Abstract
Hyperbaric oxygen treatment (HBOT)—the administration of 100% oxygen at atmospheric pressure (ATA) greater than 1 ATA—increases the proportion of dissolved oxygen in the blood five- to twenty-fold. This increase in accessible oxygen places the mitochondrion—the organelle that consumes most of the oxygen that we breathe—at the epicenter of HBOT’s effects. As the mitochondrion is also a major site for the production of reactive oxygen species (ROS), it is possible that HBOT will increase also oxidative stress. Depending on the conditions of the HBO treatment (duration, pressure, umber of treatments), short-term treatments have been shown to have deleterious effects on both mitochondrial activity and production of ROS. Long-term treatment, on the other hand, improves mitochondrial activity and leads to a decrease in ROS levels, partially due to the effects of HBOT, which increases antioxidant defense mechanisms. Many diseases and conditions are characterized by mitochondrial dysfunction and imbalance between ROS and antioxidant scavengers, suggesting potential therapeutic intervention for HBOT. In the present review, we will present current views on the effects of HBOT on mitochondrial function and oxidative stress, the interplay between them and the implications for several diseases.
Keywords: hyperbaric oxygen treatment (HBOT), mitochondrial function, reactive oxygen species (ROS), superoxide dismutase (SOD), neuroinflammation, oxidative stress, SIRT1, HIF1a, Nrf2, hyperoxic–hypoxic paradox
1. Introduction
Under normal conditions, most of the oxygen that we consume is carried by hemoglobin, which is ~98% saturated at sea-level pressure. A small fraction of oxygen that is not carried by hemoglobin is dissolved in the plasma. HBOT elevates the partial pressure of oxygen in the blood and tissues [1]. Following Henry’s Law, the percentage of dissolved oxygen increases as the partial pressure of oxygen rises. At sea level, the percentage of dissolved oxygen in the blood is 0.32, and at 2.5 ATA, it is 5.62 [1]. This 20-fold increase in dissolved oxygen in the blood reaches all body tissues, providing excess oxygen to tissues that are suffering from a lack of delivered oxygen. Hence, HBOT has been used to treat many diseases [2,3,4,5,6,7,8,9] and was shown to improve cognition in several brain disorders [10,11,12,13,14]. Although oxygen is needed for energy production in the form of adenine triphosphate (ATP), it can sometimes have deleterious effects when it interacts with other molecules, exchanges electrons and is transformed into reactive oxygen species (ROS). These ROS can damage tissues by “stealing” electrons from lipids, proteins, DNA, etc., rendering them inactive or reactive themselves. ROS have several sources and different forms, e.g., superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl (HO), alkoxy (RO) and more. These free radicals are cleared by enzymatic and nonenzymatic antioxidants. Enzymatic antioxidants change the ROS into nonharmful molecules (such as water, or back to oxygen) and they include superoxide dismutase (SOD), catalase, heme oxygenase 1 (HO-1), thioredoxin and glutathione-dependent peroxidase (GPx) and reductase(s) [15]. Alongside these enzymes, nonenzymatic antioxidants and endogenous radical scavengers such as vitamin C, vitamin E, glutathione, melatonin, uric acid and β-carotene also reduce ROS levels [16,17] by donating an electron to neutralize the unstable reactive species. Although HBOT has been found to elevate ROS production, especially via the mitochondria—the organelle that consumes most of the oxygen in an organism, studies have shown that HBOT also elevates antioxidant levels and activity, thereby efficiently reducing ROS levels. In the following review, we discuss the mechanism governing ROS production and the interplay between HBOT, mitochondrial function, ROS and antioxidative species.
2. Mitochondrial Function and Oxidative Stress
2.1. Mitochondrial Function
Mitochondria are found in most cells in which the biochemical process of respiration utilizes oxygen to create energy. The common endosymbiosis hypothesis claims that 1.5 billion years ago, mitochondria were infused into the cytoplasm, providing aerobic cellular respiration. Mitochondria also mediate other cellular processes and homeostatic mechanisms, such as apoptosis [18], autophagy [19], cell-cycle control [20], Ca2+ level regulation [21] and synaptic plasticity [22]. Mitochondria consume roughly 85–90% of the oxygen that we breathe and are not only the major source of ATP production, but also the main source for ROS generation in the cell. As HBOT elevates oxygen levels in all tissues, it is likely that its main molecular target is the mitochondrion. At the cellular level, HBOT can improve mitochondrial redox, preserve mitochondrial integrity, activate transcription factors, alleviate oxidative stress and promote neuroprotection. At the systemic level, it can mitigate harmful disease effects by reducing intracranial pressure [23], inducing angiogenesis [24] and increasing the release of neurotrophins [25].
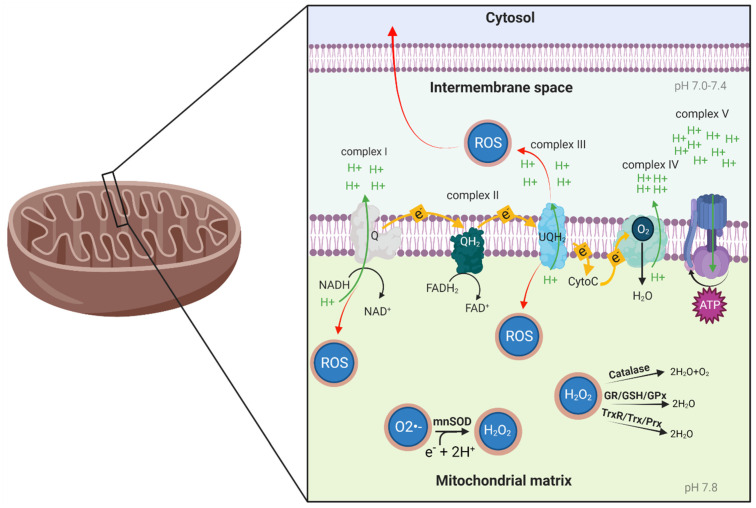
Mitochondria are double-membrane organelles that harness most of the energy required by cells to grow, function and reproduce. The production of the cell’s energy currency, ATP, occurs at the inner mitochondrial membrane. One of the key roles of this membrane is to act as a barrier for positively charged particles—protons. This separation is used by the oxidative phosphorylation complexes (Figure 1) to pump protons from the mitochondrial matrix to the intermembrane space and create an electrochemical gradient—the mitochondrial membrane potential (∆ψ). The gradient force draws protons through the protein ATP synthase (complex V), turning the rotor subunit (much like a water mill), and this movement is used to create ATP.
Figure 1.
Oxidative phosphorylation process and oxidative stress balance. NADH, a product of the citric acid cycle, is oxidized to NAD+ at complex I, transferring an electron to ubiquinone (Q). At complex II, FADH2 is oxidized to FAD+, transferring another electron to Q. When Q arrives at complex III, it transfers its electrons to cytochrome C (CytoC). At complex IV, the electrons carried by CytoC are drawn to diatomic oxygen (O2) and together with hydrogen, they form H2O. In the electron transport chain process, complexes I, III and IV pump protons from the mitochondrial matrix to the intermembrane space, creating an electrochemical proton gradient and a membrane potential (∆ψ) on the inner membrane of the mitochondria. Complex V (ATP synthase) uses the proton gradient to allow protons back into the mitochondrial matrix, synthesizing ATP in the process. Complexes I and III are the major sites of free radical production; if they release superoxide (O2•−) to the mitochondrial matrix, mnSOD will transform it to H2O2. Then, depending on cell specificity, H2O2 will be transformed to O2 and H2O by catalase, or to H2O by glutathione reductase/glutathione peroxidase (GR/GPx) or thioredoxin reductase/peroxiredoxin (TrxR/Prx) activity, in the presence of respiration products (i.e., NADPH).
The electron transport chain (ETC) begins with the arrival of the energy-rich nicotinamide adenine dinucleotide (NADH) to respiratory complex I and flavin adenine dinucleotide (FADH2) to complex II. These molecules contain electrons with high transfer potential. The electrons are then shuffled from complex I to complex IV using electron carriers, releasing energy that is utilized for proton pumping and the creation of ∆ψ. The last electron acceptor is oxygen. Complex IV transfers electron to oxygen and, along with hydrogen, creates water molecules (Figure 1). Therefore, oxygen must be present for the oxidative phosphorylation process to occur, and decreased oxygen concentration (hypoxia) is a major stressor disrupting aerobic functions, especially ATP production. There are several factors influencing the oxidative phosphorylation process, such as Ca2+ regulation, ROS production and availability and solubility of O2 (which can change under different environmental conditions, development of pathology or exercise). All of these processes affect mitochondrial function and ATP production [26].
2.2. Oxidative Stress
Oxidative stress is an imbalance between free radicals and antioxidants in the body, which can potentially cause DNA, protein and tissue damage. In some cases, the natural processes in our cells have deleterious outcomes, such as the production of partially reduced oxygen (free radicals). Free radicals are chemically unstable and highly reactive molecules that can oxidize other molecules in the cell.
A major site for these redox reactions, potentially resulting in free radicals, is the mitochondrion, and the main source for these free radicals is diatomic oxygen which is partially reduced in the mitochondria. In the normal ETC process, electrons will be carried to complex IV on the electron carrier (cytochrome C) and with hydrogen ions and oxygen, the final electron acceptor, will produce 2 water molecules. However, aberrations in this process can occur and produce ROS. There are several reasons for this, among them electron leakage from the electron carriers. In the ETC, a fraction of the oxygen can be reduced to superoxide (O2•−) [27]. Most of these sites release O2•− into the mitochondrial matrix, and a few of them release it into the intermembrane space [28] (Figure 1). When released into the mitochondrial matrix, O2•− is converted to hydrogen peroxide (H2O2) by the antioxidant enzyme manganese superoxide dismutase (mnSOD). The H2O2 is then converted to water and oxygen by the antioxidant enzyme catalase, or to water by peroxidases, which also reside in the mitochondrial matrix, depending on the cell type (Figure 1). Catalase is highly expressed in heart and liver mitochondria [29,30], while in the brain, catalase is confined to peroxisomes, and its level in the mitochondria is low [31]. Brain mitochondria are enriched with the peroxidase systems GSH/glutathione peroxidase (GPx) and Trx2/peroxiredoxin 3 and 5 (Prx3 and Prx5) that can detoxify H2O2 in the presence of respiration products (i.e., NADPH). The TrxR/Trx/Prx pathway was estimated to contribute to 70–80% H2O2 removal by brain mitochondria, and the GR/GSH/GPx pathway to an additional 10–20%. The remaining 10% is handled by non-enzymatic scavenging mechanisms [32]. If O2•− is released to the intermembrane space, it can leave the mitochondria as ROS and cause cell damage.
ROS are a frequent byproduct of all aerobic oxygen metabolism. ROS play a detrimental role in processes such as apoptosis [33], hyperglycemia, diabetes [34], protein aggregation, neurodegeneration [35] and cancer [36] and also participate in cell signaling [37].
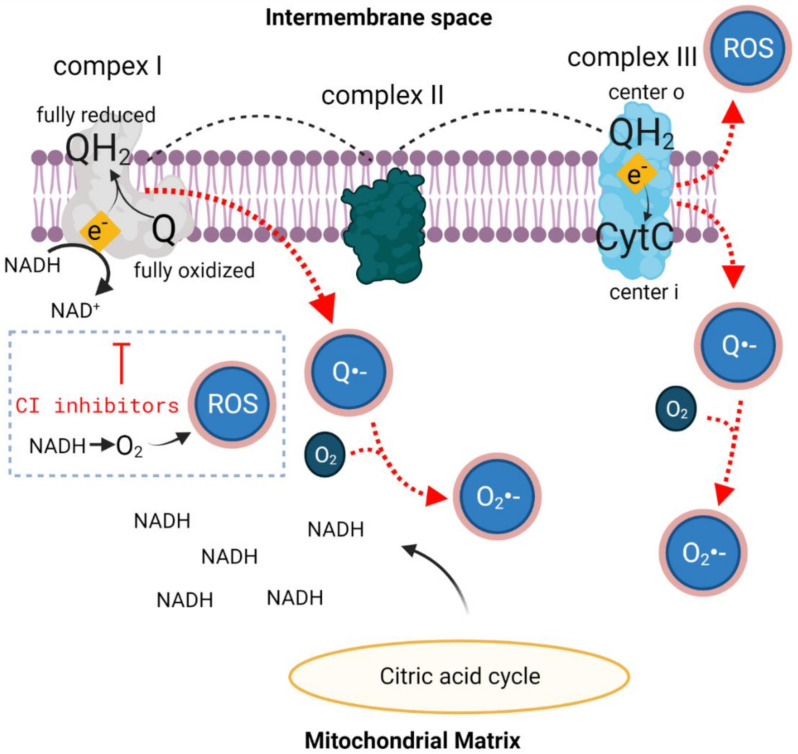
The conversion of oxygen to ROS is mainly a function of metabolic rate. Complexes I and III of the ETC are especially prone to electron leakage and O2•− production (Figure 2). Complex I activity is considered a rate-limiting step for the mitochondrial respiratory chain and is therefore an important factor in the regulation of oxidative phosphorylation. Both defective complex I activity due to lack of substrates such as pyruvate, malate, glutamate, etc. [38], and inhibition of its activity [39] elevate ROS levels. Studies on isolated complex I have shown two main ROS-producing mechanisms, involving flavin mononucleotide (FMN) at the NADH-binding site and ubiquinone (Q) at the electron carrier-binding site. FMN initiates the ETC in complex I, where it receives electrons from NADH and passes them on through a series of iron-sulfur (Fe-S) redox clusters to the first electron carrier, Q. Once FMN is fully reduced, O2•− can be produced; if there is a dwindling NADH pool that will not bind to this site, O2 will come in contact with FMN and create ROS (Figure 2) [40]. Another pathway through which complex I creates O2•− is formation of the free-radical semiquinone anion species (Q•−) (Figure 2). Mitochondrial Q transfers electrons between dehydrogenases and the redox pathways of the ETC. Once formed, Q•− is highly reactive and will transfer its electron to molecular oxygen to produce O2•−. Studies show that complex I inhibitors, such as rotenone and piericidin A, which block the transfer of electrons from the Fe-S centers to the ubiquinone pool, increase ROS production (Figure 2) [41,42,43] because NADH can react directly with O2 to create either H2O2 or O2•− (Figure 2) [44]. These findings suggest that the rate of NADH production (from the citric acid cycle) and the ratio of O2 to NADH are crucial. In support of this, when rotenone was added to cells overexpressing mnSOD, the rate of ROS production was significantly lower than in controls. However, when rotenone was added to cells with defective mitochondria, it did not change the rate of ROS production [45], supporting the notion that mitochondria are the main source of ROS.
Figure 2.
Semiquinone and ROS formation in complex I and complex III. The electron transport chain begins with the arrival of NADH to complex I from the citric acid cycle. NADH gives its electrons to ubiquinone (Q), which is the first electron carrier. This electron transfer can yield an either fully reduced ubiquinol (QH2)—the favorable outcome, or a semi reduced semiquinone (Q•−)—the ROS outcome. At complex III, electron transfer between QH2 and the next electron carrier, cytochrome C (CytC), can also result in the fully reduced CytC or Q•−. These redox reactions can yield ROS when homeostatic conditions change. Since NADH is a redox-ready molecule, it has a high potential to reduce other molecules in its surroundings. In the situation when complex I’s NADH binding site is occupied, especially when complex I inhibitors are used and there is a large pool of NADH arriving from the citric acid, NADH and O2 could react to create ROS. A plethora of O2 in the tissue, such as in HBOT, can increase interaction between O2 and redox-ready molecules such as NADH or Q•−.
An additional pivotal site for diatomic oxygen conversion to ROS is ETC complex III. At complex III Q•−, the intermediate of ubiquinol oxidation can create ROS by giving the remaining electron directly to O2 [41] (Figure 2). There are two sites of ROS production in complex III: the o center, oriented toward the intermembrane space and the i center, oriented toward the mitochondrial matrix [46]. At the matrix, ROS scavengers can neutralize these reactive molecules and reduce mitochondrial and cell damage. In contrast, the o site releases ROS toward the intermembrane space, away from matrix antioxidant defenses, facilitating ROS release to the cytosol.
3. HBOT, Mitochondrial Function and Oxidative Stress
In the last two decades, the effects of HBOT on mitochondrial function have been examined using a variety of protocols. However, these protocols apply different pressures (ranging from 1.5 ATA to 2.5 ATA), time in the chamber (1 to 4 h) and number of treatments (1 to 60), leading to different conclusions on HBOT’s effects (Table 1). Interestingly, while 1 to 5 consecutive treatments mainly lead to a reduction in mitochondrial function, 20 to 60 consecutive treatments lead to a significant improvement in mitochondrial parameters [47,48,49,50]. Animal studies have shown that following one to five treatments, HBOT induces loss of ∆ψ (indicating reduced ETC integrity) and initiation of the mitochondrial apoptotic pathway. These adverse effects do not appear when HBOT is repeated for more than 20 days, and studies that performed treatments for 20 to 30 days showed beneficial effects on mitochondrial activity and metabolism (see more details in Table 1). Hence, HBOT shows promising effects on mitochondrial activity (ETC complexes, ∆ψ, apoptosis and ATP production) and is thus predicted to improve mitochondria function in diseases and conditions that exhibit mitochondrial dysfunction, but only when the treatment is long-term.
Table 1.
Effects of HBOT on mitochondrial activity in different pathologies and protocols.
| Study | Treatment Length | Pressure | Effect on Mitochondria | Disease/Condition |
|---|---|---|---|---|
| Kurt et al., 2008 [51] | 4 weeks | 3 ATA | Increased energy production | Healthy |
| Dave et al., 2003 [52] | 30 days | 2 ATA | Improved complex IV activity | Wobbler mice (model for amyotrophic lateral sclerosis (ALS)) |
| Tian et al., 2013 [53] | 20 days of 1 h treatments | 2 ATA | Reduced mitochondria-mediated apoptosis signaling (increased Bcl-2 and decreased Bax) | Amyloid-β25-35-injected rats |
| Pan et al., 2015 [54] | 14 days of 1 h treatments | 2.5 ATA | Reduced mitochondria-mediated apoptosis signaling (increased Bcl-2 and decreased Bax) | Rat model of Parkinson’s disease |
| Botigeli Baldim et al., 2013 [47] | A single treatment of 1 h | 2 ATA | Reduced baseline mitochondrial consumption rate | Ischemia-induced rats |
| Zhou et al., 2007 [49] | A single treatment of 1 h | 1.5 ATA | Increased ATP levels | Traumatic brain injury |
| Palzur et al., 2008 [48] | 4 treatments (twice, 2 consecutive) of 45 min each | 2.8 ATA | Reduction in mitochondrial membrane potential and an increase in caspase 8 activity level | Focal brain injury |
| Zhao et al., 2020 [55] | Twice daily 90-min treatments for either 1, 2 or 3 days | 2.5 ATA | Day 1—increased mitochondrial apoptosis activation mediated by Bcl2/Bax ratio. Reduced caspase 3 and 9 activity in damaged area. Increased ATP levels. Day 2—similar results. Day 3—no activation of protein apoptosis or differences in energy production | Pancreatitis-induced rats |
| Han et al., 2017 [50] | Five 1 h treatments | 2.4 ATA | Reduction in ∆ψ after HBOT in control group, but not in the mitophagy-inhibited group | Mitophagy-inhibited rats |
| Shams et al., 2017 [56] | Five 1 h treatments | 2 ATA | Reduced apoptosis in HBOT rats (compared to nontreated sciatic nerve-damaged rats) | Rats with sciatic nerve damage |
Although HBOT is thought to lead to higher levels of oxidative stress due to excess ROS generation by the mitochondria, it is hypothesized that as part of the protective response, the cells reduce their mitochondrial activity to lower ROS production and to alleviate the oxidative stress [57]. It is therefore possible that early elevation of ROS following single (1–5) HBOTs reduces mitochondrial activity, thereby reducing additional ROS production. This might explain the previously mentioned reduction in mitochondrial activity after 1 to 5 HBOTs.
As the hyperoxic state during HBOT can increase ROS production, one can speculate that reoccurring treatments might cause excessive generation of ROS. However, until recently, it was not clear if indeed ROS accumulate following repeat HBOTs and if these ROS also have beneficial effects on the cells. As discussed below, repetitive cycles of HBOT do not necessarily cause excess generation of ROS, and it is now known that these ROS and reactive nitrogen species (RNS) also serve as signaling molecules in transduction cascades that support healing, cell survival and proliferation through the activation of major factors, such as nuclear factor erythroid 2-related factor 2 (Nrf2), HIF1α, SIRT1, vascular endothelial growth factor (VEGF) and other growth factors and hormones [58,59,60,61,62,63]. It should also be noted that in parallel to the elevation in ROS levels, antioxidant pathways are activated. Hence, the balance between free radical levels and antioxidant levels and activity will determine the extent of the oxidative stress. Bosco et al. [64] showed that under 1.5 or 2.5 ATA, changes in ROS or antioxidant scavenger activity go back to baseline within approximately a month.
Usually, for research purposes and in the clinic, HBOT is administered at between 2 to 3 ATA. Above this pressure, where the levels are referred to as supranormal, the main outcome is a clear increase in bio-oxidative products and saturation of antioxidants, leading to enhanced oxidative damage [65,66]. Exposure to high oxygen levels for a long duration can cause oxygen toxicity and may lead to systemic damages such as seizures, lung malfunction or retinopathy of prematurity [67]. The occurrence of oxygen toxicity and seizures is very low [68]; however, to minimize side effects and complications, HBOT should be provided by a certified and trained medical staff using strict operational protocols, including pre-therapy evaluations, appropriate exclusion criteria and in-chamber monitoring [68,69].
To reduce the chances of damage, exposure to HBOT in the clinic is rather brief (generally 1 h per treatment) and at pressures lower than 3 ATA, most commonly 2 ATA. In some cases, intermittent HBOT protocol is used, where oxygen levels are changed several times during a single treatment [13,70]. As a result, if HBOT is administered within therapeutic and approved limits, an antioxidation process mostly accompanies the rise in oxidation products (Table 2) and is adequate to counter the oxidative stress damage. Moreover, following the HBOT session, the reversal of oxidation products occurs approximately half an hour earlier than that of the antioxidant enzymes’ activities [71,72], which supports the safety of HBOT within therapeutic ranges.
Table 2.
Effects of HBOT on oxidative stress balance in different pathologies and protocols.
| Study | Treatment Length | Pressure | Effect on Oxidative Stress | Disease/Condition |
|---|---|---|---|---|
| Zhou et al., 2018 [73] | One treatment of 60 min | 2.7 ATA | ROS were significantly elevated in the mitochondria and in the cell | HUVEC culture |
| Dennog et al., 1996 [74] | A total of 75 min—three 20 min treatments with a 5 min break between | 2.5 ATA | Oxidative DNA damage in the leukocytes | Healthy human volunteers |
| Topuz et al., 2010 [75] | One treatment of 90 min | 2.4 ATA | Prevented elevation in lipid peroxidation observed in nontreated group and increased antioxidant activity | Spinal cord injury in mice |
| Oscarsson et al., 2017 [76] | 20 treatments of 90 min | 2 ATA | Elevated levels of DNA oxidation and of SOD2, HO-1 and Nrf2 expression | Irradiated rats |
| Matsunami et al., 2010 [77] | 7 days of 2 h treatment | 2.8 ATA | Elevated lipid peroxidation and decreased SOD activity in treated diabetic rats | Induced diabetic rats |
| Simsek et al., 2012 [65] | 1, 2, 3, 4, 6 or 8 weeks of 90 min treatments | 2.8 ATA | ROS and radical scavenger enzyme levels in rat brain were not significantly altered | Healthy rats |
| Rothfuß et al., 1998 [78] | A total of 75 min—three 20 min treatments with a 5 min break between | 2.5 ATA | Increased antioxidant activity lasting for at least 1 week | Healthy human volunteers |
| Körpınar and Uzun, 2019 [79] | 3 treatments of 1 h (within 24 h) | 2 ATA | Significant elevation of lipid peroxidation (MDA) and reduced antioxidant SOD levels in the plasma | Healthy rats |
| 3 treatments of 1 h (within 24 h) | 2.4 ATA | |||
| 15 treatments of 1 h (within 10 days) | 2 ATA | No significant change in lipid peroxidation (MDA) and antioxidant SOD levels in the plasma | ||
| 15 treatments of 1 h (within 10 days) | 2.4 ATA | |||
| Hu et al., 2014 [80] | 1–4 days of 1 h treatments twice a day | 2 ATA | Decreased levels of lipid peroxidation. GPx, SOD and Gr(glutathione reductase) levels increased after 1 treatment, and these levels increased further on days 2, 3 and 4 compared to the first day | Cerebral artery occlusion |
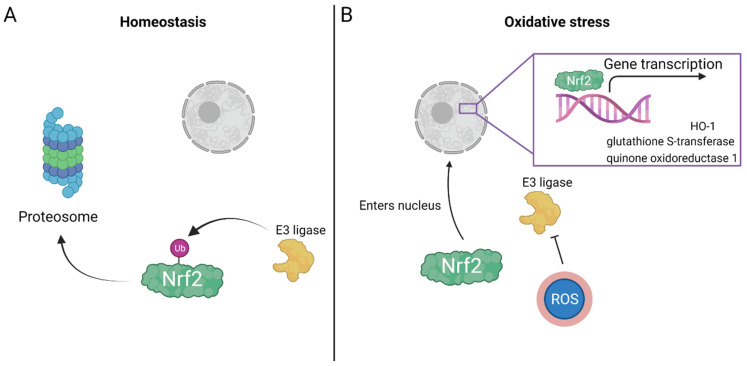
Mechanistically, HBOT induces activation of transcription factors and gene expression, evoking antioxidant enzymatic activity, especially through the Nrf2 pathway. Nrf2 is a redox-sensitive transcription factor that is involved in cellular defense mechanisms and acts on several known target genes, including HO-1, quinone oxidoreductase 1 and glutathione S-transferase [81], all of which have detoxification properties, reducing the free radical load (Figure 2). However, activation of these transcription factors depends on the partial oxygen pressure. Mild hyperoxia (30% O2) initiates activation of HIF1α [82] and decreases Nrf2 levels in the nucleus [83], and administration of 100% O2 elevates Nrf2 levels in the nucleus and upregulation of Nrf2-regulated genes [84]. One of the major molecules supporting tolerance of the organism to oxidative damage is HO-1 [85], also called heat-shock protein (HSP) 32, a ubiquitously expressed multitasking enzyme that has neuroprotective action [85]. Under basal conditions, Nrf2 is found in a ubiquitinated state and is degraded in the proteasome (Figure 3), whereas in the presence of oxidative stress and electrophiles (reactant species willing to accept electrons), the ubiquitin E3 ligase complex is modified and Nrf2 is stabilized [86]. Once stabilized, it is transported to the nucleus where it activates its known antioxidant response (Figure 3) by acting on the aforementioned gene targets [58,87]. Indeed, after 25 HBOT sessions, tissue levels of Nrf2, along with its downstream targets, were significantly increased compared to those in nontreated control patients [88].
Figure 3.
Nrf2 enters the nucleus under oxidative stress conditions. (A) At basal conditions, the E3 ligase targets Nrf2 for ubiquitination and marks it to be sent to the proteosome for degradation. Thus, Nrf2 does not enter the nucleus. Under oxidative stress conditions, free radicals can attach to the E3 ligase creating a conformational change that inhibits its binding to Nrf2 and, by this, allows Nrf2 to enter the nucleus. (B) In the nucleus, Nrf2 initiates gene transcription of protective genes, such as the antioxidants HO-1, glutathione S-transferase, quinone oxidoreductase 1.
In summary, short-term HBOT creates stress and causes mitochondria to reduce their activity, which partially decreases ROS production. However, in long-term HBOT, antioxidant scavenger activity is elevated, helping the mitochondria function without disturbing the redox balance and even enhancing their activity.
4. The Interplay between SIRT1, HIF1a and ROS during HBOT
Most HBOT protocols consist of not only breathing 100% oxygen under high atmospheric pressure, but also intermittent fluctuations in oxygen [13,70]. These fluctuations occur between the daily HBOTs as oxygen levels return from 100% to 21% at the end of the daily treatment. In addition, a unique oxygen fluctuation protocol changes oxygen levels from the physiological 21% oxygen to 100% oxygen and back to physiological oxygen levels of 21% several times during each treatment [13]. These fluctuations are interpreted by the body as a hypoxic signal—lack of oxygen (moving from 100% to 21%), although hypoxia does not actually occur. These signals and fluctuations activate specific cellular mechanisms including the activation of transcription factors [89] that enhance cell activity and the body’s natural rejuvenation potential. This phenomenon, called the hyperoxic–hypoxic paradox (HHP) or the “normobaric oxygen paradox”, is key to the effect of HBOT [13,70,82,90] and is highly effective at enhancing subjects’ performance.
During such intermittent HBOT protocol, two phenomena occur sequentially and repeatedly—hyperoxia and apparent hypoxia. This recruits two additional important factors that affect mitochondrial function, mitochondrial biogenesis and oxidative stress: hypoxia-induced factor (HIF1a) and SIRT1, a class III histone deacetylase. SIRT1 belongs to a large family of sirtuins also known as longevity proteins. HIF1a is important for many processes, including the formation of red blood cells via the transcription of erythropoietin and activation of VEGF to increase blood vessel formation, both of which are needed to combat the hypoxic state [91]. The intermittent HBOT raises HIF1a activity at a systemic level [82]. In addition, elevation of oxygen activates another transcription factor, Nrf2, that regulates the expression of antioxidant proteins [70,90] as detailed above.
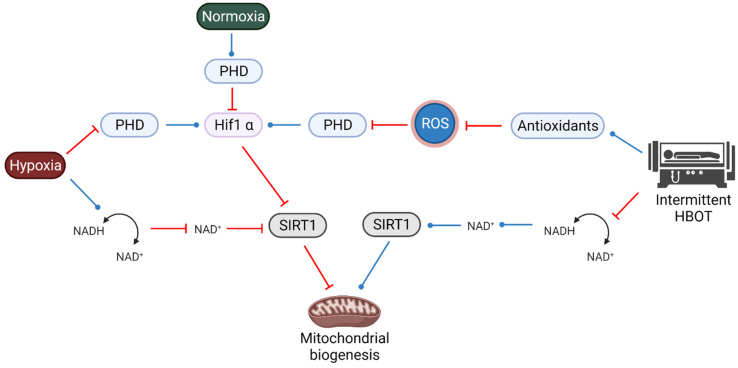
SIRT1 utilizes nicotinamide adenine dinucleotide (NAD+) as a substrate and is involved in neuronal survival, cell proliferation, metabolic dysfunction and in modulating the stress response. During normoxia, prolyl hydroxylase domain (PHD) proteins are activated and destabilize HIF1α (Figure 4). Hypoxia, through the inhibition of PHD, stabilizes HIF1α, which interacts with HIF1α to form an active HIF.
Figure 4.
Interplay between SIRT1, HIF1a and ROS during HBOT. Under normoxia, PHD senses oxygen and leads to the degradation of HIF1a. Hypoxia inhibits PHD, leading to an increase in HIF1a, while intermittent HBOT elevates antioxidants, which leads to inhibition of PHD, therefore mimicking a hypoxic state and also activating HIF1a. In a parallel by interwind pathway, hypoxia causes inhibition of SIRT1 via changes in NADH/NAD+ ratio that can lead to inhibition of mitochondrial biogenesis, while intermittent HBOT activates SIRT1, leading to an activation of mitochondrial biogenesis.
Hypoxia also reduces NADH consumption and the level of NAD+, which inhibits SIRT1 (Figure 4). Reduced SIRT1 activity reduces mitochondrial biogenesis. On the other hand, the hyperoxic state during HBOT increases NADH consumption and NAD+ levels, hence activating SIRT1 and increasing mitochondrial biogenesis. Nevertheless, the effect of the apparent hypoxia during intermittent HBOT can also inhibit SIRT1 and reduce the net effect of hyperoxia on mitochondrial biogenesis.
On a parallel but intertwined pathway, HIF1α also affects SIRT1 and is affected by ROS (Figure 4). As mentioned above, during intermittent HBOT, due to formation of excess ROS scavengers and antioxidants, the level of ROS decreases. As PHD proteins need ROS for their activity, PHD level decreases, and consequently, HIF1α level is expected to increase (even though this is not a hypoxic state). As HIF1α inhibits SIRT1, this can reduce mitochondrial biogenesis. In addition, oxidative stress elevates Nrf2 as described above [84,88]. Future studies should thoroughly investigate the interplay between various key factors, such as HIF1a and SIRT1, which are important for many processes, including neuroprotection, mitochondrial biogenesis and rejuvenation. These factors are affected not only by hypoxia, but also by the intermittent hyperoxia–hypoxia cycles that are commonly practiced in HBOT [13,70] and change according to the partial pressure subjected to the patient. A recent study suggests that at a lower pressure (30%), HIF1a is activated, but only for the adjacent time after the HBOT exposure. At a higher pressure (100%), HIF1a is also activated right after the HBOT, but above that (140%), HIF1a is no longer active [90].
Interestingly, moderate normobaric intermittent hypoxia (exposure to 10 cycle/day of 10% oxygen for 6 min for 21 days) showed improvement in the 3xTg-AD mouse model and was associated with increase in BDNF, erythropoietin (EPO) and improved spatial memory [92,93]. The exact mechanisms are still not known, but it is expected that HIF1a and the mitochondria are central in this process [94,95,96].
It should be noted that changes in SIRT1 have also been detected during and following physical exercise [97]. Similar to HBOT, physical exercise increases the consumption of oxygen and leads to elevation of ROS. This is accompanied by elevation of antioxidant and oxidative damage repair mechanisms, including the thiol antioxidants glutathione and thioredoxin. The antioxidant activity compensates for the exercise-induced ROS production and is part of the cellular and physiological processes involved in exercise [97]. Interestingly, exercise restores the level of SIRT1 in patients with neurodegenerative diseases and attenuates the severity of these diseases [98]. More research aimed to understand the effects of intermittent HBOT, physical exercise and intermittent hypoxia will lead to better understanding of the mechanisms of action and advantages and may lead to synergistic treatments or improvement in performance of these treatments.
5. Disease and Oxidative Stress
Oxidative stress is associated with the disease course of cancer, diabetes, neurodegenerative disorders and more. Many studies have shown that this association alters metabolic rate, which in turn contributes to development of the pathophysiology. Hyperglycemia induces oxidative stress by elevating ROS production and interfering with ROS-scavenging activity. Targets for oxidative stress in the beta cell are likely to include PDX-1, a transcription factor that plays an important role in pancreas development and differentiation, as well as in maintaining normal beta-cell function. Thus, when cells are exposed to H2O2 and elevated ROS production, PDX-1 is inhibited, and consequently, insulin gene expression is compromised [99,100]. These reactive species interact with transcription factors, increase endothelial dysfunction and dysregulate enzymatic activity, leading to vascular inflammation [101]. Diabetic patients receiving HBOT show increased level of lipid oxidation after the first treatment, yet after the 15th treatment, these patients returned to basal level (before the first treatment) [102]. The authors hypothesized that this effect could be explained by increased activation of antioxidant enzymes. In cancer cells, the redox homeostasis is altered, which renders the cells resilient to exogenous stressors. ROS can both initiate cancer and contribute to its progression [103]. Some of the anticancer drugs that are in clinical use today act by regulating oxidative stress and elevating ROS production to initiate apoptosis of the mutated cells [104]. While irradiation therapy increases oxidative stress markers (such as DNA oxidative damage), treatment of HBO reversed this effect by elevating the antioxidant SOD and HO-1 activity [76]. Other pathologies that go hand in hand with oxidative stress are neurodegenerative diseases. In Alzheimer’s disease, brain regions with high concentrations of the aggregated amyloid-β (either 1–40 or 1–42) show higher levels of oxidative stress markers [105], whereas brain regions with a low concentration of amyloid-β aggregates do not show elevation in oxidation products [106]. Yatin et al. [107] showed that knocking out methionine residue 35 on amyloid-β1–42 prevents the creation of free radicals in vitro, thus demonstrating the involvement of amyloid-β1–42 in the formation of ROS in Alzheimer-diseased brains. Other studies have shown the involvement of the methionine at residue 35 on amyloid-β in neurotoxicity [108]. Methionine is a readily oxidizable amino acid and can undergo 2-electron oxidation to form methionine sulfoxide and cause protein carbonylation [109]. In Huntington’s disease, inducing an antioxidant enzyme in the mitochondria attenuated disease pathophysiology: animals gained more weight, with decreased neuronal death and delayed motor impairments [110]. All of these pathologies (and more) present different etiologies, yet all present with alterations in ROS production. Many mutations and diseases disturb mitochondrial equilibrium which leads to further damage, aggravating the insult to the cells. Mitochondria are the major source of ROS in the cells. Nevertheless, in moderation, ROS are utilized for routine physiological functions. Researchers have been studying the effects of HBOT on neurodegenerative diseases and have found promising results. HBOT on neurodegenerative disease animal models show positive effects on the basal elevated oxidative stress levels. In these studies, HBOT was shown to alleviate the levels of ROS damage and increase antioxidant activity [53,54,111]. As of today, clinical trials focus on the advantageous effects of HBOT on cognitive decline and quality of life for patients suffering from neurodegenerative diseases and show that HBOT improve cognitive functions and ameliorate the reduced brain metabolism in MCI and AD patients [10,11,112,113,114] and in animal models [12,53,111,115,116,117]. To better understand the effects of oxidative stress and antioxidant scavenger activity in clinical trials, more research is needed.
In immune cells, mitochondrial function and metabolism were found to drive responses against bacterial infections and inflammation. ROS damages bacteria and parasites (as bacteria lack antioxidant defenses) and, by this, helps macrophages eliminate them [118]. The adaptive immune system uses ROS to regulate signal transduction by cell-surface receptors. Mitochondrial ROS in affected T cells induce activation and proliferation through secretion of Il-2 (T-cell survival marker), and the addition of antioxidants inhibits T-cell expansion [119]. Similar effects have been found in neutrophil activation. Asehnoune et al. [120] found that ROS initiate neutrophil activation. Once cells are exposed to ROS, NF-κB translocation to the nucleus is enhanced, and cytokine-induced activation of NF-κB could be prevented by antioxidants [121]. HBOT’s mechanism of action in infections is by elevation of ROS [122,123]; such elevation in oxidative stress renders the bacteria more susceptible to damage [124]. NF-κB activation also increases anti-apoptotic-related genes such as Bcl-2 in immune cells [125].
Taken together, this emphasizes the importance of the mitochondria and maintenance of mitochondrial equilibrium with the environment in avoiding oxidative stress. Imbalances can occur as a result of increased free radicals or a decrease in antioxidant defense; either way, inducing the mitochondria to restore normal levels of ROS is a vital step in treating any disease.
6. Summary
HBOT’s effect on oxidative stress and mitochondrial activity changes over the course of the treatment. Short-term treatment (one to five sessions) has been shown to have deleterious effects, though longer treatment (twenty to thirty sessions and more) has beneficial effects. Intermittent HBOT fluctuations induce the HHP, which increases oxidative stress scavenger transcription factors and their subsequent antioxidant enzyme production. As mitochondrial dysfunction and oxidative stress are associated with many different diseases, elevation of antioxidant activity may be a major pathway underlying HBOT’s benefit in the clinic.
Acknowledgments
All figures were created using BioRender.com (accessed on 4 November 2021).
Author Contributions
N.S., I.G. and U.A. were involved in writing—original draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
N.S. is supported by a PhD scholarship from the Tel Aviv University Center for Combatting Pandemics. U.A. was supported by The Aufzien Family Center for the Prevention and Treatment of Parkinson’s Disease at Tel Aviv University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Calvert J.W., Cahill J., Zhang J.H. Hyperbaric oxygen and cerebral physiology. Neurol. Res. 2007;29:132–141. doi: 10.1179/016164107X174156. [DOI] [PubMed] [Google Scholar]
- 2.Glik J., Cholewka A., Stanek A., Englisz B., Sieroń K., Mikuś-Zagórska K., Knefel G., Nowak M., Kawecki M. Thermal imaging and planimetry evaluation of the results of chronic wounds treatment with hyperbaric oxygen therapy. Adv. Clin. Exp. Med. 2019;28:229–236. doi: 10.17219/acem/92304. [DOI] [PubMed] [Google Scholar]
- 3.Kasprzyk-Kucewicz T., Cholewka A., Englisz-Jurgielewicz B., Mucha R., Relich M., Kawecki M., Sieroń K., Onak P., Stanek A. Thermal effects of topical hyperbaric oxygen therapy in hard-to-heal wounds—A pilot study. Int. J. Environ. Res. Public Health. 2021;18:6737. doi: 10.3390/ijerph18136737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gebala-Prajsnar K., Stanek A., Pasek J., Prajsnar G., Berszakiewicz A., Sieron A., Cholewka A. Selected physical medicine interventions in the treatment of diabetic foot syndrome. Acta Angiol. 2015;21:140–145. doi: 10.5603/AA.2015.0024. [DOI] [Google Scholar]
- 5.Lin P.Y., Sung P.H., Chung S.Y., Hsu S.L., Chung W.J., Sheu J.J., Hsueh S.K., Chen K.H., Wu R.W., Yip H.K. Hyperbaric oxygen therapy enhanced circulating levels of endothelial progenitor cells and angiogenesis biomarkers, blood flow, in ischemic areas in patients with peripheral arterial occlusive disease. J. Clin. Med. 2018;7:548. doi: 10.3390/jcm7120548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carturan D., Boussuges A., Vanuxem P., Bar-Hen A., Burnet H., Gardette B. Ascent rate, age, maximal oxygen uptake, adiposity, and circulating venous bubbles after diving. J. Appl. Physiol. 2002;93:1349–1356. doi: 10.1152/japplphysiol.00723.1999. [DOI] [PubMed] [Google Scholar]
- 7.Edwards M., Singh M., Selesny S., Cooper J.S. Hyperbaric Treatment of Thermal Burns. StatPearls Publishing; Treasure Island, FL, USA: 2020. [PubMed] [Google Scholar]
- 8.Cooper J.S., Hanley M.E. Hyperbaric Treatment of Radiation Proctitis. StatPearls Publishing; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 9.Hanley M.E., Manna B. Hyperbaric Treatment of Diabetic Foot Ulcer. StatPearls Publishing; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 10.Gottfried I., Schottlender N., Ashery U. Hyperbaric Oxygen Treatment—From Mechanisms to Cognitive Improvement. Biomolecules. 2021;11:1520. doi: 10.3390/biom11101520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapira R., Efrati S., Ashery U. Hyperbaric oxygen therapy as a new treatment approach for Alzheimer’s disease. Neural Regen. Res. 2018;13:817. doi: 10.4103/1673-5374.232475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapira R., Gdalyahu A., Gottfried I., Sasson E., Hadanny A., Efrati S., Blinder P., Ashery U. Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer’s disease mouse model and in elderly patients. Aging (Albany N. Y.) 2021 doi: 10.18632/aging.203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamat S.M., Mendelsohn A.R., Larrick J.W. Rejuvenation through Oxygen, More or Less. Rejuvenation Res. 2021;24:158–163. doi: 10.1089/rej.2021.0014. [DOI] [PubMed] [Google Scholar]
- 14.Somaa F. A Review of the Application of Hyperbaric Oxygen Therapy in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021;81:1361–1367. doi: 10.3233/JAD-210157. [DOI] [PubMed] [Google Scholar]
- 15.Ighodaro O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 16.Mates J.M., Perez-Gomez C., Nunez de Castro I. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999;32:595–603. doi: 10.1016/S0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 17.Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Susin S.A., Lorenzo K.H., Zamzami N., Marzo I., Snow B.E., Brothers G.M., Mangion J., Jacotot E., Costantini P., Loeffler M., et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-enriquez S., He L., Lemasters J.J. Role of mitochondrial permeability transition pores in mitochondrial autophagy. Int. J. Biochem. Cell Biol. 2004;36:2463–2472. doi: 10.1016/j.biocel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Mcbride H.M., Neuspiel M., Wasiak S. Mitochondria: More than Just a Powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 21.Duchen M.R. Topical Review Mitochondria and calcium: From cell signalling to cell death. J. Physiol. 2000;591:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy M., Faas G.C., Saggau P., Craigen W.J., Sweatt J.D. Mitochondrial Regulation of Synaptic Plasticity in the Hippocampus. J. Biol. Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- 23.Huang L., Obenaus A. Hyperbaric oxygen therapy for traumatic brain injury. Med. Gas Res. 2011;1:21. doi: 10.1186/2045-9912-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan S., Shao G., Yu L., Ren C., Duan S., Shao G., Yu L., Ren C. Angiogenesis contributes to the neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats Angiogenesis contributes to the neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischem. Int. J. Neurosci. 2015;125:625–634. doi: 10.3109/00207454.2014.956101. [DOI] [PubMed] [Google Scholar]
- 25.Schulze J., Kaiser O., Paasche G., Lamm H., Pich A., Hoffmann A., Lenarz T., Warnecke A. Effect of hyperbaric oxygen on BDNF-release and neuroprotection: Investigations with human mesenchymal stem cells and genetically modified NIH3T3 fibroblasts as putative cell therapeutics. PLoS ONE. 2017;12:e0178182. doi: 10.1371/journal.pone.0178182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calbet J.A.L., Martin-Rodriguez S., Martin-Rincon M., Morales-Alamo D. An integrative approach to the regulation of mitochondrial respiration during exercise: Focus on high-intensity exercise. Redox Biol. 2020:101478. doi: 10.1016/j.redox.2020.101478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radi R., Turrens J.F., Chang L.Y., Bush K.M., Crapo J.D., Freeman B.A. Detection of catalase in rat heart mitochondria. J. Biol. Chem. 1991;266:22028–22034. doi: 10.1016/S0021-9258(18)54740-2. [DOI] [PubMed] [Google Scholar]
- 30.Salvi M., Battaglia V., Brunati A.M., La Rocca N., Tibaldi E., Pietrangeli P., Marcocci L., Mondoví B., Rossi C.A., Toninello A. Catalase takes part in rat liver mitochondria oxidative stress defense. J. Biol. Chem. 2007;282:24407–24415. doi: 10.1074/jbc.M701589200. [DOI] [PubMed] [Google Scholar]
- 31.Sinet P.M., Heikkila R.E., Cohen G. Hydrogen Peroxide Production by Rat Brain In Vivo. J. Neurochem. 1980;34:1421–1428. doi: 10.1111/j.1471-4159.1980.tb11222.x. [DOI] [PubMed] [Google Scholar]
- 32.Drechsel D.A., Patel M. Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J. Biol. Chem. 2010;285:27850–27858. doi: 10.1074/jbc.M110.101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta—Mol. Cell Res. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Volpe C.M.O., Villar-Delfino P.H., Dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications review-Article. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X., Guo C., Kong J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012;7:376–385. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogrunc M., Di Micco R., Liontos M., Bombardelli L., Mione M., Fumagalli M., Gorgoulis V.G., D’Adda Di Fagagna F. Oncogene-induced reactive oxygen species fuel hyperproliferation and DNA damage response activation. Cell Death Differ. 2014;21:998–1012. doi: 10.1038/cdd.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paradies G., Petrosillo G., Pistolese M., Di Venosa N., Federici A., Ruggiero F.M. Decrease in Mitochondrial Complex I Activity in Ischemic/Reperfused Rat Heart: Involvement of Reactive Oxygen Species and Cardiolipin. Circ. Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 39.Petrosillo G., Portincasa P., Grattagliano I., Casanova G., Matera M., Ruggiero F.M., Ferri D., Paradies G. Mitochondrial dysfunction in rat with nonalcoholic fatty liver. Involvement of complex I, reactive oxygen species and cardiolipin. Biochim. Biophys. Acta—Bioenerg. 2007;1767:1260–1267. doi: 10.1016/j.bbabio.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Kussmaul L., Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fato R., Bergamini C., Bortolus M., Maniero A.L., Leoni S., Ohnishi T., Lenaz G. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim. Biophys. Acta—Bioenerg. 2009;1787:384–392. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parameshwaran K., Irwin M.H., Steliou K., Pinkert C.A. Protection by an antioxidant of rotenone-induced neuromotor decline, reactive oxygen species generation and cellular stress in mouse brain. Pharmacol. Biochem. Behav. 2012;101:487–492. doi: 10.1016/j.pbb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 43.MacKenzie E.L., Ray P.D., Tsuji Y. Role and regulation of ferritin H in rotenone-mediated mitochondrial oxidative stress. Free Radic. Biol. Med. 2008;44:1762–1771. doi: 10.1016/j.freeradbiomed.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinogradov A.D., Grivennikova V.G. Oxidation of NADH and ROS production by respiratory complex I. Biochim. Biophys. Acta—Bioenerg. 2016;1857:863–871. doi: 10.1016/j.bbabio.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J.A., Robinson J.P. Mitochondrial complex I inhibitor Rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z., Huang L., Shulmeister V.M., Chi Y.I., Kim K.K., Hung L.W., Crofts A.R., Berry E.A., Kim S.H. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 47.Botigeli Baldim L., Nejo R.J., Souza Jordani E.M., Gomes Jordani C.M., Neves Cardoso Picinato M.A., Fleury Fina C., Castro-e-silva O. Effect of hyperbaric oxygen therapy on liver function during intermittent ischemia 1 Efeito da oxigenoterapia hiperbárica sobre a função hepática na isquemia intermitente. Acta Cirúrgica Bras. 2013;28:61–65. doi: 10.1590/S0102-86502013001300012. [DOI] [PubMed] [Google Scholar]
- 48.Palzur E., Zaaroor M., Vlodavsky E., Milman F., Soustiel J.F. Neuroprotective effect of hyperbaric oxygen therapy in brain injury is mediated by preservation of mitochondrial membrane properties. Brain Res. 2008;1221:126–133. doi: 10.1016/j.brainres.2008.04.078. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Z., Daugherty W.P., Sun D., Levasseur J.E., Altememi N., Hamm R.J., Rockswold G.L., Bullock M.R. Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fluid-percussion injury. J. Neurosurg. 2007;106:687–694. doi: 10.3171/jns.2007.106.4.687. [DOI] [PubMed] [Google Scholar]
- 50.Han G., Liu K., Li L., Li X., Zhao P. Effects of hyperbaric oxygen therapy on neuropathic pain via mitophagy in microglia. Mol. Pain. 2017;13:1–10. doi: 10.1177/1744806917710862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurt B., Kurt Y., Karslioǧlu Y., Topal T., Erdamar H., Korkmaz A., Türközkan N., Yaman H., Odabaşi Z., Günhan Ö. Effects of hyperbaric oxygen on energy production and xanthine oxidase levels in striated muscle tissue of healthy rats. J. Clin. Neurosci. 2008;15:445–450. doi: 10.1016/j.jocn.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Dave K.R., Prado R., Busto R., Raval A.P., Bradley W.G., Torbati D., Perez-Pinzón M.A. Hyperbaric oxygen therapy protects against mitochondrial dysfunction and delays onset of motor neuron disease in Wobbler mice. Neuroscience. 2003;120:113–120. doi: 10.1016/S0306-4522(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 53.Tian X.Q., Zhang L.D., Wang J.M., Dai J.G., Shen S.S., Yang L., Huang P.L. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Aβ25-35-induced oxidative stress and neuronal apoptosis in rats. Behav. Brain Res. 2013;242:1–8. doi: 10.1016/j.bbr.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 54.Pan X., Chen C., Huang J., Wei H., Fan Q. Neuroprotective effect of combined therapy with hyperbaric oxygen and madopar on 6-hydroxydopamine-induced Parkinson’s disease in rats. Neurosci. Lett. 2015;600:220–225. doi: 10.1016/j.neulet.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 55.Zhao H., Ge B., Yuan Y., Wang G. Hyperbaric Oxygen Ameliorated Acute Pancreatitis in Rats via the Mitochondrial Pathway. Dig. Dis. Sci. 2020;65:3558–3569. doi: 10.1007/s10620-020-06070-3. [DOI] [PubMed] [Google Scholar]
- 56.Shams Z., Khalatbary A.R., Ahmadvand H., Zare Z., Kian K. Neuroprotective effects of hyperbaric oxygen (HBO) therapy on neuronal death induced by sciatic nerve transection in rat. BMC Neurol. 2017;17:220. doi: 10.1186/s12883-017-1004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tezgin D., Giardina C., Perdrizet G.A., Hightower L.E. The effect of hyperbaric oxygen on mitochondrial and glycolytic energy metabolism: The caloristasis concept. Cell Stress Chaperones. 2020;25:667–677. doi: 10.1007/s12192-020-01100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godman C.A., Chheda K.P., Hightower L.E., Perdrizet G., Shin D.G., Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010;15:431–442. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pi J., Zhang Q., Fu J., Woods C.G., Hou Y., Corkey B.E., Collins S., Andersen M.E. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol. Appl. Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J., Liu S., Xu F., Liu Y., Lv C., Deng Y., Shi J., Gong Q. Trilobatin Protects Against Oxidative Injury in Neuronal PC12 Cells Through Regulating Mitochondrial ROS Homeostasis Mediated by AMPK/Nrf2/Sirt3 Signaling Pathway. Front. Mol. Neurosci. 2018;11:267. doi: 10.3389/fnmol.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xian Z., Choi Y.H., Zheng M., Jiang J., Zhao Y., Wang C., Li J., Li Y., Li L., Piao H., et al. Imperatorin alleviates ROS-mediated airway remodeling by targeting the Nrf2/HO-1 signaling pathway. Biosci. Biotechnol. Biochem. 2020;84:898–910. doi: 10.1080/09168451.2019.1710107. [DOI] [PubMed] [Google Scholar]
- 62.Kim J.Y., Kim J.K., Kim H. ABCB7 simultaneously regulates apoptotic and non-apoptotic cell death by modulating mitochondrial ROS and HIF1α-driven NFκB signaling. Oncogene. 2020;39:1969–1982. doi: 10.1038/s41388-019-1118-6. [DOI] [PubMed] [Google Scholar]
- 63.Djordjevic J., Roy Chowdhury S., Snow W.M., Perez C., Cadonic C., Fernyhough P., Albensi B.C. Early Onset of Sex-Dependent Mitochondrial Deficits in the Cortex of 3xTg Alzheimer’s Mice. Cells. 2020;9:1541. doi: 10.3390/cells9061541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosco G., Paganini M., Giacon T.A., Oppio A., Vezzoli A., Dellanoce C., Moro T., Paoli A., Zanotti F., Zavan B., et al. Oxidative stress and inflammation, microRNA, and hemoglobin variations after administration of oxygen at different pressures and concentrations: A randomized trial. Int. J. Environ. Res. Public Health. 2021;18:9755. doi: 10.3390/ijerph18189755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simsek K., Ozler M., Yildirim A.O., Sadir S., Demirbas S., Oztosun M., Korkmaz A., Ay H., Oter S., Yildiz S. Evaluation of the oxidative effect of long-term repetitive hyperbaric oxygen exposures on different brain regions of rats. Sci. World J. 2012;2012:849183. doi: 10.1100/2012/849183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oter S., Korkmaz A., Topal T., Ozcan O., Sadir S., Ozler M., Ogur R., Bilgic H. Correlation between hyperbaric oxygen exposure pressures and oxidative parameters in rat lung, brain, and erythrocytes. Clin. Biochem. 2005;38:706–711. doi: 10.1016/j.clinbiochem.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 67.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. Oxford University Press; Oxford, UK: 1999. Oxygen toxicity in aerobes; pp. 17–21. [Google Scholar]
- 68.Yildiz Ş., Aktaş Ş., Cimşit M., Ay H., Toǧrol E. Seizure incidence in 80,000 patient treatments with hyperbaric oxygen. Aviat. Space. Environ. Med. 2004;75:992–994. [PubMed] [Google Scholar]
- 69.Hadanny A., Meir O., Bechor Y., Fishlev G., Bergan J., Efrati S. The safety of hyperbaric oxygen treatment–retrospective analysis in 2334 patients. Undersea Hyperb. Med. 2016;43:113–122. [PubMed] [Google Scholar]
- 70.Hadanny A., Efrati S. The Hyperoxic—Hypoxic Paradox. Biomolecules. 2020;10:958. doi: 10.3390/biom10060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ay H., Topal T., Ozler M., Uysal B., Korkmaz A., Oter S., Ogur R., Dundar K. Persistence of hyperbaric oxygen-induced oxidative effects after exposure in rat brain cortex tissue. Life Sci. 2007;80:2025–2029. doi: 10.1016/j.lfs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Ay H., Topal T., Uysal B., Ozler M., Oter S., Korkmaz A., Dundar K. Time-dependent course of hyperbaric oxygen-induced oxidative effects in rat lung and erythrocytes. Clin. Exp. Pharmacol. Physiol. 2007;34:787–791. doi: 10.1111/j.1440-1681.2007.04645.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Q., Huang G., Yu X., Xu W. A Novel Approach to Estimate ROS Origination by Hyperbaric Oxygen Exposure, Targeted Probes and Specific Inhibitors. Cell. Physiol. Biochem. 2018;47:1800–1808. doi: 10.1159/000491061. [DOI] [PubMed] [Google Scholar]
- 74.Dennog C., Hartmann A., Frey G., Speit G. Detection of DNA damage after hyperbaric oxygen (HBO) therapy. Mutagenesis. 1996;11:605–609. doi: 10.1093/mutage/11.6.605. [DOI] [PubMed] [Google Scholar]
- 75.Topuz K., Colak A., Cemil B., Kutlay M., Demircan M.N., Simsek H., Ipcioglu O., Kucukodaci Z., Uzun G. Combined hyperbaric oxygen and hypothermia treatment on oxidative stress parameters after spinal cord injury: An experimental study. Arch. Med. Res. 2010;41:506–512. doi: 10.1016/j.arcmed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Oscarsson N., Ny L., Mölne J., Lind F., Ricksten S.E., Seeman-Lodding H., Giglio D. Hyperbaric oxygen treatment reverses radiation induced pro-fibrotic and oxidative stress responses in a rat model. Free Radic. Biol. Med. 2017;103:248–255. doi: 10.1016/j.freeradbiomed.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 77.Matsunami T., Sato Y., Sato T., Yukawa M. Antioxidant status and lipid peroxidation in diabetic rats under hyperbaric oxygen exposure. Physiol. Res. 2010;59:97–104. doi: 10.33549/physiolres.931711. [DOI] [PubMed] [Google Scholar]
- 78.Rothfuß A., Dennog C., Speit G. Adaptive protection against the induction of oxidative DNA damage after hyperbaric oxygen treatment. Carcinogenesis. 1998;19:1913–1917. doi: 10.1093/carcin/19.11.1913. [DOI] [PubMed] [Google Scholar]
- 79.Körpınar S., Uzun H. The Effects of Hyperbaric Oxygen at Different Pressures on Oxidative Stress and Antioxidant Status in Rats. Medicina. 2019;55:205. doi: 10.3390/medicina55050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu Q., Liang X., Chen D., Chen Y., Doycheva D., Tang J., Tang J., Zhang J.H. Delayed hyperbaric oxygen therapy promotes neurogenesis through reactive oxygen species/hypoxia-inducible factor-1α/β-catenin pathway in middle cerebral artery occlusion rats. Stroke. 2014;45:1807–1814. doi: 10.1161/STROKEAHA.114.005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tonelli C., In I., Chio C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balestra C., Lambrechts K., Mrakic-Sposta S., Vezzoli A., Levenez M., Germonpré P., Virgili F., Bosco G., Lafère P. Hypoxic and hyperoxic breathing as a complement to low-intensity physical exercise programs: A proof-of-principle study. Int. J. Mol. Sci. 2021;22:9600. doi: 10.3390/ijms22179600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cimino F., Speciale A., Anwar S., Canali R., Ricciardi E., Virgili F., Trombetta D., Saija A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8:391–399. doi: 10.1007/s12263-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng Y., Zhang Z., Li Q., Li W., Xu J., Cao H. Hyperbaric oxygen preconditioning protects lung against hyperoxic acute lung injury in rats via heme oxygenase-1 induction. Biochem. Biophys. Res. Commun. 2015;456:549–554. doi: 10.1016/j.bbrc.2014.09.074. [DOI] [PubMed] [Google Scholar]
- 85.Chen J. Heme oxygenase in neuroprotection: From mechanisms to therapeutic implications. Rev. Neurosci. 2014;25:269–280. doi: 10.1515/revneuro-2013-0046. [DOI] [PubMed] [Google Scholar]
- 86.Taguchi K., Motohashi H., Yamamoto M. Molecular mechanisms of the Keap1—Nrf2 pathway in stress response and cancer evolution. Genes Cell. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 87.Verma A.K., Yadav A., Singh S.V., Mishra M., Singh K.S., Rath S.K. Redox Biology Isoniazid prevents Nrf2 translocation by inhibiting ERK1 phosphorylation and induces oxidative stress and apoptosis. Redox Biol. 2015;6:80–92. doi: 10.1016/j.redox.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhamodharan U., Karan A., Sireesh D., Vaishnavi A., Somasundar A., Rajesh K., Rmkumar K.M. Free Radical Biology and Medicine Tissue-speci fi c role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free Radic. Biol. Med. 2019;138:53–62. doi: 10.1016/j.freeradbiomed.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 89.Fratantonio D., Cimino F., Speciale A., Virgili F. Need (more than) two to Tango: Multiple tools to adapt to changes in oxygen availability. BioFactors. 2018;44:207–218. doi: 10.1002/biof.1419. [DOI] [PubMed] [Google Scholar]
- 90.Fratantonio D., Virgili F., Zucchi A., Lambrechts K., Latronico T., Lafère P., Germonpré P., Balestra C. Increasing oxygen partial pressures induce a distinct transcriptional response in human PBMC: A pilot study on the “normobaric oxygen paradox”. Int. J. Mol. Sci. 2021;22:458. doi: 10.3390/ijms22010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Vliet T., Casciaro F., Demaria M. To breathe or not to breathe: Understanding how oxygen sensing contributes to age-related phenotypes. Ageing Res. Rev. 2021;67:101267. doi: 10.1016/j.arr.2021.101267. [DOI] [PubMed] [Google Scholar]
- 92.Bernaudin M., Bellail A., Marti H.H., Yvon A., Vivien D., Duchatelle I., Mackenzie E.T., Petit E. Neurons and astrocytes express EPO mRNA: Oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30:271–278. doi: 10.1002/(SICI)1098-1136(200005)30:3<271::AID-GLIA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 93.Hassan A., Arnold B.M., Caine S., Toosi B.M., Verge V.M.K., Muir G.D. Acute intermittent hypoxia and rehabilitative training following cervical spinal injury alters neuronal hypoxia- and plasticity-associated protein expression. PLoS ONE. 2018;13:e0197486. doi: 10.1371/journal.pone.0197486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jain I.H., Zazzeron L., Goli R., Alexa K., Schatzman-Bone S., Dhillon H., Goldberger O., Peng J., Shalem O., Sanjana N.E., et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryou M.G., Chen X., Cai M., Wang H., Jung M.E., Metzger D.B., Mallet R.T., Shi X. Intermittent Hypoxia Training Prevents Deficient Learning-Memory Behavior in Mice Modeling Alzheimer’s Disease: A Pilot Study. Front. Aging Neurosci. 2021;13:674688. doi: 10.3389/fnagi.2021.674688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang J.L., Manaenko A., Ye Z.H., Sun X.J., Hu Q. Hypoxia therapy—A new hope for the treatment of mitochondrial dysfunctions. Med. Gas Res. 2016;6:174–176. doi: 10.4103/2045-9912.191365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Radak Z., Zhao Z., Koltai E., Ohno H., Atalay M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013;18:1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radak Z., Suzuki K., Posa A., Petrovszky Z., Koltai E., Boldogh I. The systemic role of SIRT1 in exercise mediated adaptation. Redox Biol. 2020;35:101467. doi: 10.1016/j.redox.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaneto H., Xu G., Fujii N., Kim S., Bonner-Weir S., Weir G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- 100.Ohlsson H., Karlsson K., Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robson R., Kundur A.R., Singh I. Oxidative stress biomarkers in type 2 diabetes mellitus for assessment of cardiovascular disease risk. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;12:455–462. doi: 10.1016/j.dsx.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 102.Gürdöl F., Cimşit M., Öner-Iyidoǧan Y., Körpinar S., Yalçinkaya S., Koçak H. Early and late effects of hyperbaric oxygen treatment on oxidative stress parameters in diabetic patients. Physiol. Res. 2008;57:41–47. doi: 10.33549/physiolres.931139. [DOI] [PubMed] [Google Scholar]
- 103.Halliwell B. Oxidative Stress and Cancer: Have We Moved Forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 104.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 105.Butterfield D.A., Lauderback M.C. Serial Review: Causes and Consequences of Oxidative Stress in Alzheimer’s Disease. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/S0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 106.Sultana R., Boyd-Kimball D., Poon H.F., Cai J., Pierce W.M., Klein J.B., Merchant M., Markesbery W.R., Butterfield D.A. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 107.Yatin S.M., Aksenov M., Butterfield D.A. The antioxidant vitamin E modulates amyloid beta-peptide-induced creatine kinase activity inhibition and increased protein oxidation: Implications for the free radical hypothesis of Alzheimer’s disease. Neurochem. Res. 1999;24:427–435. doi: 10.1023/A:1020997903147. [DOI] [PubMed] [Google Scholar]
- 108.Clementi M.E., Pezzotti M., Orsini F., Sampaolese B., Mezzogori D., Grassi C., Giardina B., Misiti F. Alzheimer’s amyloid β-peptide (1-42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: An intriguing role for methionine 35. Biochem. Biophys. Res. Commun. 2006;342:206–213. doi: 10.1016/j.bbrc.2006.01.137. [DOI] [PubMed] [Google Scholar]
- 109.Pogocki D., Schöneich C. Redox properties of Met(35) in neurotoxic beta-amyloid peptide. A molecular modeling study. Chem. Res. Toxicol. 2002;15:408–418. doi: 10.1021/tx0101550. [DOI] [PubMed] [Google Scholar]
- 110.Polyzos A.A., McMurray C.T. The chicken or the egg: Mitochondrial dysfunction as a cause or consequence of toxicity in Huntington’s disease. Mech. Ageing Dev. 2017;161:181–197. doi: 10.1016/j.mad.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tian X.Q., Wang J.M., Dai J.G., Yang L., Zhang L.D., Shen S.S., Huang P.L. Hyperbaric oxygen and ginkgo biloba extract inhibit Aβ25-35-induced toxicity and oxidative stress in vivo: A potential role in Alzheimer’s disease. Int. J. Neurosci. 2012;122:563–569. doi: 10.3109/00207454.2012.690797. [DOI] [PubMed] [Google Scholar]
- 112.Chen J., Zhang F., Zhao L., Cheng C., Zhong R., Dong C., Le W. Hyperbaric oxygen ameliorates cognitive impairment in patients with Alzheimer’s disease and amnestic mild cognitive impairment. Alzheimer’s Dement. 2020;6:e12030. doi: 10.1002/trc2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harch P.G., Fogarty E.F. Hyperbaric oxygen therapy for Alzheimer’s dementia with positron emission tomography imaging: A case report. Med. Gas Res. 2018;8:181–184. doi: 10.4103/2045-9912.248271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu J.J., Yang S.T., Sha Y., Ge Y.Y., Wang J.M. Hyperbaric oxygen treatment for Parkinson’s disease with severe depression and anxiety. Medicine. 2018;97:2017–2019. doi: 10.1097/MD.0000000000010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Phillips G.R., Huang J.K., Wang Y., Tanaka H., Shapiro L., Zhang W., Shan W.S., Arndt K., Frank M., Gordon R.E., et al. The presynaptic particle web: Ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/S0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- 116.Chen C., Chen W., Nong Z., Nie Y., Chen X., Pan X., Guo Y., Yao M., Deng W. Hyperbaric oxygen alleviated cognitive impairments in mice induced by repeated cerebral ischemia-reperfusion injury via inhibition of autophagy. Life Sci. 2020;241:117170. doi: 10.1016/j.lfs.2019.117170. [DOI] [PubMed] [Google Scholar]
- 117.Choi J., Kwon H., Han P.L. Hyperoxygenation treatment reduces beta-amyloid deposition via mecp2-dependent upregulation of MMP-2 and MMP-9 in the hippocampus of Tg-APP/PS1 mice. Exp. Neurobiol. 2021;30:294–307. doi: 10.5607/en21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arsenijevic D., Onuma H., Pecqueur C., Raimbault S., Manning B.S., Miroux B., Couplan E., Alves-Guerra M.C., Goubern M., Surwit R., et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 119.Dornand J., Gerber M. Inhibition of murine T-cell responses by anti-oxidants: The targets of lipo-oxygenase pathway inhibitors. Immunology. 1989;68:384–391. [PMC free article] [PubMed] [Google Scholar]
- 120.Asehnoune K., Strassheim D., Mitra S., Kim J.Y., Abraham E. Involvement of Reactive Oxygen Species in Toll-Like Receptor 4-Dependent Activation of NF-κB. J. Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 121.Kabe Y., Ando K., Hirao S., Yoshida M., Handa H. Redox Regulation of NF-kB Activation: Distinct Redox Regulation between the Cytoplasm and the Nucleus. Antioxid. Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 122.Dwyer D.J., Kohanski M.A., Collins J.J. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 2009;12:482–489. doi: 10.1016/j.mib.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paiva C.N., Bozza M.T. Are Reactive Oxygen Species always detrimental to Pathogens? Antioxid. Redox Signal. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cimsit M., Uzun G., Yildiz S. Hyperbaric oxygen therapy as an anti-infective agent. Expert Rev. Anti. Infect. Ther. 2009;7:1015–1026. doi: 10.1586/eri.09.76. [DOI] [PubMed] [Google Scholar]
- 125.Mattson M.P., Meffert M.K. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]