The research community has responded to the COVID-19 pandemic with innovative platform trials to address the need for rapid evaluation of novel agents using a common protocol, among them being RECOVERY,1 ACTIV,2 and Solidarity.3 Despite several successes with anti-SARS-CoV-2 monoclonal antibodies (mAbs) for treatment of mild or moderate COVID-19 in ambulatory patients,4, 5 an effective SARS-CoV-2-specific treatment for patients with COVID-19 who are being treated in hospital (ie, hospitalised) has remained elusive.
The ACTIV-3 Therapeutics for Inpatients with COVID-19 (TICO) platform was developed to assess multiple candidate mAbs in individuals hospitalised with moderate or severe COVID-19 within 12 days of symptom onset. In The Lancet Infectious Diseases, the ACTIV-3 TICO Study Group6 report the results of two neutralising mAb treatments (sotrovimab and BRII-196 plus BRII-198) that were provided in addition to standard of care, typically including remdesivir and corticosteroids, in a double-blind, randomised fashion, predominantly before the availability of SARS-CoV-2 vaccines, and were compared with a pooled placebo group. Enrolment into the trial was stopped early after a prespecified interim futility analysis in 536 participants in the modified intention-to-treat population found no improvement in odds of favourable pulmonary outcome scores on day 5 after infusion with either sotrovimab or BRII-196 plus BRII-198 compared with placebo. By day 90, no difference was seen in the primary endpoint of sustained clinical recovery with either sotrovimab or BRII-196 plus BRII-198 compared with placebo, and composite safety outcomes were similar across the three groups.
Based on intriguing results from the RECOVERY study on efficacy of casirivimab–imdevimab (REGN-COV2) in patients hospitalised with COVID-19, which showed benefit only in people who were retroactively determined to be seronegative for anti-spike IgG at randomisation,7 similar serostatus-dependent effects could have been seen with sotrovimab or BRII-196 plus BRII-198, despite no overall benefit. In the study by the ACTIV-3/TICO Study Group,6 513 of 536 patients in the mITT population had baseline anti-spike antibody levels measured, enabling a subgroup analysis stratified by serostatus. At the time of randomisation, 212 (41%) participants were positive for anti-spike neutralising antibodies. Non-significant heterogenous effects in time to sustained recovery by baseline anti-spike neutralising antibody status were identified in the BRII-196 plus BRII-198 group, but not in the sotrovimab group; the difference in effect was small, and all 95% CIs crossed 1 and overlapped. Notably, by contrast with the results of the RECOVERY trial, which found no treatment effect in seropositive individuals, there was a trend favouring placebo for the composite safety outcome up to day 90 among seropositive participants. Although the heterogeneity of effect for this outcome was significant, again, 95% CIs in both subgroups crossed 1 and were overlapping. Moreover, neither mAb showed benefit in analyses restricted to people with earlier disease (ie, those admitted within 5 days of symptom onset, those not on oxygen, or those on <4 L/min of supplementary oxygen).
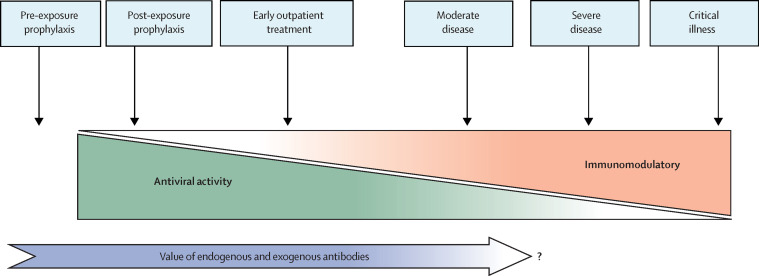
The data from this well executed platform trial contribute to accumulating evidence that anti-SARS-CoV2 mAbs do not have a role for the treatment of moderate or severe COVID-19 in general inpatients, compounding null results first seen with convalescent plasma and then with bamlanivimab and casirivimab–imdevimab.7, 8 Despite a tantalising signal of potential benefit of some agents in seronegative individuals hospitalised with COVID-19, the time-sensitive implementation of a therapy that requires baseline antibody testing, when turnaround time for in-hospital serological testing can be upwards of 48 h, is of questionable practicality, especially given the resource implications of mAb administration. Therefore, we would ask the next obvious question: is there a mechanistic rationale for use of neutralising antibodies in people who have already developed advanced COVID-19 pneumonia or acute respiratory distress syndrome (ARDS)? Whether administration of exogenous neutralising antibodies is unhelpful because most people have made endogenous antibodies by the time they develop severe disease or because neutralising antibodies have little role in mitigating the pathology driven by the hyper-inflammatory phase of COVID-19, or even exacerbate it, is as yet unknown.9 We are increasingly finding indications that targeting SARS-CoV-2, whether through mAb neutralisation or with direct-acting antivirals (eg, remdesivir), might be of little importance once clinically significant lung damage has occurred (figure ).3 At this stage of disease, pathophysiology appears to be driven by a dysregulated host innate immune response, and immunomodulatory therapies (eg, corticosteroids and anti-cytokine antibodies) targeting these processes might provide the greatest clinical benefit.10
Figure.
Role for anti-SARS-CoV-2 antibodies in the disease course of COVID-19
As disease states progress from preinfection through to critical illness (blue boxes), the potential for antibodies to mitigate illness decreases (dark blue arrow) as pathology transitions from being virally mediated, where antiviral acting therapies are most effective (green triangle), to a hyper-inflammatory state best treated with immunomodulatory therapies (orange triangle).
There remains reasonable equipoise as to whether people who might never make endogenous antibodies (eg, severely immunocompromised individuals, who, in our experience, often remain seronegative into advanced disease, even after developing ARDS) could still benefit from exogenous mAbs once admitted to hospital with severe disease. People who are unlikely to develop endogenous antibodies in response to either vaccination or infection constitute a population who can be presumed seronegative at the time of therapeutic decision making, without requiring assessment of serological status. This immunocompromised population should be the exclusive focus of ongoing investigation of anti-SARS-CoV-2 mAbs in patients hospitalised with COVID-19. The ACTIV-3 TICO trial should be the final trial of anti-SARS-CoV2 mAbs in non-immunocompromised patients hospitalised with COVID-19.
AES has received grant funding through her institution from Vir Biotechnology, as an investigator to conduct the COMET-ICE and COMET-TAIL clinical trials of Vir-7831 in non-hospitalised patients with COVID-19. RABI has received grant funding from the US National Institutes of Health through her institution to conduct COVID-19 therapeutic trials in outpatients as an investigator in the ACTIV2 platform.
References
- 1.Normand ST. The RECOVERY platform. N Engl J Med. 2021;384:757–758. doi: 10.1056/NEJMe2025674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV) 2021. https://www.nih.gov/research-training/medical-research-initiatives/activ
- 3.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 6.ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00751-9. published online Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horby PW, Mafham M, Peto L, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.15.21258542. published online June 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricke DO. Two different antibody-dependent enhancement (ADE) risks for SARS-CoV-2 antibodies. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.640093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]