Abstract
The 56-kDa major outer membrane protein antigen of Orientia tsutsugamuchi is the immunodominant antigen in human scrub typhus (ST) infections. An enzyme-linked immunosorbent assay (ELISA) using a recombinant 56-kDa protein (r56) to detect specific immunoglobulin M (IgM) produced in ST infections was developed, and its performance was evaluated using sera from patients with active ST (n = 59), spotted fever (SF) (n = 31), and murine typhus (MT) (n = 6) and from those without rickettsial infection (n = 52). The r56 ELISA was compared to an ELISA using native whole cell lysate of O. tsutsugamushi Karp or O. tsutsugamushi Gilliam as antigens. The performance of the assays using r56 was similar to that of those using native antigens. Using indirect immunoperoxidase (IIP) as the reference test, sensitivities were 86, 88, and 88% while specificities were 84, 90, and 87% in the three assays. Furthermore, cross-reactivity in confirmed cases of SF and MT was low (5.4, 2.7, and 2.7% respectively). The additional use of IgG in the r56 ELISA gave improved performance (sensitivity, 80%; specificity, 96%; cross-reactivity in SF and MT, 2.7%). The detection of high levels of IgG in some IgM-negative patients illustrates the importance of including a test for IgG in the detection of secondary or reactivated infections, since many of these patients were from regions in Thailand where these infections are endemic.
Scrub typhus is an acute febrile disease endemic in the Asia-Pacific region. The causative agent, Orientia (formerly Rickettsia) tsutsugamushi, is a gram-negative obligate intracellular bacterium which has been isolated from a variety of eukaryotic host cells and is transmitted via chiggers. O. tsutsugamuchi does not possess a lipopolysaccharide or peptidoglycan layer, and the ultrastructure of its cell wall differs significantly from those of its closest relatives, typhus and spotted fever (SF) group rickettsia (2). Orientia isolates are antigenically diverse, resulting in numerous serotypes. Gilliam, Karp, and Kato are representative strains of the antigenic variants (7). The major surface protein antigen of O. tsutsugamushi is the variable 56-kDa protein, which accounts for 10 to 15% of its total protein (2, 7). Group-specific and strain-specific epitopes have been reported in the 56-kDa protein (2, 7).
Traditionally, diagnosis has been based on clinical presentation and patient history. Typical clinical manifestations are nonspecific, including fever, headache, myalgia, and rash. An eschar is the most characteristic sign, though this is seen in only 60% of patients (12, 13). PCR amplification of the 56-kDa protein gene is a reliable diagnostic method for scrub typhus but does not lend itself to small or rural testing facilities. Current serodiagnostic assays, indirect immunoperoxidase (IIP) assay and indirect immunofluorescent antibody (IFA) assay are not without limitations (8, 10). IIP and IFA assays are time consuming, requiring specialized equipment and trained personnel. In this study, we developed and evaluated recombinant rickettsial protein antigen immunoglobulin M (IgM) and IgG indirect enzyme-linked immunosorbent assays (ELISAs), which have sensitivities and specificities similar to those of ELISAs using native rickettsia for serodiagnosis of O. tsutsugamuchi disease.
MATERIALS AND METHODS
Recombinant 56-kDa protein.
The gene encoding the immunodominant 56-kDa protein from the Karp strain was cloned into the expression vector pET11a and expressed in Escherichia coli BL21 (2). The recombinant protein (r56) was purified from an inclusion body using ion-exchange chromatography in 6 M urea and refolded by sequential dialysis into 4 M and 2 M urea (2).
Native O. tsutsugamuchi antigens for ELISA.
O. tsutsugamuchi serotypes Gilliam and Karp, sourced from the American Type Culture Collection, were grown in Vero cells at 35°C in RPMI 1640 medium containing 10% fetal calf serum. After cells were dislodged with a scraper, the medium was collected and centrifuged. The pellet was resuspended in Hank's Balanced Salt Solution and incubated at 56°C for 1 h to kill rickettsia. Antigenicity was confirmed by immunofluorescence. The cells were sonicated briefly prior to their use in coating microwells to release the rickettsial antigen.
IIP method and diagnostic criteria.
Rickettsial particles from pooled Karp, Gilliam, and Kato strains were spotted and fixed on a glass slide as antigen. If present in the test serum, O. tsutsugamushi-specific antibody was bound and subsequently detected by adding peroxidase-conjugated anti-human IgG or IgM. Peroxidase bound to the antigen-antibody complex reacted with the substrate to give a specific color. Cell components were stained with methylene blue, and the slide was viewed under a light microscope. The reaction was positive when the rickettsial particles were stained light brown. Titers of antibody were expressed as the reciprocal of the highest dilution with a positive reaction. A test was positive if IgG antibody titers were 1:1600 or greater or IgM titers were 1:400 or greater (10).
IgM and IgG indirect ELISA.
Serum was diluted 1:100 in diluent containing goat anti-human IgG to remove any competing IgG and rheumatoid factor and was transferred to rickettsial antigen-coated microwells for 20 min at 37°C (100 μl per well). After washing with phosphate-buffered saline containing 0.05% Tween 20, bound IgM was detected via a 20-min incubation with anti-human IgM-peroxidase (100 μl per well) and, after another wash, a 10-min incubation with a tetramethylbenzidine substrate (100 μl per well). The reaction was stopped by the addition of 100 μl of 1 M phosphoric acid per well, and the strips were read at 450 nm with a microtiter plate reader. Sample absorbances were divided by the mean cutoff calibrator absorbance to give an ELISA ratio. A positive sample was defined as having an ELISA ratio of ≥1.0, and a negative sample was one with a ratio of <1.0. The IgG ELISA was performed similarly, except that goat anti-human IgG was not present in the diluent and anti-human IgG-peroxidase was substituted in place of the anti-human IgM-peroxidase.
Serum.
Sera were collected from 49 Thai patients diagnosed with scrub typhus (cases were confirmed by elevated IIP IgG or IIP IgM or the presence of an eschar). A further 10 specimens were collected in Australia from O. tsutsugamushi IFA-positive patients. Fifty negative specimens were collected from residents of Thailand showing no evidence of recent illness, and a further two specimens from Australia were included. Thirty-one specimens from patients with active SF and six sera from patients with active murine typhus (MT) infection were also included in this study. Serum samples were frozen at −70°C prior to being assayed.
Data analysis.
The proportion of patients with antibody levels above the designated cutoff for ELISA was determined. Analysis of variance (ANOVA) was used to compare the mean r56 IgG and IgM assay values with IIP values. Fisher's exact test was performed to compare sensitivity, specificity, and F values. Spearman's correlation analysis was performed to compare ELISA ratios in individual sera in the r56 and native-antigen ELISAs. Receiver-operator curve (ROC) analysis was performed to compare sensitivities and specificities at different cutoff values (9). The cutoffs for optimal assay performance were determined using two-graph ROC analysis (TG-ROC) (6, 14). Statistics were performed using Instat (Graphpad Software Inc., San Diego, Calif.).
RESULTS
Comparison of r56 ELISA with IIP.
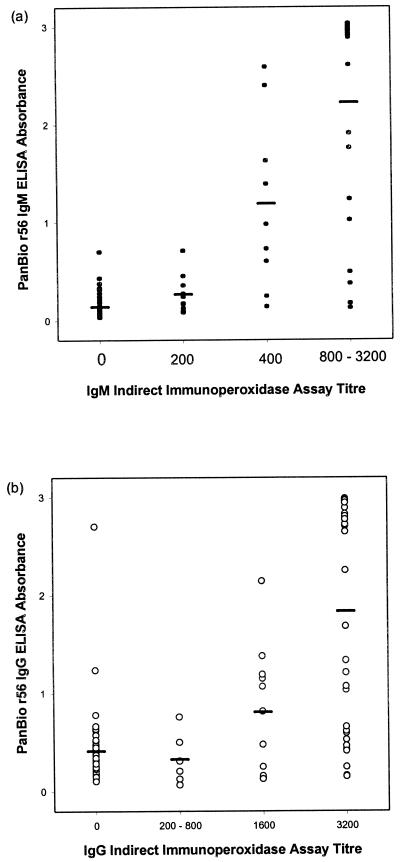
Mean ELISA absorbances were significantly correlated with increasing IIP titers for both IgM and IgG determinations (ANOVA; P < 0.0001) (Fig. 1).
FIG. 1.
Comparison of r56 ELISA and IIP assay. The mean ELISA absorbance corresponding to each IIP titer (see Materials and Methods) is represented by a horizontal bar.
Comparison of ELISA using recombinant r56 with that using native O. tsutsugamushi antigens.
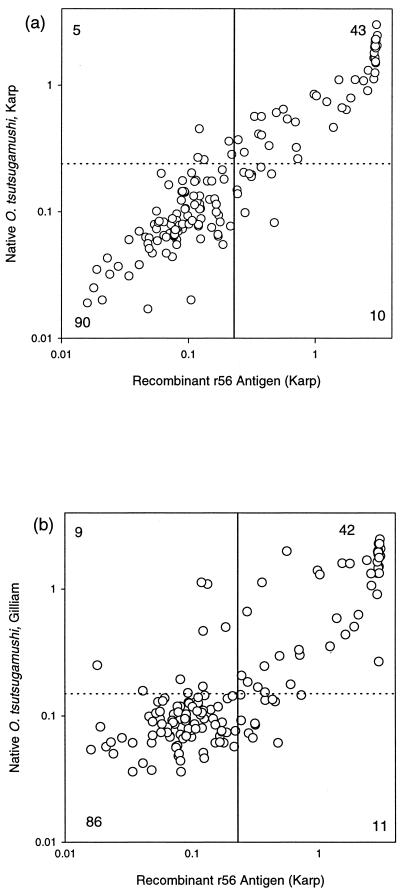
The ELISA using the 56-kDa recombinant protein of O. tsutsugamushi Karp was compared with the ELISA using native O. tsutsugamushi Karp antigens or native O. tsutsugamushi Gilliam antigens. With the IIP IgM assay as the reference test, the performances of the IgM ELISAs were not significantly different (Fischer's exact test; P > 0.05) (Table 1). The r56 IgM ELISA showed good correlation with native O. tsutsugamushi Karp and Gilliam antigen ELISAs (Spearman's r = 0.8766 and P < 0.0001 and r = 0.6970 and P < 0.0001, respectively) (Fig. 2).
TABLE 1.
Comparison of native and r56 O. tsutsugamushi IgM ELISAsa
| IgM assay | Sensitivity [no. of positive samples (%)] | Specificity [no. of negative samples (%)] |
|---|---|---|
| Native Karp ELISA | 37 (88) | 95 (90) |
| Native Gilliam ELISA | 37 (88) | 92 (87) |
| r56 ELISA | 36 (86) | 89 (84) |
| IIP (reference) | 42 | 106 |
The cutoffs in the ELISA using r56, native Karp, or native Gilliam antigens are based on TG-ROC, using IIP as the reference test.
FIG. 2.
Correlation between scrub typhus-specific IgM antibody levels with the native O. tsutsugamushi Karp IgM ELISA and the r56 IgM ELISA (a) and scrub typhus-specific IgM antibody levels with the native O. tsutsugamushi Gilliam IgM ELISA and the r56 IgM ELISA (b). The cutoffs for ELISAs using native antigen are shown by broken lines, while the cutoff for the r56 ELISA is shown by an unbroken line. The numbers of sera in each quadrant are also shown. Numbers outside the graphs represent ELISA absorbances at 450 nm.
Performance of r56 IgM ELISA.
The overall agreement between the IgM IIP assay and the r56 IgM ELISA was 85% (125 of 148). Using the IIP IgM assay as the reference test, 36 of 42 IIP-positive specimens were positive in the r56 IgM ELISA (sensitivity, 86%) and 89 of 106 IIP-negative specimens were negative in the r56 IgM ELISA (specificity, 84%). Five of 17 specimens that were IgM ELISA positive and IIP IgM negative were diagnosed with scrub typhus based on an IIP IgG titer of ≥1:1600, while 3 of 6 r56 IgM ELISA-negative, IIP IgM-positive specimens were positive in the r56 IgG ELISA. Cross-reactivity in the IgM ELISA with sera collected from patients with SF or MT was 5.4% (2 of 37).
Combined use of IgG and IgM scrub typhus-specific antibody levels.
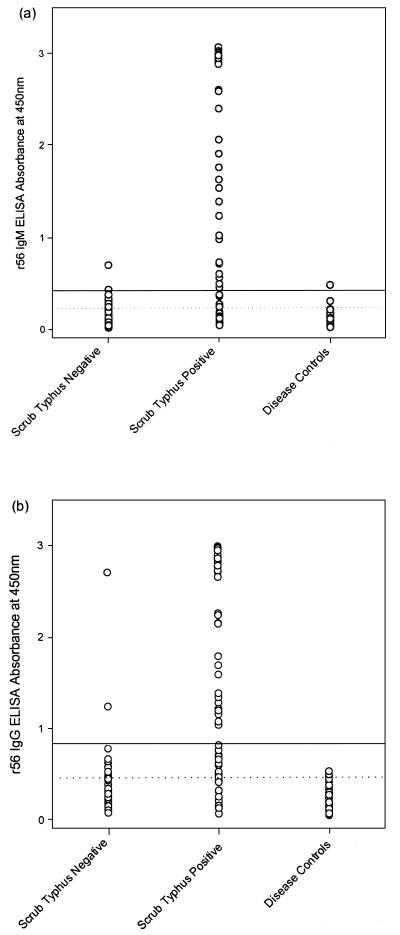
When scrub typhus infection was defined as elevated IIP IgG or IgM, positive IFA, or the presence of an eschar, the r56 IgM ELISA had a sensitivity of 70% and specificity of 87% (Table 2; Fig. 3). The r56 IgG ELISA showed a sensitivity of 76% and specificity of 74% (Table 2; Fig. 3). When the r56 IgM and r56 IgG ELISAs were used in combination (with elevation of either IgG or IgM taken as a positive result), sensitivity and specificity were 88 and 65%, respectively. Use of higher cutoff values in the IgM and IgG ELISAs (Fig. 3) improved specificity to 96% when the assays were used in combination, while sensitivity (80%) was not significantly changed (Table 2; Fig. 4). Sensitivity was 90% (9 of 10) in scrub typhus cases from Australia and 78% (38 of 49) in scrub typhus cases from Thailand. Only 1 of 37 (2.7%) of specimens from patients with SF or MT showed elevated levels when the IgM and IgG ELISAs were used in combination.
TABLE 2.
| Scrub typhus assay | Cutoff | % Sensitivityc | % Specificityc |
|---|---|---|---|
| r56 IgM ELISA | TG-ROC | 70 (41/59) | 87 (77/89) |
| Arbitrary | 59 (35/59) | 97 (86/89) | |
| r56 IgG ELISA | TG-ROC | 76 (45/59) | 74 (66/89) |
| Arbitrary | 58 (34/59) | 98 (87/89) | |
| r56 IgM and IgG ELISA | TG-ROC | 88 (52/59) | 65 (58/89) |
| Arbitrary | 80 (47/59) | 96 (85/89) |
Active infection was defined by any one of the following: IIP IgM titer of ≥400, IIP IgG titer of ≥1,600, positive IgM IFA, or the presence of an eschar.
Cutoffs used were either determined by TG-ROC analysis using IIP as the reference test (as described in Materials and Methods) or selected arbitrarily to optimize diagnostic performance when the IgM and IgG assays were used in combination.
Numbers in parentheses represent number of sera positive (sensitivity) or negative (specificity) in ELISA over total number of sera tested.
FIG. 3.
Individual assay values for the r56 IgM ELISA (a) and the r56 IgG ELISA (b). The cutoff values determined by TG-ROC analysis are shown by a broken line, while the cutoff selected to maximize diagnostic performance when the IgM and IgG ELISAs were used in combination is shown by an unbroken line. Scrub typhus-positive cases were classified by either elevation of IIP, positive IFA, or the presence of an eschar, while scrub typhus-negative cases were IIP and IFA negative, and no eschar was present. Disease controls were cases of SF or MT.
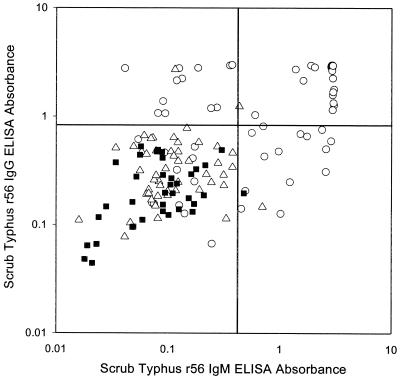
FIG. 4.
Comparison of r56 IgM ELISA and r56 IgG ELISA results in individual sera. Specimens collected from patients with active scrub typhus (circle), patients negative for laboratory reference tests for scrub typhus (triangle), and disease controls (square) were tested in the r56 IgG ELISA and the r56 IgM ELISA. Arbitrary cutoffs (unbroken lines) were identified to maximize sensitivity and specificity in diagnosis of active scrub typhus infection. ELISA absorbance at 450 nm is represented on both axes.
DISCUSSION
The routine manufacture of ELISAs for infectious diseases may be hindered by the need to use antigen preparations from microorganisms that are highly pathogenic or difficult to culture. As a result, synthetic antigens, such as recombinant proteins, have been proposed as suitable alternatives. Due to the safety issues associated with the culturing of live rickettsia, the recombinant 56-kDa immunodominant protein from O. tsutsugamushi (r56) was used to develop a serological ELISA for scrub typhus. Large quantities of this antigen can be prepared using E. coli without the need for level 3 facilities.
The r56 and IgM and IgG ELISAs showed good correlation with the ELISA utilizing native antigen (both Karp and Gilliam strains) and the IIP assay. The best correlation was shown with the ELISA using the native Karp strain, and this can be attributed to the r56 protein being derived from this strain. The r56 protein is an immunodominant outer membrane antigen containing several constant domains that may account for the broad reactivity with O. tsutsugamushi variants and the low levels of cross-reactivity with other rickettsial groups (2, 3, 7). In this study, the IgM ELISA showed 85% agreement with the IgM IIP assay, and only 2 of 37 samples from cases of SF or MT showed reactivity in the r56 IgM ELISA. Previous studies with ELISAs using native O. tsutsugamushi antigen (Gilliam, Karp, or Kato strains) have also demonstrated a high correlation with IIP results (4, 10). In this study, the infecting strain (e.g., Karp, Kato, or Gilliam) was not known, and it will be of interest to study the reactivity of this antigen with sera from patients with confirmed infections by different O. tsutsugamuchi strains.
The additional use of the r56 IgG ELISA improved performance compared with the r56 IgM ELISA alone. The use of both assays in combination allowed the use of higher cutoff values that improved specificity to 96% without seriously compromising sensitivity (80%). Twenty percent (12 of 59) of specimens collected from active cases of scrub typhus showed elevated IgG levels in the absence of specific IgM, while 22% (13 of 59) of specimens were r56 IgM ELISA positive and r56 IgG ELISA negative. Twenty-two of fifty-nine (37%) specimens were both r56 IgM and r56 IgG ELISA positive.
The improved performance observed with the additional use of IgG for diagnosis of active infection may be due to the endemic nature of scrub typhus in Thailand, since most sera used in this study were derived from this region. Secondary infection with a different variant of O. tsutsugamushi would be expected to produce lower levels of scrub typhus-specific IgM and a higher and more rapid rise in specific IgG. Two distinct antibody responses have been reported previously for active scrub typhus (1). Primary infections produced a rapid increase in specific IgM antibodies around day 8, with a slower increase in specific IgG levels at day 12. Secondary infections were characterized by a sharp rise in IgG levels with a variable IgM response. Both primary and secondary scrub typhus infections were associated with persistent IgG levels for up to 12 months, though protection from infection with a second variant was believed to be limited. This phenomenon has been reported for epidemic scrub typhus and secondary dengue infections (1, 11). The immunity induced by one strain of rickettsia may not be protective against challenge or infection by another strain.
Scrub typhus is often underreported and may also go undiagnosed (5, 8, 10), and secondary infection is often present subclinically (1). The availability of a diagnostic tool requiring minimal equipment and expertise that can be used for the determination of both IgG and IgM antibody levels would be useful, particularly in regions where the disease is endemic. A commercial dipstick (dot blot) ELISA for scrub typhus that showed good correlation with the reference IIP assay has been described previously (13). However, the ELISA developed in this study can accommodate a larger number of samples and should have utility in these regions.
ACKNOWLEDGMENT
We thank George Watt, Department of Medicine, Armed Forces Research Institute of Medical Sciences, Bangkok, for the provision of sera and IIP results from Thai patients.
REFERENCES
- 1.Bourgeois A L, Olson J M, Fanf R C Y, Huang J, Wang C L, Chow L, Bechthold D, Dennis D T, Collbaugh J C, Weiss E. Humoral and cellular responses in scrub typhus patients reflecting primary infection and reinfection with Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1982;31:532–540. doi: 10.4269/ajtmh.1982.31.532. [DOI] [PubMed] [Google Scholar]
- 2.Ching W-M, Wang H, Eamsila C, Kelly D J, Dasch G A. Expression and refolding of truncated major outer membrane protein antigen (r56) of Orientia tsutsugamushi and its use in enzyme-linked immunosorbent assays. Clin Diagn Lab Immunol. 1998;5:519–526. doi: 10.1128/cdli.5.4.519-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi M S, Seong S Y, Kang J S, Kim Y W, Huh M S, Kim I S. Homotypic and heterotypic antibody responses to a 56-kilodalton protein of Orientia tsutsugamuchi. Infect Immun. 1999;67:6194–6197. doi: 10.1128/iai.67.11.6194-6197.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasch G A, Halle S, Bourgeois A L. Sensitive microplate enzyme-linked immunosorbent assay for detection of antibodies against the scrub typhus rickettsia, Rickettsia tsutsugamushi. J Clin Microbiol. 1979;9:38–48. doi: 10.1128/jcm.9.1.38-48.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eamsila C, Singsawat P, Duangvaraporn A, Strickman D. Antibodies to Orientia tsutsugamushi in Thai soldiers. Am J Trop Med Hyg. 1996;55:556–559. doi: 10.4269/ajtmh.1996.55.556. [DOI] [PubMed] [Google Scholar]
- 6.Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 7.Kim I-S, Seong S-Y, Woo S-G, Choi M-S, Chang W-H. High-level expression of 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J Clin Microbiol. 1993;31:598–605. doi: 10.1128/jcm.31.3.598-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBride J H, Taylor C T, Pryor J A, Simpson J D. Scrub typhus in north Queensland. Med J Aust. 1999;170:318–320. doi: 10.5694/j.1326-5377.1999.tb127786.x. [DOI] [PubMed] [Google Scholar]
- 9.Metz C E. Basic principles of ROC analysis. Semin Nucl Med. 1978;38:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 10.Suwanabun N, Chouriyagune C, Eamsila C, Watcharapichat P, Dasch G, Howard R S, Kelley D J. Evaluation of an enzyme-linked immunosorbent assay in Thai scrub typhus patients. Am J Trop Med Hyg. 1997;56:38–43. doi: 10.4269/ajtmh.1997.56.38. [DOI] [PubMed] [Google Scholar]
- 11.Vaughn D W, Nisalak A, Kalayanarooj S, Solomon T, Dung N M, Kneen R, Cuzzubbo A, Devine P L. Rapid serological diagnosis of dengue virus infection using a commercial capture enzyme-linked immunosorbent assay that distinguishes primary and secondary infections. Am J Trop Med Hyg. 1999;60:693–698. doi: 10.4269/ajtmh.1999.60.693. [DOI] [PubMed] [Google Scholar]
- 12.Watt G, Stickman D. Life threatening scrub typhus in a traveller returning from Thailand. Clin Infect Dis. 1994;18:624–626. doi: 10.1093/clinids/18.4.624. [DOI] [PubMed] [Google Scholar]
- 13.Weddle J R, Chan T-C, Thompson K, Paxton H, Kelly D J, Dasch G, Strickman D. Effectiveness of a dot-blot immunoassay of anti-Rickettsia tsutsugamushi antibodies for serological analysis of scrub typhus. Am J Trop Med Hyg. 1995;53:43–46. [PubMed] [Google Scholar]
- 14.Xu H, Lohr J, Greiner M. The selection of ELISA cutoff points for testing antibody to Newcastle disease by two-graph receiver-operating characteristic (TG-ROC) analysis. J Immunol Methods. 1997;13:61–64. doi: 10.1016/s0022-1759(97)00128-2. [DOI] [PubMed] [Google Scholar]