Abstract
The majority of lung cancers are non-small-cell lung cancer (NSCLC) having a low survival rate. Recent studies have indicated the involvement of epidermal growth factor receptor (EGFR) oncogene mutations like EGFR exon 20 insertions (EGFRex20ins) mutation among NSCLC patients. The response of patients of NSCLC with the EGFRex20ins mutation to the currently available EGFR inhibitor is negligible. Mobocertinib is the first oral treatment that has been approved by the USFDA, on 15 September 2021, to treat NSCLC with the EGFRex20ins mutation. This patent review discusses the inventions and patent literature of mobocertinib that will help the scientific community to develop additional and improved inventions related to mobocertinib. The structure of mobocertinib was first reported in 2015. Therefore, this article covered the patents/patent applications related to mobocertinib from 2015 to 25 October 2021. The patent search revealed 27 patents/patent applications related to compound, method of treatment, salt, polymorph, process, composition, and drug combinations of mobocertinib. The authors foresee an exciting prospect for developing a treatment for NSCLC with EGFRex20ins mutation, and other cancers employing a combination of mobocertinib with other approved anticancer agents. The inventions related to novel dosage forms, processes, and intermediates used in the synthesis of mobocertinib are also anticipated.
Keywords: mobocertinib, TAK-788, EGFR mutation, NSCLC, inventions, patent
1. Introduction
Lung cancer is the second most common form of cancer among different kinds of cancer (11.4%) and is one of the major causes of mortality worldwide [1]. The usual risk factors [2], symptoms [3], screening tests [4], and the diagnostic tests [5] are well documented for this disease. Small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) are two major types of lung cancers. The NSCLC is the most frequent type of lung cancer, accounting for about 85% of all lung cancers, whereas SCLC accounts for about 15% of all lung cancers [6,7]. The low survival rate for SCLC (about 6%) and NSCLC (about 24%) is because these diseases are not detected at early stages. The treatment of NSCLC includes surgery, radiofrequency ablation, radiation therapy, chemotherapy, immunotherapy, palliative care, and targeted drug therapy [6].
Epidermal growth factor receptor (EGFR) is a member of the type 1 tyrosine kinase family. It plays a significant role in the growth, survival, and differentiation of cells. The overexpression of EGFR is implicated in the pathogenesis of human cancers, including NSCLC [8]. Many EGFR inhibitors are in clinical practice to treat NSCLC, for example, erlotinib [9], gefitinib [10], afatinib [11,12], dacomitinib [13], and osimertinib [14]. However, many types of mutations, including the EGFR exon 20 insertions (EGFRex20ins) mutation, have been reported in EGFR [15]. These mutations cause conformational changes in EGFR, reduce access of the EGFR inhibitors to their binding pockets, and lead to the development of therapeutic resistance [16,17]. The EGFRex20ins mutations are found in about 12% of NSCLC patients and have approximately 50 variants [18,19,20]. The NSCLC patients with EGFRex20ins mutations have a brief survival time as compared with NSCLC patients with other mutations because such cases are insensitive to EGFR inhibitors. Therefore, the NSCLC cases with EGFRex20ins mutations are considered an unmet medical need [20]. Amivantamab (Rybrevant), a monoclonal antibody, is one of the options to treat NSCLC patients with EGFRex20ins mutations. However, this injectable drug is not equally efficacious in patients suffering from this disease [21]. Recently, USFDA has approved mobocertinib (Exkivity) as the first oral small molecule to treat NSCLC patients with EGFRex20ins mutations [22,23]. Pre-clinical and some clinical studies of mobocertinib have concisely been reviewed [20]. Herein, we provided a comprehensive review of the inventions and patents related to mobocertinib. This review will be useful to the scientists of the pharmaceutical industry/academia working on the development of mobocertinib inventions.
2. Mobocertinib
Mobocertinib (Other names: AP-32788, TAK 788, TAK-788, TAK788; CAS registry number: 1847461-43-1; Molecular Formula: C32H39N7O4; Molecular weight: 585.70), chemically, is 5-Pyrimidinecarboxylic acid, 2-[[4-[[2-(dimethylamino)ethyl]methylamino]-2-methoxy-5-[(1-oxo-2-propen-1-yl)amino]phenyl]amino]-4-(1-methyl-1H-indol-3-yl)-,1-methylethyl ester (Figure 1) [24,25,26,27]. The chemical structure of this non-chiral molecule was first disclosed in 2015 [28]. The trade name of mobocertinib is Exkivity®, which is marketed as a capsule dosage form and contains mobocertinib succinate (CAS registry number: 2389149-74-8; Molecular Formula: C32H39N7O4.C4H6O4; Molecular weight: 703.8) as an active ingredient [25,29,30]. This immediate-release capsule of mobocertinib for oral administration contains 40 mg mobocertinib (free base, equivalent to 48.06 mg mobocertinib succinate) without excipients. The mobocertinib monosuccinate salt has high solubility across the physiologic pH range of pH 1.0 to pH 6.8. The highest proposed commercial dose (160 mg) of mobocertinib is soluble in 250 mL of aqueous media over the pH range of 1 to 6.8 and demonstrates a high permeability. Therefore, it exhibits the characteristics of a BCS Class 1 compound at the intended clinical doses [31].
Figure 1.
Chemical structure of mobocertinib.
Mobocertinib, discovered by Ariad Pharmaceuticals and developed by Takeda Pharmaceuticals, is an irreversible tyrosine kinase inhibitor (TKI) that forms a covalent bond with cysteine 797 in EGFR, leading to the inhibition of EGFR signaling, and stops EGFR exon 20 insertion mutations. It was approved by the USFDA, on 15 September 2021, to treat NSCLC with epidermal growth factor receptor (EGFR) exon 20 exon insertion mutations [24]. This is the first approval of an oral targeted therapy for patients with EGFR exon 20 insertion mutation-positive NSCLC. The route of administration, safety profile, objective response rate (ORR), and durable responses observed with mobocertinib provide a meaningful advantage over available treatments for this disease [32].
The mobocertinib development timeline and the summary of the Rx data/pharmacology of mobocertinib are provided in Scheme 1 and Table 1, respectively.
Scheme 1.
The development timeline for mobocertinib.
Table 1.
Rx data of mobocertinib.
| Parameter | Summary |
|---|---|
| Drug data | Proprietary name: Exkivity; Active ingredient: Mobocertinib succinate; Dosage Form: Capsule; Route: Oral; Strength: 48.06 mg of mobocertinib succinate (Equivalent to 40 mg of mobocertinib base); Applicant: Takeda Pharmaceuticals [33] |
| Application data | Application number: N215310; Review type: Priority review; Orphan status: Orphan drug; Approval date: 15 September 2021; Marketing Status: Prescription [33] |
| Exclusivity data | Exclusivity type (Expiry date): New chemical entity (September 15, 2026) [33] |
| Indication (Use Code) |
NSCLC with EGFR exon 20 exon insertion mutations whose disease has progressed on or after platinum-based chemotherapy (U-3220) [30,33] |
| Recommended Dose | 160 mg/day with/without food till disease progression/unacceptable toxicity. The dose can be reduced based on the adverse reactions [29,30,34,35] |
| Warning/Precautions | Exkivity may lead to QTc prolongation and/or torsades de pointes. If this happens, reduce the dose or discontinue Exkivity based on the severity of QTc prolongation. Discontinue Exkivity if the patient shows induction of interstitial lung disease/pneumonitis, cardiac toxicity, diarrhea, and embryo-fetal toxicity. Additionally, avoid concomitant use of CYP3A inhibitors with Exkivity that can prolong QTc [29,30] |
| Adverse effects | Diarrhoea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, musculoskeletal pain, increased amylase/lipase/creatinine, and decreased potassium/hemoglobin/magnesium/lymphocytes [29,30,34] |
| Contraindication | No data is available [29,30] |
| Drug interaction | Avoid concomitant use of CYP3A inhibitors as well as inducers like itraconazole and rifampin [24,30] |
| Absorption | Tmax = 4 h; Absolute bioavailability = 37%; Cmax 45.8 ng/mL; AUC0-inf = 862 ngh/mL [34] |
| Volume of distribution | 3509 L (38%) at steady-state [29,30] |
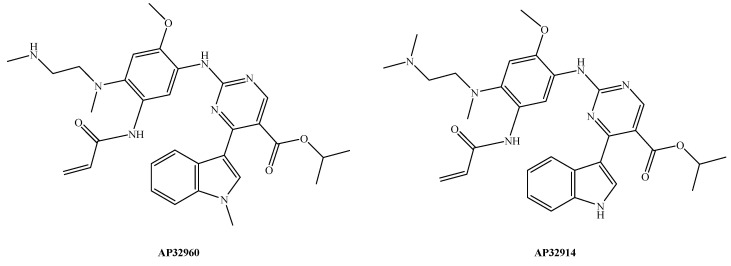
| Metabolism | Mobocertinib is metabolized by CYP3A via N-demethylation to produce two equipotent metabolites, namely, AP32960 (Figure 2) and AP32914 (Figure 2) [24] |
| Protein binding | Mobocertinib = 99.3%; AP32960 = 99.5%; AP32914 = 98.6% [25,29,30] |
| Route of elimination | Mobocertinib (Faeces, 76%; Urine, 4%), AP32960 (Faeces, 12%; Urine, 1%), AP32914 (Undetected) [29,30] |
| Half-life | Mobocertinib = 18 h; AP32960 = 24 h; AP32914 = 18 h [29,30] |
| Clearance | Mobocertinib = 138 L/h; AP32960 = 149 L/h; AP32914 = 159 L/h [29,30] |
| Toxicity | Cardiac toxicity; Ocular toxicity; embryo-fetal toxicity [29,30] Grade 3 or 4 abnormality: Decreased red blood cells (3.5%); Decreased lymphocytes (15%); Decreased platelets (0.9); Decreased leukocytes (0%); Increased creatinine (2.7%); Increased amylase (13%); Increased lipase (10%); Decreased potassium (5.3%); Increased alkaline phosphatase (1.8%); Decreased albumin (1.8%); Decreased magnesium (2.7%); Increased alanine aminotransferase (2.7%); Increased aspartate aminotransferase (1.8%); Decreased sodium (0.9%) [29,30] No data about overdose are available [29,30] |
Clinical Trials on Mobocertinib
The clinical trial studies on mobocertinib were searched on the clinicaltrials.gov database [36]. The search revealed eight clinical studies on mobocertinib. The summary of these clinical trials is provided in Table 2.
Table 2.
Clinical trials on mobocertinib.
| Title of the Study | Condition (Study Type; Phase; NCT Number; Number Enrolled) |
Status (Study Start Date (SSD); Study Completion Date (SCD); Last Update Date (LUD)) |
Sponsor/Collaborators (Funder Type; Location) |
|---|---|---|---|
| An expanded access protocol for mobocertinib in refractory NSCLC participants with EGFR Exon20 insertion mutations | NSCLC (Expanded access; Marketed; NCT04535557; Not mentioned) |
Approved for marketing (SSD: Not mentioned; SCD: Not mentioned; LUD: 27 September 2021) |
Millennium Pharmaceuticals/Takeda (Industry; USA) |
| A study of TAK-788 in adults with NSCLC in combination with pemetrexed/carboplatin | NSCLC (Interventional; Phase 1 and 2; NCT02716116; 395) |
Recruiting (SSD: 16 June 2016; SCD: 13 March 2023; LUD: 25 May 2021) |
Millennium Pharmaceuticals/Takeda (Industry; USA) |
| A study to evaluate the impact of management strategies on gastrointestinal-related adverse events in participants with NSCLC harboring EGFR Exon20 insertion mutations receiving TAK-788 | NSCLC (Interventional; Phase 2; NCT04576208; zero) |
Withdrawn (SSD: 30 November 2020; SCD: 31 July 2022; LUD: 17 December 2020) |
Takeda (Industry; not mentioned) |
| A study to evaluate drug-drug interaction of TAK-788 with itraconazole and rifampin in healthy adult participants | Healthy volunteers (Interventional; Phase 1; NCT03928327; 24) |
Completed (SSD: 2 May 2019; SCD: 16 August 2019; LUD: 21 August 2020) |
Millennium Pharmaceuticals/Takeda (Industry; USA) |
| A study to evaluate the effect of high-fat meals on TAK-788 pharmacokinetics in healthy adult participants | Healthy volunteers (Interventional; Phase 1; NCT04441255; 14) |
Completed (SSD: 1 July 2020; SCD: 10 August 2020; LUD: 5 September 2021) |
Takeda (Industry; USA) |
| A study of mobocertinib capsules in people with severe kidney problems and people with healthy kidneys | Renal impairment (Interventional; Phase 1; NCT04056455; 24) |
Recruiting (SSD: 10 March 2020; SCD: 31 August 2022; LUD: 13 July 2021) |
Millennium Pharmaceuticals/Takeda (Industry; USA) |
| A study to evaluate pharmacokinetics and safety of oral mobocertinib in participants with moderate or severe hepatic impairment and normal hepatic function | Hepatic impairment (Interventional; Phase 1; NCT04056468; 24) |
Recruiting (SSD: 23 August 2019; SCD: 31 December 2021; LUD: 16 June 2021) |
Millennium Pharmaceuticals/Takeda (Industry; USA) |
| A study to assess absolute bioavailability of mobocertinib and to characterize mass balance, pharmacokinetics, metabolism, and excretion of carbon-14 ([14C])-mobocertinib in healthy male participants | Healthy Volunteers (Interventional; Phase 1; NCT03811834; 7) |
Completed (SSD: 22 January 2019; SCD: 11 March 2019; LUD: 30 July 2021) |
Millennium Pharmaceuticals/Takeda (Industry; USA) |
3. Patent Searching
Searching was performed on 25 October 2021, utilizing different patent databases (Sci-finder, Espacenet, WIPO, and USPTO). The patent searching strategy is provided in Scheme 2. The patents/applications that did not cover mobocertinib (chemical structure or keywords) explicitly or implicitly in their claim section were excluded from the final list. The bibliographic data of the patents/patent applications which are cited in the text are provided in Table 3.
Scheme 2.
Patent searching methodology.
Table 3.
Bibliography of the cited patents.
| Patent/Patent Application Number (Assignee; Publication Date; Earliest Priority Date; Estimated Expiry Date) |
Legal Status on 25 October 2021 (International Patent Classification) |
Family Members |
|---|---|---|
|
US9796712B2 (Ariad Pharma; 24 October 2017; 19 June 2014; 13 May 2035) |
Patented case (C07D401/14, C07D403/04, C07D405/12, C07D471/04, C07F9/6558, A61K31/506, C07D403/14) |
AU2015277786B2, AU2019206024A1, BR112016029662A2, CA2949793A1, CL2016003222A1, CL2017003103A1, CN106559991B, CN110526912A, CO2017000386A2, CR20170011A, CU20160185A7, CY1121359T1, DK3157916T3, EA034691B1, ECSP17003553A, EP3157916B1, EP3409669B1, EP3778584A1, ES2715500T3, GEP20207111B, GEP20197011B, HRP20190407T1, HUE042390T2, IL248859A, JP6230205B2, JP6546630B2, KR20170016861A, LT3157916T, MA40240B1, ME03334B, MX361802B, PE20170268A1, PH12016502453A1, PL3157916T3, RS58541B1, SG10201913753VA, SG11201610517PA, SI3157916T1, TN2016000560A1, TR201903322T4, TR201903322T4, UA121657C2, WO2015195228A1, ZA201608224B |
|
US10227342B2 (Ariad Pharma; 12 March 2019; 19 June 2014; 13 May 2035) |
Patented case (C07D401/14, C07D403/04, C07D405/12, C07D471/04, C07F9/6558, A61K31/506, C07D403/14) |
|
|
US2019218212A1 (Ariad Pharma; 18 July 2019; 19 June 2014; 13 May 2035) |
Abandoned on 28 April 2020 The child continuity data state that application number 17/463,457 has been filed on 31 August 2021, which may be published soon. (C07D401/04, C07D401/14, C07D403/04, C07D403/14, C07D405/12, C07D405/14, C07D471/04, C07F9/6558) |
|
|
US2021309640A1 (Ariad Pharma; 7 October 2021; 14 May 2018; 13 May 2039) |
Ready for Examination (A61P35/00, C07D403/04) |
AU2019269372A1, BR112020023107A2, CA3099737A1, CN112313223A, EP3793993A1, JP2021523208A, KR20200144579A, TW202015687A, WO2019222093A1 |
|
CN110776495A (LUO YUN; 11 February 2020; 30 July 2018; 30 July 2038) |
Under examination (A61K31/506, A61P35/00, C07D403/04) |
None |
|
CN111643503A (Chia Tai Tianqing Pharmaceutical Group; 11 September 2020; 4 March 2019; 4 March 2039) |
Publication (A61K31/4709, A61K45/06, A61P35/00) |
None |
|
CN111840289A (Chia Tai Tianqing Pharmaceutical Group; 30 October 2020; 28 April 2019; 28 April 2039) |
Publication (A61K31/4709, A61K31/675, A61K31/704, A61K31/7048, A61K33/243, A61P29/00, A61P35/00) |
None |
|
CN111956649A (Chia Tai Tianqing Pharmaceutical Group; 20 November 2020; 20 May 2019; 20 May 2039) |
Publication (A61K31/475, A61K45/06, A61P11/00) |
None |
|
CN111973747A (Chia Tai Tianqing Pharmaceutical Group; 24 November 2020; 23 May 2019; 23 May 2039) |
Publication (A61K31/4709, A61K45/06, A61P15/08, A61P35/00) |
None |
|
CN112043702A (Chia Tai Tianqing Pharmaceutical Group; 8 December 2020; 5 June 2019; 5 June 2040) |
Publication (A61K31/4709, A61K45/06, A61P35/00) |
None |
|
CN112121048A (Chia Tai Tianqing Pharmaceutical Group; 25 December 2020; 6 June 2019; 8 June 2040) |
Publication (A61K31/337, A61K31/4709, A61K31/7048, A61K33/24, A61P35/00) |
None |
|
CN112294814A (Chia Tai Tianqing Pharmaceutical Group; 2 February 2021; 31 July 2019; 31 July 2040) |
Publication (A61K31/4188, A61K31/4709, A61K45/06, A61P35/00) |
None |
|
WO2020211860A1 (Chia Tai Tianqing Pharmaceutical Group; 22 October 2020; 19 April 2019; 19 April 2040) |
No national phase entry (A61K31/4709, A61K45/06, A61P35/00, C07D401/12) |
None |
|
WO2020228656A1 (Chia Tai Tianqing Pharmaceutical Group; 19 November 2020; 10 May 2019; 11 May 2040) |
No national phase entry (A61K31/4709, A61K31/704, A61P35/00) |
None |
|
WO2020233602A1 (Chia Tai Tianqing Pharmaceutical Group; 26 November 2020; 20 May 2019; 20 May 2040) |
No national phase entry (A61K31/4709, A61P35/00, A61P35/04) |
None |
|
US2019091229A1 (LAM Therapeutics; 28 March 2019; 27 September 2017; 27 September 2038) |
Abandoned on April 15, 2020 (A61K31/095, A61K31/337, A61K31/357, A61K31/4184, A61K31/4709, A61K31/4725, A61K31/506, A61K31/52, A61K31/5377, A61K31/553, A61K9/00, A61P35/00) |
AU2018341571A1, BR112020006009A2, CA3076915A1, CN111372588A, EP3687542A1, JP2020535173A, KR20200077518A, TW201919634A, US2020253979A1, WO2019067666A1 |
|
US2021196719A1 (Jiangsu Hengrui Medicine Company Limited; 1 July 2021; 23 May 2018; 22 May 2039) |
Ready for Examination (A61K31/506, A61K31/519, A61K31/5377, A61P35/00) |
CA3100867A1, CN111818925A, EP3811946A1, JP2021525244A, KR20210013155A, TW202002964A, WO2019223716A1 |
|
WO2020052575A1 (Jiangsu Hengrui Medicine Company Limited; 19 March 2020; 12 September 2018; 11 September 2039) |
National phase entry in China and Taiwan (A61K31/519, A61K31/675, A61P35/00) |
CN112533606A, TW202024067A |
|
WO2020177678A1 (Jiangsu Hengrui Medicine Company Limited; 10 September 2020; 4 March 2019; 3 March 2040) |
National phase entry in China and Taiwan (A61K31/404, A61P35/00, C07D403/04, C07D519/00) |
CN113301895A, TW202100150A |
|
CN111760024A (China Pharmaceutical University; 13 October 2020; 24 July 2020; 24 July 2040) |
Request for examination filed (A61K31/282, A61K31/5365, A61K33/243, A61K39/395, A61K41/00, A61K47/52, A61K47/62, A61K49/04, A61K49/08, A61K49/14, A61K49/18, A61K49/22, A61P35/00, B22F9/24, B82Y30/00, B82Y40/00) |
None |
|
WO2019178267A2 (University Of Iowa Research Foundation; 19 September 2019; 13 March 2018; 13 March 2039) |
National phase entry in USA (C12N5/07, C12N5/0789) |
US2021255170A1 |
|
WO2021163064A2 (Jounce Therapeutics; 19 August 2021; 14 February 2020; 9 February 2041) |
National phase entry in USA (A61P35/00, C07K16/28) |
US2021277129A1 |
|
WO2021183474A1 (Tract Pharmaceuticals; 16 September 2021; 9 March 2020; 9 March 2041) |
No national phase entry (A61K39/00, A61K39/395, A61P1/00, A61P1/04, A61P19/02, A61P25/00) |
None |
|
WO2019113345A1 (Mousses Spyro; 13 June 2019; 6 December 2017; 6 December 2038) |
No national phase entry (A61K31/4745, A61P35/00) |
None |
|
WO2021000912A1 (Wigen Biomedicine Technology Company Limited; 7 January 2021; 4 July 2019; 2 July 2040) |
National phase entry in China and USA (A61K31/506, A61P35/00, A61P35/02, C07D471/06) |
CN112174961A, US2021230161A1 |
|
WO2021033153A1 (Otsuka Pharmaceuticals; 25 February 2021; 20 August 2019; 20 August 2040) |
National phase entry in Taiwan (A61K31/4985, A61P35/00, C07D487/04) |
TW202124383A |
|
WO2021149817A1 (Otsuka Pharmaceuticals; 29 July 2021; 24 January 2020; 22 January 2041) |
No national phase entry (A61K31/506, A61K31/519, A61K39/395, A61K45/00, A61P35/00) |
None |
4. Patent Analysis
4.1. Compound Patent
US9796712B2 is related to heteroaryl compounds that selectively inhibit mutant EGFR proteins like EGFR having one or more mutations in the exon 20 domain. This patent specifically claims mobocertinib, its salts, and a pharmaceutical composition comprising them. The specification of this patent also discloses the chemical structures of the metabolites of mobocertinib (AP32960 and AP32914). Example 10 of US9796712B2 provides the process to prepare mobocertinib (Scheme 3) [37]. Briefly, the intermediate Methyl 2-chloro-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate (G1) was synthesized by stirring an equimolar solution of methyl 2,4-dichloropyrimidine-5-carboxylate in dichloroethane (previously treated with aluminum chloride, 20 mmol) with 1-methyl-indole at 55 °C for 90 min. The mixture was cooled to 0 °C, methanol (5 mL) and water (10 mL) were added, and stirring was further continued at ambient temperature for 30 more min. The obtained mixture was separated into layers by the addition of water and then extracted with DCM (4 × 30 mL). The combined organic layers were dried over anhydrous magnesium sulfate, filtered, concentrated, and purified using flash chromatography to obtain pure yellow crystals of G1. 4.5 mmol of G1 was heated at 100 °C for 48 h with 4-fluoro-2-methoxy-5-nitroaniline and p-toluenesulfonic acid in dioxane. The cooled and concentrated product upon flash chromatography yielded intermediate K3 (methyl 2-((4-fluoro-2-methoxy-5-nitrophenyl) amino)-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate) as a brown residue. A mixture of K3 (0.8 mmol) and a tertiary amine (N,N,N′-trimethylethylenediamine; 0.88 mmol) in acetonitrile in presence of K2CO3 (2.40 mmol) was stirred for 90 min at 80 °C. The resulting mixture was cooled and filtered through a pad of celite, rinsed with EtOAc, and then purified by flash chromatography to obtain M4 (methyl 2-((4-((2-(dimethylamino) ethyl) (methyl) amino)-2-methoxy-5-nitrophenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate). The mixture of M4 (0.21 mmol) in isopropyl alcohol (3 mL) and 0.26 mmol of sodium hydride was refluxed for 5 min and then cooled to obtain a solid red compound, isopropyl 2-((4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxy-5-nitrophenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate (Q2). The nitrobenzene group of Q2 was further reduced to aniline derivative by stirring its acetone solution with zinc powder (0.56 mmol) and saturated aqueous ammonium chloride (1.4 mmol) at room temperature for 30 min. The mixture was filtered through a pad of celite and concentrated to obtain isopropyl 2-((5-amino-4-((2-(dimethylamino) ethyl) (methyl) amino)-2-methoxyphenyl) amino)-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate (R13) as a solid product. The desired mobocertinib (isopropyl 2-((5-acrylamido-4-((2-dimethylamino) ethyl) (methyl) amino)-2-methoxyphenyl) amino)-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate) was synthesized by reacting R13 (0.14 mmol) in DCM with 1-ethyl-3 (3-dimethylpropylamine) carbodiimide (EDCI; 0.28 mmol), Hunig’s base (0.42 mmol), and acrylic acid (0.28 mmol). The resulting mixture was concentrated in vacuo and purified by preparative TLC (5% MeOH/DCM).
Scheme 3.
Process of preparing mobocertinib as disclosed in US9796712B2 [37].
The biological example records of this patent report the IC50 data of mobocertinib (IC50 ≤ 100 µM) against EGFR exon 20 ASV and NPG insertion; EGFR exon 19 deletion and T790M mutation; EGFR exon 21 L858R and T790M mutation; and Her2 exon 20 YVMA insertion employing mutated Ba/F3 and murine pro-B cell lines. The claimed compounds of US9796712B2 [37], including mobocertinib, were declared to be effective against diseases linked with mutant EGFR activity. The compounds prepared according to examples 9, 17, 30, 34, 35, 36, 56, 66, 67, and 77 of US9796712B2 [37] displayed equipotency (IC50 ≤ 100 µM) with mobocertinib. The analysis of these structures revealed the possible basic pharmacophoric structure of mobocertinib (Figure 3), wherein the indole ring can be replaced with 1H-pyrrolo[2,3-b]pyridine ring [37].
Figure 3.
Possible basic pharmacophoric structure of mobocertinib.
US10227342B2 [38], a family member of US9796712B2 [37], also claims mobocertinib and its pharmaceutical compositions. It also claims a method to treat NSCLC linked with one or more insertion or deletion mutations in the exon 20 domain of EGFR/HER2 using an effective amount of mobocertinib, wherein the NSCLC is resistant to other EGFR/HER2 inhibitors. US2019218212A1 is also a family member of US9796712B2 [37] and US10227342B2 [38]. It claims a process for preparing mobocertinib [39].
4.2. Salt Patent Application
US2021309640A1 discloses salts of mobocertinib (succinate, sulfate, mesylate, oxalate, hydrochloride, tosylate, hippurate, hydrobromide, and fumarate) [40]. It also discloses a process for preparing mobocertinib (Scheme 4), wherein the polymorphic Form I of mobocertinib was obtained after the recrystallization/purification of mobocertinib from ethyl acetate (EtOAc) as well as from dichloromethane (DCM). They prepared mobocertinib in six steps via five intermediates. In step 1, isopropyl 2,4-dichloropyrimidine-5-carboxylate (42.5 mmol) in DME was reacted with 1-methyl indole (44.9 mmol) in the presence of AlCl3 to obtain isopropyl 2-chloro-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate (intermediate 1) as a pure yellow solid in 77% yield. Intermediate 1 (258 mmol) was heated with 4-fluoro-2-methoxy-5 nitroaniline (306 mmol) and PTSA monohydrate (70 mmol) in acetonitrile under nitrogen for 19 h which, on extraction with EtOAc and usual work up, furnished isopropyl 2-((4-fluoro-2-methoxy-5-nitrophenyl) amino)-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate (intermediate 2) in 98% yield. In step 3, the 4-fluoro substituent on phenyl ring was replaced with dimethylamino) ethyl (methyl) amino group. Reaction of one equivalent of intermediate 2 and two equivalents of N,N,N-trimethylethylenediamine in acetonitrile produced dimethylamino)ethyl(methyl)amino)-2-methoxy-5-nitrophenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate (int 3). The 5-nitro phenyl group of intermediate 3 was reduced to aniline derivatives by carrying out hydrogenation with 10% Pd/C. A yellow solid in 91% yield was obtained as intermediate 4 (isopropyl 2-((5-amino-4-((2-(dimethylamino) ethyl) (methyl)-amino)-2-methoxyphenyl) amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate). In step five, a reaction mixture comprising of one equivalent of intermediate 4 and 3-(phenylsulfonyl) propionic acid (1.07 eq.) in anhydrous DCM was cooled to 2 °C, and stirred with one equivalent of N,N-diisopropylethylamine, or Hünig’s base, to yield intermediate 5 (isopropyl 2-((4-((2-(dimethylamino) ethyl)(methyl) amino)-2-methoxy-5-(3-(phenylsulfonyl) prop anamido) phenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate). The intermediate 5 in tetrahydrofuran (THF) was then reacted slowly with KOSi(CH3)3 in THF to obtain mobocertinib in 92% yield. It also states that polymorphic Form I of mobocertinib (free base compound) can also be obtained using acetone, acetonitrile, chloroform, dimethylformamide (DMF), dimethylsulfoxide (DMSO), EtOAc, isobutyl acetate, methanol, 2-methoxyethanol, 2-MeTHF, dioxane, or methyl isobutyl ketone. US2021309640A1 specifically claims succinate salt of mobocertinib along with its crystalline forms (Form-I and Form-III), pharmaceutical compositions (capsule), and a method of treating NSCLC linked with mutant EGFR/HER2 using mobocertinib succinate [40]. The patent application reports the preparation of mobocertinib succinate by the reaction of mobocertinib with succinic acid in ethanol as well as 2-methyl THF. The preparation of Form-III is reported employing a mixture of water-saturated ethyl acetate and methanol. Example 12 (phase 1/2 results), example 13 (phase 1/2 study/dose-escalation study), example 14 (expansion and extension phases), and example 15 (clinical pharmacology and pharmacokinetics) of US2021309640A1 discuss the clinical studies on the capsule formulation of the polymorphic Form-I of mobocertinib succinate (DSC melting point: 177.5 °C; Hygroscopicity: 1.3%; Chemical stability: stable, as no change in the XRPD was observed at ambient light, 40 °C + 75% relative humidity, and at 80 °C; Water solubility: 1.9 mg/mL) on patients with advanced NSCLC refractory to standard therapy having EGFR exon 20 insertions [40]. The pharmacological data are also available in the approved label of mobocertinib and are summarized in Table 2.
Scheme 4.
Process of preparing mobocertinib disclosed in US2021309640A1 [40].
4.3. Polymorph Patent Application
CN110776495A claims crystalline form A and crystalline form B of mobocertinib having a defined X-ray powder diffraction (XRPD) pattern. It also claims the use of these crystalline forms to prepare medicaments to treat cancer [27]. Example 2 of CN110776495A provides a process for preparing crystalline form A of mobocertinib, wherein mobocertinib (2 g) was dissolved in 120 mL of isopropanol at 80 °C. The mixture was slowly cooled at room temperature, stirred for 5–15 h, filtered, and dried at 50 °C [27]. Example 3 of CN110776495A mentions the preparation of form B, wherein mobocertinib (2 g) was dissolved in 50 mL of acetone at reflux temperature. The mixture was cooled to below 10 °C, stirred, and the crystals were filtered. The crystal forms A and B of mobocertinib were found to be stable and did not show any change in their XRPD after storing at 40 °C and 75% relative humidity for one week [27]. The CN110776495A also provides a new process for preparing mobocertinib (Scheme 5) but does not claim it. Overall, the synthesis of mobocertinib was achieved in five steps; 2-(dimethylamino) ethyl)-5-methoxy-N1-methyl-2-nitrobenzene-1, 4-diamine (Compound 1) was prepared by reacting 4-fluoro-2-methoxy-5-nitrobenzene and N,N,N’-trimethylethylenediamine in an organic solvent in presence of a base such as sodium or potassium carbonate. Isopropyl 2-chloro-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate (compound 2) was synthesized separately in a Lewis acid-catalyzed reaction by condensing isopropyl 2, 4-dichloropyrimidine-5-carboxylate with N-methylindole in an organic solvent. Compounds 1 and 2 were reacted together in presence of an acid to form the isopropyl 2-((4(2 (dimethylamino) ethyl) (methyl) amino)-2-methoxy-5-nitrophenyl) amino-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate (compound 3) which was further reduced with zinc dust in acidic medium (ammonium chloride) to yield Isopropyl 2-(5-amino-4-((2-(dimethylamino) ethyl) (methyl) amino)-2-methoxyphenyl) amino-4-(1-methyl-1H-indol-3-yl) pyrimidine-5-carboxylate (compound 4). Finally, the desired compound, mobocertinib, was obtained by condensing compound 4 with acrylic acid in presence of condensing agents like Dicyclohexylcarbodiimide (DCC), Diisopropylcarbodiimide (DIC), or EDCI.
Scheme 5.
Process of preparing mobocertinib disclosed in CN110776495A [27].
4.4. Drug Combinations with Anlotinib
Anlotinib is a clinically used receptor tyrosine kinase (RTK) inhibitor to treat NSCLC [41]. Many patent applications have been identified that claim a combination of anlotinib and other anticancer agents (mobocertinib, erlotinib, afatinib, crizotinib, ceritinib, gefitinib, dacomitinib, etc.) for the treatment of NSCLC with positive gene mutation (CN111643503A) [42], giant cell tumor of bone (CN111840289A) [43], interstitial lung disease (CN111956649A) [44], ovarian cancer (CN111973747A) [45], colorectal cancer (CN112043702A) [46], esophageal cancer (CN112121048A) [47], glioblastoma (CN112294814A) [48], Ewing sarcoma (WO2020211860A1) [49], soft tissue sarcoma (WO2020228656A1) [50], and SCLC (WO2020233602A1) [51]. The beneficial effects of the claimed inventions include enhanced curative effect and reduced dose/side effects of chemotherapeutics. However, none of these patent applications exemplify the effect of the combination of anlotinib and mobocertinib against any cancer type.
4.5. Other Combinations
US2019091229A1 states a method for treating cancer (NSCLC, acute myeloid leukemia, and chronic lymphocytic leukemia) having one or more activating mutations (EGFR or HER2 exon 20 insertion mutation) using a pharmaceutical composition comprising MPC-0767 (an Hsp90 inhibitor) or its salt, alone or in combination with a protein kinase inhibitor (erlotinib, afatinib, lapatinib, dacomitinib, gefitinib, mobocertinib, poziotinib, osimertinib, and EGF816) [52].
US2021196719A1 claims a method for preventing or treating EGFR mutation tumor diseases like NSCLC using a combination of a CDK4/6 inhibitor (abemaciclib, ribociclib, palbociclib, alvocidib, trilaciclib, voruciclib, gossypin, taurosporine, etc.) and an EGFR inhibitor (mobocertinib, osimertinib, gefitinib, erlotinib, olmutinib, dacomitinib, etc.). The combination is claimed to exhibit good effects in treating NSCLC [53].
WO2020052575A1 states the use of a JAK kinase inhibitor (tofacitinib, itacitinib, peficitinib, baricitinib, ruxolitinib, etc.) combined with an EGFR inhibitor (mobocertinib, osimertinib, gefitinib, erlotinib, brigatinib, dacomitinib, afatinib, neratinib, lapatinib, etc.) in the manufacture of a medicament for preventing or treating an EGFR-mutated tumor disease-like NSCLC (squamous cell carcinoma and non-squamous cell carcinoma, preferably non-phosphorous cell carcinoma). The combination is stated to possess a synergistic effect [54].
WO2020177678A1 reveals the use of a multi-target tyrosine kinase inhibitor (sunitinib, axitinib, sorafenib, nintedanib, cabozantinib, lenvatinib, regorafenib, ponatinib, pazopanib, etc.) combined with an EGFR inhibitor (mobocertinib, osiertinib, gefitinib, erlotinib, brigatinib, dacomitinib, afatinib, neratinib, lapatinib, etc.) in the preparation of a medicine for treating EGFR mutation tumor-like NSCLC (squamous cell carcinoma and non-squamous cell carcinoma). The combination is said to produce an improved therapeutic effect [55].
It is important to note that these patent applications do not exemplify the effect of MPC-0767 [52], CDK4/6 inhibitor [53], JAK kinase inhibitor [54], and multi-target tyrosine kinase inhibitor [55] in combination with mobocertinib against any cancer type.
4.6. Miscellaneous
CN111760024A claims a penetration-enhancing gold nanocluster drug-carrying targeting preparation. The preparation comprises a gold nanocluster, a cross-linking agent, and a tumor treatment drug (mobocertinib, doxorubicin, erlotinib, lapatinib, dacomitinib, gefitinib, etc.) for treating malignant tumors of breast cancer, melanoma, and prostate cancer. The claimed preparation not only promotes penetration, but also improves the targeted treatment effect on tumors and reduces the toxic and side effects of a single treatment, and has a high medical diagnosis and treatment application value [56].
WO2019178267A2 provides a composition comprising an isolated transcription factor (Lymphoid enhancer factor 1 or Lef-1) and an EGFR inhibitor (mobocertinib) to treat degenerative lung diseases and/or conditions such as asthma, COPD, cystic fibrosis, and other forms of airway epithelial damage. The composition covers a combination of a large number of active agents along with Lef-1 [57].
WO2021163064A2 reveals antibodies and fusion proteins that bind CCR8 for treatment of cancer alone or in a combination of other anticancer agents like mobocertinib. The application is silent about the experimental details of such a combination [58].
WO2021183474A1 provides a method for treating a patient suffering from chronic inflammatory injury, metaplasia, dysplasia, or cancer of gastrointestinal tissue, whose method comprises administering to the patient an agent like HSP90 inhibitor or an EGFR inhibitor (mobocertinib) or a combination thereof that selectively kills or inhibits the proliferation or differentiation of pathogenic epithelial stem cells (PESCs) in the gastrointestinal tissue relative to normal epithelial stem cells in GI tissue in which the PESC is found. This application does not exemplify any data related to mobocertinib [59].
Our search revealed some patent applications claiming novel compounds as anticancer agents, including deoxynyboquinone analogs (WO2019113345A1) [60], pyrimidine derivatives (WO2021000912A1) [61], pyrazine derivatives (WO2021033153A1) [62], and pyrimidinones (WO2021149817A1) [63]. These patent applications also state the use of the claimed compounds in combination with mobocertinib for the treatment of cancer without providing any experimental evidence.
5. Conclusions
Mobocertinib is a promising first-in-class oral treatment for an unmet medical need (NSCLC with EGFRex20ins mutations). It is expected to increase the survival rate of the patients suffering from this disease. The patents/patent applications related to compound, process, salt, polymorphs, and combination of mobocertinib have been filed by many applicants, especially in USA and China. It would be interesting to observe how many patented mobocertinib combinations with other anticancer agents will enter the clinical trial. Many more patent applications related to novel dosage forms, new drug combinations, processes, and intermediates used in the synthesis of mobocertinib are expected to come into the public domain in the future.
6. Expert Opinion
A decrease in the mortality of NSCLC patients has been observed in recent times with the arrival of targeted therapy [64]. Oncogenes, for example, EGFR genes, have been identified in NSCLC patients. Therefore, the NSCLC is treated based on the oncogene mutations [65,66,67]. Many EGFR inhibitors are available in the market to treat NSCLC with common mutations [9,10,11,12,13,14,68]. The EGFRex20ins mutation is considered an uncommon mutation and has also been acknowledged as a separate subclass of EGFR mutations [69]. The NSCLC patients with EGFRex20ins mutations receive negligible benefit from the existing therapeutic option [68], which makes it an unmet medical need. In May 2021, USFDA approved the first injectable treatment (amivantamab) for NSCLC with EGFRex20ins mutations [21]. However, mobocertinib is a recently approved first-in-class oral innovative treatment of NSCLC with EGFRex20ins mutations.
It is imperative to understand the prior inventions related to therapy to develop its improved inventions/innovations. Accordingly, the patent literature search of mobocertinib was undertaken (Scheme 2). The patent search revealed 27 patents/patent applications belonging to 25 patent families (Table 3). The first Patent Cooperation Treaty (PCT) patent application claiming mobocertinib was published in 2015 [28], wherein the compound patent of mobocertinib was granted in 2017 in the USA [37]. The method of use patent of mobocertinib was granted in 2019 in the USA [38]. The process patent application of the compound patent family has been abandoned in the USA [39]. However, a continuity application number 17/463,457 has been filed at the USPTO in 2021, which may be published soon. Most of the patent applications on mobocertinib were published in 2020 (Figure 4).
Figure 4.
Publication year of the patents/patent applications cited in the text.
The maximum number of priority patent applications have been filed in China followed by the USA (Figure 5). One patent application each has been filed in Japan and Great Britain.
Figure 5.
The country of the priority application filing of the patents/patent applications cited in the text.
The 25 patent families cited in the text were filed by different applicants (Figure 6). It is important to note that only two patent families (compound patent family and the mobocertinib succinate patent family) have been filed by Ariad Pharmaceuticals, who discovered and developed mobocertinib [37,38,39,40]. Most of the patent applications have been filed by Chia Tai Tianqing Pharmaceutical Group, which are related to combinations of anlotinib with other anticancer agents like mobocertinib (Table 3, Figure 6).
Figure 6.
The number of patent families filed by different applicants.
Different types of patents are possible for a pharmaceutical compound including patents claiming compound, salt, isomer, polymorph, process, intermediate used in the process, drug impurity, method of treatment, different dosage forms, particle size, and drug combinations [70,71,72,73]. The types of patents/patent applications related to mobocertinib are mentioned in Figure 7.
Figure 7.
The types of patents filed for mobocertinib.
The compound patent and the method of use patent of mobocertinib expire on 13 May 2035, and were filed by Ariad Pharmaceuticals (Table 3) [37,38]. In early 2017, Takeda acquired Ariad Pharmaceuticals and became the owner of the patents filed by Ariad Pharmaceuticals. These two patents are eligible for patent term extension up to five years [70,71,72,73]. If this happens, these patents will expire by 2040. Our analysis of the structure-activity relationship resulted in the possible basic pharmacophoric structure of mobocertinib (Figure 3). We trust that medicinal chemists can use the isostere and bioisotere concepts to develop more potent and safer drugs like mobocertinib. The mobocertinib succinate salt patent application was published in 2019 and is under examination at the time of writing this review [40]. If this patent application is granted, then the estimated expiry of the granted patent will be 13 May 2039. The authors trust that the mobocertinib succinate salt patent will be listed in the orange book of the USFDA [74,75]. It will also be eligible for a 5-year patent term extension and may become the governing patent for the generic drug launch of mobocertinib in the USA market. The authors also see an opportunity for a salt switch strategy [76] to develop other salts of mobocertinib with improved pharmaceutical properties. The compound patent family [37,38,39] also has published patent applications related to the process for preparing mobocertinib [39] (Scheme 3). The salt patent application [40] and polymorph patent application [27] also provide other processes for preparing mobocertinib (Scheme 4 and Scheme 5). The process patents of drugs help to reduce the price of the marketed dosage form [70]. Accordingly, we anticipate more patent filing related to the process for preparing mobocertinib from the drug suppliers. Anticancer therapy generally involves the use of a combination of drugs. Accordingly, many patent applications related to the combination of mobocertinib with other anticancer agents have been identified [42,43,44,45,46,47,48,49,50,51,52,53,54,55]. However, most of these patent applications are silent about the clinical data of the combination of mobocertinib with other drugs. Therefore, clinical studies for the combination of mobocertinib with other anticancer agents are recommended.
The patent literature revealed important inventions related to mobocertinib. However, there remains a great scope for developing inventions related to pharmaceutical compositions (different dosage forms, combinations, particle size, process intermediates, etc.). The combination of mobocertinib with pemetrexed and carboplatin is in a clinical trial for NSCLC (Table 1). There are many agents in clinical trials to treat NSCLC with EGFRex20ins mutations [15,18]. It will be worth assessing the combinations of these drugs with mobocertinib to treat this unmet need. The authors believe that there exists an exciting prospect for developing a treatment for NSCLC with EGFRex20ins mutations, giant cell tumor of the bone [43], interstitial lung disease [44], ovarian cancer [45], colorectal cancer [46], oesophageal cancer [47], glioblastoma [48], Ewing sarcoma [49], and soft tissue sarcoma [50] employing a combination of mobocertinib with other approved anticancer agents.
Figure 2.
Chemical structures of N-demethylated active metabolites of mobocertinib (AP32960 and AP32914).
Acknowledgments
The authors are thankful to the Northern Border University, Rafha, Saudi Arabia, for providing the necessary resources to complete this review article.
Author Contributions
Conceptualization, M.I., S.A.K. and F.S.; methodology, M.I. and S.A.K.; software, M.K.A.; validation, F.S., S.A. and M.M.G.; formal analysis, S.A.K. and F.S.; investigation, M.K.A. and F.A.A.; resources, F.S. and M.M.G.; data curation, R.S.A.; writing—original draft preparation, M.K.A., M.A.A., A.A.A., R.S.A. and F.A.A.; writing—review and editing, S.A., S.A.K. and M.M.G.; visualization, M.A.A. and A.A.A.; supervision, M.I.; project administration, F.S., S.A. and M.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This work did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra J., Malvezzi M., Negri E., La Vecchia C., Boffetta P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 3.Bradley S.H., Kennedy M.P.T., Neal R.D. Recognising lung cancer in primary care. Adv. Ther. 2019;36:19–30. doi: 10.1007/s12325-018-0843-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houston T. Screening for lung cancer. Med. Clin. N. Am. 2020;104:1037–1050. doi: 10.1016/j.mcna.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Latimer K.M., Mott T.F. Lung cancer: Diagnosis, treatment principles, and screening. Am. Fam. Physician. 2015;91:250–256. [PubMed] [Google Scholar]
- 6.Duma N., Santana-Davila R., Molina J.R. Non-small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Lung cancer. Breathe (Sheff) 2016;12:392–399. doi: 10.1183/20734735.ELF124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz-Serrano A., Gella P., Jiménez E., Zugazagoitia J., Paz-Ares Rodríguez L. Targeting EGFR in lung cancer: Current standards and developments. Drugs. 2018;78:893–911. doi: 10.1007/s40265-018-0916-4. [DOI] [PubMed] [Google Scholar]
- 9.Steins M., Thomas M., Geißler M. Erlotinib. Recent Results Cancer Res. 2018;211:1–17. doi: 10.1007/978-3-319-91442-8_1. [DOI] [PubMed] [Google Scholar]
- 10.Sim E.H., Yang I.A., Wood-Baker R., Bowman R.V., Fong K.M. Gefitinib for advanced non-small cell lung cancer. Cochrane Database Syst Rev. 2018;1:CD006847. doi: 10.1002/14651858.CD006847.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu S., Shih J.Y., Jang T.W., Liam C.K., Yu Y. Afatinib as first-line treatment in Asian patients with EGFR mutation-positive NSCLC: A narrative review of real-world evidence. Adv. Ther. 2021;38:2038–2053. doi: 10.1007/s12325-021-01696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masood A., Kancha R.K., Subramanian J. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: Focus on afatinib. Semin. Oncol. 2019;46:271–283. doi: 10.1053/j.seminoncol.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lavacchi D., Mazzoni F., Giaccone G. Clinical evaluation of dacomitinib for the treatment of metastatic non-small cell lung cancer (NSCLC): Current perspectives. Drug Des. Devel. Ther. 2019;13:3187–3198. doi: 10.2147/DDDT.S194231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee C.S., Milone M., Seetharamu N. Osimertinib in EGFR-Mutated lung cancer: A review of the existing and emerging clinical data. Onco Targets Ther. 2021;14:4579–4597. doi: 10.2147/OTT.S227032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F., Li C., Wu Q., Lu H. EGFR exon 20 insertion mutations in non-small cell lung cancer. Transl. Cancer Res. 2020;9:2982–2991. doi: 10.21037/tcr.2020.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirose T., Ikegami M., Endo M., Matsumoto Y., Nakashima Y., Mano H., Kohsaka S. Extensive functional evaluation of exon 20 insertion mutations of EGFR. Lung Cancer. 2021;152:135–142. doi: 10.1016/j.lungcan.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Vasconcelos P.E.N.S., Kobayashi I.S., Kobayashi S.S., Costa D.B. Preclinical characterization of mobocertinib highlights the putative therapeutic window of this novel EGFR inhibitor to EGFR exon 20 insertion mutations. JTO Clin. Res. Rep. 2021;2:100105. doi: 10.1016/j.jtocrr.2020.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyse S., Huang P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target. Ther. 2019;4:5. doi: 10.1038/s41392-019-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda H., Kobayashi S., Costa D.B. EGFR exon 20 insertion mutations in non-small-cell lung cancer: Preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–e31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S.S., Zhu V.W. Spotlight on mobocertinib (TAK-788) in NSCLC with EGFR exon 20 insertion mutations. Lung Cancer (Auckl.) 2021;12:61–65. doi: 10.2147/LCTT.S307321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syed Y.Y. Amivantamab: First approval. Drugs. 2021;81:1349–1353. doi: 10.1007/s40265-021-01561-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou C., Ramalingam S.S., Kim T.M., Kim S.W., Yang J.C., Riely G.J., Mekhail T., Nguyen D., Garcia Campelo M.R., Felip E., et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR Exon 20 insertion-positive metastatic non-small cell lung cancer: A Phase 1/2 Open-label nonrandomized clinical trial. JAMA Oncol. 2021:e214761. doi: 10.1001/jamaoncol.2021.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. Food and Drug Administration FDA Grants Accelerated Approval to Mobocertinib for Metastatic Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations. [(accessed on 15 December 2021)];2021 September 15; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20.
- 24.Zhang S., Jin S., Griffin C., Feng Z., Lin J., Venkatakrishnan K., Gupta N. Effects of itraconazole and rifampin on the pharmacokinetics of mobocertinib (TAK-788), an oral epidermal growth factor receptor inhibitor, in healthy volunteers. Clin. Pharmacol. Drug Dev. 2021;10:1044–1053. doi: 10.1002/cpdd.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DrugBank; Dec 23, 2020. [(accessed on 14 December 2021)]. Mobocertinib: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB16390. [Google Scholar]
- 26.Gonzalvez F., Vincent S., Baker T.E., Gould A.E., Li S., Wardwell S.D., Nadworny S., Ning Y., Zhang S., Huang W.S., et al. Mobocertinib (TAK-788): A targeted inhibitor of EGFR Exon 20 insertion mutants in non-small cell lung cancer. Cancer Discov. 2021;11:1672–1687. doi: 10.1158/2159-8290.CD-20-1683. [DOI] [PubMed] [Google Scholar]
- 27.Qiuwei T. Crystal Form of Kinase Inhibitor and Preparation Method and Application Thereof. CN110776495A. 2020 February 11;
- 28.Huang W.S., Gong Y., Li F., Bencivenga N.E., Dalgarno D.C., Kohlmann A., Shakespeare W.C., Thomas R.M., Zhu X., West A.V., et al. Heteroaryl Compounds for Kinase Inhibition. WO2015195228A1. 2015 December 23;
- 29.RxList, Exkivity (Mobocertinib Capsules): Uses, Dosage, Side Effects, Drug Interactions, Warnings & Precautions, Overdosage & Contraindications, Clinical Pharmacology, Medication Guide. WebMD; San Clemente, CA, USA: [(accessed on 16 October 2021)]. Available online: https://www.rxlist.com/exkivity-drug.htm/ [Google Scholar]
- 30.U.S. Food and Drug Administration EXKIVITY™ (Mobocertinib) Capsules, for Oral Use. [(accessed on 16 October 2021)];2021 September 15; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215310s000lbl.pdf.
- 31.Center for Drug Evaluation and Researc NDA OPQ Review and Evaluation NDA 215,310 EXKIVITY (Mobocertinib) [(accessed on 16 October 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215310Orig1s000ChemR.pdf.
- 32.Center for Drug Evaluation and Research NDA Multi-disciplinary Review and Evaluation NDA 215,310 Mobocertinib (formerly TAK-788, AP32788) [(accessed on 16 October 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/215310Orig1s000MultidisciplineR.pdf.
- 33.U.S. Food and Drug Administration [(accessed on 16 October 2021)];Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/
- 34.Zhang S., Jin S., Griffin C., Feng Z., Lin J., Baratta M., Brake R., Venkatakrishnan K., Gupta N. Single-dose pharmacokinetics and tolerability of the oral epidermal growth factor receptor inhibitor mobocertinib (TAK-788) in healthy volunteers: Low-fat meal effect and relative bioavailability of 2 capsule products. Clin. Pharmacol. Drug Dev. 2021;10:1028–1043. doi: 10.1002/cpdd.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riely G.J., Neal J.W., Camidge D.R., Spira A.I., Piotrowska Z., Costa D.B., Tsao A.S., Patel J.D., Gadgeel S.M., Bazhenova L., et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with EGFR exon 20 insertion mutations from a phase I/II trial. Cancer Discov. 2021;11:1688–1699. doi: 10.1158/2159-8290.CD-20-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. National Library of Medicine ClinicalTrials.gov NLM. [(accessed on 15 October 2021)]; Available online: https://clinicaltrials.gov/
- 37.Huang W.S., Gong Y., Li F., Bencivenga N.E., Dalgarno D.C., Kohlmann A., Shakespeare W.C., Thomas R.M., Zhu X., West A.V., et al. Heteroaryl Compounds for Kinase Inhibition. US9796712B2. 2017 October 24;
- 38.Huang W.S., Gong Y., Li F., Bencivenga N.E., Dalgarno D.C., Kohlmann A., Shakespeare W.C., Thomas R.M., Zhu X., West A.V., et al. Heteroaryl Compounds for Kinase Inhibition. US10227342B2. 2019 March 12;
- 39.Huang W.S., Gong Y., Li F., Bencivenga N.E., Dalgarno D.C., Kohlmann A., Shakespeare W.C., Thomas R.M., Zhu X., West A.V., et al. Heteroaryl Compounds for Kinase Inhibition. US2019218212A1. 2019 July 18;
- 40.Durak L.J., Langston M., Sharma P.K., Nguyen T.H., Li S., Zhang X. Pharmaceutical Salts of Pyrimidine Derivatives and Method of Treating Disorders. US2021309640A1. 2021 October 7;
- 41.Gao Y., Liu P., Shi R. Anlotinib as a molecular targeted therapy for tumors. Oncol. Lett. 2020;20:1001–1014. doi: 10.3892/ol.2020.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenjun G., Shanchun W., Xiquan Z. Quinoline Derivatives for the Treatment of Non-Small Cell Lung Cancer. CN111643503A. 2020 September 11;
- 43.Ping X., Chengqian W. Quinoline Compound or Pharmaceutically Acceptable Salt Thereof for Treating Giant Cell Tumor of Bone. CN111840289A. 2020 October 30;
- 44.Ping X., Chengqian W. Quinoline Derivatives or Pharmaceutically Acceptable Salts Thereof for Use in the Combined Treatment of Interstitial Lung Disease. CN111956649A. 2020 November 20;
- 45.Ping X., Chengqian W. Quinoline Derivatives for the Combined Treatment of Ovarian Cancer. CN111973747A. 2020 November 24;
- 46.Hongming L., Xiquan Z., Xunqiang W., Ding Y., Lili S., Kun L. Quinolines for the Combined Treatment on Carcinoma of Colon and Rectum. CN112043702A. 2020 December 8;
- 47.Shanchun W., Feng W. Quinolines for the Combined Treatment of Esophageal Cancer. CN112121048A. 2020 December 25;
- 48.Yuanyuan C., Chengqian W. Quinoline Derivatives for the Treatment of Glioblastoma. CN112294814A. 2021 February 2;
- 49.Xiaole Z., Lifan T., Chaoqiang Y., Xiquan Z., Xunqiang W., Jie X. Quinoline Compound or Pharmaceutically Acceptable Salt Thereof for Treating Ewing’s Sarcoma. WO2020211860A1. 2020 October 22;
- 50.Zhiming W., Shilong Z., Rongyuan Z., Xi G., Yan W., Hua Y., Weiqi L., Yuhong Z., Lei G. Quinoline Derivative Used for Soft Tissue Sarcoma Combination Therapy. WO2020228656A1. 2020 November 19;
- 51.Dong W., Nan D., Hao L., Ping X. Quinoline Derivative Used for Combination Treatment of Small Cell Lung Cancer. WO2020233602A1. 2020 November 26;
- 52.Lichenstein H., Beeharry N., Landrette S., Gayle S., Grotzke J., Hernandez M., Young P.R., Rothberg J.M. Therapeutic Methods Relating to hsp90 Inhibitors. US2019091229A1. 2019 March 28;
- 53.Zhang L., Yang C., Liao C., Zhang L. Use of cdk4/6 Inhibitor in Combination with EGFR Inhibitor in the Preparation of Medicament for Treating Tumor Diseases. US2021196719A1. 2021 July 1;
- 54.Mi T., Zhilin D., Cheng L., Changyong Y., Lianshan Z. Use of Combination of Jak Kinase Inhibitor and EGFR Inhibitor in Preparation of Medicament for Treating Tumor Diseases. WO2020052575A1. 2020 March 19;
- 55.Wenming R., Mi T., Changyong Y.N., Cheng L., Lianshan Z. Use of Multi-Target Tyrosine Kinase Inhibitor in Combination with EGFR Inhibitor in Preparing Drug for Treating Tumor. WO2020177678A1. 2020 September 10;
- 56.Yan S., Jiasheng T., Zixuan Y., Wenqian Y., Yanyan L. Penetration Enhanced Gold Nanocluster Drug-Loaded Targeting Preparation and Preparation Method and Application Thereof. CN111760024A. 2020 October 13;
- 57.Engelhardt J.F., Lynch T.J., Anderson P.J., Shahin W. Inductive Regeneration of the Airway by Transcriptional Factor Modulation of Glandular Myoepithelial Stem Cells. WO2019178267A3. 2019 October 10;
- 58.McGrath L.L., Dépis F., Hu C., Presta L.G., Buggé J.A. Antibodies and Fusion Proteins that Bind to CCR8 and Uses Thereof. WO2021163064A3. 2021 September 30;
- 59.Mckeon F., Neupane R., Xie J., Xian W., Vincent M., Wang S. Inflammatory Bowel Disease Stem Cells, Agents which Target IBD Stem Cells, and Uses Related Thereto. WO2021183474A1. 2021 September 16;
- 60.Mousses S. Combination of Cancer Therapeutics for Treatment of Cancer and Hyperproliferative Diseases. WO2019113345A1. 2019 June 13;
- 61.Xie Y., Cao G., Fan H. Compound for Inhibiting EGFR Kinase and Preparation Method and Use Thereof. WO2021000912A1. 2021 January 7;
- 62.Howard S., Liebeschuetz J.W., Shimamura T. Pyrazolo [3,4-b] Pyrazine SHP2 Phosphatase Inhibitors. WO2021033153A1. 2021 February 25;
- 63.Hoshino T., Komiya Y., Nakatsuru Y., Wallis N.G. Enhancement of Anti-Tumor Activity of SHP2 Inhibitor Pyrimidinone in Combination with Novel Cancer Medicines in Cancers. WO2021149817A1. 2021 July 29;
- 64.Howlader N., Forjaz G., Mooradian M.J., Meza R., Kong C.Y., Cronin K.A., Mariotto A.B., Lowy D.R., Feuer E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burnett H., Emich H., Carroll C., Stapleton N., Mahadevia P., Li T. Epidemiological and clinical burden of EGFR Exon 20 insertion in advanced non-small cell lung cancer: A systematic literature review. PLoS ONE. 2021;16:e0247620. doi: 10.1371/journal.pone.0247620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cardona A.F., Rojas L., Zatarain-Barrón Z.L., Freitas H.C., Granados S.T., Castillo O., Oblitas G., Corrales L., Castro C.D., Ruiz-Patiño A., et al. EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP) Lung Cancer. 2018;125:265–272. doi: 10.1016/j.lungcan.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Choudhury N.J., Schoenfeld A.J., Flynn J., Falcon C.J., Rizvi H., Rudin C.M., Kris M.G., Arcila M.E., Heller G., Yu H.A., et al. Response to standard therapies and comprehensive genomic analysis for patients with lung adenocarcinoma with EGFR Exon 20 insertions. Clin. Cancer Res. 2021;27:2920–2927. doi: 10.1158/1078-0432.CCR-20-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau S.C.M., Fares A.F., Le L.W., Mackay K.M., Soberano S., Chan S.W., Smith E., Ryan M., Tsao M.S., Bradbury P.A., et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin. Lung Cancer. 2021;22:253–259. doi: 10.1016/j.cllc.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 69.Remon J., Hendriks L.E.L., Cardona A.F., Besse B. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat. Rev. 2020;90:102105. doi: 10.1016/j.ctrv.2020.102105. [DOI] [PubMed] [Google Scholar]
- 70.Imran M., Alshrari A.S., Asdaq S.M.B., Abida Trends in the development of remdesivir based inventions against COVID-19 and other disorders: A patent review. J. Infect. Public Health. 2021;14:1075–1086. doi: 10.1016/j.jiph.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Imran M., Asdaq S.M.B., Khan S.A., Unnikrishnan Meenakshi D., Alamri A.S., Alsanie W.F., Alhomrani M., Mohzari Y., Alrashed A., AlMotairi M., et al. Innovations and patent trends in the development of USFDA approved protein kinase inhibitors in the last two decades. Pharmaceuticals. 2021;14:710. doi: 10.3390/ph14080710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imran M., Kumar Arora M., Asdaq S.M.B., Khan S.A., Alaqel S.I., Alshammari M.K., Alshehri M.M., Alshrari A.S., Mateq Ali A., Al-Shammeri A.M., et al. Discovery, development, and patent trends on Molnupiravir: A prospective oral treatment for COVID-19. Molecules. 2021;26:5795. doi: 10.3390/molecules26195795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imran M., Alshrari A.S., Thabet H.K., Abida, Bakht M.A. Synthetic molecules as DprE1 inhibitors: A patent review. Expert Opin. Ther. Pat. 2021;31:759–772. doi: 10.1080/13543776.2021.1902990. [DOI] [PubMed] [Google Scholar]
- 74.Pacheco J.M. Mobocertinib: A potential treatment for NSCLC with EGFR Exon 20 Insertions. Cancer Discov. 2021;11:1617–1619. doi: 10.1158/2159-8290.CD-21-0379. [DOI] [PubMed] [Google Scholar]
- 75.Saal C., Becker A. Pharmaceutical salts: A summary on doses of salt formers from the Orange Book. Eur. J. Pharm. Sci. 2013;49:614–623. doi: 10.1016/j.ejps.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 76.Verbeeck R.K., Kanfer I., Walker R.B. Generic substitution: The use of medicinal products containing different salts and implications for safety and efficacy. Eur. J. Pharm. Sci. 2006;28:1–6. doi: 10.1016/j.ejps.2005.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This work did not report any data.