R.Z. Production of Chitinase from Chromobacterium violaceum Using Agro Industrial Residues under Solid State Fermentation. Indian J. Pharm. Sci. 2021;83:39–44. doi: 10.36468/pharmaceutical-sciences.747. [DOI] [Google Scholar]
- 81.dos Reis C.B.L., Sobucki L., Mazutti M.A., Guedes J.V.C., Jacques R.J.S. Production of Chitinase from Metarhizium anisopliae by Solid-State Fermentation Using Sugarcane Bagasse as Substrate. Ind. Biotechnol. 2018;14:230–234. doi: 10.1089/ind.2017.0031. [DOI] [Google Scholar]
- 82.Chaiharn M., Pikomol A., Lumyoung S. Purification and Characterization of an Extracellular Chitinase from Bacillus thuringiensis R 176 using Solid State Fermentation with Shrimp Shells Waste. Chiang Mai J. Sci. 2019;46:495–509. [Google Scholar]
- 83.Wang L., Yang S.-T. Chapter 18—Solid State Fermentation and Its Applications. In: Yang S.-T., editor. Bioprocessing for Value-Added Products from Renewable Resources. Elsevier; Amsterdam, The Netherlands: 2007. pp. 465–489. [DOI] [Google Scholar]
- 84.Mutturi S., Ike M., Yamagishi K., Tokuyasu K. Isolation, characterization, and application of thermotolerant Streptomyces sp. K5 for efficient conversion of cellobiose to chitinase using pulse- feeding strategy. Process Biochem. 2020;94:58–65. doi: 10.1016/j.procbio.2020.04.009. [DOI] [Google Scholar]
- 85.Cheba B.A., Zaghloul T.I., El-Mahdy A.R., El-Massry M.H. Effect of nitrogen sources and fermentation conditions on Bacillus sp. R2 chitinase production. Procedia Manuf. 2018;22:280–287. doi: 10.1016/j.promfg.2018.03.043. [DOI] [Google Scholar]
- 86.Saima, Kuddus M., Roohi, Ahmad I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013;11:39–46. doi: 10.1016/j.jgeb.2013.03.001. [DOI] [Google Scholar]
- 87.Liu H., Cheng M., Zhao S., Lin C., Song J., Yang Q. ATP-Binding Cassette Transporter Regulates N,N′-diacetylchitobiose Transportation and Chitinase Production in Trichoderma asperellum T4. Int. J. Mol. Sci. 2019;20:2412. doi: 10.3390/ijms20102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akeed Y., Atrash F., Naffaa W. Partial purification and Characterization of chitinase produced by Bacillus licheniformis B307. Heliyon. 2020;6:e03858. doi: 10.1016/j.heliyon.2020.e03858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fenice M., Barghini P., Selbmann L., Federici F. Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003. Microb. Cell Factories. 2012;11:12. doi: 10.1186/1475-2859-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandhya C., Adapa L.K., Nampoothiri K.M., Binod P., Szakacs G., Pandey A. Extracellular chitinase production by Trichoderma harzianum in submerged fermentation. J. Basic Microbiol. 2004;44:49–58. doi: 10.1002/jobm.200310284. [DOI] [PubMed] [Google Scholar]
- 91.Tran T.N., Doan C.T., Nguyen V.B., Nguyen A.D., Wang S.-L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019;45:727–742. doi: 10.1007/s11164-018-3639-y. [DOI] [Google Scholar]
- 92.Sukalkar S.R., Kadam T.A., Bhosale H.J. Optimization of chitinase production from Streptomyces macrosporeus M1. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018;4:106–114. [Google Scholar]
- 93.Gomaa E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: Their potential in antifungal biocontrol. J. Microbiol. 2012;50:103–111. doi: 10.1007/s12275-012-1343-y. [DOI] [PubMed] [Google Scholar]
- 94.Bajpai P., editor. Xylanolytic Enzymes. Academic Press; Amsterdam, The Netherlands: 2014. Chapter 6—Purification of Xylanases; pp. 53–61. [DOI] [Google Scholar]
- 95.Asmani K.-L., Bouacem K., Ouelhadj A., Yahiaoui M., Bechami S., Mechri S., Jabeur F., Taleb-Ait Menguellet K., Jaouadi B. Biochemical and molecular Characterization of an acido-thermostable endo-chitinase from Bacillus altitudinis KA15 for industrial degradation of chitinous waste. Carbohydr. Res. 2020;495:108089. doi: 10.1016/j.carres.2020.108089. [DOI] [PubMed] [Google Scholar]
- 96.Laribi-Habchi H., Bouacem K., Allala F., Jabeur F., Selama O., Mechri S., Yahiaoui M., Bouanane-Darenfed A., Jaouadi B. Characterization of chitinase from Shewanella inventionis HE3 with bio-insecticidal effect against granary weevil, Sitophilus granarius Linnaeus (Coleoptera: Curculionidae) Process Biochem. 2020;97:222–233. doi: 10.1016/j.procbio.2020.06.023. [DOI] [Google Scholar]
- 97.Kidibule P.E., Costa J., Atrei A., Plou F.J., Fernandez-Lobato M., Pogni R. Production and Characterization of chitooligosaccharides by the fungal chitinase Chit42 immobilized on magnetic nanoparticles and chitosan beads: Selectivity, specificity and improved operational utility. RSC Adv. 2021;11:5529–5536. doi: 10.1039/D0RA10409D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sastre D.E., Reis E.A., Marques Netto C.G.C. Chapter 4 - Strategies to rationalize enzyme immobilization procedures. In: Kumar C.V., editor. Methods in Enzymology. Volume 630. Academic Press; Cambridge, MA, USA: 2020. pp. 81–110. [DOI] [PubMed] [Google Scholar]
- 99.Ismail S.A., Hassan M.E., Hashem A.M. Single step hydrolysis of chitin using thermophilic immobilized exochitinase on carrageenan-guar gum gel beads. Biocatal. Agric. Biotechnol. 2019;21:101281. doi: 10.1016/j.bcab.2019.101281. [DOI] [Google Scholar]
- 100.Prasad M., Palanivelu P. Immobilization of a thermostable, fungal recombinant chitinase on biocompatible chitosan beads and the properties of the immobilized enzyme. Biotechnol. Appl. Biochem. 2015;62:523–529. doi: 10.1002/bab.1283. [DOI] [PubMed] [Google Scholar]
- 101.Mohammadzadeh R., Agheshlouie M., Mahdavinia G.R. Expression of chitinase gene in BL21 pET system and investigating the biocatalystic performance of chitinase-loaded AlgSep nanocomposite beads. Int. J. Biol. Macromol. 2017;104:1664–1671. doi: 10.1016/j.ijbiomac.2017.03.119. [DOI] [PubMed] [Google Scholar]
- 102.Patil N.S., Jadhav J.P. Significance of Penicillium ochrochloron chitinase as a biocontrol agent against pest Helicoverpa armigera. Chemosphere. 2015;128:231–235. doi: 10.1016/j.chemosphere.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 103.Khan F.I., Bisetty K., Singh S., Permaul K., Hassan M.I. Chitinase from Thermomyces lanuginosus SSBP and its biotechnological applications. Extremophiles. 2015;19:1055–1066. doi: 10.1007/s00792-015-0792-8. [DOI] [PubMed] [Google Scholar]
- 104.Patil N., Jadhav J. Single cell protein production using Penicillium ochrochloron chitinase and its evaluation in fish meal formulations. J. Microb. Biochem. Technol. 2014;4:2. doi: 10.4172/1948-5948.S4-005. [DOI] [Google Scholar]
- 105.Revah-Moiseev S., Carroad P.A. Conversion of the enzymatic hydrolysate of shellfish waste chitin to single-cell protein. Biotechnol. Bioeng. 1981;23:1067–1078. doi: 10.1002/bit.260230514. [DOI] [Google Scholar]
- 106.Waghmare S.R., Kulkarni S.S., Ghosh J.S. Chitinase Production in Solid-State Fermentation from Oerskovia xanthineolytica NCIM 2839 and its Application in Fungal Protoplasts Formation. Curr. Microbiol. 2011;63:295. doi: 10.1007/s00284-011-9978-1. [DOI] [PubMed] [Google Scholar]
- 107.Dahiya N., Tewari R., Tiwari R.P., Hoondal G.S. Production of an Antifungal Chitinase from Enterobacter sp. NRG4 and its Application in Protoplast Production. World J. Microbiol. Biotechnol. 2005;21:1611–1616. doi: 10.1007/s11274-005-8343-6. [DOI] [Google Scholar]
- 108.Vannella K.M., Ramalingam T.R., Hart K.M., de Queiroz Prado R., Sciurba J., Barron L., Borthwick L.A., Smith A.D., Mentink-Kane M., White S., et al. Acidic chitinase primes the protective immune response to gastrointestinal nematodes. Nat. Immunol. 2016;17:538–544. doi: 10.1038/ni.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allonsius C.N., Vandenheuvel D., Oerlemans E.F.M., Petrova M.I., Donders G.G.G., Cos P., Delputte P., Lebeer S. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci. Rep. 2019;9:2900. doi: 10.1038/s41598-019-39625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saini S., Chand M., Sharma H.O., Kumar P. Role of Chitinases as a waste management to control global crisis. Int. J. Environ. Rehabil. Conserv. 2020:303–313. doi: 10.11208/essence.20.11.SP2.156. [DOI] [Google Scholar]
- 111.Wang D., Li A., Han H., Liu T., Yang Q. A potent chitinase from Bacillus subtilis for the efficient bioconversion of chitin-containing wastes. Int. J. Biol. Macromol. 2018;116:863–868. doi: 10.1016/j.ijbiomac.2018.05.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.