Abstract
Dendritic spines are small, thin, hair-like protrusions found on the dendritic processes of neurons. They serve as independent compartments providing large amplitudes of Ca2+ signals to achieve synaptic plasticity, provide sites for newer synapses, facilitate learning and memory. One of the common and severe complication of neurodegenerative disease is cognitive impairment, which is said to be closely associated with spine pathologies viz., decreased in spine density, spine length, spine volume, spine size etc. Many treatments targeting neurological diseases have shown to improve the spine structure and distribution. However, concise data on the various modulators of dendritic spines are imperative and a need of the hour. Hence, in this review we made an attempt to consolidate the effects of various pharmacological (cholinergic, glutamatergic, GABAergic, serotonergic, adrenergic, and dopaminergic agents) and non-pharmacological modulators (dietary interventions, enriched environment, yoga and meditation) on dendritic spines structure and functions. These data suggest that both the pharmacological and non-pharmacological modulators produced significant improvement in dendritic spine structure and functions and in turn reversing the pathologies underlying neurodegeneration. Intriguingly, the non-pharmacological approaches have shown to improve intellectual performances both in preclinical and clinical platforms, but still more technology-based evidence needs to be studied. Thus, we conclude that a combination of pharmacological and non-pharmacological intervention may restore cognitive performance synergistically via improving dendritic spine number and functions in various neurological disorders.
Keywords: dendritic spines, pharmacological modulators, modulators, yoga and meditation, enriched environment, diet
1. Introduction
Small, thin, special hair-like protrusions found on the dendritic processes of neurons are known as dendritic spines. Typically, dendritic spines receive inputs from excitatory synapse in brain [1]. Spines act as independent compartments providing rapid-large-amplitude Ca2+ signals and are involved in achieving synaptic plasticity [2]. Based on the morphological features, spines are categorized into stubby, mushroom, thin, and filopodial spines [3]. A significant difference between them is observed across the type of neurons, age, diseased states, dendritic locations, and laminar positions [2]. Thin and stubby spines represent initial stages of spine formation while mushroom spines represent matured and stable types [4]. Spine shape influences local Ca2++ concentration and cyclic adenosine monophosphate (cAMP)-regulated signaling proteins which are involved in synaptic plasticity [5,6,7]. Structural changes in spines contribute to the induction of long-term potentiation (LTP) or long-term depression (LTD), which plays a crucial role in synaptic transmissions. De novo spine growth also occurs in response to various triggers like LTP, two-photon glutamate uncaging or altered sensory experiences leading to new functional synapse formation [6,8,9,10]. LTP-induced changes in spines such as enlargement of spine head and shortening of the neck are widely evidenced from many experimental studies, explaining close association between the synaptic plasticity and spine morphology [11]. Likewise, as the magnitude of LTP induction increases in hippocampal cornu ammonis-1 (CA1) region there was a subsequent increase in the spine density, due to enhanced F-actin content, cofilin enrichment, and stabilization of F-actin content etc., [12,13,14]. Conversely, induction of long-term depression (LTD), glutamate uncaging, optogenetic stimulations reported to cause shrinkage of spine head and increased spine numbers loss [9,15,16,17,18]. Further, recent research explains the drawbacks in traditional way of dendritic spine classifications like the spine head area correlated to PSD may not, be applicable for thin and stubby spines. Hence to overcome such drawbacks a newer approach of clusterization of spines has been developed. This approach automatically groups spines into similar structural classes based on selected algorithm without a prior input. Each spine will be represented as set of value of parameters reflecting its morphology from neck and head size to complex geometrical parameters [19].
2. Dendritic Spines Biosynthesis
Dendritic spines exhibit a spectrum of structural reorganizations with respect to formation, maturation, and elimination, including subtle changes in size and shape modulated by neuronal activities and developmental age [20]. Current theories suggest that dendritic spines possess a chemical and electrical signaling domain that is discrete from their parent dendritic domain [21]. Most of the developing neurons form long, thin, and headless filopodia, which gets stabilized through generation of calcium transients. As filopodia are highly motile, only 10–20% of them transform to spines [22]. Filopodia formation is primarily dependent on synaptic interactions involving Rho GTPase effector P21 activated kinases (PAK). Thus, filopodia acts as precursors for spine development in the early stages [23]. Molecules like calcium/calmodulin-dependent protein kinase II (CaMKII), syndecan-2 via enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) pathway [24], and paralemmin-1 [25] enhance filopodia formation and maturation of spines. While dendritic-associated adhesion molecule like telencephalin (TLCN) slows down the spine maturation by promoting filopodia formation and negatively regulating filopodia to spine transition [26]. However newly formed spines (thin and elongated) acquire post synaptic density (PSD) leading to enlargement of the head to become classical mushroom-shaped spines. Increase in the spine volume is associated with the accumulation of additional α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and reorganization of actin cytoskeleton [27]. Cytoskeleton determines the shape of the spines, and actin is the major cytoskeletal protein in dendritic spines which polymerizes to filamentous actin (F-actin) through complex interactions with actin binding proteins (ABPs). Due to the polar nature, one end of actin filament grows rapidly than the other until adenosine-triphosphate (ATP) bound to the actin filament hydrolyses to adenosine-diphosphate (ADP), promoting the disassembly of pointed end by cofilin [28]. In addition to this treadmilling process complexes like actin-related proteins 2/3 (Arp2/3) induces branching of filaments [29]. The variation in the locations and turnover rates of the F-actin pools at the tip, base, and head regions of the spines contribute to the differences in the spine volume. Variations in the spine volume or enlargement is also linked to the destabilization of PSD, increased dynamics of PSD proteins like PSD-95, SHANK2, and phosphorylation of CaMKII [30]. Matrix metalloproteinases-9 (MMP-9) contributes to spine enlargement through beta-1 integrin receptor, cofilin phosphorylation, and actin polymerization. Further, the signaling pathways mediated by N-cadherin involving scaffolding proteins AF6/afadin, the Rho GTPase exchange factor Kalirin-7, Rac1, and PAKs regulate the size of spine head [31]. Rho GTPases like Rac1 or Cdc42 play important role in spine remodeling through signaling complexes including protein kinases like CaMKK and CaMKI associated with GTPase exchange factor beta-Pix or calcium/calmodulin serine protein kinase CASK [20]. Rate of elimination of spine is two-folds higher than that of spine formation between adolescence and adulthood, leading to net spine loss termed as pruning of existing connections [32]. Spine addition and elimination is related to net gain and loss of excitatory synapses over lifetime and is influenced by factors like PSD recruitment, sensory stimulation etc., [33]. Semaphorin 3F-induced activation of Tiam 1-Rac1-3-LIMK1/2-Cofilin1 and RhoA-ROCK1/2-Myosin II in dendritic spines regulates pruning of spines [34].
Pathological changes of spines are categorized into pathologies of distribution and structural pathologies [35]. Increase or decrease in the spine density [36], morphological changes viz reduction in size [15], alteration in shape [19,37], dendritic beading with loss of spines [38] and sprouting of spines (text Box 1) [39] are pathologies of distribution [40]. While structural pathologies include the changes observed with single spine like densification of cytoplasm [41,42], hypertrophy of organelles, decreased spine volume [43,44], and formation of aberrant synapse-like connections.
Box 1. Definitions.
Dendritic Spine sprouting: “Recurving of distal dendritic arbors, short-segment branching, growth cone-like processes initiating at the neuronal dendritic process is spine sprouting” [39].
Siddha medicine: “Siddha is an ancient traditional Indian system of medicine practiced in southern part of India. Siddha medicine is claimed to revitalize and rejuvenate dysfunctional organs that cause the disease. Kayakarpam, a special combination of medicine and life style, Varmam therapy, Vaasi (Pranayamam) and Muppu the universal Salt are the specialties of Siddha system of medicine. Thus, this system connects both spiritual and physical and treats the person as a whole i.e., it concentrates on the physical, psychological, social, and spiritual wellbeing of an individual” [180].
Prebiotics: “Are the source of food for gut’s healthy bacteria or the food that induces growth or activity of beneficial microorganisms in the gut”.
Probiotics: “Are the live microorganisms, when consumed improve or restore the gut flora”.
Synbiotics: “Are mixture of both prebiotics and probiotics viz, fermented food that synergistically improve the gut flora” [181].
Alterations in spine structure and function negatively impacts learning and memory [45,46]. Majority of the neurodegenerative diseases (such as Alzheimer’s disease (ad), Parkinson’s disease (pd), Huntington’s disease (hd)) and neuropsychiatric disorders (such as depression, autism, schizophrenia,) are linked to spine dysfunction [47,48]. Mounting evidence from experimental and clinical reports indicate that the therapeutic intervention restores spine pathologies which in turn is reflected by improved learning and memory. On the other hand, non-pharmacological approaches like dietary modifications, practice of yoga and meditation, and enriched environment habitation are shown to improve memory, but very few clinical reports explain the direct effects of non-pharmacological treatments on spines structure and distribution.
Reduced synaptic plasticity, altered dendritic spine’s structure and distribution and memory impairment are key features of neurodegeneration and underlying diseases, which has been explored extensively in the past two decades. But recent evidence suggest that targeting genes, signaling molecules, and proteins involved in the biosynthesis of dendritic spines have direct effect on the synaptic plasticity and enhancement in learning and memory. Despite of extensive research there is no clear delineation on the modulators of dendritic spines, as most of the research was focused either on the disease modification or the underpinning signaling cascade. Thus, in this review, we made an attempt to summarize the effects of pharmacological (such as cholinergic agents, glutamatergic agents, GABAergic agents, serotonergic agents, adrenergic agents and dopaminergic) and non-pharmacological modulators (such as dietary modifications, enriched environment, yoga and meditation) of dendritic spines and synaptic plasticity in various conditions affecting learning and memory. An online search was conducted in PubMed, MEDLINE, Scopus, Web of Science, and Google Scholar for the published articles either experimental or reviews effecting structure and functions of dendritic spines. The data collected were then categorized into pharmacological and non-pharmacological modulators.
3. Effects of Pharmacological Interventions on Dendritic Spines
3.1. Cholinergic System Modulators
Synaptic plasticity and cognition are significantly connected to cholinergic system in the brain [49]. Genetic disruption of muscarinic and nicotinic receptors affects learning and memory [50]. Acetylcholine and other nicotinic receptor agonists are reported morphogens exhibiting potential effects on dendritic arborization [51]. In a rat model of myocardial ischemia/reperfusion (I/R) injury, a significant loss of dendritic spine is corroborated to decreased cholinergic activity [52]. Administration of donepezil, a acetyl cholinesterase inhibitor, in rats subjected to I/R injury, increased the spine numbers [53]. Similarly, administration of donepezil produced significant increase in spine numbers in prefrontal cortex pyramidal neurons of aged rats [54]. LM11A-3, a small molecule ligand for neurotrophin receptor p75 (p75NTR), reversed cholinergic neurites dystrophy, and prevented spine loss through activation of RhoA receptors [55].
Brahmi Nei, a poly herbal Siddha formulation (text Box 1), reversed cognitive decline along with increase in the dendritic length and number of mushroom-shaped spines in rodent model of scopolamine-induced dementia. The improvement in LTP is attributed to the anti-cholinesterase activity of the herbs in the formulation [56]. Phenserine tartarate (Phen), a novel acetylcholine esterase inhibitor, reversed mild traumatic brain injury (mTBI)-induced downregulation of PSD-95, and spine loss in wild type mice. Also, Phen reversed mTBI-induced loss of synaptophysin in both wild type and transgenic mouse model of AD (APP/PSEN1) [57]. Similarly, treatment with Huperzine A, a potent acetylcholinesterase inhibitor (AChEI), improved dendritic spine density and synaptotagmin levels in the cortex and hippocampus of severe model of AD (APPswe/presenilin-1) thus improved spine density is linked to reduction in amyloid plaque burden and oligomeric beta amyloid levels [58].
Manipulation of nicotinic receptors either by chrna5 deletion or by chronic exposure to nicotine during developmental stages significantly reduced apical spine density in long neurons and basal spine density in short neurons of the prefrontal L6 pyramidal neurons in mice [59]. On the similar lines, exposure of cultured hippocampal neurons to nicotine produced spine enlargement and responsiveness mediated through glutamergic transmission via α4β2 nicotinic acetylcholine receptors(nAChRs) [60]. Moreover, mice lacking β2-nAChRs showed scarcer dendritic spines when compared to the age matched wild type mice, which indicates that β2-nAChRs play a crucial role in the restoration of dendritic spine density [61]. Studies also revealed the evidence of cholinergic system modulating structural plasticity at the sub-spine level, wherein muscarinic receptor activation led to induction of fine filopodia from spine heads in the CA1 pyramidal cells of the hippocampus. The formations of spine head filopodia represents novel structural form of sub-spine plasticity which occur due to interaction between presynaptic buttons and dendritic spines [62]. Overall, increase in both basal and apical spine numbers, increased spine density, enhanced dendritic length, alterations at sub-spine level (formation of fine filopodia), and increased synaptotagmine levels in various brain regions are regulated by the cholinergic system. Thus, cholinergic system either through activation of muscarinic or nicotinic receptors or through inhibition of acetyl choline esterase makes significant impact on the formation and functions of dendritic spines.
3.2. Effect of Glutamatergic Modulators on Dendritic Spines
Glutamate being an important excitatory neurotransmitter in the central nervous system plays a crucial role in learning and memory and synaptic plasticity. Researchers have demonstrated that the dendritic spines also receive the glutamergic input directly from the presynaptic terminals. A study demonstrated that, application of shorter pulses of glutamate resulted in elongation of spines while long pulses led to shrinkage of the same set of spines. This explains that the differential responses exerted by glutamate depends mainly on the intensity and duration of application. [63]. Calcium released from internal stores significantly changes the spine shape of the dendritic spines (elongation of spine neck) in cultured hippocampal neurons [64]. Activation of group 1 metabotropic glutamate receptors (mGLUR1) induces release of calcium from internal stores triggering dendritic protein synthesis, thereby contributing to increased spine length [65]. Furthermore, increase in the spine length and sprouting of spines is correlated to increased synaptic activation of N-methyl-D-aspartate (NMDA)-glutamate receptors [66]. In contrast, NMDA receptor overactivation also results in spine retraction [67]. Spines formed initially in response to NMDA receptor activation are further stabilized through activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Activation of AMPA receptors blocks further growth of spines and helps stabilizing spines through post synaptic membrane depolarization and calcium influx via voltage-activated calcium channels [68]. Multiple reports demonstrate the beneficial role of glutamate and its modulators in achieving growth and maintenance of the dendritic spines and its alteration in few pathological conditions. Ageing-related cognitive decline is attributed to diminished spine clustering and density in the CA1 region of hippocampus. Treatment with riluzole, a presynaptic NMDA receptor antagonist, increased thin spine density and non-linear thin spine clustering by increasing the glutamate uptake via glial transporters (EAAC1) and thus increases the synaptic glutamergic activity [69]. Moreover, positive (3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide) and negative (1-(3-chlorophenyl)-3-(3-methyl-5-oxo-4Himidazol-2-yl) urea) allosteric modulators of mGLuR5, differentially increased spine density in the pyramidal cells of medial prefrontal cortex [70]. Similarly, Gamma-aminobutyric acid (GABAergic) striatal spiny projection neurons co-cultured with glutamatergic cortical neurons showed increased LTP, spine numbers, and GluA1 cluster densities on NMDA activation. These data demonstrate the necessity of NMDA receptor activation to drive glutamatergic structural plasticity in striatal spiny projections [71].
Ketamine, an NMDA receptor antagonist, improved dendritic spine numbers by enhancing Ca2+ levels in dendritic spines of prefrontal cortex [72]. LY341495, a group II metabotropic glutamate 2/3 receptor (mGlu2/3) antagonist, acts at mammalian target of rapamycin complex 1 (mTORC1) and AMPA receptors, restored dexamethasone-induced decrease in dendritic outgrowth and spine density [73]. Thus, from the above reports it is implicated that, calcium-driven activation of NMDA-glutamate receptors and glutamate agonists is shown to increase spine length, sprouting of spines, spine density, spine clustering, and dendritic outgrowth.
3.3. Effect of GABAergic Agents on Dendritic Spines
GABA, a major inhibitory neurotransmitter, causes membrane hyperpolarization in adult neurons through opening of chloride (Cl−) channels. However, during embryonic development GABA receptor activation in the motor neurons of the spinal cord causes membrane depolarization due to increased intracellular concentration of CI−, mediated through a bumetanide-sensitive Na+/K+/2CI− cotransporter (NKCC1). Disruption of GABA receptor activation by bicuculline in vivo (chicken embryos) significantly reduced the dendritic outgrowth in terms of change in the number of branch points and number of ends at different dendritic orders [74]. Pharmacological blockade of GABAA receptors resulted in profound increase in the elimination of pre-existing spines persisting for first 4 postnatal months, without affecting new spine formation, also, resulted in structural stability of mature circuits. This demonstrates the role of GABAA receptors in preventing over-pruning of spines during spine refinement in the mouse cortex [75]. Thus, maturation of dendritic morphology and modulation of spine turnover in developing neurons is attributed to the excitatory GABA-driven activity. However, lowering the concentration of intracellular CI− (due to lowered expression of chloride exporter KCC2) shifts the excitatory effect of GABA to inhibitory effect in matured neurons. The switch in GABAA transmission from developmental depolarizing to hyperpolarizing was recapitulated in organotypic hippocampal slice cultures.
Studies have demonstrated that the application of GABAA antagonists to organotypic hippocampal neuronal cultures showed marked loss of spines. Upon administration of bicuculline, a GABAA receptor antagonist decreased spine density, explains that absence of hyperpolarizing GABAA transmission resulted in the spine loss [76]. Activation of GABA receptors suppressed overall cytosolic calcium concentration promoting spine shrinkage and elimination in CA1 region of rat hippocampus. However, observed local spread of spine shrinkage was attributed to induction of calcineurin and actin depolymerizing factor, i.e., ADF/cofilin (dephosphorylated) signaling cascade [16]. Mutations in alpha 1 subunit of GABAA receptor, specifically GABRA1 missense mutation (alpha1- A322D) resulted in significant increase in the number of mushroom-like spines and spine density in pyramidal cells. A322D expression on the GABAergic cells increased perisomatic bouton density [77]. Activation of GABAB receptors in a single dendritic spine of layer 5 pyramidal neurons inhibited NMDA receptors, said to be mediated through downregulation of cAMP and PKA, explaining the mechanism of establishment of neuromodulatory microdomains in the subcellular compartments like dendritic spines [78]. Blockade of GABA synthesis in cultured hippocampal neurons by mercaptopropionic acid resulted in a significant increase in the dendritic spine density. It is also proved that stressful experience promotes contextual fear memory and enhanced spine density, however administration of midazolam, a positive modulator of GABAA sites, prevented the influence of stress on both fear retention and hippocampal dendritic spine modelling in the basolateral amygdala complex (BLA) and dorsal hippocampus [79]. Together these studies demonstrate that the GABAergic agents modulate dendritic spine structure and functions differentially during embryonic development and in adult neurons.
3.4. Effect of Serotonergic Agents on Dendritic Spines
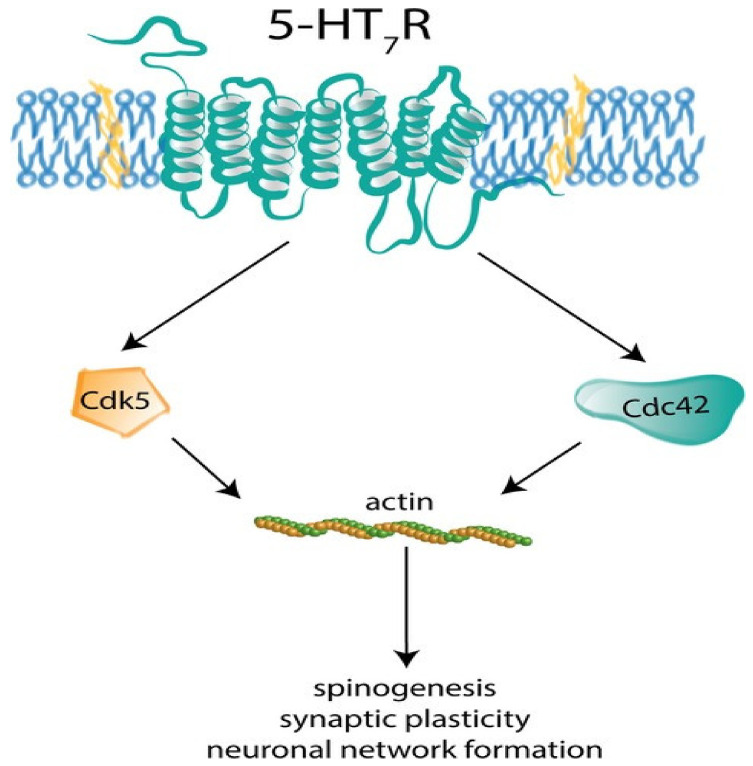
Several studies have indicated that 5-hydroxy tryptamine (5-HT) is synthesized in the fetus early in the embryonic development [80]. In addition to the endogenous 5-HT, the brain of the fetus also receives 5-HT from the mother through placenta, emphasizing its significance in the early embryonic development including brain [81]. Establishment of cortical circuits, neuronal migration, and dendritic differentiation are few of the crucial cellular processes mediated by 5-HT system [82]. In mammalian brain, serotonergic neurons execute their functions through 20 subtypes of receptors belonging to 7 subclasses. Stimulation of 5-HT7R/Gα12 signaling pathway in one-month-old mice hippocampus has shown to increase the formation of dendritic spines [83]. In addition to the role of 5-HT in the embryonic and early post-natal life, experiments performed on adult mice by administering LP-211, a potent 5-HT7 agonist, showed significant increase in the total number and density of dendritic spines in striatal neurons. Binding of LP-211 to 5-HT7R activates downstream small GTPases like Cdk5 and Cdc42 leading to actin polymerization in turn spinogenesis (Figure 1) [84]. It was also found that 5-HT7R stimulation enhances local matrix metallopeptidase-9 (MMP-9) activity in the mouse brain, triggering dendritic spine remodeling and synaptic pruning, leading to the cleavage of CD44 followed by Cdc42 activation [85]. In an experimental model of neuroblastoma cells, activation of 5-HT4R boosts phosphorylation of cofilin and maturation of dendritic spines via RhoA-dependent control of F-actin. It was found that postnatal expression of 5-HT4R and 5-HT7R in hippocampus is differently regulated. In the early postnatal stages, 5-HT7R activation is responsible for arborization of dendritic trees and spinogenesis, while in the later developmental stages, 5-HT4R is involved in the maturation and stabilization of spines [83]. Exposure of 5-HT2 agonist, DOI to cultured cortical neurons induced transient increase in dendritic spine size as well as spine remodeling by phosphorylating PAK, a downstream target of neuronal Rac guanine nucleotide exchange factor (RacGEF) kalirin-7 [86]. Studies have also shown that short-term regulation of dendritic spines occurs through acute stimulation of 5-HT2A/2C receptors by activating phospholipase-C (PLC) which induces downstream Ca2+-dependent TGase activation catalyzing G proteins of Rho family and thus, activating Rac1 and Cdc42. Rac1 and Cdc42 in turn regulate the dynamics of actin cytoskeleton leading to transient dendritic spine enlargement, and thus, contributing to spine plasticity. But chronic treatment with olanzapine, 5-HT2A receptor antagonist, increased expression of Nrg1 gene, which promotes spine maturation via JAK2/STAT3 pathway [87]. Vortioxetine, agonist at 5-HT1A and 5-HT1B receptors induced spine enlargement contributing to more functional synaptic contacts in rat hippocampus [88].
Figure 1.
5-HT7R activates downstream small molecule GTPase’s Cdk5 and Cdc42 leading to actin polymerization and spinogenesis [89] (reused as per the Journal of Neurochemistry copyright permission policy) (CdK5: Cyclin Dependent Kinase5,Cdc42: Cell Division Control Protein 42).
3.5. Effect of Adrenergic Agents on Dendritic Spines
Noradrenergic system influences multiple brain functions like arousal, perception, attention, learning, and memory by regulating synaptic plasticity and dendritic spine dynamics via the modulation of cell surface receptors (NMDARs, AMPARs), protein kinases, and phosphatases [90,91]. Noradrenergic agonists are known to elevate intracellular cAMP concentration, tropomyosin receptor kinase-B (TrKB) phosphorylation and its translocation to spines thus enabling TrKB interaction with PSD-95 [92]. Studies report that activation of β-adrenergic receptors (β-ARs) by isoproterenol, through canonical and noncanonical (Gi and Gs Coupled) pathways forms LTP through activation of Ca2+/calmodulin-activated kinase-II, protein kinase A, and exchange protein activated by cAMP (Epac) which is critical for spine formation [93]. The α(2A)-adrenoreceptors (α(2A)-ARs) are highly expressed in neurons and essential for neuronal differentiation, growth, and neurotrophy. It was found that activation of α(2A)-ARs by guanfacine enhanced the expression of PSD-95, and significantly induced stubby and mushroom spines, along with enlargement of spine head size in cultured prefrontal cortex neurons. However, co-administration of yohimbine with guanfacin blocked the effects of guanfacine, confirming the role of α(2A)-ARs in the maturation of dendritic spines [94]. Furthermore, guanfacine prevented dendritic spine loss in layer II/III pyramidal neurons of prelimbic prefrontal cortex in rats exposed to chronic restraint stress [95]. In cultured cortical neurons from C57/B6 mice, guanfacine enhanced the expression of spinophilin (a key cytoskeletal protein involved in the formation and maintenance of dendritic spines) that leads to 1.2- and 1.8-fold increase in spine length and spine density, respectively [96]. PD rats treated with bromocriptine, a α-adrenergic agonist, showed significant increase in the number of synaptic contacts with dendritic spines compared to sham-operated rats indicating the indirect effect of bromocriptine on the memory and learning [97]. These findings collectively indicate that activation of adrenergic receptors have positive impact on the growth and distribution of dendritic spines.
3.6. Effect of Dopaminergic Agents on Dendritic Spines
Dopamine (DA) is implicated in several neuropsychiatric and neurological disorders as it regulates movement, motivation, reward, learning, and memory [98]. Primary prefrontal cortex (PFC) neuronal culture on repeated treatment with SKF81297, a DA agonist, resulted in increased dendritic branching and spine density, which is believed to be mediated through activation of D1 receptors and downstream signaling molecules (Rac1 and RhoA). While administration of SCH23390, a dopamine antagonist, reversed the SKF81297-induced increase in dendritic morphogenesis, indicating the positive role of D1 receptors in dendritic morphogenesis [99]. Increased spine density in hippocampal neurons is also reported by promoting transactivation of TrKB, mediated by D1 receptors [100]. D3 receptor agonists, ropinirole and pramipexole showed dose-dependent increase in the dendritic arborization in translational model of human-inducible pluripotent stem cells (hiPSCs) derived from dopaminergic neurons [101]. PD animal models have shown marked decrease in the number of spines and increase in spine head volume in striatal dendrites [102]. 6-hydroxydopamine-lesioned rats on chronic treatment with levodopa showed significant increase in the spine density and spine head area in the pyramidal tract neurons of primary motor cortex [103]. Further, the spine enlargement in the nucleus accumbens (NAc) and medium spiny neurons (MSNs) is linked to be associated with dopamine dysregulation syndrome (DDS). These observations are corroborated to dopaminergic degeneration and loss of dopamine in striatum and NAc [104]. Loss of nigrostriatal dopamine neurons in PD induces reduction in the number of dendritic spines with smaller cell body and less profusely arborized dendritic trees on MSNs of striatum expressing both D1 and D2 receptors [105]. However, non-selective activation of dopaminergic receptor by apomorphine (D1-D5 dopamine receptor agonist) resulted in learning and memory impairment, reduced dendritic length and LTP followed by neuronal damage in CA1 region of mice hippocampus [106]. Similar results were also observed in young Mongolian gerbil’s hippocampus on apomorphine treatment [107]. Another study revealed that brain-derived neurotrophic factor (BDNF)-induced chronic activation of D3 receptors improved motor functions and increased the dendritic spines in the striatal neurons of 6-hydroxydopamine (6-OHDA)-induced Parkinson’s rat [108].
Dopamine D(4) receptor activation upregulates the cofilin activity by dephosphorylating cofilin and thus depolymerizing actin [109]. 6-hydroxydopamine lesions in the ventral tegmental area disrupts the prefrontal cortex dopamine resulting in the decreased dendritic length and spine density of layer V pyramidal cells in the prelimbic cortex [110]. High-fat-diet-fed mice displayed reduced dendritic spine density in the substantia nigra due to neuroinflammation-mediated degeneration of dopaminergic neurons in midbrain [90]. Striatal dopamine loss is shown to decrease the number of MSN dendritic spines [111]. Activation of D1R and D2R receptors in a striatal cell culture containing MSNs increased the number of spines and spinophilin expression, indicating the direct role of these receptors on spines formation. Similarly, blockade of D1R and D2R receptors reduced the number of dendritic spines in cultured striatal MSNs indicating the role of dopamine in the spine formation [112]. It was also found that there was significant decrease in the association of spinophilin with neurofilament medium (NF-M) in dopamine-depleted striatum, causing decreased number of spines, as observed in Parkinson’s disease [113]. Thus, it is clear that spinophilin-induced alterations in spine density and morphology depends on D1 and D2 receptors which act as scaffolding key synaptic proteins [114]. Further, another study showed that alcohol-dependent rats fail to perform emotional learning tasks, while administration of L-DOPA during early withdrawal showed optimal learning along with increased number of long thin spines in MSNs of NAc. Another study revealed that drebrin, a dendritic spine protein depletion reduces D1 receptor levels and in turn reduces dendritic spine morphogenesis in mouse hippocampus [115]. These results display the role of dopamine in growth, maintenance, and restoration of spines [116].
The observations for all the neurotransmitter systems from various studies are summarized in Table 1.
Table 1.
Effect of various neurochemical systems on dendritic spines.
| Sl. No | Neurochemical System | Drug/Chemical Treatment |
Effect on DS | References | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Density | Length | Size | Morphogenesis | Enlargement | ||||
| 1 | Cholinergic | Donepezil, LM11A-3, Brahmi Nei, Phenserine tartarate, Huperzine A, | ↑ | ↑ | ↑ | - | - | - | [53,55,56,57,58] |
| 2 | Glutamatergic | Riluzole, Ketamine, LY341495 | ↑ | ↑ | - | - | - | - | [69,72,73] |
| 3 | GABAergic | Bicuculline, mercaptopropionic acid | - | ↑ | - | - | - | - | [79] |
| 4 | Serotonergic | LP-211, Olanzapine, Vortioxetine, | ↑ | ↑ | - | ↑ | ↑ | ↑ | [84,87,88] |
| 5 | Adrenergic | Guanfacine, Bromocriptine, isoproterenol | - | ↑ | ↑ | ↑ | - | - | [93,94,97] |
| 6 | Dopaminergic | SKF81297, Ropinirole, Pramipexole | ↑ | ↑ | ↑ | - | ↑ | - | [99,101] |
↑ denotes increase; - denotes no information.
4. Non-Therapeutic Modulators of Dendritic Spines
4.1. Impact of Calorie Restriction on Dendritic Spine
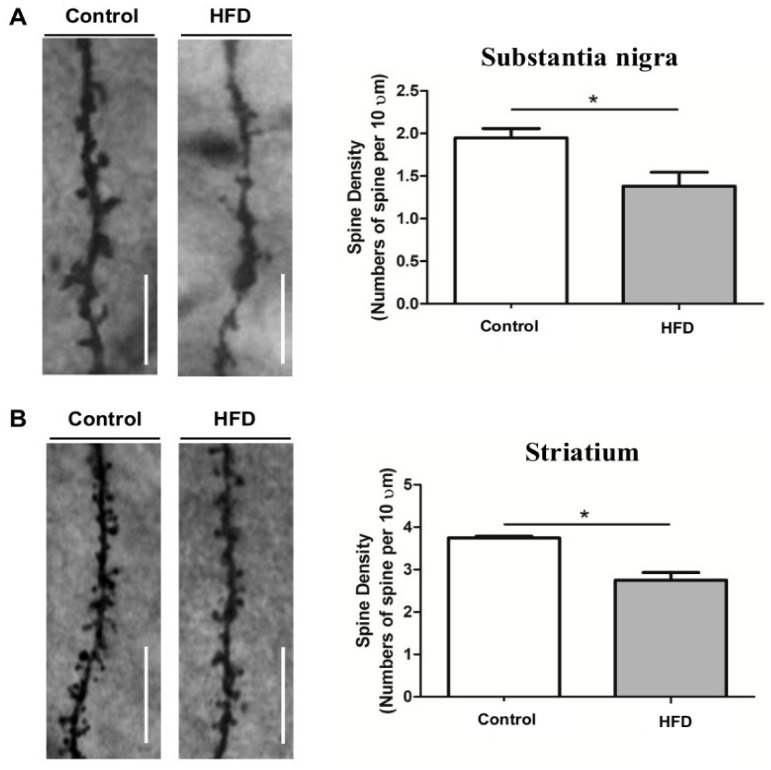
Calorie restriction (CR) has shown prominent improvement in adult neurogenesis and neural plasticity [117,118], while high saturated fat diets have shown to impair learning and memory, demonstrated by decreased spine number and density in substantia nigra and striatum of mice brain (Figure 2) [90]. Diet that mimics fasting enhanced the hippocampal neurogenesis, upregulated neurogenic differentiation factor-1 (NeuroD1), spine density, and improved cognitive performance in aged mice [119]. Stranahan et al. (2009) investigated the effect of physical exercise and dietary restriction on hippocampal neuron morphology. Increase in neurotropin, BDNF, and density of dendritic spines was attributed to the dietary restriction, but not to the physical exercise. These data indicate that dietary restriction influences the biosynthesis of new dendritic spines and hence synaptic plasticity [120]. Rats fed ad libitum displayed significant loss of L-type spines (lollipop-shaped) in the basilar tree of layer V pyramidal cells of parietal cortex with normal ageing when compared to the diet-restricted rats [121]. Similarly, a reduction in dendritic spines density in CA1 region of hippocampus was observed along with insulin resistance in the high-fat-diet-fed rats [122]. Diabetic rats maintained on dietary restriction showed significant increase in the dendritic mushroom spines in the pyramidal neurons of CA1 region of hippocampus than diabetic rats fed ad libitum [123]. Further, mice maintained with CR and low protein-high carbohydrate (LPHC) diet showed increased dendritic spines in the dentate gyrus neurons [117].
Figure 2.
Representative Golgi staining images of dopaminergic neurons in substantia nigra and striatum [90] (reused as per the International Journal of molecular Sciences copyright permission policy). (A) Represents decreased dendritic spine density in substantia nigra of high fat diet (HFD) mice compared to control. (B) Represents decreased dendritic spine density in striatum of high fat diet (HFD) mice compared to control. * indicates the significant difference with respect to control.
Similarly, rats maintained with low-protein diets showed minimal decrease in the total number of neurons in dentate gyrus, CA1 and CA3 neurons compared to ad libitum fed rats. However, the feed-restricted rats also displayed reduced segments in the dendritic arborization of granule cells. However, there was an increase in the spine density in distal segments of dendritic spines and total number of axospinous synapses in dentate gyrus region. In addition, dendritic length of granule cells was also found to be increased. This study explains that controlled diet restriction improves the dendritic spines density in turn the synaptic functions [124].
On contrary, feed restriction in crucial stages of life viz feed restriction in new born rats between post-natal day 0 and 20 decreased dendritic spines in the layer V pyramidal cells in the frontal, parietal, and occipital cortices which may be related to the perinatal undernourishment [125]. Similarly post-mortem brains of severely malnourished infants showed short apical dendrites and fewer spines [126]. These data reveal that dietary interventions play critical role in dendritic spines formation and maintenance.
4.2. Effect of Pre-, Pro-, and Syn-Biotics on Dendritic Spines
Several studies have established the correlation between gut microbiota and neurological disorders including blood brain barrier integrity [127], neurogenesis [128], maturation, and ramification of microglia [129], myelination [130], expression of neurotrophins like BDNF, oxytocin, and vasopressin [131], and release of neurotransmitters [132]. Pre- and pro-biotics (text Box 1) are well-known for their positive influence on mental health, along with supporting the growth of commensal bacteria with psychophysiological effects, and hence they are also termed as psychobiotics [133,134]. The role of psychobiotics is not only limited to the regulation of neuroimmune axis and diseases of nervous system, but also involved the enhancement of cognition, and behavior phenomena [135].
The microbiome has attracted significant attention for its impact on the synaptic plasticity by modulating the dendritic spine morphology and distribution [136]. In light of this, germ-free (GF) animals have shown decreased dendritic size and fewer synaptic connections in the hippocampal pyramidal neurons. Indeed, absence of symbionts in mice was correlated to dendritic hypertrophy with more thin, stubby, and mushroom spines in the basolateral amygdala neurons, while the ventral hippocampal pyramidal neurons of GF mice displayed shorter, less branched spines with less stubby and mushroom types. These findings reveal a casual role of gut microbiota in the realm of spine pathology [136].
Multiple reports evidenced the involvement of BDNF in dendritic arborization and spine formation [137,138]. Moreover, administration of prebiotics and probiotics enhanced brain BDNF levels, which has prompted many researchers to examine the role of pre/probiotics on spine density and dendritic arborization in different diseased conditions [139,140]. Administration of antibiotics during pregnancy is reported to affect the maternal gut microbiota and alters the behavioral pattern in offspring [141].
Pregnant rats and their female offspring exposed to lead resulted in the loss of dendritic spines in both CA1 and dentate gyrus (DG) regions irrespective of the developmental stages (PND 22 or PND 68). Long-term intervention with multispecies probiotic (Bifidobacterium longum BL986, Lactobacillus acidophilus LA43, Lactobacillus fermentum LF26, Lactobacillus helveticus LH43, Lactobacillus paracasei LPC12, Lactobacillus rhamnosus LRH10, and Streptococcus therophilus ST30) restored the spine densities in both adolescence and adulthood [142].
In an in vivo study, high-fat-diet (HFD)-fed rats displayed hippocampal dysplasticity (decreased synaptic proteins like PSD-95, synaptophysin and spinophilin) including LTP impairment along with low grade local and systemic inflammation [143,144,145]. Earlier reports also suggest the evidence of gut dysbiosis on HFD consumption by enhancing the ratio of Firmicutes to Bacteroidetes ratio, thus promoting the growth of Proteobacteria [146,147]. On the other hand, decreased spine density in high-fat-diet-fed rats was restored on treatment with prebiotic Xyloolidosaccharide (XOS), probiotic Lactobacillus paracasei HIIO1 (L. paracasei) and synbiotics (combination of XOS and L. paracasei) [143]. Drebrin, a cytoplasmic actin-binding protein expressed in dendritic spines, regulates the morphology and plasticity of dendritic spines. Aberrant expression of drebrin indicates abnormal dendritic spines [148,149,150]. In an in vitro study, exposure of hippocampal neurons to live or heat inactivated Lactobacillus rhamnosus (LGG) and Bifidobactererium bifidum (TMC3115) increased the neuronal viability, and upregulated drebrin and SYP levels. Evidence strongly supports probiotics belonging to the family Lactobacillus and Bifidobactererium influence host neuronal development, brain biochemistry, and behavioral phenomenon [151].
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism mice hippocampus showed increased expression of neuropsin, decreased expression of PSD-95 and synaptophysin along with reduced CA1 apical spine density. Administration of probiotic Bifidobacterium breve (MCC1274) reversed the MPTP-induced changes [152]. Aberrant higher induction of neuropsin in PD mice is linked to abnormal changes in the hippocampal plasticity [152]. Chronic administration of mixture of three Bifidobacteria (B. longum, B. breve and B. infantis) known as B-MIX to rats induced significant increase in thin and mushroom spine numbers in both apical and basal dendritic branches in CA1 region of hippocampus, while stubby spines remained unaltered. The mixture also positively enhanced the total dendritic length, total branch number, number of tips/neuron, and the number of pints/neurons, confirming the role of B-MIX mixture not only on spine density, but also on dendritic arborization [153].
4.3. Effect of Enriched Environment (EE) on Dendritic Spines
EE is well utilized experimentally to substantially mitigate conditions like depression and anxiety [154,155]. EE has shown to improve the rate of synaptogenesis and complex dendritic arbors formation [156]. Huntington’s disease (HD) mice exposed to EE showed the absence of dendritic spine pathology [157]. Maternal immune activation (MIA) of female rats downregulated HPA axis and glutamate signaling pathways in amygdala leading to alterations in behavior, endocrine status, and neural markers of synaptic plasticity [158]. MIA rats challenged with endotoxin lipopolysaccharide (LPS) during mid gestation (G15) induced alterations in the expression of genes critical to synaptic transmission and plasticity like Eaats, PSD-95, Gria1, and TrKB in amygdala, leading to the development of cognitive impairments. On postnatal day 50, the offspring were grouped to standard housing, communal nesting, and EE. The offspring exposed to EE rescued the deficits through reducing the activity of HPA axis, upregulating the synaptic markers like Eaat1, Eaat3, PSD-95, and TrKB, which otherwise reduced following prenatal immune activation. The upregulation in synaptic markers indirectly indicate the enhancement of dendritic spines [159]. SNK-SPAR pathway is involved in activity-dependent remodeling of synapses [160]. Similarly, studies have illustrated the mechanism of NWASP-ARP2/3 pathway in regulating spine and synapse formation via ARP2/3-mediated branching of actin for dendritic head enlargement and maturation [161]. Pregnant rats exposed to heavy metal mixtures (MM) via drinking water throughout gestation (from G0) and the offspring were continued to receive the MM throughout lactation, and were experimentally grouped into control and EE-treated groups. The offspring from control group showed impaired cognitive performance correlated to the decreased dendritic spine density, decreased number of mushroom type spines, activation of SNK-SPAR, and inhibition of NWASP-ARP2/3 pathways. However, EE-treated offspring reversed MM-induced disruptions [162]. Few studies claim the necessity of zinc for experience-dependent plasticity in brain. A study conducted by McAllister et al. reported the beneficial effects of EE are independent of zinc in terms of increased basal dendritic length and spine density in neurons of barrel cortex, striatum and amygdala [163]. Amyloid beta (Aβ) oligomer, a synaptotoxic agent, induced the impairment of LTP through downregulation of miRNA-132 expression and increased histone deacetylase (HDAC3), while EE produced beneficial effects through suppressing the effects of Aβ oligomer in hippocampus of wild-type mice [164].
In mice subjected to cerebral focal ischemia by occluding middle cerebral artery, exposure to EE increased the synapse numbers along with the upregulation of expression of synaptophysin and GAP-43, indicating the important role of EE in promoting synaptic remodeling [165]. Recognition memory impairment induced by MK-801, a NMDA antagonist was significantly reversed by EE through restoration of LTP and dendritic spines in long Evans rats [166]. Deafening protocol in rats significantly decreased apical and basal spine density indicating the absence of auditory stimulation induced neuronal atrophy in auditory cortex. Auditory environmental enrichment increased the number of action potentials recorded and glutamergic synapses in layer II/III of auditory cortex along with increase in apical dendritic length and spine density in pyramidal neurons of auditory cortex [167].
4.4. Effects of Yoga and Meditation on Dendritic Spine
Yoga and meditation offer several health benefits including enhanced memory, emotional and attention control, improvement in depressive, anxious, and stressful behavior [168,169,170]. However, the effects of yoga and meditation on central nervous system function still need to be studied more scientifically. Recently, Villemure et al. (2021) reported that yogic practitioners have greater grey matter volume compared to non-yogic practitioners [171]. Though many studies proved the beneficial role of yoga and meditation practice on memory and cognition, not much information is available on their impact on dendritic spines. Practice of Hatha yoga is corroborated to the increased grey mater volumes in the frontal, limbic, temporal, occipital, and cerebellar regions and improved performance in cognitive failure questionnaire which is linked to the neuroplastic changes in the brain [172]. Sahaja yoga facilitates emotional and attentional control which is again linked to the neuroplastic changes in the temporal and frontal areas of cerebrum [173]. Intriguingly, the larger grey matter volume, evidenced from magnetic resonance imaging, in long-term mediators is linked to the improved cognitive, emotional, and attention control [174]. Mounting evidence shed light on the benefits of different yoga and meditation techniques on the cognition, emotional balance, attention control, reward, and happiness which is correlated to increased grey matter volume [174,175,176,177,178]. Indeed, the loss of dendritic spines is positively correlated to decrease in grey matter volume in stressful conditions in mice [179]. Thus, the increased grey matter volume in yoga and meditators might be due to the positive changes in the dendritic spine numbers/density. However, imaging the morphology and distribution of dendritic spines in yoga and meditators will provide interesting evidence and studies are warranted in this line.
5. Conclusions
Spinogenesis has a functional role in memory formation and retrieval, and is regulated by multiple factors like PAK, CaMKII, syndecan-2, Ena/VASP, paralemmin-1, TLCN, PSD, AF6/afadin, Kalirin-7, Rac1, Semaphorin 3F, Tiam1-Rac1-3-LIMK1/2-Cofilin1, and RhoA-ROCK1/2-Myosin II pathways. Autism spectrum disorder (ASD), schizophrenia, and AD are the neurological disorders characterized by significant disruptions in processing information and cognition. Although multiple studies suggest synaptic dysfunctions as a leading insult to neuronal death, disease-specific disruptions in spine shape, size, or number accompany a large number of brain disorders, suggesting dendritic spines as target for many neurodegenerative disorders. Exploration of relationship between dendritic spines and neurological diseases unlocked spine dysmorphogenesis as one of the main underlying causes. This led to the identification of new windows of opportunities for therapeutic intervention.
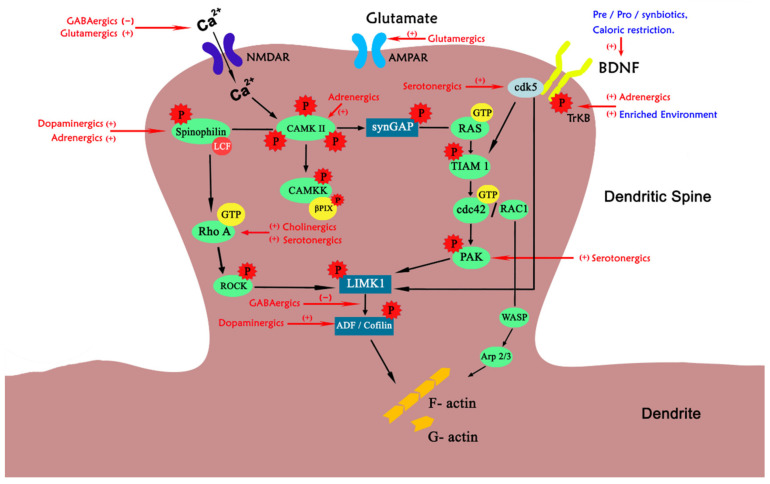
Experimental studies have demonstrated that application of neurotransmitters and chemical messengers like acetylcholine, adrenaline, dopamine, glutamate, GABA, and 5-HT elicits alterations in spine structure and functions (Figure 3). Incidentally, alteration in these chemical messengers is also one of the underpinning pathological mechanisms for most of the neurological disorders. These chemical messengers have been reported to be involved in rectifying both structural and distributional pathologies of dendritic spines and hence can be of therapeutic interest. Interestingly, various studies have shown the role of life style modifications like reduced calorie intake, inclusion of pro/pre/synbiotics in the diet, exposure to enriched environment, and regular practice of yoga and meditation in improving the dendritic spine pathologies as shown in Figure 3 As per the observations from this review it can be proposed that effect of non-pharmacological interventions on the dendritic spine morphogenesis is on par with the pharmacological interventions. Thus, non-pharmacological interventions can be advised as supplementary to neurodegenerative diseases associated with cognitive decline.
Figure 3.
Pharmacological and non-pharmacological targets on structural pathways in dendritic spine. In the figure, the descriptions in red colour indicates pharmacological modulators and blue indicates non-pharmacological modulators on various phases of dendritic spine morphogenesis. (+) denotes positive modulation; (−) indicates negative modulation. NMDAR: N-methyl-D-aspartate receptor, AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, cdK5: Cyclin dependent kinase-5, BDNF: Brain derived neurotrophic factor, CaMKII: Calcium/calmodulin-dependent protein kinase II, TrKB: Tropomyosin receptor kinase-B, TIAM1: T-cell lymphoma invasion and metastasis-inducing protein 1, cdc42: Cell division control protein 42, WASP: Wiskott-Aldrich syndrome protein.
Overall, this review robustly reinforces the application of non-pharmacological modulators to treat spine pathologies as an appropriate approach. However, new discoveries that contribute to the elucidation of beneficial effects of therapeutic vs. non-therapeutic modulators are much needed. More detailed mechanistic studies on non-therapeutic modulators are imperative because the non-therapeutic interventions may allow the development of newer avenues in treating spine pathologies and thus the underlying neurological diseases.
6. Limitations
In the present review, an attempt is made to collate information that demonstrate the influence of various neurotransmitter systems, chemical messengers, and non-pharmacological approaches on the structural and functional status of dendritic spines. Majority of the evidence on the effects of neurotransmitters on the dendritic spines are obtained from preclinical studies, while only a few clinical data are available, even in recent years. Further, in many instances the results of the in vivo studies are not specific, as the effects observed may also be contributed by the overlapping role of other neurochemical systems. Hence, specific and elaborate in vitro studies are needed. Although preliminary research data have provided significant evidence on improved cognitive performance following non-pharmacological approaches, by altering the dendritic spine turnover and shape, only limited literature are available on the spine turnover, and imaging-based structural information before and after the intervention.
These findings underpin the urgent need for intense preclinical and clinical investigations on both pharmacological and non-pharmacological modulators, the combination of which may impart synergistic effects on dendritic spines, in turn cognitive performance, and shall be of transitional importance.
Acknowledgments
S.B.C. and A.M.M. acknowledge JSS AHER for providing facilities to carry out this manuscript preparation. All the authors express their consent for the acknowledgements.
Author Contributions
Conceptualization: A.M.M. and S.B.C.; methodology: S.B.C.; software, B.R.; formal analysis, S.T., T.A.H., P.R., A.G.R., S.B.C., M.W.Q., M.M.E.; resources, A.M.M., M.M.E., M.W.Q.; writing—original draft preparation, A.M.M.; writing—review and editing, S.B.C.; visualization, S.B.C., M.W.Q., M.M.E.; supervision, S.B.C. project administration, S.B.C.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSSAHER through Faculty Research Grant and Special Interest Group (SIG)-Brain, Behavior and Cognitive Neuroscience Research (BBRC) grant number: REG/DIR (R)/URG/54/2011-12.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the literature collected from search engines, journal prints, printouts related copyright permission for reuse of figures etc., are available in the IT section of SBC lab.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGuier N.S., Uys J.D., Mulholland P.J. Chapter 9—Neural morphology and addiction. In: Torregrossa M., editor. Neural Mechanisms of Addiction. Academic Press; Cambridge, MA, USA: 2019. pp. 123–135. [Google Scholar]
- 2.Nimchinsky E.A., Sabatini B.L., Svoboda K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 3.Peters A., Proskauer C.C., Kaiserman-Abramof I.R. The small pyramidal neuron of the rat cerebral cortex. The axon hillock and initial segment. J. Cell Biol. 1968;39:604–619. doi: 10.1083/jcb.39.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochefort N.L., Konnerth A. Dendritic spines: From structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne J., Harris K.M. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Holtmaat A., Wilbrecht L., Knott G.W., Welker E., Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 7.Paspalas C.D., Wang M., Arnsten A.F.T. Constellation of HCN channels and CAMP regulating proteins in dendritic spines of the primate prefrontal cortex: Potential substrate for working memory deficits in schizophrenia. Cereb. Cortex. 2013;23:1643–1654. doi: 10.1093/cercor/bhs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engert F., Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 9.Nägerl U.V., Eberhorn N., Cambridge S.B., Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Kwon H.-B., Sabatini B.L. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuste R., Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 12.Collin C., Miyaguchi K., Segal M. Dendritic spine density and LTP induction in cultured hippocampal slices. J. Neurophysiol. 1997;77:1614–1623. doi: 10.1152/jn.1997.77.3.1614. [DOI] [PubMed] [Google Scholar]
- 13.Meng Y., Zhang Y., Tregoubov V., Janus C., Cruz L., Jackson M., Lu W.-Y., MacDonald J.F., Wang J.Y., Falls D.L., et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/S0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 14.Bosch M., Castro J., Saneyoshi T., Matsuno H., Sur M., Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q., Homma K.J., Poo M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Hayama T., Noguchi J., Watanabe S., Takahashi N., Hayashi-Takagi A., Ellis-Davies G.C.R., Matsuzaki M., Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat. Neurosci. 2013;16:1409–1416. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh W.C., Hill T.C., Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. USA. 2013;110:E305–E312. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiegert J.S., Oertner T.G. Long-Term Depression triggers the selective elimination of weakly integrated synapses. Proc. Natl. Acad. Sci. USA. 2013;110:E4510–E4519. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pchitskaya E., Bezprozvanny I. Dendritic spines shape analysis—Classification or clusterization? Perspective. Front. Synaptic Neurosci. 2020;12:31. doi: 10.3389/fnsyn.2020.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolfrey K.M., Srivastava D.P. Control of dendritic spine morphological and functional plasticity by small GTPases. Neural Plast. 2016;2016:3025948. doi: 10.1155/2016/3025948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emes R.D., Pocklington A.J., Anderson C.N.G., Bayes A., Collins M.O., Vickers C.A., Croning M.D.R., Malik B.R., Choudhary J.S., Armstrong J.D., et al. Evolutionary expansion and anatomical specialization of synapse proteome complexity. Nat. Neurosci. 2008;11:799–806. doi: 10.1038/nn.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Roo M., Klauser P., Mendez P., Poglia L., Muller D. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb. Cortex. 2008;18:151–161. doi: 10.1093/cercor/bhm041. [DOI] [PubMed] [Google Scholar]
- 23.Kayser M.S., Nolt M.J., Dalva M.B. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y.-L., Lei Y.-T., Hong C.-J., Hsueh Y.-P. Syndecan-2 induces filopodia and dendritic spine formation via the Neurofibromin-PKA-Ena/VASP pathway. J. Cell Biol. 2007;177:829–841. doi: 10.1083/jcb.200608121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arstikaitis P., Gauthier-Campbell C., Carolina Gutierrez Herrera R., Huang K., Levinson J.N., Murphy T.H., Kilimann M.W., Sala C., Colicos M.A., El-Husseini A. Paralemmin-1, a Modulator of filopodia induction is required for spine maturation. Mol. Biol. Cell. 2008;19:2026–2038. doi: 10.1091/mbc.e07-08-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuno H., Okabe S., Mishina M., Yanagida T., Mori K., Yoshihara Y. Telencephalin slows spine maturation. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:1776–1786. doi: 10.1523/JNEUROSCI.2651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honkura N., Matsuzaki M., Noguchi J., Ellis-Davies G.C.R., Kasai H. The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron. 2008;57:719–729. doi: 10.1016/j.neuron.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Basu S., Lamprecht R. The role of actin cytoskeleton in dendritic spines in the maintenance of long-term memory. Front. Mol. Neurosci. 2018;11:143. doi: 10.3389/fnmol.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borovac J., Bosch M., Okamoto K. Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018;91:122–130. doi: 10.1016/j.mcn.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Koskinen M., Hotulainen P. Measuring F-actin properties in dendritic spines. Front. Neuroanat. 2014;8:74. doi: 10.3389/fnana.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilinc D. The emerging role of mechanics in synapse formation and plasticity. Front. Cell. Neurosci. 2018;12:483. doi: 10.3389/fncel.2018.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Runge K., Cardoso C., de Chevigny A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020;12:36. doi: 10.3389/fnsyn.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry K.P., Nedivi E. Spine Dynamics: Are they all the same? Neuron. 2017;96:43–55. doi: 10.1016/j.neuron.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan B.W., Mohan V., Wade S.D., Truong Y., Kampov-Polevoi A., Temple B.R., Maness P.F. Semaphorin3F drives dendritic spine pruning through Rho-GTPase signaling. bioRxiv. 2021:1–18. doi: 10.1007/s12035-021-02373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiala J.C., Spacek J., Harris K.M. Dendritic spine pathology: Cause or consequence of neurological disorders? Brain Res. Rev. 2002;39:29–54. doi: 10.1016/S0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 36.Norrholm S.D., Ouimet C.C. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse. 2001;42:151–163. doi: 10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi K., Shirao T. Change in the Shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J. Neurosci. 1999;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenwood S.M., Mizielinska S.M., Frenguelli B.G., Harvey J., Connolly C.N. Mitochondrial dysfunction and dendritic beading during neuronal toxicity. J. Biol. Chem. 2007;282:26235–26244. doi: 10.1074/jbc.M704488200. [DOI] [PubMed] [Google Scholar]
- 39.Scheibel A.B., Tomiyasu U. Dendritic sprouting in Alzheimer’s presenile dementia. Exp. Neurol. 1978;60:1–8. doi: 10.1016/0014-4886(78)90164-4. [DOI] [PubMed] [Google Scholar]
- 40.Bloss E.B., Janssen W.G., Ohm D.T., Yuk F.J., Wadsworth S., Saardi K.M., McEwen B.S., Morrison J.H. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J. Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maiti P., Manna J., Ilavazhagan G., Rossignol J., Dunbar G.L. Molecular Regulation of dendritic spine dynamics and their potential impact on synaptic plasticity and neurological diseases. Neurosci. Biobehav. Rev. 2015;59:208–237. doi: 10.1016/j.neubiorev.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Danglot L., Freret T., Roux N.L., Nême N.N., Burgo A., Hyenne V., Roumier A., Contremoulins V., Dauphin F., Bizot J.-C., et al. Vezatin is essential for dendritic spine morphogenesis and functional synaptic maturation. J. Neurosci. 2012;32:9007–9022. doi: 10.1523/JNEUROSCI.3084-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii K., Nagaoka A., Kishida Y., Okazaki H., Yagishita S., Ucar H., Takahashi N., Saito N., Kasai H. In vivo volume dynamics of dendritic spines in the neocortex of wild-type and Fmr1 KO Mice. eNeuro. 2018;5 doi: 10.1523/ENEURO.0282-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borczyk M., Śliwińska M.A., Caly A., Bernas T., Radwanska K. Neuronal plasticity affects correlation between the size of dendritic spine and its postsynaptic density. Sci. Rep. 2019;9:1693. doi: 10.1038/s41598-018-38412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sala C., Segal M. Dendritic spines: The locus of structural and functional plasticity. Physiol. Rev. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- 46.Südhof T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatt P.C., Pathak S., Kumar V., Panda B.P. Attenuation of Neurobehavioral and neurochemical abnormalities in animal model of cognitive deficits of Alzheimer’s disease by fermented soybean nanonutraceutical. Inflammopharmacology. 2018;26:105–118. doi: 10.1007/s10787-017-0381-9. [DOI] [PubMed] [Google Scholar]
- 48.Dorostkar M.M., Zou C., Blazquez-Llorca L., Herms J. analyzing dendritic spine pathology in Alzheimer’s disease: Problems and opportunities. Acta Neuropathol. 2015;130:1–19. doi: 10.1007/s00401-015-1449-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toledano A., Alvarez M.I. Lesions and dysfunctions of the nucleus basalis as Alzheimer’s disease models: General and critical overview and analysis of the long-term changes in several excitotoxic models. Curr. Alzheimer Res. 2004;1:189–214. doi: 10.2174/1567205043332117. [DOI] [PubMed] [Google Scholar]
- 50.Champtiaux N., Changeux J.-P. Knockout and knockin mice to investigate the role of nicotinic receptors in the central nervous system. Prog. Brain Res. 2004;145:235–251. doi: 10.1016/s0079-6123(03)45016-4. [DOI] [PubMed] [Google Scholar]
- 51.Pugh P.C., Berg D.K. Neuronal Acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J. Neurosci. Off. J. Soc. Neurosci. 1994;14:889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chattipakorn S.C., Leech T., Apaijai N., Higgins L., Jinawong K., Palee S., Maneechote C., Jaiwongkam T., Chattipakorn N. Pretreatment with metformin reduced dendritic spine loss following cardiac ischaemia/reperfusion injury by preventing amyloid beta aggregation, brain inflammation and mitochondrial dysfunction. Eur. Heart J. 2020;41:ehaa946.3142. doi: 10.1093/ehjci/ehaa946.3142. [DOI] [Google Scholar]
- 53.Ongnok B., Khuanjing T., Chunchai T., Kerdphoo S., Jaiwongkam T., Chattipakorn N., Chattipakorn S.C. Donepezil provides neuroprotective effects against brain injury and Alzheimer’s pathology under conditions of cardiac ischemia/reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2021;1867:165975. doi: 10.1016/j.bbadis.2020.165975. [DOI] [PubMed] [Google Scholar]
- 54.Alcantara-Gonzalez F., Juarez I., Solis O., Martinez-Tellez I., Camacho-Abrego I., Masliah E., Mena R., Flores G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus and nucleus accumbens in old rats after chronic donepezil administration. Synapse. 2010;64:786–793. doi: 10.1002/syn.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang T., Tran K.C., Zeng A.Y., Massa S.M., Longo F.M. Small Molecule Modulation of the P75 Neurotrophin Receptor Inhibits Multiple Amyloid Beta-Induced Tau Pathologies. Sci. Rep. 2020;10:20322. doi: 10.1038/s41598-020-77210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajendran K., Chellappan D.R., Sanaranarayanan S., Ramakrishnan V., Krishnan U.M. Investigations on a Polyherbal Formulation for Treatment of Cognitive Impairment in a Cholinergic Dysfunctional Rodent Model. Neurochem. Int. 2020;141:104890. doi: 10.1016/j.neuint.2020.104890. [DOI] [PubMed] [Google Scholar]
- 57.Lecca D., Bader M., Tweedie D., Hoffman A.F., Jung Y.J., Hsueh S.-C., Hoffer B.J., Becker R.E., Pick C.G., Lupica C.R., et al. (-)-Phenserine and the Prevention of pre-programmed cell death and neuroinflammation in mild traumatic brain injury and Alzheimer’s disease challenged mice. Neurobiol. Dis. 2019;130:104528. doi: 10.1016/j.nbd.2019.104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Tang X.C., Zhang H.Y. Huperzine a Alleviates synaptic deficits and modulates amyloidogenic and nonamyloidogenic pathways in APPswe/PS1dE9 transgenic mice. J. Neurosci. Res. 2012;90:508–517. doi: 10.1002/jnr.22775. [DOI] [PubMed] [Google Scholar]
- 59.Kang L., Tian M.K., Bailey C.D.C., Lambe E.K. Dendritic Spine density of prefrontal layer 6 pyramidal neurons in relation to apical dendrite sculpting by nicotinic acetylcholine receptors. Front. Cell. Neurosci. 2015;9:398. doi: 10.3389/fncel.2015.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oda A., Yamagata K., Nakagomi S., Uejima H., Wiriyasermkul P., Ohgaki R., Nagamori S., Kanai Y., Tanaka H. Nicotine induces dendritic spine remodeling in cultured hippocampal neurons. J. Neurochem. 2014;128:246–255. doi: 10.1111/jnc.12470. [DOI] [PubMed] [Google Scholar]
- 61.Lozada A.F., Wang X., Gounko N.V., Massey K.A., Duan J., Liu Z., Berg D.K. Induction of Dendritic spines by Β2-containing nicotinic receptors. J. Neurosci. 2012;32:8391–8400. doi: 10.1523/JNEUROSCI.6247-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schätzle P., Ster J., Verbich D., McKinney R.A., Gerber U., Sonderegger P., María Mateos J. Rapid and reversible formation of spine head filopodia in response to muscarinic receptor activation in CA1 pyramidal cells. J. Physiol. 2011;589:4353–4364. doi: 10.1113/jphysiol.2010.204446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korkotian E., Segal M. Bidirectional regulation of dendritic spine dimensions by glutamate receptors. Neuroreport. 1999;10:2875–2877. doi: 10.1097/00001756-199909090-00032. [DOI] [PubMed] [Google Scholar]
- 64.Korkotian E., Segal M. Release of Calcium from stores alters the morphology of dendritic spines in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 1999;96:12068–12072. doi: 10.1073/pnas.96.21.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanderklish P.W., Edelman G.M. Dendritic Spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maletic-Savatic M., Malinow R., Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 67.Hasbani M.J., Schlief M.L., Fisher D.A., Goldberg M.P. Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. J. Neurosci. Off. J. Soc. Neurosci. 2001;21:2393–2403. doi: 10.1523/JNEUROSCI.21-07-02393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer M., Kaech S., Wagner U., Brinkhaus H., Matus A. Glutamate receptors regulate actin-based plasticity in dendritic spines. Nat. Neurosci. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- 69.Pereira A.C., Lambert H.K., Grossman Y.S., Dumitriu D., Waldman R., Jannetty S.K., Calakos K., Janssen W.G., McEwen B.S., Morrison J.H. Glutamatergic Regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc. Natl. Acad. Sci. USA. 2014;111:18733–18738. doi: 10.1073/pnas.1421285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LaCrosse A.L., Taylor S.B., Nemirovsky N.E., Gass J.T., Olive M.F. MGluR5 Positive and negative allosteric modulators differentially affect dendritic spine density and morphology in the prefrontal cortex. CNS Neurol. Disord. Drug Targets. 2015;14:476–485. doi: 10.2174/1871527314666150429112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuhlmann N., Wagner Valladolid M., Quesada-Ramírez L., Farrer M.J., Milnerwood A.J. Chronic and acute manipulation of cortical glutamate transmission induces structural and synaptic changes in co-cultured striatal neurons. Front. Cell. Neurosci. 2021;15:23. doi: 10.3389/fncel.2021.569031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ali F., Gerhard D.M., Sweasy K., Pothula S., Pittenger C., Duman R.S., Kwan A.C. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat. Commun. 2020;11:72. doi: 10.1038/s41467-019-13809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seo M., Hien L.T., Park M.K., Choi A.J., Seog D.-H., Kim S.-H., Park S.W., Lee J.G. AMPA Receptor-MTORC1 signaling activation is required for neuroplastic effects of LY341495 in rat hippocampal neurons. Sci. Rep. 2020;10:993. doi: 10.1038/s41598-020-58017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon Y.J., Gokin A.P., Martin-Caraballo M. Pharmacological manipulation of GABA-driven activity in ovo disrupts the development of dendritic morphology but not the maturation of spinal cord network activity. Neural Dev. 2010;5:11. doi: 10.1186/1749-8104-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y. GABA-A receptor-dependent mechanisms prevent excessive spine elimination during postnatal maturation of the mouse cortex in vivo. FEBS Lett. 2014;588:4551–4560. doi: 10.1016/j.febslet.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salmon C.K., Pribiag H., Gizowski C., Farmer W.T., Cameron S., Jones E.V., Mahadevan V., Bourque C.W., Stellwagen D., Woodin M.A., et al. Depolarizing GABA transmission restrains activity-dependent glutamatergic synapse formation in the developing hippocampal circuit. Front. Cell. Neurosci. 2020;14:36. doi: 10.3389/fncel.2020.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lachance-Touchette P., Choudhury M., Stoica A., Di Cristo G., Cossette P. Single-cell genetic expression of mutant GABAA receptors causing human genetic epilepsy alters dendritic spine and GABAergic bouton formation in a mutation-specific manner. Front. Cell. Neurosci. 2014;8:317. doi: 10.3389/fncel.2014.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lur G., Higley M.J. Glutamate receptor modulation is restricted to synaptic microdomains. Cell Rep. 2015;12:326–334. doi: 10.1016/j.celrep.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giachero M., Calfa G.D., Molina V.A. Hippocampal Dendritic spines remodeling and fear memory are modulated by GABAergic signaling within the basolateral amygdala complex. Hippocampus. 2015;25:545–555. doi: 10.1002/hipo.22409. [DOI] [PubMed] [Google Scholar]
- 80.Vitalis T., Ansorge M.S., Dayer A.G. Serotonin Homeostasis and serotonin receptors as actors of cortical construction: Special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front. Cell. Neurosci. 2013;7:93. doi: 10.3389/fncel.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bonnin A., Goeden N., Chen K., Wilson M.L., King J., Shih J.C., Blakely R.D., Deneris E.S., Levitt P. A Transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dayer A. Serotonin-related pathways and developmental plasticity: Relevance for psychiatric disorders. Dialogues Clin. Neurosci. 2014;16:29–41. doi: 10.31887/DCNS.2014.16.1/adayer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kobe F., Guseva D., Jensen T.P., Wirth A., Renner U., Hess D., Müller M., Medrihan L., Zhang W., Zhang M., et al. 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:2915–2930. doi: 10.1523/JNEUROSCI.2765-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hedlund P.B., Leopoldo M., Caccia S., Sarkisyan G., Fracasso C., Martelli G., Lacivita E., Berardi F., Perrone R. LP-211 Is a brain penetrant selective agonist for the serotonin 5-HT(7) receptor. Neurosci. Lett. 2010;481:12–16. doi: 10.1016/j.neulet.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bijata M., Labus J., Guseva D., Stawarski M., Butzlaff M., Dzwonek J., Schneeberg J., Böhm K., Michaluk P., Rusakov D.A., et al. Synaptic remodeling depends on signaling between serotonin receptors and the extracellular matrix. Cell Rep. 2017;19:1767–1782. doi: 10.1016/j.celrep.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 86.Jones K.A., Srivastava D.P., Allen J.A., Strachan R.T., Roth B.L., Penzes P. Rapid Modulation of spine morphology by the 5-HT2A serotonin receptor through Kalirin-7 signaling. Proc. Natl. Acad. Sci. USA. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mi Z., Si T., Kapadia K., Li Q., Muma N.A. Receptor-stimulated transamidation induces activation of Rac1 and Cdc42 and the regulation of dendritic spines. Neuropharmacology. 2017;117:93–105. doi: 10.1016/j.neuropharm.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waller J.A., Chen F., Sánchez C. Vortioxetine promotes maturation of dendritic spines in vitro: A comparative study in hippocampal cultures. Neuropharmacology. 2016;103:143–154. doi: 10.1016/j.neuropharm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Speranza L., Labus J., Volpicelli F., Guseva D., Lacivita E., Leopoldo M., Bellenchi G.C., di Porzio U., Bijata M., Perrone-Capano C., et al. Serotonin 5-HT7 Receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J. Neurochem. 2017;141:647–661. doi: 10.1111/jnc.13962. [DOI] [PubMed] [Google Scholar]
- 90.Kao Y.-C., Wei W.-Y., Tsai K., Wang L.-C. High fat diet suppresses peroxisome proliferator-activated receptors and reduces dopaminergic neurons in the substantia nigra. Int. J. Mol. Sci. 2019;21:207. doi: 10.3390/ijms21010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Dell T.J., Connor S.A., Guglietta R., Nguyen P.V. β-adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 2015;22:461–471. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji Y., Pang P.T., Feng L., Lu B. Cyclic AMP controls BDNF-induced TrkB Phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat. Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 93.Jȩdrzejewska-Szmek J., Luczak V., Abel T., Blackwell K.T. β-adrenergic signaling broadly contributes to LTP induction. PLoS Comput. Biol. 2017;13:e1005657. doi: 10.1371/journal.pcbi.1005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren W.-W., Liu Y., Li B.-M. Stimulation of α(2A)-adrenoceptors promotes the maturation of dendritic spines in cultured neurons of the medial prefrontal cortex. Mol. Cell. Neurosci. 2012;49:205–216. doi: 10.1016/j.mcn.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Hains A.B., Yabe Y., Arnsten A.F.T. Chronic stimulation of Alpha-2A-adrenoceptors with guanfacine protects rodent prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Neurobiol. Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu J., Vidovic M., Chen M.-M., Lu Q.-Y., Song Z.-M. Activation of A2A Adrenoceptors Alters Dendritic Spine Development and the Expression of Spinophilin in Cultured Cortical Neurones. Brain Res. 2008;1199:37–45. doi: 10.1016/j.brainres.2007.12.073. [DOI] [PubMed] [Google Scholar]
- 97.Avila-Costa M.R., Colín-Barenque L., Montiel-Flores E., Aley-Medina P., Valdez A.L.G., Librado J.L.O., Martínez E.F., Martínez V.A., Mussali-Galante P., Fortoul T.I. Bromocriptine treatment in a murine Parkinson’s model: Ultrastructural evaluation after dopaminergic deafferentation. Int. J. Neurosci. 2005;115:851–859. doi: 10.1080/00207450590897059. [DOI] [PubMed] [Google Scholar]
- 98.Yao W.-D., Spealman R.D., Zhang J. Dopaminergic signaling in dendritic spines. Biochem. Pharmacol. 2008;75:2055–2069. doi: 10.1016/j.bcp.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J., Gu J., Wang B., Xie M., Huang L., Liu Y., Zhang L., Xue J., Guo F., Zhang L., et al. Activation of dopamine D1 receptors regulates dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons. Mol. Neurobiol. 2015;51:1024–1037. doi: 10.1007/s12035-014-8762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zagrebelsky M., Tacke C., Korte M. BDNF signaling during the lifetime of dendritic spines. Cell Tissue Res. 2020;382:185–199. doi: 10.1007/s00441-020-03226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Collo G., Cavalleri L., Bono F., Mora C., Fedele S., Invernizzi R.W., Gennarelli M., Piovani G., Kunath T., Millan M.J., et al. Ropinirole and pramipexole promote structural plasticity in human IPSC-derived dopaminergic neurons via BDNF and MTOR signaling. Neural Plast. 2018;2018:4196961. doi: 10.1155/2018/4196961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parajuli L.K., Wako K., Maruo S., Kakuta S., Taguchi T., Ikuno M., Yamakado H., Takahashi R., Koike M. Developmental changes in dendritic spine morphology in the striatum and their alteration in an A53T α-Synuclein transgenic mouse model of Parkinson’s disease. eNeuro. 2020;7 doi: 10.1523/ENEURO.0072-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ueno T., Nishijima H., Ueno S., Tomiyama M. Spine Enlargement of pyramidal tract-type neurons in the motor cortex of a rat model of Levodopa-induced dyskinesia. Front. Neurosci. 2017;11:206. doi: 10.3389/fnins.2017.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Funamizu Y., Nishijima H., Ueno T., Ueno S., Mizukami H., Yagihashi S., Tomiyama M. Morphological dendritic spine changes of medium spiny neurons in the nucleus accumbens in 6-Hydroxydopamine-Lesioned Rats treated with Levodopa. Neurosci. Res. 2017;121:49–53. doi: 10.1016/j.neures.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 105.Gagnon D., Petryszyn S., Sanchez M.G., Bories C., Beaulieu J.M., De Koninck Y., Parent A., Parent M. Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci. Rep. 2017;7:41432. doi: 10.1038/srep41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arroyo-García L.E., Vázquez-Roque R.A., Díaz A., Treviño S., De La Cruz F., Flores G., Rodríguez-Moreno A. The effects of non-selective dopamine receptor activation by apomorphine in the mouse hippocampus. Mol. Neurobiol. 2018;55:8625–8636. doi: 10.1007/s12035-018-0991-2. [DOI] [PubMed] [Google Scholar]
- 107.Russo E., De Fazio S., De Fazio P., Amorosi A., Perrotta I., De Sarro G., Donato G. Apomorphine-Induced neurodegeneration in mongolian gerbil hippocampus. Schizophr. Res. 2007;95:223–227. doi: 10.1016/j.schres.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 108.Razgado-Hernandez L.F., Espadas-Alvarez A.J., Reyna-Velazquez P., Sierra-Sanchez A., Anaya-Martinez V., Jimenez-Estrada I., Bannon M.J., Martinez-Fong D., Aceves-Ruiz J. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 Receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS ONE. 2015;10:e0117391. doi: 10.1371/journal.pone.0117391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Graziane N.M., Yuen E.Y., Yan Z. Dopamine D4 receptors regulate GABAA receptor trafficking via an actin/cofilin/myosin-dependent mechanism. J. Biol. Chem. 2009;284:8329–8336. doi: 10.1074/jbc.M807387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H.-D., Deutch A.Y. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: Reversal by atypical antipsychotic drug treatment. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia B.G., Neely M.D., Deutch A.Y. Cortical regulation of striatal medium spiny neuron dendritic remodeling in Parkinsonism: Modulation of glutamate release reverses dopamine depletion–induced dendritic spine loss. Cereb. Cortex. 2010;20:2423–2432. doi: 10.1093/cercor/bhp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fasano C., Bourque M.-J., Lapointe G., Leo D., Thibault D., Haber M., Kortleven C., Desgroseillers L., Murai K.K., Trudeau L.-É. Dopamine facilitates dendritic spine formation by cultured striatal medium spiny neurons through Both D1 and D2 dopamine receptors. Neuropharmacology. 2013;67:432–443. doi: 10.1016/j.neuropharm.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 113.Hiday A.C., Edler M.C., Salek A.B., Morris C.W., Thang M., Rentz T.J., Rose K.L., Jones L.M., Baucum A.J. Mechanisms and consequences of dopamine depletion-induced attenuation of the spinophilin/neurofilament medium interaction. PMC. 2017;2017:4153076. doi: 10.1155/2017/4153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Areal L.B., Hamilton A., Martins-Silva C., Pires R.G.W., Ferguson S.S.G. Neuronal scaffolding protein spinophilin is integral for cocaine-induced behavioral sensitization and ERK1/2 activation. Mol. Brain. 2019;12:15. doi: 10.1186/s13041-019-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jung G., Kim E.-J., Cicvaric A., Sase S., Gröger M., Höger H., Sialana F.J., Berger J., Monje F.J., Lubec G. Drebrin depletion alters neurotransmitter receptor levels in protein complexes, dendritic spine morphogenesis and memory-related synaptic plasticity in the mouse hippocampus. J. Neurochem. 2015;134:327–339. doi: 10.1111/jnc.13119. [DOI] [PubMed] [Google Scholar]
- 116.Cannizzaro C., Talani G., Brancato A., Mulas G., Spiga S., Luca M.A.D., Sanna A., Marino R.A.M., Biggio G., Sanna E., et al. Dopamine restores limbic memory loss, dendritic spine structure, and NMDAR-dependent LTD in the Nucleus accumbens of alcohol-withdrawn rats. J. Neurosci. 2019;39:929–943. doi: 10.1523/JNEUROSCI.1377-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wahl D., Solon-Biet S.M., Wang Q.-P., Wali J.A., Pulpitel T., Clark X., Raubenheimer D., Senior A.M., Sinclair D.A., Cooney G.J., et al. Comparing the effects of low-protein and high-carbohydrate diets and caloric restriction on brain aging in mice. Cell Rep. 2018;25:2234–2243.e6. doi: 10.1016/j.celrep.2018.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pani G. Neuroprotective effects of dietary restriction: Evidence and mechanisms. Semin. Cell Dev. Biol. 2015;40:106–114. doi: 10.1016/j.semcdb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 119.Brandhorst S., Choi I.Y., Wei M., Cheng C.W., Sedrakyan S., Navarrete G., Dubeau L., Yap L.P., Park R., Vinciguerra M., et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stranahan A.M., Lee K., Martin B., Maudsley S., Golden E., Cutler R.G., Mattson M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moroi-Fetters S.E., Mervis R.F., London E.D., Ingram D.K. Dietary Restriction suppresses age-related changes in dendritic spines. Neurobiol. Aging. 1989;10:317–322. doi: 10.1016/0197-4580(89)90042-0. [DOI] [PubMed] [Google Scholar]
- 122.Sripetchwandee J., Pipatpiboon N., Pratchayasakul W., Chattipakorn N., Chattipakorn S.C. DPP-4 inhibitor and PPARγ agonist restore the loss of CA1 dendritic spines in obese insulin-resistant rats. Arch. Med. Res. 2014;45:547–552. doi: 10.1016/j.arcmed.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Torabi T., Azizzadeh Delshad A., Roghani M. Dietary Restriction prevents dendritic changes of pyramidal neurons in hippocampal and prefrontal cortex in diabetic rat. J. Basic Clin. Pathophysiol. 2019;7:28–32. doi: 10.22070/jbcp.2019.4700.1119. [DOI] [Google Scholar]
- 124.Andrade J.P., Lukoyanov N.V., Paula-Barbosa M.M. Chronic Food restriction is associated with subtle dendritic alterations in granule cells of the rat hippocampal formation. Hippocampus. 2002;12:149–164. doi: 10.1002/hipo.1102. [DOI] [PubMed] [Google Scholar]
- 125.Salas M. Effects of early undernutrition on dendritic spines of cortical pyramidal cells in the rat. Dev. Neurosci. 1980;3:109–117. doi: 10.1159/000112384. [DOI] [PubMed] [Google Scholar]
- 126.Benítez-Bribiesca L., De la Rosa-Alvarez I., Mansilla-Olivares A. Dendritic spine pathology in infants with severe protein-calorie malnutrition. Pediatrics. 1999;104:e21. doi: 10.1542/peds.104.2.e21. [DOI] [PubMed] [Google Scholar]
- 127.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Guan N.L., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Möhle L., Mattei D., Heimesaat M.M., Bereswill S., Fischer A., Alutis M., French T., Hambardzumyan D., Matzinger P., Dunay I.R., et al. Ly6C(Hi) monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 129.Matcovitch-Natan O., Winter D.R., Giladi A., Vargas Aguilar S., Spinrad A., Sarrazin S., Ben-Yehuda H., David E., Zelada González F., Perrin P., et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 130.Hoban A.E., Stilling R.M., Ryan F.J., Shanahan F., Dinan T.G., Claesson M.J., Clarke G., Cryan J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry. 2016;6:e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Desbonnet L., Clarke G., Traplin A., O’Sullivan O., Crispie F., Moloney R.D., Cotter P.D., Dinan T.G., Cryan J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 132.O’Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 133.Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 134.Sarkar A., Lehto S.M., Harty S., Dinan T.G., Cryan J.F., Burnet P.W.J. Psychobiotics and the Manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763–781. doi: 10.1016/j.tins.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arentsen T., Raith H., Qian Y., Forssberg H., Diaz Heijtz R. Host microbiota modulates development of social preference in mice. Microb. Ecol. Health Dis. 2015;26:29719. doi: 10.3402/mehd.v26.29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luczynski P., Whelan S.O., O’Sullivan C., Clarke G., Shanahan F., Dinan T.G., Cryan J.F. Adult Microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016;44:2654–2666. doi: 10.1111/ejn.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Vincenti A.P., Ríos A.S., Paratcha G., Ledda F. Mechanisms that modulate and diversify BDNF functions: Implications for hippocampal synaptic plasticity. Front. Cell. Neurosci. 2019;13:135. doi: 10.3389/fncel.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kellner Y., Gödecke N., Dierkes T., Thieme N., Zagrebelsky M., Korte M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front. Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Sullivan E., Barrett E., Grenham S., Fitzgerald P., Stanton C., Ross R.P., Quigley E.M.M., Cryan J.F., Dinan T.G. BDNF expression in the hippocampus of maternally separated rats: Does bifidobacterium breve 6330 alter BDNF levels? Benef. Microbes. 2011;2:199–207. doi: 10.3920/BM2011.0015. [DOI] [PubMed] [Google Scholar]
- 140.Ranuh R., Athiyyah A.F., Darma A., Risky V.P., Riawan W., Surono I.S., Sudarmo S.M. Effect of the probiotic lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of intestinal microbiota on the gut-brain axis. Iran. J. Microbiol. 2019;11:145–150. doi: 10.18502/ijm.v11i2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tochitani S., Ikeno T., Ito T., Sakurai A., Yamauchi T., Matsuzaki H. Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. PLoS ONE. 2016;11:e0138293. doi: 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiao J., Wang T., Xu Y., Gu X., Li D., Niu K., Wang T., Zhao J., Zhou R., Wang H.-L. Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats. Transl. Psychiatry. 2020;10:25. doi: 10.1038/s41398-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chunchai T., Thunapong W., Yasom S., Wanchai K., Eaimworawuthikul S., Metzler G., Lungkaphin A., Pongchaidecha A., Sirilun S., Chaiyasut C., et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018;15:11. doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bocarsly M.E., Fasolino M., Kane G.A., LaMarca E.A., Kirschen G.W., Karatsoreos I.N., McEwen B.S., Gould E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA. 2015;112:15731–15736. doi: 10.1073/pnas.1511593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hao S., Dey A., Yu X., Stranahan A.M. Dietary Obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun. 2016;51:230–239. doi: 10.1016/j.bbi.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 147.Kim K.-A., Gu W., Lee I.-A., Joh E.-H., Kim D.-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shirao T., Hanamura K., Koganezawa N., Ishizuka Y., Yamazaki H., Sekino Y. The role of drebrin in neurons. J. Neurochem. 2017;141:819–834. doi: 10.1111/jnc.13988. [DOI] [PubMed] [Google Scholar]
- 149.Koganezawa N., Hanamura K., Sekino Y., Shirao T. The role of drebrin in dendritic spines. Mol. Cell. Neurosci. 2017;84:85–92. doi: 10.1016/j.mcn.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 150.Sekino Y., Koganezawa N., Mizui T., Shirao T. Role of drebrin in synaptic plasticity. In: Shirao T., Sekino Y., editors. Drebrin: From Structure and Function to Physiological and Pathological Roles. Springer; Tokyo, Japan: 2017. pp. 183–201. Advances in Experimental Medicine and Biology. [Google Scholar]
- 151.Rudzki L., Szulc A. “Immune gate” of psychopathology—The role of gut derived immune activation in major psychiatric disorders. Front. Psychiatry. 2018;9:205. doi: 10.3389/fpsyt.2018.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ishii T., Furuoka H., Kaya M., Kuhara T. Oral administration of probiotic bifidobacterium breve improves facilitation of hippocampal memory extinction via restoration of aberrant higher induction of neuropsin in an MPTP-induced mouse model of Parkinson’s disease. Biomedicines. 2021;9:167. doi: 10.3390/biomedicines9020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Talani G., Biggio F., Mostallino M.C., Locci V., Porcedda C., Boi L., Saolini E., Piras R., Sanna E., Biggio G. Treatment with gut bifidobacteria improves hippocampal plasticity and cognitive behavior in adult healthy rats. Neuropharmacology. 2020;165:107909. doi: 10.1016/j.neuropharm.2019.107909. [DOI] [PubMed] [Google Scholar]
- 154.Sale A., Putignano E., Cancedda L., Landi S., Cirulli F., Berardi N., Maffei L. Enriched environment and acceleration of visual system development. Neuropharmacology. 2004;47:649–660. doi: 10.1016/j.neuropharm.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 155.Scichilone J.M., Yarraguntla K., Charalambides A., Harney J.P., Butler D. Environmental enrichment mitigates detrimental cognitive effects of ketogenic diet in weanling rats. J. Mol. Neurosci. 2016;60:1–9. doi: 10.1007/s12031-016-0753-4. [DOI] [PubMed] [Google Scholar]
- 156.Diamond M.C. Response of the brain to enrichment. An. Acad. Bras. Cienc. 2001;73:211–220. doi: 10.1590/S0001-37652001000200006. [DOI] [PubMed] [Google Scholar]
- 157.Nithianantharajah J., Barkus C., Vijiaratnam N., Clement O., Hannan A.J. Modeling brain reserve: Experience-dependent neuronal plasticity in healthy and Huntington’s disease transgenic mice. Am. J. Geriatr. Psychiatry. 2009;17:196–209. doi: 10.1097/JGP.0b013e318196a632. [DOI] [PubMed] [Google Scholar]
- 158.Estes M.L., McAllister A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao X., Rondón-Ortiz A.N., Lima E.P., Puracchio M., Roderick R.C., Kentner A.C. Therapeutic efficacy of environmental enrichment on behavioral, endocrine, and synaptic alterations in an animal model of maternal immune activation. Brain Behav. Immun. Health. 2020;3:100043. doi: 10.1016/j.bbih.2020.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gong X., Lu X., Zhan L., Sui H., Qi X., Ji Z., Niu X., Liu L. Role of the SNK-SPAR pathway in the development of Alzheimer’s disease. IUBMB Life. 2010;62:214–221. doi: 10.1002/iub.308. [DOI] [PubMed] [Google Scholar]
- 161.Wegner A.M., Nebhan C.A., Hu L., Majumdar D., Meier K.M., Weaver A.M., Webb D.J. N-WASP and the Arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou F., Yin G., Gao Y., Ouyang L., Liu S., Jia Q., Yu H., Zha Z., Wang K., Xie J., et al. Insights into Cognitive deficits caused by low-dose toxic heavy metal mixtures and their remediation through a postnatal enriched environment in rats. J. Hazard. Mater. 2020;388:122081. doi: 10.1016/j.jhazmat.2020.122081. [DOI] [PubMed] [Google Scholar]
- 163.McAllister B.B., Thackray S.E., de la Orta B.K.G., Gosse E., Tak P., Chipak C., Rehal S., Rascon A.V., Dyck R.H. Effects of enriched housing on the neuronal morphology of mice that lack zinc transporter 3 (ZnT3) and vesicular zinc. Behav. Brain Res. 2020;379:112336. doi: 10.1016/j.bbr.2019.112336. [DOI] [PubMed] [Google Scholar]
- 164.Wei Z., Meng X., El Fatimy R., Sun B., Mai D., Zhang J., Arora R., Zeng A., Xu P., Qu S., et al. Environmental enrichment prevents a beta oligomer-induced synaptic dysfunction through Mirna-132 and Hdac3 signaling pathways. Neurobiol. Dis. 2020;134:104617. doi: 10.1016/j.nbd.2019.104617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang C., Zhang Q., Yu K., Shen X., Wu Y., Wu J. Enriched Environment promoted cognitive function via bilateral synaptic remodeling after cerebral ischemia. Front. Neurol. 2019;10:1189. doi: 10.3389/fneur.2019.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Murueta-Goyena A., Morera-Herreras T., Miguelez C., Gutierrez-Ceballos A., Ugedo L., Vicente Lafuente J., Bengoetxea H. Effects of adult enriched environment on cognition, hippocampal-prefrontal plasticity and NMDAR subunit expression in MK-801-induced schizophrenia model. Eur. Neuropsychopharmacol. 2019;29:590–600. doi: 10.1016/j.euroneuro.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 167.Bose M., Muñoz-Llancao P., Roychowdhury S., Nichols J.A., Jakkamsetti V., Porter B., Byrapureddy R., Salgado H., Kilgard M.P., Aboitiz F., et al. Effect of the environment on the dendritic morphology of the rat auditory cortex. Synapse. 2010;64:97–110. doi: 10.1002/syn.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Woolery A., Myers H., Sternlieb B., Zeltzer L. A yoga intervention for young adults with elevated symptoms of depression. Altern. Ther. Health Med. 2004;10:60–63. [PubMed] [Google Scholar]
- 169.Galantino M.L., Bzdewka T.M., Eissler-Russo J.L., Holbrook M.L., Mogck E.P., Geigle P., Farrar J.T. The impact of modified hatha yoga on chronic low back pain: A pilot study. Altern. Ther. Health Med. 2004;10:56–59. [PubMed] [Google Scholar]
- 170.Epstein G.N., Halper J.P., Barrett E.A.M., Birdsall C., McGee M., Baron K.P., Lowenstein S. A pilot study of mind-body changes in adults with asthma who practice mental imagery. Altern. Ther. Health Med. 2004;10:66–71. [PubMed] [Google Scholar]
- 171.Villemure C., Ceko M., Cotton V.A., Bushnell M.C. Neuroprotective effects of yoga practice: Age-, experience-, and frequency-dependent plasticity. Front. Hum. Neurosci. 2015;9:281. doi: 10.3389/fnhum.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Froeliger B., Garland E.L., McClernon F.J. Yoga meditation practitioners exhibit greater gray matter volume and fewer reported cognitive failures: Results of a preliminary voxel-based morphometric analysis. Evid. Based Complement. Altern. Med. ECAM. 2012;2012:821307. doi: 10.1155/2012/821307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hernández S.E., Dorta R., Suero J., Barros-Loscertales A., González-Mora J.L., Rubia K. Larger whole brain grey matter associated with long-term sahaja yoga meditation: A detailed area by area comparison. PLoS ONE. 2020;15:e0237552. doi: 10.1371/journal.pone.0237552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hernández S.E., Barros-Loscertales A., Xiao Y., González-Mora J.L., Rubia K. Gray matter and functional connectivity in anterior cingulate cortex are associated with the state of mental silence during sahaja yoga meditation. Neuroscience. 2018;371:395–406. doi: 10.1016/j.neuroscience.2017.12.017. [DOI] [PubMed] [Google Scholar]
- 175.Babu M.G.R., Kadavigere R., Koteshwara P., Sathian B., Rai K.S. Rajyoga Meditation induces grey matter volume changes in regions that process reward and happiness. Sci. Rep. 2020;10:16177. doi: 10.1038/s41598-020-73221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hernández S.E., Suero J., Rubia K., González-Mora J.L. Monitoring the neural activity of the state of mental silence while practicing sahaja yoga meditation. J. Altern. Complement. Med. 2015;21:175–179. doi: 10.1089/acm.2013.0450. [DOI] [PubMed] [Google Scholar]
- 177.Hendriks T. The effects of sahaja yoga meditation on mental health: A systematic review. J. Complement. Integr. Med. 2018;15:0163. doi: 10.1515/jcim-2016-0163. [DOI] [PubMed] [Google Scholar]
- 178.Froeliger B.E., Garland E.L., Modlin L.A., McClernon F.J. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: A pilot study. Front. Integr. Neurosci. 2012;6:48. doi: 10.3389/fnint.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kassem M.S., Lagopoulos J., Stait-Gardner T., Price W.S., Chohan T.W., Arnold J.C., Hatton S.N., Bennett M.R. Stress-induced grey matter loss determined by MRI Is primarily due to loss of dendrites and their synapses. Mol. Neurobiol. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- 180.Thas J.J. Siddha medicine—Background and principles and the application for skin diseases. Clin. Dermatol. 2008;26:62–78. doi: 10.1016/j.clindermatol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 181.Pandey K.R., Naik S.R., Vakil B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the literature collected from search engines, journal prints, printouts related copyright permission for reuse of figures etc., are available in the IT section of SBC lab.