Abstract
The prevalence and severity of non-alcoholic fatty liver disease (NAFLD) is increasing, yet adequately validated tests for care paths are limited and non-invasive markers of disease progression are urgently needed. The aim of this work was to summarize the performance of Pro-C3, a biomarker of active fibrogenesis, in detecting significant fibrosis (F ≥ 2), advanced fibrosis (F ≥ 3), cirrhosis (F4) and non-alcoholic steatohepatitis (NASH) in patients with NAFLD. A sensitive search of five databases was performed in July 2021. Studies reporting Pro-C3 measurements and liver histology in adults with NAFLD without co-existing liver diseases were eligible. Meta-analysis was conducted by applying a bivariate random effects model to produce summary estimates of Pro-C3 accuracy. From 35 evaluated reports, eight studies met our inclusion criteria; 1568 patients were included in our meta-analysis of significant fibrosis and 2058 in that of advanced fibrosis. The area under the summary curve was 0.81 (95% CI 0.77–0.84) in detecting significant fibrosis and 0.79 (95% CI 0.73–0.82) for advanced fibrosis. Our results support Pro-C3 as an important candidate biomarker for non-invasive assessment of liver fibrosis in NAFLD. Further direct comparisons with currently recommended non-invasive tests will demonstrate whether Pro-C3 panels can outperform these tests, and improve care paths for patients with NAFLD.
Keywords: fatty liver, biomarker, Pro-C3, collagen type III, fibrosis, liver disease
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of metabolic liver disease that is estimated to affect around 25% of the worldwide population [1]. Its incidence has swiftly risen in recent years, along with the increase in obesity and type 2 diabetes mellitus (T2DM) [1]. Although most individuals with NAFLD are in the first stage of simple steatosis without clinical symptoms, a considerable portion will progress into non-alcoholic steatohepatitis (NASH) and/or hepatic fibrosis, which may lead to cirrhosis and liver-related mortality [2,3]. Liver fibrosis is the main predictor of mortality in patients with NAFLD [4,5].
A key question in the field is to identify patients with advanced liver fibrosis in order to ensure preventative measures before irreversible liver damage occurs. Moreover, patients in advanced and potentially active and progressive stages of NAFLD are the ones most likely to benefit from anti-NASH drugs that are currently being developed [6].
Liver biopsy is the preferred method to assess disease activity and fibrosis stage [5,7,8]. However, it is invasive, time-consuming, costly and carries a small yet significant risk of complications [9]. These factors limit its use in patients with NAFLD [3]. Therefore, liver biopsy is currently only recommended for patients at an increased risk of NASH or advanced fibrosis, to provide prognostic information, to rule out other liver diseases or for enrollment in therapeutic clinical trials [10]. These limitations of liver biopsy hamper larger-scale identification and staging of patients with NAFLD, as does the general paucity of care paths and guidelines [11,12,13]. Consequently, a significant percentage of patients with NAFLD remain undiagnosed and not staged. Imaging by multiparametric MRI has been shown to be a good non-invasive tool to monitor NAFLD and predict liver-related outcomes [14,15,16], but multiparametric MRI is expensive and not readily available on a large scale to identify all patients with advanced stages of NAFLD in need of further care and surveillance. Transient elastography by FibroScan is another non-invasive imaging tool that can be used to estimate the degree of liver fibrosis, by using shear wave velocity combined with ultrasound to determine liver stiffness [17]. However, the FibroScan tool is not available at every center, especially not in primary care. Non-invasive biomarkers of NASH and fibrosis likely offer a safer, less expensive, and more broadly accessible and applicable alternative to liver biopsy.
In recent years, several biomarkers have been developed for NAFLD, including clinical risk calculation scores, individual blood-based markers, complex panels, and new imaging modalities [18]. Some, such as the Fibrosis-4 (FIB-4) score and the Enhanced Liver Fibrosis (ELF) test, are already implemented in some clinical guidelines, for excluding patients with severe disease in the first line of care, because of their high negative predictive values [10,19]. However, the specificity of FIB-4 becomes low above the age of 65 [20,21] and the commercially available ELF test is quite costly, limiting its implementation.
A relatively new biomarker to be considered and tested for NAFLD is Pro-C3, an enzyme-linked immunosorbent assay (ELISA) towards the N-terminal propeptide of type III collagen. This ELISA method measures ADAMTS2-mediated cleavage of the propeptide from type III collagen during fibrillar assembly, making the test outcome indicative of active fibrogenesis [22]. Since a dense fibrillar collagen band can block the passage of nutrients and oxygen through the interstitial extracellular matrix, this is an important structure in the development of fibrosis [23]. Pro-C3 was developed to assess the formation of type III collagen in different pathologies, hence not specifically for NAFLD [24]. This marker has been studied in patients with fibrosis related to chronic hepatitis C [25], after liver transplantation, tumor fibrosis in pancreatic ductal adenocarcinoma [26] and lung fibrosis in patients with systemic sclerosis [27]. Several studies have assessed the performance of Pro-C3 in NAFLD, mostly as a single marker and sometimes as part of a diagnostic biomarker panel [28,29,30,31]. To date, it is unknown for which target condition within the NAFLD spectrum; in which context of use (i.e., in primary, secondary or tertiary care); and at what diagnostic threshold, that Pro-C3 has optimal performance.
Thus far, the number of studies that evaluated the diagnostic accuracy of Pro-C3 is limited, and different levels of accuracy are reported. The largest study to investigate the diagnostic accuracy of Pro-C3 included 449 subjects, in a setting with a relatively high prevalence of advanced fibrosis (37%) [28]. A meta-analysis of the accuracy of Pro-C3 based on all available studies can provide a more comprehensive evidence-base. We performed a systematic review and meta-analysis to summarize the clinical performance of Pro-C3 in detecting significant fibrosis (F ≥ 2), advanced fibrosis (F ≥ 3), cirrhosis (F4), and NASH in patients with NAFLD.
2. Materials and Methods
This work has been conducted as part of the international multi-center LITMUS project (Liver Investigation: Testing Marker Utility in Steatohepatitis), aiming to develop and validate a defined set of biomarkers for detecting of NASH and NAFLD-related fibrosis. The protocol of the full systematic review can be accessed on PROSPERO: CRD42018106821. The current report was prepared using the PRISMA-DTA statement (Supplementary Figure S1) [32].
2.1. Literature Search
We developed a sensitive search strategy to identify reports on the diagnostic accuracy of Pro-C3 in patients with NAFLD. The search strategy encompassed words in the title or abstract as well as in the full record and used the Medical Subject Heading (MeSH) terms. In January 2020, the following databases were searched: MEDLINE (via OVID), EMBASE (via OVID), PubMed, Science Citation Index and CENTRAL (the Cochrane Library). The full search strategy can be found in Supplementary Table S1. To identify additional studies, we hand searched the list of references of eligible study reports and contacted our LITMUS partners for any studies that might have been missed by our search strategy. The search was updated in July 2021. All available records that fulfilled the search criteria to that date were screened.
2.2. Eligibility Criteria
Studies that included patients with NAFLD and undertook both Pro-C3 testing and a liver biopsy as the reference standard, published in peer-reviewed journals or identified conference abstracts/presentations, were eligible. Prognostic studies were excluded. We made no further restrictions on language or study design. Only studies of adult patients (≥18 years) with NAFLD were included. Study groups consisting of patients with mixed etiologies were excluded if data for patients with NAFLD were not reported separately. Studies that included patients with co-existing liver disease (e.g., both NAFLD and hepatitis B) were excluded. Potential overlap of patient groups between studies was thoroughly investigated and checked with the authors. In case of overlapping patient groups between studies, the largest study was selected.
Only studies using a liver biopsy as the clinical reference standard were eligible. The target conditions were NASH, significant liver fibrosis (F ≥ 2), advanced liver fibrosis (F ≥ 3), and cirrhosis (F4). The index test was the Pro-C3 ELISA, developed by Nordic Biosciences in 2013 [24]. Time between Pro-C3 measurements and liver biopsies could not be more than 6 months for a study to be eligible.
Studies that provided data on true positive (TP), false positive (FP), true negative (TN) and false negative (FN) Pro-C3 test results, or data that allowed us to reconstruct the classification table, were eligible for inclusion in the meta-analysis. In case studies did not report a threshold value for Pro-C3, or did not report sufficient information to reconstruct classification tables to calculate diagnostic accuracy estimates, authors were contacted to provide the required information. All studies from authors who decided not to or failed to provide these data within four months were excluded from the meta-analysis.
2.3. Study Selection and Data Extraction
Two authors (ALM and AvD) independently screened the identified titles and abstracts for potentially eligible studies. The same two authors subsequently evaluated full-text articles reporting potentially eligible studies to make final decisions about inclusion. The title and abstract screening phases were conducted using Rayyan QCRI [33]. Any conflict of judgment was discussed and resolved between the two primary reviewers; when inconclusive, a third reviewer’s judgment (JL) was decisive.
The following data were extracted from the included studies by the first reviewer (ALM) and cross-checked by the second reviewer (AvD): study characteristics, study group characteristics, reference test features, index test features, and numbers needed for reconstructing 2 × 2 classification tables (TP, FP, TN, FN).
2.4. Risk of Bias and Applicability Assessment
The risk of bias and applicability to our review question of each included study were independently assessed by two reviewers (ALM and AvD) using the QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies) tool in Review Manager 5.4 [34,35]. Any conflict of opinion was discussed and resolved between them, or discussed with a third reviewer (JL or YV). For each of the domains of the QUADAS-2 tool, the risk of bias for each study was assigned a judgment of ‘low risk’, ‘unclear risk’ or ‘high risk’. Concerns about applicability to the review question were classified in the same manner.
2.5. Statistical Analysis
Forest plots summarizing sensitivity, specificity and threshold values reported in each study were designed using Review Manager 5.4 [35].
We conducted a meta-analysis of the accuracy of Pro-C3 in detecting the respective target conditions (i.e., significant fibrosis, advanced fibrosis, NASH or cirrhosis), whenever three or more studies reporting accuracy data for this target condition were available. A bivariate logitnormal random effects model was used to compute summary measures of the diagnostic accuracy of Pro-C3, in terms of sensitivity and specificity and area under the receiver operating characteristic curve (AUC) [36]. From studies that reported accuracy data at more than one threshold, one threshold was selected for the analysis: either the point maximizing the Youden Index or, if no Youden Index was calculated, the threshold closest to those of the other included studies.
Summary receiver operating characteristic (SROC) curves were constructed using the estimates from the bivariate random effects model, to illustrate the overall diagnostic accuracy of Pro-C3 for each target condition, including 95% confidence intervals. We further calculated 95% prediction intervals to assess between and within study heterogeneity. All analyses were performed in R version 3.6.3, using the ‘mada’ package [37,38]. Additional bootstrapping was done using the ‘dmetatools’ package to calculate the 95% confidence interval around the AUC [39].
Publication bias was not assessed in this systematic review, as no reliable methods are available for evaluating publication bias in diagnostic accuracy studies [40].
2.6. Sensitivity Analysis
Sensitivity analyses were conducted by repeating the meta-analysis excluding one primary study that included only patients with T2DM [41] and one abstract-only study [42].
3. Results
3.1. Study Characteristics
From the 35 records on Pro-C3 which were identified in the search, eight studies were eligible for inclusion in the systematic review. The study flow diagram is shown in Figure 1. Seven of the included studies were full text reports [28,29,30,41,43,44,45] and one was an abstract [42].
Figure 1.
Flow diagram of studies included in the systematic review and meta-analysis.
The study characteristics are presented in Table 1. One study had two separate groups for the current analysis because the characteristics and outcomes of the discovery and validation cohorts were reported separately [43]. Seven out of the eight studies were designed as diagnostic accuracy studies, while one was an exercise intervention trial that reported Pro-C3 values and liver biopsies at baseline [44]. Included patients were mostly from Western countries (United States and Europe); two studies also included participants from Asia and Australia [29,30].
Table 1.
Characteristics of included studies.
| Study ID | Country | Setting | Population | N (% Male) | Mean Age | BMI (SD) | Target Conditions | DM | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Daniels 2019 [29] | Australia, UK, Japan | Secondary and tertiary care | Biopsy-confirmed NAFLD | 239 (56%) | 52.2 | 33.6 (7.7) | F ≥ 2; F ≥ 3; F4 | 37% | 49.6 (34.4) * | 72.2 (54.6) * |
| Boyle 2019 [28] | 7 European countries | Tertiary care | Suspected NAFLD | 449 (59%) | 52.0 | 32.6 (6.8) | F ≥ 3; NASH + F ≥ 2; NASH + F4 | 48% | 47.0 (26.0) | 69.0 (41.0) |
| Huber 2019 [44] | Germany | Secondary or tertiary care | Biopsy-confirmed NAFLD | 27 (66%) | 41.0 † | 30.8 (5.1) | F ≥ 2; F ≥ 3 | 27% | NR | NR |
| Luo 2018 Discovery [43] | USA | Secondary or tertiary care | Suspected or biopsy-confirmed NAFLD | 164 (32%) | 53.3 | NR | F ≥ 2; F ≥ 3 | NR | 46.8 (21.3) * | 59.8 (38.1) * |
| Luo 2018 Validation [23] | USA | Secondary or tertiary care | Biopsy-confirmed NAFLD | 41 (32%) | 50.1 | NR | F ≥ 2; F ≥ 3 | 37% | 71.3 (50.6) * | 98.3 (57.5) * |
| Nielsen, Leeming 2021 [30] | USA, Australia, Belgium, France, Germany, Hong Kong, Italy, Poland, Spain, UK | Secondary or tertiary care | Biopsy-confirmed NAFLD | 517 (52%) | 55.2 † | 32.7 † | F ≥ 2; F ≥ 3; NASH | 40% | 34.8 † | 47.1 † |
| Bril 2019 [41] | USA | Primary and tertiary care | Suspected NAFLD | 125 (87%) | 58.7 | 34.4 (4.6) | F ≥ 2; F ≥ 3 | 100% | 40.4 (23.1) | 53.6 (35.6) |
| Knöchel 2021 [42] | Sweden | Secondary or tertiary care | Biopsy-confirmed NAFLD | 56 (71%) | 61.0 | 29.1 (4.7) | F ≥ 2; F ≥ 3 | NR | NR | NR |
| Erhardtsen 2021 [45] | UK and Germany | Secondary and tertiary care | Biopsy-confirmed NAFLD | 215 (52%) | 56.0 | 33 † | F ≥ 2; F ≥ 3; NASH + F ≥ 2 | 47% | 48.5 † | 64.0 † |
* not documented for all patients; † = median, not mean; NAFLD = non-alcoholic fatty liver disease, NASH = non-alcoholic steatohepatitis, AST = aspartate aminotransferase, ALT = alanine aminotransferase, BMI = body mass index, NR = not reported, SD = standard deviation, DM = diabetes mellitus.
The mean age of individuals was 54 years (SD 14.2) and the average percentage of male participants was 55%. Of note, seven out of eight studies had recruited patients from secondary or tertiary care only. One study included solely patients with T2DM [41], whereas the percentage of patients with T2DM in the other studies ranged from 27% to 48%.
Seven studies reported the accuracy of Pro-C3 in detecting significant fibrosis, eight for advanced fibrosis, three for (fibrotic) NASH, and two for cirrhosis or cirrhotic NASH. All studies used the NASH CRN criteria for histological scoring of liver biopsies, the clinical reference standard. Supplementary Table S2 details what additional information was received from authors of primary studies.
3.2. Risk of Bias Assessment
The overall judgment of methodological quality of each of the included studies is shown in Supplementary Figure S2. Overall, the risk of bias in the included studies was low. One study was deemed at a high risk of bias for patient selection for the purpose of this systematic review because of inappropriate exclusions [44]. Another abstract-only study did not describe patient selection in sufficient detail [42]. Two studies were deemed at high risk of bias for the index test, because of unclear reporting of the interpreters’ blinding, of how the Pro-C3 threshold was determined, or because the threshold was not pre-specified [29,41]. There were few applicability concerns; the only high concern judgment here was based on the inclusion solely of patients with T2DM in one primary study [41].
3.3. Accuracy of Pro-C3 in Detecting Significant Fibrosis
The meta-analysis of the accuracy of Pro-C3 in detecting significant liver fibrosis (F ≥ 2) comprised data from seven primary studies on 1568 patients with NAFLD in total. The proportion of study participants with significant liver fibrosis in the included studies ranged from 36% to 74%.
Supplementary Figure S3A shows the forest plot of the diagnostic accuracy data from primary studies in detecting significant fibrosis. As shown, included studies used different positivity threshold values for Pro-C3, ranging from 9.7 to 20.9 ng/mL.
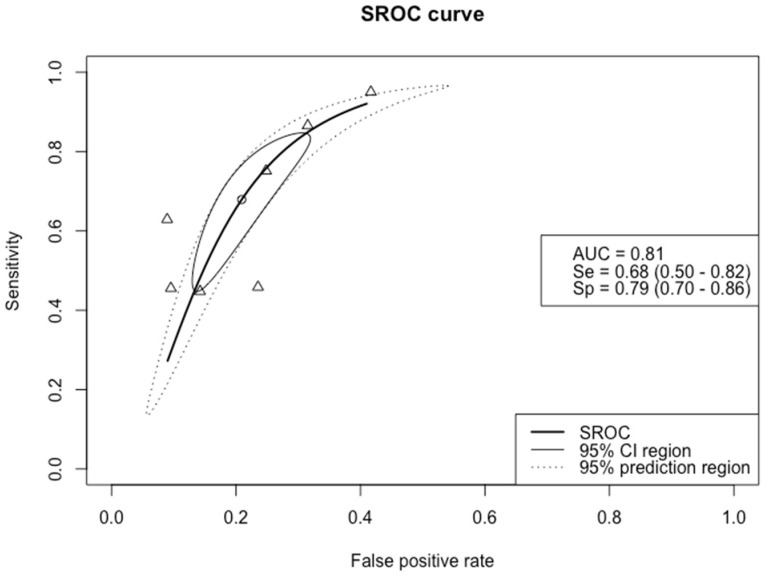
The SROC curve in Figure 2 depicts the sensitivity versus the false positive rate (1–specificity) of Pro-C3 for detecting significant fibrosis. As shown, the estimated mean specificity and sensitivity of Pro-C3 were 79% (95% CI 0.71–0.86) and 68% (95% CI 0.50–0.82), respectively. The AUC was 0.81 (95% CI 0.77–0.84) for detecting significant fibrosis.
Figure 2.
Summary receiver operating characteristic (SROC) curve of the diagnostic accuracy of Pro–C3 in detecting significant fibrosis. The solid ellipse depicts the 95% confidence interval region of diagnostic accuracy data of Pro–C3 in the included studies; the dotted ellipse depicts the prediction region in which 95% of future diagnostic accuracy study estimates of Pro–C3 will fall. Triangles represent diagnostic accuracy estimates from each included study; circle represents the Youden Index threshold value. AUC = area under the receiver operating curve, Se = sensitivity, Sp = specificity.
3.4. Accuracy of Pro-C3 in Detecting Advanced Fibrosis
Nine groups from eight primary studies were available for meta-analysis of the accuracy of Pro-C3 in the detection of advanced fibrosis. In total, these included 2058 patients with NAFLD. The proportion of study participants with advanced fibrosis in the included studies ranged from 17% to 47%.
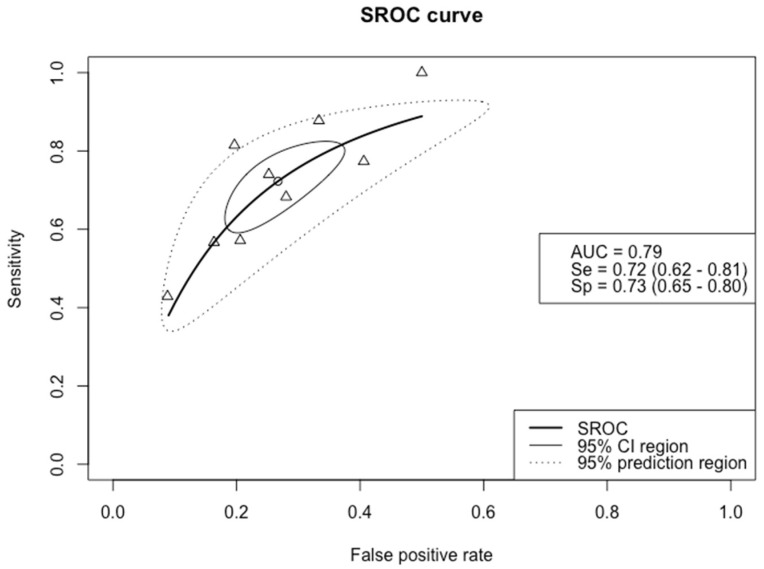
The forest plot in Supplementary Figure S3B provides an overview of the diagnostic accuracy data of Pro-C3 in detecting advanced fibrosis. The threshold values ranged from 12.7 to 21.3 ng/mL: six studies employed a threshold around 15 ng/mL, while the three remaining studies used a threshold around 21 ng/mL. Figure 3 shows the SROC curve of Pro-C3 in the detection of advanced fibrosis. For this target condition, the specificity and sensitivity were similar: 73% (95% CI 0.65–0.80) and 72% (95% CI 0.62–0.81), respectively. The summary estimate of the AUC of Pro-C3 in detecting advanced fibrosis was 0.79 (95% CI 0.73–0.82).
Figure 3.
SROC curve of the diagnostic accuracy of Pro–C3 in detecting advanced fibrosis. The solid ellipse depicts the 95% confidence interval region of diagnostic accuracy data of Pro–C3 in the included studies; the dotted ellipse depicts the prediction region in which 95% of future diagnostic accuracy study estimates of Pro–C3 will fall. Triangles represent diagnostic accuracy estimates from each included study; circle represents the Youden Index threshold value. AUC = area under the receiver operating curve, Se = sensitivity, Sp = specificity.
One point in the SROC curve is depicted at a sensitivity of 1.0, since there were no false negatives among the 56 patients with advanced fibrosis included in the study from Knöchel and colleagues [42]. Because of the small number of participants in this study, this had no substantial influence on the summary curve.
3.5. Accuracy of Pro-C3 Detecting Non-Alcoholic Steatohepatitis (NASH) or Cirrhosis
One study reported accuracy data for NASH, and reported a sensitivity of 55% and sensitivity of 82% at a threshold of 14.7 ng/mL [30]. Two studies reported accuracy data of Pro-C3 in detecting fibrotic NASH, defined as NAS ≥ 4 plus F ≥ 2, and estimated sensitivity at 66% and 68%, and specificity at 68% and 73%, for thresholds of 14.5 and 12.6, respectively [28,45]. For cirrhosis or cirrhotic NASH, defined as NAS ≥ 4 plus F4, sensitivities of 90% and 76%, and specificities of 57% and 63% were reported, at thresholds of 15.6 and 16.5 ng/mL, respectively [28,29]. Since the classification of (fibrotic) NASH differed between the three studies and only two studies reported accuracy data for cirrhosis, insufficient data were available for meta-analyses of the diagnostic accuracy of Pro-C3 for these target conditions.
3.6. Sensitivity Analyses
We performed a sensitivity analysis excluding the one study that included only patients with T2DM [41]. We observed no major differences in sensitivity or specificity for the detection of either significant or advanced fibrosis with or without the T2DM study. For significant fibrosis, the sensitivity analysis yielded an AUC of 0.81; for advanced fibrosis, the AUC was 0.78.
Another sensitivity analysis was performed excluding the data from the included abstract [42] as non-peer-reviewed data may be less reliable. The AUCs in this sensitivity analysis remained the same as the main analysis (0.81 for significant fibrosis and 0.79 for advanced fibrosis) and there were no major differences in sensitivity or specificity.
4. Discussion
4.1. Main Results
In this systematic review, we summarized the evidence on the accuracy of Pro-C3 in detecting target conditions within the NAFLD spectrum: significant fibrosis (F ≥ 2), advanced fibrosis (F ≥ 3), or NASH. We found that Pro-C3 had an overall AUC around 0.80 in detecting significant and advanced fibrosis in patients with NAFLD. Few studies have reported on the accuracy of Pro-C3 in detecting NASH or cirrhosis.
Currently, clinical guidelines recommend using non-invasive biomarkers such as FIB-4 and the ELF test to predict NAFLD fibrosis [10,19,46]. A recent systematic review showed that the AUC of the ELF test in detecting advanced fibrosis is 0.83 [47]. For FIB-4, a study with a large sample size reported an AUC of 0.76 in detecting advanced fibrosis [48]. The results of the current systematic review and meta-analysis, therefore, indicate that the diagnostic accuracy of Pro-C3 is comparable to these tests that are already mentioned in clinical guidelines, and should be seen as an important candidate in improving non-invasive diagnostics for NAFLD fibrosis. We suggest that Pro-C3, or a panel incorporating Pro-C3, may be used as an adjunct test to the widely available FIB-4 test in a two-tiered screening approach. It could replace transient elastography, especially in clinics where FibroScan is not available, although the accuracy of this suggested approach should still be investigated.
4.2. Test Performance in Detecting Liver Fibrosis
Pro-C3 is a measurement of collagen cleavage during active fibrogenesis. Other collagen cleavage particles have been evaluated in diagnosing hepatic fibrosis as well, such as PIIINP, a component of the ELF test panel. The exact epitope of PIIINP is not known, and PIIINP can be a marker of both formation and degradation of extracellular matrix [23,24]. In contrast, the Pro-C3 ELISA antibody specifically binds to the ADAMTS2 cleavage site of the N-terminal propeptide of type III collagen. Pro-C3 can thus be expected to be more specific than PIIINP for fibrogenesis. Indeed, our analyses indicate that the specificity of Pro-C3 could be high: the summary estimate was 79% (95% CI 0.71–0.86) for detecting significant fibrosis. There is some uncertainty to this result, reflected in the 95% confidence area around the mean and the 95% prediction region. False positivity of Pro-C3 can be expected in patients with fibrogenesis in other tissues, since type 3 collagen is not liver-specific, but also can be generated in muscle tissue or when lung fibrosis occurs [24,27]. We found that the mean sensitivity of Pro-C3 in detecting significant and advanced fibrosis was 68% (95% CI 0.50–0.82) and 72% (95% CI 0.62–0.81), respectively. The relatively low sensitivity may be explained by the fact that Pro-C3 is a marker of active collagen turnover, while liver biopsy as the comparison standard only gives a snapshot of fibrosis, the result of a chronic process of damage and repair in NAFLD. Therefore, based on a liver biopsy, one is likely unable to tell whether patients have been at a certain fibrosis stage for a long time or have recently progressed there. It will be highly interesting and clinically relevant to investigate the predictive potential of Pro-C3 in identifying patients that will progress in fibrosis stage, especially since it has been shown that Pro-C3 has this ability in chronic hepatitis C [49]. Bril and colleagues already reported that changes in Pro-C3 could identify patients that improved by 2 or more points in fibrosis score after treatment with pioglitazone and/or vitamin E, with an AUC of 0.85 [41]. Recent results from the phase 2B CENTAUR study on cenicriviroc indicate that Pro-C3 as a biomarker may have prognostic value as well [50].
4.3. Test Performance in Detecting NASH
From the limited data available, we observed low performance of Pro-C3 in detecting NASH; sensitivity ranged from 55% to 68%, and specificity ranged from 68% to 82%. This may be attributed to the paradigm that fibrosis progression results from repetitive periods of inflammation, as in NASH, alternated with periods of ‘repair’ of the extracellular matrix (ECM) [51]. The three studies that evaluated Pro-C3 accuracy for NASH used different criteria to establish NASH and, therefore, cannot be accurately compared [28,30,45]. All three studies used a NAFLD activity score (NAS) of ≥4 with at least 1 point each for steatosis, hepatocyte ballooning and hepatic inflammation to define NASH. However, the studies by Boyle and Erhardtsen added a fibrosis stage of ≥F2 to these criteria, and thus were purely looking at active stages of NASH.
4.4. Panels Including Pro-C3
Several studies included in this systematic review also assessed the diagnostic accuracy of a combination of Pro-C3 measurements with clinical data such as age, body mass index (BMI), T2DM status, and routine clinical tests, such as platelet count. Daniels and colleagues established the ADAPT algorithm, finding an increased AUC for detecting advanced fibrosis with ADAPT compared to Pro-C3 alone of 4% to 0.87 [29]. Boyle and colleagues found their FIB-C3 algorithm could increase diagnostic accuracy for detecting advanced fibrosis from an AUC of 0.76 for Pro-C3 alone to 0.85 for FIB-C3, similar to the performance of the ADAPT score in their study group. A simplified version of this panel that would be more easily calculated in a clinical setting, ABC3D, performed similarly to FIB-C3 with an AUC of 0.83 [28]. An overview of the accuracy of these panels for NAFLD fibrosis is given in Supplementary Table S3. The incorporation of Pro-C3 into a diagnostic panel offers the attractive combination of a direct test of active collagen turnover with clinical variables widely known to increase the risk of advanced disease. All three diagnostic panels evaluated by Boyle and colleagues (i.e., FIB-C3, ABC3D and ADAPT) were shown to outperform FIB-4 in their study group [28]. For clinical use, we think these panels show potential over Pro-C3 alone and should be further validated.
4.5. Strengths and Limitations
This study presents the first meta-analysis of the diagnostic accuracy of Pro-C3 with liver biopsy as the reference standard. Results from more than 1500 patients were included in our meta-analysis of significant fibrosis and more than 2000 in that of advanced fibrosis.
An aspect that merits consideration is that we were unable to evaluate the performance of Pro-C3 in a primary care setting. In a setting with a low prevalence of advanced disease one can expect to find undiagnosed patients with potentially advanced NAFLD that could benefit most from early detection.
From the included articles in this review, we were not able to acquire enough accuracy data at different threshold values to report accuracy at different threshold values. Future research should evaluate the diagnostic accuracy of Pro-C3 across several pre-determined thresholds with respect to the context of use, not only at the threshold identified by Youden Index, which attributes equal importance to false negatives and false positives, a condition that does not reflect the respective clinical consequences. To evaluate the potential of a marker for use in primary, secondary or tertiary care centers, the sensitivity and specificity of the test should be evaluated in the respective setting and false positives and false negatives should be differentially weighted.
It should be pointed out that availability of the Pro-C3 ELISA is still limited. It is exclusively produced by Nordic Biosciences, and at present has solely been used for research purposes. Before this test can be recommended for clinical use, its availability should be broadened. Pro-C3 tests are expected to soon become available worldwide on the Roche COBAS platform, and an assessment costs around 40–50 euros. Of note, the recent study by Erhardtsen and colleagues investigated the robustness and analyte stability of the Pro-C3 assay and found it passed the predefined acceptance criteria for precision [45].
4.6. Conclusion and Recommendations for Future Research
This review shows that Pro-C3 holds potential for the non-invasive assessment of NAFLD fibrosis. We recommend focusing future studies into Pro-C3 on both diagnostic and prognostic evaluations, and to recruit patients in the intended use setting. Firstly, it is necessary to further evaluate the diagnostic potential of Pro-C3, focusing on the diagnostic panels that incorporate Pro-C3 measurement. Further direct comparisons between these Pro-C3 panels and currently recommended tests, such as FIB-4 and ELF, will resolve whether Pro-C3 panels can, indeed, outperform these tests. Secondly, it is appealing to assess the prognostic potential of Pro-C3 in paired liver biopsy studies for progression in fibrosis stage and treatment response. Both of these directions may provide a basis for future guidelines, and for improvement of care paths for patients with NAFLD.
Acknowledgments
We sincerely thank Samuel Joseph Daniels (Institute of Clinical Research, University of Southern Denmark, Odense, Denmark) and Jane Knöchel (Clinical Pharmacology and Quantitative Pharmacology, AstraZeneca, Gothenburg, Sweden), who shared additional data from their studies, enabling us to perform these meta-analyses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biomedicines9121920/s1, Figure S1: PRISMA DTA Checklist, Figure S2: Methodological quality assessment results of primary included studies using QUADAS-2, Figure S3: Forest plots of the diagnostic accuracy of Pro-C3 in detecting significant fibrosis (A) and advanced fibrosis (B) in patients with NAFLD in included primary studies, Table S1: MEDLINE search strategy; run on 2 January 2020 and updated 30 July 2021, Table S2: Additional data received from authors of primary publications, Table S3: Incorporation of Pro-C3 into diagnostic panels for advanced fibrosis in patients with NAFLD.
Author Contributions
Conceptualization, A.L.M., A.-M.v.D., J.L., Y.V., M.H.Z., P.M.B. and A.G.H.; methodology A.L.M., J.L., Y.V., M.H.Z. and P.M.B.; formal analysis, A.L.M.; resources, G.P.A., J.M.S., Q.M.A., M.J.B., D.R., S.A.H. and M.N.; data curation, A.L.M. and A.-M.v.D.; writing—original draft preparation, A.L.M.; writing—review and editing, all authors; supervision, M.H.Z., P.M.B., M.N. and A.G.H. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented here are outcomes of the LITMUS project, which has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 777377. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Conflicts of Interest
A.G.H. is supported by the Amsterdam UMC Fellowship, the Gilead Research Scholarship and grants of Health-Holland and acts as a consultant to Boehringer Ingelheim, Echosens, Gilead Sciences, Inventiva, Novo Nordisk and Norgine. J.M.S. acts as consultant or advisory board member to BMS, Boehringer Ingelheim, Echosens, Genfit, Gilead Sciences, Intercept Pharmaceuticals, Madrigal, Nordic Bioscience, Novartis, Pfizer, Roche, Sanofi, Zydus and has received research funding from Gilead Sciences. M.J.B. is an employee and share-holder of Pfizer. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 4.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z., Sebastiani G., Ekstedt M., Hagstrom H., Nasr P., et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor R.S., Taylor R.J., Bayliss S., Hagström H., Nasr P., Schattenberg J.M., Ishigami M., Toyoda H., Wai-Sun Wong V., Peleg N., et al. Association between Fibrosis Stage and Outcomes of Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology. 2020;158:1611–1625.e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Konerman M.A., Jones J.C., Harrison S.A. Pharmacotherapy for NASH: Current and emerging. J. Hepatol. 2018;68:362–375. doi: 10.1016/j.jhep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397.e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Hultcrantz R., Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Midia M., Odedra D., Shuster A., Midia R., Muir J. Predictors of bleeding complications following percutaneous image-guided liver biopsy: A scoping review. Diagn. Interv. Radiol. 2019;25:71–80. doi: 10.5152/dir.2018.17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 11.Lazarus J.V., Ekstedt M., Marchesini G., Mullen J., Novak K., Pericàs J.M., Roel E., Romero-Gómez M., Ratziu V., Tacke F., et al. A cross-sectional study of the public health response to non-alcoholic fatty liver disease in Europe. J. Hepatol. 2020;72:14–24. doi: 10.1016/j.jhep.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus J.V., Palayew A., Carrieri P., Ekstedt M., Marchesini G., Novak K., Ratziu V., Romero-Gómez M., Tacke F., Zelber-Sagi S., et al. European ‘NAFLD Preparedness Index’—Is Europe ready to meet the challenge of fatty liver disease? JHEP Rep. 2021;3:100234. doi: 10.1016/j.jhepr.2021.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijk A.M., Schattenberg J.M., Holleboom A.G., Tushuizen M.E. Referral care paths for non-alcoholic fatty liver disease-Gearing up for an ever more prevalent and severe liver disease. United Eur. Gastroenterol. J. 2021;9:903–909. doi: 10.1002/ueg2.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayaswal A.N.A., Levick C., Selvaraj E.A., Dennis A., Booth J.C., Collier J., Cobbold J., Tunnicliffe E.M., Kelly M., Barnes E., et al. Prognostic value of multiparametric magnetic resonance imaging, transient elastography and blood-based fibrosis markers in patients with chronic liver disease. Liver Int. 2020;40:3071–3082. doi: 10.1111/liv.14625. [DOI] [PubMed] [Google Scholar]
- 15.Pavlides M., Banerjee R., Sellwood J., Kelly C.J., Robson M.D., Booth J.C., Collier J., Neubauer S., Barnes E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J. Hepatol. 2016;64:308–315. doi: 10.1016/j.jhep.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troelstra M.A., Witjes J.J., van Dijk A.M., Mak A.L., Gurney-Champion O., Runge J.H., Zwirs D., Stols-Gonçalves D., Zwinderman A.H., Ten Wolde M., et al. Assessment of Imaging Modalities against Liver Biopsy in Nonalcoholic Fatty Liver Disease: The Amsterdam NAFLD-NASH Cohort. J. Magn. Reson. Imaging. 2021;54:1937–1949. doi: 10.1002/jmri.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikolasevic I., Orlic L., Franjic N., Hauser G., Stimac D., Milic S. Transient elastography (FibroScan((R))) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease—Where do we stand? World J. Gastroenterol. 2016;22:7236–7251. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilar-Gomez E., Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J. Hepatol. 2018;68:305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 19.EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021;75:659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 20.van Kleef L.A., Sonneveld M.J., de Man R.A., de Knegt R.J. Poor performance of FIB-4 in elderly individuals at risk for chronic liver disease—Implications for the clinical utility of the EASL NIT guideline. J. Hepatol. 2021 doi: 10.1016/j.jhep.2021.08.017. [DOI] [PubMed] [Google Scholar]
- 21.McPherson S., Hardy T., Dufour J.F., Petta S., Romero-Gomez M., Allison M., Oliveira C.P., Francque S., Van Gaal L., Schattenberg J.M., et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen M.J.V.I., Sinkeviciute D., Bay-Jensen A.C., Karsdal M.A. Type III Collagen. In: Karsdal M.A., editor. Biochemistry of Collagens, Laminins and Elastin. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2016. pp. 23–36. [Google Scholar]
- 23.Karsdal M.A., Daniels S.J., Holm Nielsen S., Bager C., Rasmussen D.G.K., Loomba R., Surabattula R., Villesen I.F., Luo Y., Shevell D., et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020;40:736–750. doi: 10.1111/liv.14390. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen M.J., Nedergaard A.F., Sun S., Veidal S.S., Larsen L., Zheng Q., Suetta C., Henriksen K., Christiansen C., Karsdal M.A., et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen J.F., Juul Nielsen M., Nyström K., Leeming D.J., Lagging M., Norkrans G., Brehm Christensen P., Karsdal M. PRO-C3: A new and more precise collagen marker for liver fibrosis in patients with chronic hepatitis C. Scand. J. Gastroenterol. 2018;53:83–87. doi: 10.1080/00365521.2017.1392596. [DOI] [PubMed] [Google Scholar]
- 26.Willumsen N., Ali S.M., Leitzel K., Drabick J.J., Yee N., Polimera H.V., Nagabhairu V., Krecko L., Ali A., Maddukuri A., et al. Collagen fragments quantified in serum as measures of desmoplasia associate with survival outcome in patients with advanced pancreatic cancer. Sci. Rep. 2019;9:19761. doi: 10.1038/s41598-019-56268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo S., Siebuhr A.S., Bay-Jensen A.C., Juhl P., Karsdal M.A., Satoh Y., Todoroki Y., Nakano K., Nakayamada S., Tanaka Y. Correlation between serological biomarkers of extracellular matrix turnover and lung fibrosis and pulmonary artery hypertension in patients with systemic sclerosis. Int. J. Rheum. Dis. 2020;23:532–539. doi: 10.1111/1756-185X.13804. [DOI] [PubMed] [Google Scholar]
- 28.Boyle M., Tiniakos D., Schattenberg J.M., Ratziu V., Bugianessi E., Petta S., Oliveira C.P., Govaere O., Younes R., McPherson S., et al. Performance of the PRO-C3 collagen neo-epitope biomarker in non-alcoholic fatty liver disease. JHEP Rep. 2019;1:188–198. doi: 10.1016/j.jhepr.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels S.J., Leeming D.J., Eslam M., Hashem A.M., Nielsen M.J., Krag A., Karsdal M.A., Grove J.I., Neil Guha I., Kawaguchi T., et al. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology. 2019;69:1075–1086. doi: 10.1002/hep.30163. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen M.J., Leeming D.J., Goodman Z., Friedman S., Frederiksen P., Rasmussen D.G.K., Vig P., Seyedkazemi S., Fischer L., Torstenson R., et al. Comparison of ADAPT, FIB-4 and APRI as non-invasive predictors of liver fibrosis and NASH within the CENTAUR screening population. J. Hepatol. 2021;75:1292–1300. doi: 10.1016/j.jhep.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Eslam M., Wong G.L., Hashem A.M., Chan H.L., Nielsen M.J., Leeming D.J., Chan A.W., Chen Y., Duffin K.L., Karsdal M., et al. A Sequential Algorithm Combining ADAPT and Liver Stiffness Can Stage Metabolic-Associated Fatty Liver Disease in Hospital-Based and Primary Care Patients. Am. J. Gastroenterol. 2021;116:984–993. doi: 10.14309/ajg.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 32.McInnes M.D.F., Moher D., Thombs B.D., McGrath T.A., Bossuyt P.M., Clifford T., Cohen J.F., Deeks J.J., Gatsonis C., Hooft L., et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 33.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 35.The Cochrane Collaboration . Review Manager (RevMan) The Nordic Cochrane Centre; Copenhagen, Denmark: 2014. Version 5.3. [Google Scholar]
- 36.Reitsma J.B., Glas A.S., Rutjes A.W., Scholten R.J., Bossuyt P.M., Zwinderman A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005;58:982–990. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. [(accessed on 1 December 2021)]. Available online: https://www.R-project.org/
- 38.Doebler P. Mada: Meta-Analysis of Diagnostic Accuracy. R Package, Version 0.5.10. 2020. [(accessed on 1 December 2021)]. Available online: https://CRAN.R-project.org/package=mada.
- 39.Noma H., Matsushima Y., Ishii R. Confidence interval for the AUC of SROC curve and some related methods using bootstrap for meta-analysis of diagnostic accuracy studies. Commun. Stat. Case Stud. Data Anal. Appl. 2021;7:344–358. doi: 10.1080/23737484.2021.1894408. [DOI] [Google Scholar]
- 40.van Enst W.A., Ochodo E., Scholten R.J., Hooft L., Leeflang M.M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: A meta-epidemiological study. BMC Med. Res. Methodol. 2014;14:70. doi: 10.1186/1471-2288-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bril F., Leeming D.J., Karsdal M.A., Kalavalapalli S., Barb D., Lai J., Rabe M., Cusi K. Use of Plasma Fragments of Propeptides of Type III, V, and VI Procollagen for the Detection of Liver Fibrosis in Type 2 Diabetes. Diabetes Care. 2019;42:1348–1351. doi: 10.2337/dc18-2578. [DOI] [PubMed] [Google Scholar]
- 42.Knochel J., Kechagias S., Bergenholm L., Liljeblad M., Daniels S.J., Leeming D.J., Nasr P., Carlsson B., Ekstedt M., Hansson S., et al. Pro-C3 but not PC3X is increased with advanced fibrosis stage in a longitudinal cohort of non-alcoholic fatty liver disease (NAFLD) patients; Proceedings of the AASLD; Virtual. 13–16 November 2020. [Google Scholar]
- 43.Luo Y., Oseini A., Gagnon R., Charles E.D., Sidik K., Vincent R., Collen R., Idowu M., Contos M.J., Mirshahi F., et al. An Evaluation of the Collagen Fragments Related to Fibrogenesis and Fibrolysis in Nonalcoholic Steatohepatitis. Sci. Rep. 2018;8:12414. doi: 10.1038/s41598-018-30457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huber Y., Pfirrmann D., Gebhardt I., Labenz C., Gehrke N., Straub B.K., Ruckes C., Bantel H., Belda E., Clément K., et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment. Pharmacol. Ther. 2019;50:930–939. doi: 10.1111/apt.15427. [DOI] [PubMed] [Google Scholar]
- 45.Erhardtsen E., Rasmussen D.G.K., Frederiksen P., Leeming D.J., Shevell D., Gluud L.L., Karsdal M.A., Aithal G.P., Schattenberg J.M. Determining a healthy reference range and factors potentially influencing PRO-C3—A biomarker of liver fibrosis. JHEP Rep. 2021;3:100317. doi: 10.1016/j.jhepr.2021.100317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glen J., Floros L., Day C., Pryke R. Non-alcoholic fatty liver disease (NAFLD): Summary of NICE guidance. BMJ. 2016;354:i4428. doi: 10.1136/bmj.i4428. [DOI] [PubMed] [Google Scholar]
- 47.Vali Y., Lee J., Boursier J., Spijker R., Löffler J., Verheij J., Brosnan M.J., Böcskei Z., Anstee Q.M., Bossuyt P.M., et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2020;73:252–262. doi: 10.1016/j.jhep.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 48.Boursier J., Guillaume M., Leroy V., Irlès M., Roux M., Lannes A., Foucher J., Zuberbuhler F., Delabaudière C., Barthelon J., et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J. Hepatol. 2019;71:389–396. doi: 10.1016/j.jhep.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen M.J., Veidal S.S., Karsdal M.A., Ørsnes-Leeming D.J., Vainer B., Gardner S.D., Hamatake R., Goodman Z.D., Schuppan D., Patel K. Plasma Pro-C3 (N-terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429–437. doi: 10.1111/liv.12700. [DOI] [PubMed] [Google Scholar]
- 50.Ratziu V., Sanyal A., Harrison S.A., Wong V.W., Francque S., Goodman Z., Aithal G.P., Kowdley K.V., Seyedkazemi S., Fischer L., et al. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology. 2020;72:892–905. doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 51.Schuppan D., Surabattula R., Wang X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018;68:238–250. doi: 10.1016/j.jhep.2017.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.