Abstract
Cancer is a leading cause of death worldwide, with approximately 19 million new cases each year. Lately, several novel chemotherapeutic drugs have been introduced, efficiently inhibiting tumor growth and proliferation. However, developing a new drug is a time- and money-consuming process, requiring around 1 billion dollars and nearly ten years, with only a minority of the initially effective anti-cancer drugs experimentally finally being efficient in human clinical trials. Drug repurposing for cancer treatment is an optimal alternative as the safety of these drugs has been previously tested, and thus, in case of successful preclinical studies, can be introduced faster and with a lower cost into phase 3 clinical trials. Antipsychotic drugs are associated with anti-cancer properties and, lately, there has been an increasing interest in their role in cancer treatment. In the present review, we discussed in detail the in-vitro and in-vivo properties of the most common typical and atypical antipsychotics, along with their mechanism of action.
Keywords: cancer, antipsychotics, repurposing
1. Introduction
Cancer is a leading cause of death in the world, with around 19 million new cancer cases and 10 million cancer-related deaths in 2020. These numbers are expected to increase in the following years, with new cancer cases projected to reach 28 million worldwide in 2040 [1]. Although chemotherapy plays a pivotal role in cancer treatment, in many cancer types, despite the initial vulnerability to chemotherapy, cancer cells become drug-resistant over time through different mechanisms such as DNA mutations which lead to drug inhibition and degradation, thus necessitating new drug development [2]. However, drug development is associated with a cost of around 1 billion dollars and an estimated 10 years on average from the initial discovery to final market approval [3]. Repurposing drugs for a variety of diseases has emerged as a cost- and time-effective strategy compared with the traditional development of a new drug [4]. Antipsychotic drugs, which are classified into typical/first-generation and atypical/second-generation antipsychotics, have been in clinical practice since the mid-1950s and early 1990s, respectively; thus, their adverse effects such as extrapyramidal symptoms (parkinsonism, dystonia) in the typical antipsychotics, agranulocytosis (clozapine), and elevated serum prolactin levels (risperidone) are well-documented [5].
The antipsychotic effect of typical antipsychotics is primarily mediated through inhibition of the D2 dopamine receptors (DRD2) in the mesolimbic dopaminergic pathway, the main brain pathway hyperactivated in patients with positive psychotic symptoms such as hallucinations and delirium, while atypical antipsychotics work by inhibiting DRD2 and serotonin receptors, most commonly the 5-HT2A subtype [6]. Recently, there have been studies supporting decreased incidence of some types of cancer in patients with schizophrenia treated with antipsychotic drugs [7,8], suggesting a possible anticancer activity. In this review, we investigated the anticancer properties of antipsychotic drugs focusing mainly on experimental studies (in vitro and in vivo) and the presumptive mechanism of action behind the anti-proliferative effect of each drug.
2. Typical Antipsychotics
2.1. Haloperidol
Haloperidol is a typical antipsychotic medication of the butyrophenones class synthesized by Janssen pharmaceuticals and used for numerous psychiatric conditions such as acute psychosis, schizophrenia, bipolar disorder, Tourette syndrome, delirium, agitation, and hallucinations related to alcohol withdrawal [9,10]. The antipsychotic properties of haloperidol are attributed to the blockage of DRD2 in the mesolimbic dopaminergic pathway. However, haloperidol is a non-selective inhibitor for D2 receptors and also has antimuscarinic, anti-adrenergic (a1), and antihistaminic (H1) effects [10,11]. Except for its use as an antipsychotic drug, haloperidol has also been found, mainly at an experimental level, to have anti-neoplastic, anti-fungal, anti-viral, and immunomodulatory properties [12,13,14,15].
Regarding its antineoplastic properties, haloperidol has been found to induce autophagy, apoptosis, and cell cycle arrest [15,16]. In an in vitro study, Papadopoulos et al. observed that glioblastoma (GBM) U87, U251, and T-98 cell lines are sensitive to haloperidol. Additionally, they found that haloperidol induces G2/M cell inhibition, increases the percentage of cells found in sub G0/G1 phase, and promotes caspase-8 activation, the latter suggesting the induction of apoptosis. Except for haloperidol’s effect in the cell cycle progression, they observed that haloperidol decreases the expression of CD24, CD44 adhesion molecules, inhibits wound-generated cell migration, and enhances GBM cell death when combined with the alkylating agent temozolomide (TMZ) and radiotherapy [15]. The synergistic effect of haloperidol in the treatment of GBM with TMZ was also supported in another study [17]. In that in vitro study, the authors found that the combination of TMZ with haloperidol or risperidone enhanced the anti-tumor effect compared with TMZ alone. Specifically, the authors observed that the activation of DRD2 is essential for the proliferation of glioma cells and that the combination of TMZ with DRD2 significantly inhibits GBM cell mitotic activity, increases the induction of DNA double-strand breaks (DSBs), as evident by the formation of γH2aX (a marker of DSBs), and inhibits the autophagic flux caused by TMZ in GBM cells. Moreover, they found that dopamine receptor antagonists can inhibit the TMZ-induced extracellular signal-related kinase (ERK) activation, suggesting a potential link between ERK activation and TMZ-mediated autophagy [17].
In a recent study, He et al. examined the effect of DRD2 inhibition in patient-derived glioblastoma xenograft models. DRD2 was inhibited by using haloperidol or ONC201, a small novel molecule selectively blocking DRD2. Subsequently, the authors studied the effects of this inhibition in the expression profiles of epidermal growth factor receptor (EGFR) and DRD2, observing an anti-correlation between the expression levels of these two molecules, suggesting a compensatory EGFR-mediated hyperactivation of their shared downstream effector REK when DRD2 are blocked, and resulting to haloperidol resistance. The latter finding indicates that EGFR levels in patients with GBM could be a potential biomarker to predict the effect of DRD2 inhibition in future clinical trials [18]. Haloperidol and other antipsychotics have been found to inhibit the Sonic Hedgehog signaling (SHH) pathway in glial cells by downregulating the expression of GLI-1, a key transcription factor of the SHH signaling pathway, via a complex mechanism involving the overexpression of 7-dehydrocholesterol reductase (7-DHCR) [16,19].
Haloperidol has also been found to cause DNA demethylation in MIA PaCa-2 human pancreatic carcinoma cells. In this cell line, the expression of Dual-specificity phosphatase 6 (DUSP6), an ERK1/2-selective phosphatase, has been suppressed with intronic methylation. Kim et al. observed that haloperidol induces CpG demethylation of DUSP6 in a dose-dependent manner, and the effect of demethylation was similar to the one with the demethylated agent 5-azacytidine. The latter effect was not observed in pancreatic cell line PANC-1, where the DUSP6 transcription was not suppressed [20]. In another study, Jandaghi et al. found that the protein levels of DRD2 were significantly increased in patients with pancreatic adenocarcinoma, and the inhibition of these receptors with haloperidol in orthotopic xenograft mice models reduced neoplasm size and metastasis [21].
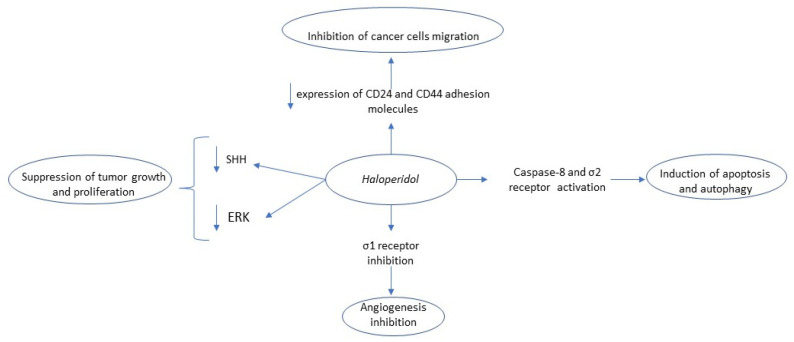
Sigma (σ) receptors are cell surface protein molecules found in multiple tissues and highly expressed in several neoplasms including prostate cancer, gliomas, breast cancer, and uveal melanoma [22,23]. Currently, two subtypes of σ receptors have been described, the σ1 a chaperon protein, found in mitochondria-associated membrane and involved in VEGF-mediated angiogenesis, and the σ2 receptor or ER-resident transmembrane protein 97, mediating cell proliferation and involved in apoptosis and autophagy. Even though their exact biologic role is currently unclear, there is mounting evidence that the inhibition of σ1 and simultaneous activation of σ2 have anti-tumor effects [22,23,24]. Additionally, the σ receptors decrease the expression of P-glycoprotein, a multidrug-resistant protein (MDR) that is an ATP-dependent efflux pump for several chemotherapeutic drugs. The haloperidol metabolite II is a molecule with such properties (σ1 antagonist/σ2 agonism) and its combination with 4-phenylbutyric acid (a Histone deacetylase inhibitor) in a single-ester molecule, the phenylbutyrate ester of haloperidol metabolite II (+/−MRJF4), or with valproic acid (+/− MRJF22) has been found to have an anti-tumor effect against prostate cancer cell lines (LNCaP, PC3) or rat C6 glioma cells [22,23,24], and uveal melanoma [25], respectively. The antitumor mechanisms of action of haloperidol are summarized in Figure 1.
Figure 1.
Summary of haloperidol’s anti-tumor mechanism of action. Key: SHH, Sonic hedgehog; ERK, extracellular signal-regulated kinase.
2.2. Trifluoperazine
Trifluoperazine (TFP) is a commonly prescribed typical antipsychotic drug of the phenothiazines group, mainly utilized for schizophrenia treatment. However, the role of trifluoperazine as a potential anti-cancer agent is suggested by several authors [26,27]. TFP has been found to reduce the phenotype conversion of glioma cells into glioma initiating cells (GICs) by decreasing the radiation induced-Nanog expression, a pluripotency maintenance factor, and Glycogen synthase kinase-3 (GSK-3) activation. Moreover, the trifluoperazine decreased the number of GICs in GBM cell lines and increased survival in patient-derived orthotopic xenograft (PDOX) mouse models of GBM [27,28]. TFP has also been found to increase the radiosensitivity of GBM both in vitro and in vivo, by impairing homologous recombination and blocking autophagy by decreasing the levels of cathepsin L, a lysosomal cysteine protease [29]. In another study, Chen et al. found that trifluoperazine enhances the chemotherapeutic effect of doxorubicin in doxorubicin-resistant SHG44/DOX glioma cell lines by stimulating Forkhead box O1 (FOXO1) nuclear translocation. The FOXO1 is a transcription factor suppressing the expression of MDR genes, including P-gp, and its cytoplasmic translocation increases the expression of MDR genes, thus inducing chemotherapy resistance against various other agents such as gefitinib in lung cancer [30,31]. Regarding the role of TFP as an anti-glioma agent, Kang et al. reported that it can impair, in vitro and in vivo, intracellular calcium signaling by causing a massive and irreversible release from intracellular stores. Particularly, TFP binds to calmodulin subtype 2 (CaM2) and induces its releasement from the endoplasmic reticulum-Ca+2 channel- inositol 1,4,5-trisphosphate receptor (IP3R) subtype 3. The increased cytotoxic effect of the drug in glioma cells is probably explained by the increased expression levels of CaM2. Consequently, TFP induces impaired homeostasis of calcium intracellular signaling that inhibits glioma proliferation, invasion, and metastasis [32]. Its anti-metastatic activity was also noted by Pulkoski-Gross et al., who described a reduction in the levels of phosphorylated AKT (Ser473 and Thr308) and β-catenin (Ser552), induced by DRD2 blockage, ultimately leading to limited angiogenic (VEGF-mediated) and metastatic potential [33].
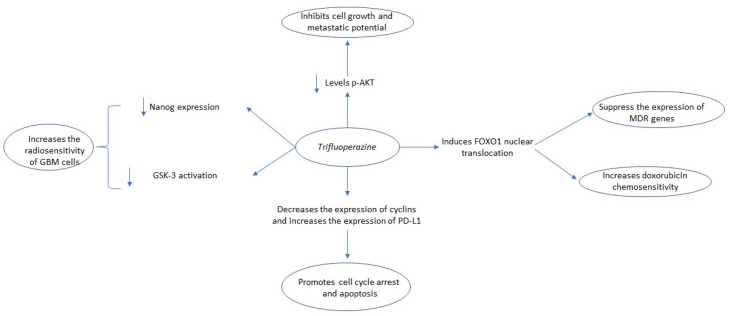
Currently, there is mounting evidence supporting that TFP can effectively induce G0/G1 cell cycle arrest and induce apoptosis [26,33,34]. Jiang et al. found that it can inhibit tumor growth in hepatocellular carcinoma (HCC) cell lines (SMMC-7721, Bel-7402), induce Go/G1 cell cycle arrest, and induce apoptosis through FOXO1 activation and increase of Bax to Bcl-2 ratio [35]. Except for its role in inducing cell cycle arrest and apoptosis in colorectal cancer (CRC), according to Xia et al., TFP increases the expression of programmed death-ligand (PD-L1), indicating that it can have a synergistic anti-cancer effect with immune checkpoint inhibitors [34]. Finally, Feng et al. observed that trifluoperazine impairs the expression of both cyclin D1/CDK4 and cyclin E/CDK2 in MDA-MB-468, MDA-MB-231, and 4T1 triple-negative breast cancer cell lines and increases survival of mice with brain metastasis [26,36]. The antitumor mechanisms of the action of trifluoperazine are summarized in Figure 2.
Figure 2.
Summary of trifluoperazine’s anti-tumor mechanism of action. Key: GSK-3, Glycogen synthase kinase-3; p-AKT, Phosphorylated Akt, PD-L1, Programmed death-ligand 1; FOXO1, Forkhead Box O3; MDR, Multiple drug resistance; GBM, glioblastoma.
2.3. Chlorpromazine
Chlorpromazine is another typical antipsychotic drug of the phenothiazines class utilized for the treatment of schizophrenia. Several in vitro and in vivo studies have shown that chlorpromazine has multifaceted anti-cancer effects, including induction of apoptosis and inhibition of several intracellular signaling pathways and DNA synthesis [37,38]. Matteoni et al. reported that chlorpromazine had in vitro anti-tumor effects in six GBM lines, the anchorage-dependent cell lines T98G, U-251 MG, U-87 MG, and the anchorage-independent cell lines TS#1, TS#83, and TS#163 neurospheres. Chlorpromazine could inhibit cell viability, cause hyperdiploidy, reduce cloning, and downregulate the expression of stemness genes such as SOX2, NANOG, Nestin, and OLIG2. Additionally, chlorpromazine exhibited a synergistic effect with TMZ in the reduction of cell cloning [39] as well as with nitrosoureas in rat GBM cell lines (RG2) [40]. In another in vitro study, chlorpromazine inhibited the proliferation of TMZ-resistant glioma cell lines and glioma stem cells by inhibiting the CcO subunit 4 isoform 1 (COX4-1), which is mainly expressed in chemotherapy-resistant glioma cell lines [41]. In rat C6 glioma cell lines, chlorpromazine resulted in cell cycle arrest and an increase of p21Waf1/Cip1 level, a cyclin E/CDK2 inhibitor, by p53-independent induction of the transcription factor early growth response-1 (Egr-1) [42]. In U87MG glioma cell lines, chlorpromazine also induces autophagic cell death, but not apoptosis, through inhibiting the Akt/mTOR pathway [43]. A similar effect of chlorpromazine was observed in human oral cancer HSC-3 and Ca9-22 cell lines [44]. Kurita et al. found that chlorpromazine inhibits the interaction between RE1-silencing transcription factor/neuron-restrictive silencer factor (REST/NRSF), a transcription repressor overexpressed in medulloblastoma, and the paired amphipathic helix domains (PAH1) domain of mSin3 thus inhibiting the in vitro growth of medulloblastoma DAOY cell lines [45].
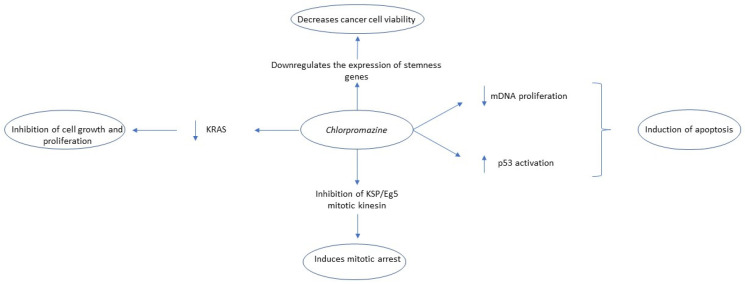
Regarding the anti-tumor effect of chlorpromazine in white blood cell malignancies, chlorpromazine appears to inhibit mitochondrial DNA polymerase and induce apoptosis selectively in several leukemia types, including acute T and acute B lymphoblastic leukemia, and Burkitt lymphoma cell lines but not in normal lymphocytes [46]. In a recent study, Rai et al. found that chlorpromazine caused the reduction, both in vitro and in vivo, in the levels of clathrin assembly lymphoid myeloid leukemia (CALM) protein and disturbed the subcellular localization of acute myeloid leukemia (AML)-mutated receptor tyrosine kinases (RTKs) such as FLT3-ITD and KIT-D816V, while sparing the non-mutated RTKs [47]. Similarly, in Ewing sarcoma, chlorpromazine impairs the clathrin mediated-insulin-like growth factor receptor 1 (IGFR1) internalization, involved in Ewing sarcoma pathogenesis, consequently decreasing proliferation rate and inducing apoptosis [48,49]. In diffuse large B-cell lymphoma, chlorpromazine promotes the expression of Sphingosine-1-phosphate receptor 2 (S1PR2), which is a G-coupled protein receptor involved in the maturation of B cells [50]. Chlorpromazine can also induce apoptosis in human colorectal cancer cell lines in a p53-dependent mechanism. Specifically, chlorpromazine induced p53 expression in CRC cell lines via activation of c-jun N-terminal kinase (JNK). The JNK promotes the degradation of sirtuin 1 (SIRT1), a histone deacetylase, and thus can block the deacetylation of p53, and its consequent deactivation [51]. The combination of chlorpromazine with pentamidine, an antiparasitic agent, seems to have a synergistic antiproliferative effect on non-small-cell lung carcinoma (NSCLC) cell line A549 in vitro and in vivo, as well as in human mice models xenografted with HCT116 colorectal carcinoma (CRC) cell line. Mechanistically, chlorpromazine inhibits mitotic kinesin KSP/Eg5 and induces mitotic arrest, while pentamidine impairs chromosome segregation [49]. The mutation of oncogenic Kirsten rat sarcoma virus (KRAS), a GTPase protein activating several key pathways involved in cell proliferation, survival, and transformation, is a major event involved in the pathogenesis of pancreatic cancer [52]. The anchoring of KRAS with the plasma membrane is necessary for the activation of KRAS-downstream signaling targets. Chlorpromazine was found to impair the association of oncogenic mutant (G12V) KRAS with the plasma membrane by modifying the electrostatic interactions of the KRAS- polybasic region with the plasma membrane, thus inducing cell cycle arrest and inhibition of cell growth and migration in PANC-1 cells [53]. Similarly, Yde et al. reported that chlorpromazine intercalates into hydrophobic regions of cell membranes and binds into negatively charged membrane surfaces, thus causing alterations in membrane permeability. Particularly, they observed that chlorpromazine had a synergistic effect with TMZ in reducing tamoxifen-resistant MCF-7/TAMR-1 breast cancer line growth by increasing tamoxifen’s cellular influx, probably through chlorpromazine-induced increased membrane permeability [54]. Finally, chlorpromazine suppressed the HIPPO signaling pathway, involved in cancer cell stemness and invasion, in a dose-dependent manner in the MCF-7 cell line, presumably by promoting proteasomal degradation, and decreased the chemotherapy resistance of cancer stem cells (CSC) [55]. The antitumor mechanisms of the action of chlorpromazine are summarized in Figure 3.
Figure 3.
Summary of chlorpromazine’s anti-tumor mechanism of action. Key: KSP, Kinesin Spindle Protein; mDNA, mitochondrial DNA.
2.4. Pimozide
Pimozide is a first-generation antipsychotic drug of the diphenylbutylpiperidines class mainly utilized for treating schizophrenia and Gilles de la Tourette disease. Numerous preclinical studies have shown that pimozide has multifaceted anti-cancer effects such as inhibition of cell cycle and proliferation, induction of apoptosis, and cell invasion by interacting with multiple biological targets [56]. Recently, Hongo et al., through in-silico drug screening, found that pimozide inhibits aurora B kinase (AURKB) and kinesin family member 20 A (KIF20A), involved in spindle formation and cytokinesis, in castration-resistant DU145CR prostate cancer cell line, and its combination with taxane cabazitaxel appears to have a synergistic effect in the same line in vivo and in vitro [57]. In another study about the role of pimozide in prostate cancer treatment, pimozide reduced cell proliferation and migration by increasing the formation of reactive oxygen species (ROS) in PC3 and DU145 prostate cancer cell lines in vitro and in vivo in TRAMP mice [58], while in the study of Cai et al., pimozide had a similar effect in osteosarcoma U2OS cell lines by downregulating the expression of catalase, probably mediated through inhibition of the activity of signal transducer and activator of transcription 3 (STAT3) [59].
The STAT protein family encompasses transcription factors which are activated by Janus Kinases (JAK) and regulate the expression of oncogenes, the progression of cell cycle, and the induction of apoptosis. In triple-negative breast cancer cell lines, pimozide inhibited the STAT-3 mediated activation of matrix metalloproteinase-9 (MMP-9) and vimentin [60], while in brain tumor cell lines, it induced a STAT-3 mediated apoptosis in vitro and in vivo [61]. However, in another study, Dakir et al. reported that pimozide induced apoptosis in MDA-MB-231 breast cancer cells by downregulating the expression of Akt [62], while according to Strobl et al., the anti-tumor effect in breast cancer could relate to the calmodulin antagonist properties of pimozide [63]. Additionally, pimozide has been found to inhibit the in vitro phosphorylation of STAT5 in BCR-ABL positive and pSTAT5 overexpressing K562 chronic lymphocytic leukemia (CLL) cell lines [64,65], as well as in mice models with acute myeloid leukemia that contain the FLT3 internal tandem duplication (ITD) mutation [66]. A similar anti-cancer effect mediated by the inhibition of STAT5 phosphorylation was observed in Rat prolactinoma MMQ cells through inhibition of the STAT5/Bcl-xL and STAT5/cyclin D1 signaling pathways, in peripheral T-lymphoma through induction of the TRAIL/DR4-dependent apoptosis [67], and in osteosarcoma cells through inhibition of the proliferation of osteosarcoma stem cells [68]. Finally, Fako et al. found that haloperidol inhibits the in vitro growth of hepatocellular carcinoma Hep3B and HepG2 cell lines through impairment of the Wnt/β-catenin signaling pathway and its downstream molecules, including EpCAM, which is involved in cancer cell stemness [69].
2.5. Fluspirilene
Fluspirilene (FSP) is an antiphychotic drug that belongs to the disphenylbutylpiperidines class. Its main indication is schizophrenia treatment, antagonizing mainly the DRD2 and also calcium channels [70]. Preliminary data suggest FSP’s antitumor activity, both in vitro and in vivo in different cancer cells. Shi et al., in their in vitro studies, found that FSP attenuated viability and proliferation of HCC (HepG2, Huh cells) cell lines by reducing the expression of CDK2, Rb, pho-CDK2, pho-Rb, and cyclin E, all responsible for the transition from the G1 to S phase of the cell cycle, thus arresting the cancerous cells in G2 phase and subsequently inducing apoptosis. These findings were also validated in vivo where FSP reduced tumor size and volume in mice xenografted with Huh cells. The latter antineoplastic effect was comparable to that of 5-fluorouracil, the standard of care drug in HCC. Additionally, the combination of those two drugs provided the highest antitumor activity, suggesting a potential synergistic action [71]. In another study, Dong et al. described that FSP inhibited GBM’s increased mitotic activity and invasiveness in vitro by reducing the activity of signal transducer and activator of STAT-3, a pivotal transcription factor contributing to increased cell proliferation as well as treatment resistance. Of note, the inhibition of STAT-3 was observed in both glioma stem cells and differentiated glioma cells. Subsequently, the drug was tested in vivo, leading to significant tumor reduction (p = 0.017) and life prolongation (p = 0.026) in mice xenografted with cells with similar characteristics to GBM cells [72]. Finally, Patil et al., based on their own in vitro studies, proposed that FSP acts as a p53-MDM2 inhibitor by binding to the p-53 binding pocket of the MDM2 protein, causing activation of the tumor suppressor protein p53 and subsequent inhibition of tumor growth in human colon cells and several other human tumor cell lines in the NCI60 cell line panel [73].
2.6. Penfluridol
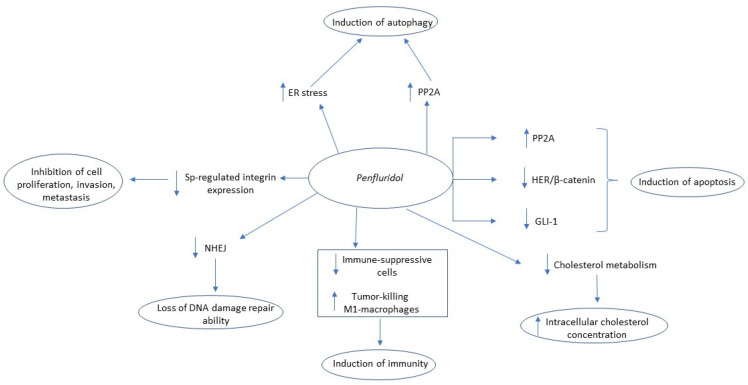
Penfluridol represents a first-generation antipsychotic drug used for treating chronic schizophrenia and other psychotic disorders. Developed in the late 1960s at Janssen Pharmaceutical, penfluridol’s antipsychotic properties result from the blockade of dopamine receptors, particularly the D2 receptors. However, the drug also inhibits other dopamine receptors (D1, D3, D5) as well as T-type calcium-channel and 5HT2B receptors [74,75,76]. Recently, penfluridol has emerged as a potential anticancer drug, showing promising results both in vitro and in vivo in different types of cancer. Ranjan et al., in their in vitro studies with three triple-negative cancer cell lines (MDA-MB-231, HCC1806, 4T1), reported that penfluridol attenuated not only the expression of integrin α6, integrin β4, integrin α4, integrin α5, integrin β1, and integrin β3 but also integrin axis’ downstream molecules including FAK, p-Paxillin (Y118), and paxillin, ultimately inhibiting cell proliferation, invasiveness, and migration [77]. The integrin signaling pathway has been described as a key factor in tumor initiation, progression, and extravasation in secondary sites [78]. After orthotopic injection of 4T1 cells (in vivo), the same authors reported a 49% tumor volume reduction in penfluridol-treated mice, while after intracardiac and intracranial injections of the same cell line, the growth of metastatic brain tumors were suppressed by 90% and 72%, respectively, after penfluridol administration. The mechanism behind the tumor reduction was the one described in their in vitro study [77]. Based on this study, Hedrick et al. attempted to elucidate the molecular mechanism of integrins’ repression. The authors utilized two breast cancer cell lines (SKBR3, MDA-MB-231) for their in vitro experiments and found that penfluridol promotes ROS formation in both cell lines, which subsequently attenuates the expression of Specificity Factors (Sp) 1,3,4 and induces cleavage of PARP/caspase-3 (induction of apoptosis) [79]. Particularly, integrin expression is regulated by Sp transcriptional factors and orphan nuclear receptor 4A1 (NR4A1) [80,81]. Penfluridol does not antagonize NR4A1 but inhibits the expression of Sp 1, Sp 3, and Sp 4 (and therefore integrin expression) by downregulating ROS-dependent epigenetic expression of cMyc, decreased expression of cMyc-regulated miR-27a and miR-20a/miR-17, and induction of the Sp repressors ZBTB10 and ZBTB4 [79]. Another in vitro study investigated the effects of penfluridol in six different pancreatic cell lines (Panc1, Panc0504, Panc0403, SU8686, MiaPaCa2, AsPc1) and observed that the drug promoted the activity of protein phosphatase 2A (PP2A), an established a tumor-suppressor protein, leading to the suppression of phosphorylation of AKT, p70S6K, GSK3β and to MYC ubiquitination and degradation, and ultimately to cell death. Additionally, in the penfluridol-sensitive cells, there was increased expression of pro-apoptotic molecules (BIM, BAX, PUMA) and, simultaneously, decreased expression of anti-apoptotic proteins (e.g., Bcl-2), indicating an apoptotic-mediated pathway of cell death [82]. Activation of PP2A was also implicated as penfluridol’s mechanism of action in reducing the viability of AML cells (HL-60, U937, MV4-11) in vitro. The authors suggested that PP2A activation led to downregulation of Akt, ERK, and JNK signals and subsequently triggered caspase-mediated cellular apoptosis. The authors also observed high levels of ROS, but in contrast to Hendick et al., ROS appeared to play a protective role in the penfluridol-induced apoptosis [83]. In another study, penfluridol suppressed GBM cell in vitro proliferation by inhibiting the phosphorylation of Akt at Ser473, thus attenuating the expression of Akt-associated GLI-1, a highly expressed transcription factor in GBM cells, partly responsible for GBM’s treatment resistance as well as the expression of GLI1-mediated stem cells markers including OCT4, Nanog, and Sox2. Additionally, the drug-enhanced cleavage of caspase-3 and PARP indicated the promotion of cellular apoptosis. These results were confirmed in vivo, where penfluridol reduced the growth of GBM tumors in mice models after subcutaneous and intracranial injection by 65% and 72%, respectively [84]. The same molecule (GLI-1) was penfluridol’s target, according to Kim et al. [85]. The authors investigated the anticancer activity of penfluridol in glioma sphere-forming cell lines (GSCs) in vitro, a subpopulation of GBM cells responsible for treatment resistance and GBM recurrence, and reported an inhibitory effect of penfluridol on GLI-1, which subsequently reduced the GLI-1-associated markers of stemness (SOX2, NESTIN, OCT4), markers of invasion (Integrin α6, uPAR), and epithelial-mesenchymal transition (EMT) factors (Vimentin, Zeb-1, N-cadherin, Snail, Slug), molecules strongly associated with increased cell motility and resistance to genotoxic agents of GSCs. These findings were confirmed in orthotopic xenograft mice models (in vivo) where penfluridol-treated mice had a 30% tumor volume decrease compared with control with reduced expression of GSC-related factors, including GLI1, SOX2, and vimentin. This percentage surged to 80% in mice treated with the combination of temozolomide and penfluridol, suggesting a potential synergistic effect of the two drugs [85]. Another suggested mechanism of penfluridol’s cytotoxicity in pancreatic cancer, both in vitro (Panc-1, AsPC-1, BxPC-3) and in vivo, was the induction of autophagy-mediated apoptosis as indicated by increased cleavage of caspase-3 and PARP. At the same time, there was an increased conversion of LC3-I to LC3-II (a specific indicator of autophagosome formation) and high levels of p62, an autophagy-specific substrate, indicating that the increased autophagosome formation is accompanied by autophagosome-reduced degradation by lysosomes [86]. Penfluridol upregulated the expression of endoplasmic reticulum stress markers, including binding protein (BIP), C/EBP homologous protein (CHOP), and inositol requiring 1a (IRE1a), implicating the penfluridol-mediated endoplasmic reticulum system stress as the molecular mechanism behind the induction of autophagy which subsequently led to apoptosis [87]. The above findings were also confirmed in vitro [86,87]. The induction of autophagy by penfluridol, as indicated by increased levels of LC3-II and p62, was also observed in another in vitro study in NSCLC cell lines (A549, HCC827). The authors described that penfluridol promotes ER stress-mediated unfolded protein response (UPR) signaling pathways and activation of p38 mitogen-activated protein kinase (MAPK), both causing autophagosome accumulation in NSCLC cells [88]. However, in contrast to [86,87], given that autophagy is a major energy supplier for the cancer cell [89], the increased autophagosome synthesis promotes cell death by energy (ATP) depletion rather than apoptosis [88]. Another penfluridol-mediated inhibition of GBM cell (U87MG) proliferation in vivo was reported by Ranjan et al., describing a reduction of immune suppressive cells (T regulatory cells, myeloid-derived suppressive cells), upregulation of tumor-killing M1 macrophages, and decreased chronic inflammation biomarkers (CC14, IFNγ) in mice subcutaneously injected with U87MG GBM cells [90]. Gupta et al., using breast cancer cell lines (MCF-7, 4T1) in vitro and in vivo, found that penfluridol inhibits cell proliferation and migration by suppressing the HER2/β-catenin signaling pathway as well as its associated molecules (TCF-4, TCF-1, Cyclin D1, C-MYC, p-GSK3β) and inducing apoptosis (increased cleavage of caspase-3 and C1-PARP). Since this pathway is implicated in breast cancer paclitaxel (chemotherapeutic drug) resistance, its inhibition led to enhanced paclitaxel efficacy and significant tumor growth suppression (p = 0.0012) compared with controls in orthotopic mouse models (in vivo) [91]. Non-homologous end joining (NHEJ) is a mechanism of repair of ionizing radiation-induced double-stranded DNA breaks. Penfluridol-treated HeLa cells (in vitro), after 8Gy radiation, showed increased levels of broken DNA and loss of DNA damage repair processes, which were thought to be the result of inhibition of DNA-PKcs activation [92]. Finally, penfluridol inhibited the proliferation of melanoma (B16/F10), lung carcinoma (LL/2), and breast cancer (4T1) cell lines, both in vitro and in vivo, causing accumulation of unesterified cholesterol in all cancer cells. However, the exact mechanism of penfluridol-induced cholesterol dysregulation was not elucidated [93]. The antitumor mechanisms of action of penfluridol are summarized in Figure 4.
Figure 4.
Summary of penfluridol’s anti-tumor mechanism of action. Key: PP2A, Protein phosphatase 2; NHEJ, Non-homologous end joining; ER, Endoplasmic reticulum; HER, Human epidermal growth factor receptor; GLI Family Zinc Finger 1, GLI-1.
2.7. Thioridazine (THD)
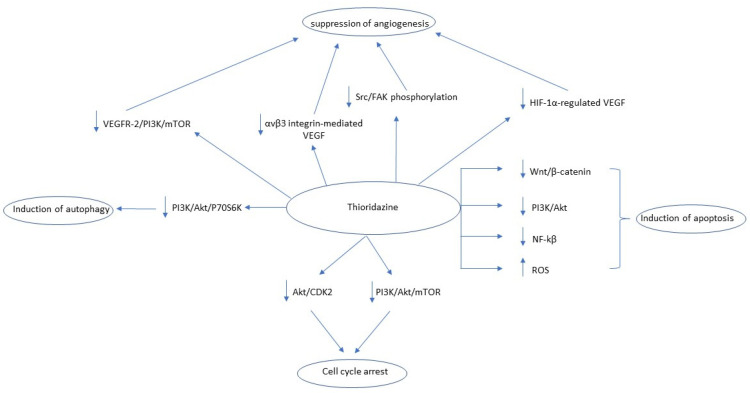
Thioridazine (THD) is a first-generation antipsychotic drug that belongs to the phenothiazines drug family, primarily blocking dopamine receptor 2 (DRD2), mainly used for the treatment of a wide range of psychotic disorders including schizophrenia and psychosis [94]. In cases of advanced cancer, THD may alleviate cancer-related sweating [95,96] and depression [97]. Regarding its potential anticancer effects, THD is one of the most studied antipsychotic drugs, being tested in various cancer cells, both in vitro and in vivo, including GBM cells [98,99,100,101,102], ovarian cancer cells [103,104,105,106,107], breast cancer cells [63,102,108,109,110], lung cancer cells [104,111], cervical cancer cells [112,113], endometrial cancer cells [112,114], melanoma cells [115,116], malignant blood cells (lymphoma, leukemia) [46,117,118,119], gastric cancer cells [120], malignant testicular germ cells [121], hepatocellular carcinoma cells [122], head and neck cancer cells [102], renal cancer cells [123], prostate cancer cells [124], neuroblastoma cells [101], and different types of cancer stem cells [125,126,127,128]. According to these studies, THD exerts its antiproliferative properties through discrepant molecular mechanisms. One of these mechanisms is the THD-induced cell cycle arrest, mainly in the G1 phase [98,103,104,105,108,109,111,112,120,122,125,126], DRD2 independently [109], through downregulation of the Akt/CDK2 pathway [125] or PI3K/Akt/mTOR/p70S6K pathway [105,112]. Another mechanism is the induction of the caspase- or mitochondrial-mediated apoptosis [46,98,100,101,102,103,104,105,107,108,110,111,112,113,114,115,116,117,118,120,123,124,125,127], which is achieved through inhibition of different pathways including the Wnt/β-catenin [98], PI3K/Akt [100,105,108,112,116], and NF-kβ pathways [110,118], while in some studies the apoptosis was ROS-mediated [102,107,108,123]. However, some studies reported that the THD’s antiproliferative effect was independent of apoptotic cell death induction [99,122]. A third mechanism was the THD-promoted autophagy [100,114,116], achieved through modulation of PI3K/Akt/P706SK pathway [100,114] while an equally important antiproliferative mechanism was the suppression of angiogenesis [103,106,107,110,116] through downregulation of the VEGFR-2/PIEK/mTOR pathway [103], suppression of αvβ3 integrin-mediated VEGF [106], suppression of Src/FAK phosphorylation [106], and attenuation of HIF-1a-regulated VEGF [107]. Finally, regarding cancer stem cells, THD was able to selectively promote the differentiation of cancer stem cells while sparing the normal stem cells [121,122,128]. The antitumor mechanisms of action of thioridazine are summarized in Figure 5.
Figure 5.
Summary of thioridazine’s anti-tumor mechanism of action. Key: VEGF, Vascular endothelial growth factor; PI3/AKT, phosphoinositide-3-kinase–protein kinase B/Akt HIF-1a, Hypoxia-inducible factor-1a; WNT, Wingless and Int-1; mTOR, Mammalian target of rapamycin; CDK2, Cyclin-dependent kinase 2; FAK, Focal adhesion kinase; ROS, Reactive oxygen species.
3. Atypical Antipsychotics
3.1. Quetiapine
Quetiapine is a commonly prescribed atypical antipsychotic drug for treating schizophrenia, depression, and bipolar disorder. So far, only limited research has been performed regarding its potential antitumor effects. Wang et al. studied the anti-tumor effect of quetiapine in glioma stem cells isolated from the GBM GL216 cell line, which were subsequently xenografted in mice orthotopically and subcutaneously. Quetiapine suppressed glioma growth by inducing oligodendrocyte differentiation of glioma stem cells. Mechanistically, this was linked with the inhibition of the Wnt/β-catenin signaling pathway, which is involved in embryogenesis and differentiation of stem cells. Moreover, they found that quetiapine has a synergistic effect with TMZ, inhibiting the growth of TMZ-resistant glioma stem cells [129]. Recently, Saki et al. found that quetiapine increases the radiosensitivity of GBM cells in GBM mice models. In this study, the glioma cells that developed resistance in the combination of quetiapine and irradiation-expressed genes involved in cholesterol synthesis, and the addition of atorvastatin, a 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA) inhibitor, enhanced the efficacy of quetiapine [130]. Moreover, epidemiological studies have shown that quetiapine reduces the risk of hepatocellular carcinoma development in patients with schizophrenia [131], while according to Lee et al., quetiapine inhibited cell proliferation and migration of Hep1 and Hep3B HCC cancer cell lines and induced their apoptosis via activation of intrinsic and extrinsic apoptotic pathways and simultaneous reduction of expression of anti-apoptotic proteins such as XIAP and survivin via inhibition of the ERK/AKT signaling pathway [132].
3.2. Olanzapine
Olanzapine is a first-line atypical antipsychotic drug that primarily acts as a DRD2 antagonist. Moreover, olanzapine inhibits various other receptors, including serotonin (5-HT2A) and muscarinic receptors, and is thus utilized for controlling chemotherapy-induced nausea and vomiting. Regarding its potential anti-cancer properties, only limited experimental studies have been performed [133]. Olanzapine inhibits the proliferation of U87MG and A172 GBM cell lines, probably by promoting apoptosis, and enhances the anti-tumor effect of TMZ. Moreover, olanzapine inhibits the anchorage-dependent growth in U87MG cell lines and inhibits cancer cell migration in the A172 GBM cell line but not in the U87MG cell line. The exact mechanism of action is currently unclear, but downregulation of β-catenin and c-jun, an oncogenic transcription factor, may be involved [134]. In vitro, Sanomachi et al. supported that olanzapine specifically inhibits various cancer stem cell lines such as lung (A549) and pancreatic (PANC-1, PSN-1) in low concentrations, around 50μΜ, without affecting normal cells and increases the efficacy of several chemotherapeutic agents possibly by decreasing the expression of survivin in these cells [135].
3.3. Risperidone
Risperidone (RIS) is a benzisoxazole derivative and the second atypical antipsychotic developed following clozapine. RIS received FDA approval for the treatment of schizophrenia in 1993 and, since then, has also been approved for the treatment of bipolar I disorder (2003) and autism-related irritability (2006) [136]. From a pharmacological perspective, RIS displays a profound affinity for 5-HT2A serotonin receptors and, to a lesser extent (around 10-times less), for the DRD2, while it also antagonizes the a1- and a2-adrenergic receptors. However, it does not inhibit muscarinic receptors, thus lacking anticholinergic effects [137].
Regarding its anti-cancer properties, Dilly et al. reported that RIS, combined with rumenic acid, can hamper prostate cancer cell (PC-3 cells) proliferation in vitro and slow down tumor growth in vivo in mice with PC-3 prostate cancer xenografts [138]. The latter effects were attributed to the potent inhibition of 17-β-Hydroxysteroid dehydrogenase 10 (17HSD10), a multifunctional mitochondrial enzyme, which, among many other properties, has been found to convert the very weak androgen 5alpha-androstanediol into the more potent androgen 5alpha-dihydrotestosterone (DHT), thus providing an alternative androgen synthesis pathway that can operate even in the absence of testosterone, eventually helping cancerous cells survive androgen ablation therapy [139,140].
In another study, Wang et al. investigated in vitro the interaction between several antipsychotic drugs and MCF7/MX100 (resistant breast cancer) and Caco-2 (colon carcinoma) cell lines, both overexpressing the breast cancer resistance protein (BCRP), an energy-dependent transporter of the adenosine triphosphate-binding cassette (ABC) transporter family. BCRP can be found in many different human cells, including the liver canaliculi, mucosal surfaces of the small intestine, and the colon, cardiac muscle, pancreas, adrenal cortex, thyroid and parathyroid endothelia, and others where it regulates the drug absorption, predominantly leading to drug excretion. Thus, inhibiting BCRP could potentially increase the efficacy of chemotherapy drugs in the abovementioned organs. Wang and colleagues reported that risperidone, at concentrations of 1–100μΜ, effectively inhibited the overexpression of BCRP transporter in both cell lines in a concentration-dependent manner [141]. On the same lines, Gundogdu et al. observed in vitro proliferation and migration suppression as well as dose-dependent DNA damage of MC7-cells with the application of risperidone. The exact genotoxic mechanisms were not discussed, yet the authors implied a possible role of the Dopamine D2- and 5-HT2a-associated ERK and Akt signaling pathways [142]. Finally, the in vitro study by Chen et al. in human hepatocellular carcinoma cell lines (Huh-7 and Hep G2) indicated that risperidone suppresses proliferation and invasion of these cells while it induces apoptosis by potent activation of caspase-3 [131,143].
3.4. Aripiprazole (ARP)
Aripiprazole (ARP) is an atypical antiphychotic drug commonly used for the treatment of schizophrenia and bipolar disorder. It has a high affinity for dopamine D2 receptors and agonizes and antagonizes, particularly the 5-HT1A and 5-HT2A, respectively [144]. Regarding its anticancer properties, Badran et al., in their in vitro studies in MCF-7 cells (breast cancer cell line), observed inhibition of cell proliferation by cell cycle arrest in subG0/G1 phase and subsequently the induction of apoptosis (downregulation of anti-apoptotic genes BCL2L1, C-myc and upregulation of pro-apoptotic genes BCL-10, BAK, active caspase-3 and caspase-9) [145]. Similarly, Lee et al., using the same cell line (MCF-7) for their in vitro studies, and also attributed the antiproliferative effect of ARP to the induction of apoptosis but, noteworthily, the D2R/AMPK activation may attenuate the latter effect [146]. In another in vitro study, ARP decreased the proliferation of pancreatic and non-small lung cancer stem cells, presumably through Wnt/β-catenin pathway inhibition. The authors also reported a significant reduction of the anti-apoptotic protein survivin as well as inhibition of P-gp pumps, both leading to reduced cells’ resistance to chemotherapeutic agents [147]. In the context of P-gp pumps, two additional in vitro studies supported that the effective P-gp inhibition by ARP increased the potency of anti-mitotic drugs when these two drugs were combined [148,149]. Finally, Kim et al., using cell culture (in vitro) and xenografted mice (in vivo), supported that ARP induced apoptosis-mediated cytotoxicity and migration suppression by direct inhibition of Src oncogenic tyrosine and therefore inhibition of the signaling cascade molecules (phosphorylated phosphatidylinositide 3-kinase, STAT3, and Akt), all involved in glioma progression [150].
3.5. Clozapine (CLZ)
Clozapine (CLZ) is an atypical antipsychotic drug, widely considered the most efficient drug for the treatment of resistant schizophrenia. However, its clinical utility remains limited due to undesirable side effects such as agranulocytosis, sedation, and hyperlipidosis. Clozapine extracts its antipsychotic activity by binding with high affinity to serotonin 2A receptor (5-HT2A) and with a weaker affinity to DRD2 [151]. Few studies have reported that clozapine may possess anti-cancer properties. In one in vitro study using human NSCLC cells (A549, H1299), clozapine exerted antiproliferative activity by arresting cancer cells in the G0/G1 phase and promoting autophagic cell death, presumably through inhibition of the PI3k/Akt/mTOR pathway [152]. In another study, the authors studied the effect of clozapine in melanoma cell lines both in vitro (WM35: Human primary melanoma cells, M1/15: Human metastatic melanoma cells) and in vivo (M1/15 cell line). Clozapine decreased cell proliferation in both cell lines by promoting cell cycle arrest in the G0/G1 phase and senescence (disruption of lysosomal function through enhanced activity of senescence-associated b-galactosidase, leading to terminal growth arrest), both through clozapine-induced phosphorylation of the ERK pathway, a crucial pathway for melanoma progression [153]. Finally, Shin et al. tested the effect of clozapine on PTEN-negative U-87MG glioblastoma cells in vitro. The authors observed that clozapine blocks the IP3-dependent calcium release and inhibits calcium uptake through inotropic and voltage-dependent calcium receptors, downregulating the Ca2+/calmodulin and subsequently the PI3K/Akt/GSK-3β pathways, inducing cell cycle arrest in the G0/G1 phase with a reduction in cyclin D1 expression and ultimately inhibiting cell cycle progression [154].
4. D3 Receptors’ Modulation and Cancer Treatment
Dopamine D3 receptors are located predominantly in the limbic system, both pre-and post-synaptically. Although they belong to the D2-like receptor family, the D3 receptors signaling pathways are discrepant. Animal studies suggest that D3 antagonism can effectively alleviate schizophrenia’s positive symptoms without the D2 antagonism-associated adverse effects, including extrapyramidal motor symptoms, hyperprolactinemia, anhedonia, weight gain, and metabolic dysregulations [155]. Additionally, buspirone, an azapirone anxiolytic drug, through its D3-receptor antagonistic properties, improved schizophrenia-related cognitive impairment in vivo [156], while in a double-blind randomized controlled trial in 200 patients with schizophrenia, the combination of buspirone and atypical antipsychotic drugs was more effective in improving cognitive function compared with atypical antipsychotics alone [157], postulating a positive effect of D3-receptor antagonism also in cancer-associated cognitive impairment [158]. In an in vitro study, Hussein et al. found that selective D3 receptor antagonists PG01037, NGB2904, SB277011A, and U99194 significantly enhanced the efficacy of the chemotherapeutic drugs mitoxantrone and doxorubicin in the ABCG2 over-expressing multidrug-resistant lung (H460-MX20, A549-MX10) and colon cancer (S1M1-80) cell lines by impairing the regulation of the ABCG2 transporters of those cells at non-toxic concentrations [159]. In another study, cariprazine, a D3-preferring D3/D2 receptor partial agonist used for the treatment of schizophrenia and bipolar disorder [160], was utilized in vitro in ABCG2-mediated multidrug-resistant non-small lung cancer (H460-MX20 cell line) and colon cancer cell line (S1M1-80). The authors reported that cariprazine synergistically enhanced the efficacy of the antineoplastic drug mitoxantrone by downregulating the expression of ABCG2 protein, with the exact molecular mechanism of this modulation remaining unknown [161].
5. Conclusions
The antitumor effect of antipsychotic drugs is mediated through multiple distinct molecular mechanisms, and there is also a significant difference in the antitumor mechanism observed between the different antipsychotic drugs. Most experimental studies in the current topic include the older typical antipsychotics, probably due to their ability to interact with several intracellular receptors, whereas the atypical ones appear to be selective for DRD2. However, there has been an increasing research trend in the last few years, focusing on the newer atypical antipsychotics with promising results. Antipsychotic drugs also appear to increase the effect of chemotherapeutic drugs such as TMZ and doxorubicin, combating the tumor’s chemo- and radio-resistance.
So far, only three clinical trials have been performed in humans, one phase II clinical trial in chlorpromazine, one phase II clinical trial in pimozide, and one phase I clinical trial in thioridazine. The details of these clinical trials are summarized in Table 1. However, due to the increasing amount of preclinical evidence gathered in the last years, especially in typical antipsychotics, there are increasing probabilities of new clinical trials being introduced within the following 5–10 years. The most studied antipsychotic drugs regarding their anti-tumor effects are haloperidol, chlorpromazine, thioridazine, and penfluridol. On the contrary, clinical trials regarding the newer atypical antipsychotics, despite their favorable adverse effects profile, are probably not expected within the next 5 years due to the limited preclinical evidence of anti-tumor activity.
Table 1.
Clinical trials studying the antitumor effect of antipsychotic drugs in different types of cancer.
| Cancer | Drug | Start Date | Title | NCT | Phase | Results | Estimated Completion Year/Date |
|---|---|---|---|---|---|---|---|
| Glioblastoma | Chlorpromazine | 15 December 2019 | Repurposing chlorpromazine for the Treatment of Glioblastoma (RACTAC) | NCT04224441 | II | Not posted | 15 December 2022 |
| Metastatic Melanoma [162] |
Pimozide | n/s | Phase II trial of pimozide in previously treated melanoma patients | n/a | II | 17% of the evaluated patients had complete or partial response for at least 8 months | Completed (1983) |
| Acute myeloid leukemia | Thioridazine (THD) | July 2014 | Safety study of Thioridazine in combination with Cytarabine to treat relapsed or refractory Acute Myeloid Leukemia (THORIDAL) | NCT02096289 | I | A reduction up to 55% in blast levels was noted in 85% of the patients | Completed (September 2016) |
Author Contributions
N.V. and M.L. equally participated in the present study. All authors M.L., N.V., S.V. and G.A.A. conceived the study, co-wrote the final manuscript, and reviewed the literature. S.V. and G.A.A. supervised the study and gave the final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., Sarkar S., Housman G., Byler S., Heerboth S., et al. Drug Resistance in Cancer: An Overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciociola A.A., Cohen L.B., Kulkarni P., Kefalas C., Buchman A., Burke C., Cain T., Connor J., Ehrenpreis E.D., Fang J., et al. How Drugs are Developed and Approved by the FDA: Current Process and Future Directions. Am. J. Gastroenterol. 2014;109:620–623. doi: 10.1038/ajg.2013.407. [DOI] [PubMed] [Google Scholar]
- 4.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 5.Shen W.W. A history of antipsychotic drug development. Compr. Psychiatry. 1999;40:407–414. doi: 10.1016/S0010-440X(99)90082-2. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto S., Duncan E.G., E Marx C., Lieberman A.J. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry. 2004;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 7.Fond G., Macgregor A., Attal J., Larue A., Brittner M., Ducasse D., Capdevielle D. Antipsychotic drugs: Pro-cancer or anti-cancer? A systematic review. Med. Hypotheses. 2012;79:38–42. doi: 10.1016/j.mehy.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Dalton O.S., Johansen C., Poulsen A.H., Nørgaard M., Sørensen H.T., McLaughlin J.K., Mortensen P.B., Friis S. Cancer risk among users of neuroleptic medication: A population-based cohort study. Br. J. Cancer. 2006;95:934–939. doi: 10.1038/sj.bjc.6603259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Muñoz F., Alamo C. The consolidation of neuroleptic therapy: Janssen, the discovery of haloperidol and its introduction into clinical practice. Brain Res. Bull. 2009;79:130–141. doi: 10.1016/j.brainresbull.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Beach S.R., Gross A.F., Hartney K.E., Taylor J.B., Rundell J.R. Intravenous haloperidol: A systematic review of side effects and recommendations for clinical use. Gen. Hosp. Psychiatry. 2020;67:42–50. doi: 10.1016/j.genhosppsych.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Saeedi H., Remington G., Christensen B.K. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr. Res. 2006;85:222–231. doi: 10.1016/j.schres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Wahba M., Messiha B.A.S., Abo-Saif A.A. Ramipril and haloperidol as promising approaches in managing rheumatoid arthritis in rats. Eur. J. Pharmacol. 2015;765:307–315. doi: 10.1016/j.ejphar.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Hoertel N., Sánchez-Rico M., Vernet R., Jannot A.-S., Neuraz A., Blanco C., Lemogne C., Airagnes G., Paris N., Daniel C., et al. Observational study of haloperidol in hospitalized patients with COVID-19. PLoS ONE. 2021;16:e0247122. doi: 10.1371/journal.pone.0247122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji C., Liu N., Tu J., Li Z., Han G., Li J., Sheng C. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect. Dis. 2019;6:768–786. doi: 10.1021/acsinfecdis.9b00197. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos F., Isihou R., Alexiou G.A., Tsalios T., Vartholomatos E., Markopoulos G.S., Sioka C., Tsekeris P., Kyritsis A.P., Galani V. Haloperidol Induced Cell Cycle Arrest and Apoptosis in Glioblastoma Cells. Biomedicines. 2020;8:595. doi: 10.3390/biomedicines8120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendouei N., Saghafi F., Shadfar F., Hosseinimehr S.J. Molecular mechanisms of anti-psychotic drugs for improvement of cancer treatment. Eur. J. Pharmacol. 2019;856:172402. doi: 10.1016/j.ejphar.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Jiang X., Gao L., Liu X., Li J., Huang X., Zeng T. Synergistic Suppression of Glioblastoma Cell Growth by Combined Application of Temozolomide and Dopamine D2 Receptor Antagonists. World Neurosurg. 2019;128:e468–e477. doi: 10.1016/j.wneu.2019.04.180. [DOI] [PubMed] [Google Scholar]
- 18.He Y., Li J., Koga T., Ma J., Dhawan S., Suzuki Y., Furnari F., Prabhu V.V., E Allen J., Chen C.C. Epidermal growth factor receptor as a molecular determinant of glioblastoma response to dopamine receptor D2 inhibitors. Neuro-Oncology. 2020;23:400–411. doi: 10.1093/neuonc/noaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauth M., Rohnalter V., Bergström Å., Kooshesh M., Svenningsson P., Toftgård R. Antipsychotic Drugs Regulate Hedgehog Signaling by Modulation of 7-Dehydrocholesterol Reductase Levels. Mol. Pharmacol. 2010;78:486–496. doi: 10.1124/mol.110.066431. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.H., Lee H.Y., Yi H., Ahn Y.M., Kim Y.S. Haloperidol induces demethylation and expression of the dual specificity phosphatase 6 gene in MIA PaCa-2 human pancreatic cancer cells. Life Sci. 2012;91:1317–1322. doi: 10.1016/j.lfs.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Jandaghi P., Najafabadi H.S., Bauer A.S., Papadakis A.I., Fassan M., Hall A., Monast A., von Knebel Doeberitz M., Neoptolemos J.P., Costello E., et al. Expression of DRD2 Is Increased in Human Pancreatic Ductal Adenocarcinoma and Inhibitors Slow Tumor Growth in Mice. Gastroenterology. 2016;151:1218–1231. doi: 10.1053/j.gastro.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Sozio P., Fiorito J., Di Giacomo V., Di Stefano A., Marinelli L., Cacciatore I., Cataldi A., Pacella S., Turkez H., Parenti C., et al. Haloperidol metabolite II prodrug: Asymmetric synthesis and biological evaluation on rat C6 glioma cells. Eur. J. Med. Chem. 2015;90:1–9. doi: 10.1016/j.ejmech.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Marrazzo A., Fiorito J., Zappalà L., Prezzavento O., Ronsisvalle S., Pasquinucci L., Scoto G.M., Bernardini R., Ronsisvalle G. Antiproliferative activity of phenylbutyrate ester of haloperidol metabolite II [(±)-MRJF4] in prostate cancer cells. Eur. J. Med. Chem. 2011;46:433–438. doi: 10.1016/j.ejmech.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Di Giacomo V., Di Valerio V., Rapino M., Bosco D., Cacciatore I., Ciulla M., Marrazzo A., Fiorito J., DI Stefano A., Cataldi A. MRJF4, a novel histone deacetylase inhibitor, induces p21 mediated autophagy in PC3 prostate cancer cells. Cell. Mol. Boil. 2015;61:17–23. [PubMed] [Google Scholar]
- 25.Barbaraci C., Giurdanella G., Leotta C.G., Longo A., Amata E., Dichiara M., Pasquinucci L., Turnaturi R., Prezzavento O., Cacciatore I., et al. Haloperidol Metabolite II Valproate Ester (S)-(−)-MRJF22: Preliminary Studies as a Potential Multifunctional Agent against Uveal Melanoma. J. Med. Chem. 2021;64:13622–13632. doi: 10.1021/acs.jmedchem.1c00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Z., Xia Y., Gao T., Xu F., Lei Q., Peng C., Yang Y., Xue Q., Hu X., Wang Q., et al. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis. 2018;9:1–15. doi: 10.1038/s41419-018-1046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat K., Saki M., Vlashi E., Cheng F., Duhachek-Muggy S., Alli C., Yu G., Medina P., He L., Damoiseaux R., et al. The dopamine receptor antagonist trifluoperazine prevents phenotype conversion and improves survival in mouse models of glioblastoma. Proc. Natl. Acad. Sci. USA. 2020;117:11085–11096. doi: 10.1073/pnas.1920154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caragher S., Shireman J., Huang M., Miska J., Atashi F., Baisiwala S., Park C.H., Saathoff M., Warnke L., Xiao T., et al. Activation of Dopamine Receptor 2 Prompts Transcriptomic and Metabolic Plasticity in Glioblastoma. J. Neurosci. 2019;39:1982–1993. doi: 10.1523/JNEUROSCI.1589-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Xu R., Zhang C., Xu Y., Han M., Huang B., Chen A., Qiu C., Thorsen F., Prestegarden L., et al. Trifluoperazine, a novel autophagy inhibitor, increases radiosensitivity in glioblastoma by impairing homologous recombination. J. Exp. Clin. Cancer Res. 2017;36:1–13. doi: 10.1186/s13046-017-0588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X., Luo X., Cheng Y. Trifluoperazine prevents FOXO1 nuclear excretion and reverses doxorubicin-resistance in the SHG44/DOX drug-resistant glioma cell line. Int. J. Mol. Med. 2018;42:3300–3308. doi: 10.3892/ijmm.2018.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh C.-T., Wu A., Chang P.M.-H., Chen K.-Y., Yang C.-N., Yang S.-C., Ho C.-C., Chen C.-C., Kuo Y.-L., Lee P.-Y., et al. Trifluoperazine, an Antipsychotic Agent, Inhibits Cancer Stem Cell Growth and Overcomes Drug Resistance of Lung Cancer. Am. J. Respir. Crit. Care Med. 2012;186:1180–1188. doi: 10.1164/rccm.201207-1180OC. [DOI] [PubMed] [Google Scholar]
- 32.Kang S., Hong J., Lee J.M., Moon H.E., Jeon B., Choi J., Yoon N.A., Paek S.H., Roh E.J., Lee C.J., et al. Trifluoperazine, a Well-Known Antipsychotic, Inhibits Glioblastoma Invasion by Binding to Calmodulin and Disinhibiting Calcium Release Channel IP3R. Mol. Cancer Ther. 2016;16:217–227. doi: 10.1158/1535-7163.MCT-16-0169-T. [DOI] [PubMed] [Google Scholar]
- 33.Pulkoski-Gross A., Li J., Zheng C., Li Y., Ouyang N., Rigas B., Zucker S., Cao J. Repurposing the Antipsychotic Trifluoperazine as an Antimetastasis Agent. Mol. Pharmacol. 2014;87:501–512. doi: 10.1124/mol.114.096941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia Y., Jia C., Xue Q., Jiang J., Xie Y., Wang R., Ran Z., Xu F., Zhang Y., Ye T. Antipsychotic Drug Trifluoperazine Suppresses Colorectal Cancer by Inducing G0/G1 Arrest and Apoptosis. Front. Pharmacol. 2019;10:1029. doi: 10.3389/fphar.2019.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang J., Huang Z., Chen X., Luo R., Cai H., Wang H., Zhang H., Sun T., Zhang Y. Trifluoperazine Activates FOXO1-Related Signals to Inhibit Tumor Growth in Hepatocellular Carcinoma. DNA Cell Biol. 2017;36:813–821. doi: 10.1089/dna.2017.3790. [DOI] [PubMed] [Google Scholar]
- 36.Ding L., Cao J., Lin W., Chen H., Xiong X., Ao H., Yu M., Lin J., Cui Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21061960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamgar-Dayhoff P., Brelidze T.I. Multifaceted effect of chlorpromazine in cancer: Implications for cancer treatment. Oncotarget. 2021;12:1406–1426. doi: 10.18632/oncotarget.28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbruzzese C., Matteoni S., Persico M., Villani V., Paggi M.G. Repurposing chlorpromazine in the treatment of glioblastoma multiforme: Analysis of literature and forthcoming steps. J. Exp. Clin. Cancer Res. 2020;39:1–3. doi: 10.1186/s13046-020-1534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matteoni S., Matarrese P., Ascione B., Buccarelli M., Ricci-Vitiani L., Pallini R., Villani V., Pace A., Paggi M.G., Abbruzzese C. Anticancer Properties of the Antipsychotic Drug Chlorpromazine and Its Synergism with Temozolomide in Restraining Human Glioblastoma Proliferation In Vitro. Front. Oncol. 2021;11:635472. doi: 10.3389/fonc.2021.635472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aas A.T., Brun A., Pero R.W., Salford L.G. Chlorpromazine in combination with nitrosourea inhibits experimental glioma growth. Br. J. Neurosurg. 1994;8:187–192. doi: 10.3109/02688699409027965. [DOI] [PubMed] [Google Scholar]
- 41.Oliva C.R., Zhang W., Langford C., Suto M.J., Griguer C.E. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget. 2017;8:37568–37583. doi: 10.18632/oncotarget.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin S.Y., Kim C.G., Kim S.H., Kim Y.S., Lim Y., Lee Y.H. Chlorpromazine activates p21Waf1/Cip1gene transcription via early growth response-1 (Egr-1) in C6 glioma cells. Exp. Mol. Med. 2010;42:395–405. doi: 10.3858/emm.2010.42.5.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin S.Y., Lee K.S., Choi Y.-K., Lim H.J., Lee H.G., Lim Y., Lee Y.H. The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis. 2013;34:2080–2089. doi: 10.1093/carcin/bgt169. [DOI] [PubMed] [Google Scholar]
- 44.Jhou A.-J., Chang H.-C., Hung C.-C., Lin H.-C., Lee Y.-C., Liu W.-T., Han K.-F., Lai Y.-W., Lin M.-Y., Lee C.-H. Chlorpromazine, an antipsychotic agent, induces G2/M phase arrest and apoptosis via regulation of the PI3K/AKT/mTOR-mediated autophagy pathways in human oral cancer. Biochem. Pharmacol. 2020;184:114403. doi: 10.1016/j.bcp.2020.114403. [DOI] [PubMed] [Google Scholar]
- 45.Kurita J.-I., Hirao Y., Nakano H., Fukunishi Y., Nishimura Y. Sertraline, chlorprothixene, and chlorpromazine characteristically interact with the REST-binding site of the corepressor mSin3, showing medulloblastoma cell growth inhibitory activities. Sci. Rep. 2018;8:13763. doi: 10.1038/s41598-018-31852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhelev Z., Ohba H., Bakalova R., Hadjimitova V., Ishikawa M., Shinohara Y., Baba Y. Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Cancer Chemother. Pharmacol. 2004;53:267–275. doi: 10.1007/s00280-003-0738-1. [DOI] [PubMed] [Google Scholar]
- 47.Rai S., Tanaka H., Suzuki M., Espinoza J.L., Kumode T., Tanimura A., Yokota T., Oritani K., Watanabe T., Kanakura Y., et al. Chlorpromazine eliminates acute myeloid leukemia cells by perturbing subcellular localization of FLT3-ITD and KIT-D816V. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-17666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M.S., Johansen L., Zhang Y., Wilson A., Keegan M., Avery W., Elliott P., Borisy A.A., Keith C.T. The Novel Combination of Chlorpromazine and Pentamidine Exerts Synergistic Antiproliferative Effects through Dual Mitotic Action. Cancer Res. 2007;67:11359–11367. doi: 10.1158/0008-5472.CAN-07-2235. [DOI] [PubMed] [Google Scholar]
- 49.Martins A.S., Ordóñez J.L., Amaral A.T., Prins F., Floris G., Debiec-Rychter M., Hogendoorn P.C.W., de Álava E. IGF1R Signaling in Ewing Sarcoma Is Shaped by Clathrin-/Caveolin-Dependent Endocytosis. PLoS ONE. 2011;6:e19846. doi: 10.1371/journal.pone.0019846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Z., Zang Y.Q., Lu Y., Dong Q.P., Dong K.T., Zhou H.F. Chlorpromazine hydrochloride plays a tumor suppressive role in diffuse large B lymphoma by promoting the expression of S1PR2. Chin. J. Ind. Hyg. Occup. Dis. 2021;39:418–423. doi: 10.3760/cma.j.cn121094-20201116-00633. [DOI] [PubMed] [Google Scholar]
- 51.Lee W.-Y., Lee W.-T., Cheng C.-H., Chen K.-C., Chou C.-M., Chung C.-H., Sun M.-S., Cheng H.-W., Ho M.-N., Lin C.-W. Repositioning antipsychotic chlorpromazine for treating colorectal cancer by inhibiting sirtuin 1. Oncotarget. 2015;6:27580–27595. doi: 10.18632/oncotarget.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buscail L., Bournet B., Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 53.Eisenberg S., Giehl K., Henis Y.I., Ehrlich M. Differential Interference of Chlorpromazine with the Membrane Interactions of Oncogenic K-Ras and Its Effects on Cell Growth. J. Biol. Chem. 2008;283:27279–27288. doi: 10.1074/jbc.M804589200. [DOI] [PubMed] [Google Scholar]
- 54.Yde C.W., Clausen M.P., Bennetzen M.V., Lykkesfeldt A.E., Mouritsen O.G., Guerra B. The antipsychotic drug chlorpromazine enhances the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells. Anti-Cancer Drugs. 2009;20:723–735. doi: 10.1097/CAD.0b013e32832ec041. [DOI] [PubMed] [Google Scholar]
- 55.Yang C.-E., Lee W.-Y., Cheng H.-W., Chung C.-H., Mi F.-L., Lin C.-W. The antipsychotic chlorpromazine suppresses YAP signaling, stemness properties, and drug resistance in breast cancer cells. Chem.-Biol. Interact. 2019;302:28–35. doi: 10.1016/j.cbi.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 56.Shaw V., Srivastava S., Srivastava S.K. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin. Cancer Biol. 2021;68:75–83. doi: 10.1016/j.semcancer.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hongo H., Kosaka T., Suzuki Y., Oya M. Discovery of a New Candidate Drug to Overcome Cabazitaxel-Resistant Gene Signature in Castration-Resistant Prostate Cancer by in Silico Screening. Prostate Cancer Prostatic Dis. 2021 doi: 10.1038/s41391-021-00426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim U., Kim C.-Y., Lee J.M., Ryu B., Kim J., Shin C., Park J.-H. Pimozide Inhibits the Human Prostate Cancer Cells through the Generation of Reactive Oxygen Species. Front. Pharmacol. 2020;10:1517. doi: 10.3389/fphar.2019.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai N., Zhou W., Ye L.-L., Chen J., Liang Q.-N., Chang G., Chen J.-J. The STAT3 inhibitor pimozide impedes cell proliferation and induces ROS generation in human osteosarcoma by suppressing catalase expression. Am. J. Transl. Res. 2017;9:3853–3866. [PMC free article] [PubMed] [Google Scholar]
- 60.Dees S., Pontiggia L., Jasmin J.-F., Mercier I. Phosphorylated STAT3 (Tyr705) as a biomarker of response to pimozide treatment in triple-negative breast cancer. Cancer Biol. Ther. 2020;21:506–521. doi: 10.1080/15384047.2020.1726718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ranjan A., Kaushik I., Srivastava S.K. Pimozide Suppresses the Growth of Brain Tumors by Targeting STAT3-Mediated Autophagy. Cells. 2020;9:2141. doi: 10.3390/cells9092141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dakir E.-H., Pickard A., Srivastava K., McCrudden C.M., Gross S., Lloyd S., Zhang S.-D., Margariti A., Morgan R., Rudland P.S., et al. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget. 2018;9:34889–34910. doi: 10.18632/oncotarget.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strobl J.S., Kirkwood K., Lantz T.K., Lewine A.M., A Peterson V., Worley J.F. Inhibition of human breast cancer cell proliferation in tissue culture by the neuroleptic agents pimozide and thioridazine. Cancer Res. 1990;50:5399–5405. [PubMed] [Google Scholar]
- 64.Rondanin R., Simoni D., Maccesi M., Romagnoli R., Grimaudo S., Pipitone R.M., Meli M., Cascio A., Tolomeo M. Effects of Pimozide Derivatives on pSTAT5 in K562 Cells. ChemMedChem. 2017;12:1183–1190. doi: 10.1002/cmdc.201700234. [DOI] [PubMed] [Google Scholar]
- 65.Nelson E.A., Walker S.R., Weisberg E., Bar-Natan M., Barrett R., Gashin L.B., Terrell S., Klitgaard J.L., Santo L., Addorio M.R., et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nelson E.A., Walker S.R., Xiang M., Weisberg E., Bar-Natan M., Barrett R., Liu S., Kharbanda S., Christie A.L., Nicolais M., et al. The STAT5 Inhibitor Pimozide Displays Efficacy in Models of Acute Myelogenous Leukemia Driven by FLT3 Mutations. Genes Cancer. 2012;3:503–511. doi: 10.1177/1947601912466555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao Z., Liang J., Deng Q., Song C., Yang X., Liu Z., Shao Z., Zhang K., Wang X., Li Z. Pimozide augments bromocriptine lethality in prolactinoma cells and in a xenograft model via the STAT5/cyclin D1 and STAT5/Bcl-xL signaling pathways. Int. J. Mol. Med. 2020;47:113–124. doi: 10.3892/ijmm.2020.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subramaniam D., Angulo P., Ponnurangam S., Dandawate P., Ramamoorthy P., Srinivasan P., Iwakuma T., Weir S.J., Chastain K., Anant S. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020;11:1–15. doi: 10.1038/s41419-020-2335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fako V., Yu Z., Henrich C.J., Ransom T., Budhu A.S., Wang X.W. Inhibition of wnt/β-catenin Signaling in Hepatocellular Carcinoma by an Antipsychotic Drug Pimozide. Int. J. Biol. Sci. 2016;12:768–775. doi: 10.7150/ijbs.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gould R.J., Murphy K.M., Reynolds I.J., Snyder S.H. Antischizophrenic drugs of the diphenylbutylpiperidine type act as calcium channel antagonists. Proc. Natl. Acad. Sci. USA. 1983;80:5122–5125. doi: 10.1073/pnas.80.16.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi X.-N., Li H., Yao H., Liu X., Li L., Leung K.-S., Kung H.-F., Lu D., Wong M.-H., Lin M.C.-M. In Silico Identification and In Vitro and In Vivo Validation of Anti-Psychotic Drug Fluspirilene as a Potential CDK2 Inhibitor and a Candidate Anti-Cancer Drug. PLoS ONE. 2015;10:e0132072. doi: 10.1371/journal.pone.0132072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dong Y., Furuta T., Sabit H., Kitabayashi T., Jiapaer S., Kobayashi M., Ino Y., Todo T., Teng L., Hirao A., et al. Identification of antipsychotic drug fluspirilene as a potential anti-glioma stem cell drug. Oncotarget. 2017;8:111728–111741. doi: 10.18632/oncotarget.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patil S.P., Pacitti M.F., Gilroy K.S., Ruggiero J.C., Griffin J.D., Butera J.J., Notarfrancesco J.M., Tran S., Stoddart J.W. Identification of antipsychotic drug fluspirilene as a potential p53-MDM2 inhibitor: A combined computational and experimental study. J. Comput. Mol. Des. 2014;29:155–163. doi: 10.1007/s10822-014-9811-6. [DOI] [PubMed] [Google Scholar]
- 74.Janssen P.A., Niemegeers C.J., Schellekens K.H., Lenaerts F.M., Verbruggen F.J., Van Nueten J.M., Schaper W.K. The pharmacology of penfluridol (R 16341) a new potent and orally long-acting neuroleptic drug. Eur. J. Pharmacol. 1970;11:139–154. doi: 10.1016/0014-2999(70)90043-9. [DOI] [PubMed] [Google Scholar]
- 75.Santi C.M., Cayabyab F.S., Sutton K.G., McRory J.E., Mezeyova J., Hamming K.S., Parker D., Stea A., Snutch T.P. Differential Inhibition of T-Type Calcium Channels by Neuroleptics. J. Neurosci. 2002;22:396–403. doi: 10.1523/JNEUROSCI.22-02-00396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaman A.U., Sajib S., Cucullo L., Mikelis C.M., German N.A. Analogs of penfluridol as chemotherapeutic agents with reduced central nervous system activity. Bioorganic Med. Chem. Lett. 2018;28:3652–3657. doi: 10.1016/j.bmcl.2018.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ranjan A., Gupta P., Srivastava S.K. Penfluridol: An Antipsychotic Agent Suppresses Metastatic Tumor Growth in Triple-Negative Breast Cancer by Inhibiting Integrin Signaling Axis. Cancer Res. 2015;76:877–890. doi: 10.1158/0008-5472.CAN-15-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamidi H., Ivaska J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hedrick E., Li X., Safe S. Penfluridol Represses Integrin Expression in Breast Cancer through Induction of Reactive Oxygen Species and Downregulation of Sp Transcription Factors. Mol. Cancer Ther. 2016;16:205–216. doi: 10.1158/1535-7163.MCT-16-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedrick E., Cheng Y., Jin U.-H., Kim K., Safe S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget. 2016;7:22245–22256. doi: 10.18632/oncotarget.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hedrick E., Lee S.-O., Doddapaneni R., Singh M., Safe S. NR4A1 Antagonists Inhibit β1-Integrin-Dependent Breast Cancer Cell Migration. Mol. Cell. Biol. 2016;36:1383–1394. doi: 10.1128/MCB.00912-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chien W., Sun Q.-Y., Lee K.L., Ding L.-W., Wuensche P., Torres-Fernandez L.A., Tan S.Z., Tokatly I., Zaiden N., Poellinger L., et al. Activation of protein phosphatase 2A tumor suppressor as potential treatment of pancreatic cancer. Mol. Oncol. 2015;9:889–905. doi: 10.1016/j.molonc.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu S.-Y., Wen Y.-C., Ku C.-C., Yang Y.-C., Chow J.-M., Yang S.-F., Lee W.-J., Chien M.-H. Penfluridol triggers cytoprotective autophagy and cellular apoptosis through ROS induction and activation of the PP2A-modulated MAPK pathway in acute myeloid leukemia with different FLT3 statuses. J. Biomed. Sci. 2019;26:1–13. doi: 10.1186/s12929-019-0557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ranjan A., Srivastava S.K. Penfluridol suppresses glioblastoma tumor growth by Akt-mediated inhibition of GLI1. Oncotarget. 2017;8:32960–32976. doi: 10.18632/oncotarget.16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim H., Chong K., Ryu B.K., Park K.J., Yu M.O., Lee J., Chung S., Choi S., Park M.J., Chung Y.G., et al. Repurposing Penfluridol in Combination with Temozolomide for the Treatment of Glioblastoma. Cancers. 2019;11:1310. doi: 10.3390/cancers11091310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ranjan A., Srivastava S.K. Penfluridol suppresses pancreatic tumor growth by autophagy-mediated apoptosis. Sci. Rep. 2016;6:26165. doi: 10.1038/srep26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ranjan A., German N., Mikelis C., Srivenugopal K., Srivastava S.K. Penfluridol induces endoplasmic reticulum stress leading to autophagy in pancreatic cancer. Tumor Biol. 2017;39:1010428317705517. doi: 10.1177/1010428317705517. [DOI] [PubMed] [Google Scholar]
- 88.Hung W.-Y., Chang J.-H., Cheng Y., Cheng G.-Z., Huang H.-C., Hsiao M., Chung C.-L., Lee W.-J., Chien M.-H. Autophagosome accumulation-mediated ATP energy deprivation induced by penfluridol triggers nonapoptotic cell death of lung cancer via activating unfolded protein response. Cell Death Dis. 2019;10:1–17. doi: 10.1038/s41419-019-1785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo J.Y., White E. Autophagy, Metabolism, and Cancer. Cold Spring Harb. Symp. Quant. Biol. 2016;81:73–78. doi: 10.1101/sqb.2016.81.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ranjan A., Wright S., Srivastava S.K. Immune consequences of penfluridol treatment associated with inhibition of glioblastoma tumor growth. Oncotarget. 2017;8:47632–47641. doi: 10.18632/oncotarget.17425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta N., Gupta P., Srivastava S.K. Penfluridol overcomes paclitaxel resistance in metastatic breast cancer. Sci. Rep. 2019;9:5066. doi: 10.1038/s41598-019-41632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Du J., Shang J., Chen F., Zhang Y., Yin N., Xie T., Zhang H., Yushuo Z., Liu F. A CRISPR/Cas9–Based Screening for Non-Homologous End Joining Inhibitors Reveals Ouabain and Penfluridol as Radiosensitizers. Mol. Cancer Ther. 2017;17:419–431. doi: 10.1158/1535-7163.MCT-17-0090. [DOI] [PubMed] [Google Scholar]
- 93.Wu L., Liu Y.-Y., Li Z.-X., Zhao Q., Wang X., Yu Y., Wang Y.-Y., Wang Y.-Q., Luo F. Anti-tumor effects of penfluridol through dysregulation of cholesterol homeostasis. Asian Pac. J. Cancer Prev. 2014;15:489–494. doi: 10.7314/APJCP.2014.15.1.489. [DOI] [PubMed] [Google Scholar]
- 94.Thanacoody R.H. Thioridazine: The good and the bad. Recent Pat. Anti-Infect. Drug Discov. 2011;6:92–98. doi: 10.2174/157489111796064588. [DOI] [PubMed] [Google Scholar]
- 95.Cowap J., Hardy J. Thioridazine in the management of cancer-related sweating. J. Pain Symptom Manag. 1998;15:266. [PubMed] [Google Scholar]
- 96.Zhukovsky D.S. Fever and sweats in the patient with advanced cancer. Hematol. Clin. N. Am. 2002;16:579–588. doi: 10.1016/S0889-8588(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 97.Ly K.L., Chidgey J., Addington-Hall J., Hotopf M. Depression in palliative care: A systematic review. Part 2. Treatment. Palliat. Med. 2002;16:279–284. doi: 10.1191/0269216302pm570oa. [DOI] [PubMed] [Google Scholar]
- 98.Chu C.-W., Ko H.-J., Chou C.-H., Cheng T.-S., Cheng H.-W., Liang Y.-H., Lai Y.-L., Lin C.-Y., Wang C., Loh J.-K., et al. Thioridazine Enhances P62-Mediated Autophagy and Apoptosis through Wnt/β-Catenin Signaling Pathway in Glioma Cells. Int. J. Mol. Sci. 2019;20:473. doi: 10.3390/ijms20030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johannessen T., Hasan-Olive M., Zhu H., Denisova O., Grudic A., Latif A., Saed H., Varughese J.K., Røsland G.V., Yang N., et al. Thioridazine inhibits autophagy and sensitizes glioblastoma cells to temozolomide. Int. J. Cancer. 2018;144:1735–1745. doi: 10.1002/ijc.31912. [DOI] [PubMed] [Google Scholar]
- 100.Cheng H.-W., Liang Y.-H., Kuo Y.-L., Chuu C.-P., Lin C.-Y., Lee M.-H., Wu A.T.H., Yeh C.-T., Chen E.I.-T., Whangpeng J., et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6:e1753. doi: 10.1038/cddis.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gil-Ad I., Shtaif B., Levkovitz Y., Dayag M., Zeldich E., Weizman A. Characterization of Phenothiazine-Induced Apoptosis in Neuroblastoma and Glioma Cell Lines: Clinical Relevance and Possible Application for Brain-Derived Tumors. J. Mol. Neurosci. 2004;22:189–198. doi: 10.1385/JMN:22:3:189. [DOI] [PubMed] [Google Scholar]
- 102.Seo S.U., Cho H.K., Min K.-J., Woo S.M., Kim S., Park J.-W., Kim S.H., Choi Y.H., Keum Y.S., Hyun J.W., et al. Thioridazine enhances sensitivity to carboplatin in human head and neck cancer cells through downregulation of c-FLIP and Mcl-1 expression. Cell Death Dis. 2017;8:e2599. doi: 10.1038/cddis.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park M.S., Dong S.M., Kim B.-R., Seo S.H., Kang S., Lee E.-J., Lee S.-H., Rho S.B. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5:4929–4934. doi: 10.18632/oncotarget.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qian G., Dai L., Yu T. Thioridazine Sensitizes Cisplatin against Chemoresistant Human Lung and Ovary Cancer Cells. DNA Cell Biol. 2019;38:718–724. doi: 10.1089/dna.2019.4715. [DOI] [PubMed] [Google Scholar]
- 105.Rho S.B., Kim B.-R., Kang S. A gene signature-based approach identifies thioridazine as an inhibitor of phosphatidylinositol-3′-kinase (PI3K)/AKT pathway in ovarian cancer cells. Gynecol. Oncol. 2011;120:121–127. doi: 10.1016/j.ygyno.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 106.Byun H.-J., Lee J.H., Kim B.-R., Kang S., Dong S.M., Park M.S., Lee S.-H., Park S.H., Rho S.B. Anti-angiogenic effects of thioridazine involving the FAK-mTOR pathway. Microvasc. Res. 2012;84:227–234. doi: 10.1016/j.mvr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Yong M., Yu T., Tian S., Liu S., Xu J., Hu J., Hu L. DR2 blocker thioridazine: A promising drug for ovarian cancer therapy. Oncol. Lett. 2017;14:8171–8177. doi: 10.3892/ol.2017.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song Y., Li L., Chen J., Chen H., Cui B., Feng Y., Zhang P., Zhang Q., Xia Y., Luo M. Thioridazine hydrochloride: An antipsychotic agent that inhibits tumor growth and lung metastasis in triple-negative breast cancer via inducing G0/G1 arrest and apoptosis. Cell Cycle. 2020;19:3521–3533. doi: 10.1080/15384101.2020.1850969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tegowski M., Fan C., Baldwin A.S. Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G1 arrest independent of DRD2. J. Biol. Chem. 2018;293:15977–15990. doi: 10.1074/jbc.RA118.003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin T., He S., Shen G., Ye T., Guo F., Wang Y. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol. Med. Rep. 2015;12:4103–4108. doi: 10.3892/mmr.2015.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gong L., Wang Y., Tong S., Liu L., Niu L., Yuan Y., Bao Y. Mechanism of Killing Effect of Thioridazine on Human Lung Cancer PC9 Cells. Chin. J. Lung Cancer. 2015;18:727–733. doi: 10.3779/j.issn.1009-3419.2015.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang S., Dong S.M., Kim B.-R., Park M.S., Trink B., Byun H.-J., Rho S.B. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis. 2012;17:989–997. doi: 10.1007/s10495-012-0717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mao M., Yu T., Hu J., Hu L. Dopamine D2 receptor blocker thioridazine induces cell death in human uterine cervical carcinoma cell line SiHa. J. Obstet. Gynaecol. Res. 2015;41:1240–1245. doi: 10.1111/jog.12691. [DOI] [PubMed] [Google Scholar]
- 114.Meng Q., Sun X., Wang J., Wang Y., Wang L. The important application of thioridazine in the endometrial cancer. Am. J. Transl. Res. 2016;8:2767–2775. [PMC free article] [PubMed] [Google Scholar]
- 115.Gil-Ad I., Shtaif B., Levkovitz Y., Nordenberg J., Taler M., Korov I., Weizman A. Phenothiazines induce apoptosis in a B16 mouse melanoma cell line and attenuate in vivo melanoma tumor growth. Oncol. Rep. 2006;15:107–112. doi: 10.3892/or.15.1.107. [DOI] [PubMed] [Google Scholar]
- 116.Jiang X., Chen Z., Shen G., Jiang Y., Wu L., Li X., Wang G., Yin T. Psychotropic agent thioridazine elicits potent in vitro and in vivo anti-melanoma effects. Biomed. Pharmacother. 2018;97:833–837. doi: 10.1016/j.biopha.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 117.Spengler G., Molnar J., Viveiros M., Amaral L. Thioridazine induces apoptosis of multidrug-resistant mouse lymphoma cells transfected with the human ABCB1 and inhibits the expression of P-glycoprotein. Anticancer. Res. 2011;31:4201–4205. [PubMed] [Google Scholar]
- 118.Nagel D., Spranger S., Vincendeau M., Grau M., Raffegerst S., Kloo B., Hlahla D., Neuenschwander M., von Kries J.P., Hadian K., et al. Pharmacologic Inhibition of MALT1 Protease by Phenothiazines as a Therapeutic Approach for the Treatment of Aggressive ABC-DLBCL. Cancer Cell. 2012;22:825–837. doi: 10.1016/j.ccr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 119.Aslostovar L., Boyd A.L., Almakadi M., Collins T.J., Leong D.P., Tirona R.G., Kim R.B., Julian J.A., Xenocostas A., Leber B., et al. A Phase 1 Trial Evaluating Thioridazine in Combination with Cytarabine in Patients with Acute Myeloid Leukemia. Blood Adv. 2018;2:1935–1945. doi: 10.1182/bloodadvances.2018015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mu J., Xu H., Yang Y., Huang W., Xiao J., Li M., Tan Z., Ding Q., Zhang L., Lü J., et al. Thioridazine, an antipsychotic drug, elicits potent antitumor effects in gastric cancer. Oncol. Rep. 2014;31:2107–2114. doi: 10.3892/or.2014.3068. [DOI] [PubMed] [Google Scholar]
- 121.Loehr A., Pierpont T., Gelsleichter E., Galang A., Fernandez I., Moore E., Guo M., Miller A., Weiss R. Targeting Cancer Stem Cells with Differentiation Agents as an Alternative to Genotoxic Chemotherapy for the Treatment of Malignant Testicular Germ Cell Tumors. Cancers. 2021;13:2045. doi: 10.3390/cancers13092045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu M., Li J., Luo Z., Zhang S., Xue S., Wang K., Shi Y., Zhang C., Chen H., Li Z. Roles of dopamine receptors and their antagonist thioridazine in hepatoma metastasis. OncoTargets Ther. 2015;8:1543–1552. doi: 10.2147/OTT.S77373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Min K.-J., Seo B.R., Bae Y.C., Yoo Y.H., Kwon T.K. Antipsychotic agent thioridazine sensitizes renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated inhibition of Akt signaling and downregulation of Mcl-1 and c-FLIP(L) Cell Death Dis. 2014;5:e1063. doi: 10.1038/cddis.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh V., Jaiswal P.K., Ghosh I., Koul H.K., Yu X., De Benedetti A. Targeting the TLK1/NEK1 DDR axis with Thioridazine suppresses outgrowth of androgen independent prostate tumors. Int. J. Cancer. 2019;145:1055–1067. doi: 10.1002/ijc.32200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shen J., Ma B., Zhang X., Sun X., Han J., Wang Y., Chu L., Xu H., Yang Y. Thioridazine has potent antitumor effects on lung cancer stem-like cells. Oncol. Lett. 2017;13:1563–1568. doi: 10.3892/ol.2017.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yue H., Huang D., Qin L., Zheng Z., Hua L., Wang G., Huang J., Huang H. Targeting Lung Cancer Stem Cells with Antipsychological Drug Thioridazine. BioMed Res. Int. 2016;2016:1–7. doi: 10.1155/2016/6709828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang C., Gong P., Liu P., Zhou N., Zhou Y., Wang Y. Thioridazine elicits potent antitumor effects in colorectal cancer stem cells. Oncol. Rep. 2016;37:1168–1174. doi: 10.3892/or.2016.5313. [DOI] [PubMed] [Google Scholar]
- 128.Sachlos E., Risueño R.M., Laronde S., Shapovalova Z., Lee J.-H., Russell J., Malig M., McNicol J.D., Fiebig-Comyn A., Graham M., et al. Identification of Drugs Including a Dopamine Receptor Antagonist that Selectively Target Cancer Stem Cells. Cell. 2012;149:1284–1297. doi: 10.1016/j.cell.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 129.Wang Y., Huang N., Li H., Liu S., Chen X., Yu S., Wu N., Bian X.-W., Shen H.-Y., Li C., et al. Promoting oligodendroglial-oriented differentiation of glioma stem cell: A repurposing of quetiapine for the treatment of malignant glioma. Oncotarget. 2017;8:37511–37524. doi: 10.18632/oncotarget.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bhat K., Saki M., Cheng F., He L., Zhang L., Ioannidis A., Nathanson D., Tsang J., Bensinger S.J., Nghiemphu P.L., et al. Dopamine Receptor Antagonists, Radiation, and Cholesterol Biosynthesis in Mouse Models of Glioblastoma. J. Natl. Cancer Inst. 2021;113:1094–1104. doi: 10.1093/jnci/djab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen V.C.-H., Chan H.-L., Hsu T.-C., Lu M.-L., Lee Y.-C., Lee Y., Siow J.Y., McIntyre R.S., Zhou A.J., Tzang B.-S., et al. New use for old drugs: The protective effect of atypical antipsychotics on hepatocellular carcinoma. Int. J. Cancer. 2018;144:2428–2439. doi: 10.1002/ijc.31980. [DOI] [PubMed] [Google Scholar]
- 132.Lee Y.-J., Chung J.-G., Tan Z.-L., Hsu F.-T., Liu Y.-C., Lin S.-S. ERK/AKT Inactivation and Apoptosis Induction Associate With Quetiapine-inhibited Cell Survival and Invasion in Hepatocellular Carcinoma Cells. Vivo. 2020;34:2407–2417. doi: 10.21873/invivo.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Navari R.M., Qin R., Ruddy K.J., Liu H., Powell S.F., Bajaj M., Dietrich L., Biggs D., Lafky J.M., Loprinzi C.L. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016;375:134–142. doi: 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Karpel-Massler G., Kast R.E., Westhoff M.-A., Dwucet A., Welscher N., Nonnenmacher L., Hlavac M., Siegelin M.D., Wirtz C.R., Debatin K.-M., et al. Olanzapine inhibits proliferation, migration and anchorage-independent growth in human glioblastoma cell lines and enhances temozolomide’s antiproliferative effect. J. Neuro-Oncol. 2014;122:21–33. doi: 10.1007/s11060-014-1688-7. [DOI] [PubMed] [Google Scholar]
- 135.Sanomachi T., Suzuki S., Kuramoto K., Takeda H., Sakaki H., Togashi K., Seino S., Yoshioka T., Okada M., Kitanaka C. Olanzapine, an Atypical Antipsychotic, Inhibits Survivin Expression and Sensitizes Cancer Cells to Chemotherapeutic Agents. Anticancer Res. 2017;37:6177–6188. doi: 10.21873/anticanres.12067. [DOI] [PubMed] [Google Scholar]
- 136.Chopko T.C., Lindsley C.W. Classics in Chemical Neuroscience: Risperidone. ACS Chem. Neurosci. 2018;9:1520–1529. doi: 10.1021/acschemneuro.8b00159. [DOI] [PubMed] [Google Scholar]
- 137.Möller H.-J. Risperidone: A review. Expert Opin. Pharmacother. 2005;6:803–818. doi: 10.1517/14656566.6.5.803. [DOI] [PubMed] [Google Scholar]
- 138.Dilly S.J., Clark A.J., Marsh A., Mitchell D.A., Cain R., Fishwick C.W., Taylor P.C. A chemical genomics approach to drug reprofiling in oncology: Antipsychotic drug risperidone as a potential adenocarcinoma treatment. Cancer Lett. 2017;393:16–21. doi: 10.1016/j.canlet.2017.01.042. [DOI] [PubMed] [Google Scholar]
- 139.Yang S.-Y., He X.-Y., Schulz H. Multiple functions of type 10 17β-hydroxysteroid dehydrogenase. Trends Endocrinol. Metab. 2005;16:167–175. doi: 10.1016/j.tem.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 140.He X., Yang X.Y.H.A.S.Y. Roles of Type 10 17beta-Hydroxysteroid Dehydrogenase in Intracrinology and Metabolism of Isoleucine and Fatty Acids. Endocr. Metab. Immune Disord.-Drug Targets. 2006;6:95–102. doi: 10.2174/187153006776056639. [DOI] [PubMed] [Google Scholar]
- 141.Wang J.-S., Zhu H.-J., Markowitz J.S., Donovan J.L., Yuan H.-J., DeVane C.L. Antipsychotic Drugs Inhibit the Function of Breast Cancer Resistance Protein. Basic Clin. Pharmacol. Toxicol. 2008;103:336–341. doi: 10.1111/j.1742-7843.2008.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gündoğdu U.D., Çoban F., Liman R., Şelli M.E. Investigation of Risperidone’s anti-tumor activity on MCF-7 breast cancer cells. Ann. Clin. Anal. Med. 2021;12:1267–1271. doi: 10.4328/acam.20724. [DOI] [Google Scholar]
- 143.Persad R., Liu C., Wu T.-T., Houlihan P.S., Hamilton S.R., Diehl A.M., Rashid A. Overexpression of Caspase-3 in Hepatocellular Carcinomas. Mod. Pathol. 2004;17:861–867. doi: 10.1038/modpathol.3800146. [DOI] [PubMed] [Google Scholar]
- 144.Prommer E. Aripiprazole. Am. J. Hosp. Palliat. Med. 2015;34:180–185. doi: 10.1177/1049909115612800. [DOI] [PubMed] [Google Scholar]
- 145.Badran A., Tul-Wahab A., Zafar H., Mohammad N., Imad R., Khan M.A., Baydoun E., Choudhary M.I. Antipsychotics drug aripiprazole as a lead against breast cancer cell line (MCF-7) in vitro. PLoS ONE. 2020;15:e0235676. doi: 10.1371/journal.pone.0235676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee H., Kang S., Sonn J.K., Lim Y. Dopamine receptor D2 activation suppresses the radiosensitizing effect of aripiprazole via activation of AMPK. FEBS Open Bio. 2019;9:1580–1588. doi: 10.1002/2211-5463.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Suzuki S., Okada M., Kuramoto K., Takeda H., Sakaki H., Watarai H., Sanomachi T., Seino S., Yoshioka T., Kitanaka C. Aripiprazole, an Antipsychotic and Partial Dopamine Agonist, Inhibits Cancer Stem Cells and Reverses Chemoresistance. Anticancer Res. 2016;36:5153–5162. doi: 10.21873/anticanres.11085. [DOI] [PubMed] [Google Scholar]
- 148.Kim J.Y., Tae I.H., Lee B.-M., Kim H.S., Yoon S. Low Doses of the Anti-psychotic Drug Aripiprazole Have Strong P-gp-inhibitory Activity and Sensitize Anti-mitotic Drug-resistant Cancer Cells. Anticancer Res. 2018;38:5101–5108. doi: 10.21873/anticanres.12830. [DOI] [PubMed] [Google Scholar]
- 149.Jiang C., Lee S.H., Park J.H., Lee J.-S., Park J.W., Kim J.R., Lee S.H., Kim H.S., Yoon S. A Low Dose of Aripiprazole Has the Strongest Sensitization Effect among 19 Repositioned Bipolar Drugs in P-gp-overexpressing Drug-resistant Cancer Cells. Anticancer Res. 2021;41:687–697. doi: 10.21873/anticanres.14820. [DOI] [PubMed] [Google Scholar]
- 150.Kim M.S., Yoo B.C., Yang W.S., Han S.Y., Jeong D., Song J.M., Kim K.-H., Aravinthan A., Kim J.H., Kim J.-H., et al. Src is the primary target of aripiprazole, an atypical antipsychotic drug, in its anti-tumor action. Oncotarget. 2017;9:5979–5992. doi: 10.18632/oncotarget.23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmid C.L., Streicher J.M., Meltzer H.Y., Bohn L.M. Clozapine Acts as an Agonist at Serotonin 2A Receptors to Counter MK-801-Induced Behaviors through a βArrestin2-Independent Activation of Akt. Neuropsychopharmacology. 2014;39:1902–1913. doi: 10.1038/npp.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yin Y.-C., Lin C.-C., Chen T.-T., Chen J.-Y., Tsai H.-J., Wang C.-Y., Chen S.-Y. Clozapine Induces Autophagic Cell Death in Non-Small Cell Lung Cancer Cells. Cell. Physiol. Biochem. 2015;35:945–956. doi: 10.1159/000369751. [DOI] [PubMed] [Google Scholar]
- 153.Massari N.A., Medina V.A., Cricco G.P., Lamas D.J.M., Sambuco L., Pagotto R., Ventura C., Ciraolo P.J., Pignataro O., Bergoc R.M., et al. Antitumor activity of histamine and clozapine in a mouse experimental model of human melanoma. J. Dermatol. Sci. 2013;72:252–262. doi: 10.1016/j.jdermsci.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 154.Shin S.Y., Choi B.H., Ko J., Kim S.H., Kim Y.S., Lee Y.H. Clozapine, a neuroleptic agent, inhibits Akt by counteracting Ca2+/calmodulin in PTEN-negative U-87MG human glioblastoma cells. Cell. Signal. 2006;18:1876–1886. doi: 10.1016/j.cellsig.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 155.Geyer M.A. Gerhard Gross. Handbook of Experimental Pharmacology. Vol. 213, Novel Antischizophrenia Treatments. Springer; Heidelberg, Germany: 2012. [Google Scholar]
- 156.Torrisi S.A., Salomone S., Geraci F., Caraci F., Bucolo C., Drago F., Leggio G.M. Buspirone Counteracts MK-801-Induced Schizophrenia-Like Phenotypes through Dopamine D3 Receptor Blockade. Front. Pharmacol. 2017;8:710. doi: 10.3389/fphar.2017.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y., Yang X., Song X., Zhao L., Wei J., Wang J., Tian H., Zheng C., Wei M., Wang Q., et al. Co-treatment of buspirone with atypical antipsychotic drugs (AAPDs) improved neurocognitive function in chronic schizophrenia. Schizophr. Res. 2019;209:135–140. doi: 10.1016/j.schres.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 158.Pendergrass J.C., Targum S.D., Harrison J.E. Cognitive Impairment Associated with Cancer: A Brief Review. Innov. Clin. Neurosci. 2018;15:36–44. [PMC free article] [PubMed] [Google Scholar]
- 159.Hussein N., Amawi H., Karthikeyan C., Hall F.S., Mittal R., Trivedi P., Ashby C.R., Tiwari A.K. The dopamine D 3 receptor antagonists PG01037, NGB2904, SB277011A, and U99194 reverse ABCG2 transporter-mediated drug resistance in cancer cell lines. Cancer Lett. 2017;396:167–180. doi: 10.1016/j.canlet.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 160.Edinoff A., Ruoff M.T., Ghaffar Y.T., Rezayev A., Jani D., Kaye A.M., Cornett E.M., Kaye A.D., Viswanath O., Urits I. Cariprazine to Treat Schizophrenia and Bipolar Disorder in Adults. Psychopharmacol. Bull. 2020;50:83–117. doi: 10.64719/pb.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hussein N., Ashby J.C.R., Amawi H., Nyinawabera A., Vij A., Khare V.M., Karthikeyan C., Tiwari A.K. Cariprazine, A. Dopamine D2/D3 Receptor Partial Agonist, Modulates ABCG2-Mediated Multidrug Resistance in Cancer. Cancers. 2018;10:308. doi: 10.3390/cancers10090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Neifeld J.P., Tormey D.C., A Baker M., Meyskens F.L., Taub R.N. Phase II trial of the dopaminergic inhibitor pimozide in previously treated melanoma patients. Cancer Treat. Rep. 1983;67:155–157. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.