Abstract
Background:
Consolidative autologous hematopoietic stem cell transplantation (AHCT) is commonly used for multiple myeloma (MM) patients. We studied AHCT utilization and outcomes in MM patients ≥75 years.
Methods:
MM patients ≥75 years receiving AHCT between 2013 and 2017 in the United States (US) were identified using the CIBMTR database. Relapse and/or progression (REL), progression-free survival (PFS), and overall survival (OS) were modeled using Cox proportional hazards models. Covariates used were age, sex, Karnofsky performance score (KPS), HCT-comorbidity index (HCT-CI), International Staging System and/or Durie-Salmon stage, high-risk cytogenetics, melphalan (Mel) dose, and disease status at and 1 year after transplant. AHCT utilization rate using the SEER database was used to estimate specific incidence among ≥75 years by race and gender.
Results:
Of 360 patients; 63% male, 84% white, 56% had KPS <90 and 57% had HCT-CI ≥3. The 100-day transplant-related mortality (TRM) was 1% (0–2%) with two-year REL rate of 27% (95% CI, 22–33%), PFS of 66% (95% CI, 60–72%) and OS of 83% (95% CI, 78–87%). On multivariate analysis, only high-risk cytogenetics were associated with REL risk and decreased PFS. In white males, transplant utilization rate was 5.2–5.8% compared to 3.5–4.0% in African-American males (P 0.02). There was 3.37–3.79% transplant utilization in white females compared to 1.88–2.12% in African-American females (P <0.01).
Conclusions:
The use of AHCT was associated with excellent 2-year outcomes in this selected MM population ≥75 years. Transplant utilization for ≥75 years remains low with significant racial and gender disparities.
Keywords: Elderly, myeloma, hematopoietic, transplantation, utilization
Precis:
Among newly diagnosed myeloma patients ≥75 years, the stem cell transplant utilization rate was significantly lower in African Americans - females>males, compared to whites, while overall outcomes remain excellent for this selected population. Only high-risk cytogenetics were associated with inferior outcomes.
Introduction
Multiple myeloma is a hematologic malignancy of older adults with a median age at diagnosis of 66–70 years in the US.1, 2 The Center for International Blood and Marrow Transplant (CIBMTR®) database shows that the utilization of transplants over the age of 70 has increased annually from 2000 to 2018 in MM and other hematologic malignancies.3 We recently reported the effectiveness of upfront AHCT in the current era of novel therapies with similar progression-free survival (PFS) and non-relapse mortality (NRM) in patients aged 70 years or older compared with MM patients 60–69 years old. 4
However, the utilization of AHCT for MM in older age groups remains low.5–7 Data regarding the outcomes of upfront AHCT in MM patients ≥75 years and older are few.8–12 Aging may lead to a decline in organ function and, at times, cognitive function as well as concerns for drug tolerability. Many clinical trials of AHCT have upper age limits and consequently, the safety and outcomes of transplant in these patients outside of single center series are largely unknown.13 Further, concerns regarding the use of standard dose melphalan 200mg/m2 may limit AHCT to a younger population by physician choice or frailty concerns, though this has not been prospectively evaluated. Single center studies show low rates of TRM from AHCT with improved supportive care in this elderly population.14 Thus, AHCT can be a safe option in the treatment of selected fit elderly patients.15 We therefore studied AHCT utilization and outcomes in MM patients aged ≥75 years in the US. We used the CIBMTR database as described below.
Materials and Methods
Data Source
The CIBMTR is a working group of more than 500 transplantation centers worldwide that contribute detailed data on HCT to a statistical center at the Medical College of Wisconsin (MCW). Participating centers are required to report all transplantations consecutively and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Data are collected at two levels: transplant essential data (TED) and comprehensive report form (CRF) data. TED data include disease type, age, gender, pre-HCT disease stage and chemotherapy-responsiveness, date of diagnosis, graft type, conditioning regimen, post-transplant disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR centers contribute to TED data. More detailed disease and pre- and post-transplant clinical information is collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED- and CRF-level data are collected pre-transplant, 100-days, and 6 months post-HCT and annually thereafter or until death. Data for the current analysis were retrieved from TED report forms as our intent was to capture all patients registered with the CIBMTR. Reporting of autologous HCT to the CIBMTR is voluntary, however, it is estimated that over 80–90% of MM AHCT activity in the US was reported to the CIBMTR during this period.16
Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The MCW Institutional Review Board approved this study.
Patients
Included in this analysis were consented adult (≥ 75 years) MM patients undergoing a single AHCT within 12 months from diagnosis between 2013 and 2017 in the US after melphalan conditioning. The TED dataset was used in this study and provided data on patient (age, gender, race, Karnofsky performance score [KPS], HCT comorbidity index [HCT-CI]), disease (immunoglobulin subtype, International staging system [ISS], cytogenetics) and transplant (time from diagnosis to transplant, disease status at transplant, Mel conditioning dose and year of transplant) related covariates.
The incidence of MM was obtained from the Surveillance, Epidemiology and End Result (SEER) Program of the US National Cancer Institute. SEER data are derived from registries covering approximately 27.8% of the US population; we used SEER 18 database, which contains patients diagnosed from 2002–2016. Using publicly available software which also provides US population estimates (SEER*Stat, version 8.3.2), we calculated incidence rates per 100,000 persons for the years 2013–2016 by age, race, and sex. We combined MM incidence derived from the SEER program with transplantation activity reported to the Center for International Blood and Marrow Transplant Research for the period 2013–2016.
Definitions and study endpoints
Non-relapse mortality (NRM) was defined as death from any cause in the absence of relapse/progression. Relapse/Progression (REL) was defined by IMWG criteria. Progression-free survival (PFS) was defined as the time from transplantation to relapse, disease progression or death from any cause. Overall survival (OS) was defined as the time from transplantation to death from any cause.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics. Cumulative incidences of NRM and disease REL were calculated accounting for competing risks. Kaplan-Meier estimates were used to calculate the probabilities of PFS and OS. Multivariate analysis of PFS and OS was conducted using the Cox proportional hazards regression analysis to assess the main effect, age at transplant, adjusting for key patient-, disease-, and transplant-related covariates. The assumption of proportional hazards for each covariate in the Cox model was tested using time-dependent variables. A stepwise model selection approach was used to identify covariates associated with outcomes. Factors significant at the 5% level of significance (P <0.05) were kept in the final model. Hazard ratio (HR) with 95% confidence intervals (CI) were shown. Statistical analysis was performed using SAS v9.2 (Cary, NC).
Next, an estimate of the US transplant rate was calculated – this was defined as new AHCT in a given year by newly diagnosed number of MM for that year, The number of new AHCT each year was calculated as the number of AHCT reported to the CIBMTR divided by the CIBMTR autologous transplant capture rate. Since, the estimate of the CIBMTR capture rate during this time was 80–90%, a sensitivity analysis was performed to provide a range to the rate for +/−5% for the transplant utilization rates each year.
Results
Table 1 shows the overall patient population included in this study (N=360), who were 75 years or older. The median patient age was 76.3 years (range, 75–83.2 years). The majority of patients were white (84%) and 12% were African American. Sixty-three percent were male. Majority had a KPS <90 (56%), or HCT-CI >=3 (57%), and stage III disease (Durie-Salmon/International Staging System, 53%). High-risk cytogenetics [t(4;14), t(14;16), t(14;20), del 17p, +1q or 1p del, del 13q or hypodiploidy on cytogenetics)] were reported in 32%, with 19% patients missing this data. At pre-transplant timepoint, 57% of patients were in very good partial response (VGPR) or better. Nearly 29% of the patients received Mel 200 mg/m2; 71% received Mel 140 mg/m2. Supplemental Table 1 shows the breakdown of Mel dose by age at AHCT. Additionally, post-AHCT maintenance therapy was planned in 27% patients. The median follow-up of survivors was 24 (3–63) months. At a median follow up of 24 months (3–63), second cancers were seen in 16 patients (4.4%).
Table 1.
Baseline Characteristics
| Characteristic | Total N (%) |
|---|---|
| Number of patients | 360 |
| Number of Centers | 83 |
| Age at AHCT, Median age (range) | 76.25 (75.01–83.17) |
| Sex, Male | 227 (63) |
| Self-reported race | |
| Caucasian | 303 (84) |
| African American | 44 (12) |
| Other | 3 (1) |
| Missing | 10 (3) |
| Karnofsky score | |
| ≥ 90 | 156 (43) |
| < 90 | 201 (56) |
| Missing | 3 (1) |
| HCT-CI | |
| 0 | 70 (19) |
| 1 | 36 (10) |
| 2 | 51 (14) |
| 3 | 67 (19) |
| 4 | 54 (15) |
| 5 | 33 (9) |
| ≥ 6 | 49 (14) |
| Stage (ISS/DSS) at diagnosis | |
| Stage III | 190 (53) |
| Stage I-II | 166 (46) |
| Missing | 4 (1) |
| Cytogenetics | |
| No abnormal/Standard risk | 177 (49) |
| High risk | 116 (32) |
| Test not done/unknown | 67 (19) |
| Melphalan dose | |
| MEL 140 | 254 (71) |
| MEL 200 | 106 (29) |
| Disease status prior to transplant | |
| sCR/CR | 53 (15) |
| VGPR | 151 (42) |
| PR | 129 (36) |
| SD/PD/Relapse | 26 (7) |
| Missing | 1 (0) |
| Maintenance therapy planned | 96 (27) |
| Median follow-up of survivors (range), months | 24 (3–63) |
Legend: HCT-CI: hematopoietic cell transplant comorbidity index; ISS: International staging system; DSS: Durie-Salmon staging; VGPR: Very good partial response.
Outcomes
The 100-day NRM was 1 (95% CI, 0–2)%. At 2 years, the rate of REL/progression was 27% (95% CI, 22–33%), PFS was 66% (95% CI, 60–72%) and OS was 83% (95% CI, 78–87%) (Figure 1, Table 2). Multivariate analyses for relapse/progression, PFS and OS are shown in Table 3. Only high-risk cytogenetics were associated with relapse/progression (HR 2.15, 95%CI, 1.29–3.58, P 0.003) and decreased PFS (HR 1.63, 95%CI, 1.04–2.57, P 0.033). No difference was seen in outcomes between Mel 140 or Mel 200 conditioning dose. Other factors including stage (I/II versus III ISS/DS) disease, and disease status at HCT were not associated with outcomes.
Figure 1:
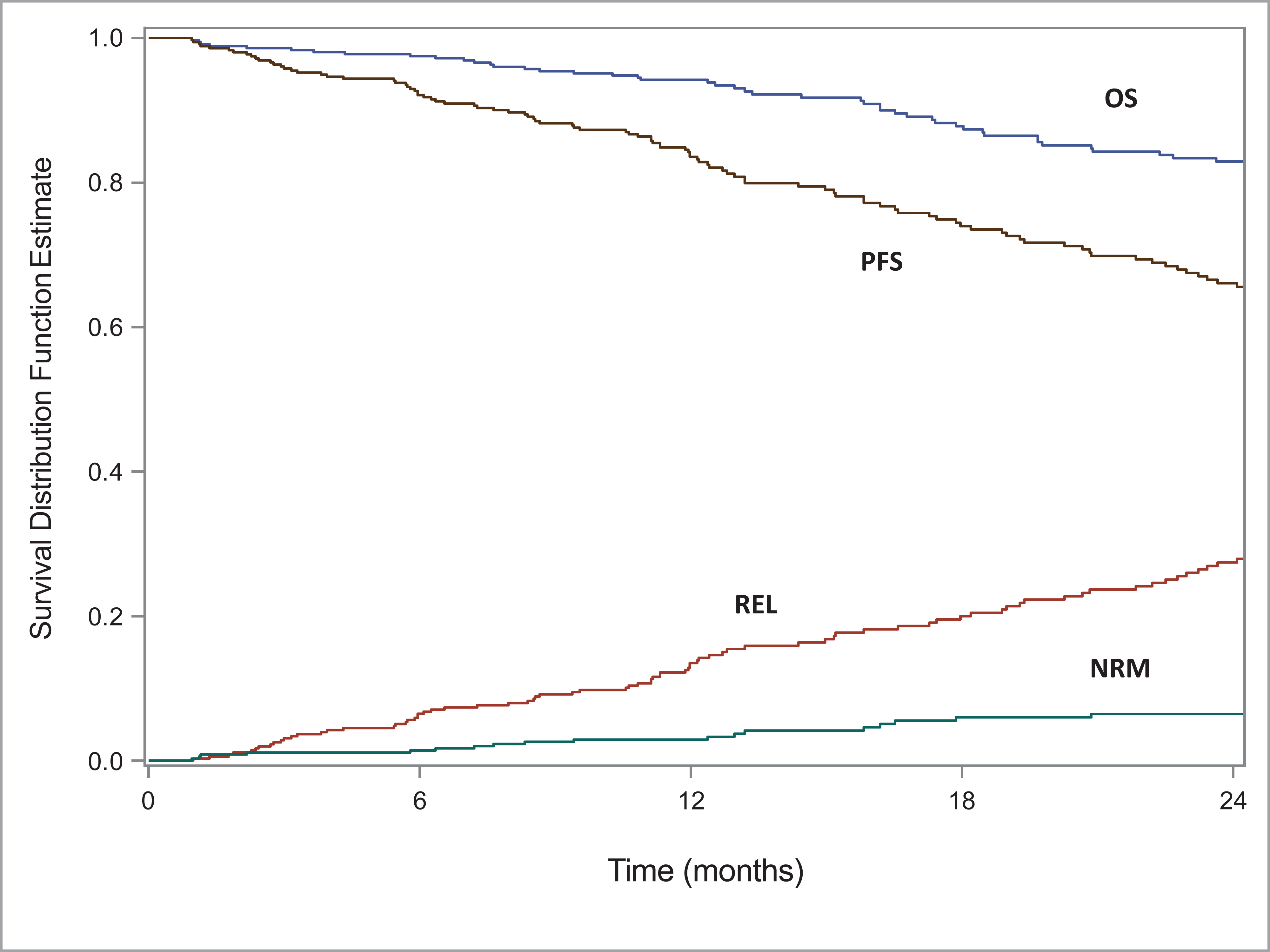
Outcomes for multiple myeloma patients ≥75 years undergoing AHCT at 2 years.
Table 2.
Post-AHCT univariate outcomes
| N = 360 | ||
|---|---|---|
| Outcomes | N | Prob (95% CI) |
|
| ||
| Treatment related mortality | 356 | |
| 100-day | 1 (0–2)% | |
| 1-year | 3 (1–5)% | |
| 2-year | 6 (4–10)% | |
| Relapse | 356 | |
| 100-day | 3 (2–5)% | |
| 1-year | 14 (10–17)% | |
| 2-year | 27 (22–33)% | |
| Disease free survival | 356 | |
| 100-day | 96 (93–97)% | |
| 1-year | 84 (79–87)% | |
| 2-year | 66 (60–72)% | |
| Overall survival | 360 | |
| 100-day | 98 (97–99)% | |
| 1-year | 94 (92–96)% | |
| 2-year | 83 (78–87)% | |
Table 3.
Multivariate analysis of outcomes
| Outcome | N (Events/Evaluable) | Hazard Ratio (95% CI) | p-value |
|---|---|---|---|
| Relapse/Progression | |||
| Cytogenetics | |||
| No abnormality/Standard risk | 28/174 | 1.00 | 0.01 |
| High risk | 32/115 | 2.15 (1.29–3.58) | 0.003 |
| Not tested/unknown | 19/67 | 1.47 (0.82–2.65) | 0.19 |
| ISS/DSS | |||
| I-II | 32/163 | 1.00 | 0.55 |
| III | 47/189 | 1.29 (0.82–2.65) | 0.19 |
| Disease status pre-AHCT | |||
| sCR/CR | 15/53 | 1.00 | 0.57 |
| VGPR | 30/150 | 0.75 (0.40–1.4) | 0.36 |
| PR | 27/127 | 0.66 (0.35–1.25) | 0.20 |
| SD/PD | 8/25 | 1.17 (0.49–2.76) | 0.73 |
| Mel dose | |||
| 200 | 17/104 | 1.00 | |
| 140 | 65/252 | 1.43 (0.83–2.45) | 0.20 |
| Progression-free survival | |||
| Cytogenetics | |||
| No abnormality/Standard risk | 41/174 | 1.00 | 0.09 |
| High risk | 36/115 | 1.63 (1.04–2.57) | 0.03 |
| Not tested/unknown | 20/67 | 1.08 (0.63–1.85) | 0.78 |
| ISS/DSS | |||
| I-II | 38/163 | 1.00 | 0.34 |
| III | 59/189 | 1.36 (0.90–2.05) | 0.14 |
| Disease status pre-AHCT | |||
| sCR/CR | 16/53 | 1.00 | 0.88 |
| VGPR | 36/150 | 0.84 (0.46–1.52) | 0.56 |
| PR | 36/127 | 0.85 (0.47–1.54) | 0.60 |
| SD/PD | 9/25 | 1.20 (0.53–2.72) | 0.66 |
| Mel dose | |||
| 200 | 21/104 | 1.00 | |
| 140 | 76/252 | 1.42(0.87–2.31) | 0.16 |
| Overall survival | |||
| Cytogenetics | |||
| No abnormality/Standard risk | 20/177 | 1.00 | 0.48 |
| High risk | 17/116 | 1.49(0.78–2.85) | 0.23 |
| Not tested/unknown | 11/67 | 1.13(0.54–2.37) | 0.74 |
| ISS/DSS | |||
| I-II | 18/166 | 1.00 | 0.42 |
| III | 30/190 | 1.49 (0.83–2.68) | 0.19 |
| Disease status pre-AHCT | |||
| sCR/CR | 8/53 | 1.00 | 0.93 |
| VGPR | 15/151 | 0.79 (0.33–1.89) | 0.60 |
| PR | 21/129 | 1.08 (0.48–2.45) | 0.86 |
| SD/PD | 4/26 | 1.03 (0.31–3.43) | 0.96 |
| Mel dose | |||
| 200 | 10/106 | 1.00 | |
| 140 | 38/254 | 1.52 (0.75–3.06) | 0.25 |
ISS/DSS- International Staging System/Durie Salmon Stage, sCR- stringent complete response, CR- complete response, VGPR- very good partial response, PR- partial response, SD- stable disease, PD- progression, Mel- high dose melphalan
Transplant Utilization Rates
The MM incidence rates, by sex and race, in the age group 75–79 years from 2013–2016 using SEER data are reported in Table 4. For a white male aged 75–79 years, transplant utilization rate estimate was 5.19–5.84% compared to 3.53–3.97% for an African American male in the same age group (P 0.02). Among females, there was 3.37–3.79% transplant utilization in whites compared to 1.88–2.12% in African American females (P <0.01).
Table 4.
Sensitivity analysis of transplant utilization rate estimate, by sex and race
| Sex | Capture rate | Black | White | P-valuea |
|---|---|---|---|---|
|
| ||||
| Female | 80% | 2.12 (2.00–2.24)% | 3.79 (3.68–3.91)% | <0.01 |
| 85% | 2.00 (1.89–2.11)% | 3.58 (3.48–3.69)% | <0.01 | |
| 90% | 1.88 (1.78–1.99)% | 3.37 (3.27–3.47)% | <0.01 | |
|
| ||||
| Male | 80% | 3.97 (3.75–4.21)% | 5.84 (5.70–5.99)% | 0.01 |
| 85% | 3.75 (3.54–3.97)% | 5.52 (5.38–5.66)% | 0.01 | |
| 90% | 3.53 (3.33–3.74)% | 5.19 (5.06–5.32)% | 0.02 | |
Test CIBMTR AUTO HCT transplant capture rate at each year: 80–90% from 2013–2016
Chi-square test
Discussion
Myeloma is a disease of those aged 70 or above but the predominant age group receiving AHCT are in the 60–69 age range.1, 2 A large CIBMTR study analyzing 15,999 MM patients receiving upfront AHCT from 2013–2017, recently showed that patients ≥70 years have comparable outcomes to patients aged 60–69 years.4 This analysis also revealed that age ≥70 years was not associated with adverse outcomes and that the use of Mel 200 mg/m2 was associated with superior outcomes. The current analysis of patients aged 75 years or older represents the largest number of older MM patients receiving upfront AHCT. Based on this the following conclusions can be drawn: 1. Selected patients >=75 years can undergo transplant safely with an acceptable TRM, 2. Outcomes in AHCT recipients are excellent and comparable to those in 60–69 years reported in the prior study [4], 3. Overall transplant utilization among the 75–79-year population remains extremely low with significant racial and gender disparities.
A prior CIBMTR analysis of 11,430 MM patients (>70 years = 946) receiving AHCT within 24 months of diagnosis showed that older patients were less likely to receive transplantation within the first year of diagnosis and more likely to have Mel dose reduction.17 In this study, 146 patients were ≥75 years and 8 patients were ≥80 years. Two-year survival in patients 75–79-years old and ≥80-years-old was 91% (83% to 95%) and 100%, respectively. The perception that advanced age by itself bars eligibility to AHCT is therefore not true. Several studies now show that physiological fitness rather than chronological age should be a criterion for patient selection for high dose MEL based AHCT in general.9, 18 Our data similarly show relapse and PFS is affected by presence of high-risk cytogenetics. Our results do not show any difference in outcomes based on Mel dose, potentially due to low power, patient selection and low numbers of patients ≥75 years. The majority of patients had dose reductions in Mel as expected in this older patient population while a subset of predominantly 75 or 76 years old patients received Mel 200 mg/m2.
The smaller number of transplant centers in this study when compared to centers reporting to the CIBMTR reflects that fewer centers offer AHCT to elderly myeloma patients. Several smaller studies also show the safety and feasibility of AHCT with high dose Mel in the elderly patients.10, 11, 19 Muta et al. compared results of 25 elderly (ages 65–76 years) MM patients undergoing AHCT with a younger control group (aged 51–64 years) receiving Mel 100–120mg/m2 or Mel 180–200mg/m2 showing similar outcomes.10 Colleagues from the Mayo Clinic, Rochester recently reported data on 50 newly diagnosed MM patients ≥ 75 years receiving first AHCT between 2005 through 2020.11 Fifty percent of patients received lower dose Mel140 mg/m2 while 48% patients safely completed AHCT without requiring hospitalization. Fever or infection (32%), cardiac arrhythmia (36%), and dehydration (32%) were the most common reasons requiring hospitalization, (n = 26, 52%). Median OS and PFS were 82 months and 33 months, respectively showing feasibility of this treatment. 100-day TRM was 2% (n=1).
Another important finding in our study includes a significant gender and race disparate utilization rates for AHCT utilization in MM. In a CIBMTR study from 1995–2005, it was noted that African American recipients were younger but more likely to be transplanted later in their disease course compared to whites.20 The overall outcomes after AHCT were similar for both races despite the delay in administration of the AHCT. In a recent study of 28,450 MM patients receiving AHCT, lower stem cell utilization rate (STUR) was noted amongst Hispanics (8.6%−16.9%) and non-Hispanic Blacks (12.2%−20.5%) than non-Hispanic whites (22.6%−37.8%).5 Fewer patients ≥60 years received AHCT among Hispanics (39%) and non-Hispanic blacks (42%) versus non-Hispanic whites (56%). Costa et al. show 13.8% of AHCT utilizations were affected by racial ethic disparities from 2005–2009.6 In our study, we also note that utilization rates of AHCT is lower in African American females (1.88–2.12%, P <0.01) compared to African American males (3.53–3.97%, P 0.01). While there are increasing trends in utilization of early AHCT in the treatment of MM from 1995–2010,7 the SEER-18 study6 showed lower relative utilization of AHCTs to new cases in non-Hispanic blacks, Hispanics, and Asians. The reasons for underutilization of AHCT in older females are beyond the scope of this study but perhaps reflect differences in referral patterns, lack of caregiver support or patient preferences.
With the advent of multiple newer agents and combinations in the treatment of multiple myeloma, the role of transplant needs to be balanced with other combinations. Induction regimens that include novel agents like daratumumab has become a standard induction regimen in ‘transplant-ineligible’ MM with a PFS at 1-year of 83.5%21 which is comparable to the 1-year PFS after AHCT in our current study of elderly patients (Table 2, Figure 1). Other advantages that may favor one treatment option over another would include cost and ongoing treatment burden for patients. Furthermore, patients would still have the option of using these at post-transplant relapse rather than using AHCT versus DRd.
The main limitation of our study is that we are only studying patients who underwent AHCT and do not have a comparison group of similar patients who did not receive AHCT. As shown by the transplant utilization rate, this is a highly selected patient population. Since we restricted our analysis to upfront AHCT within 12 months of diagnosis, it is possible, though unlikely, that there are patients ≥75 years who receive delayed AHCT at relapse. Our study does not include details of maintenance therapies and can only postulate that 27% patients may have received some form of maintenance based on what was reported as an intent to use maintenance therapy on the data collection forms though this cannot be confirmed. Finally, geriatric assessment and/or frailty testing as well patient-reported outcomes data would have added immense value to the data, but these are not routinely practiced across all centers nor captured in the CIBMTR data forms at this time.
In conclusion, our data demonstrate that AHCT is safe and effective in selected MM patients ≥ 75 years and that age by itself should not be a criterion to avoid AHCT referral. These patients should be referred to transplant centers, just as their younger counterparts, and be given the opportunity for thorough evaluation to determine transplant eligibility.22 The use of comprehensive geriatric assessments needs to be studied to better understand functional capabilities of these patients and their ability to utilize full dose Mel when appropriate.
Data Sharing:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Supplementary Material
Acknowledgements
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); U24HL138660 from NHLBI and NCI; OT3HL147741, and U01HL128568 from the NHLBI; HHSH250201700006C, and HHSH250201700007C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Additional federal support is provided by P01CA111412, R01CA152108, R01CA215134, R01CA218285, R01CA231141, R01AI128775, R01HL126589, R01HL129472, R01HL130388, R01HL131731, U01AI069197, U01AI126612, UG1HL06924, and BARDA. Support is also provided by Be the Match Foundation, Boston Children’s Hospital, Dana Farber, St. Baldrick’s Foundation, Stanford University, the Medical College of Wisconsin the National Marrow Donor Program, and from the following commercial entities: Actinium Pharmaceuticals, Inc.; Adienne SA; Allovir, Inc.; Amgen, Inc.; Angiocrine Bioscience; Astellas Pharma US; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Incyte Corporation; Janssen/Johnson & Johnson; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Merck Sharp & Dohme Corp.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Sanofi Genzyme; Stemcyte; Takeda Pharma; Vor Biopharma; Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Conflicts of Interest:
Dr. Munshi reports honorarium from KITE and Incyte as a participant on the speaker’s bureau, none of which are relevant to this work.
Dr. Shah reports grants from Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, other from GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm, Oncopeptides, outside the submitted work.
Dr. Hari reports grants and personal fees from BMS, grants and personal fees from Takeda, grants and personal fees from Amgen, grants and personal fees from Sanofi, grants and personal fees from Janssen, outside the submitted work.
Dr. Kumar reports grants and other from BMS/Celgene, grants and other from Takeda, grants and other from Abbvie, grants and other from Roche, grants from Medimmune, grants from Tenebio, grants from Carsgen, personal fees from Oncopeptides, grants and other from Janssen, outside the submitted work.
Dr. Qazilbash reports other from Janssen, other from Bioline, other from Angiocrine, other from Bioclinica, during the conduct of the study.
Dr. D’Souza reports grants from Sanofi, Takeda, Tenebio, fees from Akcea, Pfizer, Janssen, outside the submitted work.
REFERENCES
- 1.SEER Cancer Statistics Review (CSR) 1975-2016. https://seer.cancer.gov/archive/csr/1975_2016/results_merged/sect_18_myeloma.pdf.
- 2.Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood Adv. 2017;1: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Souza A, Fretham C, Lee SJ, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2020;26: e177–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munshi PN, Vesole D, Jurczyszyn A, et al. Age no bar: A CIBMTR analysis of elderly patients undergoing autologous hematopoietic cell transplantation for multiple myeloma. Cancer. 2020;126: 5077–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schriber JR, Hari PN, Ahn KW, et al. Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: A CIBMTR report. Cancer. 2017;123: 3141–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa LJ, Zhang MJ, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19: 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa LJ, Huang JX, Hari PN. Disparities in utilization of autologous hematopoietic cell transplantation for treatment of multiple myeloma. Biol Blood Marrow Transplant. 2015;21: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar SK, Dingli D, Lacy MQ, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: Results from a matched pair analysis. Am J Hematol. 2008;83: 614–617. [DOI] [PubMed] [Google Scholar]
- 9.El Cheikh J, Kfoury E, Calmels B, et al. Age at transplantation and outcome after autologous stem cell transplantation in elderly patients with multiple myeloma. Hematol Oncol Stem Cell Ther. 2011;4: 30–36. [DOI] [PubMed] [Google Scholar]
- 10.Muta T, Miyamoto T, Fujisaki T, et al. Evaluation of the feasibility and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Intern Med. 2013;52: 63–70. [DOI] [PubMed] [Google Scholar]
- 11.Vaxman I, Visram A, Kumar S, et al. Autologous stem cell transplantation for multiple myeloma patients aged >/= 75 treated with novel agents. Bone Marrow Transplant. 2020. [DOI] [PubMed] [Google Scholar]
- 12.Bashir Q, Chamoun K, Milton DR, et al. Outcomes of autologous hematopoietic cell transplantation in myeloma patients aged >/=75 years. Leuk Lymphoma. 2019;60: 3536–3543. [DOI] [PubMed] [Google Scholar]
- 13.Mohyuddin GR, Koehn K, Costa L, Kumar SK, McClune B. Enrolment of racial minorities across 15 years of multiple myeloma randomised trials; calling on researchers to become agents of change. Lancet Haematol. 2020;7: e704–e706. [DOI] [PubMed] [Google Scholar]
- 14.Bashir Q, Shah N, Parmar S, et al. Feasibility of autologous hematopoietic stem cell transplant in patients aged >/=70 years with multiple myeloma. Leuk Lymphoma. 2012;53: 118–122. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118: 4519–4529. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2017;23: 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma M, Zhang MJ, Zhong X, et al. Older patients with myeloma derive similar benefit from autologous transplantation. Biol Blood Marrow Transplant. 2014;20: 1796–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy PL Jr., Hahn T, Hassebroek A, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19: 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhakal B, Nelson A, Guru Murthy GS, et al. Autologous Hematopoietic Cell Transplantation in Patients With Multiple Myeloma: Effect of Age. Clin Lymphoma Myeloma Leuk. 2017;17: 165–172. [DOI] [PubMed] [Google Scholar]
- 20.Hari PN, Majhail NS, Zhang MJ, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2010;16: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016;375: 1319–1331. [DOI] [PubMed] [Google Scholar]
- 22.Mikhael J, Ismaila N, Cheung MC, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019;37: 1228–1263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


