Abstract
In a panel of four human melanoma cell lines, equitoxic doses of cisplatin induced the proapoptotic conformation of the Bcl-2 family protein Bak prior to the execution phase of apoptosis. Because cisplatin-induced modulation of the related Bax protein was seen in only one cell line, a degree of specificity in the signal to Bak is indicated. Little is known about upstream regulation of Bak activity. In this study, we examined whether the apoptosis-specific pathway mediated by a kinase fragment of MEKK1 (ΔMEKK1) is involved in the observed Bak modulation. We report that expression of a kinase-inactive fragment of MEKK1 (dominant negative MEKK [dnMEKK]) efficiently blocked cisplatin-induced modulation of Bak and cytochrome c release and consequently also reduced DEVDase activation and nuclear fragmentation. Accordingly, expression of a kinase-active MEKK1 fragment (dominant positive MEKK) was sufficient to induce modulation of Bak in three cell lines and to induce apoptosis in two of these. dnMEKK did not block cisplatin-induced c-Jun N-terminal kinase (JNK) activation, in agreement with a specifically proapoptotic role for the ΔMEKK1 pathway. Finally, we show that reduction of Bak expression by antisense Bak reduced cisplatin-induced loss of mitochondrial integrity and caspase cleavage activity in breast cancer cell lines. In summary, we have identified Bak as a cisplatin-regulated component downstream in a proapoptotic, JNK-independent ΔMEKK1 pathway.
Increased mitochondrial permeability is a crucial event in many types of drug-induced apoptosis and leads to release of a number of apoptosis-related proteins, e.g., AIF, DIABLO, and cytochrome c, from the mitochondrial intermembrane space. Cytochrome c is a component of the apoptosome complex which activates procaspase 9, which in turn activates the effector caspases, notably, caspase 3 and caspase 7 (DEVDases) (20).
Regulation of mitochondrial permeability during apoptosis is complex, but key roles are played by Bak and Bax, two proapoptotic Bcl-2 family members, which contribute to mitochondrial pore formation, allowing release of cytochrome c (16, 25). In staurosporine-induced apoptosis, this function of Bak and Bax was recently shown to involve conformational changes in both proteins rather than an increase in expression (5, 10). Similar results have been obtained with other drugs and with Bax mutants (10, 18). However, little is known about how the upstream apoptotic signal(s) is relayed to these proteins.
The stress-activated protein kinase (SAPK) pathways have long been of interest in apoptosis research. The roles of the c-Jun N-terminal kinase (JNK)/SAPK isoforms are, however, not yet clear, since they are reported to have survival-promoting functions as well as proapoptotic ones, depending on the signaling context (3). Activators upstream of JNK/SAPK include ASK1, MEKK1, and Tpl-2 (3). Apoptosis induced by such divergent agents as genotoxins, anti-Fas ligation, and loss of cell adherence (anoikis) all involve activation of JNK/SAPK and proteolytic cleavage of MEKK1, leading to a constitutively active kinase fragment (ΔMEKK1) (1, 4, 27).
A model for the roles of full-length and cleaved MEKK1 has been proposed (1, 27). According to this model, activated full-length MEKK1 may be part of a survival pathway involving JNK/SAPK, whereas ΔMEKK1 is involved in amplification of the apoptotic signal. Overexpression of ΔMEKK1 is reported to be lethal to several cell types, and it can also sensitize cells to genotoxic damage, e.g., by cisplatin (13, 27, 28). Although the model indicates interaction of ΔMEKK1 with molecules involved in the control of apoptosis (27), no downstream components of the ΔMEKK1 pathway have been identified.
Cisplatin is a widely used DNA-damaging anticancer drug which activates JNK (23, 30, 31) and induces MEKK1 cleavage and caspase activation (27). As with most chemotherapeutic drugs, tumors may show inherent or acquired resistance against cisplatin. The clinical problem of response variability has engendered research and knowledge of a number of resistance mechanisms, e.g., increased DNA repair (21). However, considering the reactivity of cisplatin and the complexity of the cellular responses to DNA damage, cisplatin-induced apoptotic signaling likely involves several pathways.
Because of its suggested role in caspase activation, in this study we investigated the proapoptotic ΔMEKK1 pathway and the involvement of Bak and Bax in cisplatin-induced apoptosis in human metastatic melanoma cells.
MATERIALS AND METHODS
Cells and cisplatin treatment.
We screened a panel of four human metastatic melanoma cell lines (224, FM55, AA, and DFW) for cisplatin sensitivity by assessing nuclear fragmentation after 24 h of treatment with different doses (Table 1). All four cell lines had similar levels of Bcl-2 protein as determined by Western blotting. Only the 224 cell line expresses mutant p53. Being the most sensitive cell line, 224 cells were chosen for experiments involving blocking of cisplatin-induced apoptosis. The cisplatin concentration used was 20 μM. MCF-7 breast carcinoma cells and two sublines stably expressing antisense Bak (6) were also used for assessment of the role of Bak in cisplatin-induced apoptosis. All cell lines were maintained at 37°C in 5% CO2 in RPMI medium supplemented with fetal calf serum (10%), l-glutamate, penicillin, and streptomycin.
TABLE 1.
Effects of cisplatin on human melanoma cell lines
| Cell line | LD50a (μM) | Modulationb (mean ± SD)
|
|
|---|---|---|---|
| Bak | Bax | ||
| 224 | 20 | 2.7 ± 1.0 | 1.0 ± 0.3 |
| FM55 | 23 | 2.4 ± 1.4 | 1.4 ± 0.2 |
| AA | 40 | 3.5 ± 0.2 | 1.0 ± 0.1 |
| DFW | 60 | 2.3 ± 0.8 | 0.7 ± 0.5 |
Dose required to elicit 50% nuclear fragmentation after 24 h.
Induced by 20 μM (224 and FM55) or 40 μM cisplatin (AA and DFW), expressed as fold increase in median Bak- or Bax-associated immunofluorescence, using antibodies specific for an N-terminal epitope. All samples were prepared at 16 h after cisplatin addition.
Assessment of apoptosis.
Cells were harvested with cell dissociation solution (Sigma Aldrich), resuspended in 50 to 100 μl of hypotonic salt solution with 30 mM glycerol, and smeared on glass slides. Air-dried smears were fixed in acetone-methanol (2:1) for 5 min and then covered with ethidium bromide (5 ng/ml in distilled water) for 5 min. After being rinsed in tap water, stained cells were examined by UV microscopy. At least 100 cells per sample were counted, and the percentage of cells with condensed and fragmented nuclei was assessed in each sample.
In addition to cytochrome c release and DNA-dependent protein kinase (DNA-PK) cleavage (see Results), apoptosis was also assessed by quantitation of DEVDase activity against the Ac-DEVD-AMC substrate (CaspACE assay; Promega) and by a similar caspase 9 assay using Ac-LEHD-AMC (Enzyme Systems Products Inc.) as the substrate. Harvested cells were centrifuged, washed in ice-cold phosphate-buffered saline (PBS), and resuspended in lysis buffer (25 mM HEPES [pH 7.5], 5 mM MgCl2, 5 mM EDTA, 5 mM dithiothreitol, 2 mM phenylmethylsulfonyl fluoride, 10 μg each of pepstatin and leupeptin per ml). Cells were lysed by three cycles of freeze-thawing in liquid nitrogen. Lysates were centrifuged (16,000 × g; 20 min), and supernatant fractions were collected. Caspase activity was assayed according to manufacturer's instructions. The fluorescence produced upon cleavage of the labeled substrate is proportional to the caspase activity in the sample. Addition of DEVD or LEHD inhibitor to all samples with caspase activity abrogated the whole signal.
Inducible expression of dnMEKK and dpMEKK.
The cDNA of a dominant negative mutant of MEKK1 (dnMEKK; 37-kDa kinase fragment with a K432M point mutation) or a dominant positive MEKK1 mutant (dpMEKK; 37-kDa active kinase fragment) (both generously provided by T. Maniatis) were cloned into a recombinant adenovirus vector for use in an inducible adenovirus gene transfer system (17), consisting of two recombinant adenovirus vectors, one encoding a transactivating protein (adeno-rtTA) and another encoding the protein of interest. The constitutively expressed activator protein rtTA requires an inducer (doxycycline [DOX], a tetracycline derivative) to undergo a conformational change which allows it to bind to and activate the promoter of the gene of interest.
Cell (106/dish) were seeded and after 6 h infected with adeno-dnMEKK and adeno-rtTA constructs (5 to 10 PFU of each per cell). After 20 h in the presence of 1 μM DOX, cells were either postincubated with freshly added DOX or treated with cisplatin in the presence of freshly added DOX. Western blotting confirmed dnMEKK expression. Because DOX at 5 μM or more was initially found to induce apoptosis on its own, in accordance with similar effects on several types of cells, e.g., osteosarcoma (7), 1 μM DOX was subsequently included in all samples. The procedure for induction of dpMEKK expression was identical to that for induction of dnMEKK.
Western blot analysis.
Cell extract proteins (20 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane for Western blotting. The following antibodies were used: anti-phospho-JNK (Biolabs), anti-MEKK1 (Santa Cruz Biotechnology), anti-DNA-PK (Labvision), anti-cytochrome c (PharMingen), anti-cytochrome c oxidase subunit IV (Molecular Probes), anti-caspase 9 (Oncogene Research Products), and antitubulin (Sigma Aldrich). Tubulin was used as an internal standard for loading.
Cytochrome c release.
Cells (224 cell line) were treated with 20 μM cisplatin for 20 h in the presence or absence of dnMEKK. After harvesting (107 cells per sample), cells were centrifuged (600 × g, 5 min, 4°C), washed in ice-cold PBS, and resuspended in 3 volumes of isolation buffer (20 mM HEPES-KOH [pH 7.5], 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM each EDTA, EGTA, and dithiothreitol, 2 mM phenylmethylsulfonyl fluoride, 20 μg of leupeptin per ml, 10 μg of aprotinin and pepstatin per ml). After chilling on ice for 15 min, cells were disrupted by 40 strokes in a glass homogenizer. Homogenates were centrifuged at 700 × g and thereafter at 2,500 × g for 10 min at 4°C. After centrifugation at 12,000 × g (30 min, 4°C), cytosolic proteins (25 μg) in supernatant fractions were analyzed by Western blotting for cytochrome c. Possible mitochondrial contamination in the same samples was monitored using an antibody against mitochondrial cytochrome c oxidase subunit IV.
Flow cytometric analysis of Bak- and Bax-associated IFL.
Upon induction of apoptosis, the proapoptotic Bax and Bak proteins undergo conformational changes which expose otherwise inaccessible N-terminal epitopes (10, 11). In the present study, we have used the same two antibodies that were shown to specifically recognize these epitopes. Using a fluorescein isothiocyanate (FITC)-conjugated secondary antibody, the increases in accessibility of these epitopes can be monitored by flow cytometry (fluorescence-activated cell sorting [FACS] analysis) (10). At specific time points after cisplatin treatment, cells were detached using cell dissociation solution (Sigma). Cells were then fixed in paraformaldehyde (0.25%, 5 min), washed three times in PBS, and incubated for 30 min with a mouse monoclonal antibody against amino acids 1 to 52 of Bak (AM03, clone TC100; Oncogene Research Products) or against amino acids 12 to 24 of Bax (clone 6A7; PharMingen). Antibodies were diluted 1:50 in PBS containing digitonin (100 μg/ml). After three washes in PBS, cells were incubated with FITC-labeled anti-mouse antibody for 30 min, washed twice in PBS, and resuspended in PBS. Negative controls using an irrelevant primary antibody (rabbit anti-MEKK1) were also prepared. Cells (10,000/sample) were analyzed on a FACSCalibur flow cytometer, using Cell Quest software. To quantitate the results, cell debris was first excluded from the analysis by electronic gating. The median fluorescence of the irrelevant antibody sample (fI) was subtracted from the median Bak/Bax-associated immuno fluorescence (IFL) in cells with fluorescence above fI. Data are presented as fold increase in IFL from control levels.
RESULTS
Cisplatin induces the proapoptotic conformation of Bak but not Bax in the melanoma cell line 224.
The human melanoma cell line 224 is sensitive to cisplatin (50% lethal dose [LD50], 20 μM [Table 1]), which induces apoptosis, as seen by nuclear fragmentation as well as cytochrome c release, activation of DEVDases (caspases 3 and 7, which have similar substrate specificities) and caspase 9, and by DNA-PK cleavage (see below).
In vivo, cytochrome c release is promoted by the proapoptotic Bcl-2 family proteins Bak and Bax (25). To examine proapoptotic conformational changes in Bak and Bax, we incubated control and cisplatin-treated cells, in the presence of digitonin, with monoclonal antibodies recognizing the N-terminal epitopes of Bak and Bax. An FITC-conjugated secondary antibody allowed detection of bound anti-Bak/Bax antibodies by flow cytometry. A shift to the right in the resulting graph or histogram indicates an increase in Bak/Bax-associated IFL.
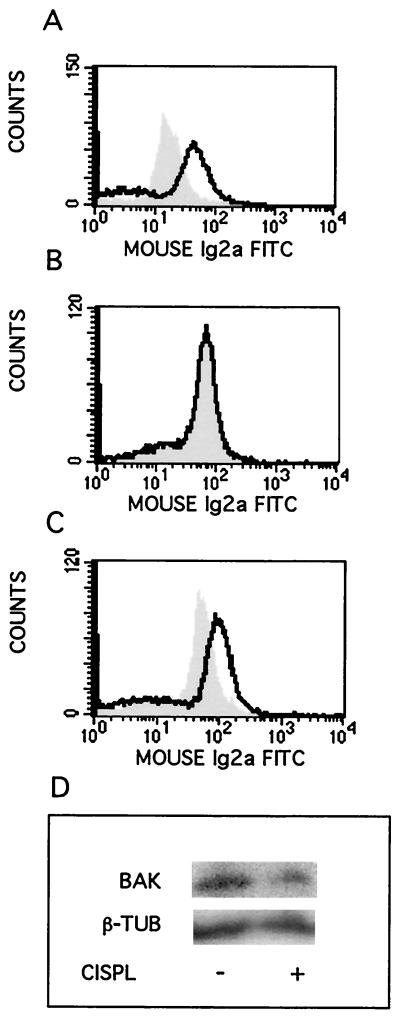
With this method, we show for the first time that cisplatin induces an increase in the proapoptotic conformation of Bak, as seen in 224 cells treated with 20 μM cisplatin for 16 h (Fig. 1A). Similar shifts were seen also at 14 and 18 h (not shown). By contrast, cisplatin treatment did not lead to Bax modulation at 16 h (Fig. 1B). Staurosporine treatment (1 μM; 5 h) did lead to Bax modulation (Fig. 1C), showing that this effect was not constitutively abrogated in 224 cells. Western blotting confirmed that Bak protein levels were similar in control and cisplatin-treated cells (Fig. 1D). Approximately equitoxic doses of cisplatin were found to induce Bak modulation also in three other human melanoma cell lines, whereas Bax modulation was not a general feature, being seen only in FM55 cells (Table 1).
FIG. 1.
Cisplatin-induced modulation of Bak but not Bax. Human melanoma 224 cells were treated with cisplatin (20 μM, 16 h) or staurosporine (1 μM, 5 h), as indicated, before samples were prepared for FACS analysis of Bak or Bax-associated IFL. Control cells, gray peak; treated cells, black line. (A) Bak-associated IFL after cisplatin treatment; (B) Bax-associated IFL after cisplatin treatment; (C) Bax-associated IFL after staurosporine treatment; (D) Western blot showing Bak protein levels in control cells and after 16 h of cisplatin (CISPL) treatment. Tubulin (β-TUB) was used as an internal loading control. Ig2a, immunoglobulin 2a.
dnMEKK blocks cisplatin-induced apoptosis and cytochrome c release but not JNK activation.
Since Bak and Bax functions ultimately lead to caspase activation, and since an apoptosis-specific ΔMEKK1 pathway is implied in caspase activation (1, 27, 28), we examined the effects of kinase-active and -inactive ΔMEKK1 mutants on Bak/Bax modulation, caspase activation, and nuclear fragmentation in cisplatin-induced apoptosis. An adenovirus vector system (17) was used to inducibly express dpMEKK, consisting of the kinase domain of MEKK1, and dnMEKK, carrying an inactivating point mutation (K432M).
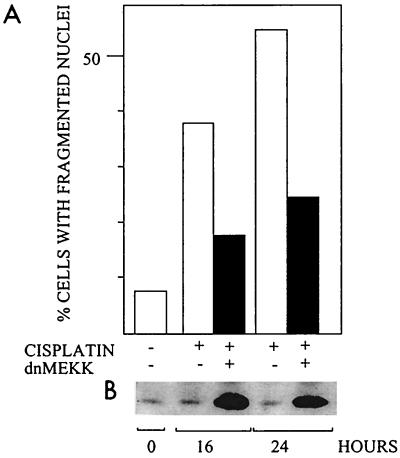
In 224 cells preinduced to express dnMEKK, cisplatin-induced nuclear fragmentation was significantly decreased (Fig. 2). Similarly, dnMEKK inhibited nuclear fragmentation by 50 to 75% in the other three cell lines (not shown). Expression of dnMEKK did not per se cause nuclear fragmentation, nor did it activate caspases or lead to DNA-PK cleavage (see below). Infection with adeno-rtTA only and subsequent DOX treatment did not block nuclear fragmentation, showing that blocking was not an unspecific adenovirus-mediated effect.
FIG. 2.
Effect of dnMEKK on cisplatin-induced nuclear fragmentation. Cells were treated with 20 μM cisplatin in the presence or absence of adeno-dnMEKK. Expression of dnMEKK was induced with DOX at 20 h before cisplatin treatment. (A) Nuclear fragmentation in 224 cells after 16 and 24 h of cisplatin treatment; (B) Western blot showing expression of dnMEKK (37 kDa) in the same samples. A faint endogenous band is seen also in the extracts from cells which were not infected and is thus not due to leakage. The experiment was repeated with similar results.
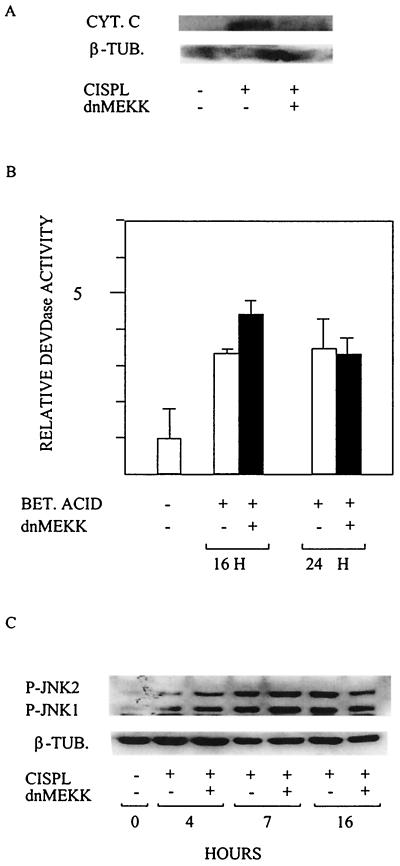
Cisplatin-induced release of cytochrome c at 20 h was abrogated in 224 cells expressing dnMEKK (Fig. 3A). To confirm that dnMEKK blocks upstream of cytochrome c release, we used betulinic acid (BA) as an apoptosis-inducing agent. BA is reported to have a direct effect on mitochondria, leading to mitochondrial permeability transition followed by cytochrome c release, caspase activation, and nuclear fragmentation (8). In agreement with dnMEKK blocking an event upstream of cytochrome c release, dnMEKK did not block BA-induced apoptosis (not shown), nor did it block BA-induced DEVDase activation seen at 16 and 24 h (Fig. 3B).
FIG. 3.
dnMEKK blocks upstream of cytochrome c release without blocking JNK activation. 224 cells were treated with 20 μM cisplatin or 15 μg of BA per ml in the presence or absence of adeno-dnMEKK. Expression of dnMEKK was induced with DOX at 20 h before drug treatment. (A) Western blot for cytochrome c (CYT. C) in cytosolic extracts at 20 h after addition of cisplatin (CISPL). The membrane was also probed with an antibody against mitochondrial cytochrome oxidase subunit IV to ensure lack of mitochondrial contamination of the extracts (not shown). β-TUB., tubulin. (B) BA (BET. ACID)-induced DEVDase activity measured in extracts (50 μg) of cells at the indicated time points. Results are shown as fold activation relative to control cells. (C) Cisplatin-induced activation of JNK1 and -2, as seen by phosphorylation at the indicated time points in the presence or absence of dnMEKK, was assessed by Western blotting using an antibody specific for phosphorylated JNK1 and -2 (P-JNK1 and -2). Tubulin was used as an internal loading control.
Because sustained JNK activation between 3 and 12 h of treatment is implied in cisplatin-induced apoptosis (23), JNK activity at 4, 7, and 16 h was assessed with an antibody specific for phosphorylated JNK1 and JNK2. Cisplatin-induced JNK1 and -2 phosphorylation at these time points and expression of dnMEKK did not block this effect (Fig. 3C).
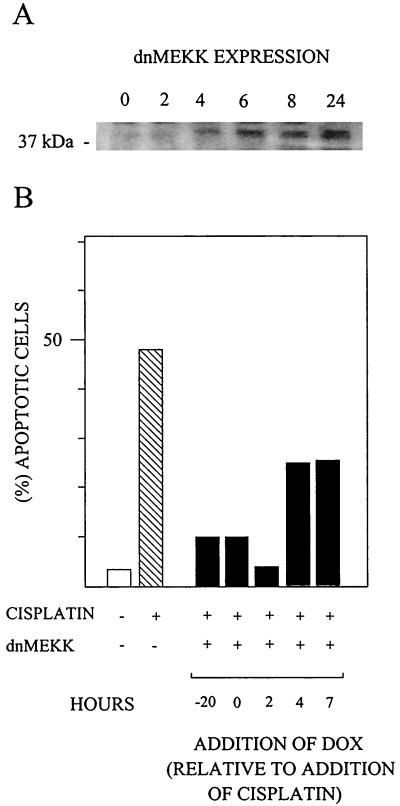
Time course experiments were carried out to determine when expression of dnMEKK was required to block apoptosis. At 2 h of DOX treatment, there was no expression of the mutant, while some expression was seen after 4 h (Fig. 4A). When DOX was added at 2 h after cisplatin, apoptosis was blocked to the same extent as in cells which expressed dnMEKK already at the time of cisplatin addition (Fig. 4B). Even later addition of DOX also afforded some protection against apoptosis (Fig. 4B). This experiment shows that dnMEKK inhibition of apoptosis occurs at a stage later than 4 to 6 h after addition of cisplatin.
FIG. 4.
Delayed induction of dnMEKK expression blocks nuclear fragmentation. (A) Western blot showing 37-kDa dnMEKK expression at different time points after DOX addition. (B) 224 cells infected with adeno-dnMEKK were induced to express dnMEKK by addition of DOX at the indicated time points, which are relative to the time of cisplatin addition (20 μM) at 0 h. Nuclear fragmentation levels were assessed at 24 h and compared to fragmentation induced in the absence of adeno-dnMEKK.
dnMEKK blocks caspase activation.
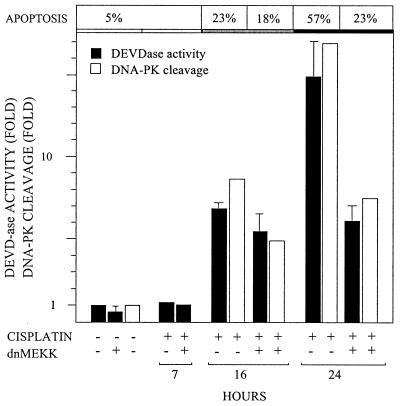
If dnMEKK blocks cytochrome c release, it should also block caspase activation. Cisplatin-induced DEVDase activity in 224 cells exhibited a 15-fold increase at 24 h (Fig. 5). Expression of dnMEKK partly inhibited this activation (Fig. 5). Blocking of DEVDase activity was confirmed in the same samples by a reduction in cisplatin-induced cleavage of DNA-PK, a nuclear caspase 3 substrate (Fig. 5).
FIG. 5.
dnMEKK blocks cisplatin-induced DEVDase activity. 224 cells were treated with 20 μM cisplatin for the indicated time periods, in the presence or absence of adeno-dnMEKK. Expression of dnMEKK was induced with DOX at 20 h before cisplatin treatment. After harvest, cells were aliquoted for apoptosis and DEVDase assays and for western blotting. Apoptosis levels, seen as nuclear fragmentation, are shown above the relevant bars. Filled bars, DEVDase activity against a synthetic substrate (Ac-DEVD-AMC), measured in cell extracts at the indicated time points. Results are shown as induction of activity relative to that in control samples. Empty bars, cleavage of DNA-PK, as assessed by densitometric scanning of the 160-kDa proteolytic fragment on Western blot films. Results are shown as fragment levels relative to that in control samples.
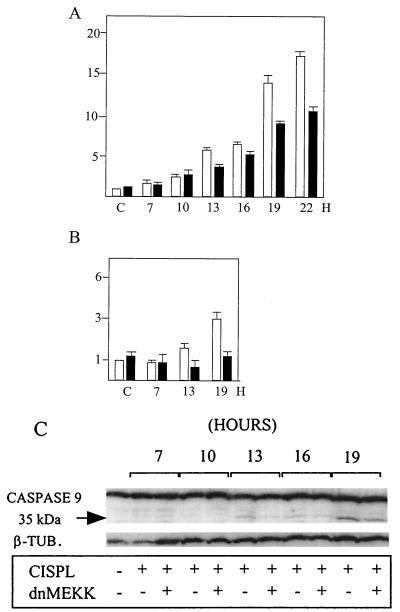
Cell extracts made at several time points after cisplatin treatment were then assayed for DEVDase and caspase 9/LEHDase activity in the presence and absence of dnMEKK (Fig. 6). These experiments confirmed that a major increase in DEVDase activity occurred at 13 h and beyond and that this increase was partly inhibitable by dnMEKK (Fig. 6A). An enzymatic assay of caspase 9 seen as LEHDase activity showed 1.6- and 3-fold activation at 13 and 19 h, respectively (Fig. 6B). This caspase 9/LEHDase activity was furthermore inhibited in the presence of dnMEKK (Fig. 6B). To confirm this result, we performed Western blotting of caspase 9. The 35-kDa active cleavage fragment of procaspase 9 appeared as a faint band at 13 to 16 h and was easily observed at 19 h of cisplatin treatment (Fig. 6C). In accordance with the enzymatic assay, dnMEKK was found to reduce the fragment signal at all three time points (Fig. 6C). In attempts to examine DEVDase cleavage fragments in these cells by Western blotting, we consistently failed to detect any with a range of commercially available antibodies against caspases 3 and 7.
FIG. 6.
Kinetics of cisplatin-induced DEVDase and caspase 9/ LEHDase activities. 224 cells were treated with 20 μM cisplatin for the indicated time periods in the absence or presence of adeno-dnMEKK. Expression of dnMEKK was induced with DOX at 20 h before cisplatin treatment. Empty bars, cisplatin only; filled bars, cisplatin treatment in the presence of dnMEKK. (A) DEVDase activity on the synthetic substrate Ac-DEVD-AMC was assessed twice for each duplicate sample. The inhibitor DEVD-CHO reduced all activity to background in all parallel samples. Results are shown as fold increase in activity in untreated (control [C]) cells. (B) Caspase 9/LEHDase activity on the synthetic substrate Ac-LEHD-AMC. The inhibitor LEHD-CHO reduced all activity to background in all parallel samples. (C) Cell extracts (30 μg/lane) were analyzed by Western blotting of caspase 9. The 35-kDa cleavage fragment represents the active form. β-TUB., tubulin; CISPL, cisplatin.
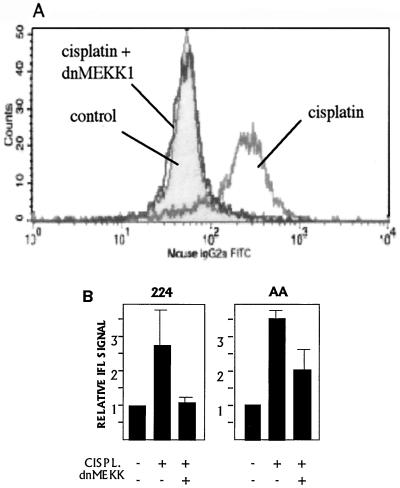
dnMEKK blocks cisplatin-induced Bak modulation.
In accordance with a blocking effect upstream of cytochrome c release and caspase 9 activation in 224 cells, dnMEKK was found to block cisplatin-induced Bak modulation in 224 and AA cells (Fig. 7). dnMEKK did not block staurosporine-induced Bak modulation (not shown), suggesting a degree of specificity for this pathway.
FIG. 7.
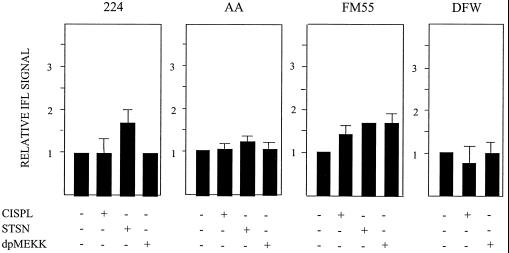
dnMEKK blocks cisplatin-induced modulation of Bak. (A) 224 cells were treated with 20 μM cisplatin for 16 h in the absence or presence of adeno-dnMEKK. Expression of dnMEKK was induced with DOX at 20 h before cisplatin treatment. Shown are overlaid FACS graphs indicating Bak-associated IFL. Gray peak, control cells; dark lines, cisplatin treatment in the presence or absence of dnMEKK, as indicated. (B) Quantitation of cisplatin-induced Bak modulation in the presence or absence of dnMEKK in 224 and AA cell lines. Cells were treated with LD50s of cisplatin (CISPL.). Results are shown as fold increase in median IFL signal relative to controls.
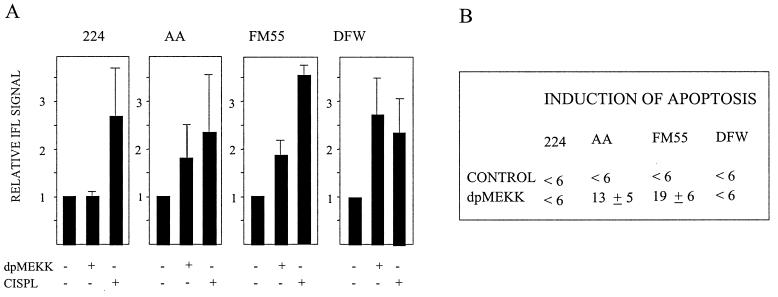
Induction of Bak modulation and apoptosis by dpMEKK.
The constitutively active kinase fragment of MEKK1 has been shown to be important for amplification of the apoptotic signal (1, 27, 28). Using the same protocol as with dnMEKK, cells were infected with an adenovirus vector expressing the constitutively active 37-kDa kinase fragment of MEKK1 (dpMEKK). At 24 h postinduction, dpMEKK had induced Bak modulation in AA, FM55, and DFW cells but not in 224 cells (Fig. 8A). dpMEKK did not elicit apoptosis in 224 or DFW cells, while in FM55 and AA cells it induced 19 and 13% apoptosis, respectively (Fig. 8B).
FIG. 8.
Induction of Bak modulation and apoptosis by dpMEKK. (A) Cells were infected with adeno-dpMEKK, and Bak-associated IFL was assessed at 24 h after addition of DOX. For comparison, Bak modulation at 16 h induced by LD50s of cisplatin (CISPL) is also shown. Results are shown as fold increase in median IFL signal compared to controls. (B) Cells were infected with adeno-dpMEKK, and nuclear fragmentation was assessed at 24 h after addition of DOX. Background levels in control cells varied but did not exceed 6%.
Modulation of Bax by cisplatin and dpMEKK.
In three out of four cell lines, neither cisplatin treatment for 16 h nor dpMEKK could induce Bax modulation. FM55 cells were thus unique in that they were the only cells to show Bax modulation by either treatment (Fig. 9). Staurosporine treatment of 224 and AA cells showed that Bax modulation is not constitutively abrogated in these cell lines (Fig. 9).
FIG. 9.
Induction of Bax modulation. Bax-associated IFL was assessed at 16 h after cisplatin (CISPL) addition or at 24 h after addition of DOX to induce dpMEKK expression in adeno-dpMEKK-infected cells. The effect of staurosporine (STSN; 1 μM, 5 h) on Bax modulation in 224, AA, and FM55 cells is also shown.
Antisense Bak reduces cisplatin-induced apoptotic responses.
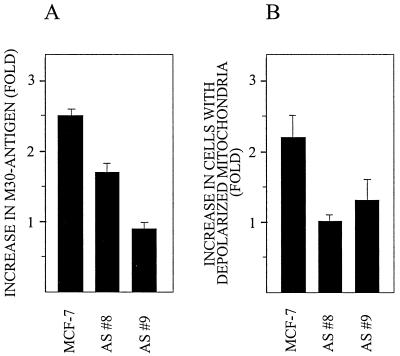
Since dnMEKK blocked cisplatin-induced Bak modulation as well as apoptosis, we also wished to examine the effect of cisplatin in cells expressing antisense Bak. We therefore compared the effects of cisplatin on parental MCF-7 breast carcinoma cells and two sublines stably expressing antisense Bak (6). Because MCF-7 cells lack caspase 3 expression and thus may have aberrant DNA fragmentation, apoptosis was assessed using an antibody, M30, specifically recognizing caspase-cleaved fragments of cytokeratin 18, which can be cleaved by activated caspases other than caspase 3 (2, 6). The M30 antibody is used for immunohistochemical detection of apoptosis or, as we have done here, in an enzyme-linked immunosorbent assay (ELISA) system (Peviva AB, Stockholm, Sweden) (14). We found that at 21 h after addition of 20 μM cisplatin, caspase cleavage of cytokeratin 18 was significantly reduced in the cell lines expressing antisense Bak compared to parental cells (Fig. 10A). Cisplatin treatment also induced mitochondrial depolarization, and this effect was blocked in cells expressing antisense Bak but not in parental cells (Fig. 10B).
FIG. 10.
Reduced apoptotic responses in cells expressing antisense Bak. Parental MCF-7 and two subclones with stable expression of antisense Bak were treated with 20 μM cisplatin for 21 h. (A) Caspase activity was assessed by ELISA-based quantitation of the caspase-cleaved fragment of cytokeratin 18 in cell lysates as instructed by the manufacturer (Apoptosense ELISA kit; Peviva AB). Results are shown as fold increase in the levels of fragment specifically recognized by the antibody M30. (B) The mitochondrial transmembrane potential was determined in duplicate samples as retention of tetramethylrhodamine ethyl ester (TMRE; Molecular Probes Inc.), a cationic, lipophilic fluorochrome dye that accumulates in the negatively charged mitochondrial matrix. Depolarization of mitochondria, as seen during apoptosis, is quantitated as loss of TMRE fluorescent signal assessed by flow cytometry. Results are shown as fold increase in the fraction of cells with depolarized mitochondria, i.e., cells showing a median TMRE signal less than 5% of the median signal in controls. AS #8 and AS #9 are clones stably expressing antisense Bak.
DISCUSSION
Bax and Bak, proapoptotic members of the Bcl-2 family, are involved in cytochrome c release (16, 25), and their proapoptotic function is dependent on a specific conformational change (5, 10, 11, 18). This is the first report to show that cisplatin-induced apoptosis involves induction of the apoptotic conformation of Bak. Thus, in the four cell lines tested, approximately equitoxic cisplatin treatment led to Bak modulation prior to the onset of nuclear fragmentation. Bak modulation was seen both in the presence of mutant p53 (224 cells) and in the other three cell lines, expressing wild-type p53. In contrast to Bak, an effect on Bax was seen only in one of the cell lines (FM55). This suggests a degree of specificity in the signal to Bak.
A specific pathway involving a constitutively active MEKK1 fragment (ΔMEKK1) in preapoptotic cells has been implied in apoptosis induced by genotoxic stress or by anti-Fas treatment (1, 4, 27), likely by leading to caspase activation (24), although the molecular details are not known. We have here used kinase-active and -inactive MEKK1 fragment mutants to study the relationship(s) between Bak and Bax, caspase activation, and the proapoptotic MEKK1 pathway in cisplatin-induced apoptosis. We show that a 37-kDa kinase-inactive MEKK1 mutant (dnMEKK) inhibits cisplatin-induced modulation of Bak, cytochrome c release, caspase activation, and nuclear fragmentation.
Because cisplatin-induced Bak modulation and nuclear fragmentation were affected by dnMEKK, we examined whether dpMEKK would have effects similar to those of cisplatin. First, we found that the dpMEKK signal is potentially sufficient to induce apoptosis. Thus, in AA and FM55 cells, dpMEKK induced some apoptosis; this was higher in FM55 cells, in which dpMEKK modulated both Bak and Bax. Although dpMEKK has been shown to inhibit growth in a range of cell lines in a clonogenic assay over 2 weeks (24), this is the first study to show an actual ability to induce apoptosis within 24 h. Second, we found that, like cisplatin, dpMEKK expression may be sufficient to induce Bak modulation, as seen in the AA, FM55, and DFW cell lines.
The exceptions to these findings suggest that in some cell lines, Bak modulation and/or its effects require a signal in addition to that provided by dpMEKK and that cisplatin likely induces such a signal. This may explain the resistance of 224 cells to dpMEKK-induced responses. The weak apoptotic response of DFW cells to dpMEKK-induced Bak modulation may in turn be explained by the recent finding that some metastatic melanomas have lost expression of Apaf-1 by gene inactivation due to methylation (26). Because of the role of Apaf-1 in caspase activation downstream of mitochondrial events, reduced Apaf-1 levels will block caspase activation but not mitochondrial events such as Bak modulation.
The existence of additional cisplatin-induced signals, other than via ΔMEKK1, were indicated also by the findings that dnMEKK very efficiently blocked Bak modulation and cytochrome c release in 224 cells, whereas DEVDase activation and nuclear fragmentation were reduced only by half. This suggests the presence of a Bak-independent, dnMEKK-insensitive DEVDase activation which should be cytochrome c independent. Another possibility is the existence of different intracellular pools of caspase 3 with different activation mechanisms, since it has been reported that the caspase 3 precursor has both a cytosolic and a mitochondrial distribution, likely with different modes of activation (12, 15, 32).
As cisplatin-induced apoptosis has been reported to require JNK activation (23, 30), it is interesting that dnMEKK inhibited apoptosis without inhibiting JNK activation. Apoptosis inhibition was seen also when the inducer (DOX) was added later than cisplatin. DOX treatment for approximately 4 h was required to induce dnMEKK expression, which means that dnMEKK was not expressed at time points when JNK was already activated. The blocking effect of dnMEKK in this system is thus independent at least of early JNK activation.
JNK activation by apoptosis-inducing agents is not necessarily MEKK1 dependent, as JNK activation by UV was not impaired in MEKK1-deficient cells (29) and dnMEKK1 did not block JNK activation by tumor necrosis factor alpha (27). Cisplatin-mediated JNK activation has been reported to be independent of SEK1 (19). If so, it is independent of the classical MEKK1 pathway and dnMEKK should not block. It is interesting to speculate whether dnMEKK-insensitive JNK activation is responsible for dnMEKK-insensitive, Bak-independent DEVDase activation.
In conclusion, we here demonstrate that cisplatin-induced apoptosis involves induction of the apoptotic conformation of Bak, whereas a similar modulation of Bax is not a general feature. In accordance with a role for Bak in cisplatin-induced apoptosis, we also show that MCF-7 subclones stably expressing antisense Bak display a significantly reduced apoptotic response to cisplatin compared to parental MCF-7 cells. Bak modulation and the subsequent cytochrome c release and caspase 9 activation are all blocked by a kinase-negative MEKK1 fragment (dnMEKK). Conversely, a kinase-active MEKK1 fragment (dpMEKK) was able to induce Bak modulation and apoptosis. Our findings thus connect an upstream kinase activity to mitochondrial events in the execution phase of apoptosis.
ACKNOWLEDGMENTS
K.V. and A.M. contributed equally to this work.
We thank T. Maniatis for generously providing the plasmid constructs of dn- and dpMEKK, and we gratefully acknowledge M. Varsanyi for analyzing p53 status.
This work was funded by grants from the Swedish Cancer Society and by Cancerföreningen Stockholm. K.V. was supported by a grant from the Swedish Foundation for Strategic Research.
REFERENCES
- 1.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 2.Caulin C, Salvesen G S, Oshima R G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis R J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 4.Deak J, Cross J V, Lewis M, Qian Y, Parrott L A. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc Natl Acad Sci USA. 1998;95:5595–5600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J-C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eguchi H, Suga K, Saji H, Toi M, Nakachi K, Hayashi S I. Different expression patterns of Bcl-2 family genes in breast cancer by estrogen receptor status with special reference to pro-apoptotic Bak gene. Cell Death Differ. 2000;7:439–446. doi: 10.1038/sj.cdd.4400675. [DOI] [PubMed] [Google Scholar]
- 7.Fife R S, Rougraff B T, Proctor C, Sledge G W. Inhibition of proliferation and induction of apoptosis by doxycycline in cultured human osteosarcoma cells. J Lab Clin Med. 1997;130:530–534. doi: 10.1016/s0022-2143(97)90130-x. [DOI] [PubMed] [Google Scholar]
- 8.Fulda S, Scaffidi C, Susin A, Krammer P H, Kroemer G, Peter M E, Debatin K. Activation of mitochondria and release of mitochondrial apoptogenic factors by betulinic acid. J Biol Chem. 1998;273:33942–33948. doi: 10.1074/jbc.273.51.33942. [DOI] [PubMed] [Google Scholar]
- 9.Gibson S, Widmann C, Johnson G L. Differential involvement of MEK kinase 1 (MEKK1) in the induction of apoptosis in response to microtubule-targeted drugs versus DNA damaging agents. J Biol Chem. 1999;274:10916–10922. doi: 10.1074/jbc.274.16.10916. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths G J, Dubrez L, Morgan C P, Jones N A, Whitehouse J, Corfe B M, Dive C, Hickman J A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu Y-T, Youle R J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 12.Krebs J F, Armstrong R C, Srinivasan A, Aja T, Wong A M, Aboy A, Sayers R, Pham B, Vu T, Hoang K, Karanewsky D S, Leist C, Schmitz A, Wu J C, Tomaselli K J, Fritz L C. Activation of membrane-associated procaspase-3 is regulated by Bcl-2. J Cell Biol. 1999;144:915–931. doi: 10.1083/jcb.144.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lassignal Johnson N, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 14.Leers M P G, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers F C S, Bjorklund B, Nap M, Jornvall H, Schutte B. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Mancini M, Nicholson D W, Roy S, Thornberry N A, Peterson E P, Casciola-Rosen L A, Rosen A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: implications for apoptotic signaling. J Cell Biol. 1998;140:1486–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzo I, Brenner C, Zamzami N, Jurgensmeier J M, Susin S A, Vieira H L, Prevost M C, Xie Z, Matsuyama S, Reed J C, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 17.Molin M, Shoshan M C, Öhman-Forslund K, Linder S, Akusjärvi G. Two novel adenovirus vector systems permitting regulated protein expression in gene transfer experiments. J Virol. 1998;72:8358–8361. doi: 10.1128/jvi.72.10.8358-8361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nechushtan A, Smith L, Hsu Y T, Youle R J. Conformation of the Bax C terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehmé A, Baskarna R, Aebi S, Fink D, Nebel S, Cenni B, Wang J Y J, Howell S B, Christen R D. Differential induction of c-Jun NH2-terminal kinase and c-Abl kinase in DNA mismatch repair-proficient and -deficient cells exposed to cisplatin. Cancer Res. 1997;57:3253–3257. [PubMed] [Google Scholar]
- 20.Nunez G, Benedict M A, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 21.Perez R P. Cellular and molecular determinants of cisplatin resistance. Eur J Cancer. 1998;34:1535–1542. doi: 10.1016/s0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 22.Porter A G, Jänicke R U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Pérez I, Rámon Murguia J, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 24.Seimiya H, Mashima T, Toho M, Tsuruo T. c-Jun NH-terminal kinase-mediated activation of interleukin 1β converting enzyme/CED-3-like protease during anticancer drug-induced apoptosis. J Biol Chem. 1997;272:4631–4636. doi: 10.1074/jbc.272.7.4631. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 26.Soengas M S, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman J G, Gerald W L, Lazebnik Y A, Cordón-Cardó C, Lowe S W. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 27.Widmann C, Lassignal Johnson N, Gardner A M, Smith R J, Johnson G L. Potentiation of apoptosis by low dose stress stimuli in cells expressing activated MEK kinase 1. Oncogene. 1997;15:2439–2447. doi: 10.1038/sj.onc.1201421. [DOI] [PubMed] [Google Scholar]
- 28.Widmann C, Gerwins P, Lassignal Johnson N, Jarpe M B, Johnson G L. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol Cell Biol. 1998;18:2416–2429. doi: 10.1128/mcb.18.4.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 30.Zanke B, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon I, Kyriakis J, Liu F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhao R, Bendix Rabo Y, Egyházi S, Andersson A, Edgren M, Linder S, Hansson J. Apoptosis and c-jun induction by cisplatin in a human melanoma cell line and a drug-resistant daughter cell line. Anti-Cancer Drugs. 1995;6:657–668. doi: 10.1097/00001813-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Zhivotovsky B, Samali A, Gahm A, Orrenius S. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]