Abstract
Accurate and sensitive quantification of human immunodeficiency virus type 1 (HIV-1) RNA has been invaluable as a marker for disease prognosis and for clinical monitoring of HIV-1 disease. The first generation of commercially available HIV-1 RNA tests were optimized to detect the predominant HIV-1 subtype found in North America and Europe, subtype B. However, these tests are frequently suboptimal in detecting HIV-1 genetic forms or subtypes found in other parts of the world. The goal of the present study was to evaluate the performance of a new viral load assay with non-subtype B viruses. A transcription-mediated amplification method for detection and quantitation of diverse HIV-1 subtypes, called the Gen-Probe HIV-1 viral load assay, is under development. In this study we examined the performance of the Gen-Probe HIV-1 viral load assay relative to that of the commonly used commercial HIV-1 RNA assays using a panel of primary isolates from Kenya. For comparison, we included several subtype B cloned viruses, and we quantified each virus using an in-house quantitative-competitive reverse transcriptase PCR (QC-RT-PCR) method and gagp24 antigen capture. The Gen-Probe HIV-1 viral load assay and a version of the Roche AMPLICOR HIV-1 MONITOR test (version 1.5) that was designed to detect a broader range of subtypes were both sensitive for the quantification of Kenyan primary isolates, which represented subtype A, C, and D viruses. The Gen-Probe HIV-1 viral load assay was more sensitive for the majority of viruses than the Roche AMPLICOR HIV-1 MONITOR test version 1.0, the Bayer Quantiplex HIV RNA 3.0 assay, or a QC-RT-PCR method in use in our laboratory, suggesting that it provides a useful method for quantifying HIV-1 RNAs from diverse parts of the world, including Africa.
Human immunodeficiency virus type 1 (HIV-1) RNA levels provide a marker for virus replication, a measure of the effects of antiretroviral therapy, and a prognostic indicator of disease (18, 24, 25, 33, 34). Because the monitoring of HIV-1 RNA in patients for these purposes has been almost exclusively applied in developed countries, principally in the United States and Europe, the initial methods for quantifying HIV-1 RNA were designed with strains from these countries in mind. As a result, these assays are not optimized to quantify the variants of HIV-1 that are found in other parts of the world. HIV-1 has been categorized into groups M (main) and O (outlier) (28, 36) and, most recently, group N (39). Group M, which is most common, has been further divided into subtypes or clades based on the relatedness of viral gag and/or envelope (env) gene sequences. The group M and O viruses differ by as much as 50% in their overall sequences, and the subtypes differ by as much as 15 and 30% from each other in gag and env, respectively (22, 23, 40). The vast majority of all HIV-1 infections in the United States and Europe are with subtype B, although there have been more recent case reports of infections with other subtypes (1, 4, 5, 15, 20). However, worldwide, most infections are with non-subtype B HIV-1 (6, 14, 40). In sub-Saharan Africa, where more than three-quarters of HIV-1 infections have occurred, almost all of the known subtypes can be found, including representatives from groups M, O, and N (6, 14, 39, 40). For example, the majority of the HIV-1 infections in Kenya are with subtype A (70%), but subtypes D and C are also represented in a significant number of infections (20 and 7%, respectively) (30, 37). In addition, intersubtype recombinants have been identified in Kenya and may represent as many as 20% of all viruses (30). Thus, in these settings, existing first-generation technologies for determination of HIV-1 RNA viral loads may not be dependable.
The first commercial assays for detection and quantification of HIV-1 RNA were based on nucleic acid sequence-based amplification (NASBA) (Organon Teknika) (41), branched-DNA signal amplification (bDNA) (Bayer) (19), and reverse transcriptase PCR (RT-PCR) (Roche AMPLICOR MONITOR) (27). Of these three first-generation HIV-1 RNA tests which were designed to detect subtype B HIV-1, the bDNA assay seems to be the most adaptable for use with viruses from non-subtype B strains of HIV-1 (35). However, this assay is somewhat less sensitive for detection of different subtypes in samples with low viral RNA levels (7, 13, 32), and it does not appear to be as reliable as the newer AMPLICOR MONITOR assay, version 1.5 (35). The NASBA assay, which detects gag HIV-1 sequence, appears to be somewhat better than the first-generation AMPLICOR MONITOR assay (version 1.0) for quantification of different subtypes in most cases (11), but it has been shown to be suboptimal for quantification of subtype A, G, and H viruses (1, 8, 11). The AMPLICOR MONITOR 1.0 assay is suboptimal for detecting HIV-1 viruses of the A, E, F, G, and H subtypes (7, 9, 32). None of these assays are able to detect HIV-1 group O viruses (2, 8, 11). Some of the limitations of these assays are likely due to primer mismatches that result from the extensive diversity between viral subtypes. A developmental NASBA assay, which is long terminal repeat based as opposed to gag based, is able to detect and quantify non-B subtype viruses, as well as group O viruses (8). For studies of HIV-1 infection outside the United States and Europe, it is important that commercial viral load assays be modified to accurately detect and quantitate viral RNAs from all HIV-1 subtypes.
The goal of the present study was to evaluate the performance of a new viral load assay with non-subtype B viruses found frequently in Africa. The Gen-Probe HIV-1 viral load assay, which is under development, was designed to detect all presently known HIV-1 group M, N, and O subtypes (3). The Kenyan viruses that we analyzed should provide an initial indication of the general utility of this assay for African isolates, since subtype A, D, and C viruses are the most common HIV-1 subtypes throughout most of sub-Saharan Africa (16, 22, 37).
MATERIALS AND METHODS
Viral isolates.
Primary viral isolates were obtained from 19 adults and 5 infants from Nairobi, Kenya. Blood from these individuals was collected in EDTA tubes, separated into cellular and plasma fractions by standard methods, and then stored and transported in liquid nitrogen. In two cases, two primary isolates were obtained from the same individual but from two different dates of examination (M2664 P32 and M2664 W6; B2062 W6 and B2062 W15). In the case of M2664, we also examined an isolate for her infant, B2664. In four cases, two isolates from different time points after culture of the primary isolate were obtained (M7376, M1462, M15742, and M2059). These were designated p1 or p2. A total of 30 Kenyan isolates from 24 different individuals were examined in this study.
Virus was obtained by coculturing 106 peripheral blood mononuclear cells (PBMCs) from these individuals with 106 uninfected phytohemagglutinin (PHA)-stimulated PBMCs. Cells were maintained at 106 per ml by either addition of medium or addition of 106 phytohemagglutinin-stimulated PBMCs per ml in fresh medium. Cultures were expanded and maintained in this manner and then tested for HIV-1 gagp24 after about 2 weeks in culture or when the cell number did not increase. Fresh medium was added to positive cultures, and they were harvested several days later, typically after a total of 3 to 4 weeks in culture. Cultures were spun at 1,000 rpm for 5 min in a Beckman GPR centrifuge, and the supernatant was collected, passed through a 0.2-μm syringe filter, aliquoted into 1-ml samples, and stored at −70°C. Subtype B viruses were generated in a similar manner, but infection was initiated using virus generated by transient transfection of HIV-1 proviral clones in 293T cells.
The subtype of the virus with which each individual was infected had previously been determined using heteroduplex mobility assays of envelope sequences amplified directly from their PBMCs prior to culturing (30). The subtypes of HIV-1 with which the mothers had been infected have been described previously (30), and we assumed that the infants were infected with the same subtypes as their mothers. The viruses analyzed here include 17 subtype A, 7 subtype D, and 4 subtype C primary isolates. The subtype for two of the isolates could not be resolved, suggesting they may be intersubtype recombinants. Seven subtype B viruses, obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, were also included for comparison.
Antigen detection.
The HIVAG-1 Monoclonal test (Abbott Laboratories, Chicago, Ill.), an assay to determine gagp24 antigen levels, was performed in accordance with the manufacturer's instructions at the Clinical Retrovirus Lab at the University of Washington.
QC-RT-PCR.
RNA was extracted from 140 μl of cell-free viral supernatant using the QIAmp viral RNA extraction kit (Qiagen, Inc., Valencia, Calif.) and eluted with 50 μl of RNase-free water in the final step of the protocol. An additional 30 μl of diethyl pyrocarbonate-treated water was added to each sample prior to treatment with DNase I. Twenty microliters of 5× RQ1 buffer was added, the sample was vortexed, 2 μl of RQ1 RNase-free DNase (Promega, Madison, WI) were added, and the sample was gently tapped and then incubated at 37°C for 30 min followed by heat inactivation at 75°C for 15 min. A previously described quantitative-competitive RT-PCR (QC-RT-PCR) (21) was performed to quantitate the amount of HIV-1 RNA.
Commercial HIV-1 RNA tests.
The Quantiplex HIV-1 RNA 3.0 test (Bayer Diagnostics, Walpole, Mass.), an enhanced-sensitivity bDNA assay, was performed in accordance with the manufacturer's instructions at the Clinical Retrovirus Lab at the University of Washington.
The Roche AMPLICOR HIV-1 MONITOR tests, versions 1.0 and 1.5 (Roche Diagnostics, Inc., Branchburg, N.J.), RT-PCR tests with an internal quantitation standard, were performed in accordance with the manufacturer's instructions (26). Six specimens that were diluted into heparinized plasma (JRCSF, NL4-3, YU2, Bru, ADA, and 8002) were extracted using the silica extraction method (NucliSens lysis and extraction kits, catalog no. 84047 and 84039; Organon Teknika Corp.).
Gen-Probe HIV-1 viral load assay.
The HIV-1 viral load assay (Gen-Probe Incorporated, San Diego, Calif.), a transcription-mediated amplification (TMA) assay under development, was performed in our laboratory using the methods recommended by Gen-Probe. In this assay, sample preparation, amplification, and detection are performed in a single tube. Three steps are involved in the assay protocol: (i) sample preparation and target capture, (ii) amplification by TMA, and (iii) detection of the amplicon with the hybridization protection assay. This integrated approach allows processing of 200 samples in 6 to 8 h (10). The viral RNA is released and stabilized during specimen processing. The RNA is then captured on magnetic particles which contain poly(dT) oligonucleotides as well as oligonucleotides with sequences complementary to the viral RNA. A magnetic field is then applied to the sample to separate the target viral RNA from other plasma components. Amplification of HIV-1 viral RNA sequences that are captured is performed by TMA (17). TMA is an exponential isothermal reaction that utilizes reverse transcriptase and T7 RNA polymerase. The reaction is initiated by the annealing of a chimeric primer that contains a T7 polymerase promoter coupled to an HIV-1-specific primer that primes DNA synthesis via reverse transcriptase. The RNA in this resulting RNA-DNA duplex is degraded by the RNase H activity of reverse transcriptase. An HIV-1-specific primer binds to the single stranded, antisense DNA, and a sense strand of the DNA is synthesized by reverse transcriptase. This DNA, which was engineered to include 5′ promoter sequences, then serves as a template for RNA synthesis by T7 polymerase. The reaction proceeds at an exponential rate, leading to a greater-than-109-fold amplification of the specific target nucleic acid. Detection is achieved by the addition of oligonucleotide probes with chemiluminescent labels. The label on unhybridized probes is chemically destroyed, and the label on hybridized probes is detected (31). In the hybridization protection assay, photons are measured with a luminometer and reported as relative light units. The results (in relative light units) are converted to copies of HIV-1 virus per milliliter by interpolation against an external standard curve run at the same time as the samples.
For the Gen-Probe assay, cell-free viral supernatants were diluted in HIV-1-seronegative human plasma to approximately 10,000 copies/ml as judged by QC-RT-PCR results. The diluted supernatants were tested in duplicate in the Gen-Probe HIV-1 quantitative test. The viral load was calculated from the duplicate data points if the values fell between 100 and 100,000 copies/ml. For those samples that fell above the range of the assay, further dilutions were made and samples were again tested in duplicate. In all cases, the duplicate data fell within twofold of each other.
Statistical methods.
To obtain approximately normal distributions, we used log10 transformations for gagp24 antigen levels and HIV-1 RNA viral load results for all assay analyses. Paired t tests were used to compare HIV-1 RNA viral load results using the Gen-Probe assay versus results using the QC-RT-PCR, Quantiplex 3.0, AMPLICOR 1.0, and AMPLICOR 1.5 assays. All reported correlations are Pearson's correlation coefficients. Although there were two cases in which two samples from the same individual were included in the analyses, these samples were from independent time points. Hence, all individual samples were treated as independent in the analyses.
RESULTS
Comparative analysis of different viral assays.
Primary isolates were obtained from 19 mothers and 5 infants who were part of a randomized clinical trial of breastfeeding and formula feeding in Nairobi, Kenya (29). A total of 30 primary isolates were examined because in some cases different isolates from the same individuals were tested, as described in Materials and Methods. This collection of viruses was isolated from individuals from several tribes and many parts of central and western Kenya (30). Seven HIV-1 subtype B viruses, all of which were generated from proviral clones, were included in this analysis for comparative purposes. The viral isolates and the corresponding subtypes are shown in Table 1. For each isolate, we determined the level of HIV-1 gagp24 antigen and the level of HIV-1 RNA using a variety of assays (Table 1).
TABLE 1.
Quantitative viral measurements
| Virus | Subtype | p24 (ng/ml) | HIV-1 RNA (log10 copies/ml)a
|
||||
|---|---|---|---|---|---|---|---|
| Gen-Probe | QC-RT-PCR | Quantiplex 3.0 | Amplicor 1.0 | Amplicor 1.5 | |||
| M1399 | A | 35.06 | 9.19 | 8.45 | 7.67 | 7.43 | 8.84 |
| B2062 (W6) | A | 127.50 | 9.65 | 8.85 | 8.22 | 7.36 | 9.03 |
| B2062 (W15) | A | 0.48 | 7.04 | 6.63 | 5.73 | 5.57 | 6.41 |
| M2201 | A | 15.23 | 8.92 | 7.38 | 7.62 | 7.99 | 8.92 |
| M2539 | A | 31.96 | 8.59 | 8.56 | 7.36 | NDb | 8.27 |
| B2664 | A | 852.06 | 10.36 | 9.68 | 9.35 | 10.26 | 10.38 |
| M2664 (P32) | A | 515.67 | 10.25 | 9.43 | 9.45 | 10.13 | 9.95 |
| M2664 (W6) | A | 1,050.45 | 10.19 | 9.68 | 9.38 | 10.06 | 10.45 |
| B2847 | A | 4.32 | 8.16 | 8.38 | 7.38 | 7.56 | 7.93 |
| B2897 | A | 12.96 | 8.24 | 7.85 | 7.09 | 7.90 | 8.16 |
| M8002 | A | 108.30 | 8.98 | 8.77 | 8.60c | 9.52 | 9.39 |
| M11879 | A | 58.93 | 9.33 | 8.85 | 7.92 | 7.48 | 9.14 |
| M15393 | A | 0.66 | 7.44 | 7.38 | 6.42 | 6.16 | 7.16 |
| M15834 | A | 1.10 | 7.79 | 7.38 | 6.45 | 5.94 | 7.35 |
| M16417 | A | 4.78 | 8.03 | 7.20 | 6.86 | 6.71 | 8.01 |
| M17415 | A | 0.47 | 7.36 | 6.85 | 6.53 | 7.19 | 7.43 |
| M18080 | A | 6.55 | 8.54 | 7.85 | 7.19 | 8.48 | 8.50 |
| ADA | B | 89.01 | 8.83 | 8.52 | 8.19 | 8.82 | 8.54 |
| Bru | B | 271.89 | 10.06 | 9.36 | 9.05 | 9.67 | 9.81 |
| JRCSF | B | 34.86 | 8.97 | 8.36 | 8.52 | 8.71 | 8.69 |
| NL4-3 | B | 35.39 | 8.74 | 8.36 | 8.35 | 8.61 | 8.55 |
| YU2 | B | 56.51 | 9.10 | 8.85 | 8.54 | 9.05 | 8.97 |
| SF33 | B | 280.40 | 9.77 | 9.20 | 9.15c | 9.41 | 9.51 |
| SF162 | B | 135.80 | 9.39 | 9.00 | 8.69c | 9.18 | 9.27 |
| M1462 (p1) | C | 0.60 | 7.31 | 7.38 | 6.20 | 6.84 | 7.18 |
| M1462 (p2) | C | 24.64 | 8.99 | 8.85 | 8.40 | 8.71 | 9.03 |
| M7376 (p1) | C | 0.33 | 6.85 | 5.85 | 6.12 | 6.45 | 6.50 |
| M7376 (p2) | C | 0.16 | 7.14 | 6.38 | 6.08 | 6.66 | 6.73 |
| B2059 (p1) | D | 111.67 | 9.71 | 8.85 | 8.58 | 9.43 | 9.89 |
| B2059 (p2) | D | 19.64 | 9.25 | 8.68 | 7.93 | 8.95 | 9.01 |
| M2965 | D | 5.19 | 8.17 | 7.85 | 7.21 | 8.05 | 7.93 |
| M15742 (p1a) | D | 6.72 | 8.80 | 7.85 | 7.26 | 8.31 | 8.52 |
| M15742 (p1b) | D | 36.96 | 8.80 | 8.68 | 7.46 | 8.53 | 8.64 |
| M16275 | D | 30.18 | 8.69 | 7.85 | 7.63 | 8.43 | 8.42 |
| M16731 | D | 3.41 | 7.96 | 7.38 | 6.62 | 7.54 | 7.81 |
| M13898 | ? | 16.24 | 8.85 | 8.38 | 7.51 | 8.29 | 8.25 |
| M16241 | ? | 24.37 | 9.52 | 7.85 | 8.18 | 6.09 | 9.26 |
Samples were diluted as needed to obtain values in the linear range of the assay.
ND, not detected in this assay.
Version 2.0.
We examined the Pearson correlation coefficient between the HIV-1 RNA viral load results from the Gen-Probe assay and other assays by HIV-1 subtype (Table 2 and Fig. 1). For HIV-1 subtype B viruses, the Gen-Probe assay exhibited a high correlation with all other assays. This correlation ranged from 0.95 with the Quantiplex 3.0 and QC-RT-PCR assays to 0.99 with the AMPLICOR 1.5 assay. For HIV-1 non-B subtypes, the Gen-Probe assay was again most highly correlated with the AMPLICOR 1.5 assay (r = 0.98) but also was correlated well with the Quantiplex 3.0, QC-RT-PCR, and AMPLICOR 1.0 assays (Table 2).
TABLE 2.
Correlation coefficients between log10 HIV-1 RNA copies per milliliter from the Gen-Probe HIV-1 viral load assay and other assays by HIV-1 subtype
| Assay | Correlation with Gen-Probe results
|
||
|---|---|---|---|
| All subtypes | Subtype B | Subtypes A, C, and D | |
| QC-RT-PCR | 0.91 | 0.95 | 0.93 |
| Quantiplex 3.0 | 0.95 | 0.95 | 0.96 |
| Amplicor 1.0 | 0.78 | 0.98 | 0.84 |
| Amplicor 1.5 | 0.98 | 0.99 | 0.98 |
FIG. 1.
Comparison of log10 RNA plasma viral load results from Gen-Probe assay versus other assays by HIV-1 clade. ◊, clade A; ✠, clade B; +, clade C; ▿, clade D; ○, unknown clade.
The Gen-Probe assay yielded statistically significant higher HIV-1 RNA viral load results than all other assays for all HIV-1 subtypes (Tables 3 and 4). For HIV-1 subtype B viruses, the Gen-Probe results were 0.2 to 0.6 log10 unit higher than those of the other assays, with the smallest difference being between the Gen-Probe and AMPLICOR 1.0 and 1.5 assays. For HIV-1 non-B subtypes, the Gen-Probe results were 0.2 to 1.1 log10 units higher than those of the other assays. The largest difference was between the Gen-Probe assay and the Quantiplex 3.0 assay, and the smallest was between the Gen-Probe assay and the AMPLICOR 1.5 assay (Tables 3 and 4).
TABLE 3.
Average log10 HIV-1 RNA copies per milliliter by assay and HIV-1 subtype
| Assay | Mean log10 HIV-1 RNA copies/ml for:
|
||
|---|---|---|---|
| All subtypes | Subtype B | Subtypes A, C, and D | |
| Gen-Probe | 8.7 | 9.3 | 8.6 |
| QC-RT-PCR | 8.2 | 8.8 | 8.0 |
| Quantiplex 3.0 | 7.7 | 8.6 | 7.5 |
| Amplicor 1.0 | 8.1 | 9.1 | 7.9 |
| Amplicor 1.5 | 8.5 | 9.1 | 8.4 |
TABLE 4.
Average difference between log10 HIV-1 RNA copies per milliliter obtained by Gen-Probe and other assays by HIV-1 subtype
| Assay | Avg difference from Gen-Probe results (P value for difference)
|
||
|---|---|---|---|
| All subtypes | Subtype B | Subtypes A, C, and D | |
| QC-RT-PCR | 0.55 (<0.001) | 0.46 (<0.001) | 0.53 (<0.001) |
| Quantiplex 3.0 | 1.03 (<0.001) | 0.63 (<0.001) | 1.10 (<0.001) |
| Amplicor 1.0 | 0.64 (<0.001) | 0.20 (0.01) | 0.65 (<0.001) |
| Amplicor 1.5 | 0.19 (<0.001) | 0.22 (<0.001) | 0.17 (0.001) |
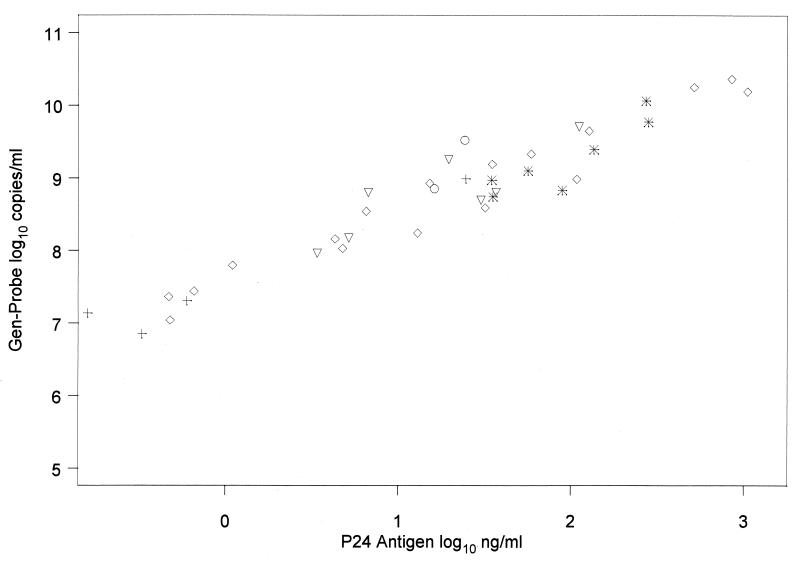
We looked at the correlation between p24 antigen levels and HIV-1 RNA viral load levels from each assay. The p24 assay measures virus levels based on the amount of viral gag protein. Thus, this provides an independent measure of virus using an antigen-antibody interaction to quantitate virus. Overall, all assays had a high correlation with p24. The correlation was lowest with the AMPLICOR 1.0 assay (r = 0.83) and highest with the Gen-Probe assay (r = 0.96) (Fig. 2). Correlations between p24 and the other assays were 0.93 with QC-RT-PCR, 0.95 with Quantiplex 3.0, and 0.95 with AMPLICOR 1.5.
FIG. 2.
p24 antigen levels versus log10 RNA plasma viral load by HIV-1 clade. ◊, clade A; ✠, clade B; +, clade C; ▿, clade D; ○, unknown clade.
Analyses of multiple blood samples from the same or linked individuals allowed us to examine how the assays performed using genetically related virus populations. For example, in comparing the Gen-Probe assay with the AMPLICOR 1.5 assay for viral isolates derived from B2062 blood samples taken at week 6 and week 15 of life, the Gen-Probe assay consistently yielded higher viral RNA concentrations (0.62 log unit for W6 and 0.63 log unit for W15). In contrast, the AMPLICOR 1.5 assay gave results very comparable to those of the Gen-Probe assay for the two isolates from a mother (M2664 p32 and W6) and the isolate from her baby (B2664). In cases in which we examined different passages of the same primary isolate (M1462 and M7376, etc.), we saw consistent performance between assays for genetically related isolates.
Reproducibility of the Gen-Probe HIV-1 viral load assay.
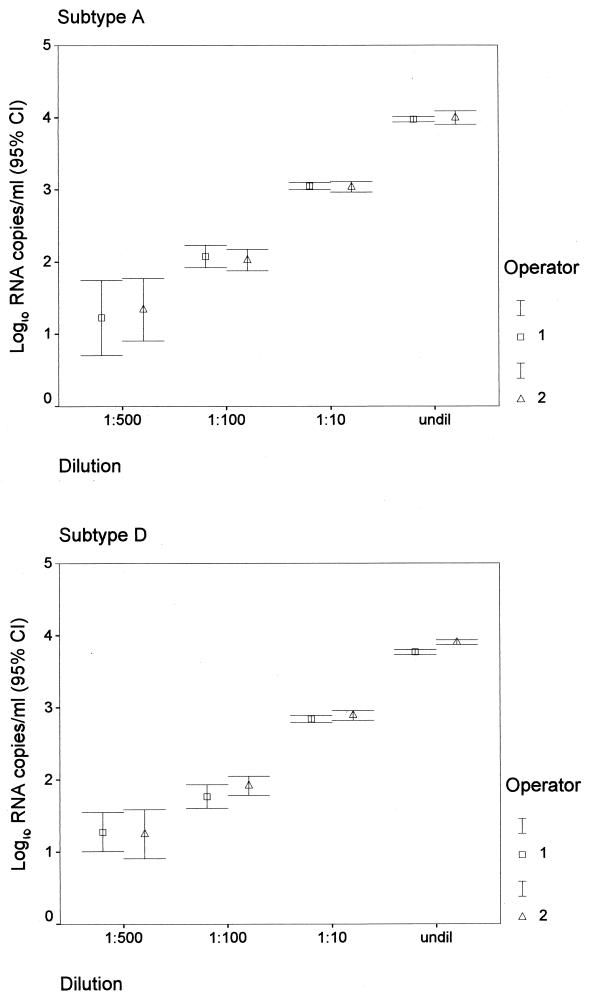
The Gen-Probe HIV-1 viral load assay can reliably quantify 50 to 100,000 HIV-1 RNA copies per ml of plasma (3). As shown previously (31), the test has higher variability at low RNA levels. To verify the reproducibility and linear range of the Gen-Probe assay for the Kenya isolates, representative viruses of A and D subtypes were serially diluted and tested in quadruplicate at each dilution. These results suggest that samples can be tested at a range of dilutions and yield similar numbers of RNA copies per milliliter (Fig. 3). To examine between-run reproducibility, we performed repetitive testing, including testing by two different operators using the same two viruses at various dilutions (Fig. 3). The standard deviation and coefficient of variation at each dilution are shown in Table 5. These data show good reproducibility, particularly at high RNA copy levels.
FIG. 3.
Reproducibility of Gen-Probe assay by operator, dilution, and clade. Cl, confidence limit; undil, undiluted.
TABLE 5.
Mean, standard deviation, and coefficient of variation for log10 copies per milliliter from Gen-Probe assay by subtype, dilution, and operator
| Subtype | Dilution and operator | Mean log10 copies/ml | SD | Coefficient of variation (%) |
|---|---|---|---|---|
| A | 1:500 | |||
| Operator 1 | 1.23 | 0.68 | 55.1 | |
| Operator 2 | 1.34 | 0.61 | 45.3 | |
| 1:100 | ||||
| Operator 1 | 2.08 | 0.24 | 11.8 | |
| Operator 2 | 2.03 | 0.23 | 11.5 | |
| 1:10 | ||||
| Operator 1 | 3.05 | 0.08 | 2.8 | |
| Operator 2 | 3.04 | 0.11 | 3.5 | |
| Undiluted | ||||
| Operator 1 | 3.97 | 0.06 | 1.4 | |
| Operator 2 | 3.99 | 0.15 | 3.7 | |
| D | 1:500 | |||
| Operator 1 | 1.28 | 0.40 | 31.5 | |
| Operator 2 | 1.25 | 0.53 | 42.9 | |
| 1:100 | ||||
| Operator 1 | 1.77 | 0.26 | 14.6 | |
| Operator 2 | 1.92 | 0.21 | 10.9 | |
| 1:10 | ||||
| Operator 1 | 2.84 | 0.07 | 2.6 | |
| Operator 2 | 2.89 | 0.11 | 3.7 | |
| Undiluted | ||||
| Operator 1 | 3.77 | 0.05 | 1.4 | |
| Operator 2 | 3.90 | 0.05 | 1.3 |
DISCUSSION
Most of the people presently infected with HIV-1 reside in Africa. The diversity of HIV-1 subtypes that are found in this region of the world necessitates the development of methods capable of quantifying all subtypes equivalently. Here we show that the Gen-Probe HIV-1 viral load assay is highly sensitive for detection of HIV-1 subtypes A, C, and D found in Africa. Although there was a good correlation between the Gen-Probe assay and other commercial assays and a QC-RT-PCR method, the Gen-Probe assay yielded higher values in many cases. The Gen-Probe assay and the newest version of the AMPLICOR HIV-1 MONITOR assay, which has been designed to minimize subtype differences (version 1.5), yielded very comparable data using primary isolates from Kenya. Our analyses suggest that the AMPLICOR HIV-1 MONITOR assay version 1.5 is also highly sensitive for the HIV-1 subtypes found in Kenya and in this regard represents an improvement over its predecessor, version 1.0, that is now in commercial use. These data are consistent with previous findings using a panel of viruses from different parts of the world (26). The improved subtype sensitivity likely reflects the fact that the Gen-Probe HIV-1 viral load assay and the AMPLICOR HIV-1 MONITOR 1.5 assay were developed at a time when more non-subtype B HIV-1 sequences were available, allowing probe and primer design specific for these new viruses. This is especially notable in the design of the assay oligomers and the demonstrated ability of the Gen-Probe assay to quantitatively detect HIV-1 group O viruses (M. Sanders, L. Gurtler, F. Simon, M. Bott, E. Dise, C. Giachetti, K. Nunomura, and S. Bodrug, Abstr. 5th Conf. Retroviruses Opportun. Infect., abstr. 117, 1998).
The Gen-Probe assay gave consistently higher values than other assays. The most notable difference was between the Gen-Probe and Quantiplex 3.0 assays, in which the calculated viral loads were 1 log unit higher overall with the Gen-Probe assay. This cannot simply be attributed to differences in the ability of these assays to detect a broad range of subtypes, since the Gen-Probe assay was found to yield RNA values 0.6 log unit higher for subtype B isolates. However, this difference is in keeping with previous studies showing that RNA levels as determined by the Quantiplex assay are low relative to those with the other commercial assays available, even for subtype B isolates (7, 9, 32). The Gen-Probe assay gave only slightly higher values than the AMPLICOR 1.5 assay (0.19 log unit). This 0.19-log-unit difference may reflect subtle differences in the standardization references between the two assays, but the basis for these differences was not actually explored in our study. In general, it is important to keep in mind that there may be subtle differences between assays when comparing results generated with any of the available quantitative HIV-1 RNA methods, and the same assay should be used for sequential samples.
The virion core protein, gagp24, provides an independent measure of the amount of virus in a sample. One would expect that the levels of a viral structural protein should correlate closely with the levels of virion-associated RNA in replication-competent viruses such as those examined here. Our analyses suggest that the Gen-Probe RNA levels correlate closely with the levels of gagp24 and that this correlation is independent of viral subtype. There was also a correlation between the levels of viral RNA determined by the other RNA assays and gagp24 protein levels (results not shown), although the correlation was less robust, particularly with the AMPLICOR 1.0 test. These findings are in keeping with the suggestion that the Gen-Probe assay is able to more efficiently detect and quantify genetically diverse viral RNAs, particularly relative to the AMPLICOR version 1.0 assay.
The Gen-Probe assay provides a rapid, sensitive, and highly reproducible HIV-1 quantitative assay. Dilution of the viruses under study here suggests that as few as 10 copies could be detected with this method. However, as is true with all amplification methods, the copy number at these low levels cannot be determined with the same precision as with higher copy numbers (S. Bodrug, M. Sanders, T. Bixby, E. Dise, B. Eguchi, E. Lasalita, D. Weiner, and M. Bott, Abstr. 6th Conf. Retroviruses Opportun. Infect., abstr. 151, 1999). Similarly, as is true for other assays, dilutions may occasionally be required for samples with very high viral levels to generate data in the linear range of the assay. Although the assay is sensitive to detect as few as 10 copies, it is being developed to quantitatively detect down to 50 copies/ml. The assay combines a lysis and purification step in the same tube as an amplification reaction, minimizing labor and the chance for contamination. Thus, this assay can be performed on large numbers of samples in a single day, which makes it suitable for large studies. Moreover, the use of target capture to purify target RNA from the specimen reduces the impact of inhibitors such as heparin, expanding the reliability and usefulness of the assay (Bodrug et al., Abstr. 6th Conf. Retroviruses Opportun. Infect.).
Rapid, sensitive methods for detecting RNAs from a broad range of HIV-1 subtypes will be important for monitoring HIV-1 infection and disease progression in a variety of settings. Such analyses will allow comparisons of viral levels in persons infected with different subtypes and in persons in different regions of the world. Moreover, the evaluation of vaccine efficacy will likely entail analyses of the effects of immunization on disease progression (12, 38) in vaccinated individuals who become infected. For example, using the Gen-Probe method, we have demonstrated differences in viral load in women infected with HIV-1 subtype C versus subtypes A and D (30). Because using mortality as an HIV-1 disease end point will require many years of follow-up, intermediate measures for monitoring disease progression are crucial. Studies of United States cohorts suggest that plasma viral RNA levels provide the best surrogate for disease progression (25, 33, 34). Thus, HIV-1 viral load assays that quantitatively detect non-B subtypes of HIV-1 are essential for vaccine studies and studies of the natural history of HIV-1 disease in Africa and other parts of the world. Moreover, RNA analyses are particularly important for vaccinated individuals when serological testing cannot be applied to detect infection. Our data suggest that the Gen-Probe HIV-1 viral load assay will provide a useful tool for such studies.
ACKNOWLEDGMENTS
We thank Grace John and Dorothy Mbori-Ngacha for provision of samples; Stephanie Rainwater, Mary Welch, Joel Neilson, Lyle Rudensey, Joan Dragavon, David Weiner, Elvie Lasalita, Debra Wiggins, and John Cooley for technical assistance; the Viral Quality Assurance Program for their HIV-1 standard; and the Nairobi HIV/STD Research Project. Samples JRCSF, NL4-3, and YU2 were obtained from the NIH AIDS Research and Reference Reagent Program.
This work was supported by NIH grants AI 38518 and HD 23412 and in part by Cooperative Agreement no. DAMD17-93-V-3004 between the U.S. Army Medical Research and Materiel Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine. Julie Overbaugh is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
REFERENCES
- 1.Alaeus A, Lidman K, Sonnerborg A, Albert J. Subtype-specific problems with quantification of plasma HIV-1 RNA. AIDS. 1997;11:859–865. doi: 10.1097/00002030-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Berger A, Braner J, Doerr H W, Weber B. Quantification of viral load: clinical relevance for human immunodeficiency virus, hepatitis B virus and hepatitis C virus infection. Intervirology. 1998;41:24–34. doi: 10.1159/000024912. [DOI] [PubMed] [Google Scholar]
- 3.Bodrug S, Domingo R, Holloway J, Sanders M, Nunomura K, Sloan C, Billyard B. Gen-Probe single tube quantitative HIV assay. J Clin Microbiol Infect. 1997;3:1050. [Google Scholar]
- 4.Brodine S K, Mascola J R, Weiss P J, Ito S I, Porter K R, Artenstein A W, Garland F C, McCutchan F E, Burke D S. Detection of diverse HIV-1 genetic subtypes in the USA. Lancet. 1995;346:1198–1199. doi: 10.1016/s0140-6736(95)92901-0. [DOI] [PubMed] [Google Scholar]
- 5.Brodine S K, Shaffer R A, Starkey M J, Tasker S A, Gilcrest J L, Louder M K, Barile A, VanCott T C, Vahey M T, McCutchan F E, Birx D L, Richman D D, Mascola J R. Drug resistance patterns, genetic subtypes, clinical features, and risk factors in military personnel with HIV-1 seroconversion. Ann Intern Med. 1999;131:502–506. doi: 10.7326/0003-4819-131-7-199910050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Burke D S, McCutchan F E. Global distribution of human immunodeficiency virus-1 clades. In: DeVita T Jr, Vincent S H, Rosenberg S A, editors. AIDS: biology, diagnosis, treatment and prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 119–126. [Google Scholar]
- 7.Coste J, Montes B, Reynes J, Peeters M, Segarra C, Vendrell J P, Delaporte E, Segondy M. Comparative evaluation of three assays for the quantitation of human immunodeficiency virus type 1 RNA in plasma. J Med Virol. 1996;50:293–302. doi: 10.1002/(SICI)1096-9071(199612)50:4<293::AID-JMV3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.de Baar M, van der Schoot A, Goudsmit J, Jacobs F, Ehren R, van der Horn K, Oudshoorn P, de Wolf F, de Ronde A. Design and evaluation of a human immunodeficiency virus type 1 RNA assay using nucleic acid sequence-based amplification technology able to quantify both group M and O viruses by using the long terminal repeat as target. J Clin Microbiol. 1999;37:1813–1818. doi: 10.1128/jcm.37.6.1813-1818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunne A L, Crowe S M. Comparison of branched DNA and reverse transcriptase polymerase chain reaction for quantifying six different HIV-1 subtypes in plasma. AIDS. 1997;11:126–127. [PubMed] [Google Scholar]
- 10.Giachetti C, Kolk D, Dockter J, Knowlton J, Wang R, Hotaling H, McDonough S. The 12th World AIDS Conference. Bologna, Italy: Monduzzi Editore S. p. A.; 1998. High throughput assay for sensitive detection of HIV-1 RNA of diverse origins, including type O strains; pp. 151–155. [Google Scholar]
- 11.Gobbers E, Fransen K, Oosterlaken T, Janssens W, Heyndrickx L, Ivens T, Vereecken K, Schoones R, van de Wiel P, van der Groen G. Reactivity and amplification efficiency of the NASBA HIV-1 RNA amplification system with regard to different HIV-1 subtypes. J Virol Methods. 1997;66:293–301. doi: 10.1016/s0166-0934(97)00072-4. [DOI] [PubMed] [Google Scholar]
- 12.Hoff R, Barker L F. Trial objectives and end points for measuring the efficacy of HIV vaccines. Infect Agents Dis. 1995;4:95–101. [PubMed] [Google Scholar]
- 13.Holguin A, de Mendoza C, Soriano V. Comparison of three different commercial methods for measuring plasma viraemia in patients infected with non-B HIV-1 subtypes. Eur J Clin Microbiol Infect Dis. 1999;18:256–259. doi: 10.1007/s100960050273. [DOI] [PubMed] [Google Scholar]
- 14.Hu D J, Dondero T J, Rayfield M A, George J R, Schochetman G, Jaffe H W, Luo C C, Kalish M L, Weniger B G, Pau C P, Schable C A, Curran J W. The emerging genetic diversity of HIV. The importance of global surveillance for diagnostics, research, and prevention. JAMA. 1996;275:210–216. [PubMed] [Google Scholar]
- 15.Irwin K L, Pau C P, Lupo D, Pienazek D, Luo C C, Olivo N, Rayfield M, Hu D J, Weber J T, Respess R A, Janssen R, Minor P, Ernst J. Presence of human immunodeficiency virus (HIV) type 1 subtype A infection in a New York community with high HIV prevalence: a sentinel site for monitoring HIV genetic diversity in North America. J Infect Dis. 1997;176:1629–1633. doi: 10.1086/517343. [DOI] [PubMed] [Google Scholar]
- 16.Janssens W, Buve A, Nkengasong J N. The puzzle of HIV-1 subtypes in Africa. AIDS. 1997;11:705–712. doi: 10.1097/00002030-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Kacian, D. L., and T. J. Fultz. 1995. Nucleic acid sequence amplification methods. U.S. patent 5,399,491.
- 18.Katzenstein D A, Hammer S M, Hughes M D, Gundacker H, Jackson J B, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour R, Merigan T C, Hirsch M S. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimeter. N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 19.Kern D, Collins M, Fultz T, Detmer J, Hamren S, Peterkin J J, Sheridan P, Urdea M, White R, Yeghiazarian T, Todd J. An enhanced-sensitivity branched-DNA assay for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34:3196–3202. doi: 10.1128/jcm.34.12.3196-3202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leitner T, Escanilla D, Marquina S, Wahlberg J, Brostrom C, Hansson H B, Uhlen M, Albert J. Biological and molecular characterization of subtype D, G and A/D recombinant HIV-1 transmission in Sweden. Virology. 1995;209:136–146. doi: 10.1006/viro.1995.1237. [DOI] [PubMed] [Google Scholar]
- 21.Lewis P, Nduati R, Kreiss J K, John G C, Richardson B, Mbori-Ngacha D, Ndinya-Achola J, Overbaugh J. Cell-free HIV-1 in breast milk. J Infect Dis. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louwagie J, Janssens W, Mascola J, Heyndrickx L, Hegerich P, van der Groen G, McCutchan F E, Burke D S. Genetic diversity of the envelope glycoprotein from human immunodeficiency virus type 1 isolates of African origin. J Virol. 1995;69:263–271. doi: 10.1128/jvi.69.1.263-271.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louwagie J, McCutchan F E, Peeters M, Brennan T P, Sanders-Buell E, Eddy G A, van-der-Groen G, Fransen K, Gershy-Damet G M, Deleys R, Burke D S. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Mellors J W, Kingsley L A, Rinaldo C R, Jr, Todd J A, Hoo B S, Kokka R P, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 25.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 26.Michael N L, Herman S A, Kwok S, Dreyer K, Wang J, Christopherson C, Spadoro J P, Young K K, Polonis V, McCutchan F E, Carr J, Mascola J R, Jagodzinski L L, Robb M L. Development of calibrated viral load standards for group M subtypes of human immunodeficiency virus type 1 and performance of an improved AMPLICOR HIV-1 MONITOR test with isolates of diverse subtypes. J Clin Microbiol. 1999;37:2557–2563. doi: 10.1128/jcm.37.8.2557-2563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder J, McKinney N, Christopherson C, Sninsky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers G, Korber B, Berzofsky J A, Smith R F, Pavlakis G N. Human retroviruses and AIDS. Theoretical Biology and Biophysics Group T-10. Los Alamos, N.Mex: Los Alamos National Laboratory; 1997. [Google Scholar]
- 29.Nduati R W, John G, Mbori-Ngacha D, Richardson B A, Overbaugh J, Mwatha A, Ndinya-Achola J O, Bwayo J, Onyango F E, Hughes J, Kreiss J K. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 30.Neilson J, John G, Carr J K, Lewis P, Kreiss J K, Jackson S, Nduati R W, Mbori-Ngacha D, Panteleeff D, Bodrug S, Giachetti C, Bott M, Richardson B A, Bwayo J, Ndinya-Achola J, Overbaugh J. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol. 1999;73:4393–4403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson N C, Reynolds M A, Arnold L J. Detection of acridinium esters by chemiluminescence. In: Kricka L, editor. Nonisotopic probing, blotting, and sequencing. San Diego, Calif: Academic Press; 1995. pp. 391–428. [Google Scholar]
- 32.Nolte F S, Boysza J, Thurmond C, Clark W S, Lennox J L. Clinical comparison of an enhanced-sensitivity branched-DNA assay and reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:716–720. doi: 10.1128/jcm.36.3.716-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien T R, Blattner W A, Waters D, Eyster E, Hilgartner M W, Cohen A R, Luban N, Hatzakis A, Aledort L M, Rosenberg P S, Miley W J, Kroner B L, Goedert J J. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA. 1996;276:105–110. [PubMed] [Google Scholar]
- 34.O'Brien W A, Hartigan P M, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff M S, Hamilton J D the Veterans Affairs Cooperative Study Group on AIDS. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 35.Parekh B, Phillips S, Granade T C, Baggs J, Hu D J, Respess R. Impact of HIV type 1 subtype variation on viral RNA quantitation. AIDS Res Hum Retroviruses. 1999;15:133–142. doi: 10.1089/088922299311556. [DOI] [PubMed] [Google Scholar]
- 36.Peeters M, Gueye A, Mboup S, Bibollet-Ruche F, Ekaza E, Mulanga C, Ouedrago R, Gandji R, Mpele P, Dibanga G, Koumare B, Saidou M, Esu-Williams E, Lombart J P, Badombena W, Luo N, Vanden-Haesevelde M, Delaporte E. Geographical distribution of HIV-1 group O viruses in Africa. AIDS. 1997;11:493–498. doi: 10.1097/00002030-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Poss M, Gosink J, Thomas E, Kreiss J K, Ndinya-Achola J, Mandaliya K, Bwayo J, Overbaugh J. Phylogenetic evaluation of Kenyan human immunodeficiency virus type 1 isolates. AIDS Res Hum Retroviruses. 1997;13:493–499. doi: 10.1089/aid.1997.13.493. [DOI] [PubMed] [Google Scholar]
- 38.Rida W, Fast P, Hoff R, Fleming T. Intermediate-size trials for the evaluation of HIV vaccine candidates: a workshop summary. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:195–203. doi: 10.1097/00042560-199711010-00009. [DOI] [PubMed] [Google Scholar]
- 39.Simon J H, Gaddis N C, Fouchier R A, Malim M H. Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat Med. 1998;4:1397–1400. doi: 10.1038/3987. [DOI] [PubMed] [Google Scholar]
- 40.Subbarao S, Schochetman G. Genetic variability of HIV-1. AIDS. 1996;10:S13–S23. doi: 10.1097/00002030-199601001-00003. [DOI] [PubMed] [Google Scholar]
- 41.Vandamme A M, Van Dooren S, Kok W, Goubau P, Fransen K, Kievits T, Schmit J C, De Clercq E, Desmyter J. Detection of HIV-1 RNA in plasma and serum samples using the NASBA amplification system compared to RNA-PCR. J Virol Methods. 1995;52:121–132. doi: 10.1016/0166-0934(94)00151-6. [DOI] [PubMed] [Google Scholar]