Summary
Lung cancer (LC) is the leading cause of cancer death. Barriers to the early presentation for LC include lack of symptom awareness, symptom misappraisal, poor relationship with doctors and lack of access to healthcare services. Addressing such barriers can help detect LC early. This systematic review describes the effect of recent interventions to improve LC awareness, help-seeking and early detection. This review was guided by the Cochrane Handbook for Systematic Reviews of Interventions. Electronic databases MEDLINE, CINAHL, ERIC, APA PsycARTICLES, APA PsycInfo and Psychology and Behavioral Sciences Collection were searched. Sixteen studies were included. Knowledge of LC was successfully promoted in most studies using educational sessions and campaigns. LC screening uptake varied with most studies successfully reducing decision conflicts using decision aids. Large campaigns, including UK-based campaign ‘Be Clear on Cancer’, were instrumental in enhancing LC awareness, promoting help-seeking and yielding an increase in chest X-rays and a decrease in the number of individuals diagnosed with advanced LC. Multimodal public health interventions, such as educational campaigns are best suited to raise awareness, reduce barriers to help-seeking and help detect LC early. Future interventions ought to incorporate targeted information using educational resources, face-to-face counselling and video- and web-based decision aids.
Keywords: cancer, prevention, intervention, health education, community based intervention
INTRODUCTION
Lung cancer (LC) is the leading cause of cancer incidence and mortality in men and women globally, with 2.1 million new cases (11.6% of the total cancer cases) and 1.8 million deaths (18.4% of the total cancer deaths) in the year 2018 alone (Bray et al., 2018). More than half of LC cases (53%) are diagnosed among men and women aged 55–74 years (median age = 70 years) (Torre et al., 2016). In contrast to the increase in survival rates for most cancers, LC is typically diagnosed at advanced stages with a five‐year survival rate of 5% (Siegel et al., 2018).
Screening individuals at risk for LC with low dose computed tomography (LDCT) has been shown to reduce LC mortality by up to 20% (National Lung Screening Trial Research Team, 2011; Marcus et al., 2016). The European Union stressed the importance of starting LC screening using LDCT throughout Europe (Oudkerk et al., 2017). However, to date, very few countries possess screening programs for LC (Siegel et al., 2018), and the uptake of LC screening in countries like the United States of America (USA) remains low, with only 4% of 6.8 million eligible individuals reporting having undergone LDCT (Jemal and Fedewa, 2017). This highlights the importance of raising awareness of LC, supporting at-risk individuals in making a decision regarding LC screening and promoting early presentation for symptoms indicative of LC.
A persistent cough, a change in a pre-existing cough, and shortness of breath are common symptoms of early-stage LC (Chowienczyk et al., 2020). Haemoptysis remains the strongest symptom predictor of LC, yet it occurs in only a fifth of patients (Walter et al., 2015). Patients with LC can also be asymptomatic until systemic symptoms, such as unexplained weight loss and fatigue occur, signalling advanced disease (American Cancer Society, 2019). Therefore, the symptom signature of LC is considered to be broad (Koo et al., 2018) in comparison to cancers that have a narrow symptom signature with single identifiable symptoms, such as breast (O'Mahony et al., 2013) and testicular (Saab et al., 2017a) cancers. This may lead to delay in early presentation and LC diagnosis (Holmberg et al., 2010).
Early help-seeking for symptoms indicative of LC is key for timely and early diagnosis and improved survivorship. However, patients diagnosed with LC experience, on average, a 6-month delay between symptom onset and initiation of treatment (Ellis and Vandermeer, 2011). This is known to have detrimental effects on early diagnosis, quality of life, cost of healthcare, and patients’ eligibility for curative treatment (Walter et al., 2015; World Health Organisation, 2020). Several barriers to help-seeking and early detection of LC exist, such as lack of symptom awareness, poor relationship with physicians and lack of healthcare access (Carter‐Harris, 2015; Koo et al., 2018; Cassim et al., 2019; Cunningham et al., 2019). Symptom misappraisal is another key contributor to help-seeking delay, especially in the presence of risk factors like smoking (Smith et al., 2016) and comorbidities, such as chronic obstructive pulmonary disease (COPD) (Cunningham et al., 2019). For instance, a survey of 2042 participants found that being a smoker was associated with a reduced likelihood of help-seeking for symptoms indicative of LC, potentially due to pre-existing respiratory symptoms associated with chronic smoking (Smith et al., 2016). Similarly, in their qualitative study, Cunningham et al. (2019) found that individuals with COPD attributed changes in their respiratory symptoms to their COPD and failed to mention LC, despite having a significantly greater risk for LC. LC stigma also impacts negatively on help-seeking for LC ‘alarm’ symptoms. Indeed, a survey of 93 symptomatic individuals found that higher levels of perceived LC stigma were associated with a median waiting time of 41 days prior to seeking medical help for symptoms of concern (Carter‐Harris, 2015). Therefore, raising awareness and promoting early presentation for symptoms indicative of LC can help detect LC early and improve survival.
The international literature has highlighted the importance of interventions that target awareness, symptom evaluation and early help-seeking for LC (Dlamini et al., 2019). For example, a national campaign in the UK entitled ‘Be Clear on Cancer’ resulted in a significant increase in LC awareness, respiratory consultations, number of physician-prescribed chest X-rays and CT scans, and number of LC cases diagnosed at early stages (Ironmonger et al., 2015). Interventions often vary in terms of modalities, intended mechanisms, theoretical basis and target area/groups. This systematic review aims to describe the effect of recent interventions to improve (i) knowledge and/or awareness of LC; (ii) help-seeking intentions and/or behaviours for LC and (iii) early detection of LC.
METHODS
This systematic review was guided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2019) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (Moher et al., 2009).
Eligibility criteria
The review eligibility criteria were predetermined using the PICO (Population, Intervention, Comparison, Outcomes) framework (Moher et al., 2009). Population: conducted among individuals of any age including at-risk populations; Intervention: included any intervention, programme, or campaign; Comparison: incorporated within- or between-group comparison; and Outcomes: reported on at least one of the review outcomes (i.e. knowledge/awareness of LC, help-seeking intentions/behaviours for LC and/or early detection of LC). Studies were excluded if they included patients with LC, used LC screening as the intervention, did not incorporate a comparator, and used any nonexperimental design.
Search strategy
A search was conducted using the electronic databases MEDLINE, CINAHL, ERIC, APA PsycARTICLES, APA PsycInfo and Psychology and Behavioral Sciences Collection. Keywords were truncated and combined using Boolean operators ‘OR’ and ‘AND’ and the proximity indicator ‘N.’ The following keywords were searched on title or abstract: (lung* OR pulmo*) N3 (cancer* OR neoplas* OR malignan* OR tumo*) AND (know* OR aware* OR detect* OR help-seek*) AND (interven* OR program* OR campaign* OR trial* OR experiment* OR educat*).
The search was conducted on 15 January 2020 and, for pragmatic reasons, was limited to studies published in English between January 2015 and January 2020. Of note, there is no gold standard for limiting the search by year of publication, though studies published within a 10-year timeframe are broadly considered to be recent (Wilhelm and Kaunelis, 2005). However, knowledge decay is common in public health interventions and is one of the reasons researchers frequently develop and refine health promotion interventions, whilst older interventions and campaigns become increasingly obsolete over time (Nimmons et al., 2017; Saab et al., 2018). Therefore, it had been agreed a priori to limit the current search to evidence published within a five-year timeframe in order to source and synthesize the most up-to-date evidence relating to the latest interventions and educational LC campaigns.
Study selection
Records were transferred to Covidence, an online software used to produce systematic reviews of interventions (The Cochrane Collaboration, 2020). Titles and abstracts were screened, and irrelevant records were excluded. The full text of potentially eligible records was then screened and reasons for exclusion were recorded. Title, abstract and full-text screenings were conducted in pairs. For a screening decision to be made, each record was screened twice by two independent reviewers. Screening conflicts were resolved by a third reviewer.
Data extraction and synthesis
Data were extracted using a standardized data extraction table (Supplementary Table 1S) as follows: author(s); year; country; aim(s); design; theoretical underpinning; sample; setting; relevant outcomes; intervention; procedures; instruments; follow-up times and findings. Data extraction was conducted by one reviewer. Each extracted study was then cross-checked by the rest of the review team.
A meta-analysis with summary measures of intervention effect requires that the included studies be sufficiently homogenous (Higgins et al., 2019). Therefore, given the heterogeneity of the studies in terms of design, outcomes and outcome measures, a meta-analysis was not plausible. Instead, a narrative synthesis of study findings was conducted, and findings were synthesized and discussed according to the review aims under the headings (i) knowledge and awareness, (ii) help-seeking and (iii) early detection.
Quality and level of evidence
The Mixed Methods Appraisal Tool (MMAT) helps appraise the methodological quality of five study categories: qualitative studies, randomized controlled trials (RCTs), nonrandomized studies, quantitative descriptive studies and mixed methods studies (Hong et al., 2018). In line with the current review aim and eligibility criteria, the methodological quality of three study categories was appraised, namely RCTs (seven quality appraisal items), nonrandomized studies (seven quality appraisal items) and mixed methods studies (17 quality appraisal items). Each of the quality appraisal items was judged on a ‘Yes’, ‘No’ and ‘Can’t tell’ basis. The clarity of research questions and the use of appropriate data collection methods to address those were assessed for all study categories. For RCTs and nonrandomized studies, sample representativeness and similarities between participant groups at baseline were assessed. Other items related to blinding the outcome assessor, reporting of complete outcome data, accounting for confounders, and ensuring that interventions have been administered as intended. For mixed methods studies, additional items assessed the integration of quantitative and qualitative methods and explored whether divergences and inconsistencies between quantitative and qualitative results have been adequately addressed.
The Scottish Intercollegiate Guidelines Network’s (Scottish Intercollegiate Guidelines Network, 2019) guidelines were used to assess the level of evidence per study. This assesses the study design and how well a study was carried out and helps judge whether research conclusions are accurate. Level of evidence scores range from 1++, 1+, 1–, 2++, 2+, 2–, 3, to 4. A score of 1++ corresponds to high quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias, whereas a score of 4 is assigned to expert opinions (Scottish Intercollegiate Guidelines Network, 2019).
Level of evidence and quality assessments were conducted by one reviewer and verified independently by the review team. Discrepancies in quality appraisal ratings and level of evidence assessment scores were then discussed among the review team until consensus was reached. When consensus was not reached between two reviewers, a third reviewer was asked to resolve conflicts. Studies were included in the present review regardless of their methodological quality and level of evidence to minimize the risk of study selection and reporting bias (Higgins et al., 2019).
RESULTS
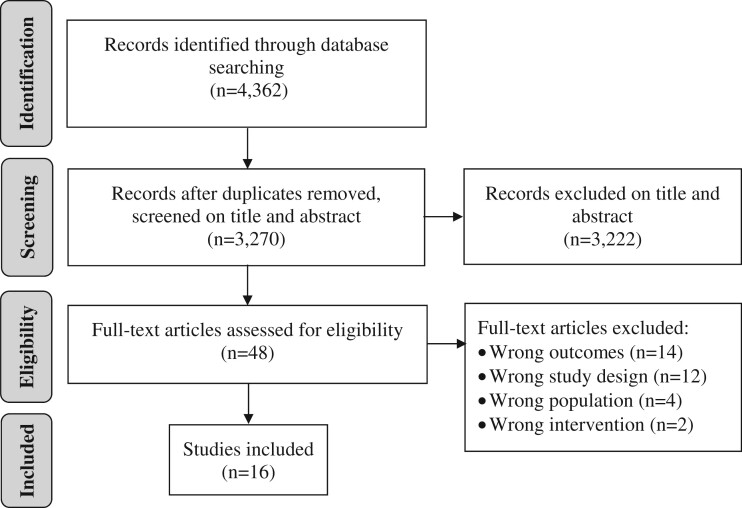
Database searching yielded 4362 records. Following deletion of duplicates, 3270 records were screened on title and abstract and 3222 irrelevant records were excluded. Full texts of the remaining 48 records were screened. Of those, 16 studies were included in this review (Figure 1).
Fig. 1:
Study identification, screening and selection process.
Study characteristics
Most studies were conducted in the USA (n = 8) and the UK (n = 6), with the majority being uncontrolled before–after studies (n = 8) and RCTs (n = 4). Half of the studies (n = 8) used multiple researcher-designed instruments to collect data and collected data from rural/underprivileged areas. Five studies were underpinned by theory including the Health Belief Model (Fung et al., 2018; Williams et al., 2021); elements of Self-Regulation Theory, Theory of Planned Behaviour, and Implementation Intentions (Emery et al., 2019); Ottawa Decision Support Framework (Lau et al., 2015) and Theory of Planned Behaviour (Mueller et al., 2019). Six studies used large or national multimodal campaigns as their intervention with three studies reporting on the same campaign namely the ‘Be Clear on Cancer’ UK-based campaign (Ironmonger et al., 2015; Moffat et al., 2015; Power and Wardle, 2015) (Table 1).
Table 1:
Study characteristics (n = 16)
| Country | USA (n = 8) UK (n = 6) Australia (n = 1) Colombia (n = 1) |
|---|---|
| Design |
Uncontrolled before–after (n = 8) Randomized controlled trial (n = 4) Controlled before–after (n = 1) Mixed methods (n = 1) Retrospective (n = 1) Time-trend (n = 1) |
| Theorya |
Health Belief Model (n = 2) Elements of Self-Regulation Theory, Theory of Planned Behaviour, and Implementation Intentions (n = 1) Ottawa Decision Support Framework (n = 1) Theory of Planned Behaviour (n = 1) |
| Sample (min–max) | 30–2090 participants |
| Settings |
Community including rural/underprivileged areas (n = 8) Acute care (n = 3) General practice (n = 1) Lung cancer screening programme (n = 1) Online (n = 1) Primary care records (n = 1) Public health centre (n = 1) |
| Outcomesb |
Knowledge/awareness of LC (n = 12) Help-seeking intentions/behaviours for LC (n = 10) Early detection of LC (n = 7) |
| Intervention |
Large/national campaigns (n = 6) Face-to-face counselling, video slideshow, and web-based decision aid (n = 1) Information film and booklet (n = 1) Lung cancer screening education class (n = 1) Research education seminars (n = 1) School educational sessions (n = 1) Self-help manual (n = 1) Tailored information and Theory of Planned Behaviour components (n = 1) Video-based decision aid (n = 1) Web-based decision aid (n = 1) Weekly sessions and lung cancer eligibility checklist (n = 1) |
| Instrumentsc |
Researcher-designed (n = 8) Cancer Awareness Measure (n = 4) Decision Conflict Scale (n = 3) Electronic medical records (n = 3) Others (n = 18) |
| Follow-up (min–max) | Immediately post-test–5 years post-test |
n = 5 studies underpinned by theory.
n = number of times an outcome was measured.
n = number of times an instrument was used.
Quality and level of evidence
All 16 studies had clear research questions and used appropriate data collection methods. RCTs (n = 4) performed appropriate randomization, had comparable groups at baseline, presented complete data outcomes, and had participants adhere to the assigned intervention; however, only one RCT reported on blinding the outcome assessor (Emery et al., 2019) (Supplementary Table 2S). As for non-RCTs (n = 11), only three studies reported that participants were representative of the target population (Power and Wardle, 2015; Sakoda et al., 2020; Williams et al., 2021), and one study accounted for confounders (Williams et al., 2021). Otherwise, all non-RCTs met the remaining MMAT criteria (Supplementary Table 3S). The only mixed methods study (Cardarelli et al., 2017) met most of the MMAT criteria; however, it was unclear as to how data were synthesized and whether there were divergences and inconsistencies between quantitative and qualitative results (Supplementary Table 4S).
Half of the studies (n = 8) scored 2+ on the SIGN level of evidence criteria, indicating well-conducted non-RCTs with a low risk of confounding or bias and a moderate probability that the relationship is causal. Only one RCT (Emery et al., 2019), and one non-RCT (Williams et al., 2021) had a low risk of bias.
Findings from individual studies are reported in Table 2.
Table 2:
Findings from individual studies (n = 16)
| Reference country | Design theory | Sample | Outcome | Intervention | Instrument | Follow-up | Findingsa |
|---|---|---|---|---|---|---|---|
|
USA |
Sequential mixed-methods | n = 145 high-risk individuals (IG = 2 regions, CG = 1 region) | LDCT uptake | ‘Terminate Lung Cancer’ campaign | Telephone survey and LDCTs | 12 months |
O2. 73(50.3%) came across the campaign: 5 (3.4%) considered LDCT and 2(1.4%) sought information about LDCT. O3. 5(3.4%) got LDCT. Significant uptake of LDCT in IG (p = NR). |
|
Australia |
RCT SRT, TPB, Implementation Intentions |
n = 551 high-risk individuals (IG = 274, CG = 277) | LC symptom knowledge, appraisal, help-seeking, consult rates, health service use | IG: Spirometry, self-help manual, discussions, tailored monthly prompts; CG: Spirometry, discussion on lung health | Questionnaire and GP record review |
1 month 12 months |
O1. No significant change in knowledge between IG and CG at 1 and 12 mon post-test (MD = –0.2, p = 0.3954 and MD = –0.1, p = 0.6083, respectively) O2. 40% increase in consults (95% CI IG 0.57 (0.47–0.70), CG 0.41 (0.32–0.52), RR = 1.40 (1.08–1.82), p = 0.0123). not statistically significant difference in time to first (p = 0.207) or all consults (p = 0.147). |
|
USA |
RCT HBM |
n = 395 Chinese Americans (IG = 202, CG = 193) | Knowledge of cancer prevention | IG: Education on cancer prevention; CG: Education on biospecimen collection | Questionnaire | Immediate | O1. Awareness of LC early detection at post-test increased by 6.4% for IG (p = 0.18) and decreased by 2.1% in CG p = 0.49, with no statistically significant differences between groups (p = 0.13). |
|
USA |
Uncontrolled before–after | n = 30 high-risk individuals | LCS knowledge | Video-based patient DA on LCS | Online survey | Immediate | O1. Knowledge increased by 33% post-test (M = 3.97, SD = 2.87, 95% CI 2.90–5.04, p < 0.001). |
|
Ironmonger et al. (2015) UK |
Controlled before–after | n = 1153 high-risk individuals (pre-test); n = 1121 (post-test) | Awareness, consults, urgent LC referral, CXRs, CTs, LC diagnosis, stage, treatment | IG: ‘Be Clear on Cancer’ national campaign; CG: No campaign | Survey |
Immediate 6 months 8 months |
O1. Increased awareness of cough (p < 0.001), breathlessness (p = 0.024), haemoptysis (p < 0.001), chest pain (p = 0.015), weight loss (p < 0.001). O2. Help-seeking increased by 63% during and 46% 8 weeks post-test (p < 0.001). CG vs IG p < 0.001. GP referrals increased by 31.8% (p < 0.001). O3. CXRs and CTs increased by 18.6% and 15.7%, respectively (p < 0.001). Diagnosis increased by 9.1% (p < 0.001) in IG and 1.5% in CG (p = 0.373). 30% increase in stage I LC and 3.5% decrease in stage IV (both p < 0.001). |
|
USA |
Retrospective study | n = 927 Twitter; N = 595 LinkedIn; N = 13 341 Facebook; N = 1522 Google | LDCT uptake | 20-Week pay-per-click campaign targeting patients, caregivers, and healthcare providers | Social media data, medical records for data on LDCT | 20 weeks | O3. Mean scheduled LDCT per week increased by 3% during and 5.8% 1-week post-campaign (p = 0.001). |
|
UK |
Time-trend study | n = NR cases of LC | CXRs, stage at diagnosis | Regional/local campaign | Referral for CXR and LC stage | 5 years | O3. 80.8% increase in CXRs. 8.8% increase in patients diagnosed with stage I/II LC and 9.3% reduction in stage III/IV LC (χ2(1) = 32.2, p < 0.0001). |
|
USA |
Uncontrolled before–after ODSF |
n = 60 high-risk individuals | Knowledge of LCS benefits and harms, decisional conflict | Web-based DA | Online survey | 4 months |
O1. Knowledge of risk factors, benign lumps, and screening benefits, harms, eligibility increased (pre-test M = 7.52/14, SD = 1.89; post-test M = 10.93/14, SD = 2.19, p < 0.001). O2. Decisional conflict decreased (pre-test M = 46.33, SD = 29.69; post-test M = 15.08, SD = 25.78, p < 0.001). |
|
USA |
Uncontrolled before–after |
n = 125 high-risk individuals pre-test; n = 122 post-test n = 113 1 month post-test |
LCS knowledge | Face-to-face counselling, video, web-based DA | Survey |
Immediate 1 month |
O1. Increased knowledge of LCS eligibility for age (p < 0.0001), smoking history (p < 0.0001), benefits (p = 0.03), harms (p = <0.0001). O3. 423 had shared decision-making visit: 23(5.4%) did not go have LDCT. |
|
Colombia |
Uncontrolled before–after | n = 243 female adolescents | LC awareness | Two 90-min school educational sessions | Survey |
1 month 3 months 6 months |
O1. Awareness of LC warning signs and risk factors increased across all timepoints (p < 0.001). |
|
UK |
Uncontrolled before–after | n = 1412 at-risk individuals pre-test; n = 1246 post-test | Awareness of LC signs and symptoms, GP attendance | ‘Be Clear on Cancer’ national campaign | Survey, medical record review |
Immediate NR weeks |
O1. Mentions of cough/hoarseness increased by 9% (p = 0.001). Proportion identifying ‘cough for 3 weeks or more that doesn’t go away’ as LC symptom increased by 15% (p < 0.001). O2. 63% increase in GP attendance for symptoms weeks post-test. |
|
UK |
Feasibility study using RCT with block randomization TPB |
n = 130 individuals with potential LC symptoms | LC help-seeking | IG: Tailored information and TPB; CG-TPB: Untailored information and TPB; CG-TAIL: Tailored information without TPB; CG: Usual care | Questionnaire | NR | O2. 98.5% (n = 128) considered help-seeking appropriate. 65.4% (n = 85) said they would make a GP appointment. Highest intention reported in IG (χ2(3) = 8.14, p = 0.04). |
|
UK |
Uncontrolled before–after | n = 2090 from households in 2010; n = 2001 in 2012 | LC knowledge, awareness, help-seeking | ‘Be Clear on Cancer’ national campaign | Computer-assisted survey | 2 years |
O1. Recall of LC symptoms increased by 8% (p < 0.001). Recognition of cough or hoarseness as signs of LC increased by 11% (p < 0.001). O2. No statistically significant change in barriers targeted by the campaign (p > 0.05). Barriers not targeted by the campaign were less endorsed post-campaign (p < 0.05). |
|
UK |
Nested RCT | n = 229 high-risk individuals (IG = 120, CG = 109) | LC knowledge, decisional conflict, LDCT completion | IG: Information film, booklet, discussion; CG: Booklet only | Questionnaire | Immediate |
O1. Mean objective knowledge increased by 2.16 (SD = 1.8) for IG and 1.84 (SD = 1.9) for CG (p < 0.001). Mean subjective knowledge increased by 0.92 (SD = 1) for IG and 0.55 (SD = 1.0) for CG (p < 0.001). O2. Decisional conflict higher in IG (M = 8.5/9, SD = 1.3) than CG (M = 8.2/9, SD = 1.5, p = 0.007). O3. No statistical significance in LDCT uptake between groups (p = 0.66). |
|
USA |
Uncontrolled before–after | n = 680 LCS class attendees | LCS knowledge, decision-making capacity, interest | Educational class on LCS | Survey | Immediate |
O1. Knowledge increased by 51% post-test (M = 0.09, SD = 2.29, p < 0.0001). O2. Willingness to get screened decreased by 15% post-test. |
|
USA |
Uncontrolled before–after HBM |
n = 481 African American majority | knowledge of LC risk factors, symptoms, LCS, screening uptake | c-Care intervention with weekly sessions on harms of smoking and LCS | Survey |
Immediate 3 months |
O1. Post-test increase in knowledge (p < 0.001) and 54.5% increase in correct answers regarding LCS. O2. Perceived susceptibility, threat, intent, and cue to action showed no significant changes between pre- and post-test. O3. 38% of those who met screening eligibility (n = 14) complete LCS. |
CG, control group; CI, confidence interval; CT, computed tomography; CXR, chest X-ray; DA, decision aid; GP, general practitioner; IG, intervention group; LC, lung cancer; LCS, lung cancer screening; LDCT, low dose computed tomography; M, mean; MD, mean difference; NR, not reported; O, objective; ODSF, Ottawa decision support framework; RCT, randomized controlled trial; RR, relative rate; SD, standard deviation; SRT, self-regulation theory; TPB, theory of planned behaviour; UK, United Kingdom; USA, United States of America; χ2, chi-square test.
Findings according to review objectives: O1: knowledge and/or awareness of LC; O2: help-seeking intentions and/or behaviours for LC; O3: early detection of LC, including clinical outcomes.
Knowledge and awareness
Subjective and objective knowledge of LC were promoted in 12 studies using approaches, such as decision aids (Lau et al., 2015; Mazzone et al., 2017; Housten et al., 2018); film and booklet (Ruparel et al., 2019) and educational sessions (Williams et al., 2021; Sakoda et al., 2020). Lau et al. (2015) evaluated the effectiveness of a web-based decision aid (www.shouldiscreen.com) among 60 at-risk individuals and found that knowledge of risk factors, benefits and harms of screening, screening eligibility and percentage of benign lumps increased significantly 4 months post-test [pre-test: mean = 7.52/14, standard deviation (SD) = 1.89; post-test: mean = 10.93/14, SD = 2.19; p < 0.001]. A second study used the same decision aid and also reported statistically significant increases in knowledge of screening-eligible ages (p < 0.0001), smoking history eligibility criteria (p < 0.0001), benefits (p = 0.03) and harms (p < 0.0001) of LC screening 1 month post-test (Mazzone et al., 2017).
An information film and booklet (intervention group [IG]) compared with booklet only (control group [CG]) yielded a statistically significant increase in knowledge in both groups, with a greater improvement among IG (p < 0.001) (Ruparel et al., 2019). Educational interventions in the form of LC screening classes (Sakoda et al., 2020) and a 4-week educational intervention (Williams et al., 2021) were also instrumental in increasing objective knowledge of LC screening immediately post-test and 3 months post-test (both p < 0.001).
As for knowledge of LC signs, symptoms, and risk factors, a 90-min educational session significantly increased awareness of warning signs for LC and LC risk factors 1 month, 3 months and 6 months post-test (p < 0.001) (Meneses-Echávez et al., 2018). Three before–after studies evaluated the impact of the UK campaign ‘Be Clear on Cancer’ on knowledge of LC signs and symptoms (Ironmonger et al., 2015; Moffat et al., 2015; Power and Wardle, 2015). The campaign was successful in increasing awareness of cough (p < 0.001), breathlessness (p = 0.024), haemoptysis (p < 0.001), chest pain (p = 0.015) and unexplained weight loss (p < 0.001) as symptoms of LC (Ironmonger et al., 2015). Recall and recognition of a persistent cough or hoarseness as signs of LC also increased significantly from 67% pre-campaign to 78% post-campaign (p < 0.001) (Power and Wardle, 2015). The increase in unprompted awareness of cough/hoarseness was significantly lower among men as compared with women (45% vs 55%; p = 0.001) (Moffat et al., 2015), and there was no statistically significant change in pre- and post-campaign results for individuals aged 75 years or more (p = 0.721) as compared with 11% increase for the 55–74 years age group (p = 0.001). As for prompted awareness, the proportion of participants identifying a ‘cough for 3 weeks or more that doesn’t go away’ as definite warning sign of LC increased from 18% pre-campaign to 33% post-campaign (p < 0.001), with no statistically significant difference between men and women pre-campaign (p = 0.389) and post-campaign (p = 0.587) (Moffat et al., 2015).
In contrast, a spirometry, self-help manual, action and coping plans and tailored monthly prompts (SMS, emails, post-cards, phone calls, and fridge magnets) (IG) yielded no statistically significant changes in knowledge in comparison to spirometry and brief general discussion about lung health (CG) 1 and 12 months post-test (mean difference = -0.2, p = 0.3954 vs. mean Difference = –0.1, p = 0.6083, respectively) (Emery et al., 2019). Similarly, a four-day research education seminar on cancer prevention (IG) and biospecimen collection (CG) did not yield a statistically significant increase in awareness of LC early detection immediately post-education (p = 0.18 and p = 0.49, respectively; group comparison p = 0.13) (Fung et al., 2018).
Help-seeking
Ten studies addressed help-seeking for LC including seeking help from a General Practitioner (GP) and deciding to undertake LC screening. Spirometry, self-help manual, action and coping plans, and tailored monthly prompts which initially failed to raise LC awareness, were successful in increasing respiratory consultations by 40% among the IG [95% Confidence Interval (CI) IG 0.57 (0.47–0.70), CG 0.41 (0.32–0.52), Relative Rate 1.40 (1.08–1.82); p = 0.0123] (Emery et al., 2019).
Mueller et al. (2019) conducted a feasibility RCT with block randomization to four groups: tailored information and Theory of Planned Behaviour components (IG); untailored information with Theory of Planned Behaviour components; tailored information without Theory of Planned Behaviour components; and usual care (CG). It was found that the four groups differed significantly in scores on the help-seeking intention variable, (χ2(3) = 8.14, p = 0.04), with the highest intention reported in the IG (Mueller et al., 2019). In contrast, an uncontrolled before–after study using the Health Belief Model reported no statistically significant changes in intent and cue to action immediately and 3 months following a 4-week educational intervention (Williams et al., 2021).
The campaign ‘Be Clear on Cancer’ was instrumental in increasing help-seeking for LC symptoms and reducing barriers to help-seeking (Ironmonger et al., 2015; Moffat et al., 2015; Power and Wardle, 2015). Help-seeking for cough increased by 63% during the campaign and by 46% 8 weeks later among at-risk groups (p < 0.001) (Ironmonger et al., 2015). The campaign was also associated with a 63% increase in GP attendances for symptoms linked to the campaign, with no difference between genders (p = 0.107) (Moffat et al., 2015). The largest increase was seen in the 50–59-year age group in comparison to older age groups (88%, p < 0.001). As for perceived barriers to help-seeking, there was no statistically significant change in barriers targeted by the campaign, such as being ‘worried about wasting the doctor’s time’ (26% in 2010 and 24% in 2012, p = 0.158) or believing that the ‘doctor would be difficult to talk to’ (14% in 2010 and 13% in 2012, p = 0.617). However, barriers not targeted by the campaign, such as being ‘too scared’ (p = 0.016), being ‘worried about what the doctor might find’ (p = 0.002), ‘difficulty arranging transport’ (p = 0.002), ‘difficulty making an appointment’ (p = 0.025) and being ‘too busy’ (p = 0.009) were less endorsed post-campaign (Power and Wardle, 2015).
In terms of screening decisions, Cardarelli et al. (2017) conducted a multimodal campaign titled ‘Terminate Lung Cancer’ and found that, out of 145 high-risk individuals, 73 (50.3%) came across the campaign. Of those, 5 (3.4%) thought about getting an LDCT and 2 (1.4%) sought information about LDCT. Three studies used the Decision Conflict Scale (Lau et al., 2015; Ruparel et al., 2019; Sakoda et al., 2020). A web-based decision aid yielded a decrease in Decision Conflict Scale scores indicating lower decisional conflict (pre-test mean = 46.33, SD = 29.69; post-test mean = 15.08, SD = 25.78; p < 0.001) (Lau et al., 2015). In contrast, participants who watched an information film and read a booklet (IG) had higher decisional conflict (mean = 8.5/9, SD = 1.3) in comparison to those who read a booklet only (CG) (mean = 8.2/9, SD = 1.5; p = 0.007) (Ruparel et al., 2019). Moreover, an LC screening class led to a decrease in the proportion of at-risk participants who wanted to be screened from 80% pre-test to 65% immediately post-test (Sakoda et al., 2020).
Early detection
The effect of interventions on early detection of LC (i.e. screening uptake and clinical outcomes) was addressed in seven studies. LDCT uptake varied widely between 38% after a 4-week educational intervention (Williams et al., 2021) and 94.6% following face-to-face counselling and shared web-based decision-making (Mazzone et al., 2017). The multimodal ‘Terminate Lung Cancer’ campaign yielded a significant uptake of LDCT in the two intervention regions as compared with the control region (p-value not reported) (Cardarelli et al., 2017). Another social media-based campaign was linked to a 3% increase in LDCT per week immediately post-campaign and a further 5.8% increase a week later (p = 0.001) (Jessup et al., 2018). In contrast, LDCT completion rates showed no statistical significance between those who watched an information film and read a booklet (IG) and those who read a booklet only (CG) (p = 0.66) (Ruparel et al., 2019).
Clinical outcomes reported following large campaigns included the number of chest X-rays and CT scans ordered, new LC cases, stage at diagnosis and LC treatments (Ironmonger et al., 2015; Kennedy et al., 2018). The ‘Be Clear on Cancer’ campaign was associated with an increase in GP-referred chest X-rays and CT scans by 18.6% and 15.7%, respectively (p < 0.001) (Ironmonger et al., 2015). Moreover, LC diagnosis increased by 9.1% (p < 0.001) for IG and 1.5% for the CG (p = 0.373) and the proportion of nonsmall cell LC diagnosed at stage I increased from 14.1% to 17.3% (p < 0.001) and decreased from 52.5% to 49% (p < 0.001) for stage IV. As for treatments, there was a 2.3%-point increase (p < 0.001) in resections for patients seen (IG), with no evidence that these proportions changed in CG pre-campaign (p = 0.404) and post-campaign (p = 0.425) (Ironmonger et al., 2015). A local UK campaign which overlapped with ‘Be Clear on Cancer’ also resulted in an 80.8% increase in community-ordered chest X-rays between the 3 years pre-campaign and 3 years post-campaign and yielded an 8.8% increase in patients diagnosed with stage I/II LC as opposed to a 9.3% reduction in cases of stage III/IV LC (χ2(1) = 32.2, p < 0.0001) (Kennedy et al., 2018).
DISCUSSION
A wide range of educational interventions were implemented across the reviewed studies, with several studies testing large national and multimodal campaigns. Most interventions explored knowledge and awareness of LC and its screening, while others examined help-seeking behaviours and early detection of LC, including screening uptake and clinical outcomes, such as stage of LC at diagnosis and treatments received.
Overall, participants were poorly informed about LC at baseline. However, web-based decision aids (Lau et al., 2015; Mazzone et al., 2017; Housten et al., 2018), information resources (Ruparel et al., 2019) and educational sessions (Sakoda et al., 2020; Williams et al., 2021) yielded a significant increase in knowledge and awareness of LC risk factors, warning signs, benefits and harms of screening, and screening eligibility. Notably, tailored monthly prompts (i.e. SMS, emails, post-cards, phone calls, fridge magnets) did not significantly increase LC awareness, detection, or screening uptake (Emery et al., 2019).
In terms of participants’ sociodemographic profiles, men demonstrated lower awareness than women (Moffat et al., 2015). Gender disparity in knowledge is well documented in other malignancies including colorectal (Clarke et al., 2016) and skin (Christoph et al., 2016) cancers. Age also played a role in increased LC awareness, with a significant improvement in unprompted awareness in the 55–74 years age group (Moffat et al., 2015). This finding is encouraging since LC is mainly diagnosed in older generations. In the USA, for example, LC is most common among those aged 65 years or older, with a median age of 71 years at diagnosis (National Cancer Institute, 2020). In an Irish study, Ryan et al. (2015) emphasized the importance of age as a significant risk factor in cancer diagnosis and highlighted that, even though age is a nonmodifiable risk factor, researchers must target information to increase LC awareness and promote consultation among at-risk age groups (McCutchan et al., 2019).
In keeping with high-risk groups, half of the studies were conducted in rural/underprivileged areas. A pooled analysis of case–control studies found that socioeconomic deprivation and lack of healthcare access among at-risk populations were associated with advanced LC at diagnosis (Hovanec et al., 2018). Therefore, McCutchan et al. (2019) identified the need for multi-faceted community-based interventions to encourage high-risk individuals, living in deprived areas, to seek LC information outside of the GP setting. This may promote better relationships between high-risk groups and trained intervention facilitators, subsequently improving engagement in LC screening and help-seeking (McCutchan et al., 2019).
Interventions aiming to increase help-seeking intentions ought to consider incorporating tailored information based on, for example, the components of the Theory of Planned Behaviour (Mueller et al., 2019). Such theory-based interventions should address individuals’ attitudes, social norms, and perceived behaviour control as well as integrating measures to ensure effective decision-making skills (Ruparel et al., 2019). Moreover, at-risk individuals should be encouraged to consider the benefits and harms of health screening in order to make informed decisions and improve health outcomes (Bell et al., 2017). Current evidence suggests that the use of decision aids can increase knowledge of the benefits and harms of LC screening, whilst providing a better understanding of the nature of screening (Reuland et al., 2018). Therefore, methods to help dissipate LC screening decisional conflicts, such as video- and web-based decision aids should be considered (Lau et al., 2015; Mazzone et al., 2017; Housten et al., 2018). Notably, the use of such aids proved successful in reducing decision conflict and cancer-related distress among individuals at risk for breast (Metcalfe et al., 2017), prostate (Reidy et al., 2018) and colorectal (Perestelo-Perez et al., 2019) cancers, inclusive of those with low literacy and health literacy levels.
Three studies reported on a successful national campaign in the UK titled ‘Be Clear on Cancer’ which resulted in a significant increase in awareness of LC symptoms (Ironmonger et al., 2015; Moffat et al., 2015; Power and Wardle, 2015). This campaign also helped reduce barriers to help-seeking, increase GP consultations for at-risk individuals, increase in individuals requesting chest X-rays, and increase in GP-referred chest X-rays and CT scans. Moreover, there was an encouraging increase in early-stage LC diagnosis as a result of this campaign (Ironmonger et al., 2015). Alternative strategies, such as the use of social media campaigns could be modified to drive engagement with health services in people with minor/early symptoms (Jessup et al., 2018). Freeman et al. (2015) reported on lessons learned from the use of social media in public health campaigns and identified positive changes in motivation and action. The use of social media makes it easier to connect with specific population cohorts, increase information visibility, and potentially deliver successful health promotion campaigns. It is worth noting, however, the age profile of at-risk individuals and the learning strategies that appeal to high-risk age groups (Chelf et al., 2002; Saab et al., 2017b).
There are several complex barriers that can affect an individual’s understanding of a disease and impede decision-making and help-seeking. It is evident from this review that multimodal public health campaigns would best suit high-risk populations. Approaching health from a population perspective, future interventions and campaigns should consider including a structured theoretical framework, such as the Theory of Planned Behaviour (Ajzen and Manstead, 2007). Moreover, future interventions ought to incorporate targeted information through the use of educational resources, face-to-face counselling, and video- and web-based decision aids, while being cognizant of the preferred learning strategies and the key characteristics of health-promoting messages that would appeal to at-risk groups.
Limitations
Rigour was sought in the conduct and reporting of this systematic review. However, several threats to generalizability are worthy of note. While some of the review team members were multilingual, none of the languages used in non-English language papers was spoken by the research team and no resources were available to professionally translate non-English papers to English, which resulted in excluding those. Moreover, while the five-year search limit helped source the latest evidence, older interventions were omitted. Generalizability of findings is also hindered by the small number of studies included and the fact that almost half of the reviewed studies (n = 7) did not meet two key quality assessment criteria namely ‘participants representative of target population’ and ‘confounder accounted for in the design and analysis’ and only two studies had a low risk of bias (Emery et al., 2019; Williams et al., 2021). Study selection bias could have occurred, since only outcomes that were in line with the review aims were reported and no records were sought from the grey literature.
Despite this being a systematic review of interventions, a meta‐analysis was not plausible primarily due to heterogeneity in study designs, outcomes and outcome measures. The reliability and generalizability of the review results are limited further by the presence of three sources of bias: (i) study designs: the included studies used six different study designs; (ii) study instruments: half of the studies (n = 8) used researcher-designed instruments and failed to report on the validity and reliability of those instruments and (iii) follow-up periods: diverse follow-up periods of data collection were evident, with some studies not having baseline data and others measuring outcomes either immediately post-test or at multiple points post-test. The implication is that findings relating to subjective data measured objectively would change over time and repeat measures at different points in time give different results. Hence, a consistent prepost repeat measures approach is key to minimizing this bias in future research.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Health Promotion International online.
FUNDING
This study was funded by the National Cancer Control Programme, Health Service Executive, Ireland.
Supplementary Material
REFERENCES
- Ajzen I., Manstead A. S. (2007) 4 Changing health-related behaviours. The Scope of Social Psychology, 43-, 43–63. [Google Scholar]
- American Cancer Society (2019) Signs and symptoms of lung cancer. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/signs-symptoms.html (last accessed 7 June 2020).
- Bell N. R., Grad R., Dickinson J. A., Singh H., Moore A. E., Kasperavicius D.. et al. (2017) Better decision making in preventive health screening: balancing benefits and harms. Canadian Family Physician Medecin de Famille Canadien, 63, 521–524. [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. (2018) Cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68, 394–424. [DOI] [PubMed] [Google Scholar]
- Cardarelli R., Roper K. L., Cardarelli K., Feltner F. J., Prater S., Ledford K. M.. et al. (2017) Identifying community perspectives for a lung cancer screening awareness campaign in Appalachia Kentucky: the Terminate Lung Cancer (TLC) study. Journal of Cancer Education, 32, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter‐Harris L. (2015) Lung cancer stigma as a barrier to medical help‐seeking behavior: practice implications. Journal of the American Association of Nurse Practitioners, 27, 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassim S., Chepulis L., Keenan R., Kidd J., Firth M., Lawrenson R. (2019) Patient and carer perceived barriers to early presentation and diagnosis of lung cancer: a systematic review. BMC Cancer, 19, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelf J. H., Deshler A., Thiemann K., Dose A. M., Quella S. K., Hillman S. (2002, June) Learning and support preferences of adult patients with cancer at a comprehensive cancer center. Oncology Nursing Forum, 29, 863–867. [DOI] [PubMed] [Google Scholar]
- Chowienczyk S., Price S., Hamilton W. (2020) Changes in the presenting symptoms of lung cancer from 2000–2017: a serial cross-sectional study of observational records in UK primary care. British Journal of General Practice, 70, e193–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph S., Cazzaniga S., Hunger R. E., Naldi L., Borradori L., Oberholzer P. A. (2016) Ultraviolet radiation protection and skin cancer awareness in recreational athletes: a survey among participants in a running event. Swiss Medical Weekly, 146, w14297. [DOI] [PubMed] [Google Scholar]
- Clarke N., Gallagher P., Kearney P. M., McNamara D., Sharp L. (2016) Impact of gender on decisions to participate in faecal immunochemical test‐based colorectal cancer screening: a qualitative study. Psycho-oncology, 25, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Cunningham Y., Wyke S., Blyth K. G., Rigg D., Macdonald S., Macleod U.. et al. (2019). Lung cancer symptom appraisal among people with chronic obstructive pulmonary disease: A qualitative interview study. Psycho‐oncology, 28, 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlamini S. B., Sartorius B., Ginindza T. (2019) Mapping the evidence on interventions to raise awareness on lung cancer in resource poor settings: a scoping review protocol. Systematic Reviews, 8, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P. M. and , Vandermeer R. (2011) Delays in the diagnosis of lung cancer. Journal of Thoracic Disease, 3, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery J. D., Murray S. R., Walter F. M., Martin A., Goodall S., Mazza D.. et al. (2019) The Chest Australia Trial: a randomised controlled trial of an intervention to increase consultation rates in smokers at risk of lung cancer. Thorax, 74, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B., Potente S., Rock V., McIver J. (2015) Social media campaigns that make a difference: what can public health learn from the corporate sector and other social change marketers. Public Health Research & Practice, 25, e2521517. [DOI] [PubMed] [Google Scholar]
- Fung L. C., Nguyen K. H., Stewart S. L., Chen, JrM. S., Tong E. K. (2018) Impact of a cancer education seminar on knowledge and screening intent among Chinese Americans: results from a randomized, controlled, community‐based trial. Cancer, 124, 1622–1630. [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., Welch V. A. (eds) (2019). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Chichester UK. [Google Scholar]
- Holmberg L., Sandin F., Bray F., Richards M., Spicer J., Lambe M.. et al. (2010) National comparisons of lung cancer survival in England, Norway and Sweden 2001–2004: differences occur early in follow-up. Thorax, 65, 436–441. [DOI] [PubMed] [Google Scholar]
- Hong Q. N., Fàbregues S., Bartlett G., Boardman F., Cargo M., Dagenais P.. et al. (2018) The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Education for Information, 34, 285–291. [Google Scholar]
- Housten A. J., Lowenstein L. M., Leal V. B., Volk R. J. (2018) Responsiveness of a brief measure of lung cancer screening knowledge. Journal of Cancer Education, 33, 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanec J., Siemiatycki J., Conway D. I., Olsson A., Stücker I., Guida F.. et al. (2018) Lung cancer and socioeconomic status in a pooled analysis of case–control studies. PLoS One, 13, e0192999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironmonger L., Ohuma E., Ormiston-Smith N., Gildea C., Thomson C. S., Peake M. D. (2015) An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. British Journal of Cancer, 112, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Fedewa S. A. (2017) Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncology, 3, 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup D. L., Glover, IVM., Daye D., Banzi L., Jones P., Choy G.. et al. (2018) Implementation of digital awareness strategies to engage patients and providers in a lung cancer screening program: retrospective study. Journal of Medical Internet Research, 20, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. P., Cheyne L., Darby M., Plant P., Milton R., Robson J. M.. et al. (2018) Lung cancer stage-shift following a symptom awareness campaign. Thorax, 73, 1128–1136. [DOI] [PubMed] [Google Scholar]
- Koo M. M., Hamilton W., Walter F. M., Rubin G. P., Lyratzopoulos G. (2018) Symptom signatures and diagnostic timeliness in cancer patients: a review of current evidence. Neoplasia, 20, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. K., Caverly T. J., Cao P., Cherng S. T., West M., Gaber C.. et al. (2015) Evaluation of a personalized, web-based decision aid for lung cancer screening. American Journal of Preventive Medicine, 49, e125–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. M., Doria-Rose V. P., Gareen I. F., Brewer B., Clingan K., Keating K.. et al. (2016) Did death certificates and a death review process agree on lung cancer cause of death in the National Lung Screening Trial? Clinical Trials: Journal of the Society for Clinical Trials, 13, 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone P. J., Tenenbaum A., Seeley M., Petersen H., Lyon C., Han X.. et al. (2017) Impact of a lung cancer screening counseling and shared decision-making visit. Chest, 151, 572–578. [DOI] [PubMed] [Google Scholar]
- McCutchan G., Hiscock J., Hood K., Murchie P., Neal R. D., Newton G.. et al. (2019) Engaging high-risk groups in early lung cancer diagnosis: a qualitative study of symptom presentation and intervention preferences among the UK’s most deprived communities. BMJ Open, 9, e025902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneses-Echávez J. F., Alba-Ramírez P. A., Correa-Bautista J. E. (2018) Raising awareness for lung cancer prevention and healthy lifestyles in female scholars from a low-income area in Bogota, Colombia: evaluation of a national framework. Journal of Cancer Education, 33, 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe K. A., Dennis C. L., Poll A., Armel S., Demsky R., Carlsson L.. et al. (2017) Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Genetics in Medicine, 19, 330–336. [DOI] [PubMed] [Google Scholar]
- Moffat J., Bentley A., Ironmonger L., Boughey A., Radford G., Duffy S. (2015) The impact of national cancer awareness campaigns for bowel and lung cancer symptoms on sociodemographic inequalities in immediate key symptom awareness and GP attendances. British Journal of Cancer, 112, S14–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., Grp P. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Reprinted from Annals of Internal Medicine). Physical Therapy, 89, 873–880. [PubMed] [Google Scholar]
- Mueller J., Davies A., Jay C., Harper S., Blackhall F., Summers Y.. et al. (2019) Developing and testing a web‐based intervention to encourage early help‐seeking in people with symptoms associated with lung cancer. British Journal of Health Psychology, 24, 31–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2020) Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html (last accessed 5 June 2020).
- National Lung Screening Trial Research Team (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine, 365, 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmons K., , BeaudoinC. E. and , St. John J. A. (2017) The outcome evaluation of a CHW cancer prevention intervention: testing individual and multilevel predictors among hispanics living along the Texas-Mexico Border. Journal of Cancer Education, 32, 183–189. [DOI] [PubMed] [Google Scholar]
- O'Mahony M., McCarthy G., Corcoran P., Hegarty J. (2013) Shedding light on women's help seeking behaviour for self discovered breast symptoms. European Journal of Oncology Nursing, 17, 632–639. [DOI] [PubMed] [Google Scholar]
- Oudkerk M., Devaraj A., Vliegenthart R., Henzler T., Prosch H., Heussel C. P.. et al. (2017) European position statement on lung cancer screening. The Lancet Oncology, 18, e754–e766. [DOI] [PubMed] [Google Scholar]
- Perestelo-Perez L., Rivero-Santana A., Torres-Castaño A., Ramos-Garcia V., Alvarez-Perez Y., Gonzalez-Hernandez N.. et al. (2019) Effectiveness of a decision aid for promoting colorectal cancer screening in Spain: a randomized trial. BMC Medical Informatics and Decision Making, 19, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power E., Wardle J. (2015) Change in public awareness of symptoms and perceived barriers to seeing a doctor following Be Clear on Cancer campaigns in England. British Journal of Cancer, 112, S22–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M., Saab M. M., Hegarty J., Von Wagner C., O’Mahony M., Murphy M.. et al. (2018) Promoting men’s knowledge of cancer risk reduction: a systematic review of interventions. Patient Education and Counseling, 101, 1322–1336. [DOI] [PubMed] [Google Scholar]
- Reuland D. S., Cubillos L., Brenner A. T., Harris R. P., Minish B., Pignone M. P. (2018) A pre-post study testing a lung cancer screening decision aid in primary care. BMC Medical Informatics and Decision Making, 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel M., Quaife S. L., Ghimire B., Dickson J. L., Bhowmik A., Navani N.. et al. (2019) Impact of a lung cancer screening information film on informed decision-making: a randomized trial. Annals of the American Thoracic Society, 16, 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A. M., Cushen S., Schellekens H., Bhuachalla E. N., Burns L., Kenny U.. et al. (2015) Poor awareness of risk factors for cancer in Irish adults: results of a large survey and review of the literature. The Oncologist, 20, 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab M. M., , LandersM., , CookeE., , MurphyD., , DavorenM. and , Hegarty J. (2018) Enhancing men’s awareness of testicular disorders using a virtual reality intervention. Nursing Research, 67, 349–358. [DOI] [PubMed] [Google Scholar]
- Saab M. M., Landers M., Hegarty J. (2017a) Exploring awareness and help-seeking intentions for testicular symptoms among heterosexual, gay, and bisexual men in Ireland: a qualitative descriptive study. International Journal of Nursing Studies, 67, 41–50. [DOI] [PubMed] [Google Scholar]
- Saab M. M., Landers M., Hegarty J. (2017b) Exploring men's preferred strategies for learning about testicular disorders inclusive of testicular cancer: a qualitative descriptive study. European Journal of Oncology Nursing, 26, 27–35. [DOI] [PubMed] [Google Scholar]
- Sakoda L. C., Meyer M. A., Chawla N., Sanchez M. A., Blatchins M. A., Nayak S.. et al. (2020) Effectiveness of a patient education class to enhance knowledge about lung cancer screening: a quality improvement evaluation. Journal of Cancer Education, 35, 897–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network (2019) SIGN 100: a handbook for patients and carer representatives. https://www.sign.ac.uk/assets/sign100.pdf (last accessed 7 June 2020).
- Siegel R. L., Miller K. D., Jemal A. (2018) Cancer statistics, 2018. CA: A Cancer Journal for Clinicians, 68, 7–30. [DOI] [PubMed] [Google Scholar]
- Smith C. F., Whitaker K. L., Winstanley K., Wardle J. (2016) Smokers are less likely than non-smokers to seek help for a lung cancer ‘alarm’ symptom. Thorax, 71, 659–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaboration (2020) About Covidence. https://community.cochrane.org/help/tools-and-software/covidence/about-covidence (last accessed 6 June 2020).
- Torre L. A., Siegel R. L., Jemal A. (2016) Lung cancer statistics. Advances in Experimental Medicine and Biology, 893, 1–19. [DOI] [PubMed] [Google Scholar]
- Walter F. M., Rubin G., Bankhead C., Morris H. C., Hall N., Mills K.. et al. (2015) Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. British Journal of Cancer, 112, S6–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm W. J., Kaunelis D. (2005) Literature reviews: analysis, planning, and query techniques. Delta Pi Epsilon Journal, 47-, 91–106. [Google Scholar]
- Williams L. B., Looney S. W., Joshua T., McCall A., Tingen M. S. (2021) Promoting community awareness of lung cancer screening among disparate populations: results of the cancer-community awareness access research and education project. Cancer Nursing,, 44, 89–97. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (2020) WHO report on cancer: setting priorities, investing wisely and providing care for all. https://www.who.int/publications-detail/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all (last accessed 20 October 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.