Abstract
Simple Summary
Cell–cell communication is an important mechanism in biological processes. Extracellular vesicles (EVs), also referred to as exosomes, microvesicles, and prostasomes, are microvesicles secreted from a variety of cells. Importantly, EVs contribute to cancer malignancy mechanisms such as carcinogenesis, proliferation, angiogenesis, metastasis, and escape from the immune system. As EVs are thought to be secreted into body fluids, they have the potential to serve as diagnostic markers for liquid biopsy. In addition, the characteristics of EVs make them suitable for use in drug delivery systems and novel cancer treatments. In this review, the potential of EVs as anti-cancer therapeutics is discussed.
Abstract
Cell–cell communication is an important mechanism in biological processes. Extracellular vesicles (EVs), also referred to as exosomes, microvesicles, and prostasomes, are microvesicles secreted by a variety of cells. EVs are nanometer-scale vesicles composed of a lipid bilayer and contain biological functional molecules, such as microRNAs (miRNAs), mRNAs, and proteins. In this review, “EVs” is used as a comprehensive term for vesicles that are secreted from cells. EV research has been developing over the last four decades. Many studies have suggested that EVs play a crucial role in cell–cell communication. Importantly, EVs contribute to cancer malignancy mechanisms such as carcinogenesis, proliferation, angiogenesis, metastasis, and escape from the immune system. EVs derived from cancer cells and their microenvironments are diverse, change in nature depending on the condition. As EVs are thought to be secreted into body fluids, they have the potential to serve as diagnostic markers for liquid biopsy. In addition, cells can encapsulate functional molecules in EVs. Hence, the characteristics of EVs make them suitable for use in drug delivery systems and novel cancer treatments. In this review, the potential of EVs as anti-cancer therapeutics is discussed.
Keywords: cancer, extracellular vesicles, exosome, cancer therapy
1. Introduction
Extracellular vesicles (EVs), which also go by exosomes, microvesicles, and prostasomes, are secreted from a variety of cells [1,2] (Table 1). EVs are nanometer-scale vesicles composed of a lipid bilayer and contain biologically functional molecules, such as microRNAs (miRNAs), mRNAs, and proteins [3]. In recent years, EVs have been recognized as a cell–cell communication tool. The basic idea of cell–cell communication using EVs is that EVs secreted from donor cells are taken up by recipient cells in a paracrine or autocrine manner. EVs modify the condition of recipient cells by the biological molecules contained within them. The history of EVs dates back approximately 40 years, when Hans Lutz et al. reported the release of vesicles from old sheep erythrocytes [4]. Ronquist reported a functional fraction in the supernatant of prostatic fluid in the same year [5]. It has been reported that red blood cells secrete vesicles containing proteins and lipids during maturation [6,7,8]. Some research groups have reported that the transferrin receptor is internalized in vesicles made by multivesicular bodies [9,10,11]. However, in 1991, Johnstone et al. concluded that vesicles secreted from cells were “a garbage bin” for unnecessary membrane proteins [12]. Importantly, the vesicles secreted from cells contain mRNA and miRNA, which can be transferred to other cells and be functional in them [13]. Vesicles secreted from cells were removed from the garbage bin and moved into the limelight as a new cell–cell communication tool.
Table 1.
The features of extracellular vesicles.
| Extracellular Vesicles (EVs) | |||
|---|---|---|---|
| Terminology | Exosomes | Microvesicles | Apoptotic Bodies |
| Origin | Multivesicle body | Plasma membrane | Plasma membrane |
| Size | 50–150 nm | 100–1000 nm | 100–5000 nm |
| Marker proteins | CD9, CD63, Tsg101 etc. | Integrins, Selectins, CD40 etc. | Annexin V, thrombospondin, C3b etc. |
| References | [1,3] | [1,23] | [1,24] |
Until the establishment of the International Society of Extracellular Vesicles (ISEV) in 2011, researchers used different names for the vesicles secreted from cells. Hence, to avoid confusion in nomenclature, ISEV encouraged the use of the term “extracellular vesicles (EVs)” for all vesicles secreted from cells [14,15] and offered three other suggestions for nomenclature in their article: (1) State their use of terms explicitly, (2) clearly state their methods, and (3) respect scientific freedom to choose of the nomenclature.
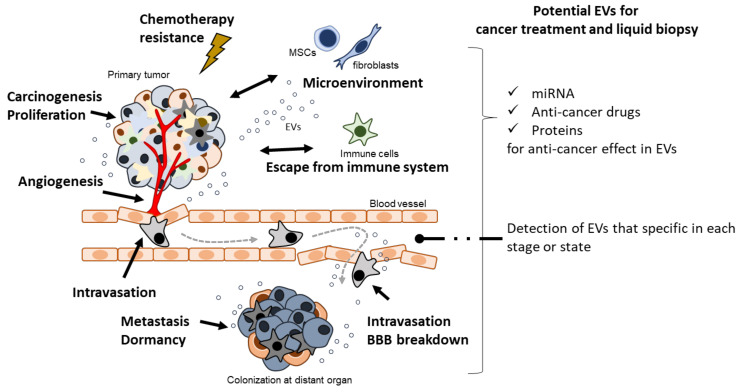
In the past two decades, EVs have been shown to play a crucial role in cancer biology. Accumulating evidence indicates the importance of cell–cell communication through EVs in cancer malignancy mechanisms, such as cancer cell proliferation [16], immune modulation [17], angiogenesis [18], metastasis [19], and pro-metastasis niche formation [20] (Figure 1). Importantly, in the early history of EV research, EVs derived from dendritic cells (DCs) pulsed with tumor peptides were proposed as a cell-free vaccine method [21]. Furthermore, a potential cancer therapeutic strategy based on the suppression of cancer metastasis via the removal of EVs that contribute to cancer malignancy has been reported [22]. Herein, we summarize and discuss the importance of EVs in cancer biology (summarized in Table 2), as well as their anti-cancer role and therapeutic potential (summarized in Table 3).
Figure 1.
EVs contribute to cancer malignancy and they have an emerging role of therapeutic potential in cancer malignancy.
Table 2.
Cancer malignancy-related EV contents.
| Proteins | miRNAs | lncRNAs | Other | |
|---|---|---|---|---|
| Carcinogenesis | let-7 [25] | EBER2 [26] | ||
| miR-23a, miR-155 [27] | ||||
| Proliferation | CLIC1 [28] | miR-410 [29] | TU399 [30] | |
| EphA2 [31] | miR-142-3p [32] | lncRNA-VLDLR [33] | ||
| L1CAM [34] | miR-95 [35] | lncRNA-H19 [36] | ||
| ZIP4 [37] | miR-30e [38] | EWSAT1 [39] | ||
| hypoxia-induced miR-155 [40] | ||||
| miR-365 [41] | ||||
| miR-130b-3p [42] | ||||
| miR-497 [43] | ||||
| Angiogenesis | Rac1, PAK2 [44] | miR-584-5p [45] | ||
| VEGF90K [46] | miR-23b, miR-320b [47] | |||
| angiopoietin-2 [48] | miR-23a [49] | |||
| laminin γ2 [50] | miR143-3p, miR145-5p [51] | |||
| miR-141-3p [52] | ||||
| miR-81b-5p [53] | ||||
| miR-4488 [54] | ||||
| miR-10a-5p [55] | ||||
| le-7b-5p [56] | ||||
| Metastasis | TTLL4 [57] | miR-370-3p [58] | HLA-F-AS1 [59] | orphan RNA [60] |
| PKM2 [61] | miR-181c [19] | HUCL [62] | ||
| miR-30a-3p [63] | ||||
| miR-185-2p [64] | ||||
| MSC-miR222 [65] | ||||
| miR-30e [38] | ||||
| miR-23b [66] | ||||
| miR-193a [67] | ||||
| miR-622 [62] | ||||
| miR-224-5p [68] | ||||
| Escape from immune system | PD-1 [69,70] | miR-222 [65] | ||
| Chemotherapeutic stress | ALK [71] | miR-30b-3p [72] | H19 [73] | |
| Vasconcelos, Chitinase 3-like-1 and fibronectin [74] | VLDLR [33,75] | |||
| HOTPIT [76] | ||||
| RP11-838N2.4 [77] | ||||
| PART1 [78] | ||||
| SNHG14 [79] |
Table 3.
Liquid biopsy and cancer treatment-related EV contents.
| Proteins | miRNAs | lncRNAs | Other | |
|---|---|---|---|---|
| Liquid biopsy | FABp5 [80] | miR-21, miR-375, miR-204 [81] | - | cell-free DNA [82,83] |
| Androgen-receptor splice vairiant 7 [84] | miR-221-3p, miR-222-3p, miR-31-5p [85] | |||
| Lipocalin-2 [86] | miR-375, miR-200c-3p, miR-21-5p [87] | |||
| UCHL1 [88] | miR-200 [86] | |||
| GPC1 [89,90] | miR-505-5p [91] | |||
| mucins, CFTR, MDR1 [92,93] | miR-193a-5p, miR-551b-5p [94] | |||
| ZIP4 [37] | miR-133b [95] | |||
| CKAP4, DKK1 receptor [96] | miR-150-3p [97] | |||
| Annexin A1 [98] | ||||
| Cancer treatment | anti-CD63 antibody, anti-CD9 antibody [22] | miR-134 [99] | - | - |
| CD9 Fab fragment [100] | miR-355-5p [101] | |||
| cell-free vaccine [21,102] | miR-124, miR-128, and miR137 [103] | |||
| mir-1252-5p [104] | ||||
| miR-320a [105] | ||||
| miR-375 [106] | ||||
| miR-424 [107] | ||||
| miR-203 [108] | ||||
| miR-30a [109] | ||||
| miR199a-3p [110] | ||||
| miR-21-sponge construct [111] | ||||
| miR-206 [112] | ||||
| miR-193a [67] | ||||
| miR-144-3p [113] | ||||
| miR-125b [114] | ||||
| mi-185 [115] | ||||
| miR-16-5p [116] |
2. Carcinogenesis
Cancer cells emerge from cells damaged by various factors, such as inflammation, chemical stress, radiation, oxidative stress, and aging [117]. These stresses, especially aging, affect the formation of malignant tumors through the accumulation of genetic and epigenetic changes in genes. It is likely that there is a relationship between these causes of carcinogenesis. Chronic inflammation, such as “inflammaging,” is a risk factor for carcinogenesis, and is caused by cytokines and chemokines [118]. It is predicted that elderly people probably have chronic inflammation without infection caused by senescent cells. The senescence-associated secretory phenotype is a feature of senescent cells that leads to chronic inflammation in elderly people, with factors such as interleukin (IL) -6, and IL-8 secreted by senescent cells [119]. EVs are also secreted from senescent cells, and they may exert detrimental effects [120,121]. Accumulating evidence suggests that EVs contribute to carcinogenesis or precancerous conditions, such as inflammation [26,122,123,124], fibrosis [25,125,126], double-strand breaks in DNA [27,126], and endoplasmic reticulum (ER) stress [127].
Bladder cancer cell-derived EVs induce neoplastic transformation of nonmalignant cells through the induction of the unfolded protein response in the ER [127]. EVs have been suggested to affect tumor recurrence and the potential for carcinogenesis. The Epstein–Barr virus M81-infected B cells release EVs that contain non-coding Epstein–Barr virus-encoded RNA, which are then taken up by B cells [26], which results in chronic inflammation. Inflammation and carcinogenesis caused by viral infection may be linked. EVs derived from macrophages have been reported to upregulate TBC1 domain family member 3 by downregulating stanniocalcin-1-mediated inflammation [122]. A relationship between cholangiocarcinoma and liver fluke infection has been suggested. IL-6 secretion from cholangiocytes is upregulated, and cholangiocytes proliferate after uptake of EVs derived from the liver fluke Opisthorchis viverrini [123]. EVs derived from neutrophils, such as miR-23a and miR-155, that infiltrate injured tissue have contributed to double-stranded breaks in DNA [27] increasing inflammation, replication stress, and genomic instability after up taken by surrounding cells. Fibrosis is also known as a precancerous condition [125]. Interestingly, it has been reported that osteosarcoma-derived EVs promote proliferation, migration, adhesion, and sphere formation through MMP-9, TNF-α, IL-6, and transforming growth factor (TGF)-β mRNA expression [128]. EVs may contribute to carcinogenesis by inducing inflammation.
3. Proliferation
“Enabling replicative immortality” is a hallmark of cancer [129]. HeLa cells were established in 1953 and contribute greatly to cancer research because of their proliferation on a dish [130]. It is well known that activation of telomerase is one of the causes of proliferation, as it is not subject to the Hayflick limit. Uncontrolled proliferation is a fundamental feature of cancer that leads to gene mutations, metabolic changes, and epigenetic alterations possibly resulting in malignancy. Cancer cell-derived EVs contribute to cell proliferation and growth [131,132,133,134,135].
EVs from mesenchymal stem cells (MSCs) play a dual role in cancer biology. They exhibit potential as anti-cancer treatments but also contribute to cancer malignancy [131,136]. Importantly, MSCs are educated by cancer-derived EVs to contribute to cancer malignancy. It has been reported that EVs derived from cholangiocarcinoma-educated bone marrow MSCs enhance the secretion of C-X-C motif chemokine ligand (CXCL)-1, C-C motif chemokine ligand 2 (CCL2), and IL-6, which affect cancer proliferation [131]. MiR-410 containing EVs derived from human umbilical cord MSCs decreases phosphatase and tensin homolog (PTEN) expression in lung adenocarcinoma. These results suggests that the uptake of EVs by lung adenocarcinoma increases proliferation and decreases apoptosis. Cancer-associated fibroblasts (CAFs) also play a crucial role in cancer proliferation [29]. EV secretion from CAFs increases after gemcitabine (an anticancer drug) treatment and promotes cancer proliferation and drug resistance. Interestingly, it has been reported that EPH receptor A2-enriched EVs from senescent cells promote cancer proliferation [31]. EVs containing miRNAs play important roles in cancer cell proliferation [32].
Long non-coding RNA (LncRNA) is a type of RNA [137]. LncRNAs have many functions, such as the regulation of chromatin states and transcription. TU339, a type of lncRNA, was found in EVs derived from hepatocellular cancer [30]. TU339-containing EVs mediate tumor cell growth and adhesion after transfer to cancer cells. lncRNA-VLDLR also contains EVs that contribute to cellular stress responses [33].
As discussed above, miRNAs, proteins, and lncRNAs in EVs play a critical role in cancer cell proliferation.
4. Angiogenesis and Intravasation
Angiogenesis and lymphangiogenesis are important for the survival and progression of cancer cells, which are activated by signals from cancer cells that are growing [138]. These are important steps for the supply of oxygen, nutrients, and metabolism in cancer cells [139]. Vascular endothelial growth factor (VEGF), basic fibroblast growth factor, angiogenin, and TGF-α play an important role in angiogenesis [139]. Cancer-derived EVs also play an important role in angiogenesis [134,140,141,142,143,144,145,146] and lymph-angiogenesis [147]. EVs derived from colorectal cancer cells activate early growth response protein-1 in endothelial cells, causing the migration of endothelial cells [34]. Cancer-derived EVs stimulate MSCs to form vessel-like formations [148]. It has been reported that miRNAs in EVs also plays an important role in angiogenesis [45]. Rac1-, PAK2- [44], VEGF- [46], and angiopoietin-2-containing [48] EVs are related to angiogenesis. These results suggest that cancer-derived EVs promote angiogenesis. Lymph nodes are a route of cancer metastasis [149]. It is reported that laminin γ2-containing EVs promote lymphangiogenesis [50]; however, mechanisms underlying lymphatic intravasation remain unclear.
5. Metastasis
Cancer cells can metastasize to any part of the body; however, sites such as bone, the liver, and the lungs are the most common. Brain metastasis is a critical cause of death. Uncontrolled cancer metastasis is a major cause of cancer-related deaths. Cancer metastasis involves multiple steps, such as epithelial–mesenchymal transition (EMT), intravasation, extravasation, and proliferation at the metastatic organ. The seed-and-soil theory is well accepted as a mechanism of metastasis [150]. Using the metastatic efficiency index, Weiss suggested that 65% of metastasis seems to be caused by the amount of organ blood flow [150,151]. In contrast, there are common sites of cancer metastasis and sites that are specific to the cancer type. In such complicated mechanisms of metastasis, EVs contribute to EMT [152,153,154], migration [63,155,156,157], metastasis niche formation [20,158,159], metastasis promotion [158], and the tumor microenvironment [160].
Hypoxia in the tumor environment affects cancer behavior. Secretion of EVs from colorectal cancer cell lines increases under hypoxic conditions [161]. These EVs stimulate the motility, invasiveness, and stemness of colorectal cancer cell lines. Cancer cells communicate with the microenvironment for progression through EVs [61,162,163,164]. MiR-370-3p-containing EVs from breast cancer cells induce IL-6, IL-8, and IL-1β secretion by suppressing the cylindromatosis-/NF-κB-signaling pathway in fibroblasts [58]. It has been suggested that the microenvironment of cancer contributes to cancer progression through education by EVs. EMT is a feature of cancer metastasis in which epithelial cells transition from the epithelial state to the mesenchymal state, and EVs affecting EMT of cancer cells may lead to cancer metastasis [152,153,154]. The migration/invasion step in cancer metastasis is important for migrating to other organs [62,67,68,165,166]. EVs derived from CAFs promote migration and invasion of oral squamous cell carcinoma cells [167]. EVs derived from endothelial cells that are associated with tumors enhance the invasion of cancer cells by changing the cancer microenvironment [156]. The pre-metastatic niche is an idea that distant organs can form a microenvironment that can metastasize cancer cells before they can reach them [20,159,168,169]. EVs derived from cancer cells can change the microenvironment of distant organs and may allow cancer cells to metastasize to distant organs. Brain metastasis is known as a poor prognosis. EVs derived from breast cancer cells break down the blood–brain barrier, making it possible for cancer cells to pass through the biological barrier [19]. MiR-181c plays a role in changing the state of brain blood vessels after being taken up by brain endothelial cells through EVs. It has been reported that tubulin tyrosine ligase-like (TTLL4) is related to EV biogenesis and brain metastasis [57]. Contents of EVs such as miRNAs [63] and lncRNAs [59] play critical roles in cancer invasion and migration.
There is considerable evidence that EVs contribute to metastasis by changing cancer cells and their microenvironment [135,170,171,172,173,174]. EVs derived from breast cancer cells contain small noncoding (nc) RNAs, named orphan ncRNAs, which originate from the 3′ end of the telomerase RNA [60]. Small ncRNAs promote breast cancer metastasis. EVs derived from breast cancer cells upregulate EV secretion via chemotherapy [175]. Drug-induced EVs promote lung metastasis. Interestingly, it has been reported that EVs derived from bovine milk induce cell senescence in cancer cells but promote metastasis by inducing EMT in the primary tumor [176]. In contrast, EVs derived from bone marrow MSCs induce dormancy in metastatic breast cancer cells via miR-23b in EVs [66]. These results indicate that EVs play a critical role in cancer metastasis.
6. Escape from Immune System
The immune system consists of macrophages, B cells, T cells, and DCs. It protects the body from invaders such as bacteria, viruses, and toxins; contributes to the recovery of the body; and removes cancer cells. However, cancer cells can escape the immune system by modifying them through EVs. Programmed death ligand-1 (PD-L1) is a receptor that suppresses or stops T-cell reactions by binding to programmed cell death-1 (PD-1). EVs derived from glioblastoma promote immune evasion through PD-1 binding to PD-L1 on EVs [69,70]. Ovarian cancer cell-derived EVs have been reported to inhibit T-cell receptor-dependent activation in T-cells [177]. EVs derived from tumor-associated macrophages have immunosuppressive effects; conversely, these cells have the potential to activate anti-tumor immunity [178]. MSC-derived EVs containing miR-222 contribute to immune escape in colorectal cancer by downregulating the AKT pathway [65]. These reports suggest that EVs derived from cancer cells and cells in the microenvironment suppress the immune system.
7. Chemotherapeutic Stress
Cancer chemotherapy has been developed for the treatment of the whole body since 1960s [179], and chemotherapy using cytotoxic drugs has been the main form of therapy for cancer in recent years. Currently, antibodies are used to kill cancer cells directly or via immune cells [180]. Unfortunately, EVs contribute to the evasion of chemotherapeutic agents.
Bone marrow MSC-derived EVs increase the viability of multiple myeloma cells and drug resistance [181]. Non-small-cell lung cancer cell-derived EVs increase gefitinib-induced apoptosis [182]. In EVs, lncRNAs contribute to drug resistance [33,75,76,77,78,79], with lncRNA H19 in EVs increasing gefitinib resistance in non-small-cell lung cancer cells [73]. EVs derived from MSCs increase drug resistance [157]. MSC-EVs are collected under stress using a non-serum culture medium. EVs derived from under-stressed MSCs decrease doxorubicin-induced apoptosis in osteosarcoma cells, and those derived from melanoma cells containing anaplastic lymphoma kinase could transfer drug resistance to other melanoma cells [71]. EVs derived from tumor-associated macrophages, which are components of the cancer microenvironment, increase resistance to the anticancer drug gemcitabine [74]. MiR-30b-3p in EVs derived from hypoxic glioma cells contributes to drug resistance by decreasing the expression of ras homolog family member B [72]. Furthermore, there is a mechanism to resist antibody therapy. Bevacizumab is an antibody used for cancer treatment because of its anti-angiogenic effect, which is discarded through EVs derived from glioblastoma cells after being captured by glioblastoma cells. Interestingly, EVs derived from breast cancer cells showed increased drug resistance in the non-tumorigenic epithelial cell line MCF10A [183]. Cells secrete EVs to resist stress environments, and this ability is acquired by cancer cells.
8. Potential of EVs for Liquid Biopsy
EVs secreted into the extracellular environment may be related to mechanisms of cancer malignancy. Many reports have suggested that EVs contain specific molecules that contribute to cancer malignancy or related cancer types. Therefore, it is possible that EVs can be used for diagnosis. A liquid biopsy is a test that uses body fluids such as blood, bone marrow, saliva, urine, and tears. A minimally invasive method is required in liquid biopsy as much as possible to avoid pain. Bone marrow biopsy is an invasive method, as is blood biopsy. On the other hand, saliva, urine, and tears are non-invasive methods of biopsy. Liquid biopsy tests can identify early stage, progression, and metastasis of cancer by detecting specific molecules. The idea of a liquid biopsy using EVs is to detect cancer-specific EV molecules such as miRNAs, DNAs, and proteins for the diagnosis of cancer [184]. As described above, tears have great potential as a non-invasive method in liquid biopsy. EVs in tears can be used for the diagnosis of cancer [185]. However, further evidence is required for its clinical application. Liquid biopsy of prostate cancer uses urine, and is hence a non-invasive method [186,187,188,189,190]. MiR-21, miR-375, and miR-204 have been detected in the urine of prostate cancer patients but not in healthy donors [81]. It has been reported that the levels of miR-221-3p, miR-222-3p, and miR31-5p are higher in high-risk patients compared to low-risk patients [85]. EV proteins are also useful as biomarkers of prostate cancer. FABP5 [80] and androgen receptor splice variant 7 [84] on EVs collected from urine may be used as cancer progression markers. Analyses with blood samples indicate that miR-375, miR-200c-3p, and miR-21-5p are useful for the diagnosis of prostate cancer [87]. There is a combination of EV miRNAs and proteins from the blood [191]. Saliva is a non-invasive bioliquid. EVs collected from saliva may be useful for the diagnosis of lung cancer [192,193,194]. It has been reported that the contents of EVs collected from serum, such as miR-200, lipocalin-2 [86], miR-505-5p [91], ubiquitin C-terminal hydrolase-L1 (UCHL1) [88], cell-free DNA [82,83], and proteins [195,196,197,198,199], are useful for the diagnosis of lung cancer. MiR-193a-5p, and miR-551b-5p have been suggested as biomarkers of malignant pleural mesothelioma. Pancreatic cancer is one of the leading causes of cancer-related mortality [94]. The five-year survival rate of pancreatic cancer is approximately 2%. It is important to detect it at an early stage; however, no diagnostic method for clinical use to identify early-stage tumors or pre-malignant conditions exists. Liquid biopsy using EVs has potential for early diagnosis. Glypican-1 on EVs derived from cancer cells has been found to be a biomarker of early-stage pancreatic cancer [89,90]. Mucins, CFTR, and MDR1 found in EVs from pancreatic juice and cancer cell lines are candidate biomarkers [92,93]. Zinc transporter ZIP4 [37], cytoskeleton-associated protein 4, a novel Dickkopf1 receptor [96], and annexin A1 [200] are also potential biomarkers. A panel of miRNAs, mRNAs, and proteins derived from EVs is useful for the early diagnosis of pancreatic cancer [201,202,203,204,205,206,207]. It may be possible to predict treatment outcomes using EVs [208]. It has also been suggested that miR-133b is a potential biomarker for pancreatic cancer [95]. Interestingly, bacteria-derived EVs collected from blood samples can be used as biomarkers for pancreatic cancer [209]. It has been reported that there are differences in the microbiome between patients with pancreatic cancer and healthy donors and between patients with cancer and the precancerous state in liver disease [210,211]. MiR-150-3p has been suggested as a prognostic biomarker for HCC [97]. MicroRNAs are important diagnostic biomarkers for breast cancer [212,213]. It is important to identify not only miRNAs but also lncRNAs for diagnosis [214]. Thus, evidence suggests that EVs have potential as diagnostic markers for cancer.
9. Potential of EVs for Cancer Treatment
As discussed above, EVs from cancer cells and their microenvironment contribute to cancer malignancy. The idea of cancer treatment using EVs has three aspects—(1) inhibition of EV production, (2) disruption of EV uptake, and (3) elimination of EVs [215]. In addition, there is a strategy for the application of EVs that carry a druggable molecule for cancer treatment [216]. CD63 and CD9 are used as markers for targeting EVs in the circulation. EV elimination from the circulation using anti-CD63 and CD9 antibodies can reduce cancer metastasis [22]. The CD9 Fab fragment also inhibits EV internalization [100]. These reports suggest that targeting proteins on EVs can be used for the neutralization and elimination of EVs from the blood. It has also been suggested that potential therapeutics exert anti-cancer effects by blocking EVs containing TGF-β [217]; however, the therapeutics strategy of “inhibition of EV production” is still unclear. Many studies have suggested that EVs can be used as drug delivery systems [218,219,220,221]. EVs derived from a macrophage cell line were loaded with the anticancer drugs paclitaxel or doxorubicin [222] and were then used to inhibit cancer growth. EVs containing hyaluronic acid grafted with 3-(diethylamino)propylamine and the anti-cancer drug doxorubicin can bind to CD44; they exhibit an anti-cancer effect [223]. Organ tropism is required when EVs are used in drug delivery systems. Rabies viral glycoprotein [98], iRGD peptide [224], and integrins [225] have been reported as factors of the organ tropism of EVs. CD47-over express EVs are reduced EV-uptake by macrophages, which can enable the deriver of distant organs [226]. However, the mechanism of organ tropism in EVs remains largely unknown. Interestingly, artificial EVs can be used as drug carriers. An artificial EV was made such that an aptamer carrying bone marrow DCs was extruded from a filter [227]. Numerous reports have shown that EVs containing miRNAs [99,101] exhibit anti-cancer effects. It is a good strategy to load miRNAs that exhibit anti-cancer effects, because EVs containing miRNAs are known. MiR-21 is a critical factor in cancer malignancy [228,229]. EVs containing miR-21 contribute to cancer malignancy and a poor prognosis [159,230]. Anti-cancer effects of miRNAs related to cancer malignancy can be disrupted by EVs containing a miRNA sponge structure. EVs were collected from HEK293T cells expressing the miR-21 sponge construct. These EVs exhibited anti-cancer effects [111]. MSCs are multipotent stem cells used in cell replacement therapy [231]. MSCs have been used to treat multiple diseases to date. Therefore, MSCs are also used as a source of EVs for cancer treatment. MSC-derived EVs containing miRNAs such as miR-206 [112], miR-193a [67], miR-144-3p [113], and miR-125b [114] exhibit anti-cancer effects. MSC-derived EVs contain mi-185 [115]. EVs exert multiple anti-cancer effects through the AKT pathway, which is targeted by miR-185. EVs from adipose-derived MSCs have been suggested to enhance cell apoptosis [232]. In addition, miR-16-5p-loaded EVs inhibited tumor growth in vivo. In contrast, it has been suggested that conditioned media from bone marrow mesenchymal stromal cells also have anti-tumor effects [116]. EVs have been used as a cell-free vaccine for cancer treatment. DCs were cultured with tumor peptides, and then EVs were collected from them [21,102]. EVs derived from tumor-antigen-activated DCs suppressed tumor growth in a T-cell-dependent manner. In addition, it is a good strategy to change the properties of EVs. Depleting CD99 in cells affects the amount of CD99 in EVs, and the effect of EVs can be altered by depleting CD99 in Ewing sarcoma cells [110]. It has been reported that 4T1 cells treated with antibacterial drugs secrete EVs that can inhibit osteoclastogenesis [233]. A natural killer cell line treated with IL-15-secreted EVs can inhibit cancer growth [234]. It is possible that cells treated with reagents secrete EVs that can function as a cancer treatment or inhibit cancer progression. Moreover, EVs from NFAT3-expressed breast cancer cells exert anti-cancer effects [235]. These reports suggest the possibility that EVs derived from specific protein-expressing cancer cells also have anti-cancer effects.
10. Conclusions
EVs are microscale vesicles secreted from a variety of cells. EVs contain lipids, proteins, and nucleic acids. EVs participate in cell–cell communication via the molecules they contain. As discussed above, EVs may contribute to cancer malignancy. It is thought that cancer-derived EVs that contribute to cancer malignancy can be detected in body fluids. Many studies have suggested that the detection of EVs may be useful for cancer diagnosis. As described above, EVs are carriers of molecules. Characteristics of EVs indicate their potential for use in drug delivery systems. In addition, many miRNAs exhibiting anti-cancer effects have been identified. EVs seem to be advantageous as carriers of miRNAs because EVs naturally contain miRNAs. Proteins are also naturally contained in EVs. The strategy of “overexpression” of proteins is used to load proteins on EVs. Some reports suggest that it is possible to load anticancer compounds. These methods are important for drug delivery systems. On the other hand, it is necessary to pay attention to EVs because they are a double-edged sword. Considerable evidence suggests that MSCs are a useful source of EVs; however, their mechanisms have also been found to contribute to cancer malignancy in some MSC-derived EVs. Importantly, it has been suggested that standard therapeutics such as anti-cancer drugs can change the nature of cancer-derived EVs from contributing to cancer malignancy to exhibiting an anti-cancer effect. In other words, secreted EVs are heterogeneous and have different contents and proteins. The purification of “druggable” EVs (or removing “wicked” EVs) is important to apply to treatment. Furthermore, the organ specificity of EVs is a key to applying its therapeutics strategy. Transmembrane proteins in EVs responsible for organ tropism are crucial; further elucidation of the mechanisms of organ tropism is required. The mechanisms of EV, such as loading and changing contents in each situation, and the amount of EVs being secreted, are still unknown. Hence, it is necessary to gain deeper understanding of the nature of EVs. In particular, it requires target specificity and the unity of contents of EVs that exclude unnecessary content. Evidence suggests that EVs have great potential for cancer treatment; however, a deeper understanding and research of EVs may be needed for their application to such therapies.
Funding
This work was supported in part by a Grant-in-Aid for Research Activity Start-up (No. 21K21219), Yamaguchi University Fund, and Start-up Research Fund (Yamaguchi University original—University Fund).
Conflicts of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 2.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutz H.U., Lomant A.J., McMillan P., Wehrli E. Rearrangements of integral membrane components during in vitro aging of sheep erythrocyte membranes. J. Cell Biol. 1977;74:389–398. doi: 10.1083/jcb.74.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronquist G., Hedström M. Restoration of detergent-inactivated adenosine triphosphatase activity of human prostatic fluid with concanavalin A. [(accessed on 15 December 2021)];Biochim. Biophys. Acta. 1977 483:483–486. doi: 10.1016/0005-2744(77)90078-X. Available online: https://www.sciencedirect.com/science/article/pii/000527447790078X?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- 6.Müller H., Schmidt U., Lutz H.U. On the mechanism of vesicle release from ATP-depleted human red blood cells. Biochim. Biophys. Acta. 1981;649:462–470. doi: 10.1016/0005-2736(81)90437-5. [DOI] [PubMed] [Google Scholar]
- 7.Zweig S.E., Tokuyasu K.T., Singer S.J. Member-associated changes during erythropoiesis. On the mechanism of maturation of reticulocytes to erythrocytes. J. Supramol. Struct. Cell. Biochem. 1981;17:163–181. doi: 10.1002/jsscb.380170207. [DOI] [PubMed] [Google Scholar]
- 8.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 9.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding C., Heuser J., Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. [(accessed on 15 December 2021)];Eur. J. Cell Biol. 1984 35:256–263. Available online: https://www.ncbi.nlm.nih.gov/pubmed/?term=6151502%5Buid%5D. [PubMed] [Google Scholar]
- 11.Pan B.T., Teng K., Wu C., Adam M., Johnstone R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnstone R.M., Mathew A., Mason A.B., Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins. J. Cell. Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 13.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 14.Gould S.J., Raposo G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles. 2013;2:3–5. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 17.Clayton A., Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells. Mol. Dis. 2005;34:206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic micrornas regulate cancer cell metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K., Lötvall J., Nakagama H., Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C.M., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 22.Nishida-Aoki N., Tominaga N., Takeshita F., Sonoda H., Yoshioka Y., Ochiya T. Disruption of Circulating Extracellular Vesicles as a Novel Therapeutic Strategy against Cancer Metastasis. Mol. Ther. 2017;25:181–191. doi: 10.1016/j.ymthe.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camussi G., Deregibus M.-C., Bruno S., Grange C., Fonsato V., Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 24.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuura K., de Giorgi V., Schechterly C., Wang R.Y., Farci P., Tanaka Y., Alter H.J. Circulating let-7 levels in plasma and extracellular vesicles correlate with hepatic fibrosis progression in chronic hepatitis C. Hepatology. 2016;64:732–745. doi: 10.1002/hep.28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z., Tsai M.-H., Shumilov A., Baccianti F., Tsao S.W., Poirey R., Delecluse H.-J. Epstein-Barr virus ncRNA from a nasopharyngeal carcinoma induces an inflammatory response that promotes virus production. Nat. Microbiol. 2019;4:2475–2486. doi: 10.1038/s41564-019-0546-y. [DOI] [PubMed] [Google Scholar]
- 27.Butin-Israeli V., Bui T.M., Wiesolek H.L., Mascarenhas L., Lee J.J., Mehl L.C., Knutson K.R., Adam S.A., Goldman R.D., Beyder A., et al. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J. Clin. Investig. 2019;129:712–726. doi: 10.1172/JCI122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setti M., Osti D., Richichi C., Ortensi B., del Bene M., Fornasari L., Beznoussenko G., Mironov A., Rappa G., Cuomo A., et al. Extracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growth. Oncotarget. 2015;6:31413–31427. doi: 10.18632/oncotarget.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kogure T., Yan I.K., Lin W.-L., Patel T. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer. 2013;4:261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takasugi M., Okada R., Takahashi A., Chen D.V., Watanabe S., Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017;8:15729. doi: 10.1038/ncomms15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson J., Dickman C., Towle R., Jabalee J., Javer A., Garnis C. Extracellular vesicle secretion of miR-142-3p from lung adenocarcinoma cells induces tumor promoting changes in the stroma through cell-cell communication. Mol. Carcinog. 2019;58:376–387. doi: 10.1002/mc.22935. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K., Yan I.K., Wood J., Haga H., Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon Y.J., Kim D.-K., Yoon C.M., Park J., Kim Y.-K., Roh T.-Y., Gho Y.S. Egr-1 activation by cancer-derived extracellular vesicles promotes endothelial cell migration via ERK1/2 and JNK signaling pathways. PLoS ONE. 2014;9:e115170. doi: 10.1371/journal.pone.0115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan H., Peng R., Fang F., Mao L., Chen Z., Yang S., Dai C., Wu H., Wang C., Feng N., et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020;235:9729–9742. doi: 10.1002/jcp.29784. [DOI] [PubMed] [Google Scholar]
- 36.Iempridee T. Long non-coding RNA H19 enhances cell proliferation and anchorage-independent growth of cervical cancer cell lines. Exp. Biol. Med. 2017;242:184–193. doi: 10.1177/1535370216670542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin H., Liu P., Wu Y., Meng X., Wu M., Han J., Tan X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018;109:2946–2956. doi: 10.1111/cas.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xi C., Wang J., Sun H., Zhang X., Kang H. Loss of microRNA-30e induced by extracellular vesicles from cancer-associated fibroblasts promotes breast cancer progression by binding to CTHRC1. Exp. Mol. Pathol. 2021;118:104586. doi: 10.1016/j.yexmp.2020.104586. [DOI] [PubMed] [Google Scholar]
- 39.Tao S.-C., Huang J.-Y., Wei Z.-Y., Li Z.-X., Guo S.-C. EWSAT1 Acts in Concert with Exosomes in Osteosarcoma Progression and Tumor-Induced Angiogenesis: The “Double Stacking Effect”. Adv. Biosyst. 2020;4:e2000152. doi: 10.1002/adbi.202000152. [DOI] [PubMed] [Google Scholar]
- 40.Meng L., Xing Z., Guo Z., Qiu Y., Liu Z. Hypoxia-induced microRNA-155 overexpression in extracellular vesicles promotes renal cell carcinoma progression by targeting FOXO3. Aging. 2021;13:9613–9626. doi: 10.18632/aging.202706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Xu H., Yi J., Dong C., Zhang H., Wang Z., Miao L., Zhou W. miR-365 secreted from M2 Macrophage-derived extracellular vesicles promotes pancreatic ductal adenocarcinoma progression through the BTG2/FAK/AKT axis. J. Cell. Mol. Med. 2021;25:4671–4683. doi: 10.1111/jcmm.16405. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Zhang Y., Meng W., Yue P., Li X. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J. Exp. Clin. Cancer Res. 2020;39:134. doi: 10.1186/s13046-020-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Zheng Y., Li J.-X., Chen C.-J., Lin Z.-Y., Liu J.-X., Lin F.-J. Extracellular vesicle-derived circ_SLC19A1 promotes prostate cancer cell growth and invasion through the miR-497/septin 2 pathway. Cell Biol. Int. 2020;44:1037–1045. doi: 10.1002/cbin.11303. [DOI] [PubMed] [Google Scholar]
- 44.Gopal S.K., Greening D.W., Hanssen E.G., Zhu H.-J., Simpson R.J., Mathias R.A. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget. 2016;7:19709–19722. doi: 10.18632/oncotarget.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao Z., Pan Q., Zhang Y. Hepatocellular carcinoma cell-derived extracellular vesicles encapsulated microRNA-584-5p facilitates angiogenesis through PCK1-mediated nuclear factor E2-related factor 2 signaling pathway. Int. J. Biochem. Cell Biol. 2020;125:105789. doi: 10.1016/j.biocel.2020.105789. [DOI] [PubMed] [Google Scholar]
- 46.Feng Q., Zhang C., Lum D., Druso J.E., Blank B., Wilson K.F., Welm A., Antonyak M.A., Cerione R.A. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat. Commun. 2017;8:14450. doi: 10.1038/ncomms14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannafon B.N., Carpenter K.J., Berry W.L., Janknecht R., Dooley W.C., Ding W.-Q. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA) Mol. Cancer. 2015;14:133. doi: 10.1186/s12943-015-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie J.-Y., Wei J.-X., Lv L.-H., Han Q.-F., Yang W.-B., Li G.-L., Wang P.-X., Wu S.-B., Duan J.-X., Zhuo W.-F., et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun. Signal. 2020;18:46. doi: 10.1186/s12964-020-00535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y., Liu L., Chen C., Ming P., Huang Q., Li C., Cao D., Xu X., Ge W. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ. 2017;5:e3627. doi: 10.7717/peerj.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S.-H., Liou G.-G., Liu S.-H., Chang J.S., Hsiao J.-R., Yen Y.-C., Chen Y.-L., Wu W.-L., Chang J.-Y., Chen Y.-W. Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. Int. J. Cancer. 2019;144:2795–2810. doi: 10.1002/ijc.32027. [DOI] [PubMed] [Google Scholar]
- 51.Lawson J., Dickman C., MacLellan S., Towle R., Jabalee J., Lam S., Garnis C. Selective secretion of microRNAs from lung cancer cells via extracellular vesicles promotes CAMK1D-mediated tube formation in endothelial cells. Oncotarget. 2017;8:83913–83924. doi: 10.18632/oncotarget.19996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masoumi-Dehghi S., Babashah S., Sadeghizadeh M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J. Cell Commun. Signal. 2020;14:233–244. doi: 10.1007/s12079-020-00548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., Lu J., Chen L., Bian H., Hu J., Li D., Xia C., Xu H. Tumor-Derived EV-Encapsulated miR-181b-5p Induces Angiogenesis to Foster Tumorigenesis and Metastasis of ESCC. Mol. Ther. Nucleic Acids. 2020;20:421–437. doi: 10.1016/j.omtn.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng X., Lu S., He Z., Huang H., Yao Z., Miao Y., Cai C., Zou F. MCU-dependent negative sorting of miR-4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene. 2020;39:6975–6989. doi: 10.1038/s41388-020-01514-6. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X., Wang Y., Wang X., Zou B., Mei J., Peng X., Wu Z. Extracellular vesicles-encapsulated microRNA-10a-5p shed from cancer-associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via Hedgehog signaling pathway. Cancer Gene Ther. 2021;28:529–542. doi: 10.1038/s41417-020-00238-9. [DOI] [PubMed] [Google Scholar]
- 56.Aday S., Hazan-Halevy I., Chamorro-Jorganes A., Anwar M., Goldsmith M., Beazley-Long N., Sahoo S., Dogra N., Sweaad W., Catapano F., et al. Bioinspired artificial exosomes based on lipid nanoparticles carrying let-7b-5p promote angiogenesis in vitro and in vivo. Mol. Ther. 2021;29:2239–2252. doi: 10.1016/j.ymthe.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold J., Schattschneider J., Blechner C., Krisp C., Schlüter H., Schweizer M., Nalaskowski M., Oliveira-Ferrer L., Windhorst S. Tubulin Tyrosine Ligase Like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis. J. Exp. Clin. Cancer Res. 2020;39:205. doi: 10.1186/s13046-020-01712-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren Z., Lv M., Yu Q., Bao J., Lou K., Li X. MicroRNA-370-3p shuttled by breast cancer cell-derived extracellular vesicles induces fibroblast activation through the CYLD/Nf-κB axis to promote breast cancer progression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021;35:e21383. doi: 10.1096/fj.202001430RR. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Li S., Zhang X., Li C., Zhang J., Zhou W. LncRNA HLA-F-AS1 promotes colorectal cancer metastasis by inducing PFN1 in colorectal cancer-derived extracellular vesicles and mediating macrophage polarization. Cancer Gene Ther. 2021 doi: 10.1038/s41417-020-00276-3. [DOI] [PubMed] [Google Scholar]
- 60.Fish L., Zhang S., Yu J.X., Culbertson B., Zhou A.Y., Goga A., Goodarzi H. Cancer cells exploit an orphan RNA to drive metastatic progression. Nat. Med. 2018;24:1743–1751. doi: 10.1038/s41591-018-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou P.-P., Luo L.-J., Chen H.-Z., Chen Q.-T., Bian X.-L., Wu S.-F., Zhou J.-X., Zhao W.-X., Liu J.-M., Wang X.-M., et al. Ectosomal PKM2 Promotes HCC by Inducing Macrophage Differentiation and Remodeling the Tumor Microenvironment. Mol. Cell. 2020;78:1192–1206.e10. doi: 10.1016/j.molcel.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K., Koyama K., Ota Y., Iwamoto H., Yamakita K., Fujii S., Kitano Y. The Interaction Between Long Non-coding RNA HULC and MicroRNA-622 via Transfer by Extracellular Vesicles Regulates Cell Invasion and Migration in Human Pancreatic Cancer. Front. Oncol. 2020;10:1013. doi: 10.3389/fonc.2020.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C., Zhou X., Long Q., Zeng H., Sun Q., Chen Y., Wu D., Liu L. Small extracellular vesicles containing miR-30a-3p attenuate the migration and invasion of hepatocellular carcinoma by targeting SNAP23 gene. Oncogene. 2021;40:233–245. doi: 10.1038/s41388-020-01521-7. [DOI] [PubMed] [Google Scholar]
- 64.Lu C., Zhao Y., Wang J., Shi W., Dong F., Xin Y., Zhao X., Liu C. Breast cancer cell-derived extracellular vesicles transfer miR-182-5p and promote breast carcinogenesis via the CMTM7/EGFR/AKT axis. Mol. Med. 2021;27:78. doi: 10.1186/s10020-021-00338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S., Yan G., Yue M., Wang L. Extracellular vesicles-derived microRNA-222 promotes immune escape via interacting with ATF3 to regulate AKT1 transcription in colorectal cancer. BMC Cancer. 2021;21:349. doi: 10.1186/s12885-021-08063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ono M., Kosaka N., Tominaga N., Yoshioka Y., Takeshita F., Takahashi R., Yoshida M., Tsuda H., Tamura K., Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014;7:ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 67.Ying H., Lin F., Ding R., Wang W., Hong W. Extracellular vesicles carrying miR-193a derived from mesenchymal stem cells impede cell proliferation, migration and invasion of colon cancer by downregulating FAK. Exp. Cell Res. 2020;394:112144. doi: 10.1016/j.yexcr.2020.112144. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Y., Zeng J., Lin D., Xia H., Wang X., Chen L., Chen H., Huang L., Zeng C. Extracellular vesicles derived from cancer-associated fibroblast carries miR-224-5p targeting SLC4A4 to promote the proliferation, invasion and migration of colorectal cancer cells. Carcinogenesis. 2021 doi: 10.1093/carcin/bgab055. [DOI] [PubMed] [Google Scholar]
- 69.Ricklefs F.L., Alayo Q., Krenzlin H., Mahmoud A.B., Speranza M.C., Nakashima H., Hayes J.L., Lee K., Balaj L., Passaro C., et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018;4:eaar2766. doi: 10.1126/sciadv.aar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Himes B.T., Peterson T.E., de Mooij T., Garcia L.M.C., Jung M.-Y., Uhm S., Yan D., Tyson J., Jin-Lee H.J., Parney D., et al. The role of extracellular vesicles and PD-L1 in glioblastoma-mediated immunosuppressive monocyte induction. Neuro-Oncology. 2020;22:967–978. doi: 10.1093/neuonc/noaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cesi G., Philippidou D., Kozar I., Kim Y.J., Bernardin F., van Niel G., Wienecke-Baldacchino A., Felten P., Letellier E., Dengler S., et al. A new ALK isoform transported by extracellular vesicles confers drug resistance to melanoma cells. Mol. Cancer. 2018;17:145. doi: 10.1186/s12943-018-0886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin J., Ge X., Shi Z., Yu C., Lu C., Wei Y., Zeng A., Wang X., Yan W., Zhang J., et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p. Theranostics. 2021;11:1763–1779. doi: 10.7150/thno.47057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei Y., Guo W., Chen B., Chen L., Gong J., Li W. Tumor-released lncRNA H19 promotes gefitinib resistance via packaging into exosomes in non-small cell lung cancer. Oncol. Rep. 2018;40:3438–3446. doi: 10.3892/or.2018.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xavier C.P.R., Castro I., Caires H.R., Ferreira D., Cavadas B., Pereira L., Santos L.L., Oliveira M.J., Vasconcelos M.H. Chitinase 3-like-1 and fibronectin in the cargo of extracellular vesicles shed by human macrophages influence pancreatic cancer cellular response to gemcitabine. Cancer Lett. 2021;501:210–223. doi: 10.1016/j.canlet.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y., Liu L., Li J., Du Y., Wang J., Liu J. Effects of long noncoding RNA (linc-VLDLR) existing in extracellular vesicles on the occurrence and multidrug resistance of esophageal cancer cells. Pathol. Res. Pract. 2019;215:470–477. doi: 10.1016/j.prp.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 76.Chen X., Liu Y., Zhang Q., Liu B., Cheng Y., Zhang Y., Sun Y., Liu J., Gen H. Exosomal Long Non-coding RNA HOTTIP Increases Resistance of Colorectal Cancer Cells to Mitomycin via Impairing MiR-214-Mediated Degradation of KPNA3. Front. Cell Dev. Biol. 2020;8:582723. doi: 10.3389/fcell.2020.582723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang W., Cai X., Yu J., Lu X., Qian Q., Qian W. Exosome-mediated transfer of lncRNA RP11-838N2. 4 promotes erlotinib resistance in non-small cell lung cancer. Int. J. Oncol. 2018;53:527–538. doi: 10.3892/ijo.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Kang M., Ren M., Li Y., Fu Y., Deng M., Li C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J. Exp. Clin. Cancer Res. 2018;37:171. doi: 10.1186/s13046-018-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Dong H., Wang W., Chen R., Zhang Y., Zou K., Ye M., He X., Zhang F., Han J. Exosome-mediated transfer of lncRNA-SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int. J. Oncol. 2018;53:1013–1026. doi: 10.3892/ijo.2018.4467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Fujita K., Kume H., Matsuzaki K., Kawashima A., Ujike T., Nagahara A., Uemura M., Miyagawa Y., Tomonaga T., Nonomura N. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci. Rep. 2017;7:42961. doi: 10.1038/srep42961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koppers-Lalic D., Hackenberg M., de Menezes R., Misovic B., Wachalska M., Geldof A., Zini N., de Reijke T., Wurdinger T., Vis A., et al. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget. 2016;7:22566–22578. doi: 10.18632/oncotarget.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hur J.Y., Lee J.S., Kim I.A., Kim H.J., Kim W.S., Lee K.Y. Extracellular vesicle-based EGFR genotyping in bronchoalveolar lavage fluid from treatment-naive non-small cell lung cancer patients. Transl. Lung Cancer Res. 2019;8:1051–1060. doi: 10.21037/tlcr.2019.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abe T., Nakashima C., Sato A., Harada Y., Sueoka E., Kimura S., Kawaguchi A., Sueoka-Aragane N. Origin of circulating free DNA in patients with lung cancer. PLoS ONE. 2020;15:e0235611. doi: 10.1371/journal.pone.0235611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woo H.-K., Park J., Ku J.Y., Lee C.H., Sunkara V., Ha H.K., Cho Y.-K. Urine-based liquid biopsy: Non-invasive and sensitive AR-V7 detection in urinary EVs from patients with prostate cancer. Lab Chip. 2018;19:87–97. doi: 10.1039/C8LC01185K. [DOI] [PubMed] [Google Scholar]
- 85.Ruiz-Plazas X., Altuna-Coy A., Alves-Santiago M., Vila-Barja J., García-Fontgivell J.F., Martínez-González S., Segarra-Tomás J., Chacón M.R. Liquid Biopsy-Based Exo-oncomiRNAs Can Predict Prostate Cancer Aggressiveness. Cancers. 2021;13:250. doi: 10.3390/cancers13020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hydbring P., de Petris L., Zhang Y., Brandén E., Koyi H., Novak M., Kanter L., Hååg P., Hurley J., Tadigotla V., et al. Exosomal RNA-profiling of pleural effusions identifies adenocarcinoma patients through elevated miR-200 and LCN2 expression. Lung Cancer. 2018;124:45–52. doi: 10.1016/j.lungcan.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Endzeliņš E., Berger A., Melne V., Bajo-Santos C., Soboļevska K., Ābols A., Rodriguez M., Šantare D., Rudņickiha A., Lietuvietis V., et al. Detection of circulating miRNAs: Comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17:730. doi: 10.1186/s12885-017-3737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimada Y., Kudo Y., Maehara S., Matsubayashi J., Otaki Y., Kajiwara N., Ohira T., Minna J.D., Ikeda N. Ubiquitin C-terminal hydrolase-L1 has prognostic relevance and is a therapeutic target for high-grade neuroendocrine lung cancers. Cancer Sci. 2020;111:610–620. doi: 10.1111/cas.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N., et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lewis J.M., Vyas A.D., Qiu Y., Messer K.S., White R., Heller M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano. 2018;12:3311–3320. doi: 10.1021/acsnano.7b08199. [DOI] [PubMed] [Google Scholar]
- 91.Fang H., Liu Y., He Y., Jiang Y., Wei Y., Liu H., Gong Y., An G. Extracellular vesicle-delivered miR-505-5p, as a diagnostic biomarker of early lung adenocarcinoma, inhibits cell apoptosis by targeting TP53AIP1. Int. J. Oncol. 2019;54:1821–1832. doi: 10.3892/ijo.2019.4738. [DOI] [PubMed] [Google Scholar]
- 92.Osteikoetxea X., Benke M., Rodriguez M., Pálóczi K., Sódar B.W., Szvicsek Z., Szabó-Taylor K., Vukman K.V., Kittel Á., Wiener Z., et al. Detection and proteomic characterization of extracellular vesicles in human pancreatic juice. Biochem. Biophys. Res. Commun. 2018;499:37–43. doi: 10.1016/j.bbrc.2018.03.107. [DOI] [PubMed] [Google Scholar]
- 93.Yang K.S., Ciprani D., O’Shea A., Liss A.S., Yang R., Fletcher-Mercaldo S., Mino-Kenudson M., Castillo C.F., Weissleder R. Extracellular Vesicle Analysis Allows for Identification of Invasive IPMN. Gastroenterology. 2021;160:1345–1358.e11. doi: 10.1053/j.gastro.2020.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi K., Ota Y., Kogure T., Suzuki Y., Iwamoto H., Yamakita K., Kitano Y., Fujii S., Haneda M., Patel T., et al. Circulating extracellular vesicle-encapsulated HULC is a potential biomarker for human pancreatic cancer. Cancer Sci. 2020;111:98–111. doi: 10.1111/cas.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kimura H., Yamamoto H., Harada T., Fumoto K., Osugi Y., Sada R., Maehara N., Hikita H., Mori S., Eguchi H., et al. CKAP4, a DKK1 Receptor, Is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019;25:1936–1947. doi: 10.1158/1078-0432.CCR-18-2124. [DOI] [PubMed] [Google Scholar]
- 97.Yugawa K., Yoshizumi T., Mano Y., Itoh S., Harada N., Ikegami T., Kohashi K., Oda Y., Mori M. Cancer-associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR-150-3p. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021;47:384–393. doi: 10.1016/j.ejso.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 98.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 99.O’Brien K., Lowry M.C., Corcoran C., Martinez V.G., Daly M., Rani S., Gallagher W.M., Radomski M.W., MacLeod R.A.F., O’Driscoll L. miR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget. 2015;6:32774–32789. doi: 10.18632/oncotarget.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santos M.F., Rappa G., Karbanová J., Vanier C., Morimoto C., Corbeil D., Lorico A. Anti-human CD9 antibody Fab fragment impairs the internalization of extracellular vesicles and the nuclear transfer of their cargo proteins. J. Cell. Mol. Med. 2019;23:4408–4421. doi: 10.1111/jcmm.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang F., Li L., Piontek K., Sakaguchi M., Selaru F.M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67:940–954. doi: 10.1002/hep.29586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santos P., Almeida F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021;12:711565. doi: 10.3389/fimmu.2021.711565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhaskaran V., Nowicki M.O., Idriss M., Jimenez M.A., Lugli G., Hayes J.L., Mahmoud A.B., Zane R.E., Passaro C., Ligon K.L., et al. The functional synergism of microRNA clustering provides therapeutically relevant epigenetic interference in glioblastoma. Nat. Commun. 2019;10:442. doi: 10.1038/s41467-019-08390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodrigues-Junior D.M., Pelarin M.F.d., Nader H.B., Vettore A.L., Pinhal M.A.S. MicroRNA-1252-5p Associated with Extracellular Vesicles Enhances Bortezomib Sensitivity in Multiple Myeloma Cells by Targeting Heparanase. OncoTargets Ther. 2021;14:455–467. doi: 10.2147/OTT.S286751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang N., Wang Y., Liu H., Shen W. Extracellular vesicle encapsulated microRNA-320a inhibits endometrial cancer by suppression of the HIF1α/VEGFA axis. Exp. Cell Res. 2020;394:112113. doi: 10.1016/j.yexcr.2020.112113. [DOI] [PubMed] [Google Scholar]
- 106.Ding F., Liu J., Zhang X. microRNA-375 released from extracellular vesicles of bone marrow mesenchymal stem cells exerts anti-oncogenic effects against cervical cancer. Stem Cell Res. Ther. 2020;11:455. doi: 10.1186/s13287-020-01908-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Li P., Xin H., Lu L. Extracellular vesicle-encapsulated microRNA-424 exerts inhibitory function in ovarian cancer by targeting MYB. J. Transl. Med. 2021;19:4. doi: 10.1186/s12967-020-02652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ma T., Hu Y., Guo Y., Zhang Q. Human umbilical vein endothelial cells-derived microRNA-203-containing extracellular vesicles alleviate non-small-cell lung cancer progression through modulating the DTL/p21 axis. Cancer Gene Ther. 2021 doi: 10.1038/s41417-020-00292-3. [DOI] [PubMed] [Google Scholar]
- 109.Tao K., Liu J., Liang J., Xu X., Xu L., Mao W. Vascular endothelial cell-derived exosomal miR-30a-5p inhibits lung adenocarcinoma malignant progression by targeting CCNE2. Carcinogenesis. 2021;42:1056–1067. doi: 10.1093/carcin/bgab051. [DOI] [PubMed] [Google Scholar]
- 110.De Feo A., Sciandra M., Ferracin M., Felicetti F., Astolfi A., Pignochino Y., Picci P., Carè A., Scotlandi K. Exosomes from CD99-deprived Ewing sarcoma cells reverse tumor malignancy by inhibiting cell migration and promoting neural differentiation. Cell Death Dis. 2019;10:471. doi: 10.1038/s41419-019-1675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Monfared H., Jahangard Y., Nikkhah M., Mirnajafi-Zadeh J., Mowla S.J. Potential Therapeutic Effects of Exosomes Packed With a miR-21-Sponge Construct in a Rat Model of Glioblastoma. Front. Oncol. 2019;9:782. doi: 10.3389/fonc.2019.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang H., Wang J., Ren T., Huang Y., Liang X., Yu Y., Wang W., Niu J., Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi: 10.1016/j.canlet.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 113.Meng Q., Zhang B., Zhang Y., Wang S., Zhu X. Human bone marrow mesenchymal stem cell-derived extracellular vesicles impede the progression of cervical cancer via the miR-144-3p/CEP55 pathway. J. Cell. Mol. Med. 2021;25:1867–1883. doi: 10.1111/jcmm.15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baldari S., di Rocco G., Magenta A., Picozza M., Toietta G. Extracellular Vesicles-Encapsulated MicroRNA-125b Produced in Genetically Modified Mesenchymal Stromal Cells Inhibits Hepatocellular Carcinoma Cell Proliferation. Cells. 2019;8:1560. doi: 10.3390/cells8121560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang L., Yin P., Wang J., Wang Y., Sun Z., Zhou Y., Guan X. Delivery of mesenchymal stem cells-derived extracellular vesicles with enriched miR-185 inhibits progression of OPMD. Artif. Cells Nanomed. Biotechnol. 2019;47:2481–2491. doi: 10.1080/21691401.2019.1623232. [DOI] [PubMed] [Google Scholar]
- 116.Gladkova N., Umezu T., Imanishi S., Kawana C., Ohyashiki J.H., Ohyashiki K. Effect of the extracellular component of bone marrow mesenchymal stromal cells from healthy donors on hematologic neoplasms and their angiogenesis. Hum. Cell. 2020;33:599–609. doi: 10.1007/s13577-020-00332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valavanidis A., Vlachogianni T., Fiotakis K., Loridas S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health. 2013;10:3886–3907. doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franceschi C., Campisi J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. J.Gerontol. Ser. A Biol. Sci. Med. Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 119.Coppé J.-P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., Nelson P.S., Desprez P.-Y., Campisi J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008;6:e301. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Loarca L., de Assuncao T.M., Jalan-Sakrikar N., Bronk S., Krishnan A., Huang B., Morton L., Trussoni C., Bonilla L.M., Krueger E., et al. Development and characterization of cholangioids from normal and diseased human cholangiocytes as an in vitro model to study primary sclerosing cholangitis. Lab. Investig. 2017;97:1385–1396. doi: 10.1038/labinvest.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jakhar R., Crasta K. Exosomes as emerging pro-tumorigenic mediators of the senescence-associated secretory phenotype. Int. J. Mol. Sci. 2019;20:2547. doi: 10.3390/ijms20102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leung C.C.T., Wong C.K.C. Characterization of stanniocalcin-1 expression in macrophage differentiation. Transl. Oncol. 2021;14:100881. doi: 10.1016/j.tranon.2020.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaiyadet S., Sotillo J., Smout M., Cantacessi C., Jones M.K., Johnson M.S., Turnbull L., Whitchurch C.B., Potriquet J., Laohaviroj M., et al. Carcinogenic liver fluke secretes extracellular vesicles that promote cholangiocytes to adopt a tumorigenic phenotype. J. Infect. Dis. 2015;212:1636–1645. doi: 10.1093/infdis/jiv291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iuliano M., Mangino G., Chiantore M.V., Zangrillo M.S., Accardi R., Tommasino M., Fiorucci G., Romeo G. Human Papillomavirus E6 and E7 oncoproteins affect the cell microenvironment by classical secretion and extracellular vesicles delivery of inflammatory mediators. Cytokine. 2018;106:182–189. doi: 10.1016/j.cyto.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 125.Kim J.H., Lee C.H., Lee S.-W. Exosomal Transmission of MicroRNA from HCV Replicating Cells Stimulates Transdifferentiation in Hepatic Stellate Cells. Mol. Ther. Nucleic Acids. 2019;14:483–497. doi: 10.1016/j.omtn.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X., Deeke S.A., Ning Z., Starr A.E., Butcher J., Li J., Mayne J., Cheng K., Liao B., Li L., et al. Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-05357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu C.H., Silvers C.R., Messing E.M., Lee Y.F. Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells. J. Biol. Chem. 2019;294:3207–3218. doi: 10.1074/jbc.RA118.006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Urciuoli E., Giorda E., Scarsella M., Petrini S., Peruzzi B. Osteosarcoma-derived extracellular vesicles induce a tumor-like phenotype in normal recipient cells. J. Cell. Physiol. 2018;233:6158–6172. doi: 10.1002/jcp.26464. [DOI] [PubMed] [Google Scholar]
- 129.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 130.Scherer W.F., Syverton J.T., Gey G.O. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haga H., Yan I.K., Takahashi K., Wood J., Zubair A., Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J. Extracell. Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou X., Li T., Chen Y., Zhang N., Wang P., Liang Y., Long M., Liu H., Mao J., Liu Q., et al. Mesenchymal stem cell-derived extracellular vesicles promote the in vitro proliferation and migration of breast cancer cells through the activation of the ERK pathway. Int. J. Oncol. 2019;54:1843–1852. doi: 10.3892/ijo.2019.4747. [DOI] [PubMed] [Google Scholar]
- 133.Czystowska-Kuzmicz M., Sosnowska A., Nowis D., Ramji K., Szajnik M., Chlebowska-Tuz J., Wolinska E., Gaj P., Grazul M., Pilch Z., et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 2019;10:3000. doi: 10.1038/s41467-019-10979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsutsui T., Kawahara H., Kimura R., Dong Y., Jiapaer S., Sabit H., Zhang J., Yoshida T., Nakada M., Hanayama R. Glioma-derived extracellular vesicles promote tumor progression by conveying WT1. Carcinogenesis. 2020;41:1238–1245. doi: 10.1093/carcin/bgaa052. [DOI] [PubMed] [Google Scholar]
- 135.Li J., Xue J., Ling M., Sun J., Xiao T., Dai X., Sun Q., Cheng C., Xia H., Wei Y., et al. MicroRNA-15b in extracellular vesicles from arsenite-treated macrophages promotes the progression of hepatocellular carcinomas by blocking the LATS1-mediated Hippo pathway. Cancer Lett. 2021;497:137–153. doi: 10.1016/j.canlet.2020.10.023. [DOI] [PubMed] [Google Scholar]
- 136.Dong L., Pu Y., Zhang L., Qi Q., Xu L., Li W., Wei C., Wang X., Zhou S., Zhu J., et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9:218. doi: 10.1038/s41419-018-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Geisler S., Coller J. RNA in unexpected places: Long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 139.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baj-Krzyworzeka M., Mytar B., Weglarczyk K., Szatanek R., Kijowski J., Siedlar M. Protumorogenic Potential of Pancreatic Adenocarcinoma-Derived Extracellular Vesicles. Folia Biol. 2020;66:104–110. [PubMed] [Google Scholar]
- 141.De Andrade A., de Oliveira C.E., Dourado M.R., Macedo C., Winck F.V., Leme A.F.P., Salo T., Coletta R.D., Freitas R.d., Galvão H.C. Extracellular vesicles from oral squamous carcinoma cells display pro- and anti-angiogenic properties. Oral Dis. 2018;24:725–731. doi: 10.1111/odi.12765. [DOI] [PubMed] [Google Scholar]
- 142.Ren J.G., Zhang W., Liu B., Man Q.W., Xiong X.P., Li C., Zhu J.Y., Wang W.M., Jia J., Sun Z.J., et al. Clinical Significance and Roles in Angiogenesis of Circulating Microparticles in Oral Cancer. J. Dent. Res. 2016;95:860–867. doi: 10.1177/0022034516641037. [DOI] [PubMed] [Google Scholar]
- 143.Zarfati M., Avivi I., Brenner B., Katz T., Aharon A. Extracellular vesicles of multiple myeloma cells utilize the proteasome inhibitor mechanism to moderate endothelial angiogenesis. Angiogenesis. 2019;22:185–196. doi: 10.1007/s10456-018-9649-y. [DOI] [PubMed] [Google Scholar]
- 144.Lucero R., Zappulli V., Sammarco A., Murillo O.D., Cheah P.S., Srinivasan S., Tai E., Ting D.T., Wei Z., Roth M.E., et al. Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep. 2020;30:2065–2074.e4. doi: 10.1016/j.celrep.2020.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li J., Liu X., Zang S., Zhou J., Zhang F., Sun B., Qi D., Li X., Kong J., Jin D., et al. Small extracellular vesicle-bound vascular endothelial growth factor secreted by carcinoma-associated fibroblasts promotes angiogenesis in a bevacizumab-resistant manner. Cancer Lett. 2020;492:71–83. doi: 10.1016/j.canlet.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 146.Garcia-Hernandez A., Leal-Orta E., Ramirez-Ricardo J., Cortes-Reynosa P., Thompson-Bonilla R., Salazar E.P. Linoleic acid induces secretion of extracellular vesicles from MDA-MB-231 breast cancer cells that mediate cellular processes involved with angiogenesis in HUVECs. Prostaglandins Other Lipid Mediat. 2021;153:106519. doi: 10.1016/j.prostaglandins.2020.106519. [DOI] [PubMed] [Google Scholar]
- 147.Carrasco-Ramírez P., Greening D.W., Andrés G., Gopal S.K., Martín-Villar E., Renart J., Simpson R.J., Quintanilla M. Podoplanin is a component of extracellular vesicles that reprograms cell-derived exosomal proteins and modulates lymphatic vessel formation. Oncotarget. 2016;7:16070–16089. doi: 10.18632/oncotarget.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lindoso R.S., Collino F., Camussi G. Extracellular vesicles derived from renal cancer stem cells induce a pro-tumorigenic phenotype in mesenchymal stromal cells. Oncotarget. 2015;6:7959–7969. doi: 10.18632/oncotarget.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weigelt B., Peterse J.L., Veer L.J.v. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 150.Tominaga N., Katsuda T., Ochiya T. Micromanaging of tumor metastasis by extracellular vesicles. Semin. Cell Dev. Biol. 2015;40:52–59. doi: 10.1016/j.semcdb.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 151.Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin. Exp. Metastasis. 1992;10:191–199. doi: 10.1007/BF00132751. [DOI] [PubMed] [Google Scholar]
- 152.Galindo-Hernandez O., Serna-Marquez N., Castillo-Sanchez R., Salazar E.P. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins. Leukot. Essent. Fatty Acids. 2014;91:299–310. doi: 10.1016/j.plefa.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 153.Schweiger M.W., Li M., Giovanazzi A., Fleming R.L., Tabet E.I., Nakano I., Würdinger T., Chiocca E.A., Tian T., Tannous B.A. Extracellular Vesicles Induce Mesenchymal Transition and Therapeutic Resistance in Glioblastomas through NF-κB/STAT3 Signaling. Adv. Biosyst. 2020;4:e1900312. doi: 10.1002/adbi.201900312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lucchetti D., Perelli L., Colella F., Ricciardi-Tenore C., Scoarughi G.L., Barbato G., Boninsegna A., de Maria R., Sgambato A. Low-intensity pulsed ultrasound affects growth, differentiation, migration, and epithelial-to-mesenchymal transition of colorectal cancer cells. J. Cell. Physiol. 2020;235:5363–5377. doi: 10.1002/jcp.29423. [DOI] [PubMed] [Google Scholar]
- 155.Chiba M., Watanabe N., Watanabe M., Sakamoto M., Sato A., Fujisaki M., Kubota S., Monzen S., Maruyama A., Nanashima N., et al. Exosomes derived from SW480 colorectal cancer cells promote cell migration in HepG2 hepatocellular cancer cells via the mitogen-activated protein kinase pathway. Int. J. Oncol. 2016;48:305–312. doi: 10.3892/ijo.2015.3255. [DOI] [PubMed] [Google Scholar]
- 156.Lopatina T., Favaro E., Danilova L., Fertig E.J., Favorov A.V., Kagohara L.T., Martone T., Bussolati B., Romagnoli R., Albera R., et al. Extracellular Vesicles Released by Tumor Endothelial Cells Spread Immunosuppressive and Transforming Signals Through Various Recipient Cells. Front. Cell Dev. Biol. 2020;8:698. doi: 10.3389/fcell.2020.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vallabhaneni K.C., Hassler M.-Y., Abraham A., Whitt J., Mo Y.-Y., Atfi A., Pochampally R. Mesenchymal Stem/Stromal Cells under Stress Increase Osteosarcoma Migration and Apoptosis Resistance via Extracellular Vesicle Mediated Communication. PLoS ONE. 2016;11:e0166027. doi: 10.1371/journal.pone.0166027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Macklin R., Wang H., Loo D., Martin S., Cumming A., Cai N., Lane R., Ponce N.S., Topkas E., Inder K., et al. Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget. 2016;7:43570–43587. doi: 10.18632/oncotarget.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shao Y., Chen T., Zheng X., Yang S., Xu K., Chen X., Xu F., Wang L., Shen Y., Wang T., et al. Colorectal cancer-derived small extracellular vesicles establish an inflammatory premetastatic niche in liver metastasis. Carcinogenesis. 2018;39:1368–1379. doi: 10.1093/carcin/bgy115. [DOI] [PubMed] [Google Scholar]
- 160.Dörsam B., Bösl T., Reiners K.S., Barnert S., Schubert R., Shatnyeva O., Zigrino P., Engert A., Hansen H.P., von Strandmann E.P. Hodgkin Lymphoma-Derived Extracellular Vesicles Change the Secretome of Fibroblasts Toward a CAF Phenotype. Front. Immunol. 2018;9:1358. doi: 10.3389/fimmu.2018.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Endzeliņš E., Ābols A., Bušs A., Zandberga E., Palviainen M., Siljander P., Linē A. Extracellular Vesicles Derived from Hypoxic Colorectal Cancer Cells Confer Metastatic Phenotype to Non-metastatic Cancer Cells. Anticancer Res. 2018;38:5139–5147. doi: 10.21873/anticanres.12836. [DOI] [PubMed] [Google Scholar]
- 162.Ito A., Kagawa S., Sakamoto S., Kuwada K., Kajioka H., Yoshimoto M., Kikuchi S., Kuroda S., Yoshida R., Tazawa H., et al. Extracellular vesicles shed from gastric cancer mediate protumor macrophage differentiation. BMC Cancer. 2021;21:102. doi: 10.1186/s12885-021-07816-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ito M., Kudo K., Higuchi H., Otsuka H., Tanaka M., Fukunishi N., Araki T., Takamatsu M., Ino Y., Kimura Y., et al. Proteomic and phospholipidomic characterization of extracellular vesicles inducing tumor microenvironment in Epstein-Barr virus-associated lymphomas. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021;35:e21505. doi: 10.1096/fj.202002730R. [DOI] [PubMed] [Google Scholar]
- 164.Dumontet E., Pangault C., Roulois D., Desoteux M., Léonard S., Marchand T., Latour M., Legoix P., Loew D., Dingli F., et al. Extracellular vesicles shed by follicular lymphoma B cells promote polarization of the bone marrow stromal cell niche. Blood. 2021;138:57–70. doi: 10.1182/blood.2020008791. [DOI] [PubMed] [Google Scholar]
- 165.Rocha S., Teles S.P., Azevedo M., Oliveira P., Carvalho J., Oliveira C. Gastric Cancer Extracellular Vesicles Tune the Migration and Invasion of Epithelial and Mesenchymal Cells in a Histotype-Dependent Manner. Int. J. Mol. Sci. 2019;20:2608. doi: 10.3390/ijms20112608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ramírez-Ricardo J., Leal-Orta E., Martínez-Baeza E., Ortiz-Mendoza C., Breton-Mora F., Herrera-Torres A., Elizalde-Acosta I., Cortes-Reynosa P., Thompson-Bonilla R., Salazar E.P. Circulating extracellular vesicles from patients with breast cancer enhance migration and invasion via a Src-dependent pathway in MDA-MB-231 breast cancer cells. Mol. Med. Rep. 2020;22:1932–1948. doi: 10.3892/mmr.2020.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dourado M.R., Korvala J., Åström P., de Oliveira C.E., Cervigne N.K., Mofatto L.S., Bastos D.C., Messetti A.C.P., Graner E., Leme A.F.P., et al. Extracellular vesicles derived from cancer-associated fibroblasts induce the migration and invasion of oral squamous cell carcinoma. J. Extracell. Vesicles. 2019;8:1578525. doi: 10.1080/20013078.2019.1578525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sceneay J., Smyth M.J., Möller A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 169.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Qiao Z., Zhang Y., Ge M., Liu S., Jiang X., Shang Z., Liu H., Cao C., Xiao H. Cancer Cell Derived Small Extracellular Vesicles Contribute to Recipient Cell Metastasis Through Promoting HGF/c-Met Pathway. Mol. Cell. Proteom. 2019;18:1619–1629. doi: 10.1074/mcp.RA119.001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Casadei L., Calore F., Braggio D.A., Zewdu A., Deshmukh A.A., Fadda P., Lopez G., Wabitsch M., Song C., Leight J.L., et al. MDM2 Derived from Dedifferentiated Liposarcoma Extracellular Vesicles Induces MMP2 Production from Preadipocytes. Cancer Res. 2019;79:4911–4922. doi: 10.1158/0008-5472.CAN-19-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Barenholz-Cohen T., Merkher Y., Haj J., Shechter D., Kirchmeier D., Shaked Y., Weihs D. Lung mechanics modifications facilitating metastasis are mediated in part by breast cancer-derived extracellular vesicles. Int. J. Cancer. 2020;147:2924–2933. doi: 10.1002/ijc.33229. [DOI] [PubMed] [Google Scholar]
- 173.Henrich S.E., McMahon K.M., Plebanek M.P., Calvert A.E., Feliciano T.J., Parrish S., Tavora F., Mega A., de Souza A., Carneiro B.A., et al. Prostate cancer extracellular vesicles mediate intercellular communication with bone marrow cells and promote metastasis in a cholesterol-dependent manner. J. Extracell. Vesicles. 2020;10:e12042. doi: 10.1002/jev2.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.López A.A., Mazariegos M.S., Capuano A., Ximénez-Embún P., Hergueta-Redondo M., Recio J.Á., Muñoz E., Al-Shahrour F., Muñoz J., Megías D., et al. Inactivation of EMILIN-1 by Proteolysis and Secretion in Small Extracellular Vesicles Favors Melanoma Progression and Metastasis. Int. J. Mol. Sci. 2021;22:7406. doi: 10.3390/ijms22147406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wills C.A., Liu X., Chen L., Zhao Y., Dower C.M., Sundstrom J., Wang H.-G. Chemotherapy-Induced Upregulation of Small Extracellular Vesicle-Associated PTX3 Accelerates Breast Cancer Metastasis. Cancer Res. 2021;81:452–463. doi: 10.1158/0008-5472.CAN-20-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Samuel M., Fonseka P., Sanwlani R., Gangoda L., Chee S.H., Keerthikumar S., Spurling A., Chitti S.V., Zanker D., Ang C.-S., et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021;12:3950. doi: 10.1038/s41467-021-24273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shenoy G.N., Loyall J., Maguire O., Iyer V., Kelleher R.J.J., Minderman H., Wallace P.K., Odunsi K., Balu-Iyer S.V., Bankert R.B. Exosomes Associated with Human Ovarian Tumors Harbor a Reversible Checkpoint of T-cell Responses. Cancer Immunol. Res. 2018;6:236–247. doi: 10.1158/2326-6066.CIR-17-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cianciaruso C., Beltraminelli T., Duval F., Nassiri S., Hamelin R., Mozes A., Gallart-Ayala H., Torres G.C., Torchia B., Ries C.H., et al. Molecular Profiling and Functional Analysis of Macrophage-Derived Tumor Extracellular Vesicles. Cell Rep. 2019;27:3062–3080.e11. doi: 10.1016/j.celrep.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Crawford S. Is it time for a new paradigm for systemic cancer treatment? Lessons from a century of cancer chemotherapy. Front. Pharmacol. 2013;4:68. doi: 10.3389/fphar.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Scott A.M., Wolchok J.D., Old L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 181.Wang J., Hendrix A., Hernot S., Lemaire M., de Bruyne E., van Valckenborgh E., Lahoutte T., de Wever O., Vanderkerken K., Menu E. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124:555–566. doi: 10.1182/blood-2014-03-562439. [DOI] [PubMed] [Google Scholar]
- 182.Choi D.-Y., You S., Jung J.H., Lee J.C., Rho J.K., Lee K.Y., Freeman M.R., Kim K.P., Kim J. Extracellular vesicles shed from gefitinib-resistant nonsmall cell lung cancer regulate the tumor microenvironment. Proteomics. 2014;14:1845–1856. doi: 10.1002/pmic.201400008. [DOI] [PubMed] [Google Scholar]
- 183.Ozawa P.M.M., Alkhilaiwi F., Cavalli I.J., Malheiros D., Ribeiro E.M.d.F., Cavalli L.R. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res. Treat. 2018;172:713–723. doi: 10.1007/s10549-018-4925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hoshino A., Kim H.S., Bojmar L., Gyan K.E., Cioffi M., Hernandez J., Zambirinis C.P., Rodrigues G., Molina H., Heissel S., et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020;182:1044–1061.e18. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Takeuchi T., Mori K., Sunayama H., Takano E., Kitayama Y., Shimizu T., Hirose Y., Inubushi S., Sasaki R., Tanino H. Antibody-Conjugated Signaling Nanocavities Fabricated by Dynamic Molding for Detecting Cancers Using Small Extracellular Vesicle Markers from Tears. J. Am. Chem. Soc. 2020;142:6617–6624. doi: 10.1021/jacs.9b13874. [DOI] [PubMed] [Google Scholar]
- 186.Biggs C.N., Siddiqui K.M., Al-Zahrani A.A., Pardhan S., Brett S.I., Guo Q.Q., Yang J., Wolf P., Power N.E., Durfee P.N., et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget. 2016;7:8839–8849. doi: 10.18632/oncotarget.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bijnsdorp I.V., Maxouri O., Kardar A., Schelfhorst T., Piersma S.R., Pham T.V., Vis A., van Moorselaar R.J., Jimenez C.R. Feasibility of urinary extracellular vesicle proteome profiling using a robust and simple, clinically applicable isolation method. J. Extracell. Vesicles. 2017;6:1313091. doi: 10.1080/20013078.2017.1313091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Skotland T., Ekroos K., Kauhanen D., Simolin H., Seierstad T., Berge V., Sandvig K., Llorente A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer. 2017;70:122–132. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 189.Smith J.T., Wunsch B.H., Dogra N., Ahsen M.E., Lee K., Yadav K.K., Weil R., Pereira M.A., Patel J.V., Duch E.A., et al. Integrated nanoscale deterministic lateral displacement arrays for separation of extracellular vesicles from clinically-relevant volumes of biological samples. Lab Chip. 2018;18:3913–3925. doi: 10.1039/C8LC01017J. [DOI] [PubMed] [Google Scholar]
- 190.Erdbrügger U., Blijdorp C.J., Bijnsdorp I.V., Borràs F.E., Burger D., Bussolati B., Byrd J.B., Clayton A., Dear J.W., Falcón-Pérez J.M., et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2021;10:e12093. doi: 10.1002/jev2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mercadal M., Herrero C., López-Rodrigo O., Castells M., de la Fuente A., Vigués F., Bassas L., Larriba S. Impact of Extracellular Vesicle Isolation Methods on Downstream Mirna Analysis in Semen: A Comparative Study. Int. J. Mol. Sci. 2020;21:5949. doi: 10.3390/ijms21175949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sun Y., Xia Z., Shang Z., Sun K., Niu X., Qian L., Fan L.-Y., Cao C.-X., Xiao H. Facile preparation of salivary extracellular vesicles for cancer proteomics. Sci. Rep. 2016;6:24669. doi: 10.1038/srep24669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thakur A., Qiu G., Ng S.-P., Guan J., Yue J., Lee Y., Wu C.-M.L. Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens. Bioelectron. 2017;94:400–407. doi: 10.1016/j.bios.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 194.Testa A., Venturelli E., Brizzi M.F. Extracellular Vesicles as a Novel Liquid Biopsy-Based Diagnosis for the Central Nervous System, Head and Neck, Lung, and Gastrointestinal Cancers: Current and Future Perspectives. Cancers. 2021;13:2792. doi: 10.3390/cancers13112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vykoukal J., Sun N., Aguilar-Bonavides C., Katayama H., Tanaka I., Fahrmann J.F., Capello M., Fujimoto J., Aguilar M., Wistuba I.I., et al. Plasma-derived extracellular vesicle proteins as a source of biomarkers for lung adenocarcinoma. Oncotarget. 2017;8:95466–95480. doi: 10.18632/oncotarget.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Urban S.K., Sänger H., Krawczyk M., Julich-Haertel H., Willms A., Ligocka J., Azkargorta M., Mocan T., Kahlert C., Kruk B., et al. Synergistic effects of extracellular vesicle phenotyping and AFP in hepatobiliary cancer differentiation. Liver Int. Off. J. Int. Assoc. Study Liver. 2020;40:3103–3116. doi: 10.1111/liv.14585. [DOI] [PubMed] [Google Scholar]
- 197.Dobra G., Bukva M., Szabo Z., Bruszel B., Harmati M., Gyukity-Sebestyen E., Jenei A., Szucs M., Horvath P., Biro T., et al. Small Extracellular Vesicles Isolated from Serum May Serve as Signal-Enhancers for the Monitoring of CNS Tumors. Int. J. Mol. Sci. 2020;21:5359. doi: 10.3390/ijms21155359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yoh K.E., Lowe C.J., Mahajan S., Suttmann R., Nguy T., Reichelt M., Yang J., Melendez R., Li Y., Molinero L., et al. Enrichment of circulating tumor-derived extracellular vesicles from human plasma. J. Immunol. Methods. 2021;490:112936. doi: 10.1016/j.jim.2020.112936. [DOI] [PubMed] [Google Scholar]
- 199.Wu F., Gu Y., Kang B., Heskia F., Pachot A., Bonneville M., Wei P., Liang J. PD-L1 detection on circulating tumor-derived extracellular vesicles (T-EVs) from patients with lung cancer. Transl. Lung Cancer Res. 2021;10:2441–2451. doi: 10.21037/tlcr-20-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Novizio N., Belvedere R., Pessolano E., Tosco A., Porta A., Perretti M., Campiglia P., Filippelli A., Petrella A. Annexin A1 Released in Extracellular Vesicles by Pancreatic Cancer Cells Activates Components of the Tumor Microenvironment, through Interaction with the Formyl-Peptide Receptors. Cells. 2020;9:2719. doi: 10.3390/cells9122719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ko J., Bhagwat N., Black T., Yee S.S., Na Y.-J., Fisher S., Kim J., Carpenter E.L., Stanger B.Z., Issadore D. miRNA Profiling of Magnetic Nanopore-Isolated Extracellular Vesicles for the Diagnosis of Pancreatic Cancer. Cancer Res. 2018;78:3688–3697. doi: 10.1158/0008-5472.CAN-17-3703. [DOI] [PubMed] [Google Scholar]
- 202.Hu J., Kwak K.J., Shi J., Yu B., Sheng Y., Lee L.J. Overhang molecular beacons encapsulated in tethered cationic lipoplex nanoparticles for detection of single-point mutation in extracellular vesicle-associated RNAs. Biomaterials. 2018;183:20–29. doi: 10.1016/j.biomaterials.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 203.Jiao Y.-J., Jin D.-D., Jiang F., Liu J.-X., Qu L.-S., Ni W.-K., Liu Z.-X., Lu C.-H., Ni R.-Z., Zhu J., et al. Characterization and proteomic profiling of pancreatic cancer-derived serum exosomes. J. Cell. Biochem. 2019;120:988–999. doi: 10.1002/jcb.27465. [DOI] [PubMed] [Google Scholar]
- 204.Yu S., Li Y., Liao Z., Wang Z., Wang Z., Li Y., Qian L., Zhao J., Zong H., Kang B., et al. Plasma extracellular vesicle long RNA profiling identifies a diagnostic signature for the detection of pancreatic ductal adenocarcinoma. Gut. 2020;69:540–550. doi: 10.1136/gutjnl-2019-318860. [DOI] [PubMed] [Google Scholar]
- 205.Kumar S.R., Kimchi E.T., Manjunath Y., Gajagowni S., Stuckel A.J., Kaifi J.T. RNA cargos in extracellular vesicles derived from blood serum in pancreas associated conditions. Sci. Rep. 2020;10:2800. doi: 10.1038/s41598-020-59523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang Z., LaRiviere M.J., Ko J., Till J.E., Christensen T., Yee S.S., Black T.A., Tien K., Lin A., Shen H., et al. A Multianalyte Panel Consisting of Extracellular Vesicle miRNAs and mRNAs, cfDNA, and CA19-9 Shows Utility for Diagnosis and Staging of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:3248–3258. doi: 10.1158/1078-0432.CCR-19-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li P., Wang J., Gao M., Wang J., Ma Y., Gu Y. Membrane Feature-Inspired Profiling of Extracellular Vesicles for Pancreatic Cancer Diagnosis. Anal. Chem. 2021;93:9860–9868. doi: 10.1021/acs.analchem.1c01712. [DOI] [PubMed] [Google Scholar]
- 208.Bernard V., Kim D.U., Lucas F.A.S., Castillo J., Allenson K., Mulu F.C., Stephens B.M., Huang J., Semaan A., Guerrero P.A., et al. Circulating Nucleic Acids Are Associated With Outcomes of Patients with Pancreatic Cancer. Gastroenterology. 2019;156:108–118.e4. doi: 10.1053/j.gastro.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kim J.R., Han K., Han Y., Kang N., Shin T.-S., Park H.J., Kim H., Kwon W., Lee S., Kim Y.-K., et al. Microbiome Markers of Pancreatic Cancer Based on Bacteria-Derived Extracellular Vesicles Acquired from Blood Samples: A Retrospective Propensity Score Matching Analysis. Biology. 2021;10:219. doi: 10.3390/biology10030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Julich-Haertel H., Urban S.K., Krawczyk M., Willms A., Jankowski K., Patkowski W., Kruk B., Krasnodębski M., Ligocka J., Schwab R., et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2017;67:282–292. doi: 10.1016/j.jhep.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 211.Arbelaiz A., Azkargorta M., Krawczyk M., Santos-Laso A., Lapitz A., Perugorria M.J., Erice O., Gonzalez E., Jimenez-Agüero R., Lacasta A., et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017;66:1125–1143. doi: 10.1002/hep.29291. [DOI] [PubMed] [Google Scholar]
- 212.Bao S., Hu T., Liu J., Su J., Sun J., Ming Y., Li J., Wu N., Chen H., Zhou M. Genomic instability-derived plasma extracellular vesicle-microRNA signature as a minimally invasive predictor of risk and unfavorable prognosis in breast cancer. J. Nanobiotechnol. 2021;19:22. doi: 10.1186/s12951-020-00767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ahmed S.H., Espinoza-Sánchez N.A., El-Damen A., Fahim S.A., Badawy M.A., Greve B., El-Shinawi M., Götte M., Ibrahim S.A. Small extracellular vesicle-encapsulated miR-181b-5p, miR-222-3p and let-7a-5p: Next generation plasma biopsy-based diagnostic biomarkers for inflammatory breast cancer. PLoS ONE. 2021;16:e0250642. doi: 10.1371/journal.pone.0250642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dong L., Lin W., Qi P., Xu M.-D., Wu X., Ni S., Huang D., Weng W.-W., Tan C., Sheng W., et al. Circulating Long RNAs in Serum Extracellular Vesicles: Their Characterization and Potential Application as Biomarkers for Diagnosis of Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2016;25:1158–1166. doi: 10.1158/1055-9965.EPI-16-0006. [DOI] [PubMed] [Google Scholar]
- 215.Kosaka N., Yoshioka Y., Tominaga N., Hagiwara K., Katsuda T., Ochiya T. Dark side of the exosome: The role of the exosome in cancer metastasis and targeting the exosome as a strategy for cancer therapy. Future Oncol. 2014;10:671–681. doi: 10.2217/fon.13.222. [DOI] [PubMed] [Google Scholar]
- 216.Moloudizargari M., Asghari M.H., Goel A. The therapeutic triad of extracellular vesicles: As drug targets, as drugs, and as drug carriers. Biochem. Pharmacol. 2021;192:114714. doi: 10.1016/j.bcp.2021.114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Baglio S.R., Lagerweij T., Pérez-Lanzón M., Ho X.D., Léveillé N., Melo S.A., Cleton-Jansen A.-M., Jordanova E.S., Roncuzzi L., Greco M., et al. Blocking Tumor-Educated MSC Paracrine Activity Halts Osteosarcoma Progression. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:3721–3733. doi: 10.1158/1078-0432.CCR-16-2726. [DOI] [PubMed] [Google Scholar]
- 218.Tominaga N., Yoshioka Y., Ochiya T. A novel platform for cancer therapy using extracellular vesicles. Adv. Drug Deliv. Rev. 2015;95:50–55. doi: 10.1016/j.addr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 219.Hatzidaki E., Vlachou I., Elka A., Georgiou E., Papadimitriou M., Iliopoulos A., Papasotiriou I. The use of serum extracellular vesicles for novel small molecule inhibitor cell delivery. Anticancer Drugs. 2019;30:271–280. doi: 10.1097/CAD.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 220.Pomatto M.A.C., Bussolati B., D’Antico S., Ghiotto S., Tetta C., Brizzi M.F., Camussi G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019;13:133–144. doi: 10.1016/j.omtm.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Garofalo M., Villa A., Brunialti E., Crescenti D., Dell’Omo G., Kuryk L., Vingiani A., Mazzaferro V., Ciana P. Cancer-derived EVs show tropism for tissues at early stage of neoplastic transformation. Nanotheranostics. 2021;5:1–7. doi: 10.7150/ntno.47226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Haney M.J., Zhao Y., Jin Y.S., Li S.M., Bago J.R., Klyachko N.L., Kabanov A.V., Batrakova E.V. Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2020;15:487–500. doi: 10.1007/s11481-019-09884-9. [DOI] [PubMed] [Google Scholar]
- 223.Lee H., Park H., Noh G.J., Lee E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018;202:323–333. doi: 10.1016/j.carbpol.2018.08.141. [DOI] [PubMed] [Google Scholar]
- 224.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 225.Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Mark M.T., Molina H., Kohsaka S., di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wei Z., Chen Z., Zhao Y., Fan F., Xiong W., Song S., Yin Y., Hu J., Yang K., Yang L., et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials. 2021;275:121000. doi: 10.1016/j.biomaterials.2021.121000. [DOI] [PubMed] [Google Scholar]
- 227.Wan Y., Wang L., Zhu C., Zheng Q., Wang G., Tong J., Fang Y., Xia Y., Cheng G., He X., et al. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018;78:798–808. doi: 10.1158/0008-5472.CAN-17-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 229.Asangani I.A., Rasheed S.A.K., Nikolova D.A., Leupold J.H., Colburn N.H., Post S., Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 230.Shi R., Wang P.-Y., Li X.-Y., Chen J.-X., Li Y., Zhang X.-Z., Zhang C.-G., Jiang T., Li W.-B., Ding W., et al. Exosomal levels of miRNA-21 from cerebrospinal fluids associated with poor prognosis and tumor recurrence of glioma patients. Oncotarget. 2015;6:26971–26981. doi: 10.18632/oncotarget.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Katsuda T., Kosaka N., Takeshita F., Ochiya T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics. 2013;13:1637–1653. doi: 10.1002/pmic.201200373. [DOI] [PubMed] [Google Scholar]
- 232.Li T., Zhou X., Wang J., Liu Z., Han S., Wan L., Sun X., Chen H. Adipose-derived mesenchymal stem cells and extracellular vesicles confer antitumor activity in preclinical treatment of breast cancer. Pharmacol. Res. 2020;157:104843. doi: 10.1016/j.phrs.2020.104843. [DOI] [PubMed] [Google Scholar]
- 233.Vracar T.C., Zuo J., Park J., Azer D., Mikhael C., Holliday S.A., Holsey D., Han G., VonMoss L., Neubert J.K., et al. Enoxacin and bis-enoxacin stimulate 4T1 murine breast cancer cells to release extracellular vesicles that inhibit osteoclastogenesis. Sci. Rep. 2018;8:16182. doi: 10.1038/s41598-018-34698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhu L., Kalimuthu S., Oh J.M., Gangadaran P., Baek S.H., Jeong S.Y., Lee S.-W., Lee J., Ahn B.-C. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials. 2019;191:38–50. doi: 10.1016/j.biomaterials.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 235.De Camargo L.C.B., Guaddachi F., Bergerat D., Ourari N., Coillard L., Parietti V., le Bras M., Lehmann-Che J., Jauliac S. Extracellular vesicles produced by NFAT3-expressing cells hinder tumor growth and metastatic dissemination. Sci. Rep. 2020;10:8964. doi: 10.1038/s41598-020-65844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]