Abstract
Trichomonas vaginalis remains the most common sexually transmitted parasite in the world and is considered a major risk factor in the transmission of the human immunodeficiency virus. A PCR technique using primers targeting a specific region of the 18S rRNA gene of T. vaginalis was developed. The PCR test was standardized using 15 reference strains, giving a single product of 312 bp in all strains. No amplification was observed when DNA from related organisms or human DNA was used as a target. The test was evaluated on 372 vaginal swab specimens and 361 urine samples from women attending infertility and obstetric clinics at two separate hospitals in Lima, Peru. Compared to T. vaginalis culture, the overall sensitivity and specificity of PCR of vaginal swab samples was 100% and 98%, respectively. The PCR of urine samples was 100% sensitive and 99.7% specific compared to culture of vaginal swab, but the sensitivity drops to 83.3% when compared to PCR of vaginal swabs. All culture-positive samples were found to be positive by PCR in either urine or vaginal secretion. None of the PCR-negative samples were positive by culture. The origin of the amplification was confirmed by digestion of PCR products with HaeIII. This PCR assay, which is easy to perform and has a high sensitivity and specificity, should be useful for routine diagnosis of T. vaginalis infection.
Trichomoniasis is the most common parasitic sexually transmitted disease in the world (5, 8, 33). In addition to reproductive tract symptoms, infection with Trichomonas vaginalis is increasingly being recognized as having an association with reproductive complications including premature rupture of membranes, premature birth, low birth weight, and infections occurring after abortion and caesarean delivery (6). Even more important is its role as a risk factor for the transmission of the human immunodeficiency virus (11, 27).
As with other sexually transmitted diseases, symptoms and signs of trichomoniasis are not adequately sensitive or specific for diagnosis (32). Thus, diagnostic laboratory testing is usually required to confirm the presence of the organism. Routine clinical diagnosis usually depends on microscopic observation of motile parasites in wet-mount preparations. Although rapid and inexpensive, the wet mount may not be highly sensitive, especially when a delay in examining the sample occurs, detecting only about 60% of culture positive samples (10, 29). Culture is considered the most reliable method of diagnosis but requires a special medium and frequent microscopic observation for up to 7 days (12, 14, 20). The sensitivity of culture is less than 90%, allowing for false negatives due to lack of detection of nonviable or small numbers of parasites (3, 10, 25). Cytology preparations, such as Papanicolaou's stain test (Pap smear) (10, 19, 29), also lack both sensitivity and specificity. Other diagnostic techniques, such as monoclonal antibodies, in situ hybridization, and immunological assays, are time-consuming and expensive and lack sensitivity (1, 10, 18, 23).
Several assays for the diagnosis of trichomoniasis based on PCR have recently been developed (9, 13, 22) and evaluated (15, 29, 30), the most common of which use DNA repetitive sequences as the target. This technique, however, allows for the production of nonspecific products due to the presence of insertion segments in some strains. Thus, different strains produce bands that migrate differently. In addition, repetitive sequences or amplification of the β-tubulin gene fails to detect some strains due to strain variation (15).
Recently, a comparative analysis of the 5.8S rRNA gene and the internal transcribed spacer regions of trichomonad protozoa demonstrated a high degree of intraspecies conservation of these sequences (2). Coding regions such as the 5.8S, 18S, and 28S genes are more conserved than the internal transcribed spacer regions. Ribosomal genes are highly conserved in their primary structure. This characteristic and their highly repetitive nature in the genome of most organisms make these genes good targets for detection by PCR. We have designed primers that are based on conserved regions of the 16S-like gene of T. vaginalis. In this study we determined the sensitivity and specificity of these primers using clinical samples from infertile and pregnant women in Lima, Peru.
MATERIALS AND METHODS
Strains.
Fifteen T. vaginalis strains were isolated from Peruvian patients. Strains were grown in Diamond's modified TYM medium and axenized (14). These strains were used as reference strains to standardize the method.
Previously extracted DNA from Trichomonas tenax ATCC 30207, Trichomonas gallinae ATCC 30002, Giardia lamblia ATCC SF-741 30888, Chilomastix sulcatus ATCC 50562, Dientamoeba fragilis ATCC 30948, Entamoeba histolytica ATCC SF-31-90015, Chlamydia trachomatis serovar E ATCC VR3488, and Neisseria gonorrhoeae ATCC 19424 was used to assess the specificity of the PCR primers.
Sample collection.
A total of 378 samples of vaginal secretions were obtained after informed consent from women attending the obstetric and infertility clinics of the Dos de Mayo and Arzobispo Loayza hospitals in Lima, Peru (Table 1). These two Ministry of Health public hospitals serve lower-middle-class populations of the city of Lima. The institutional ethics committees of Asociacion Benefica PRISMA and the Johns Hopkins University approved the protocol.
TABLE 1.
Sample source, results of culture, PCR techniques, and kappa values for vaginal secretions tested for T. vaginalis
| Hospital | Clinica | No. of patients | No. (%) culture positive | No. (%) PCR positive (swabs) | Kappa |
|---|---|---|---|---|---|
| A. Loayza | Infertility | 109 | 7 (6.4) | 12 (11.0) | 0.714 |
| Obstetric | 179 | 8 (4.5) | 9 (5.0) | 0.938 | |
| Dos de Mayo | Infertility | 34 | 4 (11.8) | 4 (11.8) | 1.000 |
| Obstetric | 50 | 5 (10.0) | 6 (12.0) | 0.898 | |
| Total | 372 | 24 (6.5) | 31 (8.3) | 0.868 |
No statistical differences were observed in incidence among patients from the different clinics (P > 0.05).
Two endocervical samples were collected from each woman using a sterile calcium alginate swab (Fisher Scientific, Pittsburgh, Pa.). One swab was placed in 3 ml of 0.15 M NaCl–0.05 M–Na2HPO4-NaH2PO4 (PBS, pH 7.2) plus antibiotics (penicillin G [200,000 U/ml], gentamicin [10 μg/ml], and amphotericin B [20 μg/ml]) and used for culturing. The other swab was placed in 500 μl of 0.01 M Tris-HCl (pH 8) and used for the PCR assay.
Patients were asked to collect urine specimens, which were kept at 4°C until they reached the laboratory, where they were processed immediately.
Culture.
The PBS tube containing the vaginal swab was vortexed, the swab was discarded under sterile conditions, and the remaining liquid was centrifuged at 3,000 × g for 5 min. The supernatant was discarded, and the pellet was transferred into a tube containing 8 ml of Diamond's modified TYM (14). The tube was then incubated at 37°C for 8 days and observed microscopically every 2 days. The sample was considered negative if no motile trichomonads were observed in the culture medium after 10 days of incubation. Culture of a vaginal sample was used as the gold standard for PCR. Urine samples were not cultured.
DNA extraction of culture strains.
Mid-log-phase axenic T. vaginalis cultures (106 parasites/ml) were chilled on ice for 10 min and then pelleted by centrifugation at 3,000 × g for 5 min at 4°C. The cellular pellet was washed twice with PBS. DNA was then extracted according to a previously described procedure (23). Briefly, the cell pellet was resuspended in 1 ml of lysis buffer (450 mM NaCl, 15 mM sodium citrate, 0.2% sodium dodecyl sulfate) plus 200 μg of proteinase K per ml and incubated at 65°C for 1 h. DNA was then phenol-chloroform extracted, precipitated using ethanol and ammonium acetate, resuspended in 500 μl of buffer TE (0.01 M Tris-HCl, 0.001 M EDTA [pH 8]) containing 20 μg of DNase-free RNase per ml and incubated at 37°C for 30 min. The suspension was phenol-chloroform extracted, precipitated, and finally resuspended in 200 μl of DNase- and RNase-free water (Sigma, St. Louis, Mo.). The DNA concentration was spectrophotometrically determined (24).
Human DNA was extracted from leukocytes from a healthy volunteer using the QIAamp Tissue kit (Qiagen Inc., Chatsworth, Calif.). The DNA from Toxoplasma gondii strain RH was extracted using the same procedure.
DNA extraction from clinical samples.
The Tris-HCl tube containing the swab was vortexed, and after removal of the swab, a 100-μl aliquot was transferred to a 1.5-ml tube and pelleted in a microcentrifuge at 12,000 × g for 15 min at room temperature. The supernatant was discarded, and the pellet was resuspended in 200 μl of a 5% (wt/vol) suspension of Chelex 100 (Sigma) in 0.01 M Tris-HCl buffer (pH 8). The suspension was gently vortexed and then incubated at 56°C for 45 min, during which time it was vortexed at 15-min intervals. The samples were then boiled for 10 min and centrifuged at 12,000 × g for 30 s. They were either used immediately or stored at −20°C. The supernatant (5 μl) was directly employed in a 25-μl total volume of PCR mix. The original tube was stored at −70°C to confirm positive samples.
Five milliliters of urine sample was centrifuged for 5 min at 3,500 × g; the sediment was washed twice with 0.01 M Tris-HCl (pH 8) buffer. The final sediment was resuspended in 500 μl of Tris-HCl buffer. An aliquot of 200 μl was processed as described above for vaginal swab samples.
Five milliliters of urine sample was centrifuged for 5 min at 3,500 × g; the sediment was washed twice with 0.01 M Tris-HCl (pH 8) buffer. The final sediment was resuspended in 500 μl of Tris-HCl buffer. An aliquot of 200 μl was processed as described above for vaginal swab samples.
Primers.
A set of primers targeting conserved regions of the 18S ribosomal gene (16S-like ribosomal gene; GenBank accession number U17510) of T. vaginalis was designed. The sequences were selected from regions of the 18S ribosomal gene that differed from those of Trichomonas tenax (accession no. U37711) Tritrichomonas foetus (U17509) Entamoeba gingivalis (D28490), Trypanosoma brucei (AJ009149), Candida albicans (M60302), Giardia lamblia (U09492), and Homo sapiens (U13369). The primer sequences were as follows: Tv1, 5′ TAA TGG CAG AAT CTT TGG AG 3′, and Tv2, 5′ GAA CTT TAA CCG AAG GAC TTC 3′.
PCR.
PCR was performed with a thermal cycler Gene Amp PCR system 2400 (Perkin-Elmer Cetus, Norwalk, Conn.). A standard PCR was carried out in a total volume of 25 μl. The master mix consisted of 1× PCR buffer (Gibco-BRL, Gaithersburg, Md.), 2.5 mM MgCl2, 200 μM each of the four deoxynucleoside triphosphates, 0.4 μM each primer, and 0.025 U of Taq DNA polymerase (Perkin-Elmer Cetus) per ml. A total of 2.5 ng of DNA from cultured organisms was used per 25 μl of PCR mix. The amplification procedure included 3 min of denaturation at 94°C, followed by 40 cycles each consisting of 10 s of denaturation at 94°C, 45 s of annealing at 58°C, and 15 s of extension at 72°C. A final extension step at 72°C for 5 min was included. Included with each amplification cycle were a T. vaginalis-positive and -negative vaginal sample, a sample with DNA extracted from T. vaginalis, and a blank containing water.
Each clinical sample was tested twice by PCR. All PCR-positive samples were confirmed by a third amplification after preparing a new sample from the backup tube.
Determination of analytical sensitivity.
Analytical sensitivity was performed using one clinical isolate of T. vaginalis. Twofold dilutions of the parasites were made until 1 organism per 25 μl of PCR mix was achieved. Dilutions were then processed using the Chelex technique as described above for clinical samples. With a similar purpose, T. vaginalis purified DNA was 10-fold diluted starting from 10 ng.
Clinical specificity determination.
Trichomonas tenax is a trichomonad of the oral cavity that is the protozoan most closely related to T. vaginalis. To determine if the primer set Tv1 and Tv2 cross-reacted with T. tenax, the primers were tested using 58 human dental plaque samples obtained from volunteers attending the dental clinic at Universidad Peruana Cayetano Heredia.
Detection of PCR inhibitors.
For each clinical sample, a PCR for the human β-globin gene was carried out as a control for the presence of inhibitors using primers PCO4 (accession no. A26623) and GH20 (A26624); if inhibition was observed, the sample was diluted 1:10 and 1:100 and tested again. Cycling parameters were as follows: 4 min at 95°C and 30 cycles each of 15 s at 94°C, 30 s at 55°C, and 30 s at 72°C.
Detection of PCR products.
A 10-μl aliquot of PCR product was separated by horizontal gel electrophoresis at 50 V on a 2.0% agarose gel in Tris-acetate-EDTA buffer. Gels were stained with ethidium bromide (0.5 μg/ml; Sigma), and PCR amplifications were visualized using a UV light transilluminator. The size of the amplified product (312 bp) was determined by comparison with a commercial 100-bp DNA ladder (Gibco-BRL).
Restriction enzyme analysis.
To confirm if PCR products were derived from the 18S RNA gene, all positive products were digested with the restriction enzyme HaeIII. Then, 13.5 μl of the PCR product was digested in 1× enzyme buffer using 1 U of enzyme. Tubes containing the reaction mixture were incubated for 3 h at 37°C. Finally, 15 μl of the reaction mixture was separated by horizontal electrophoresis in 2.5% agarose, and gels were ethidium bromide stained and visualized as described above.
Statistical analysis.
Sensitivity and specificity for PCR in vaginal samples or urine samples were calculated using as the gold standard vaginal swab samples that were culture positive in Diamond's modified TYM medium. Agreement between the culture of the vaginal sample for T. vaginalis and the PCR test of either the vaginal swab or urine samples was calculated using the kappa test. Differences in prevalence among samples from the different clinics were calculated using the chi-squared test. Statistical analysis was done using the SPSS 7.5 software (SPSS Inc., Chicago, Ill.).
RESULTS
All reference strains used to standardize the PCR gave a positive amplification of 312 bp, as expected. In all the reference strains, the origin of the PCR products was derived from the 18S ribosomal gene, since digestion of the PCR products with restriction enzyme HaeIII yielded, as expected, two fragments, one of 101 bp and the other of 211 bp (Fig. 1).
FIG. 1.
Agarose gel electrophoresis of PCR products and REA. Lanes 1 and 2, amplification products of two strains of T. vaginalis. Lanes 3 and 4, REA of amplified products of two different strains of T. vaginalis. Lane 5, REA of amplified product of T. tenax. Lane 6, REA of PCR product performed using water as the sample. Lane M, 100-bp DNA ladder.
No amplification was observed when DNA from Trichomonas tenax ATCC 30207, Trichomonas gallinae ATCC 30002, Giardia lamblia ATCC SF-741 30888, Chilomastix sulcatus ATCC 50562, Dientamoeba fragilis ATCC 30948, Entamoeba histolytica ATCC SF-31-90015, Chlamydia trachomatis serovar E ATCC VR3488, Neisseria gonorrhoeae ATCC 19424, Toxoplasma gondii strain RH, or human leukocyte DNA was tested.
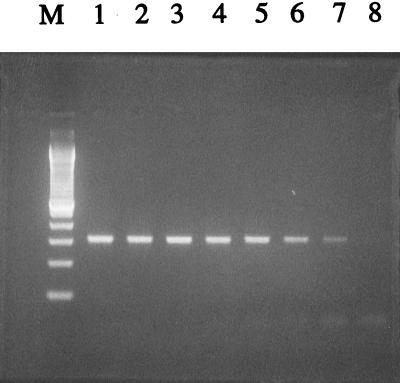
The analytical sensitivity of the primer set Tv1 and Tv2 was 10 fg for purified T. vaginalis chromosomal DNA (Fig. 2). Using the Chelex 100 method, the detection limit of the test was 1 whole flagellated cell per 25 μl of PCR mixture.
FIG. 2.
PCR sensitivity based on T. vaginalis genomic DNA quantity, Lanes 1 to 7, 10 ng, 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, and 10 fg, respectively. Lane 8, water control. Lane M, 100-bp DNA ladder.
Presence of inhibitors.
Of 372 vaginal swabs, only 1 sample, which had a high content of blood, demonstrated significant inhibition. This sample first gave a negative amplification when the PCR for β-globin was performed but became positive after being diluting 1:10 or 1:100. This sample also gave a false-negative result when tested directly for T. vaginalis, but a positive result was observed when the diluted sample was tested.
Clinical specificity.
None of the 58 dental plaque samples gave a positive amplification when PCR was performed using primer set Tv1 and Tv2, although wet-mount examination revealed the presence of T. tenax in eight samples and Entamoeba gingivalis in 15 samples.
Clinical samples.
In clinical vaginal swab samples, T. vaginalis was detected by PCR in 8.3% (31 of 372) of the samples, compared to 6.5% (24 of 372) detected by culture. All of the 24 isolates were detected by PCR in both vaginal secretions and urine (Table 1). When urine samples were tested for PCR, 6.9% (25 of 361) of the samples gave a positive amplification for T. vaginalis. One of the PCR-positive, culture-negative patients did not have her urine sample tested by PCR. One woman with a vaginal sample positive for T. vaginalis by PCR but negative by culture had a positive urine sample on testing by PCR.
There was a high degree of concordance between PCR and culture when vaginal samples were tested for the presence of T. vaginalis (kappa = 0.86, P < 0.001) (Table 1). Similar high concordance was observed when PCR of urine samples was compared to the PCR results of vaginal specimens (kappa = 0.94, P < 0.001) (Table 2).
TABLE 2.
PCR results for vaginal and urine samples tested for T. vaginalis
| Hospital | Clinic | No. (%) of PCR-positive vaginal swabs | No. of PCR-positive urine samples/no. tested (% positive) urine | Kappa |
|---|---|---|---|---|
| A. Loayza | Infertility | 12 (11.0) | 8/107 (7.5) | 0.827 |
| Obstetric | 9 (5.0) | 8/176 (4.7) | 0.938 | |
| Dos de Mayo | Infertility | 4 (11.8) | 4/31 (12.9) | 0.839 |
| Obstetric | 6 (12.0) | 5/45 (11.1) | 0.897 | |
| Total | 31 (8.3) | 25/361 (6.9) | 0.902 |
One patient with a PCR-positive, culture-negative vaginal secretion was not tested by PCR for urine.
Using the culture results for T. vaginalis as a gold standard, the overall sensitivity and specificity of PCR for vaginal samples using primer set Tv1 and Tv2 were 100 and 98%, respectively (Table 2). The PCR for urine samples, considering vaginal culture as the gold standard, was 100% sensitive and 99.7% specific.
All culture-positive clinical samples gave a single 312-bp band on PCR. The same band was observed with samples that were only positive for T. vaginalis by PCR. In all PCR-positive samples, the origin of the PCR product was shown to be derived from the 18S ribosomal gene of T. vaginalis, since digestion of PCR products with HaeIII gave two bands, one of 101 and the other of 211 bp.
The majority of T. vaginalis isolates came from the Dos de Mayo Hospital (Table 2), while the lowest prevalence was observed among patients attending the obstetric clinic at Arzobispo Loayza Hospital. There was no statistical difference in the prevalence of T. vaginalis infection detected among patients attending the two hospitals, nor was there a difference in prevalence in either hospital between patients attending the obstetric versus the infertility clinic (P > 0.05).
DISCUSSION
Detection of sexually transmitted diseases has become important in the present context of the global spread of the human immunodeficiency virus. In order to reduce obstetric and gynecological complications associated with T. vaginalis infection, early diagnosis and treatment is important. The PCR methodology we have developed is highly sensitive for the detection of T. vaginalis. It is unique in that it uses the 18S ribosomal gene as the target for the primers Tv1 and Tv2.
The specificity of the Tv1 and Tv2 primers for T. vaginalis was examined by testing dental plaque specimens that were positive on microscopy for T. tenax. No cross-reaction was observed. Although primer Tv1 is highly homologous to the DNA of T. tenax, no amplification was observed when DNA from this organism was used, probably because of the high specificity of the second primer, Tv2, and the high temperature used during the annealing step.
As demonstrated, no unusual media or buffers are needed to transport the samples. Tris-HCl was successfully used, making the PCR less expensive and easily accessible for routine diagnosis. Chelex 100 has been reported to be useful for PCR sample preparation (31). Although high levels of nucleases within the parasite have been reported (16, 21), Chelex 100 was able to extract the DNA of T. vaginalis without problems, as previously demonstrated (15). Chelex DNA extracts stored at −20°C, however, did degenerate over time (H. Mayta, personal observation).
In this study, primers Tv1 and Tv2 were also able to detect T. vaginalis in urine samples. PCR of urine samples gave results comparable to those obtained by culturing vaginal specimens. In contrast, PCR of urine samples rarely detected T. vaginalis from patients who had T. vaginalis PCR-positive and culture-negative vaginal specimens. If all PCR-positive specimens are considered (whether culture positive or negative), then the sensitivity of the urine PCR was 80%. When urine is used to detect trichomonas infection in large-scale population samples, the decrease in urine PCR sensitivity needs to be taken into consideration.
Molecular techniques for the diagnosis of T. vaginalis have been previously reported but are not as sensitive or specific as the PCR we described in the present study. The use of a DNA probe (1, 17, 23) has the disadvantage of cross-reacting with DNA from Pentatrichomonas hominis and also being relatively insensitive, since its detection limit is 200 axenically cultivated protozoa. The first PCR described for T. vaginalis (22) has a similar sensitivity to culture (7, 28) and also misses some axenically cultivated strains (15). Targeting another repetitive sequences, Kegne et al. (9) could amplify one axenically cultivated parasite, but this PCR has not been tested under clinical conditions. A nested PCR and a colorimetric nested PCR also were described which used as their target a repetitive DNA sequence (13, 18, 26). Both PCR protocols produce nonspecific bands, which may cause false-positive results. Nested PCR techniques, although generally sensitive, have a higher cost, are more labor intensive and are also more prone to contamination than a simple PCR. More recently, a PCR based on the β-tubulin genes (15) was described. However, due to the high degree of variation among T. vaginalis strains, it lacks sensitivity since it misses some culture-positive strains. The β-tubulin gene PCR also lacks specificity, since it cross-reacts with T. tenax.
The PCR described here is highly sensitive. All culture-positive specimens were detected by PCR. This technique is also highly specific, as demonstrated by the lack of cross-reaction with the closely related trichomonad T. tenax. The high sensitivity of primer set Tv1 and Tv2 may permit the detection of small numbers of T. vaginalis organisms, which may not grow in culture. Moreover, culture of T. vaginalis may not be successful, since between 300 and 500 organisms are required to obtain a positive result (4). The PCR using primer set Tv1 and Tv2 was indeed reproducible and, as demonstrated by restriction enzyme analysis (REA), the product obtained from clinical samples was concordant with those obtained from T. vaginalis axenic strains. The PCR assay for the detection of T. vaginalis that we have designed is simple, easy to perform, and highly sensitive and specific. While it is optimal when using vaginal secretions, the test will perform, albeit at lower levels of sensitivity, with urine samples, permitting an easy and noninvasive method of specimen collection.
While culturing of samples takes at least 7 days, the PCR assay described here takes about 4 to 5 h from the time of sample collection until electrophoresis of enzyme-restricted PCR products. Restriction analysis of PCR products is not a necessary step after it has been performed once; thus, on a routine basis, PCR for the diagnosis of T. vaginalis can be performed within a 3-h period.
ACKNOWLEDGMENTS
We thank Diana Williams for her comments and Martin McCann, Daniel Roth, and Anna Culotta for their assistance with the manuscript. We also thank Maria, Isaac, J. P. Cal, J. B. Phu, and D. Sara for their technical assistance.
This study was partially supported by an ITREID training grant from the NIH and the anonymous charitable RG + ER fund.
REFERENCES
- 1.DeMeo L R, Draper D L, McGregor J A, Moore D F, Peter C R, Kapernick P S, McCormack W M. Evaluation of a desoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol. 1996;174:1339–1342. doi: 10.1016/s0002-9378(96)70682-8. [DOI] [PubMed] [Google Scholar]
- 2.Felleisen R. Comparative sequence analysis of 5.8S rRNA genes and internal transcribed spacers (ITS) regions of trichomonadid protozoa. Parasitology. 1997;115:111–119. doi: 10.1017/s0031182097001212. [DOI] [PubMed] [Google Scholar]
- 3.Foust A C, Kraus S J. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis. 1980;141:137–143. doi: 10.1093/infdis/141.2.137. [DOI] [PubMed] [Google Scholar]
- 4.Garber G E, Sibau L, Ma R, Proctor E M, Bowie W R. Cell culture compared with broth for detection of Trichomonas vaginalis. J Clin Microbiol. 1987;25:1275–1279. doi: 10.1128/jcm.25.7.1275-1279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graves A, Gardner W A. Pathogenicity of Trichomonas vaginalis. Clin Obstet Gynecol. 1993;36:145–152. doi: 10.1097/00003081-199303000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Heine P, McGregor J A. Trichomonas vaginalis: a reemerging pathogen. Clin Obstet Gynecol. 1993;36:137–144. doi: 10.1097/00003081-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Heine R P, Weisenfeld H C, Sweet R L, Witkin S S. Polymerase chain reaction analysis of distal vaginal specimens: a less invasive strategy for detection of Trichomonas vaginalis. Clin Infect Dis. 1997;24:985–987. doi: 10.1093/clinids/24.5.985. [DOI] [PubMed] [Google Scholar]
- 8.Honigberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Spring Verlag; 1989. pp. 311–324. [Google Scholar]
- 9.Kengne P, Veas F, Vidal N, Rey J, Cuny G. Trichomonas vaginalis: repeated DNA target for highly sensitive and specific polymerase chain reaction diagnosis. Cell Mol Biol. 1994;40:819–831. [PubMed] [Google Scholar]
- 10.Krieger J N, Tam M R, Stevens C E, Nielsen I O, Hale J H, Kiviat N B, Holmes K K. Diagnosis of trichomoniasis: comparison of conventional wet-mount examinations with cytological studies, cultures and monoclonal antibody staining of direct specimens. JAMA. 1988;259:1223–1227. doi: 10.1001/jama.259.8.1223. [DOI] [PubMed] [Google Scholar]
- 11.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets F, Batter V, Alary M, Heyward W L, Rider R W, Piot P. Non ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in woman: results for a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Levi M H, Torres J, Piña C, Klein R S. Comparison of the InPouch TV culture system and Diamond's modified medium for detection of Trichomonas vaginalis. J Clin Microbiol. 1997;35:3308–3310. doi: 10.1128/jcm.35.12.3308-3310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin P R, Shaio M F, Liu J Y. One-tube, nested-PCR assay for the detection of Trichomonas vaginalis in vaginal discharges. Ann Trop Med Parasitol. 1997;91:61–65. doi: 10.1080/00034983.1997.11813112. [DOI] [PubMed] [Google Scholar]
- 14.Linstead D. Cultivation. In: Honimberg B M, editor. Trichomonads parasitic in humans. New York, N.Y: Spring Verlag; 1989. pp. 91–111. [Google Scholar]
- 15.Madico G, Quinn T C, Rompalo A, Mackee K T, Jr, Gaydos C A. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J Clin Microbiol. 1998;36:3205–3210. doi: 10.1128/jcm.36.11.3205-3210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller M. Biochemical cytology of trichomonad flagellates. J Cell Biol. 1973;57:453–474. doi: 10.1083/jcb.57.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muresu R, Rubino S, Rizzu P, Baldini A, Colombo M, Cappuccinelli P. A new method for identification of Trichomonas vaginalis by fluorescent DNA in situ hybridization. J Clin Microbiol. 1994;32:1018–1022. doi: 10.1128/jcm.32.4.1018-1022.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paces J, Urbánková V, Urbánek P. Cloning and characterization of a repetitive DNA sequence specific for Trichomonas vaginalis. Mol Biochem Parasitol. 1992;54:247–256. doi: 10.1016/0166-6851(92)90116-2. [DOI] [PubMed] [Google Scholar]
- 19.Petersen C S, Carl L, Alnor D, Thomsen U, Thomsen H K. Ignored trichomonal infestation diagnosed by Papanicolaou smear. Genitourin Med. 1995;71:257–258. doi: 10.1136/sti.71.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philip A, Carter-Scott P, Rogers C. An agar culture technique to quantitate Trichomonas vaginalis from women. J Infect Dis. 1987;155:304–307. doi: 10.1093/infdis/155.2.304. [DOI] [PubMed] [Google Scholar]
- 21.Riley D E, Krieger J N. Rapid and practical isolation from Trichomonas vaginalis and other nuclease-rich protozoa. Mol Biochem Parasitol. 1992;51:161–164. doi: 10.1016/0166-6851(92)90212-3. [DOI] [PubMed] [Google Scholar]
- 22.Riley D E, Roberts M C, Takayama T, Krieger J N. Development of a polymerase chain reaction-based diagnosis of Trichomonas vaginalis. J Clin Microbiol. 1992;30:465–472. doi: 10.1128/jcm.30.2.465-472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubino S, Muresu R, Rappelli P, Fiori P L, Rizzu P, Erre G, Cappuccinelli P. Molecular probe for identification of Trichomonas vaginalis DNA. J Clin Microbiol. 1991;29:702–706. doi: 10.1128/jcm.29.4.702-706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schmidt G P, Matneny L C, Zaidi A A, Kraus S J. Evaluation of six media for the growth of Trichomonas vaginalis from vaginal secretions. J Clin Microbiol. 1989;27:1230–1233. doi: 10.1128/jcm.27.6.1230-1233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaio M F, Lin P R, Liu J Y. Colorimetric one-tube nested PCR for detection of Trichomonas vaginalis in vaginal discharge. J Clin Microbiol. 1997;35:132–138. doi: 10.1128/jcm.35.1.132-138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- 28.Tabrizi Z N, Paterson B A, Fairley C K, Bowde F J, Garland S M. Comparison of tampon and urine as self-administered methods of specimen collection in the detection of Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis in women. Int J STD AIDS. 1998;9:347–349. doi: 10.1258/0956462981922386. [DOI] [PubMed] [Google Scholar]
- 29.Thomason J L, Gelbart S M, Sobun J F, Schulien M B, Hamilton P R. Comparison of four methods to detect Trichomonas vaginalis. J Clin Microbiol. 1988;26:1869–1870. doi: 10.1128/jcm.26.9.1869-1870.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Schee C, Van Belkun A, Zwijgers L, Van Der Brugge E, O'neill E L, Luijendijk A, Van Rijsoort-Vos T, Van Der Meijden W I, Verbrugh H, Sluiters H J F. Improved diagnosis of Trichomonas vaginalis infection by PCR using vaginal swabs and urine specimens compared to diagnosis by wet mount microscopy, culture, and fluorescent staining. J Clin Microbiol. 1999;37:4127–4130. doi: 10.1128/jcm.37.12.4127-4130.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh P S, Metzger D A, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10:506–513. [PubMed] [Google Scholar]
- 32.Wolner-Hanssen P, Krieger J N, Stevens C E, Kiviat N B, Koutsky L, Critchlow C, DeRouen T, Hillier S, Holmes K K. Clinical manifestations of vaginal trichomoniasis. JAMA. 1989;261:571–576. doi: 10.1001/jama.1989.03420040109029. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. Geneva, Switzerland: World Health Organization; 1995. [Google Scholar]