Abstract
Two Borrelia isolates (CA434 and CA435) cultured from the soft tick Ornithodoros coriaceus were analyzed by contour-clamped homogeneous electric field gel electrophoresis of unrestricted and ApaI-restricted DNA, standard electrophoresis of BamHI- and HindIII-restricted DNA, Southern hybridization, restriction fragment length polymorphism and sequencing of the 16S rRNA gene, and amplification of the 5S-23S intergenic spacer region. These isolates were compared with Borrelia coriaceae type strain Co53, B. burgdorferi sensu stricto strain CA4, and the relapsing-fever spirochete B. parkeri (undesignated). The 16S rRNA region of CA434 and CA435 differed from that of B. coriaceae type strain Co53 by the presence of 1 base (C) at position 367 (GenBank accession no. U42286). The linear plasmid profile of CA434 was similar to that of Co53, and the ApaI, BamHI, and HindIII restriction fingerprints of the total cellular DNA of CA434 and Co53 were similar. In contrast, CA435 differed somewhat from CA434 and Co53, which demonstrates that B. coriaceae is genetically diverse. Southern hybridization showed that the DNAs of CA434 and CA435 hybridized strongly with the digoxigenin-labeled DNA of Co53. Low homology was found between the DNA of Co53 and that of B. parkeri. The 16S rRNA sequence of B. parkeri was identical to previously published results for B. parkeri strain M3001 (GenBank accession number U42296). CA434 and CA435 represent only the second and third isolates of B. coriaceae obtained from any source since its initial isolation from an O. coriaceus tick in 1985. All three B. coriaceae isolates were derived from adult ticks collected from the same locality in northwestern California. Difficulties encountered in detecting B. coriaceae in, and isolating this spirochete from, the tissues of O. coriaceus are discussed. The lack of concordance between different detection or isolation methods suggests that reliance upon a single technique may grossly underestimate the true prevalence of spirochetal infection in wild-caught O. coriaceus ticks.
In 1985, a previously unrecognized Borrelia-like spirochete was detected in all three trophic stages of the human-biting soft tick Ornithodoros coriaceus from northern California, and a single isolate from a male tick was partially characterized morphologically and immunochemically (15). Shortly thereafter, this isolate, designated Co53, was characterized genetically and phenotypically and named Borrelia coriaceae after its tick vector (14, 19). B. coriaceae was found to be passed transstadially and occasionally via eggs in its tick vector (15, 17), and the Columbian black-tailed deer (Odocoileus hemionus columbianus) was implicated as a probable natural host of the spirochete (16). Furthermore, circumstantial evidence suggested that B. coriaceae is the cause of epizootic bovine abortion (EBA), a significant disease of rangeland cattle in some areas of the far-western United States, particularly California (15). Subsequent studies, while not ruling out the possibility that B. coriaceae or another spirochete is involved in the epizoology of EBA, have not established a firm link between the presence of spirochetes and bovine abortion (28, 29, 37, 38, 41).
To our knowledge, no more isolates of B. coriaceae have been isolated since 1985. Here we genetically characterize two more recently acquired isolates of B. coriaceae that were derived from O. coriaceus ticks and compare them with the Co53 type strain of B. coriaceae, the relapsing-fever spirochete B. parkeri, and the Lyme disease spirochete B. burgdorferi sensu stricto (s.s.). These new isolates of B. coriaceae, designated CA434 and CA435, represent only the second and third isolates of this little-known borrelial genospecies. As such, they provide an opportunity to evaluate the genetic variability inherent in B. coriaceae from the same locality as strain Co53.
MATERIALS AND METHODS
Tick collection and dissection.
Nymphal and adult O. coriaceus ticks were collected in enamelware pitfall traps baited with dry ice in chaparral and woodland-grass plant communities at the University of California Hopland Research and Extension Center (HREC) in Mendocino County, Calif., from July to September 1995. Ticks were surface disinfected for approximately 30 s each in 3% hydrogen peroxide and 70% ethanol and then rinsed in sterile phosphate-buffered saline. Next, they were embedded in paraffin and dissected in sterile phosphate-buffered saline and tissue smears were prepared from the central ganglion and portions of the midgut diverticula and salivary glands of each tick. After smears were air dried and fixed in acetone (10 min), they were examined for the presence of spirochetes by direct immunofluorescence (DI) assay with a fluorescein isothiocyanate-labeled rabbit anti-B. burgdorferi conjugate (4). The anti-B. burgdorferi conjugate was prepared with type strain B31 and purified on a protein A-Sepharose column (4).
Isolation procedures.
After tissue smears had been prepared for the DI assay, the remaining tissues from each tick were subdivided and placed into two 1.5-ml Eppendorf tubes filled with 1.25 ml of BSK-II medium (2) containing rifampin at 25 μg/ml. One member of each pair of culture tubes was incubated at 30°C, the other was incubated at 36°C, and all cultures were examined for the presence of spirochetes weekly for 6 weeks by dark-field microscopy. Prior to genetic characterization, the borrelial isolates obtained were kept frozen at −74°C in a mixture of 3 parts glycerol and 7 parts BSK-II medium. Two of five borrelial isolates were regrown successfully in BSK-H medium (30) at 34.5°C, whereas attempts to regrow them in BSK-II medium were unsuccessful.
Isolates.
The isolates compared were the type strain of B. coriaceae (Co53) from the HREC, Mendocino County, Calif., two borrelial isolates obtained from O. coriaceus ticks from the HREC during the present study (CA434 and CA435), an isolate of the relapsing-fever spirochete B. parkeri (undesignated) from an O. parkeri tick, and the CA4 strain of B. burgdorferi s.s. from an Ixodes pacificus tick from Sonoma County, Calif. (18, 21). The B. parkeri isolate was obtained from the Rocky Mountain Laboratories Bacterial Collection through the courtesy of Tom Schwan, Hamilton, Mont. No information is available about the geographical origin of the latter isolate. The passage numbers for CA434, CA435, and CA4 are six, six, and five, respectively. The passage numbers for B. parkeri and Co53 are unknown, but that of Co53 is greater than 10.
DNA extraction, restriction digestion, and agarose gel electrophoresis.
Bacterial DNA was extracted using aerosol-resistant tips and following the cetyltrimethylammonium bromide minipreparation method described by Wilson (39). Cetyltrimethylammonium bromide-purified DNAs were restricted separately with BamHI and HindIII (New England Biolabs, Beverly, Mass.) in accordance with the manufacturer's instructions. Restriction DNA fragments were separated in a 1% TAE agarose gel (23) at 1.5 V/cm for 20 h. After electrophoresis, the gel was stained in a 0.5-μg/ml ethidium bromide bath for 30 min. DNA was illuminated with UV light and photographed.
CHEF gel electrophoresis.
DNA for contour-clamped homogeneous electric field (CHEF) gel electrophoresis was prepared by a modification of the method of Cooksey and Graham (5). Bacterial cells were washed with SE buffer (75 mM NaCl, 25 mM EDTA, pH 7.5), and the cell density was adjusted to ∼108 CFU/ml in SE buffer. A 0.5-ml sample of the bacterial suspension was mixed with 0.5 ml of 2% agarose heated to 60°C (Pulsed Field Certified Agarose; Bio-Rad, Richmond, Calif.) in 10 mM Tris-HCl–10 mM MgCl2–0.1 mM EDTA (pH 7.5). Plugs were cast in plug molds (1.0 by 0.5 cm; Bio-Rad) and held at 4°C for 5 min to allow the agarose to solidify. Ten plugs were placed in 5 ml of lysis solution consisting of proteinase K (0.5 mg/ml; Bethesda Research Laboratories, Gaithersburg, Md.), 1% N-lauroylsarcosine, and 0.5 M EDTA (pH 9.5). After overnight incubation at 50°C, the plugs were washed with gentle agitation four times for 1 h each time with 20 mM Tris-HCl (pH 8.0)–50 mM EDTA at room temperature. The plugs then were washed five times with TE (10 mM Tris, 10 mM EDTA, pH 8.0) at room temperature with a 10-min wash cycle. Prior to restriction digestion, the plugs were equilibrated in 1× restriction buffer A (Boehringer Mannheim, Indianapolis, Ind.) at room temperature for 1 h. DNA restrictions were carried out in 1× restriction buffer A–1.0 mg of bovine serum albumin per ml–10 U of ApaI (Boehringer Mannheim) overnight at 37°C. CHEF gel electrophoresis of undigested and ApaI-restricted DNA was carried out in a 1% agarose gel (13 by 14 cm) prepared with 0.5× TBE buffer (23). Electrophoresis of ApaI-digested DNA was carried out in 0.5× TBE at 14°C for 18 h at 6 V/cm with a 1- to 30-s switch time ramp at an included angle of 120° using a CHEF-DRIII pulsed-field electrophoresis system (Bio-Rad). Electrophoretic conditions for undigested DNA were the same, except that the switch time ramp was 0.5 to 3.5 s for 16 h. Agarose plugs containing concatemers of lambda DNA (Bio-Rad) were used as molecular weight standards. The gel was stained and photographed as described above.
Southern blotting and hybridization.
Restricted fragments were transferred by capillary action to Magnagraph nylon membranes (MSI, Westborough, Mass.) by Southern blotting as described previously (23). After transfer, DNA was UV cross linked to the membrane using a Stratalinker (Stratagene, La Jolla, Calif.). The probe was prepared by restricting total DNA of strain Co53 with HindIII and labeling the fragments with digoxigenin using the Genius DNA labeling kit as described by the manufacturer (Boehringer Mannheim). Southern hybridization and detection of DNA which hybridized to the probe was carried out using the Genius detection kit (Boehringer Mannheim).
Amplification and restriction polymorphism of the 16S rRNA region.
The 16S rRNA region of all isolates was amplified using primers fD3 and T50 (26). The DNA Thermal Cycler (Perkin-Elmer) was used with 1 cycle of 1 min at 94°C followed by 35 cycles of 1 min at 94°C, 1 min at 54°C, and 1 min at 72°C and a final extension cycle of 10 min at 72°C.
Ten microliters of the amplification products was restricted separately with BfaI, HphI, and MseI (New England Biolabs, Beverly, Mass.). Restriction fragments were resolved in a 2.5% NuSieve GTG agarose gel (FMC BioProducts, Rockland, Maine) with 1× TBE (23) at 5 V/cm for 3.5 h.
Sequencing of the 16S rRNA region.
The 16S rRNA amplification products of all isolates were purified using Millipore ultrafree tubes (Millipore, Bedford, Mass.). Both DNA strands were sequenced by cycle sequencing using the Terminator Ready Reaction Kit (Perkin-Elmer/Applied Biosystems, Foster City, Calif.) as recommended by the manufacturer. Primers fD3, 400F, 500R, 800F, 800R, 1200F, 1200R, and T50 (26) (Table 1) were used for sequencing. Amplification using primers 800F and 800R was carried out with 1 cycle of 96°C for 2 min and 25 cycles of 96°C for 30s, 48°C for 30s, and 60°C for 4 min. For primers fD3, 400F, 500R, 1200F, 1200R, and T50, the cycling parameters were the same except that an annealing temperature of 52°C was used. Sequencing products were electrophoresed and scanned using an ABI 377 automated sequencer (Perkin-Elmer/Applied Biosystems). Sequencher software (Version 3.0; Gene Codes Corp., Ann Arbor, Mich.) was used to align and analyze the DNA sequences.
TABLE 1.
Primers used for PCR amplification and sequencing of the 16S rRNA region (26)
| Primer | Sequence (5′-3′) | Position (E. coli numbering) |
|---|---|---|
| fD3 | AGAGTTTGATCCTGGCTTAG | 8–27 |
| T50 | GTTACGACTTCACCCTCCT | 1540–1522 |
| 400F | GGAGCGACACTGCGTG | 414–429 |
| 500R | CTGCTGGCACGTAATTAGCC | 548–529 |
| 800F | ATTAGATACCCTGGTAG | 812–828 |
| 800R | CTACCAGGGTATCTAAT | 828–812 |
| 1200F | TATGTCCTGGGCTACACACG | 1248–1267 |
| 1200R | CGTGTGTAGCCCAGGACATA | 1267–1248 |
Amplification for the specific detection of relapsing-fever borreliae and of the rrf (5S)-rrl (23S) intergenic spacer region.
Primers REC4 and REC9 were used as described by Marti Ras et al. (26) for the identification of relapsing-fever borrelial isolates and B. coriaceae. To determine whether isolates carried tandemly duplicated 5S (rrf)-23S (rrl) rRNA regions as found among strains of B. burgdorferi sensu lato (s.l.), amplification of the rrf-rrl intergenic region was carried out as described previously (31).
Nucleotide sequence accession numbers.
The sequences of isolates CA434, CA435, Co53, B. parkeri, and CA4 have been submitted to GenBank. Their accession numbers are AF210134 to AF210138, respectively.
RESULTS
Tick studies.
A total of 193 nymphs and adults of O. coriaceus were collected from chaparral and woodland-grass plant communities between 26 July and 22 September 1995. Seventy-one late-instar nymphs or adults were examined for the presence of spirochetes by DI and culture techniques. Spirochetes were detected, isolated, or both in 1 (3.0%) of 33 nymphs, 3 (18.8%) of 16 females, and 5 (22.7%) of 22 males. Spirochetes were detected in only four of the nine positive ticks by DI, two of which had disseminated infections involving two or three tissue types (Table 2). In contrast, spirochetes were isolated in BSK-II medium from six ticks at 30 or 36°C or at both temperatures. An isolate from a male tick (no. 6) was lost during cultivation and before aliquots could be frozen. Three of the four DI assay-positive ticks (no. 7 to 9) did not yield borrelial isolates at either temperature, and both isolates characterized in the present study were DI test negative (Table 2).
TABLE 2.
Distribution of B. coriaceae in tissuesa of nymphal and adult O. coriaceus ticks and results of isolation attemptsb at two temperatures
| Specimen no. and stagec | Spirochetal infection
|
Result of isolation attempt at:
|
Isolate no. | |||
|---|---|---|---|---|---|---|
| Central ganglion | Midgut | Salivary gland | 30°C | 36°C | ||
| 1F | − | − | − | + | + | CA432 |
| 2M | − | − | − | − | + | CA433 |
| 3F | − | − | − | + | + | CA434 |
| 4M | − | − | − | − | + | CA435 |
| 5F | + | + | + | + | − | CA436 |
| 6M | − | − | − | + | − | −d |
| 7M | + | − | − | − | − | − |
| 8M | − | + | − | − | − | − |
| 9N | + | − | + | − | − | − |
Determined by DI examination of tissue smears.
In BSK-II medium.
N, nymph; F, female; M, male.
Isolate lost before culture stocks could be prepared.
Attempts to regrow the five remaining isolates from the frozen stock cultures about 2.5 years later in order to characterize them met with partial success. Two isolates (CA434 and CA435) were successfully recultured in BSK-H medium but not in BSK-II medium.
Agarose gel electrophoresis of total DNA and Southern hybridization.
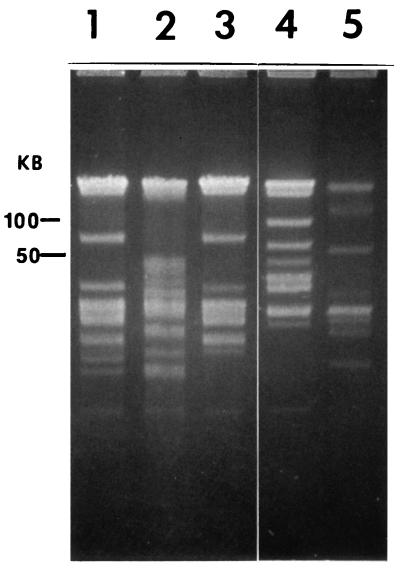
CHEF gel electrophoresis of undigested DNA revealed a number of linear plasmids in all of the isolates (Fig. 1). Except for two linear plasmid bands that were absent from Co53, the plasmid profiles of CA434 and Co53 were identical (Fig. 1, lanes 1 and 3). All of the other isolates (CA435, B. parkeri, and CA4) had unique plasmid profiles. Gel electrophoresis of BamHI-, HindIII-, and ApaI-digested (results not shown) DNA indicated that all of the isolates had unique restriction fingerprints, with the exception of CA434 and Co53, which were similar.
FIG. 1.
CHEF gel electrophoresis of undigested DNAs of Borrelia isolates. Lanes: 1, CA434; 2, CA435; 3, Co53; 4, B. parkeri; 5, CA4. Linear plasmid DNA was resolved in 1% agarose–0.5× TBE (23) at 14°C for 16 h at 6 V/cm with a 0.5- to 3.5-s switch time ramp using a CHEF-DRIII pulsed-field electrophoresis system.
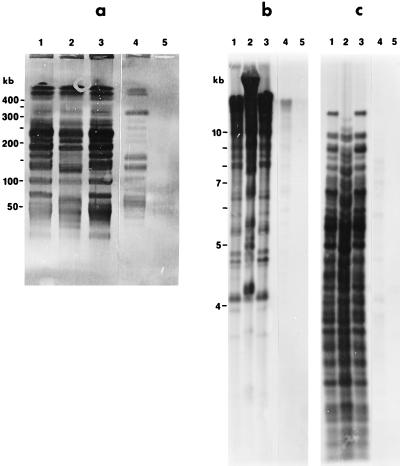
Southern hybridization using Co53 total DNA as a probe demonstrated that the DNAs of CA434 and CA435 shared strong homology with the DNA of Co53. A low degree of homology was observed with B. parkeri, as indicated by fainter bands (Fig. 2), and no homology was observed with CA4.
FIG. 2.
Southern hybridization of ApaI (a)-, BamHI (b)-, and HindIII (c)-digested DNAs of Borrelia isolates with digoxigenin-labeled Co53 DNA as a probe. Lanes: 1, CA434; 2, CA435; 3, Co53; 4, B. parkeri; 5, CA4. Southern hybridization and detection of DNA which hybridized to the probe were done as recommended by Boehringer Mannheim for the digoxigenin DNA labeling and detection kit.
Restriction fragment length polymorphism analysis of the 16S rRNA region.
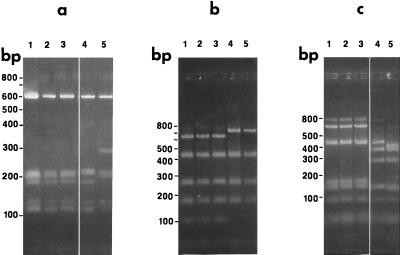
The 16S rRNA regions of isolates restricted with BfaI, HphI, and MseI are shown in Fig. 3. Isolates CA434, CA435, and Co53 had identical BfaI, HphI, and MseI restriction patterns, and they were identical to that previously reported for strain Co53 (26). The restriction patterns of isolate B. parkeri and B. parkeri strain M3001 were identical (26). The BfaI restriction pattern of B. parkeri (undesignated) resembled that of CA4.
FIG. 3.
16S rRNA amplification products restricted with HphI (a), BfaI (b), and MseI (c). Lanes: 1, CA434; 2, CA435; 3, Co53; 4, B. parkeri; 5, CA4. Restriction fragments were resolved in 2.5% NuSieve-GTG agarose–1× TBE at 5 V/cm for 3.5 h.
Sequence analysis of the 16S rRNA gene.
Approximately 1,450 bases of the 16S rRNA gene of all isolates were sequenced. The sequences of isolates CA434, CA435, and Co53 were identical. These sequences were found to differ slightly from the previously reported 16S rRNA sequence of B. coriaceae Co53 (GenBank accession number U42286) by the addition of one base (cytosine) at position 367. The sequence of B. parkeri was identical to that of B. parkeri strain M3001 (GenBank accession number U42296).
Amplification of a region specific to relapsing-fever spirochetes and the rrf(5S)-rrl(23S) intergenic spacer region.
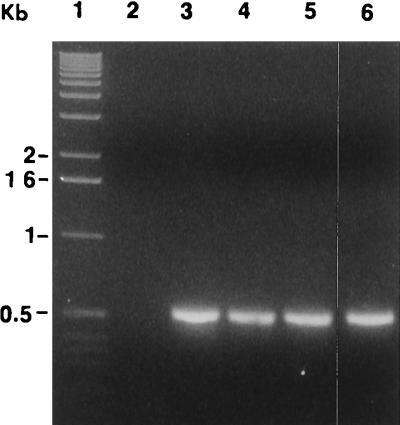
Primers REC4 and REC9 (26) were used to amplify a region in the 16S rRNA gene specific to relapsing-fever spirochetes. A 523-bp amplification product was synthesized from the genomic DNAs of isolates CA434, CA435, Co53, and B. parkeri (Fig. 4). No amplification product was present for isolate CA4.
FIG. 4.
Amplification products of Borrelia isolates using primers REC4 and REC9. Lanes: 1, molecular size marker ladder; 2, CA4; 3, CA434; 4, CA435; 5, Co53; 6, B. parkeri. Amplification products were electrophoresed in 1.4% agarose–1× TAE at 5 V/cm for 3 h.
An amplification product of approximately 250 bp was obtained for isolate CA4 using rrf-rrl primers 1 and 2 (31), whereas no amplification products were detected for isolates CA434, CA435, Co53, or B. parkeri (data not shown).
DISCUSSION
Molecular characteristics identify isolates CA434 and CA435 from the soft tick O. coriaceus as B. coriaceae. This is based on the 99.99% homology between the 16S rRNA gene sequences of CA434 and CA435 and that of the Co53 type strain of B. coriaceae (GenBank accession number U42286) (26). The use of the 16S rRNA gene locus to identify and confirm various bacterial groups, including the genus Borrelia, is well documented (1, 24, 40). Southern hybridization showed that Co53, CA434, and CA435 share DNA homology. Moreover, an amplification product was synthesized from the genomic DNAs of isolates CA434, CA435, and Co53 in PCRs with the REC4 and REC9 primers, the sequences of which are unique to the relapsing-fever spirochetes and B. coriaceae (26). An rrf-rrl (5S-23S rRNA) intergenic spacer region was absent from isolates CA434, CA435, and Co53, which indicates the presence of only one copy of the 5S-23S rRNA gene. In this respect, isolates CA434 and CA435 differ from the various genospecies of B. burgdorferi s.l., which possess a unique arrangement of ribosomal genes with one copy of the 16S (rrs) rRNA gene and two copies each of the 5S (rrf)-23S (rrl) rRNA genes (36). This kind of organization is not found in other members of the genus Borrelia or in other eubacteria (7).
The ApaI, BamHI, and HindIII restriction digestion patterns of the total DNAs of isolates Co53 and CA434 were similar but distinct from that of CA435. CHEF electrophoresis of undigested linear plasmid DNA (6) revealed that isolate CA434 carried the same-size linear plasmids as Co53, except that two plasmids were absent from Co53. It is possible that the two plasmids missing in Co53 were lost during prolonged laboratory cultivation, which occurs commonly among B. burgdorferi isolates subjected to many laboratory passages (35).
The fact that isolate CA435 differs from Co53 and CA434 indicates that genetic variability exists among B. coriaceae strains. In this regard only, B. coriaceae resembles B. burgdorferi s.l., in which considerable genetic heterogeneity has been observed with various techniques (20, 27, 34, 35). Further, our findings concur with those of LeFebvre and Perng (19), who found that Co53 shares very little DNA homology with genomes of the B. burgdorferi s.l. complex, which is not unexpected because of the similarity of Co53 to the relapsing-fever spirochetes (26). We compared all three known B. coriaceae isolates with the relapsing-fever spirochete B. parkeri because of their genetic relatedness (26) and with the Lyme disease spirochete B. burgdorferi s.s. because the geographical distributions of their primary tick vectors overlap in California (10).
CA434 and CA435 represent only the second and third isolates of B. coriaceae since this spirochete was first isolated and characterized over a decade ago (14, 15). Both isolates were obtained from the same species of human-biting soft tick and at the same locality in northwestern California as strain Co53. Repeated attempts to cultivate B. coriaceae-like spirochetes from DI test-positive O. coriaceus ticks collected in the Hopland area since the mid-1980s were unsuccessful (R. S. Lane, unpublished data) until 1995. The fact that only two of five borrelial isolates obtained from O. coriaceus ticks in 1995 could be regrown less than 3 years later from frozen stock cultures, and then only in BSK-H medium, exemplifies the difficulties encountered in culturing this organism. Our inability to routinely cultivate B. coriaceae from the tissues of spirochete-infected O. coriaceus ticks in BSK-II medium suggests either that this spirochete is a more fastidious organism than B. burgdorferi or that it has different maintenance and growth requirements.
Two methods, DI assay and culture, were used to detect spirochetes in O. coriaceus tissues, but the concordance between these methods was poor (Table 2). In fact, only one of six ticks that initially yielded an isolate was also DI test positive and, conversely, just one of four DI test-positive ticks produced an isolate. Furthermore, spirochetes from four of the six original isolates cultivated in BSK-II medium grew at either 30 or 36°C but not at both temperatures. In striking contrast, the culture-adapted B-31 type strain of B. burgdorferi s.s. grows well, albeit at variable rates, in BSK-H medium at temperatures ranging between 25 and 37°C (12). We tried to cultivate spirochetes from tick tissues at both 30 and 36°C in the present study because of the minimal previous success achieved in growing B. coriaceae from DI test-positive ticks in BSK-II medium at ∼35°C (15, Lane, unpublished data). Our data demonstrate the value of using more than one method to detect spirochetes when determining infection prevalences in tick populations. They also suggest that reliance upon a single detection method or the use of a single temperature regimen or type of liquid growth medium during efforts to isolate B. coriaceae may result in a gross underestimate of the true infection prevalence.
Despite the fact that B. coriaceae was known from a single isolate before the present study, researchers have investigated many aspects of the biology of this poorly known spirochete in relation to other spirochetes, including B. burgdorferi. For example, its growth in BSK-H culture medium (30), phylogenetic relatedness to other Borrelia spp. (8, 9, 26), proteins (3, 11, 13, 25, 32, 33), and enzyme activity (22) have been studied just since 1993. B. coriaceae spirochetes also have been shown to be of low infectivity for infant Swiss White mice, infant BALB/c mice, and adult New Zealand White rabbits (15). The availability of two additional low-passage isolates of B. coriaceae provides the scientific community with an opportunity to expand these studies and perhaps to determine definitively whether B. coriaceae is somehow involved in the etiology of EBA in the far-western United States.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant AI22501 from the National Institutes of Health and by Cooperative Agreement U50/CCU906594 from the Centers for Disease Control and Prevention to R.S.L.
We thank J. E. Kleinjan, K. A. Padgett, and May M. Kuo for technical assistance; M. E. Peot for technical assistance and for reviewing an early draft of the manuscript; and T. G. Schwan for kindly supplying the B. parkeri isolate used in this study.
REFERENCES
- 1.Adam T, Graf B, Neubert U, Gobel U B. Detection and classification of Borrelia burgdorferi by direct sequencing of 16S rRNA amplified after reverse transcription. Med Microbiol Lett. 1992;1:120–126. [Google Scholar]
- 2.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 3.Bunikis J, Luke C J, Bunikiene E, Bergstrom S, Barbour A G. A surface-exposed region of a novel outer membrane protein (P66) of Borrelia spp. is variable in size and sequence. J Bacteriol. 1998;180:1618–1623. doi: 10.1128/jb.180.7.1618-1623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgdorfer W, Lane R S, Barbour A G, Gresbrink R A, Anderson J R. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am J Trop Med Hyg. 1985;34:925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- 5.Cooksey D A, Graham J H. Genomic fingerprinting of two pathovars of phytopathogenic bacteria by rare-cutting restriction enzymes and field inversion gel electrophoresis. Phytopathology. 1989;79:745–750. [Google Scholar]
- 6.Ferdows M S, Barbour A G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci USA. 1989;86:5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukunaga M, Sohnaka M. Tandem repeat of the 23S and 5S ribosomal RNA genes in Borrelia burgdorferi, the etiological agent of Lyme disease. Biochem Biophys Res Commun. 1992;183:952–957. doi: 10.1016/s0006-291x(05)80282-7. [DOI] [PubMed] [Google Scholar]
- 8.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 9.Fukunaga M, Okada K, Nakao M, Konishi T, Sato Y. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int J Syst Bacteriol. 1996;46:898–905. doi: 10.1099/00207713-46-4-898. [DOI] [PubMed] [Google Scholar]
- 10.Furman D P, Loomis E C. The ticks of California (Acari: Ixodida) Bull Calif Insect Surv. 1984;25:1–239. [Google Scholar]
- 11.Gilmore R D, Jr, Kappel K J, Johnson B J B. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heroldova M, Nemec M, Hubalek Z. Growth parameters of Borrelia burgdorferi sensu stricto at various temperatures. Zentbl Bakteriol. 1998;288:451–455. doi: 10.1016/s0934-8840(98)80058-3. [DOI] [PubMed] [Google Scholar]
- 13.Hu L T, Pratt S D, Perides G, Katz L, Rogers R A, Klempner M S. Isolation, cloning, and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect Immun. 1997;65:4989–4995. doi: 10.1128/iai.65.12.4989-4995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R C, Burgdorfer W, Lane R S, Barbour A G, Hayes S F, Hyde F W. Borrelia coriaceae sp. nov.: putative agent of epizootic bovine abortion. Int J Syst Bacteriol. 1987;37:72–74. [Google Scholar]
- 15.Lane R S, Burgdorfer W, Hayes S F, Barbour A G. Isolation of a spirochete from the soft tick, Ornithodoros coriaceus: a possible agent of epizootic bovine abortion. Science. 1985;230:85–87. doi: 10.1126/science.3898367. [DOI] [PubMed] [Google Scholar]
- 16.Lane R S, Burgdorfer W. Spirochetes in mammals and ticks (Acari: Ixodidae) from a focus of Lyme borreliosis in California. J Wildl Dis. 1988;24:1–9. doi: 10.7589/0090-3558-24.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Lane R S, Manweiler S A. Borrelia coriaceae in its tick vector, Ornithodoros coriaceus (Acari: Argasidae), with emphasis on transstadial and transovarial infection. J Med Entomol. 1988;25:172–177. doi: 10.1093/jmedent/25.3.172. [DOI] [PubMed] [Google Scholar]
- 18.Lane R S, Pascocello J A. Antigenic characteristics of Borrelia burgdorferi isolates from ixodid ticks in California. J Clin Microbiol. 1989;27:2344–2349. doi: 10.1128/jcm.27.10.2344-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeFebvre R B, Perng G-C. Genetic and antigenic characterization of Borrelia coriaceae, putative agent of epizootic bovine abortion. J Clin Microbiol. 1989;27:389–393. doi: 10.1128/jcm.27.3.389-393.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeFebvre R B, Perng G-C, Johnson R C. Characterization of Borrelia burgdorferi isolates by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol. 1989;27:636–639. doi: 10.1128/jcm.27.4.636-639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeFebvre R B, Lane R S, Perng G-C, Brown J A, Johnson R C. DNA and protein analyses of tick-derived isolates of Borrelia burgdorferi from California. J Clin Microbiol. 1990;28:700–707. doi: 10.1128/jcm.28.4.700-707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manafi M, Stanek G. Enzyme activities of Lyme disease and relapsing fever borreliae. Lett Appl Microbiol. 1994;19:149–152. [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 24.Marconi R T, Garon C F. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. J Gen Microbiol. 1992;138:533–536. doi: 10.1099/00221287-138-3-533. [DOI] [PubMed] [Google Scholar]
- 25.Marconi R T, Samuels D S, Schwan T G, Garon C F. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993;31:2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marti Ras N, Lascola B, Postic D, Cutler S J, Rodhain F, Baranton G, Raoult D. Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol. 1996;46:859–865. doi: 10.1099/00207713-46-4-859. [DOI] [PubMed] [Google Scholar]
- 27.Mathiesen D A, Oliver J H, Jr, Kolbert C P, Tullson E D, Johnson B J B, Campbell G L, Mitchell P D, Reed K D, Telford III S R, Anderson J F, Lane R S, Persing D H. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 28.Osebold J W, Spezialetti R, Jennings M B, Pritchett R F, Bushnell R B. Congenital spirochetosis in calves: association with epizootic bovine abortion. J Am Vet Med Assoc. 1986;188:371–376. [PubMed] [Google Scholar]
- 29.Osebold J W, Osburn B I, Spezialetti R, Bushnell R B, Stott J L. Histopathologic changes in bovine fetuses after repeated reintroduction of a spirochete-like agent into pregnant heifers: association with epizootic bovine abortion. Am J Vet Res. 1987;48:627–633. [PubMed] [Google Scholar]
- 30.Pollack R J, Telford III S R, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Postic D, Assous M V, Grimont P A D, Baranton G. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int J Syst Bacteriol. 1994;44:743–752. doi: 10.1099/00207713-44-4-743. [DOI] [PubMed] [Google Scholar]
- 32.Sambri V, Massaria F, Ardizzoni M, Stefanelli C, Cevenini R. Glycoprotein patterns in Borrelia spp. Zentbl Bakteriol. 1993;279:330–335. doi: 10.1016/s0934-8840(11)80365-8. [DOI] [PubMed] [Google Scholar]
- 33.Sambri V, Stefanelli C, Rossoni C, La Placa M, Cevenini R. Acylated proteins in Borrelia hermsii, Borrelia parkeri, Borrelia anserina, and Borrelia coriaceae. Appl Environ Microbiol. 1993;59:3938–3940. doi: 10.1128/aem.59.11.3938-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid G P, Steigerwalt A G, Johnson S E, Barbour A G, Steere A C, Robinson I M, Brenner D J. DNA characterization of the spirochete that causes Lyme disease. J Clin Microbiol. 1984;20:155–158. doi: 10.1128/jcm.20.2.155-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwan T G, Burgdorfer W, Garon C F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz J J, Gazumyan A, Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992;174:3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spezialetti R, Osebold J W. Lymphocyte blastogenesis and cellular cytotoxicity in a congenital infection of bovine fetuses related to epizootic bovine abortion. Res Vet Sci. 1989;46:160–167. [PubMed] [Google Scholar]
- 38.Spezialetti R, Osebold J W. Surface markers on bovine fetal lymphocytes and immunoglobulin synthesis in a congenital infection related to epizootic bovine abortion. Res Vet Sci. 1991;51:239–245. doi: 10.1016/0034-5288(91)90070-5. [DOI] [PubMed] [Google Scholar]
- 39.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates & Wiley-Interscience; 1987. pp. 2.4.1–2.4.2. [Google Scholar]
- 40.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zingg B C, LeFebvre R B. Polymerase chain reaction for detection of Borrelia coriaceae, putative agent of epizootic bovine abortion. Am J Vet Res. 1994;55:1509–1515. [PubMed] [Google Scholar]