Abstract
Purpose
Regarding epidemiological studies, the role of vitamin D in musculoskeletal functionality (muscle weakness and physical performance) among elderly people is still controversial. The purpose of the present study was to investigate the associations between 25-hydroxyvitamin D [25(OH)D] and physical performance among community-dwelling middle-aged and old Japanese men and women.
Methods
The subjects were community-dwelling 297 men and 415 women aged 50 years and over. Data on height (m) and weight (kg) were collected. Serum 25(OH)D, parathyroid hormone, calcium, and albumin levels were measured. Serum 25(OH)D was classified into deficiency group: < 20 ng/mL, insufficiency group: 20–30 ng/mL, and sufficiency group: ≧ 30 ng/mL. Physical performance was assessed by grip strength, chair stand time, and functional reach. Information on current smoking, alcohol drinking, regular exercise, any comorbidities (hypertension, heart disease, diabetes mellitus, lung disease, and stroke), and pain (lumbar and knee) was collected.
Results
Vitamin D deficiency and insufficiency based on serum 25(OH)D levels were observed in 15.2% and 56.9% of men and 52.0% and 43.6% of women, respectively. In men, higher serum 25(OH)D levels were associated with better grip strength (p for trend = 0.003), chair stand time (p for trend = 0.042), and functional reach (p for trend <0.001). On the other hand, these parameters were not associated with serum 25(OH)D levels in women.
Conclusion
A higher level of serum 25(OH)D was associated with better physical performance in men but not in women.
Introduction
Vitamin D plays an important role in increasing the absorption of calcium and phosphate for the mineralization of the skeleton [1]. Vitamin D is produced in the skin by exposure to ultraviolet light or can be obtained orally which is then hydroxylated by the liver to the primary circulating form of 25-hydroxyvitamin D [25(OH)D], and further hydroxylated by the kidney to the active form of 1,25-dihydroxyvitamin D [1,25(OH)2D] [2]. Serum levels of 25(OH)D are a good marker of vitamin D levels in the body. Vitamin D deficiency causes secondary hyperparathyroidism, high bone turnover, bone loss, mineralization defects, and hip and other fractures [1]. Furthermore, vitamin D deficiency has been associated with increased risks of cancers, cardiovascular disease [2], and dementia [3].
In vitro studies have shown that the active form of vitamin D, calcitriol, drives cellular differentiation and proliferation by activating vitamin D receptors (VDR) in the nucleus of myocytes I [4, 5]. Endo et al. [6] have shown that VDR gene-deleted mice exhibited abnormal skeletal muscle development.
Regarding epidemiological studies, the role of vitamin D in musculoskeletal functionality (muscle weakness and physical performance) among elderly people is still controversial. Some studies reported the association between vitamin D deficiency and muscle weakness or poor physical performance [7–9]. The others did not show any associations [10–12].
The purpose of this study is to examine the associations of 25(OH)D with physical performance among community-dwelling people in Japan.
Materials and methods
Subjects
The subjects were community-dwelling men and women aged 50 years and over in Unzen city, Nagasaki Prefecture, Japan. The target population was approximately 13,000. Unzen City is located at latitude (N 32° 50’, E 130° 11’) and the residence area is almost located at the seaside. The main industries are agriculture, fishery, and tourism. This cross-sectional study used data obtained from periodic health examinations conducted from 2011 to 2013 (May to November; The Unzen Study). A total of 730 subjects (301 men and 429 women) participated in this study. Among those, participants with missing values were excluded, leaving 297 men [mean (standard deviation [SD]) age; 68.0 (8.3) years, range: 50–92 years] and 415 women [mean (SD) age; 67.7 (7.7) years, range: 50–89 years] for the final data analysis. In this study, 7 participants (1 man: 6 women) used vitamin D derivatives. Informed consent was obtained from all individual participants included in the study. This study was approved by the Ethics Committee of Nagasaki University Graduate School of Biomedical Sciences (11072739–2). All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Measurements
Participants’ height was measured while shoeless using a wall-mounted stadiometer and weight was measured with the participant in light clothing, shoeless using a daily calibrated standard scale. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Physical performance was assessed by handgrip strength, chair stand time, and functional reach. Grip strength was evaluated as an index of muscle strength in the upper limbs and was measured using a hydraulic hand dynamometer (Jamar hydraulic hand dynamometer; Lafayette Instrument Company, Inc., Lafayette, IN, US). Chair stand time was evaluated as an index of muscle strength in the lower limbs. It was quantified as the time taken to stand up from a standard chair and sit down five times, without the assistance of their arms. Functional reach was evaluated as an index of balance and was quantified as the difference between the initial point (standing comfortably upright, facing forward, hand in a fist, with the arm extended) and the reaching point (reaching forward as far as possible) without stepping or losing balance. All physical performance measures were performed twice and their excellent values were analyzed. When the test was conducted only once, the value was adopted.
Questionnaire
Information on current smoking (yes/no), alcohol drinking (≧ 40 g/day in men and ≧ 20 g/day in women), regular exercise (at least 30 min twice per week) was collected by interview. Participants were asked if they had any comorbidities (hypertension, heart disease, diabetes mellitus, lung disease, and stroke) and pain (lumbar and knee). Comorbidity was defined as per diagnosis by a physician.
Blood data
Fasting blood samples were collected, and 25(OH)D, parathyroid hormone (PTH), calcium, and albumin levels were measured. Serum 25(OH)D was measured by chemiluminescence enzyme immunoassay (CLEIA) and PTH by electrochemiluminescence immunoassay (ECLIA). Serum 25(OH)D levels ≥ 30 ng/mL, 20–30 ng/mL, and < 20 ng/mL were defined as vitamin D sufficiency, insufficiency, and deficiency, respectively.
Statistical analysis
The student’s t-test was used to compare continuous variables and the chi- squared test to compare categorical variables between men and women. Simple correlation analysis and simple regression analysis were performed to examine the correlation between serum 25(OH)D and physical performance measures (grip strength, chair stand time, and functional reach). Differences in means by vitamin D status (deficiency, insufficiency, and sufficiency) were examined using one-way analysis of variance (ANOVA) and linear regression analysis. Analysis of covariance (ANCOVA) and multiple linear regression analysis were used to compare serum 25(OH)D and physical performance measures adjusting for age, BMI, PTH, albumin, calcium, current smoking, alcohol drinking, exercise, comorbidities (hypertension, heart disease, diabetes mellitus, lung disease, and stroke) and pain (lumbar and knee). A p-value of less than 0.05 was considered significant. The data were analyzed using IBM SPSS statistics version 25 (Armonk, NY, US).
Results
Table 1 shows the characteristics of the participants. Serum 25(OH)D levels in men (25.9±5.8 ng/mL) were significantly higher compared to women (20.1±5.5 ng/mL) (p<0.001). The proportions of subjects with vitamin D deficiency and insufficiency were 15.2% and 56.9% in men and 52.0% and 43.6% in women, respectively. The prevalence of vitamin D deficiency was significantly higher in women than that in men.
Table 1. Characteristics of the participants.
| Variable | Men (n = 297) | Women (n = 415) | p-value |
|---|---|---|---|
| Age (years) | 68.0±8.3 | 67.7±7.7 | .639 |
| Height (cm) | 163.3±6.6 | 151.3±5.7 | <.001 |
| Weight (kg) | 62.4±9.9 | 50.6±7.9 | <.001 |
| Body mass index (kg/m2) | 23.3±2.9 | 22.1±3.1 | <.001 |
| Serum | |||
| 25(OH)D (ng/mL) | 25.9±5.8 | 20.1±5.5 | <.001 |
| Parathyroid hormone (pg/mL) | 37.7±16.2 | 41.0±17.2 | .010 |
| Albumin (g/mL) | 4.3±0.2 | 4.3±0.2 | .999 |
| Calcium (mg/mL) | 9.2±0.4 | 9.2±0.3 | .174 |
| Physical performance measures | |||
| Grip strength (kg) | 37.1±8.6 | 24.4±5.3 | <.001 |
| Chair stand time (sec) | 7.2±2.8 | 7.3±2.2 | .891 |
| Functional reach (cm) | 35.8±8.2 | 33.8±7.1 | <.001 |
| n (%) | n (%) | ||
| Classification of 25(OH)D | <.001 | ||
| deficiency | 45 (15.2) | 216 (52.0) | |
| insufficiency | 169 (56.9) | 181 (43.6) | |
| sufficiency | 83 (27.9) | 18 (4.3) | |
| Comorbidities | |||
| Hypertension | 131 (44.1) | 169 (40.7) | .367 |
| Heart disease | 27 (9.1) | 22 (5.3) | .049 |
| Diabetes mellitus | 35 (11.8) | 13 (3.1) | <.001 |
| Lung disease | 13 (4.4) | 12 (2.9) | .288 |
| Stroke | 14 (4.7) | 15 (3.6) | .464 |
| Lumbar pain | 162 (54.5) | 248 (59.8) | .165 |
| Knee pain | 120 (40.4) | 167 (40.2) | .965 |
| Current smoking | 51 (17.2) | 5 (1.2) | <.001 |
| Alcohol drinking | 192 (64.6) | 51 (12.3) | <.001 |
| Exercise | 91 (30.6) | 146 (35.2) | .205 |
Data are shown as means±standard deviation or n (%).
25(OH)D: 25-hydroxyvitamin D.
Student’s t-test for continuous variables.
Chi-square test for categorical variables.
Figs 1 and 2 show the scatter plots between serum 25(OH)D and physical performance measures among middle-aged and old Japanese men and women. The correlation coefficients between serum 25(OH)D and physical performances (grip strength, chair stand time, and functional reach) were 0.208 (p<0.001), -0.153 (p = 0.008), and 0.254 (p<0.001) among men, respectively (Fig 1). Alternatively, there were no significant correlations between serum 25(OH)D or any physical performance measure among women (Fig 2).
Fig 1. The scatter plot between serum 25(OH)D and physical performance measures in men.
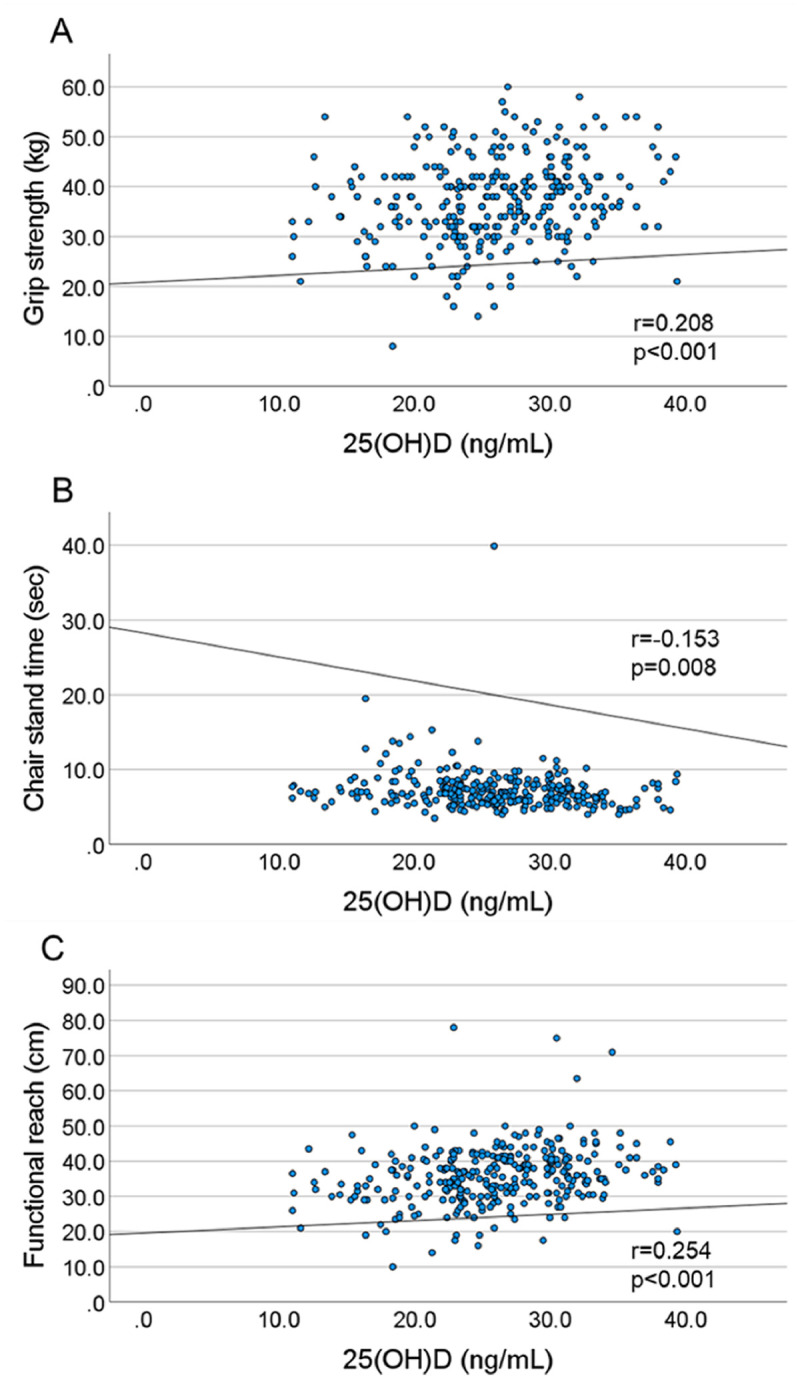
(A) Correlation between serum 25(OH)D and grip strength (r = 0.208, p<0.001). (B) Correlation between serum 25(OH)D and chair stand time (r = -0.153, p = 0.008). (C) Correlation between serum 25(OH)D and functional reach (r = 0.254, p<0.001).
Fig 2. The scatter plot between serum 25(OH)D and physical performance measures in women.
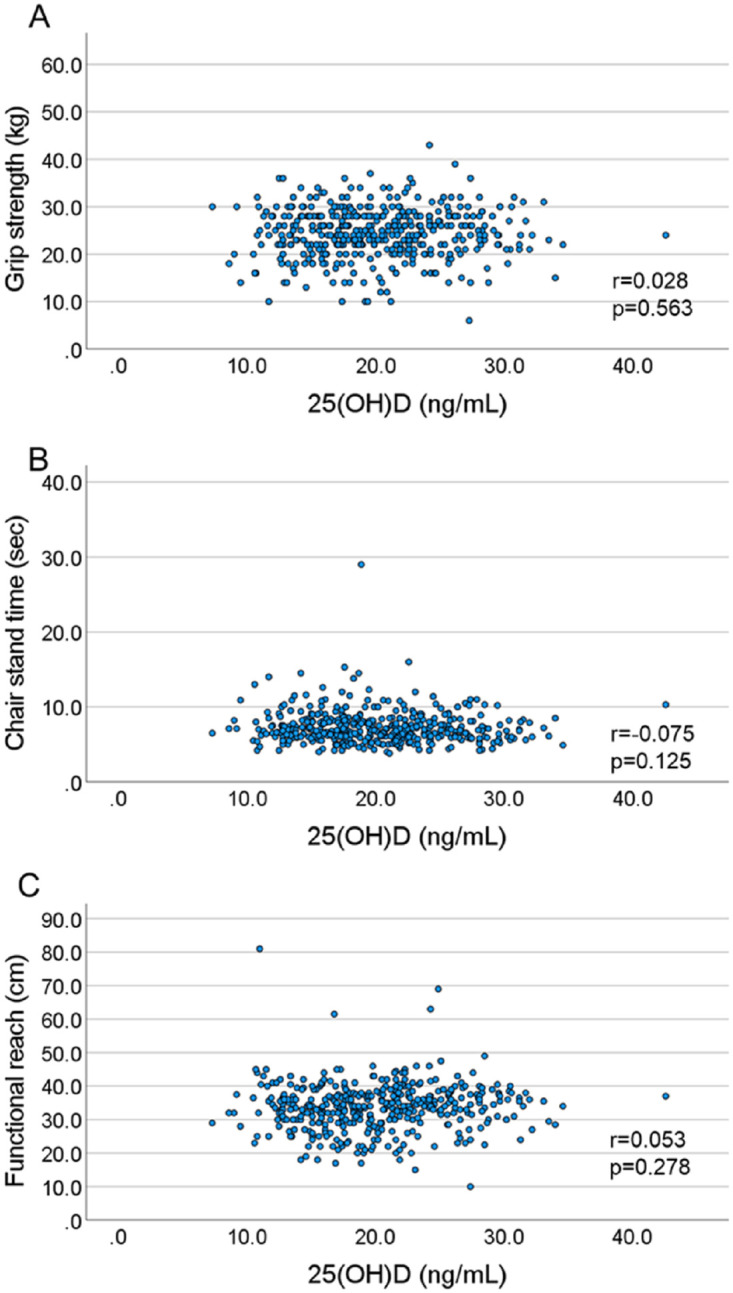
(A) Correlation between serum 25(OH)D and grip strength in women (r = 0.028, p = 0.563). (B) Correlation between serum 25(OH)D and chair stand time in women (r = -0.075, p = 0.125). (C) Correlation between serum 25(OH)D and functional reach in women (r = 0.053, p = 0.278).
Table 2 shows the means (SDs) of physical performance measures according to serum 25(OH)D levels. In men, all physical performance measures were significantly associated with serum 25(OH)D levels (grip strength, chair stand time, and functional reach: p for trend = 0.002, 0.006, and <0.001, respectively). In women, chair stand time (p for trend = 0.022) and functional reach (p for trend = 0.048) were significantly associated with serum 25(OH)D levels, whereas grip strength was not associated.
Table 2. Means (standard deviations) of physical performance measures according to 25-hydroxyvitamin D [25(OH)D] levels.
| Variable | Deficiency | Insufficiency | Sufficiency | p for trend |
|---|---|---|---|---|
| Men | (n = 45) | (n = 169) | (n = 83) | |
| Grip strength (kg) | 34.8±8.3 | 36.5±8.8 | 39.4±7.9 | .002 |
| Chair stand time (sec) | 8.2±2.9 | 7.2±3.1 | 6.7±1.5 | .006 |
| Functional reach (cm) | 32.0±7.3 | 35.5±7.8 | 38.5±8.5 | <.001 |
| Women | (n = 216) | (n = 181) | (n = 18) | |
| Grip strength (kg) | 24.3±5.3 | 24.6±5.4 | 24.1±4.7 | .746 |
| Chair stand time (sec) | 7.5±2.5 | 7.0±1.8 | 6.9±1.4 | .022 |
| Functional reach (cm) | 33.0±7.3 | 34.7±7.0 | 33.8±4.5 | .048 |
One-way analysis of variance (ANOVA) and linear regression analysis.
Table 3 shows the adjusted means (standard errors) for physical performance measures according to 25(OH)D levels. In men, all physical performance measures (grip strength, chair stand time, and functional reach) were significantly associated with serum 25(OH)D levels after adjusting for covariates (age, BMI, PTH, albumin, calcium, current smoking, alcohol drinking, exercise, hypertension, heart disease, diabetes mellitus, lung disease, stroke, lumbar pain, and knee pain). Higher serum 25(OH)D levels were associated with better physical functioning in grip strength (p for trend = 0.003), chair stand time (p for trend = 0.042), and functional reach (p for trend < 0.001). On the other hand, grip strength, chair stand time, and functional reach were not associated with serum 25(OH)D levels in women (p for trend = 0.825, 0.085, and 0.368, respectively).
Table 3. Adjusted means (standard error) of physical performance measures according to 25-hydroxyvitamin D [25(OH)D] levels.
| Variable | Deficiency | Insufficiency | Sufficiency | p for trend |
|---|---|---|---|---|
| Men | (n = 45) | (n = 169) | (n = 83) | |
| Grip strength (kg) | ||||
| age adjusted | 34.8 (1.1) | 36.8 (0.6) | 38.7 (0.8) | .003 |
| model 1 | 35.0 (1.1) | 36.8 (0.6) | 38.6 (0.8) | .007 |
| model 2 | 34.7 (1.1) | 36.9 (0.5) | 38.7 (0.8) | .003 |
| Chair stand time (sec) | ||||
| age adjusted | 8.2 (0.4) | 7.2 (0.2) | 6.9 (0.3) | .012 |
| model 1 | 8.1 (0.4) | 7.1 (0.2) | 7.0 (0.3) | .050 |
| model 2 | 8.1 (0.4) | 7.1 (0.2) | 7.0 (0.3) | .042 |
| Functional reach (cm) | ||||
| age adjusted | 32.0 (1.1) | 35.8 (0.6) | 38.0 (0.8) | <.001 |
| model 1 | 31.7 (1.1) | 35.8 (0.6) | 38.0 (0.8) | <.001 |
| model 2 | 31.7 (1.1) | 35.9 (0.6) | 38.0 (0.8) | <.001 |
| Women | (n = 216) | (n = 181) | (n = 18) | |
| Grip strength (kg) | ||||
| age adjusted | 24.4 (0.3) | 24.4 (0.4) | 24.0 (1.1) | .846 |
| model 1 | 24.4 (0.3) | 24.4 (0.4) | 24.3 (1.1) | .902 |
| model 2 | 24.5 (0.3) | 24.3 (0.4) | 24.4 (1.1) | .825 |
| Chair stand time (sec) | ||||
| age adjusted | 7.5 (0.1) | 7.1 (0.2) | 7.0 (0.5) | .049 |
| model 1 | 7.5 (0.1) | 7.1 (0.2) | 7.0 (0.5) | .061 |
| model 2 | 7.4 (0.1) | 7.1 (0.2) | 7.0 (0.5) | .085 |
| Functional reach (cm) | ||||
| age adjusted | 33.2 (0.5) | 34.5 (0.5) | 33.7 (1.6) | .099 |
| model 1 | 33.3 (0.5) | 34.5 (0.5) | 33.2 (1.5) | .234 |
| model 2 | 33.4 (0.5) | 34.3 (0.5) | 33.2 (1.5) | .368 |
Analysis of covariance (ANCOVA) and multiple linear regression analysis.
model 1: adjusted for age, BMI, parathyroid hormone, albumin, calcium, current smoking, alcohol drinking, and exercise.
model 2: adjusted for age, BMI, parathyroid hormone, albumin, calcium, current smoking, alcohol drinking and exercise, hypertension, heart disease, diabetes mellitus, lung disease, stroke, lumbar pain, and knee pain.
Discussion
We showed that serum 25(OH)D was associated with grip strength, chair stand time, and functional reach in men, after adjusting for age, BMI, and the other covariates. Men with 25(OH)D level < 20 ng/mL had poorer physical performances compared to those with level ≧ 30 ng/mL. In CHIANTI Study, vitamin D levels were significantly associated with short physical performance battery (SPPB) score in men and handgrip strength in both men and women [7]. In the Health, Aging, and Body Composition Study, participants with 25(OH)D < 50 nmol/L had significantly poorer physical performance and slower gait speed, lower knee extensor, and grip strength than those with 25(OH)D ≧75 nmol/L [13]. These reports support our results observed in men.
On the other hand, we did not observe the association between serum 25(OH)D and physical performance in women. Sufficiency of vitamin D may be a possible explanation for these contradictory findings between the sexes. Prevalence of sufficiency (25(OH)D≧ 30 ng/ml) was 27.9% in men, whereas only 4.3% in women. Serum 25(OH)D concentrations tended to be lower in women than in men [14]. Women in the present study often used long sleeves, hats, and gloves, and were more likely to use sunscreen during outdoor activities. Several studies showed that serum 25(OH)D was not associated with physical performance among populations with a sufficiency rate of < 10% [11, 15]. When vitamin D sufficiency is very low, the effect of vitamin D on muscle strength and physical performance may not be measured. However, Iolascon et, al [16] reported that serum 25(OH)D value ≧ 30n g/mL sufficiency was 49.4% among a group of Italian postmenopausal women, and a significant association between serum 25(OH)D concentration and grip strength, knee extension muscle strength, and short physical performance battery (SPPB) score. If vitamin D sufficiency was high in women, it may have affected the results of this study.
Muscle mass has been reported to be greater in men than in women [17]. Muscle mass in women in our study may be lower than the threshold at which vitamin D asserts effects, contributing to the lack of an association of vitamin D with muscle strength and physical performance. Further study is needed to include muscle mass measurement.
Low serum 25(OH)D is prevalent among the elderly. In this study, the overall prevalence of vitamin D insufficiency and deficiency was 49.2% and 36.7%, respectively, and was higher in women than in men (vitamin D insufficiency: men, 56.9%; women, 43.6%; vitamin D deficiency: men, 15.2%; women, 52.0%). Another study in Japan reported that the overall prevalence of vitamin D insufficiency and deficiency was 81.3% and 1.2%, respectively, and was higher in women than in men (vitamin D insufficiency: men, 72.1%; women, 86.3%; vitamin D deficiency: men, 0.3%; women, 1.7%) [18]. Previous studies have suggested that vitamin D supplementation results in less severe functional limitations, fewer falls, and fractures [19]. Treatment of low serum 25(OH)D may improve quality of life among the elderly through the prevention of poor physical functioning.
Serum 25(OH)D has been reported to vary seasonally [20–23]. Our study was conducted from May to Nov. Nakamura et al. [23] reported that serum 25(OH)D concentrations in May-Nov were relatively high. The results of our study may reflect serum 25(OH)D during the time of the year when they are generally higher, possibly as a result of sun exposure.
Vitamin D3 signaling enhanced the effect of physical exercise and increased locomotive ability in mice [24]. Mice with inactivation of VDR in myocytes (mVDR) had a lower grip strength and slower running speed than controls [25]. Levels of 25(OH)D3 were inversely associated with HbA1c levels, and for patients with diabetes, were lower 25(OH)D than controls [26]. Higher HbA1c was related to weaker grip strength in patients with diabetes [27, 28]. Since the prevalence of diabetes was higher among men in this study, some men with diabetes with vitamin D deficiency might induce weaker muscle strength and poor physical performance.
Some previous studies reported an association between vitamin D deficiency and sarcopenia (low muscle mass, weak muscle strength, and poor physical performance) [29–31], while some others did not [32, 33]. Kim et al. [31] reported that vitamin D levels were significantly lower in the sarcopenia group among both men and women. Conversely, there was no difference in the serum vitamin D level between participants with or without sarcopenia among both men and women [32, 33]. Li et al. [34] reported that their sarcopenia group had significantly lower 25(OH)D than the non-sarcopenia group among men, but not women, which was similar to our results. Women in the present study had lower serum 25(OH)D than men; thus relationships might be estimated for different ranges of serum 25(OH)D for men and women [35].
This study has several limitations. First, since this is a cross-sectional study, we could not provide a causal relationship between vitamin D and physical performance. Second, there is a possibility of selection bias because our subjects were periodic health examination participants. Third, we could not assess protein intake, calcium/vitamin supplementation, hours of sun exposure, or renal function which may affect vitamin D metabolism and cognitive function which may affect outdoor physical activity.
Conclusion
The present study showed that a higher level of serum 25(OH)D was associated with better physical performance in men but not women. These differences between the sexes may be due to the sufficiency of vitamin D or muscle mass. Further studies are needed to explore the association between vitamin D and physical performance.
Supporting information
(CSV)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This work was supported by JSPS KAKENHI Grant Number JP23370105.
References
- 1.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4): 477–501. doi: 10.1210/edrv.22.4.0437 . [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6) suppl: 1678S–1688S. doi: 10.1093/ajcn/80.6.1678S . [DOI] [PubMed] [Google Scholar]
- 3.Shen L, Ji HF. Vitamin D deficiency is associated with increased risk of Alzheimer’s disease and dementia: evidence from meta-analysis. Nutr J. 2015;14: 76. doi: 10.1186/s12937-015-0063-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. BioMed Res Int. 2015;2015: 953241. doi: 10.1155/2015/953241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem. 1985;260(15): 8882–8891. Epub 1985/07/25. doi: 10.1016/S0021-9258(17)39433-4, [DOI] [PubMed] [Google Scholar]
- 6.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144(12): 5138–5144. doi: 10.1210/en.2003-0502 . [DOI] [PubMed] [Google Scholar]
- 7.Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, et al. Association between vitamin D status and physical performance: the InCHIANTI study In J Gerontol A Biol Sci Med Sci. 2007;62(4): 440–446. doi: 10.1093/gerona/62.4.440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aspell N, Laird E, Healy M, Lawlor B, O’Sullivan M. Vitamin D Deficiency Is Associated With Impaired Muscle Strength And Physical Performance In Community-Dwelling Older Adults: Findings From The English Longitudinal Study Of Ageing. Clin Interv Aging. 2019;14: 1751–1761. doi: 10.2147/CIA.S222143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toffanello ED, Perissinotto E, Sergi G, Zambon S, Musacchio E, Maggi S, et al. Vitamin D and physical performance in elderly subjects: the Pro. V. PLOS ONE. 2012;7(4): e34950. doi: 10.1371/journal.pone.0034950 , [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaes AMM, Brouwer-Brolsma EM, Toussaint N, de Regt M, Tieland M, van Loon LJC, et al. The association between 25-hydroxyvitamin D concentration, physical performance and frailty status in older adults. Eur J Nutr. 2019;58(3): 1173–1181. doi: 10.1007/s00394-018-1634-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annweiler C, Beauchet O, Berrut G, Fantino B, Bonnefoy M, Herrmann FR, et al. Is there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the EPIDOS study. J Nutr Health Aging. 2009;13(2): 90–95. doi: 10.1007/s12603-009-0013-1 . [DOI] [PubMed] [Google Scholar]
- 12.Matheï C, Van Pottelbergh G, Vaes B, Adriaensen W, Gruson D, Degryse JM. No relation between vitamin D status and physical performance in the oldest old: results from the Belfrail study. Age Ageing. 2013;42(2): 186–190. doi: 10.1093/ageing/afs186 . [DOI] [PubMed] [Google Scholar]
- 13.Houston DK, Tooze JA, Neiberg RH, Hausman DB, Johnson MA, Cauley JA, et al. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: the Health, Aging, and Body Composition Study. Am J Epidemiol. 2012;176(11): 1025–1034. doi: 10.1093/aje/kws147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11): 1807–1820. doi: 10.1007/s00198-009-0954-6 . [DOI] [PubMed] [Google Scholar]
- 15.Kim BJ, Kwak MK, Lee SH, Koh JM. Lack of Association Between Vitamin D and Hand Grip Strength in Asians: A Nationwide Population-Based Study. Calcif Tissue Int. 2019;104(2): 152–159. doi: 10.1007/s00223-018-0480-7 . [DOI] [PubMed] [Google Scholar]
- 16.Iolascon G, Mauro GL, Fiore P, Cisari C, Benedetti MG, Panella L, et al. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicenter retrospective study. Eur J Phys Rehabil Med. 2018;54(5): 676–682. doi: 10.23736/S1973-9087.17.04533-6 . [DOI] [PubMed] [Google Scholar]
- 17.Seino S, Shinkai S, Iijima K, Obuchi S, Fujiwara Y, Yoshida H, et al. Reference Values and Age Differences in Body Composition of Community-Dwelling Older Japanese Men and Women: A Pooled Analysis of Four Cohort Studies. PLOS ONE. 2015;10(7): e0131975. doi: 10.1371/journal.pone.0131975 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura N, Muraki S, Oka H, Morita M, Yamada H, Tanaka S, et al. Profiles of vitamin D insufficiency and deficiency in Japanese men and women: association with biological, environmental, and nutritional factors and coexisting disorders: the ROAD study. Osteoporos Int. 2013;24(11): 2775–2787. doi: 10.1007/s00198-013-2372-z . [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20(2): 315–322. doi: 10.1007/s00198-008-0662-7 . [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Won Woo H, Kim J, Shin MH, Koh I, Youl Choi B, et al. Independent and interactive associations of season, dietary vitamin D, and vitamin D-related genetic variants with serum 25(OH)D in Korean adults aged 40 years or older. Endocr J. 2021;68(6): 701–711. doi: 10.1507/endocrj.EJ20-0519 . [DOI] [PubMed] [Google Scholar]
- 21.Karacan M, Usta A, Biçer S, Baktir G, İpek Gündogan G, Sancakli Usta C, et al. Serum vitamin D levels in healthy urban population at reproductive age: effects of age, gender and season. Cent Eur J Public Health. 2020;28(4): 306–312. doi: 10.21101/cejph.a5947 . [DOI] [PubMed] [Google Scholar]
- 22.Miyauchi M, Hirai C, Nakajima H. The solar exposure time required for vitamin D3 synthesis in the human body estimated by numerical simulation and observation in Japan. J Nutr Sci Vitaminol (Tokyo). 2013;59(4): 257–263. doi: 10.3177/jnsv.59.257 . [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Kitamura K, Takachi R, Saito T, Kobayashi R, Oshiki R, et al. Impact of demographic, environmental, and lifestyle factors on vitamin D sufficiency in 9084 Japanese adults. Bone. 2015;74: 10–17. doi: 10.1016/j.bone.2014.12.064 . [DOI] [PubMed] [Google Scholar]
- 24.Sakai S, Suzuki M, Tashiro Y, Tanaka K, Takeda S, Aizawa K, et al. Vitamin D receptor signaling enhances locomotive ability in mice. J Bone Miner Res. 2015;30(1): 128–136. doi: 10.1002/jbmr.2317 . [DOI] [PubMed] [Google Scholar]
- 25.Girgis CM, Cha KM, So B, Tsang M, Chen J, Houweling PJ, et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J Cachexia Sarcopenia Muscle. 2019;10(6): 1228–1240. doi: 10.1002/jcsm.12460 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostoglou-Athanassiou I, Athanassiou P, Gkountouvas A, Kaldrymides P. Vitamin D and glycemic control in diabetes mellitus type 2. Ther Adv Endocrinol Metab. 2013;4(4): 122–128. doi: 10.1177/2042018813501189 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama H, Shiraiwa T, Takahara M, Iwamoto M, Kuribayashi N, Nomura T, et al. Applications of physical performance measures to routine diabetes care for frailty prevention concept: fundamental data with grip strength, gait speed, timed chair stand speed, standing balance, and knee extension strength. BMJ Open Diabetes Res Care. 2020;8(1). doi: 10.1136/bmjdrc-2020-001562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou L, Liu Y, Li X, Huo C, Jia X, Yang J, et al. Changes and Risk Factors of Skeletal Muscle Mass and Strength in Patients with Type 2 Diabetes over 60 Years Old: A Cross-Sectional Study from China. J Diabetes Res. 2020;2020: 9815485. doi: 10.1155/2020/9815485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MK, Baek KH, Song KH, Il Kang M, Park CY, Lee WY, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. 2011;96(10): 3250–3256. doi: 10.1210/jc.2011-1602 . [DOI] [PubMed] [Google Scholar]
- 30.Bae EJ, Kim YH. Factors Affecting Sarcopenia in Korean Adults by Age Groups. Osong Public Health Res Perspect. 2017;8(3): 169–178. doi: 10.24171/j.phrp.2017.8.3.03 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, Hwang HJ, Kim SH. Relationship between serum ferritin levels and sarcopenia in Korean females aged 60 years and older using the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PLOS ONE. 2014;9(2): e90105. doi: 10.1371/journal.pone.0090105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JE, Lee YH, Huh JH, Kang DR, Rhee Y, Lim SK. Early-stage chronic kidney disease, insulin resistance, and osteoporosis as risk factors of sarcopenia in aged population: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008–2009. Osteoporos Int. 2014;25(9): 2189–2198. doi: 10.1007/s00198-014-2745-y . [DOI] [PubMed] [Google Scholar]
- 33.Tay L, Ding YY, Leung BP, Ismail NH, Yeo A, Yew S, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr). 2015;37(6): 121. doi: 10.1007/s11357-015-9860-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li CW, Yu K, Shyh-Chang N, Li GX, Yu SL, Liu HJ, et al. Sterol metabolism and protein metabolism are differentially correlated with sarcopenia in Asian Chinese men and women. Cell Prolif. 2021;54(4): e12989. doi: 10.1111/cpr.12989 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shardell M, Hicks GE, Miller RR, Kritchevsky S, Andersen D, Bandinelli S, et al. Association of low vitamin D levels with the frailty syndrome in men and women. J Gerontol A Biol Sci Med Sci. 2009;64(1): 69–75. doi: 10.1093/gerona/gln007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.


