Abstract
The Lassa virus (an arenavirus) is found in West Africa, where it sometimes causes a severe hemorrhagic illness called Lassa fever. Laboratory diagnosis has traditionally been by the indirect fluorescent-antibody (IFA) test. However, enzyme-linked immunosorbent assays (ELISAs) for Lassa virus antigen and immunoglobulin M (IgM) and G (IgG) antibodies have been developed that are thought to be more sensitive and specific. We compared ELISA and IFA testing on sera from 305 suspected cases of Lassa fever by using virus isolation with a positive reverse transcription-PCR (RT-PCR) test as the “gold standard.” Virus isolation and RT-PCR were positive on 50 (16%) of the 305 suspected cases. Taken together, Lassa virus antigen and IgM ELISAs were 88% (95% confidence interval [CI], 77 to 95%) sensitive and 90% (95% CI, 88 to 91%) specific for acute infection. Due to the stringent gold standard used, these likely represent underestimates. Diagnosis could often be made on a single serum specimen. Antigen detection was particularly useful in providing early diagnosis as well as prognostic information. Level of antigenemia varied inversely with survival. Detection by ELISA of IgG antibody early in the course of illness helped rule out acute Lassa virus infection. The presence of IFA during both acute and convalescent stages of infection, as well as significant interobserver variation in reading the slides, made interpretation difficult. However, the assay provided useful prognostic information, the presence of IFA early in the course of illness correlating with death. The high sensitivity and specificity, capability for early diagnosis, and prognostic value of the ELISAs make them the diagnostic tests of choice for the detection of Lassa fever.
Lassa virus is an arenavirus known to cause a severe hemorrhagic fever in West Africa. An estimated 100,000 infections and 5,000 deaths occur yearly across the region (10, 24). Early clinical manifestations are often indistinguishable from those of many other febrile illnesses, making clinical diagnosis difficult (17). The antiviral drug ribavirin is effective, but only if administered early in the course of illness (12, 18). Because of its expense, need for intravenous administration, potential toxicity, and teratogenicity, empiric therapy with ribavirin is undesirable (7, 15, 18). As Lassa virus has been associated with nosocomial outbreaks with high mortality (19, 28), early identification of infected individuals is important for the prompt implementation of appropriate barrier nursing guidelines (8). Thus, for both therapeutic and preventive considerations, a rapid and accurate laboratory test for the diagnosis of acute Lassa fever early in the course of the disease is imperative.
Laboratory diagnosis of acute Lassa virus infection has traditionally employed the indirect fluorescent-antibody (IFA) test (17, 30). This technique has been criticized, however, for a perceived lack of specificity in populations with a low apparent risk of infection (27). In recent years, enzyme-linked immunosorbent assays (ELISAs) for Lassa virus antigen and Lassa virus-specific immunoglobulin M (IgM) and G (IgG) antibodies that are thought to be more sensitive and specific have been developed (11, 13, 22). However, to date, testing with this technique has been conducted only on animals and on a small number of patients known to be infected with Lassa virus. A thorough evaluation of the assay on field-collected samples has not been conducted to assess its true sensitivity and specificity. We therefore performed the Lassa virus antibody and antigen ELISAs on sera from a large number of patients suspected of having Lassa fever from West Africa. As the “gold standard” test of Lassa virus infection, we used isolation of virus as detected by immunofluorescent staining for viral antigen along with a positive reverse transcription-PCR (RT-PCR) test on the isolate. Genetic sequencing was performed on most RT-PCR products to confirm the viruses as strains of Lassa virus. Lastly, recognizing that much previous research on Lassa fever has employed the IFA technique, we examined the relationship between antibodies detected by ELISA and IFA testing. We report here the results and interrelationships of the varied components of these assays and explore how they might best be used in both diagnosis and management of Lassa fever.
MATERIALS AND METHODS
Patient identification and specimen collection.
Specimens were collected from patients suspected of having Lassa fever who presented to Kenema Government Hospital in Sierra Leone or to one of four surveillance hospitals in Guinea, West Africa, from October 1996 through February 1998. Blood was drawn immediately upon clinical suspicion of Lassa fever (which was most often at the time of admission) and then at various intervals over the following weeks. Samples were collected in 10-ml syringes and allowed to clot at ambient temperature for no more than 2 h before the serum was separated by personnel wearing standard protective clothing consisting of masks, gowns, and gloves. Separated serum and clots were labeled and stored in a −15°C freezer in Sierra Leone and in liquid nitrogen in Guinea until transported on dry ice in specialized safety shippers to the Centers for Disease Control and Prevention (CDC) in Atlanta, Ga. Although the cold chain was maintained reliably in Guinea, conditions related to the civil war in Sierra Leone resulted in frequent power outages with subsequent freeze-thawing and suboptimal storage conditions for the specimens from that country. At CDC, samples were sorted on dry ice and stored in gas-phase liquid nitrogen freezers until testing.
Samples were first taken into a biosafety level 4 (BSL-4) laboratory and thawed, and an aliquot for serologic testing was placed into a designated well of a microtiter format tube holder (Bio-Rad, Emeryville, Calif.). This was then sealed with paraffin film, and the holder and contents were double heat sealed in plastic bags. The sealed bags were then passed out of the BSL-4 laboratory through a 3% Lysol immersion tank and kept at −70°C until being irradiated with 2 × 106 rads (20,000 Gy) of gamma in a cobalt irradiator (Gammacel 220; Nordion International, Kanata, Canada) while chilled on dry ice. Aliquots for virus isolation were kept in the BSL-4 laboratory.
ELISA. (i) General procedure.
All sera were tested for Lassa virus antigen and Lassa virus-specific IgM and IgG antibodies. The Josiah strain of Lassa virus was used in the preparation of antigens. Ninety-six-well microtiter plates (Becton-Dickinson, Oxnard, Calif.) were used in each assay, and a volume of 100 μl was added to each well at every step. In each assay, the plates were divided evenly into positive (Lassa virus) and negative (control) halves and were coated with the appropriate reagent (see below). Plates were incubated at 37°C for 1 h in a humidified chamber at each step unless otherwise specified. Between incubations, plates were washed with phosphate-buffered saline (PBS) (pH 7.4) containing 0.1% Tween by using a Skatron ELISA plate washer (Skatron Instruments, As, Norway) with three 2-s, 250-μl wash cycles and a 4-s hold and rinse. Sera were diluted in 0.01 M PBS-skim milk containing 0.1% Tween 20, pH 7.2. The substrate consisted of equal-volume amounts of preformulated 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) peroxidase and hydrogen peroxidase (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, Md.). The wells were measured at 410 nm by using a Dynatech MR5000 ELISA plate reader (Dynatech Laboratories, Chantilly, Va.). An adjusted optical density at 410 nm (OD410) was calculated by subtracting the OD410 of the control antigen from the OD410 of the specific antigen for each dilution. For the antigen detection and IgM assays, an adjusted OD410 ≥ 0.1 was required at a given dilution for it to be considered positive and a titer assigned accordingly. For the IgG assay, which had a generally lower signal-to-noise ratio, an adjusted OD410 ≥ 0.2 was required. Designation of a sample as positive was based on both the titer and the sum of the adjusted OD410. Positive and negative control sera from known Lassa virus-infected patients and healthy West African controls were run with each assay. The specific details of the antigen detection, IgM-capture, and IgG ELISAs are described below.
(ii) Antigen detection.
The positive-capture antibodies consisted of an equal-volume mix of five monoclonal antibodies directed at the nucleoprotein of Lassa virus. The dilution of this mix was determined by checkerboard titration. The negative-capture control was composed of ascites from the parent myeloma cell line.
Microtiter plates were divided evenly, and the top and bottom halves were coated with the respective positive- and negative-capture antibodies, each diluted to 1:1,000 in coating buffer (PBS, pH 7.4, with 0.01% thimerosol). Plates were kept overnight at 4°C and then washed, and fourfold serial dilutions (1:4 to 1:256) of test sera were added. After incubation and washing, a hyperimmune rabbit anti-Lassa virus antibody at a dilution of 1:2,000 was added. After another round of incubation and washing, horseradish peroxidase-labeled goat anti-rabbit IgG antibody (Kirkegaard and Perry Laboratories) at a dilution of 1:10,000 was added and the plates were again incubated. Finally, the plates were washed, substrate was added, and the plates were incubated for 30 min and read. A specimen was considered positive if it had a titer ≥1:16 and a sum OD410 of ≥0.6.
(iii) IgM capture.
A Lassa virus-Vero E6 cell slurry was prepared in the BSL-4 laboratory in the following manner. Vero E6 cells grown in roller bottles were infected with Lassa virus and harvested when virtually all the cells had become infected as determined by immunofluorescence (usually about 6 days). Harvesting was performed by first adding 0.1 M Tris, pH 8.5, to buffer the medium and then freeze-thawing the roller bottles. The medium and cells were then frozen in plastic bottles, heat sealed in double bags, passed out of the BSL-4 laboratory as described above, and gamma irradiated with 5 × 106 rads (50,000 Gy). This material was then thawed, sonicated, and dispensed into small vials for use as the antigen in IgM-capture assays. A mock-infected Vero E6 control antigen was similarly prepared.
Microtiter plates were coated with goat anti-Mu chain antibody (Biosource International, Camarillo, Calif.) in coating buffer at a dilution of 1:500, held overnight at 4°C, and then washed. Fourfold serial dilutions of test sera (1:100 to 1:6,400) in diluent were added, and the plates were incubated and washed. The Lassa virus-Vero E6 cell slurry and mock-infected control were added to the top and bottom halves of the plate, respectively, at a dilution of 1:4. After incubation and washing, Lassa virus hyperimmune mouse ascitic fluid was added at a dilution of 1:2,000 and the plates were again incubated and washed. Horseradish peroxidase-labeled anti-mouse IgG antibody (Biosource International) was added at a dilution of 1:2,000 before incubating and washing the plates once more. The addition of substrate and the final incubation and wash steps were identical to those described above. A specimen was considered positive if it had a titer of ≥1:400 and a sum OD410 of ≥0.6.
(iv) IgG detection.
A Lassa virus-Vero E6 cell lysate was prepared in the following manner: Lassa virus-infected Vero E6 cells were grown and harvested as described above. The cell suspension was then centrifuged at 10,000 × g at 4°C for 10 min and was washed once in 0.01 M borate saline, pH 9.0. The pellet was resuspended in borate saline with 1% Triton X-100 (Sigma, St. Louis, Mo.), was sonicated in a chilled cup horn for 10 min, and was again centrifuged at 10,000 × g at 4°C for 5 min. The supernatant was then passed out of the BSL-4 laboratory and gamma irradiated as described above. Unbound antigen was removed by washing three times with PBS containing 0.1% Tween 20. A mock-infected Vero E6 control antigen was similarly prepared.
Microtiter plates were again divided into positive and negative halves and coated with the respective antigens, both at a dilution of 1:1,000 as determined by checkerboard titration. Plates were kept overnight at 4°C and were washed, and fourfold serial dilutions of test sera (1:100 to 1:6,400) were added. After incubation and washing, horseradish peroxidase-labeled mouse gamma chain-specific anti-human IgG antibody (Accurate Chemical, Grand Island, N.Y.) was added at a dilution of 1:10,000. The addition of substrate, final incubation, and wash steps were performed as described above. A specimen was considered positive if it had a titer ≥1:400 and a sum OD410 of ≥1.0.
IFA testing.
In the BSL-4 laboratory, Lassa virus was propagated in a Vero E6 monolayer cell culture with Eagle's minimal essential medium with Earle's salts (GIBCO, Grand Island, N.Y.) supplemented with 2% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 50 μg of gentamycin per ml. The cells were harvested at 8 days postinoculation and were resuspended in a freezing medium (95% fetal calf serum plus 5% dimethyl sulfoxide). A suspension of uninfected Vero E6 cells was prepared in an identical manner, and both cell suspensions were passed out of the BSL-4 laboratory as described above, gamma irradiated with 5 × 106 rads (20,000 Gy), and stored at −70°C until use. Spot slides were made from infected and uninfected cell suspensions which were thawed and mixed in PBS to a concentration of approximately one million cells/ml, with a ratio of infected to uninfected cells of 3:2. Twenty microliters of this suspension was placed in each well of 12-well Teflon-coated slides (Cell-Line Associates, New-Field, N.J.) which were then air dried, fixed in cold acetone, and stored at −70°C until use.
Spot slides were thawed and dried by using an electric blow-dryer. The slides were similarly dried after each wash. Twofold serial dilutions (1:50 to 1:1,600) of test sera were placed on the slides, which were then incubated for 30 min at 37°C in a humidified chamber and washed for 15 min in PBS, pH 7.2. Fluorescein isothiocyanate (FITC)-conjugated sheep anti-human Ig (Murex Diagnostics Ltd., Dartford, England) at a dilution of 1:50 mixed with 10 μl of 1% Evans blue for counterstaining was added. This conjugate recognizes IgM, IgG, and IgA, and can thus be used to detect all three. The slides were again incubated for 30 min and washed, and a coverslip was mounted by using 10% glycerol in PBS, pH 8.2.
Slides were read immediately after preparation by using an Axioskop microscope (Zeiss, Oberkochen, Germany) with appropriate barrier and excitation filters for FITC visualization. Fluorescent intracytoplasmic inclusions seen at any dilution constituted a positive specimen. Samples still positive at a dilution of 1:1,600 were rediluted with a twofold dilution starting at 1:200 (1:200 to 1:6,400) and were retested. Positive and negative controls were included with each assay. To assess interobserver variation, slides were read independently by three different observers who were blinded to the ELISA, virus isolation, and RT-PCR results. Observers of varying degrees of experience with the IFA technique were chosen: (i) extensive experience (>10 years); (ii) intermediate experience (2 years); and (iii) no previous experience.
Virus isolation.
Qualitative virus isolation was performed in the BSL-4 laboratory. Fifty microliters of clot from the first blood draw of each patient was inoculated onto Vero E6 confluent cell monolayers in 25-cm2 plastic tissue culture flasks. For some patients, no blood clot was available, in which case serum was substituted. Inocula were incubated for 30 min at 37°C to allow adsorption to the cell monolayer. Seven milliliters of maintenance medium, as described above in the IFA section, was then added, and the flasks were returned to the incubator. Medium was changed at 1 week postinoculation. Initially, portions of the monolayers were scraped at both 1 and 2 weeks postinoculation. As the study progressed, however, it became apparent that a single scraping at week 2 would detect all the positives, and this approach was then followed for the remainder of the study. After scraping, 1 ml of the cell suspension was added to 14 ml of PBS in a 15-ml conical tube that was then centrifuged at 1,000 × g for 5 min. The supernatant was discarded, and spot slides were made from the resuspended pellet. After air drying, these slides were double bagged, passed out of the BSL-4 laboratory through the Lysol immersion tank as previously described, gamma irradiated with 2 × 106 rads (20,000 Gy), fixed in cold acetone for 10 min, and stored at 4°C until staining. The unused portion of the cell suspension from this step was stored at −70°C for subsequent RT-PCR analysis (see below).
For staining, slides were blow-dried, Lassa virus hyperimmune mouse ascitic fluid in PBS-skim milk at a dilution of 1:100 was added to each spot well, and the slides were incubated at 37°C in a humidified chamber for 1 h. After being washed for 10 min in PBS, pH 7.2, and redried, FITC-conjugated goat anti-mouse IgG (Cappel, Durham, N.C.) in PBS-skim milk at a dilution of 1:50 mixed with 10 μl of 1% Evans blue was added, and the slides were again incubated for 30 min. Finally, slides were washed, mounted, and read as described above. Positive and negative controls were included.
RT-PCR and sequencing.
RT-PCR amplification was performed on all specimens from which Lassa virus was isolated. In the BSL-4 laboratory, 300 μl of the remaining cell suspension was added to 1 ml of Tripure reagent (Boehringer Mannheim, Indianapolis, Ind.) to inactivate virus and protect viral RNA from nuclease digestion. The samples were then passed out of the BSL-4 laboratory through the Lysol immersion tank for RNA extraction and RT-PCR. Genetic sequence analysis was performed on most isolates by using a published nucleotide primer pair targeting a region near the 3′ end of the arenavirus N gene (3).
Data analysis.
Data were analyzed using SPSS version 8.0 statistical software. To account for their nonnormal distribution, viral titers were converted to the log10 of their value for statistical analysis. Student's t test, Fisher's exact test, and the kappa correlation coefficient were applied where appropriate.
RESULTS
Patients and specimen collection.
A total of 590 serum specimens collected from 305 patients suspected of having acute Lassa virus infection, 224 from Guinea and 81 from Sierra Leone, were evaluated. The mean number of specimens collected per patient was 1.9 (range, 1 to 5 days). The first blood draw was performed a mean of 9.6 days (range, 0 to 57 days) after the reported onset of illness. Patients with confirmed Lassa fever presented to the hospital significantly earlier after the reported onset of illness than those who were virus isolation negative, 7.9 versus 9.9 days (P < 0.05), suggesting that they were more seriously ill.
Virus isolation and RT-PCR.
Viral antigens were observed in 12 of 18 specimens scraped 1 week after inoculation and in all 18 after 2 weeks. Combined with the specimens that were scraped only at 2 weeks, Lassa virus infection was confirmed by virus isolation in 50 (16.4%) of the 305 suspected cases, 7 from Guinea and 43 from Sierra Leone. Viral RNA was amplified from all 50 isolates. Genetic sequence analysis performed on 41 (82%) of the 50 isolates confirmed them to be Lassa viruses with moderate heterogeneity. Viruses were not identical to the reference Josiah strain. Details on the molecular analysis will be presented in a subsequent publication. A total of 112 serum specimens were collected from the 50 culture-confirmed cases.
ELISA. (i) Sensitivity and specificity.
Figure 1 shows the distribution of ELISA titers and ODs from the first two blood specimens obtained from confirmed cases of Lassa fever. A summary of the serologic results is presented in Table 1. ELISA of IgM proved to be the single most sensitive assay in detecting acute infection overall, identifying 36 (72%) of the 50 cases. However, antigen detection was more sensitive early in the course of the disease, identifying 15 (30%) of the 50 on the first blood draw (see below). In practice, ELISAs for antigen and IgM are used in tandem, with a case considered positive if ELISA antigen and/or IgM antibody are present. Performed on all 305 suspected cases, ELISA for antigen and IgM (ELISA Ag/IgM) detected 44 (88%) of the 50 culture-confirmed cases for a sensitivity and specificity of 88% (95% confidence interval [CI], 77 to 95%) and 90% (95% CI, 88 to 91), respectively (Table 2).
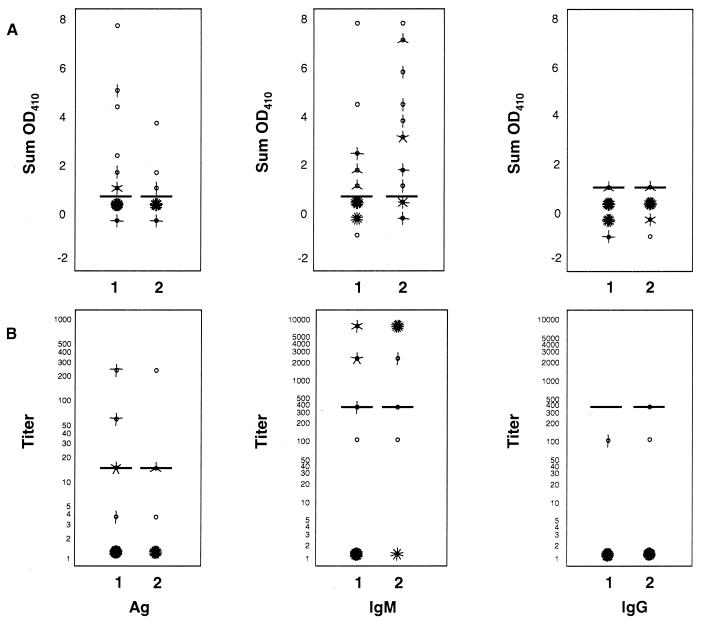
FIG. 1.
Lassa virus antigen (Ag), IgM, and IgG antibody findings from first and second samples drawn from 50 patients with culture-confirmed Lassa fever. (A) Adjusted optical density (Sum OD410). (B) Titers assigned to each serum specimen. Horizontal bars represent the cutoff for a positive result for each assay. Each “petal” of a “sunflower” ( ) represents a patient. Sunflowers without petals represent a single case. Titers are expressed on a log10 scale. The numbers of specimens in various categories are not equal because not all patients had two blood samples drawn.
) represents a patient. Sunflowers without petals represent a single case. Titers are expressed on a log10 scale. The numbers of specimens in various categories are not equal because not all patients had two blood samples drawn.
TABLE 1.
Serologic results for 50 culture-confirmed cases of Lassa fever
| Assay | No. of patients positive (% total)
|
|
|---|---|---|
| First blood draw | All blood draws | |
| ELISA antigen | 15 (30) | 18 (36) |
| ELISA IgM | 13 (26) | 36 (72) |
| ELISA IgG | 0 (0) | 9 (18) |
| IFA | 13 (26) | 35 (70) |
TABLE 2.
Results of combined ELISA Ag/IgM testing compared with virus isolation for 305 suspected cases of Lassa fever (numbers in parentheses represent the percent of total cases)a
| Virus isolation | ELISA Ag/IgMb
|
||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 229 (75.1) | 26 (8.5) | 255 (83.6) |
| Positive | 6 (2.0) | 44 (14.4) | 50 (16.4) |
| Total | 235 (77.1) | 70 (22.9) | 305 (100) |
A positive result is defined as an individual positive for ELISA antigen and/or IgM antibody.
Sensitivity (95% CI), 88% (range, 77 to 95%); specificity (95% CI), 90% (range, 88 to 91%); positive predictive value (95% CI), 63% (range, 55 to 68%); negative predictive value (95% CI), 97% (range 95 to 99%).
ELISA IgG antibody was detected in 50 (16%) of the 305 suspected cases, but in only 9 (18%) of the 50 confirmed acute cases of Lassa virus infection. These nine had multiple blood draws (mean 3.6) but were never found to be ELISA IgG antibody positive early in the course of their disease. Titers were significantly higher in the presumed convalescent cases than in the acutely infected cases (mean log10 titer, 3.3 versus 2.7, respectively, P = 0.016). Thus, the presence of ELISA IgG antibody confirms previous exposure to Lassa virus and, conversely, can be used to exclude acute infection if found in high titer early in the course of illness. Six (12%) of the 50 patients from whom Lassa virus was isolated were completely negative by all ELISA and IFA testing.
(ii) Time to diagnosis.
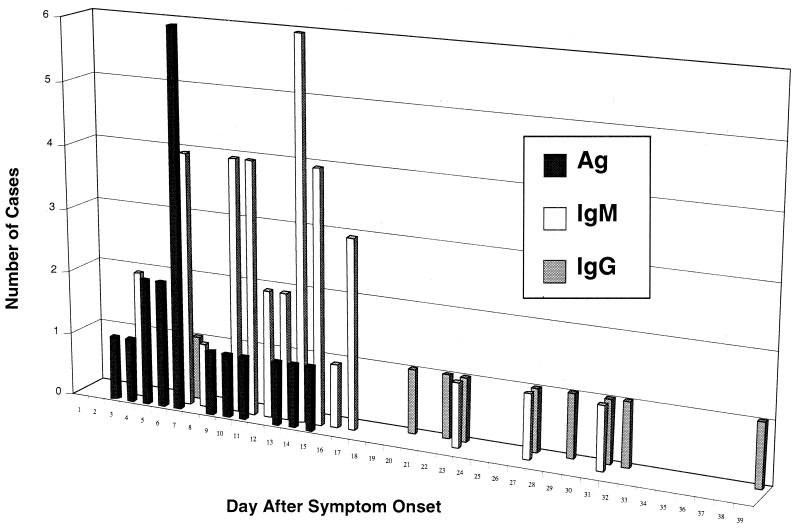
The timing of antigen and antibody responses relative to symptom onset is shown in Fig. 2. ELISA Ag/IgM detected 56% of the cases on the first serum specimen drawn. Antigen usually appeared in the first week of illness, to be subsequently replaced by IgM antibody in the second week. IgM antibody then typically remained positive through the last drawn sample, which was a mean of 16.4 days (range, 4 to 40 days) after symptom onset. Antigen and IgM almost never appeared concurrently in a single blood draw (1 of 590 specimens), suggesting that IgM either clears viral antigen or masks it from detection. ELISA IgG began to appear around week 3. The mean time from symptom onset until the first positive serologic result for the various diagnostic assays is shown in Table 3.
FIG. 2.
Graph of day after symptom onset for the first positive ELISA results for 50 culture-confirmed cases of Lassa fever.
TABLE 3.
Time from reported symptom onset until the first blood draw positive for Lassa fever with various diagnostic modalities
| Assay | No. cases positive | Mean no. days (+ SEM) | Range (days) |
|---|---|---|---|
| ELISA antigen | 18 | 7.9 (0.8)a | 3–15 |
| ELISA IgM | 36 | 13.1 (0.9)b | 4–31 |
| ELISA IgG | 9 | 25.6 (3.0)c | 7–39 |
| IFA | 34 | 13.1 (1.0)c | 4–31 |
| Virus isolation | 50 | 8.0 (0.6)a | 3–22 |
P < 0.001 for comparison with ELISA IgM.
P < 0.005 for comparison with ELISA IgG.
P < 0.01 for comparison between IFA and ELISA IgG.
(iii) ELISA results and prognosis.
High titers of Lassa virus have been associated with a poor prognosis (14). However, viral culture and quantitative assay are too time-consuming, expensive, and dangerous in most settings to make this information of practical clinical benefit. We examined whether level of antigenemia could be used as a surrogate, and also investigated the relationship between ELISA IgM and IgG antibodies and prognosis.
Seventeen (24%) of the 50 patients with confirmed Lassa fever died. Death occurred a mean of 13.2 days (range, 4 to 31 days) after symptom onset. Qualitatively, only the absence of IgM antibody was associated with death (Table 4), most likely indicating that the fatalities occurred before the time when IgM antibody usually appears. However, one could alternatively interpret this as a specific defect in IgM antibody response that resulted in death. Although the absolute numbers were perhaps too small to reach statistical significance, patients who were antigen positive were almost twice as likely to die than those who were antigen negative.
TABLE 4.
Presence of Lassa virus antigen or antibody and outcome in 50 patients with virus culture-confirmed Lassa fever (percentages are of the total in each column)
| Positive assay | No. positive samples from patients that died (n = 17) (%) | No. positive samples from patients that survived (n = 33) (%) |
|---|---|---|
| ELISA antigen | 9 (53) | 9 (27) |
| ELISA IgM | 7 (41) | 29 (88)a |
| ELISA IgG | 1 (6) | 8 (24) |
| IFA | 9 (53) | 26 (79) |
P < 0.001 between survivors and deaths.
The relationship between titer level and survival is presented in Table 5. Mean log10 antigen titers were significantly higher in patients who died. Conversely, there was an inverse relationship between death and levels of both IgM and IgG antibodies. This result again most likely simply reflects that death occurred before the usual time of antibody appearance. That more of the survivors were not ELISA IgG positive can be attributed to the fact that serum was taken only during the acute phase of illness. There were no significant differences between deaths and survivors with regard to day of presentation after symptom onset or days until becoming antigen or ELISA IgM antibody positive. Because ribavirin was only intermittently available in most of the hospitals, no conclusions can be made regarding its potential effects on outcome, antigen, or antibody titer. Lastly, due to the lack of laboratory facilities in the study hospitals, we were not able to investigate the relationship between Lassa virus antigen or antibody and the level of aspartate aminotransferase. This relationship would be important to explore, as aspartate aminotransferase levels over 150 IU/liter have also been shown to correlate inversely with survival (14).
TABLE 5.
Mean log10 peak titer of antigen or antibody and outcome in 50 patients with virus culture-confirmed Lassa fever
| Assay | Mean log10 peak titer (SEM) for patients that:
|
|
|---|---|---|
| Died (n = 17) | Survived (n = 33) | |
| ELISA antigen | 0.99 (0.24) | 0.41 (0.12)a |
| ELISA IgM | 1.47 (0.40) | 3.25 (0.22)b |
| ELISA IgG | 0.31 (0.22) | 0.98 (0.25)a |
| IFA | 1.38 (0.33) | 2.27 (0.22)a |
P < 0.05 between survivors and deaths, independent samples two-tailed t test.
P < 0.001 between survivors and deaths, independent sample two-tailed t test.
IFA.
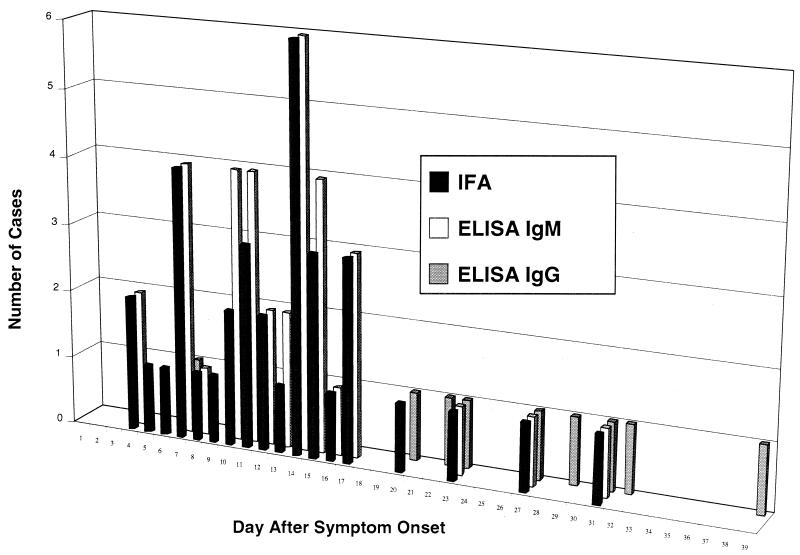
IFA testing was less sensitive than ELISA Ag/IgM, detecting 35 (70%) of the 50 culture-confirmed cases of Lassa fever (Table 6). The profile of IFA responses was quite similar to that of the combined ELISA IgM and IgG results, indicating the cross specificity of the IFA conjugate for IgM, IgG, and IgA isotypes (kappa = 0.686; P < 0.001) (Fig. 3). The time from symptom onset until appearance of IFA was also similar to that of ELISA IgM antibody (Table 3). Interestingly, IFA appeared significantly earlier in patients with fatal cases than in survivors (9.3 versus 14.1 days, respectively; P < 0.05).
TABLE 6.
Results of IFA compared with virus isolation for 305 suspected cases of Lassa fever
| Virus isolation | No. IFA (% total cases)
|
||
|---|---|---|---|
| Negative | Positive | Total | |
| Negative | 223 (73.1) | 32 (10.5) | 255 (83.6) |
| Positive | 15 (4.9) | 35 (11.5) | 50 (16.4) |
| Total | 238 (78.0) | 67 (22.0) | 305 (100) |
Sensitivity (95% CI), 70% (range, 57 to 81%); specificity (95% CI), 88% (range 85 to 90%); positive predictive value (95% CI), 52% (range 43 to 60%); negative predictive value (95% CI), 94% (range 91 to 96%).
FIG. 3.
Graph of day after symptom onset for the first positive IFA and ELISA IgM and IgG results for 50 culture-confirmed cases of Lassa fever.
The interpretation of IFA results was considerably observer dependent. While sensitivity and specificity were maintained with the observer of intermediate experience, they fell to 62% (95% CI, 49 to 74%) and 71% (95% CI, 68 to 73%), respectively, when the slides were read by novices. However, this difference appears to be largely overcome as observers gain experience. The interpretations of the expert and intermediately experienced observer were almost identical. Exactly how this would translate to the field and at what point a novice observer becomes “competent” would obviously vary with the setting and personnel. Although we did not formally examine this in our study, it is our observation that there is often significant intraobserver variation as well. Lastly, it should be noted that, short of photographing each microscopic field, no objective permanent record is produced from IFA testing. Thus, results cannot be reviewed should future questions arise.
DISCUSSION
Our results show the combined ELISA Ag/IgM assay to be highly sensitive and specific for the diagnosis of Lassa fever. The antigen detection assay offers a particular advantage in providing early diagnosis as well as prognostic information.
Although we used virus isolation as the gold standard for detection of Lassa virus infection, virus cannot be uniformly isolated from all acute cases, especially when the sample is obtained late in the course of illness (14). Furthermore, although Lassa virus is relatively hearty when stored in serum (D. G. Bausch, unpublished observation), breaks in the cold chain in Sierra Leone, where the majority of the cases were from, may have resulted in the deterioration of Lassa virus while the more heat-stable antibodies remained. This would result in apparent false-positive serologic results.
IgM antibody, but not Lassa virus antigen, was detected in 23 (88%) of the 26 cases reported as false positives in Table 2. A strong argument can be made that these were, in fact, true positives from whom blood was obtained only after the period of viremia. Twelve (63%) of the 19 cases from this group who had more than one specimen obtained showed rising IgM titers consistent with recent exposure to Lassa virus. Furthermore, ELISA IgG seroconversion was documented in 9 (82%) of the 11 who had three or more blood draws. If we consider these 23 cases as true positives, the sensitivity and specificity of the ELISA Ag/IgM would increase to 92% (95% CI, 86 to 95%) and 99% (95% CI, 97 to 100%), with positive and negative predictive values of 96% (95% CI, 89 to 99%) and 97% (95% CI, 96 to 98%), respectively. We believe these numbers to be more reflective of the true capabilities of the assay.
It was probably too late in the illness for these virus-negative, IgM-positive patients to benefit from ribavirin therapy. However, the exact mechanism of action of ribavirin in Lassa fever is unknown, and immunomodulatory effects of the drug have been reported that may confer benefit independently of any direct effects on virus replication (23). Twenty-one (91%) of the 23 virus-negative, IgM-positive patients survived. Five (22%) received ribavirin. One of the two patients who died received the drug.
Even if specific antiviral therapy would no longer be efficacious, confirming the diagnosis of Lassa fever is imperative. It allows the clinician to abandon an extensive search for other entities, to initiate rodent control measures in the patient's home to prevent infection of other family members, and to counsel the patient regarding behavior after discharge from the hospital. This latter point is important because Lassa virus has been isolated from throat swabs taken after viremia has ended (14, 20) and from semen as long as 6 weeks after acute disease (9). Anecdotal cases of Lassa virus transmission through sexual contact weeks to months after hospital discharge have been noted (A. H. Demby, personal communication). Lastly, ruling out Lassa fever spares the patient the expense and potential toxicity of ribavirin therapy.
ELISA Ag/IgM testing affords the advantage of often providing a diagnosis via a single serum specimen. Thus, diagnoses can be made on patients who would otherwise be missed because of short hospital stays, infrequent outpatient follow-up, or refusal of repeated phlebotomy. Furthermore, the costs and risks associated with repeated needle sticks and laboratory evaluation are avoided. It should be noted, however, that the surest diagnosis is still provided by the testing of both acute- and convalescent-phase sera.
Since the samples for this study were collected under field conditions, we are limited in the conclusions that can be drawn regarding the profiles of antigen and antibody responses. The timing of patient presentation and phlebotomy were variable, as was the reliability of patient histories from which the date of onset of illness was obtained. These limitations may have affected our conclusions. For example, using the results of the first blood draw, we found that antigen detected by ELISA appeared a mean of 7.9 days after symptom onset. However, 10 (31%) of the 32 antigen negative cases did not have blood drawn until after day 10. Thus, the prevalence of antigen-positive patients would likely be much higher, and the time from symptom onset until presentation of antigen would be shorter, if patients could have presented or been tested earlier in the course of their illness. Lastly, as samples for this study were drawn only within a few weeks after symptom onset in an attempt to diagnose acute infection, no conclusions can be made regarding the duration of IgM or IgG antibodies.
Of interest are the six seronegative patients from whom Lassa virus was nevertheless isolated. The reasons for this finding are unclear. Serologic testing and virus isolation were repeated multiple times to ensure accurate results. No obvious common factors were apparent in these cases with respect to age, sex, time from symptom onset until presentation, survival, or receipt of ribavirin. Host genetic factors, such as those resulting in vigorous cytotoxic T-lymphocyte responses, or concomitant immunocompromised states such as AIDS or malnutrition may have altered the profile of the antigen and antibody responses.
We considered that direct cross contamination of tubes during collection in the field could account for this finding. However, genetic sequencing performed on four of the six isolates revealed each to be unique among the 41 sequenced genomic fragments. Variation at the nucleotide level ranged from 0.3 to 5.3%. Conversely, it seems unlikely that this relatively minor degree of genetic variation would result in these viruses presenting epitopes unrecognized by the antigen detection ELISA developed with the Josiah reference strain of Lassa virus.
We did not determine viral titers in our study. However, we postulate that some samples drawn early in the course of illness, before the onset of antibody production, may have contained levels of virus below the threshold of detection of the antigen-capture ELISA but which could nevertheless be propagated by culture. This possibility could be the explanation for two of these six seronegative patients who had only single specimens drawn at days 5 and 7. However, the others each had two samples drawn, at times ranging from days 4 to 18, which would seem to be adequate to detect antigen and/or antibody.
A last possibility is that these six patients had an equilibrium of antigen and IgM antibody and thus no available binding sites for detection on the ELISAs. Perhaps a disassociation step is necessary to allow detection.
Various measures might be employed to improve the sensitivity and specificity of the IFA assay, such as the use of IgM- and IgG-specific conjugates (29), although high background often makes interpretation difficult, and even conjugates from reputable suppliers may not be sufficiently isotype specific (P. E. Rollin, personal communication). In contrast to ELISA, the IgG antibody detected by the IFA assay is produced during the course of acute Lassa virus infection (17, 29), which may make the distinction between acute and previous exposure difficult. This risk is particularly high in areas of eastern Sierra Leone, southeastern Guinea, western Liberia, and the Jos plateau of Nigeria, where Lassa virus transmission is highly endemic (1, 2, 21, 24). Criteria such as a fourfold increase in titer between acute- and convalescent-phase sera or a single serum specimen with high titer have been used to help distinguish between acute infection and previous exposure (17, 30). However, these measures would still be unlikely to render IFA testing as sensitive as ELISA, whose main sensitivity advantage is conferred by the detection of antigen. The IFA test might prove useful should one want to perform a single-assay survey for any previous exposure to Lassa virus, including very recently infected individuals along with those exposed in the past.
The association between death and the early appearance of IFA antibody was unexpected. No such association was noted between survival and ELISA antibodies. If the association is real, it might suggest an immunopathogenic mechanism of severe Lassa fever, as has been well described in animal models of another arenavirus infection, lymphocytic choriomeningitis virus (4, 5). If this is the case, antibody may either be the primary effector or, perhaps more likely, represent a surrogate for a cell-mediated immunopathologic response.
Various diagnostic methods are available for the study of Lassa fever. Although virus isolation remains the most sensitive, it is still uniquely a research tool. Sensitive RT-PCR assays exist, but issues of strain variation, cross contamination, and expense pose practical problems for use in the developing countries where Lassa fever is endemic (6, 16, 26). A rapid diagnostic immunoblot assay for Lassa fever has recently been developed, but its usefulness is limited by its low sensitivity (25), nor would it provide the prognostic information available from the antigen ELISA or IFA. The interpretation of IFA results is complicated by the presence of IFA during both acute and convalescent stages of infection and by the subjective nature of the assay. However, the appearance of IFA antibody early in the course of Lassa infection may be useful in identifying patients with poor prognoses. ELISA Ag/IgM comprises the most sensitive and specific serologic test for acute Lassa virus infection and provides useful prognostic information. An additional advantage of ELISA-based diagnostics is their applicability to the diagnosis of numerous other diseases prevalent in developing countries, such as AIDS, typhoid fever, and the hepatitis viruses.
ACKNOWLEDGMENTS
This work was supported by a grant from the American Association of Schools of Public Health in conjunction with the Tulane School of Public Health and Tropical Medicine, Department of Tropical Medicine, New Orleans, La.
We thank Alpha Bah, Rilvane Bah, Adama Seray Barry, Fatoumata Binta Baldet, Ibrahima Camara, Sekou Cissé, Mohammed Ciré Diallo, Rouguiatou Diallo, Ishmael Doukare, Djécondé Fofona, Steven Gborie, Augustine Goba, Barry Mahy, Kabba Sidibé, Robert Tamba Tolno, and Michel Tounkara for their laboratory, clinical, and logistic support in Guinea; Sidiki Saffa for laboratory support in Sierra Leone; Debbie Cannon, Heather Hustad, Rich Meyer, Pat Stockton, Kathy Veilleaux, Linda Pezzanite, and Thomas Stevens for their laboratory support at CDC; Kathy Cavallaro, Barney Cline, and Ethleen Lloyd for administrative support; and Laura Morgan for help with data management.
REFERENCES
- 1.Arnold R B, Gary G W., Jr A neutralization test survey for Lassa fever activity in Lassa, Nigeria. Trans R Soc Trop Med Hyg. 1977;71:152–154. doi: 10.1016/0035-9203(77)90085-2. [DOI] [PubMed] [Google Scholar]
- 2.Bloch A. A serological survey of Lassa fever in Liberia. Bull W H O. 1978;56:811–813. [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen M D, Peters C J, Nichol S T. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between Arenaviruses and their rodent hosts. Mol Phylogenet Evol. 1997;8:301–316. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier M, Welsh R, Dutko F, Oldstone M. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 5.Byrne J, Ahmed R, Oldstone M. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. I. Generation and recognition of virus strains and H-2b mutants. J Immunol. 1984;133:433–439. [PubMed] [Google Scholar]
- 6.Demby A H, Chamberlain J, Brown D W G, Clegg C S. Early diagnosis of Lassa fever by reverse transcription-PCR. J Clin Microbiol. 1994;32:2898–2903. doi: 10.1128/jcm.32.12.2898-2903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher-Hoch S P, Gborie S, Parker L, Huggins J. Unexpected adverse reactions during a clinical trial in rural West Africa. Antivir Res. 1992;19:139–147. doi: 10.1016/0166-3542(92)90073-e. [DOI] [PubMed] [Google Scholar]
- 8.Fisher-Hoch S P, Tomori O, Nasidi A, Perez-Oronoz G I, Fakile Y, Hutwagnor L, McCormick J B. Review of cases of nosocomial Lassa fever in Nigeria: the high price of poor medical practice. Br Med J. 1995;311:857–859. doi: 10.1136/bmj.311.7009.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frame J D, Baldwin J M, Gocke D J, Troup J M. Lassa fever, a new disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- 10.Frame J D. Clinical features of Lassa fever in Liberia. Rev Infect Dis. 1989;11:S783–S789. doi: 10.1093/clinids/11.supplement_4.s783. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov A P, Tkachenko E A, van der Groen G, Butneko A M, Konstantinov O K. Indirect enzyme-immunoassay for the laboratory diagnosis of Lassa and Ebola hemorrhagic fevers. Vopr Virusol. 1986;2:186–190. [PubMed] [Google Scholar]
- 12.Jahrling P B, Hesse R A, Eddy G A, Johnson K M, Callis R T, Stephen E L. Lassa virus infection of rhesus monkeys: pathogenesis and treatment with ribavirin. J Infect Dis. 1980;141:580–589. doi: 10.1093/infdis/141.5.580. [DOI] [PubMed] [Google Scholar]
- 13.Jahrling P B, Niklasson B S, McCormick J B. Early diagnosis of human Lassa fever by ELISA detection of antigen and antibody. Lancet. 1985;i:250–252. doi: 10.1016/s0140-6736(85)91029-3. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K M, McCormick J B, Webb P A, Smith E S, Elliott L H, King I J. Clinical virology of Lassa fever in hospitalized patients. J Infect Dis. 1987;155:456–464. doi: 10.1093/infdis/155.3.456. [DOI] [PubMed] [Google Scholar]
- 15.Kochhar D M. Effects of exposure to high concentrations of ribavirin in developing embryos. Pediatr Infect Dis. 1990;9:S88–S90. doi: 10.1097/00006454-199009001-00008. [DOI] [PubMed] [Google Scholar]
- 16.Lunkenheimer K, Hufert F T, Schmitz H. Detection of Lassa virus RNA specimens from patients with Lassa fever by using the polymerase chain reaction. J Clin Microbiol. 1990;28:2689–2692. doi: 10.1128/jcm.28.12.2689-2692.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormick J B, King I J, Webb P A, Johnson K M, O'Sullivan R, Smith E S, Trippel S, Tong T C. A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis. 1987;155:445–455. doi: 10.1093/infdis/155.3.445. [DOI] [PubMed] [Google Scholar]
- 18.McCormick J B, King I J, Webb P A, Scribner C L, Craven R B, Johnson K M, Elliott L H, Belmont-Williams R. Lassa fever: effective therapy with Ribavirin. N Eng J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 19.Mertens P E, Patton R, Baum J J, Monath T P. Clinical presentation of Lassa fever cases during the hospital epidemic at Zorzor, Liberia, March-April 1972. Am J Trop Med Hyg. 1973;22:780–784. doi: 10.4269/ajtmh.1973.22.780. [DOI] [PubMed] [Google Scholar]
- 20.Monath T P, Maher M, Casals J, Kissling R E, Cacciapuoti A. Lassa fever in the eastern province of Sierra Leone, 1970–1972. II. Clinical observations and virological studies on selected hospital cases. Am J Trop Med Hyg. 1974;23:1140–1149. doi: 10.4269/ajtmh.1974.23.1140. [DOI] [PubMed] [Google Scholar]
- 21.Monson M H, Frame J D, Jahrling P B, Alexander K. Endemic Lassa fever in Liberia. I. Clinical and epidemiologic aspects at Curran Lutheran Hospital, Zorzor, Liberia. Trans R Soc Med Hyg. 1984;78:549–553. doi: 10.1016/0035-9203(84)90082-8. [DOI] [PubMed] [Google Scholar]
- 22.Niklasson B S, Jahrling P B, Peters C J. Detection of Lassa virus antigens and Lassa virus-specific immunoglobulins G and M by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984;20:239–244. doi: 10.1128/jcm.20.2.239-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding J W, Liu M F, Rotstein O, Phillips J M, Levy G. Ribavirin inhibits viral-induced macrophage production of TNG, IL-1, the procoagulant fg12 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 24.Stephenson E H, Larson E W, Dominik J W. Effect of environmental factors on aerosol-induced Lassa virus infection. J Med Virol. 1984;14:295–303. doi: 10.1002/jmv.1890140402. [DOI] [PubMed] [Google Scholar]
- 25.Ter Meulan J, Koulemou K, Wittekindt T, Windisch K, Strigl S, Conde S, Schmitz H. Detection of Lassa virus antinucleoprotein immunoglobulin G (IgG) and IgM antibodies by a simple recombinant immunoblot assay for field use. J Clin Microbiol. 1998;36:3143–3148. doi: 10.1128/jcm.36.11.3143-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trappier S G, Conaty A L, Farrar B B, Auperin D D, McCormick J B, Fisher-Hoch S P. Evaluation of the polymerase chain reaction for diagnosis of Lassa virus infection. Am J Trop Med Hyg. 1993;49:214–221. doi: 10.4269/ajtmh.1993.49.214. [DOI] [PubMed] [Google Scholar]
- 27.Van der Waals F W, Pomeroy K L, Goudsmit J, Asher D M, Gajdusek D C. Hemorrhagic fever virus infections in an isolated rainforest area of central Liberia. Limitations of the indirect immunofluorescence slide test for antibody screening in Africa. Trop Geogr Med. 1986;38:209–214. [PubMed] [Google Scholar]
- 28.White H A. Lassa fever: a study of 23 hospital cases. Trans R Soc Med Hyg. 1972;66:390–398. doi: 10.1016/0035-9203(72)90269-6. [DOI] [PubMed] [Google Scholar]
- 29.Wulff H, Johnson K M. Immunoglobulin M and G responses measured by immunofluorescence in patients with Lassa or Marburg virus infections. Bull W H O. 1979;57:631–635. [PMC free article] [PubMed] [Google Scholar]
- 30.Wulff H, Lange J V. Indirect immunofluorescence for the diagnosis of Lassa fever infection. Bull W H O. 1975;52:429–436. [PMC free article] [PubMed] [Google Scholar]