Abstract
Background
We aimed to assess the efficacy and safety of two neutralising monoclonal antibody therapies (sotrovimab [Vir Biotechnology and GlaxoSmithKline] and BRII-196 plus BRII-198 [Brii Biosciences]) for adults admitted to hospital for COVID-19 (hereafter referred to as hospitalised) with COVID-19.
Methods
In this multinational, double-blind, randomised, placebo-controlled, clinical trial (Therapeutics for Inpatients with COVID-19 [TICO]), adults (aged ≥18 years) hospitalised with COVID-19 at 43 hospitals in the USA, Denmark, Switzerland, and Poland were recruited. Patients were eligible if they had laboratory-confirmed SARS-CoV-2 infection and COVID-19 symptoms for up to 12 days. Using a web-based application, participants were randomly assigned (2:1:2:1), stratified by trial site pharmacy, to sotrovimab 500 mg, matching placebo for sotrovimab, BRII-196 1000 mg plus BRII-198 1000 mg, or matching placebo for BRII-196 plus BRII-198, in addition to standard of care. Each study product was administered as a single dose given intravenously over 60 min. The concurrent placebo groups were pooled for analyses. The primary outcome was time to sustained clinical recovery, defined as discharge from the hospital to home and remaining at home for 14 consecutive days, up to day 90 after randomisation. Interim futility analyses were based on two seven-category ordinal outcome scales on day 5 that measured pulmonary status and extrapulmonary complications of COVID-19. The safety outcome was a composite of death, serious adverse events, incident organ failure, and serious coinfection up to day 90 after randomisation. Efficacy and safety outcomes were assessed in the modified intention-to-treat population, defined as all patients randomly assigned to treatment who started the study infusion. This study is registered with ClinicalTrials.gov, NCT04501978.
Findings
Between Dec 16, 2020, and March 1, 2021, 546 patients were enrolled and randomly assigned to sotrovimab (n=184), BRII-196 plus BRII-198 (n=183), or placebo (n=179), of whom 536 received part or all of their assigned study drug (sotrovimab n=182, BRII-196 plus BRII-198 n=176, or placebo n=178; median age of 60 years [IQR 50–72], 228 [43%] patients were female and 308 [57%] were male). At this point, enrolment was halted on the basis of the interim futility analysis. At day 5, neither the sotrovimab group nor the BRII-196 plus BRII-198 group had significantly higher odds of more favourable outcomes than the placebo group on either the pulmonary scale (adjusted odds ratio sotrovimab 1·07 [95% CI 0·74–1·56]; BRII-196 plus BRII-198 0·98 [95% CI 0·67–1·43]) or the pulmonary-plus complications scale (sotrovimab 1·08 [0·74–1·58]; BRII-196 plus BRII-198 1·00 [0·68–1·46]). By day 90, sustained clinical recovery was seen in 151 (85%) patients in the placebo group compared with 160 (88%) in the sotrovimab group (adjusted rate ratio 1·12 [95% CI 0·91–1·37]) and 155 (88%) in the BRII-196 plus BRII-198 group (1·08 [0·88–1·32]). The composite safety outcome up to day 90 was met by 48 (27%) patients in the placebo group, 42 (23%) in the sotrovimab group, and 45 (26%) in the BRII-196 plus BRII-198 group. 13 (7%) patients in the placebo group, 14 (8%) in the sotrovimab group, and 15 (9%) in the BRII-196 plus BRII-198 group died up to day 90.
Interpretation
Neither sotrovimab nor BRII-196 plus BRII-198 showed efficacy for improving clinical outcomes among adults hospitalised with COVID-19.
Funding
US National Institutes of Health and Operation Warp Speed
Introduction
Finding effective therapies for patients admitted to hospital (hereafter referred to as hospitalised) for COVID-19 remains an important priority. Remdesivir, corticosteroids, and other anti-inflammatory medications have shown efficacy among subsets of patients hospitalised with COVID-19.1, 2, 3 However, morbidity and mortality from COVID-19 remain substantial, creating an urgent need for more effective therapies for severely ill patients with COVID-19. Neutralising monoclonal antibody therapies targeting SARS-CoV-2 accelerate reduction in viral loads and reduce the risk of disease progression for outpatients with mild COVID-19.4, 5, 6, 7, 8, 9, 10 However, whether neutralising monoclonal antibody therapy can provide benefit for more severely ill patients hospitalised with COVID-19 remains a question of active investigation.
Research in context.
Evidence before this study
Neutralising monoclonal antibody therapies targeting SARS-CoV-2 have been considered promising potential therapies for COVID-19 since the beginning of the pandemic. Three anti-SARS-CoV-2 monoclonal antibody therapies have received emergency use authorisation by the US Food and Drug Administration for treatment of outpatients: sotrovimab, bamlanivimab plus etesevimab, and casirivimab plus imdevimab. However, efficacy for anti-SARS-CoV-2 monoclonal antibody therapies for patients admitted to hospital (hereafter referred to as hospitalised) with more severe COVID-19 has not been established and no trials to date have reported results for patients hospitalised with COVID-19 treated with either of sotrovimab or BRII-196 plus BRII-198. Both sotrovimab and BRII-196 plus BRII-198 are investigational human neutralising IgG monoclonal antibodies that potently inhibit SARS-CoV-2 replication. We searched PubMed for research articles published between database inception and Oct 30, 2021, for clinical trials of anti-SARS-CoV-2 monoclonal antibody therapies among patients hospitalised with COVID-19 using various combinations of the terms “COVID-19”, “SARS-CoV-2”, “monoclonal antibody”, and “clinical trial.” No language restrictions were applied. Two trials, described in three publications (two of which were preprint pieces), reported results of treatment with anti-SARS-CoV-2 monoclonal antibodies among patients hospitalised with COVID-19: bamlanivimab in a trial conducted by the ACTIV-3 investigators, and casirivimab plus imdevimab in a trial conducted by the RECOVERY investigators. Both of these trials reported no clinical benefit for anti-SARS-CoV-2 monoclonal antibody therapy overall but suggested potential benefit for patients without endogenous anti-SARS-CoV-2 antibodies at the time of treatment, including a significant survival benefit for casirivimab plus imdevimab.
Added value of this study
This study is the first clinical trial to report results of two anti-SARS-CoV-2 monoclonal antibody therapies with unique mechanisms (sotrovimab and BRII-196 plus BRII-198) for the treatment of patients hospitalised with COVID-19. Neither therapy showed efficacy over placebo for the overall trial population; results for BRII-196 plus BRII-198 suggested potential heterogeneity of treatment effect, possibly with more favourable treatment effects among patients without than with endogenous neutralising anti-SARS-CoV-2 antibodies.
Implications of all the available evidence
Clinical trials completed to date do not support indiscriminate use of anti-SARS-CoV-2 monoclonal antibody therapies for patients hospitalised with COVID-19. This trial, along with other recent trials, suggest targeted therapy using anti-SARS-CoV-2 monoclonal antibodies for patients hospitalised with COVID-19 who have not mounted an endogenous antibody response against SARS-CoV-2 could be a beneficial approach, although larger studies in this population are neeed.
The US National Institutes of Health (NIH) established the third Accelerating COVID-19 Therapeutic Interventions and Vaccines platform (ACTIV-3) to rapidly test antiviral therapies among patients hospitalised with COVID-19.11 In the first ACTIV-3 trial, we reported that the neutralising monoclonal antibody bamlanivimab did not have clinical efficacy in this setting.12 Therefore, we retired bamlanivimab from the platform trial and added two different neutralising monoclonal antibody therapies that target different SARS-CoV-2 epitopes: sotrovimab (VIR-7831; Vir Biotechnology and GlaxoSmithKline) and BRII-196 paired with BRII-198 administered as a two-antibody cocktail (BRII-196 plus BRII-198; Brii Biosciences).
Both sotrovimab and BRII-196 plus BRII-198 are investigational human neutralising IgG monoclonal antibodies that potently inhibit SARS-CoV-2 replication and have shown efficacy among outpatients with COVID-19 for preventing disease progression to death or hospitalisation.6, 10, 13 Sotrovimab was derived from a SARS-CoV survivor and tightly binds a highly conserved epitope of the SARS-CoV and SARS-CoV-2 spike protein outside the angiotensin-converting enzyme 2 (ACE2) receptor-binding motif. Sotrovimab has a two amino acid modification in its fragment crystallisable (Fc) domain (Met428Leu and Asn434Ser) designed to increase lung penetration and half-life while maintaining Fc effector function.14, 15 BRII-196 and BRII-198 are two monoclonal antibodies isolated from COVID-19 survivors that bind distinct and complementary epitopes of the SARS-CoV-2 spike protein.16 The Fc regions of BRII-196 and BRII-198 are engineered with triple amino acid modifications (Met252Tyr, Ser254Thr, and Thr256Glu) to extend half-life and reduce the binding affinity to Fc-γ receptors with the goal of reducing the potential for antibody-dependent enhancement.
Here, we report the results of the ACTIV-3 trial comparing sotrovimab versus placebo and BRII-196 plus BRII-198 versus placebo among adults hospitalised with COVID-19.
Methods
Study design and participants
Therapeutics for Inpatients with COVID-19 (TICO) is a double-blind, randomised, controlled trial within the ACTIV-3 programme focused on testing antiviral therapies for severely ill adults hospitalised with COVID-19. The rationale and design of TICO have been previously described11, 12 and the protocol is in the appendix (pp 65–238). Briefly, TICO facilitates the simultaneous testing of multiple agents using a common placebo group. Before an initial futility assessment, patients with moderate or severe COVID-19, defined as admitted to hospital with COVID-19 without organ failure or major extrapulmonary manifestations of COVID-19, are randomly assigned to an active agent or placebo. After approximately 150 patients in each group have been followed-up for at least 5 days, an early futility assessment is done for each agent using two seven-category ordinal outcome scales measured at day 5: the pulmonary and pulmonary-plus ordinal scales. If an agent does not pass the futility assessment, enrolment to that agent ceases. If an agent passes the futility assessment, enrolment expands without delay and without data unblinding to include patients with moderate, severe, and critical COVID-19 and continues until approximately 843 patients combined in the active group and the pooled concurrent placebo group have the primary outcome (sustained clinical recovery). Further methodological detail is in the appendix (pp 11–20).
For TICO, we recruited patients from 43 hospital in the USA, Denmark, Switzerland, and Poland (appendix p 27). We enrolled adults (aged ≥18 years) admitted to hospital for acute medical care for COVID-19 with laboratory-confirmed SARS-CoV-2 infection (PCR or nucleic acid test) and COVID-19 symptoms for up to 12 days. If patients had received any SARS-CoV-2 neutralising monoclonal antibodies, hyperimmune immunoglobulin to SARS-CoV-2, or convalescent plasma from a person recovered from COVID-19 any time before admission to hospital they were excluded. Before the early futility assessment, patients were eligible for enrolment if they were receiving no oxygen therapy or standard oxygen therapy via a nasal cannula or mask, but were excluded if they were receiving high-flow oxygen via nasal cannula, non-invasive ventilation, or invasive mechanical ventilation, or met any of the other criteria for acute organ failure or major extrapulmonary manifestations of COVID-19, as detailed in the protocol. Full eligibility criteria and relevant definitions are in the appendix (pp 13–15).
The protocol was approved by a governing institutional review board for each enrolling site. Written informed consent for trial participation was obtained from each enrolled patient or a legally authorised representative, as applicable. The trial was overseen by a data and safety monitoring board (DSMB) appointed by the National Institute of Allergy and Infectious Diseases.
Randomisation and masking
Participants were randomly assigned to one of two active therapies (sotrovimab or BRII-196 plus BRII-198) or placebo using a web-based application that verified eligibility before randomisation. For sites consenting patients to both investigational agents (all but one site), randomisation allocation was 2:1:2:1 to sotrovimab, matching placebo for sotrovimab, BRII-196 plus BRII-198, or matching placebo for BRII-196 plus BRII-198. One site did not obtain regulatory approval for BRII-196 plus BRII-198, and so participants were randomly assigned 1:1 to sotrovimab or matching placebo. For the analysis, the concurrent placebo groups (placebo matching sotrovimab and placebo matching BRII-196 plus BRII-198) were pooled, resulting in approximately a 1:1:1 allocation of sotrovimab to BRII-196 plus BRII-198 to placebo. Randomisation was stratified by trial site pharmacy (geographically close clinical sites shared a single trial pharmacy in some cases). The study infusion was prepared by unmasked study pharmacists. The following people were masked to treatment allocation: the patient, the patient's family, clinical personnel caring for the patient, investigators in the trial, study personnel who collected data, and all outcome assessors.
Procedures
Each study product was administered as a one-time intravenous dose over 60 min as soon as possible after randomisation. Patients randomly assigned to the sotrovimab group received a single 500 mg dose of sotrovimab (Vir Biotechnology and GlaxoSmithKline). Patients randomly assigned to the BRII-196 plus BRII-198 group received 1000 mg of BRII-196 immediately followed by 1000 mg of BRII-198 (Brii Biosciences). The doses of sotrovimab and BRII-196 plus BRII-198 were selected on the basis of in-vitro and in-vivo animal model data suggesting that these doses would provide lung concentrations with maximal antiviral activity for at least 3 weeks.17 Patients randomly assigned to the placebo groups received an intravenous infusion of 0·9% sodium chloride solution in a manner that mimicked administration of either sotrovimab or BRII-196 plus BRII-198, depending on their matched group assignment. Infusion of the placebo solution was visibly indistinguishable from the solutions containing active agents.
The trial protocol instructed investigators to administer remdesivir (Gilead Sciences) 200 mg intravenously on the first day, then 100 mg daily for up to 10 days while the participant was in hospital for those who did not have a contraindication to it; remdesivir was provided by the trial. Participants were not treated with other passive immunotherapies, such as COVID-19 convalescent plasma or open-label neutralising monoclonal antibodies. Other treatments for COVID-19, including oxygen, respiratory support, and corticosteroids, were administered at the discretion of the treating clinician per local standard of care.
Participants were assessed for study data, including outcomes and adverse events, daily from randomisation until day 7 (inclusive), and then on days 14, 28, 60, and 90. Longer-term assessments, including at 6 months, 12 months, and 18 months, are planned for the future and are not reported here. Blood samples were collected from participants before administration of the study infusion for plasma measurement of neutralising IgG antibody concentrations against the receptor binding domain of the SARS-CoV-2 spike protein (GenScript SARS-CoV-2 Surrogate Virus Neutralisation assay; GenScript, Piscataway, NJ, USA), total IgG concentration against SARS-CoV-2 nucleocapsid antigen (BioRad Platelia SARS-CoV-2 Total Ab assay; BioRad, Hercules, CA, USA), and SARS-CoV-2 nucleocapsid antigen concentrations (Quanterix assay; Quanterix, Billerica, MA, USA). These assays are described in detail in the appendix (pp 15–16).
Adverse events were graded for severity using the toxicity table of the Division of AIDS from the National Institute of Allergy and Infectious Diseases. Adverse events were categorised according to codes in the Medical Dictionary for Regulatory Activities (MedDRA; version 23.1) and grouped by System Organ Class. Infusion-related reactions were reported on a checklist during and for 2 h after infusion. After the infusion and for the first 7 days of hospitalisation, patients were assessed in-person daily and adverse event data were collected via direction interaction between the study team and the patient and via medical record review. After hospital discharge, adverse event data were collected via in-person visits and telephone visits. Relatedness of adverse events to study procedures was assessed. The protocol specified some serious events anticipated to be common in COVID-19, including death, that were exempt from being reported as serious adverse events, except when the event was classified as related or possibly related to study procedures.
Outcomes
The early futility assessment included two intermediate efficacy outcomes assessed at day 5 after randomisation: the seven-category pulmonary ordinal outcome scale, which classifies patients on the basis of the intensity of respiratory support, and the seven-category pulmonary-plus ordinal outcome scale, which is the same as the pulmonary scale but with added extrapulmonary manifestations of COVID-19 (appendix pp 16–17). These ordinal scales were derived from COVID-19 outcome scales recommended by WHO and previously used in COVID-19 trials.1, 18, 19 The pulmonary ordinal outcome scale includes the following seven categories: (1) able to independently undertake usual activities with minimal or no symptoms; (2) symptomatic and currently unable to independently undertake usual activities but no need for supplemental oxygen (or not above premorbid requirements); (3) need for supplemental oxygen at less than 4 L/min or less than 4 L/min above premorbid requirements; (4) need for supplemental oxygen at 4 L/min or higher or 4 L/min or higher than premorbid requirements but no need for high-flow oxygen; (5) need for non-invasive ventilation or high-flow oxygen (ie, high-flow nasal cannula); (6) need for invasive ventilation, extracorporeal membrane oxygenation, mechanical circulatory support, or renal replacement therapy; and (7) death. The pulmonary-plus ordinal scale includes the same classifications as the pulmonary scale with additional extrapulmonary manifestations that result in a category 4 classification (stroke with NIH Stroke Scale [NIHSS] score of ≤14, meningitis, encephalitis, myelitis, myocardial infarction, myocarditis, pericarditis, new onset congestive heart failure of New York Heart Association class III or IV or worsening to class III or IV, or arterial or deep venous thromboembolic events), a category 5 classification (stroke with NIHSS score of >14), or a category 6 classification (vasopressor therapy).
The primary efficacy outcome was time to sustained clinical recovery up to day 90, defined as time between randomisation and return to home (with home defined as the patient's residence before COVID-19 or a location that provided similar or less intensive medical care) for 14 consecutive days. Key secondary outcomes were all-cause death up to day 90 after randomisation and time to discharge from index hospitalisation.
Composite safety outcomes were ascertained on day 5, day 28, and day 90. On days 5 and 28 the composite included all-cause death, serious adverse events, grade 3 or 4 adverse events, incident organ failure, and serious coinfection. On day 90, the composite included all outcomes on days 5 and 28 except grade 3 and 4 adverse events. Definitions and additional outcomes are described in the appendix (pp 16–20).
Statistical analysis
The prespecified criteria used by the DSMB to assess futility stated that an agent would pass the early futility assessment if it showed more favourable odds ratios (OR) for both the pulmonary and pulmonary-plus ordinal scales than placebo with one-sided p values of less than 0·30. In the early futility assessment, comparison of 150 patients in an active group with 150 patients in the placebo group provided 95% power to detect an OR of 1·60 or higher for a more favourable outcome on the pulmonary or pulmonary-plus ordinal scales in the active group versus placebo group with a one-sided type 1 error of 0·30 (appendix p 22).
The population for all efficacy and safety analyses was the modified intention-to-treat (mITT) population, which included patients randomly assigned to treatment who received all or part of the assigned study product infusion. We analysed outcomes among patients with non-missing data for each outcome, and report denominators for each analysis; we did not impute missing outcome data. We did separate analyses to compare the sotrovimab group with the placebo group and the BRII-196 plus BRII-198 group with the placebo group for each outcome.
We analysed the pulmonary and pulmonary-plus ordinal outcome scales at day 5 with proportional odds models adjusted for two prespecified variables: baseline pulmonary ordinal scale category and trial pharmacy, with trial pharmacies with fewer than 15 participants grouped by geographical region (appendix p 28). In a post-hoc sensitivity analysis, we varied the strategy for grouping of trial pharmacies. We also fit proportional odds models with the same covariates for the ordinal outcomes at days 1–4, 6, 7, 14, and 28. We did a score test for the proportional odds assumption. The test for proportional odds was done by comparing the results of fitting two models, one in which a common odds ratio was assumed for each of the six dichotomised analyses of seven-category ordinal scale and compared with a second model that allowed the odds ratios to vary. The score test refers to the comparison of the two models with the six degree of freedom χ2 statistic, which was used to calculate a p value.
We analysed time to sustained recovery up to day 90, time to discharge, and time to the two most favourable categories of the pulmonary ordinal scale using a Fine-Gray model to account for the competing risk of death, stratified by trial pharmacy. We refer to summary statistics from the stratified analyses as adjusted analyses. The primary analyses were pooled over strata defined by study site pharmacy to obtain an overall summary statistic. The pooling takes into account the size of each stratum. We analysed time to death up to day 90 using a proportional hazards model stratified by trial pharmacy. We analysed the composite safety outcome at day 5 using logistic regression adjusted for trial pharmacy and we analysed the day 28 and day 90 composite safety outcomes using proportional hazards regression. Additional analyses for each outcome are described in the appendix (pp 17–20).
We analysed heterogeneity of treatment effect by adding an interaction term in the models for sustained clinical recovery and the composite safety outcome up to day 90. The baseline characteristics we considered in the analyses of heterogeneity of treatment effect included antibody and antigen levels, demographics, and duration of symptoms; a full list is in the appendix (pp 20–21). Our primary hypothesis regarding baseline antibody levels, which was added as an addendum to the statistical analysis plan after completion of enrolment and before any antibody results were revealed, was that patients without endogenous neutralising anti-SARS-CoV-2 antibodies would benefit more from sotrovimab and BRII-196 plus BRII-198 compared with placebo than would patients with these neutralising antibodies. For the subgroup analysis based on SARS-CoV-2 antigen levels, we used a single threshold at the median value for participants in this trial (1450 pg/mL) to dichotomise the population into those with high and low antigen levels, with a hypothesis that patients with higher antigen levels would be more likely to benefit from monoclonal antibody therapy than those with lower antigen levels.
Reported p values are two-sided unless otherwise stated. Unless otherwise stated, we considered p values of less than 0·05 to be significant. We did all statistical analyses using SAS (version 9.4) and R (version 4.0). This study is registered with ClinicalTrials.gov, NCT04501978
Role of the funding source
Investigators from NIH were directly involved in all aspects of this study, including study design, data collection, data analysis, data interpretation, and writing of the report.
Results
On March 1, 2021, the DSMB reviewed data for the early futility assessment for both sotrovimab and BRII-196 plus BRII-198. Based on DSMB recommendations, which were consistent with prespecified criteria for futility, enrolment to both agents was halted (appendix pp 23–26).
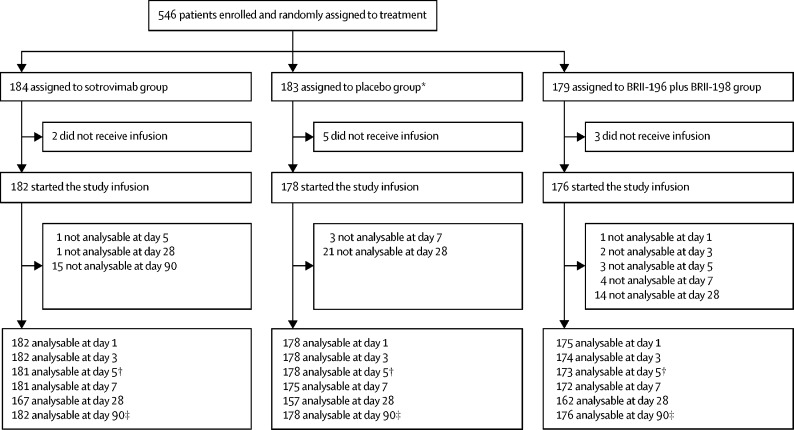
Between Dec 16, 2020, and March 1, 2021, 546 patients were enrolled and randomly assigned to treatment at 43 sites in the USA, Denmark, Switzerland, and Poland. Ten patients who were randomly assigned to treatment did not receive any volume of study product infusion, resulting in 536 patients being included in the mITT population for analysis, including 182 patients in the sotrovimab group, 176 in the BRII-196 plus BRII-198 group, and 178 in the placebo group (figure 1 ). One patient in the placebo group was enrolled at a site that was not open to the BRII-196 plus BRII-198 agent; this patient was not included in analyses of BRII-196 plus BRII-198. Among the 536 patients in the mITT population, 527 (98%) received the full volume of study product. Nine participants received a partial infusion due to adverse events (five patients), staffing error (three patients), or technical problems with the infusion pump (one patient).
Figure 1.
Study profile
*One patient who was randomly assigned to the placebo group was enrolled at a site not using the BRII-196 plus BRII-198 agent. This patient was not included in the placebo group for comparisons with BRII-196 plus BRII-198. †Day 5 outcomes were used for the early futility assessment. ‡Day 90 outcome of sustained clinical recovery was the primary efficacy outcome; patients lost to follow-up were censored.
In the mITT population, the median age was 60 years (IQR 50–72) and 228 (43%) patients were female and 308 (57%) were male (table 1 ). At the time of randomisation, median duration of COVID-19 symptoms was 8 days (IQR 5–9), 360 (67%) patients were receiving supplemental oxygen, 340 (63%) had received remdesivir, 327 (61%) had received corticosteroids, and 41 (8%) had received at least one dose of a COVID-19 vaccine (table 1). Baseline endogenous anti-SARS-CoV-2 antibody and antigen levels were available for 513 (96%) patients, among whom 212 (41%) were positive for neutralising anti-spike protein antibodies, 312 (61%) were positive for anti-nucleocapsid antibodies, 178 (35%) were negative for both antibody assays, and 488 (95%) had detectable plasma nucleocapsid antigens. Neutralising anti-spike antibodies and anti-nucleocapsid antibodies at baseline were detected less frequently in patients with shorter duration of symptoms, with higher SARS-CoV-2 plasma antigen levels, and who were older (appendix p 30).
Table 1.
Baseline characteristics of patients (modified intention-to-treat population)
| Sotrovimab group (n=182) | Placebo group (n=178)* | BRII-196 plus BRII-198 group (n=176) | ||
|---|---|---|---|---|
| Age, years | 61 (50–74) | 60 (49–70) | 61 (50–71) | |
| Sex | ||||
| Female | 75 (41%) | 75 (42%) | 78 (44%) | |
| Male | 107 (59%) | 103 (58%) | 98 (56%) | |
| Race or ethnicity† | ||||
| Asian | 7 (4%) | 9 (5%) | 12 (7%) | |
| Non-Hispanic Black | 40 (22%) | 37 (21%) | 37 (21%) | |
| Hispanic | 30 (16%) | 32 (18%) | 34 (19%) | |
| Non-Hispanic White | 101 (55%) | 92 (52%) | 87 (49%) | |
| Other | 4 (2%) | 8 (4%) | 6 (3%) | |
| Body-mass index category | ||||
| ≥30 kg/m2 (obese) | 102 (56%) | 99 (56%) | 90 (51%) | |
| ≥40 kg/m2 (severely obese) | 31 (17%) | 25 (14%) | 26 (15%) | |
| Co-existing chronic illness | ||||
| Any | 134 (74%) | 136 (76%) | 133 (76%) | |
| Hypertension treated with medication | 103 (57%) | 104 (58%) | 97 (55%) | |
| Diabetes treated with medication | 71 (39%) | 62 (35%) | 59 (3%) | |
| Renal impairment | 27 (15%) | 19 (11%) | 13 (7%) | |
| Asthma | 19 (10%) | 17 (10%) | 23 (13%) | |
| Heart failure | 13 (7%) | 8 (4%) | 15 (9%) | |
| Chronic supplemental oxygen use before COVID-19 | 2 (1%) | 5 (3%) | 4 (2%) | |
| Previous receipt of ≥1 dose of a COVID-19 vaccine | 15 (8%) | 10 (6%) | 16 (9%) | |
| Time since symptom onset, days | 8 (5–9) | 8 (5–9) | 8 (6–9) | |
| Medication use before randomisation | ||||
| Remdesivir | 120 (66%) | 112 (63%) | 108 (61%) | |
| Antibacterial agent | 46 (2%) | 54 (30%) | 42 (24%) | |
| Corticosteroid | 109 (60%) | 120 (67%) | 98 (56%) | |
| Therapeutic anticoagulation‡ | 25 (14%) | 16 (9%) | 18 (10%) | |
| Prophylactic or intermediate anticoagulation | 122 (67%) | 126 (71%) | 115 (65%) | |
| Pulmonary ordinal scale category§ | ||||
| Category 2: unable to do usual activities and no supplemental oxygen | 64 (35%) | 52 (29%) | 60 (34%) | |
| Category 3: supplemental oxygen <4 L/min | 76 (42%) | 80 (45%) | 74 (42%) | |
| Category 4: supplemental oxygen ≥4 L/min | 42 (23%) | 46 (26%) | 42 (24%) | |
| Neutralising anti-spike antibody positive | 68/173 (39%) | 76/171 (44%) | 68/169 (40%) | |
| Anti-nucleocapsid antibody positive | 100/173 (58%) | 108/171 (63%) | 104/169 (62%) | |
| Negative on both anti-nucleocapsid and neutralising anti-spike antibody assays | 67/173 (39%) | 54/171 (32%) | 57/169 (34%) | |
| Nucleocapsid antigen concentration >1450 pg/mL | 84/173 (49%) | 80/171 (47%) | 82/169 (49%) | |
Data are n (%), n/N (%), or median (IQR).
One patient in the common placebo group was enrolled at a site that was not enrolling patients into the BRII-196 plus BRII-198 group of the trial; this patient was not included in the placebo group for comparison with BRII-196 plus BRII-198.
Race or ethnicity was self-reported.
Therapeutic anticoagulation was defined as receipt of therapeutic doses of heparin, warfarin, or a direct-acting oral anticoagulant.
For patients on chronic supplemental oxygen therapy before COVID-19, categorisation on the pulmonary ordinal scale was based on oxygen flow rates above the pre-COVID-19 oxygen flow rate. For example, a patient who chronically used supplemental oxygen at 2 L/min before COVID-19 would be categorised as category 2 if using 2 L/min at randomisation, category 3 if using >2 L/min and <6 L/min, and category 4 if using ≥6 L/min of supplemental oxygen.
During the entire stay in hospital, including time both before and after randomisation, 487 (91%) of 536 patients received at least one dose of remdesivir (appendix p 31).
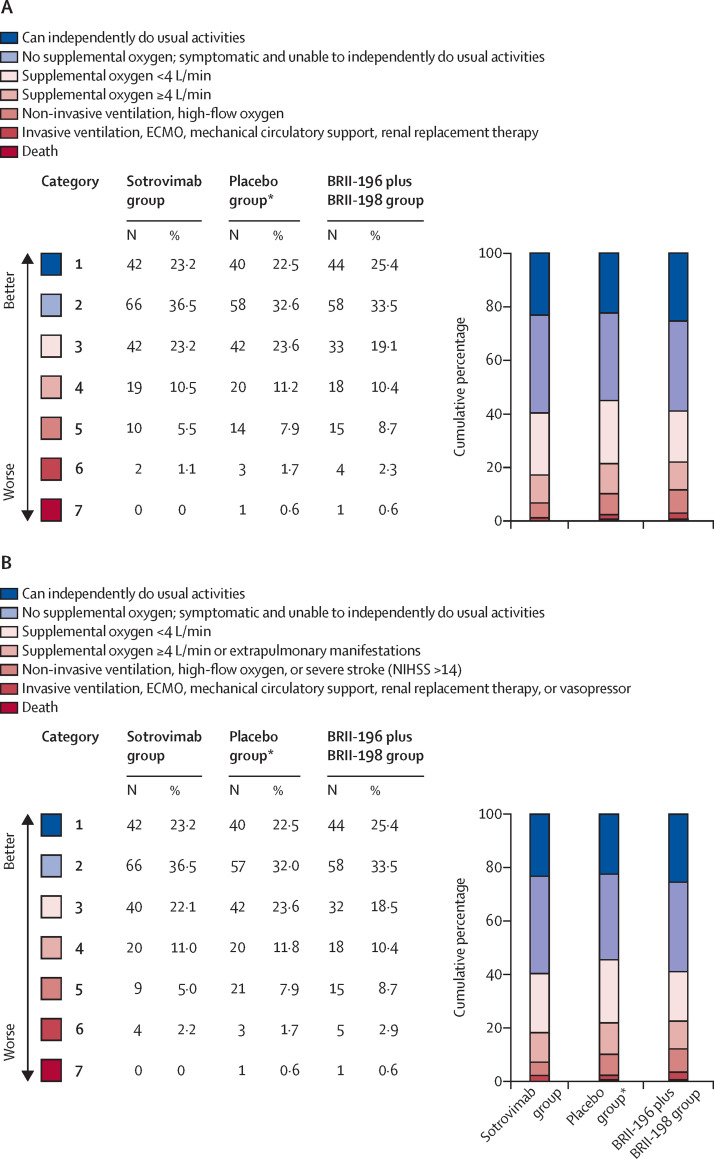
The number of patients who had an improvement in the seven-category pulmonary ordinal scale between baseline and day 5 was 85 (47%) of 181 in the sotrovimab group, 77 (45%) of 173 in the BRII-196 plus BRII-198 group, and 91 (51%) of 178 in the placebo group. Compared with placebo, the adjusted OR (active treatment vs placebo) for patients being in a more favourable category on the pulmonary scale on day 5 was 1·07 (95% CI 0·74 to 1·56) for sotrovimab and 0·98 (0·67 to 1·43) for BRII-196 plus BRII-198 (table 2 ; figure 2A ). Adjusted ORs for the pulmonary ordinal scale on other days during the first week of follow-up and days 14 and 28 also did not differ significantly (appendix p 35). Outcomes for the pulmonary-plus ordinal outcome scale were nearly identical to the pulmonary ordinal outcome scale; the number of patients with improvement between baseline and day 5 on the pulmonary-plus scale was 85 (47%) of 181 in the sotrovimab group, 77 (45%) of 173 in the BRII-196 plus BRII-198 group, and 91 (51%) of 178 in the placebo group. The adjusted OR for a more favourable day 5 pulmonary-plus category with active treatment than with placebo was 1·08 (95% CI 0·74 to 1·58) for sotrovimab and 1·00 (0·68 to 1·46) for BRII-196 plus BRII-198 (table 2; figure 2B). Adjusted ORs for the pulmonary-plus ordinal scale on other days during the first week of follow-up and days 14 and 28 also did not differ (appendix p 35). We found no evidence that the proportional odds assumption was not met for the proportional odds models. In a post-hoc sensitivity analysis, varying how trial pharmacies with small numbers of participants were pooled in each stratum did not have a substantive effect on the results (appendix p 36).
Table 2.
Summary of key clinical outcomes
| Sotrovimab group (n=182) | Placebo group (n=178)* | BRII-196 plus BRII-198 group (n=176) |
Sotrovimab vs placebo† |
BRII-196 plus BRII-198 vs placebo† |
||||
|---|---|---|---|---|---|---|---|---|
| Point estimate (95% CI) | p value | Point estimate (95% CI) | p value | |||||
| Efficacy outcomes | ||||||||
| Pulmonary ordinal outcome scale at day 5 (futility assessment) | .. | .. | .. | aOR 1·07 (0·74–1·56)‡ | 0·72 | aOR 0·98 (0·67–1·43)‡ | 0·91 | |
| Category 1: can independently do usual activities | 42/181 (23%) | 40/178 (22%) | 44/173 (25%) | .. | .. | .. | .. | |
| Category 2: no supplemental oxygen; symptomatic and unable to do usual activities | 66/181 (36%) | 58/178 (33%) | 58/173 (34%) | .. | .. | .. | .. | |
| Category 3: supplemental oxygen <4 L/min | 42/181 (23%) | 42/178 (24%) | 33/173 (19%) | .. | .. | .. | .. | |
| Category 4: supplemental oxygen ≥4 L/min | 19/181 (10%) | 20/178 (11%) | 18/173 (10%) | .. | .. | .. | .. | |
| Category 5: high-flow nasal canula or non-invasive ventilation | 10/181 (6%) | 14/178 (8%) | 15/173 (9%) | .. | .. | .. | .. | |
| Category 6: invasive ventilation, ECMO, mechanical circulatory support, or RRT | 2/181 (1%) | 3/178 (2%) | 4/173 (2%) | .. | .. | .. | .. | |
| Category 7: death | 0/181 (0%) | 1/178 (1%) | 1/173 (1%) | .. | .. | .. | .. | |
| Pulmonary-plus ordinal outcome scale at day 5 (futility assessment) | .. | .. | .. | aOR 1·08 (0·74–1·58)‡ | 0·68 | aOR 1·00 (0·68–1·46)‡ | 0·99 | |
| Category 1: can independently do usual activities | 42/181 (23%) | 40/178 (22%) | 44/173 (25%) | .. | .. | .. | .. | |
| Category 2: no supplemental oxygen; symptomatic and unable to do usual activities | 66/181 (36%) | 57/178 (32%) | 58/173 (34%) | .. | .. | .. | .. | |
| Category 3: supplemental oxygen <4 L/min | 40/181 (22%) | 42/178 (24%) | 32/173 (18%) | .. | .. | .. | .. | |
| Category 4: supplemental oxygen ≥4 L/min or extrapulmonary manifestations | 20/181 (11%) | 21/178 (12%) | 18/173 (10%) | .. | .. | .. | .. | |
| Category 5: high-flow nasal canula or non-invasive ventilation or severe stroke | 9/181 (5%) | 14/178 (8%) | 15/173 (9%) | .. | .. | .. | .. | |
| Category 6: invasive ventilation, ECMO, mechanical circulatory support, RTT, or vasopressor | 4/181 (2%) | 3/178 (2%) | 5/173 (3%) | .. | .. | .. | .. | |
| Category 7: death | 0/181 (0%) | 1/178 (1%) | 1/173 (1%) | .. | .. | .. | .. | |
| Sustained clinical recovery up to day 90 (primary outcome) | 160 (88%) | 151 (85%) | 155 (88%) | aRR 1·12 (0·91–1·37)§ | 0·29 | aRR 1·08 (0·88–1·32)§ | 0·48 | |
| Death up to day 90 | 14 (8%) | 13 (7%) | 15 (9%) | aHR 1·02 (0·48–2·17)§ | 0·96 | aHR 1·15 (0·54–2·41)§ | 0·72 | |
| Safety outcomes | ||||||||
| Composite safety outcomes | ||||||||
| Up to day 5 | 36 (20%) | 44 (25%) | 46 (26%) | aOR 0·75 (0·44–1·26)§ | 0·28 | aOR 1·14 (0·69–1·86)§ | 0·62 | |
| Up to day 28 | 51 (28%) | 57 (32%) | 58 (33%) | aHR 0·87 (0·60 – 1·27)§ | 0·48 | aHR 1·10 (0·76–1·59)§ | 0·62 | |
| Up to day 90 | 42 (23%) | 48 (27%) | 45 (26%) | aHR 0·84 (0·55–1·27)§ | 0·40 | aHR 1·00 (0·66–1·51)§ | >0·99 | |
| Infusion reaction | 18 (10%) | 14 (8%) | 23 (13%) | aOR 1·30 (0·61–2·76)§ | 0·50 | aOR 1·83 (0·89–3·77)§ | 0·10 | |
Data are n/N (%) or n (%) unless otherwise stated. aOR=adjusted odds ratio. aHR=adjusted hazard ratio. aRR=adjusted rate ratio. ECMO=extracorporeal membrane oxygenation. HR=hazard ratio. RRT=renal replacement therapy.
One patient in the common placebo group was enrolled at a site that was not enrolling patients into the BRII-196 plus BRII-198 group of the trial; this patient was not included in the placebo group for comparison with BRII-196 plus BRII-198; thus, the placebo group in the analyses of BRII-196 plus BRII-198 included 177 patients.
The difference between the active agent (sotrovimab or BRII-196 plus BRII-198) group and the placebo group was calculated as an odds ratio, rate ratio, or hazard ratio. A ratio >1·0 indicated more favourable results with the active agent compared with placebo for the following outcomes: pulmonary ordinal outcome scale, and sustained clinical recovery. A ratio >1·0 indicated more favourable results with placebo compared with the active agent for the following outcomes: death, composite safety outcomes, and infusion reaction.
Adjusted for baseline pulmonary ordinal scale category and trial pharmacy, with trial pharmacies with fewer than 15 participants grouped by geographical region.
Adjusted for trial pharmacy.
Figure 2.
Distribution of patients on the pulmonary ordinal scale (A) and the pulmonary-plus ordinal scale (B) on day 5
The sotrovimab group and the BRII-196 plus BRII-198 group were each compared with the placebo group. ECMO=extracorporeal membrane oxygenation. NIHSS=National Institutes of Health Stroke Scale. *One patient in the common placebo group was enrolled at a site that was not enrolling patients into the BRII-196 plus BRII-198 group of the trial; this patient was not included in the placebo group for comparison with BRII-196 plus BRII-198.
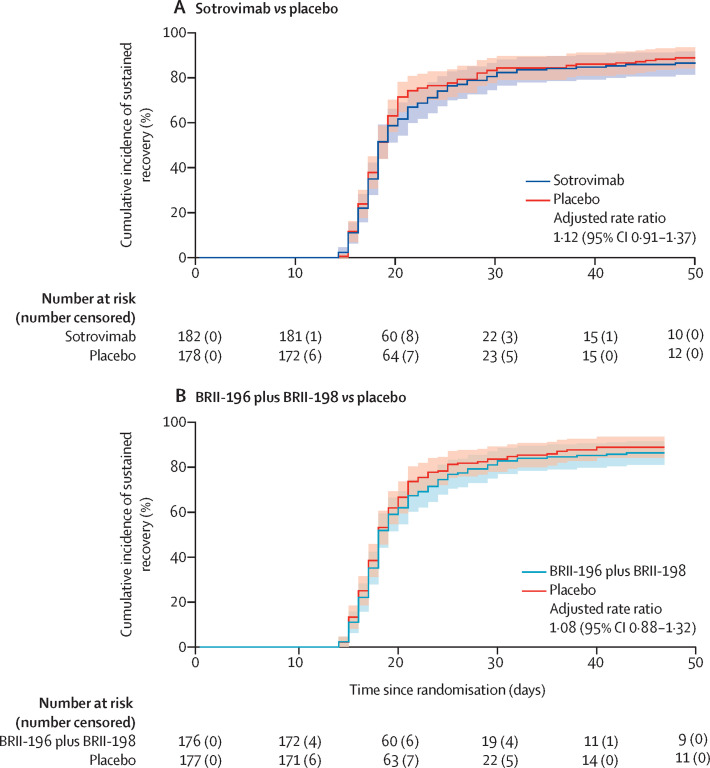
By day 90, sustained clinical recovery was seen in 151 (85%) of 178 patients in the placebo group, compared with 160 (88%) of 182 in the sotrovimab group (rate ratio 1·12 [95% CI 0·91–1·37]) and 155 (88%) of 176 in the BRII-196 plus BRII-198 group (1·08 [0·88–1·32]; table 2; figure 3 ). Time to hospital discharge and time to the two most favourable categories on the pulmonary ordinal scale for patients on supplemental oxygen at entry were not significantly different between sotrovimab and placebo or between BRII-196 plus BRII-198 and placebo (appendix pp 58–59). Among those discharged within 14 days, 37 (26%) of 142 patients in the placebo group, 31 (22%) of 144 in the sotrovimab group, and 39 (26%) of 148 in the BRII-196 plus BRII-198 group continued to receive supplemental oxygen above pre-morbid levels at home.
Figure 3.
Time to sustained clinical recovery up to day 90 for sotrovimab versus placebo (A) and BRII-196 plus BRII-198 versus placebo (B)
The rate ratios were calculated with Fine-Gray models to account for the competing risk of death and stratified according to trial pharmacy. Reasons for censoring included death before sustained recovery was reached (11 patients in the sotrovimab and placebo groups and 13 in the BRII-196 plus BRII-198 group), loss to follow-up (five patients in the sotrovimab and BRII-196 plus BRII-198 groups and nine in the placebo group), and not reaching sustained recovery by day 90 (six patients in the sotrovimab group, three in the BRII-196 plus BRII-198 group, and seven in the placebo group) .
By day 90, 42 (8%) patients had died, including 14 (8%) in the sotrovimab group, 15 (9%) in the BRII-196 plus BRII-198 group, and 13 (7%) in the placebo group (table 2). Median time between randomisation and death was 24 days (IQR 15–31). Compared with the placebo group, the hazard ratio (HR) for death for sotrovimab was 1·02 (95% CI 0·48–2·17) and for BRII-196 plus BRII-198 was 1·15 (0·54–2·41; table 2; appendix p 60).
At least one infusion-related adverse event was reported for 18 (10%) patients in the sotrovimab group, 23 (13%) patients in the BRII-196 plus BRII-198 group, and 14 (8%) patients in the placebo group (table 2). Epinephrine was used to treat anaphylaxis for one (1%) patient in the sotrovimab group, one (1%) patient in the BRII-196 plus BRII-198 group, and no patients in the placebo group.
The composite safety outcome up to day 90 was met by 48 (27%) patients in the placebo group compared with 42 (23%) patients in the sotrovimab group (adjusted HR 0·84 [95% CI 0·55–1·27]) and 45 (26%) patients in the BRII-196 plus BRII-198 group (1·00 [0·66–1·51]; table 2). Respiratory failure was the most common incident organ failure up to day 28, experienced by 19 (10%) patients in the sotrovimab group, 24 (14%) in the BRII-196 plus BRII-198 group, and 21 (12%) in the placebo group (appendix p 41). Additional safety outcome results are detailed in the appendix (pp 37–50).
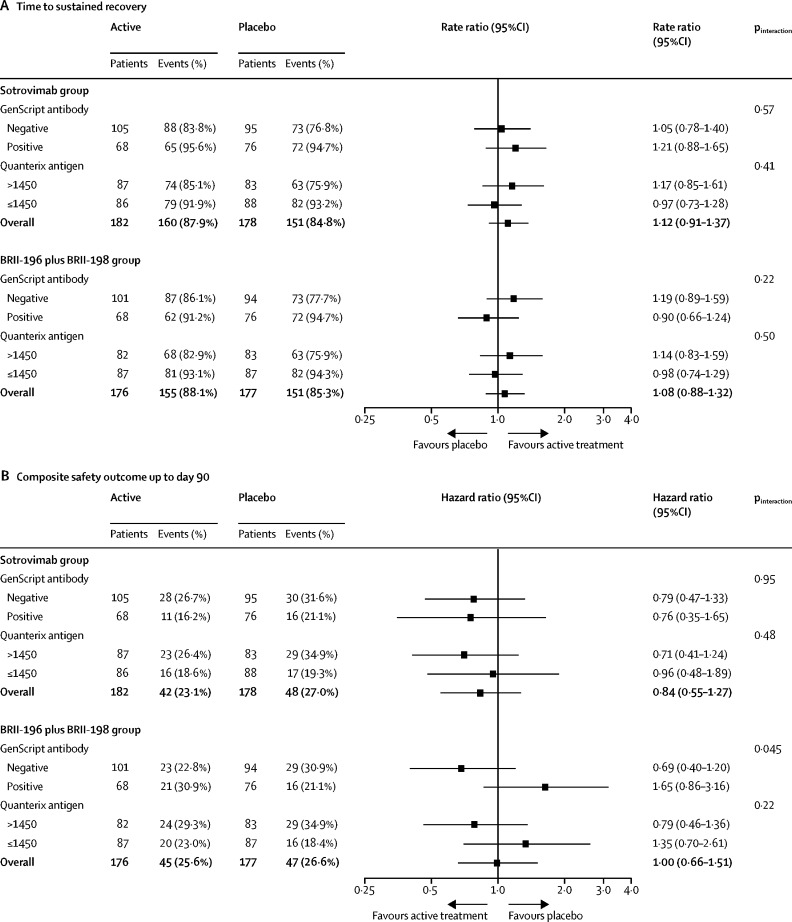
For sotrovimab, point estimates favoured active agent over placebo among both patients with and without endogenous neutralising antibodies for both the sustained recovery and composite safety outcomes, with no heterogeneity of treatment effect observed (figure 4 ). For BRII-196 plus BRII-198, point estimates for patients without endogenous neutralising antibodies favoured active agent over placebo for both the sustained recovery outcome and the composite safety outcome, whereas point estimates for patients with endogenous neutralising antibodies favoured placebo over the active agent for both outcomes with overlapping 95% CIs. For BRII-196 plus BRII-198, an interaction was observed between baseline neutralising antibody status and the composite safety outcome at 90 days (figure 4). For BRII-196 plus BRII-198, point estimates also favoured active agent over placebo for patients with high antigen levels and favoured placebo over active agent for patients with low antigen levels. Comparisons of individual components of the composite day 90 safety outcome by endogenous neutralising antibody status and treatment are summarised in the appendix (pp 49–50). We did not find evidence of heterogeneity of treatment effect for sotrovimab or BRII-196 plus BRII-198 on the basis of demographic or other clinical characteristics (appendix pp 51–56).
Figure 4.
Subgroup analysis for time to sustained recovery up to day 90 (A) and the composite safety outcome at day 90 (B) by antibody status and antigen levels at baseline
These analyses tested for heterogeneity of treatment effects for sotrovimab and BRII-196 plus BRII-198 by baseline measurements of endogenous neutralising antibodies against the SARS-CoV-2 receptor binding domain and concentrations of SARS-CoV-2 nucleocapsid antigens. Antibody and antigen measurements were done on plasma collected before study drug infusion. Samples with >30% binding inhibition against the SARS-CoV-2 receptor binding domain on the GenScript SARS-CoV-2 Surrogate Virus Neutralization Test assay were classified as positive for endogenous neutralising antibodies. Nucleocapsid antigen concentration >1450 pg/mL on a Quanterix assay, which was approximately the median value in the trial population, was considered a high antigen concentration.
Discussion
In this randomised, double-blind, clinical trial among adults hospitalised with COVID-19 without organ failure and with symptoms for up to 12 days, treatment with either of two anti-SARS-CoV-2 neutralising monoclonal antibody therapies (sotrovimab or BRII-196 plus BRII-198) that have been shown to potently inhibit SARS-CoV-2 replication13, 20 did not improve day 5 pulmonary status, time to clinical recovery, or other clinical outcomes compared with placebo. Both therapies had reassuring findings for safety.
In other clinical trials, both sotrovimab and BRII-196 plus BRII-198 have shown efficacy for reducing disease progression to hospitalisation or death among outpatients with mild or moderate COVID-19 when treatment was given within 5 days (sotrovimab in the COMET-ICE trial21) or 10 days (BRII-196 plus BRII-198 in the ACTIV-2 platform10) of symptom onset. Similarly, other anti-SARS-CoV-2 neutralising monoclonal antibody therapies, including bamlanivimab plus etesevimab5, 12, 22 and casirivimab plus imdevimab,4, 23 have exhibited benefit among outpatients with early COVID-19 in both peer-reviewed and preprint studies. This resulted in emergency use authorisation by the US Food and Drug Administration for sotrovimab,24 bamlanivimab plus etesevimab,25 and casirivimab plus imdevimab26 for the treatment of outpatients with COVID-19.
Meanwhile, a treatment strategy using neutralising monoclonal antibodies for patients hospitalised with severe COVID-19 remains under development. In addition to the overall null results reported here for sotrovimab and BRII-196 plus BRII-198, previous trials of bamlanivimab12 and casirivimab plus imdevimab23 have also reported null results for the overall population of patients hospitalised with COVID-19. However, evidence is emerging that patients hospitalised with COVID-19 without endogenous anti-SARS-CoV-2 antibodies might benefit from neutralising monoclonal antibody therapy. In our study, approximately 301 (58%) of 513 patients were negative for neutralising anti-SARS-CoV-2 antibodies at the time of randomisation and, among these patients, point estimates suggested treatment with BRII-196 plus BRII-198 could potentially be beneficial for both time to sustained clinical recovery and the composite safety outcome. Meanwhile, point estimates for patients positive at baseline for neutralising antibodies who were treated with BRII-196 plus BRII-198 were found to usually indicate worse outcomes. These results for BRII-196 plus BRII-198, which were based on underpowered subgroup analyses and should be interpreted with caution, are similar to findings reported for bamlanivimab27 and casirivimab plus imdevimab,23 in which subgroup analyses by endogenous antibody status indicated that patients hospitalised with COVID-19 without endogenous anti-SARS-CoV-2 antibodies potentially benefited from neutralising monoclonal antibody therapies, whereas those with endogenous antibodies did not. Future studies assessing neutralising monoclonal antibody therapies among patients hospitalised with COVID-19 should assess antibody status to understand if these findings are broadly applicable to monoclonal antibody therapies and if only patients without endogenous anti-SARS-CoV-2 antibodies should be targeted for treatment. Notably, although rapid SARS-CoV-2 antibody testing is not routinely available in all clinical settings, substantial progress has been made towards making rapid antibody testing a feasible route for guiding early treatment decisions in the near future.28
Results for sotrovimab in this trial did not suggest heterogeneity of treatment effect by baseline endogenous antibody status. This observation could be related to different antibody characteristics of sotrovimab, including a target epitope outside the ACE2 receptor-binding motif and differences in Fc modifications,15 or it might be due to random chance alone.
In addition to trials of patients hospitalised with COVID-19 probably having a greater proportion of patients with endogenous anti-SARS-CoV-2 antibodies than outpatient trials, increased use of concomitant COVID-19 therapies for inpatients might be an alternative or supplementary explanation for null overall results in inpatient trials and beneficial results in outpatient trials.29 For example, in our trial, more than 90% of patients received remdesivir during their hospital stay and more than 60% received corticosteroids before randomisation, which have both shown benefit for some patients hospitalised with COVID-19.1, 2 The additional antiviral activity from neutralising monoclonal antibody therapies might not provide incremental benefit above background therapy with remdesivir and corticosteroids. Additionally, inpatients with COVID-19 usually have longer duration of symptoms than do outpatients with COVID-19 seeking care,10, 21 and neutralising monoclonal antibody therapies might have reduced efficacy later in the disease course, regardless of endogenous antibody status and concomitant medications.
A potential harm signal for treatment with neutralising monoclonal antibody therapies among patients with endogenous anti-SARS-CoV-2 antibodies needs to be better understood. Among patients with endogenous anti-SARS-CoV-2 antibodies, trends toward worse clinical outcomes among patients treated with BRII-196 plus BRII-198 than treated with placebo were observed in this trial, although the 95% CIs crossed unity and the study was underpowered for subgroup analyses. This finding is consistent with findings reported in other recent trials for bamlanivimab27 and casirivimab plus imdevimab.23 Theoretically, neutralising monoclonal antibodies might cause harm in some patients through antibody-dependent enhancement of inflammation or viral replication, or both;30, 31 however, evidence of clinically important antibody-dependent enhancement has not been clearly observed in trials to date.
Limitations of this trial include its small sample size because of early stopping due to futility, which prevented robust subgroup analyses, including a well powered assessment of patients without endogenous anti-SARS-CoV-2 antibodies. Small beneficial effects from sotrovimab and BRII-196 plus BRII-198 could not be ruled out by this trial, which was designed to rapidly assess multiple agents for large clinical effects among a heterogenous population of patients hospitalised with COVID-19 and without consideration of serostatus. Early futility in this trial was based on two ordinal outcome scales (the pulmonary and pulmonary-plus scales) measured 5 days after randomisation; these are intermediate clinical outcomes and their correlation with long-term outcomes that are relevant to the patient has not been established. Critically ill patients, such as those treated with high-flow oxygen therapy or mechanical ventilation, were not included in this trial, and so the results are not generalisable to this more severely ill population. Although the doses of sotrovimab and BRII-196 plus BRII-198 used in this trial were selected on the basis of preclinical studies showing strong antiviral activity in the lungs in animals, robust dose-finding studies have not been completed and it is possible that higher doses could have been more efficacious than the doses used in this study.17 Additionally, the effect of sotrovimab and BRII-196 plus BRII-198 on SARS-CoV-2 clearance was not analysed.
In conclusion, in this multinational, double-blind, placebo-controlled, randomised clinical trial, the neutralising anti-SARS-CoV-2 monoclonal antibodies sotrovimab and BRII-196 plus BRII-198 did not show efficacy over placebo for improving clinical outcomes among adults hospitalised with COVID-19. Prespecified subgroup analyses suggested heterogeneity of treatment effect in the BRII-196 plus BRII-198 group by baseline endogenous neutralising antibody status, suggesting potential opportunity for monoclonal antibody therapies to be targeted to patients hospitalised with COVID-19 who have not developed endogenous antibodies.
Data sharing
Deidentified data from TICO trials will be made available 1 year after publication of final results from the platform. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted using the Research Proposal Form on the INSIGHT website, www.insight-trials.org.
Declaration of interests
WHS reports grants from National Heart, Lung and Blood Institute (NHLBI) during the conduct of the study and personal fees from Aerpio Pharmaceuticals outside of the submitted work. US reports grants from NIH, Cytodin, and Regeneron outside of the submitted work. RLG reports personal fees from GSK Pharmaceuticals during the conduct of the study, and personal fees and non-financial support from Gilead Sciences and personal fees from Johnson & Johnson and Roivant Sciences, outside of the submitted work. TWR reports grants from NHLBI of the NIH during the conduct of the study and personal fees from Cumberland Pharmaceuticals and Sanofi outside of the submitted work. JDC reports grants from NIH outside the submitted work. IDP reports grants from NIH during the conduct of the study, and grants from Janssen Pharmaceuticals, NIH, and US Centers for Disease Control and Prevention (CDC), research funding support from Regeneron and Asahi Kasei Pharma, and grants from Intermountain Research and Medical Foundation outside the submitted work. AJR reports personal fees from Merck outside of the submitted work. MNG reports research funding support from Regeneron outside of the submitted work. BWT reports salary support from INSIGHT during the conduct of the study, and grants from NIH, Agency for Healthcare Research and Quality, CDC, Veterans Affairs Health Services Research and Development, grants and personal fees from Zambon pharmaceuticals, and personal fees and research funding support from Genentech outside of the submitted work. MKJ reports payment to their employer for clinical trial activities from Regeneron Pharmaceuticals, Gilead Sciences, Janssen Pharmaceuticals, and Merck, and payments for advisory board meetings from Gilead Sciences outside of the submitted work. AK reports grants from United Therapeutics, Johnson & Johnson, 4D Medical, Lung LLC, and Reata Pharmaceuticals outside of the submitted work. MAM reports grants from Roche/Genentec and personal fees from Pliant Therapeutics, Novartis, Johnson & Johnson, and Citius Pharmaceuticals outside of the submitted work. AAG reports grants from NIH during the conduct of the study and grants from AbbVie and Faron Pharmaceuticals outside of the submitted work. SMB reports grants from CDC during the conduct of the study and personal fees from Hamilton, payment to their employer for service on a trial steering committee from Faron and Sedana; grants from Janssen, NIH, and US Department of Defense; and book royalties from Oxford University and Brigham Young University outside of the submitted work. SP reports grants to their university from the University of Minnesota during the conduct of the study, and other grants to their university from University of Minnesota, European and Developing Countries Clinical Trials Partnership, UK Research and Innovation (UKRI), Academy of Medical Sciences, ViiV Healthcare, UK Medical Research Council (MRC), and Gilead Sciences outside of the submitted work. HFG reports grants from Swiss National Science Foundation, NIH, Swiss HIV Cohort Study, and Yvonne Jacob Foundation; unrestricted grants from Gilead Sciences; and personal fees from Merck, Gilead Sciences and ViiV, for being an advisor, consultant, or DSMB member, outside of the submitted work. SS reports grants from NIH during the conduct of the study. HC reports being a Gilead employee. RG reports being a full-time employee of Vir Biotechnology and owned stock in Vir Biotechnology during the conduct of the study. SO reports being an employee of GlaxoSmithKline Pharmaceuticals. DM reports support from Brii Biosciences during the conduct of the study. AGB reports grants from University of Minnesota during the conduct of the study and grants from MRC and UKRI outside of the submitted work. BTT reports receiving consulting fees from Bayer, Novartis, and Thetis outside the submitted work. JDN reports grants from NIAID NIH during the conduct of the study. All other members of the writing committee declare no competing interests.
Acknowledgments
Acknowledgments
Medications used in the trial were supplied by Vir/GlaxoSmithKline (sotrovimab), Brii Biosciences (BRII-196 plus BRII-198), and Gilead Sciences (remdesivir). This trial was supported by the US Operation Warp Speed programme, NIAID and Leidos Biomedical Research for the INSIGHT Network, NHLBI, the Research Triangle Institute for the Prevention and Early Treatment of Acute Lung Injury Network, Cardiothoracic Surgical Trials Network, the Office of Research & Development, and US Department of Veterans Affairs, and grants from the governments of Denmark (number 126 from the National Research Foundation), Australia (from the National Health and Medical Research Council), and the United Kingdom (MRC_UU_12023/23 from the MRC). This research was, in part, funded by the NIH (agreement 1OT2HL156812-01). AV was supported in part by grants provided by NHLBI (5T32HL069749-17). JMS was supported in part by grants from National Center for Advancing Translational Sciences (UL1TR003015 and KL2TR003016). JDC was supported in part by a grant from NHLBI (K23HL153584). BWT was supported in part by the US Department of Veterans Affairs, Office of Research & Development, and the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413). The work at Massachusetts General Hospital was supported in part by the Harvard Catalyst/Harvard Clinical and Translational Science Center (NCATS, NIH awards UL1TR001102 and UL1TR002541–01). This research was, in part, funded by the NIH, including agreement 1OT2HL156812-01. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing official policies, either expressed or implied, of the NIH or Department of Veterans Affairs.
ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group
Writing committee: Wesley H Self*, Uriel Sandkovsky*, Cavan S Reilly, David M Vock, Robert L Gottlieb, Michael Mack, Kevin Golden, Emma Dishner, Andrew Vekstein, Emily R Ko, Tatyana Der, John Franzone, Eyad Almasri, Mohamed Fayed, Michael R Filbin, Kathryn A Hibbert, Todd W Rice, Jonathan D Casey, J Awori Hayanga, Vinay Badhwar, Bradley G Leshnower, Milad Sharifpour, Kirk U Knowlton, Ithan D Peltan, Elizieta Bakowska, Justyna Kowalska, Michael E Bowdish, Jeffrey M Sturek, Angela J Rogers, D Clark Files, Jarrod M Mosier, Michelle N Gong, David J Douin, R Duncan Hite, Barbara W Trautner, Mamta K Jain, Edward M Gardner, Akram Khan, Jens-Ulrik Jensen, Michael A Matthay, Adit A Ginde, Samuel M Brown, Elizabeth S Higgs, Sarah Pett, Amy C Weintrob, Christina C Chang, Daniel D Murrary, Huldrych F Günthard, Ellen Moquete, Greg Grandits, Nicole Engen, Birgit Grund, Shweta Sharma, Huyen Cao, Rajesh Gupta, Suzette Osei, David Margolis, Qing Zhu, Mark N Polizzotto, Abdel G Babiker, Victoria J Davey, Virginia Kan, B Taylor Thompson, Annetine C Gelijns, James D Neaton, H Clifford Lane, Jens D Lundgren
*Contributed equally
Affiliations
Department of Emergency Medicine (W H Self MD), Vanderbilt Institute for Clinical and Translational Research (W H Self), and Division of Allergy, Pulmonary and Critical Care Medicine (T W Rice MD, J D Casey, MD), Vanderbilt University Medical Center; Nashville, TN, USA; Division of Infectious Diseases (U Sandkovsky MD, K Golden MD, E Dishner MD), Center for Advanced Heart and Lung Disease (R L Gottlieb PhD), and Cardiothoracic Surgery (M Mack MD), Baylor University Medical Center, Dallas, TX, USA; Division of Biostatistics, School of Public Health (Prof C S Reilly PhD, D M Vock PhD, G Grandits MS, N Engen MS, S Sharma MS, Prof J D Neaton PhD), School of Statistics (Prof B Grund PhD), University of Minnesota, Minneapolis, MN, USA; Baylor Heart and Vascular Hospital, Dallas, TX, USA (R L Gottlieb); Baylor Scott and White, The Heart Hospital – Plano, Plano, TX, USA (R L Gottlieb); Division of Cardiovascular and Thoracic Surgery, Department of Surgery, Duke University Medical Center, Durham, NC, USA (A Vekstein MD); Duke Regional Hospital (E R Ko MD), Duke Raleigh Hospital (T Der MD), Division of General Internal Medicine, Department of Medicine, Duke Health System, Durham, NC, USA (J Franzone, MD); Pulmonary and Critical Care Division, University of California San Francisco, Fresno MEP, Fresno, CA, USA (E Almasri MD, M Fayed MD); Division of Pulmonary and Critical Care Medicine (K A Hibbert MD), Department of Emergency Medicine, Massachusetts General Hospital, Boston, MA, USA (M R Filbin MD); Department of Cardiovascular and Thoracic Surgery, WVU Heart and Vascular Institute, West Virginia University, Morgantown, WV, USA (Prof J A Hayanga MD, Prof V Badhwar MD); Division of Cardiothoracic Surgery, Department of Surgery (B G Leshnower MD) and Department of Anesthesia and Critical Care (M Sharifpour MD), Emory University, Atlanta, GA, USA; Cardiovascular Department (Prof K U Knowlton MD) and Division of Pulmonary and Critical Care Medicine, Department of Medicine (I D Peltan MD, Prof S M Brown MD), Intermountain Medical Center, Salt Lake City, UT, USA; Division of Pulmonary and Critical Care Medicine, Department of Medicine (I D Peltan, Prof S M Brown) and Department of Internal Medicine (Prof S M Brown), University of Utah School of Medicine, Salt Lake City, UT, USA; Wojewódzki Szpital Zakaźny, Warsaw, Poland (E Bakowska MD); Department of Adults' Infectious Diseases, Medical University of Warsaw, Warsaw, Poland (Prof J Kowalska MD); Department of Surgery, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA, USA (M E Bowdish MD); Department of Medicine, Division of Pulmonary and Critical Care Medicine, University of Virginia School of Medicine, Charlottesville, VA, USA (J M Sturek MD); Division of Pulmonary, Allergy, and Critical Care Medicine, Stanford University, Stanford, CA, USA (A J Rogers MD); Section on Pulmonary, Critical Care, Allergy and Immunologic Disease, Wake Forest Baptist Health, Winston-Salem, NC, USA (D C Files MD); Department of Emergency Medicine and Department of Medicine, Division of Pulmonary, Allergy, Critical Care and Sleep, University of Arizona College of Medicine, Tucson, AZ, USA (J M Mosier MD); Division of Pulmonary Medicine, Department of Medicine, Montefiore Medical Center, Bronx, NY USA (Prof M N Gong MD); Department of Anesthesiology (D J Douin MD) and Department of Emergency Medicine (Prof A A Ginde MD), University of Colorado School of Medicine, Aurora, CO, USA; Division of Pulmonary, Critical Care and Sleep Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, USA (Prof R D Hite MD); Center for Innovations in Quality, Effectiveness, and Safety, Michael E DeBakey Veterans Affairs Medical Center, Houston, TX, USA (Prof B W Trautner MD); Section of Health Services Research, Department of Medicine, Baylor College of Medicine, Houston, TX, USA (Prof B W Trautner); Division of Infectious Diseases, Department of Internal Medicine, UT Southwestern Medical Center, Dallas, TX, USA (Prof M K Jain MD); Infectious Diseases Clinic and Center for Positive Health, Denver Health and Hospital Authority, Denver, CO, USA (E M Gardner MD); Division of Pulmonary and Critical Care Medicine, Oregon Health and Science University, Portland, OR, USA (A Khan MD); Section of Respiratory Medicine, Department of Medicine Herley-Gentofte Hospital, Hellerup, Denmark (J-U Jensen MD); CHIP Center of Excellence for Health, Immunity, and Infections and Department of Infectious Diseases, Rigshospitalet, Copenhagen, Denmark (J-U Jensen, D D Murray PhD, Prof J D Lundgren MD); Department of Clinical Medicine, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark (J-U Jensen MD); Cardiovascular Research Institute, Department of Medicine and Department of Anesthesia, University of California, San Francisco, CA, USA (Prof M A Matthay MD); National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA (E S Higgs MD, H C Lane MD); The Medical Research Council Clinical Trials Unit at UCL, University College London, London, UK (Prof S Pett MD, Prof A G Babiker PhD); Infectious Diseases Section, VA Medical Center, Washington, DC, USA (A C Weintrob MD, Prof V Kan MD); The Kirby Institute (C C Hang MD, Prof M N Polizzotto MD) and St Vincent's Hospital (Prof M N Polizzotto), UNSW, Sydney, NSW, Australia; Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zurich, Zurich, Switzerland (Prof H F Günthard MD); Zurich and Institute of Medical Virology, University of Zurich, Zurich, Switzerland (Prof H F Günthard); Department of Population Heath Science and Policy (Prof A C Gelijns PhD), Icahn School of Medicine at Mount Sinai, New York, NY, USA (E Moquete BSN); Gilead Sciences, Foster City, CA USA (H Cao MD); Vir Biotechnology, San Francisco, CA, USA (R Gupta MD); GlaxoSmithKline, Collegeville, PA, USA (S Osei MD); Brii Biosciences, Durham, NC USA (D Margolis MD, Q Zhu, PhD); United States Department of Veterans Affairs, Washington, DC, USA (V J Davey PhD); Division of Pulmonary and Critical Care, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA (Prof B T Thompson MD); Department of Medicine, Harvard Medical School, Boston, MA, USA (Prof B T Thompson MD)
Contributors
Author and collaborator contributions, including responsibility for decision to submit the manuscript, drafting of the initial manuscript, study conceptualisation, investigation, data curation, formal analysis, study supervision, and review and editing of the manuscript, are provided in the appendix (pp 4–11). JDN and GG directly accessed and verified the underlying study data. JDN had access to all the study data and had final responsibility for the decision to submit the paper for publication.
Contributor Information
ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group:
Wesley H. Self, Uriel Sandkovsky, Cavan S. Reilly, David M. Vock, Robert L. Gottlieb, Michael Mack, Kevin Golden, Emma Dishner, Andrew Vekstein, Emily R. Ko, Tatyana Der, John Franzone, Eyad Almasri, Mohamed Fayed, Michael R. Filbin, Kathryn A. Hibbert, Todd W. Rice, Jonathan D. Casey, J. Awori Hayanga, Vinay Badhwar, Bradley G. Leshnower, Milad Sharifpour, Kirk U. Knowlton, Ithan D. Peltan, Elizieta Bakowska, Justyna Kowalska, Michael E. Bowdish, Jeffrey M. Sturek, Angela J. Rogers, D. Clark Files, Jarrod M. Mosier, Michelle N. Gong, David J. Douin, R. Duncan Hite, Barbara W. Trautner, Mamta K. Jain, Edward M. Gardner, Akram Khan, Jens-Ulrik Jensen, Michael A. Matthay, Adit A. Ginde, Samuel M. Brown, Elizabeth S. Higgs, Sarah Pett, Amy C. Weintrob, Christina C. Chang, Daniel D. Murrary, Huldrych F. Günthard, Ellen Moquete, Greg Grandits, Nicole Engen, Birgit Grund, Shweta Sharma, Huyen Cao, Rajesh Gupta, Suzette Osei, David Margolis, Qing Zhu, Mark N. Polizzotto, Abdel G. Babiker, Victoria J. Davey, Virginia Kan, B. Taylor Thompson, Annetine C. Gelijns, James D. Neaton, H. Clifford Lane, Jens D. Jundgren, John Tierney, Kevin Barrett, Betsey R. Herpin, Mary C. Smolskis, Susan E. Voge, Laura A. McNay, Kelly Cahill, Page Crew, Matthew Kirchoff, Ratna Sardana, Sharon Segal Raim, Joseph Chiu, Lisa Hensley, Josua Lorenzo, Rebecca Mock, Katy Shaw-Saliba, Judith Zuckerman, Stacey J. Adam, Judy Currier, Sarah Read, Eric Hughes, Laura Amos, Amy Carlsen, Anita Carter, Bionca Davis, Eileen Denning, Alain DuChene, Merrie Harrison, Payton Kaiser, Joseph Koopmeiners, Sue Meger, Thomas Murray, Kien Quan, Siu Fun Quan, Greg Thompson, Jamie Walski, Deborah Wentworth, Alan J. Moskowitz, Emilia Bagiella, Karen O'Sullivan, Mary E. Marks, Evan Accardi, Emily Kinzel, Gabriela Bedoya, Lopa Gupta, Jessica R. Overbey, Maria L. Padillia, Milerva Santos, Marc A. Gillinov, Marissa A. Miller, Wendy C. Taddei-Peters, Kathleen Fenton, Mezgebe Berhe, Clinton Haley, Christopher Bettacchi, Erin Duhaime, Madison Ryan, Sarah Burris, Felecia Jones, Samantha Villa, Samantha Want, Raven Robert, Tanquinisha Coleman, Laura Clariday, Rebecca Baker, Marian Hurutado-Rodriguez, Nazia Iram, Michelle Fresnedo, Allyson Davis, Kiara Leonard, Noelia Ramierez, Jon Thammavong, Krizia Duque, Emma Turner, Tammy Fisher, Dianna Robinson, Desirae Ransom, Erica Lusk, Aaron Killian, Adriana Palacious, Edilia Solis, Janet Jerrow, Matthew Watts, Heather Whitacre, Elizabeth Cothran, Peter K. Smith, Christina E. Barkauskas, Grace R. Dreyer, Marie Witte, Nilima Mosaly, Ahmad Mourad, Thomas L. Holland, Kathleen Lane, Andrew Bouffler, Lauren M. McGowan, Marry Motta, Gregory Tipton, Ben Stallings, Gennifer Stout, Beth McLendon-Arvik, Beth A. Hollister, Dana M. Giangiacomo, Sunil Sharma, Brian Pappers, Paul McCarthy, Troy Krupica, Arif Sarwari, Rebecca Reece, Lisa Fornaresio, Chad Glaze, Raquel Evans, Katarina Preamble, Lisa Giblin Sutton, Sabrina Buterbaugh, Elizabeth Berry Bartolo, Roger Williams, Robin Bunner, William Bender, Jeffrey Miller, Kim T. Baio, Mary K. McBride, Michele Fielding, Sonya Mathewson, Kristina Porte, Missy Maton, Chari Ponder, Elizabeth Haley, Christine Spainhour, Susan Rogers, Derrick Tyler, Noah Wald-Dickler, Douglass Hutcheon, Amytis Towfighi, May M. Lee, Meghan R. Lewis, Brad Spellberg, Linda Sher, Aniket Sharma, Anna P. Olds, Chris Justino, Edward Lozano, Chris Romero, Janet Leong, Valentina Rodina, Tammie Possemato, Jose Escobar, Charlene Chiu, Kevin Weissman, Andrew Barros, Kyle B. Enfield, Alexandra Kadl, China J. Green, Rachel M. Simon, Ashley Fox, Kara Thornton, Patrick E. Parrino, Stephen Spindel, Aditya Bansal, Katherine Baumgarten, Jonathan Hand, Derek Vonderhaar, Bobby Nossaman, Sylvia Laudun, DeAnna Ames, Shane Broussard, Nilmo Hernandez, Geralyn Isaac, Huan Dinh, Yiling Zheng, Sonny Tran, Hunter McDaniel, Nicolle Crovetto, Leslie Miller, Beth Schelle, Sherry McLean, Howard R. Rothbaum, Michael S. Alvarez, Shivam P. Kalan, Heather H. Germann, Jennifer Hendershot, Karen Maroney, Karen Herring, Sharri Cook, Pam Paul, Ronson J. Madathil, Joseph Rabin, Andrea Levine, Kapil Saharia, Ali Tabatabai, Christine Lau, James S. Gammie, Maya-Loren Peguero, Kimberley McKernan, Matthew Audette, Emily Fleischmann, Freshta Akbari, Maia Lee, Myounghee Lee, Andrew Chi, Hanna Salehi, Alan Pariser, Phuong Tran Nguyen, Jessica Moore, Adrienne Gee, Shelika Vincent, Richard A. Zuckerman, Alexander Iribarne, Sara Metzler, Samantha Shipman, Taylor Caccia, Haley Johnson, Crystallee Newton, Doug Parr, Vicente Rodriguez, Gordon Bokhart, Sharon M. Eichman, Crystal North, Cathryn Oldmixon, Nancy Ringwood, Laura Fitzgerald, Haley D. Morin, Ariela Muzikansky, Richard Morse, Roy G. Brower, Lora A. Reineck, Neil R. Aggarwal, Karen Bienstock, Peter Hou, Jay Steingrub, Mark A. Tidswell, Lori-Ann Kozikowski, Cynthia Kardos, Leslie DeSouza, Sherell Thornton-Thompson, Daniel Talmor, Nathan Shapiro, Valerie Banner-Goodspeed, Katherine L. Boyle, Sharon Hayes, Alan E. Jones, James Galbraith, Utsav Nandi, Rebekah K. Peacock, Blair Alden Parry, Justin D. Margolin, Kelsey Brait, Caroline Beakes, Kirsten N. Kangelaris, Kimberly J. Yee, Kimia Ashktorab, Alejandra E. Jauregui, Hanjing Zhuo, Gregory Hendey, Kinsley A. Hubel, Alyssa R. Hughes, Rebekah L. Garcia, Jennifer G. Wilson, Rosemary Vojnik, Jonasel Roque, Cynthia Perez, George W. Lim, Steven Y. Chang, Rebecca Beutler, Trisha Agarwal, Julia Vargas, Marc Moss, Amiran Baduashvili, Lakshmi Chauhan, Lani L. Finck, Michelle Howell, Robert C. Hyzy, Pauline K. Park, Kristine Nelson, Jake I. McSparron, Ivan N. Co, Bonnie R. Wang, Shijing Jia, Barbara Sullins, Sinan Hanna, Norman Olbrich, Lynne D. Richardson, Rahul Nair, Obiageli Offor, Brenda Lopez, Omowunmi Amosu, Hiwet Tzehaie, Thomas E. Terndrup, Herbert P. Wiedemann, Abhijit Duggal, Nirosshan Thiruchelvam, Kiran Ashok, Alexander H. King, Omar Mehkri, Kristin Hudock, Simra Kiran, Harshada More, Tammy Roads, Jamie Martinkovic, Sarah Kennedy, Bryce H. Robinson, Catherine L. Hough, Olivia F. Krol, Mistry Kinjal, Emmanuel Mills, Madeline McDougal, Rupali Deshmukh, Peter Chen, Sam S. Torbati, Yuri Matusov, June Choe, Niree A. Hindoyan, Susan E. Jackman, Emad Bayoumi, Timothy Wynter, Antonina Caudill, Ethan Pascual, Gregg J. Clapham, Lisa Herrera, Cristabelle Ojukwu, Shaunt Mehdikhani, D. Shane O'Mahony, Sonam T. Nyatsatsang, David M. Wilson, Julie A. Wallick, Chadwick Miller, Keven W. Gibbs, Lori S. Flores, Mary E. LaRose, Leigha D. Landreth, Peter E. Morris, Jamie L. Sturgill, Evan P. Cassity, Sanjay Dhar, Ashley A. Montgomery-Yates, Sara N. Pasha, Kirby P. Mayer, Brittany Bissel, Joseph Bledsoe, Samuel Brown, Michael Lanspa, Lindsey Leither, Brent P. Armbruster, Quinn Montgomery, Darrin Applegate, Naresh Kumar, Melissa Fergus, Erna Serezlic, Karah Imel, Ghazal Palmer, Brandon Webb, Valerie T. Aston, Jakea Johnson, Christopher Gray, Margaret Hays, Megan Roth, Adriana Sánchez, Laura Popielski, Heather Rivasplata, Melissa Turner, Michael Vjecha, Tianna Petersen, Dena Kamel, Laura Hansen, Claudia Sanchez Lucas, Natalie DellaValle, Sonia Gonzales, James Scott, David Wyles, Ivor Douglas, Jason Haukoos, Kevin Kamis, Caitlin Robinson, Jason V. Baker, Anne Frosch, Rachael Goldsmith, Hodan Jibrell, Melanie Lo, Jonathan Klaphake, Shari Mackedanz, Linh Ngo, Kelly Garcia-Myers, Norman Markowitz, Erika Pastor, Mayur Ramesh, Indira Brar, Emanuel Rivers, Princy Kumar, Maximiliano Menna, Kousick Biswas, Cristin Harrington, Alex Delp, Lavannya Pandit, Casey Hines-Munson, John Van, Laura Dillon, Yiqun Want, Paola Lichtenberger, Gio Baracco, Carol Ramos, Lauren Bjork, Melyssa Sueiro, Phyllis Tien, Heather Freasier, Theresa Buck, Hafida Nekach, Stephanie Nagy-Agren, Shikha Vasudeva, Tracy Ochalek, Brentin Roller, Chinh Nguyen, Amani Mikail, Dorthe Raben, Tomas O. Jensen, Bitten Aagaard, Charlotte B. Nielsen, Katharina Krapp, Bente Rosdahl Nykjær, Katja Lisa Kanne, Anne Louise Grevsen, Zillah Maria Joensen, Tina Bruun, Ane Bojesen, Frederik Woldbye, Nick E. Normand, Frederik V.L. Esmann, Clara Lundetoft Clausen, Nichlas Hovmand, Karen Brorup Pedersen, Louise Thorlacius-Ussing, Michaela Tinggaard, Dorthe S. Høgsberg, Ema Rastoder, Thobias Kamstrup, Christina Marisa Bergsøe, Lars Østergaard, Nina Breinholt Stærke, Isik S. Johansen, Fredrikke C. Knudtzen, Lykke Larsen, Mathias A. Hertz, Thilde Fabricius, Marie Helleberg, Jan Gerstoft, Tomas Østergaard Jensen, Birgitte Lindegaard, Thomas Ingemann Pedersen, Birgit Thorup Røge, Sandra Valborg Løfberg, Thomas Michael Hansen, Ariella Denize Nielsen, Sebastian Leicht von Huth, Henrik Nielsen, Rikke Krog Thisted, Daria Podlekareva, Stine Johnsen, Helle Frost Andreassen, Lars Pedersen, Cecilia Ebba Clara Ellinor Lindnér, Lothar Wiese, Lene Surland Knudsen, Nikolaj Julian Skrøder Nytofte, Signe Ravn Havmøller, Roger Paredes, Maria Exposito, Eduardo Fernández-Cruz, José Muñoz, Jose R. Arribas, Vicente Estrada, Juan P. Horcajada, Joaquin Burgos, Jose Luis Morales-Rull, Dominique L. Braun, Emily West, Khadija M'Rabeth-Bensalah, Mareile L. Eichinger, Manuela Grüttner-Durmaz, Christina Grube, Veronika Zink, Andrzej Horban, Agnieszka Bednarska, Natalia Jurek, Gerd Fätkenheuer, Jakob J. Malinm, Gail Matthews, Anthony Kelleher, Gesalit Cabrera, Catherine Carey, Sally Hough, Sophie Virachit, Amy Zhong, Barnaby E. Young, Po Ying Chia, Tau Hong Lee, Ray J. Lin, David Lye, Sean Ong, Ser Hon Puah, Tsin Wen Yeo, Shiau Hui Diong, Juwinda Ongko, Fleur Hudson, Mahesh KB Parmar, Anna Goodman, Jonathan Badrock, Adam Gregory, Nicola Harris, Giota Touloumi, Nikos Pantaz, Vicky Gioukari, Joseph Lutaakome, Cissy M. Kityo, Henry Mugerwa, Francis Kiweewa, Anu Osinusi, Craig Tipple, Angela Willis, Amanda Peppercorn, Helen Watson, Elizabeth Alexander, Erik Mogalian, Leo Lin, Xiao Ding, Li Yan, Jean-Luc Girardet, Ji Ma, Zhi Hong, Amy Adams, Sara Albert, Abby Balde, Michelle Baracz, Beth Baseler, Nancy Becker, Mona Bielica, Shere Billouin-Frazier, Jennifer Cash, Jay Choudhary, Suzanne Dolney, Mary Dixon, Carolyn Eyler, Leanna Frye, Michael Galcik, Jensen Gertz, Lisa Giebeig, Neelam Gulati, Liz Hankinson, Debbie Hissey, Debi Hogarty, Matt Hohn, H Preston Holley, Lisa Hoopengardner, Lynda Huber, Shirley Jankelevich, Gary Krauss, Eileen Lake, Jessica Linton, Leah MacDonald, Meryan Manandhar, Mary Spinelli-Nadzam, Charles Oluremi, Calvin Proffitt, Erin Rudzinski, Jen Sandrus, Marylu Schaffhauser, Adam Schechner, Connie Suders, Norman P. Gerry, Kenneth Smith, Courtney Solomon, Amanda Kubernac, Marium Rashid, Bhakti Patel, Robert Kubernac, Joseph Murphy, Marie L. Hoover, Craig Brown, Nadine DuChateau, Adam Flosi, Les Johnson, Amy Treagus, and Christine Wenner
Supplementary Material
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GlaxoSmithKline Vir Biotechnology and GSK announce VIR-7831 reduces hospitalisation and risk of death in early treatment of adults with COVID-19. March 10, 2021. https://www.gsk.com/en-gb/media/press-releases/vir-biotechnology-and-gsk-announce-vir-7831-reduces-hospitalisation-and-risk-of-death-in-early-treatment-of-adults-with-covid-19/
- 7.Lilly Lilly's bamlanivimab and etesevimab together reduced hospitalizations and death in phase 3 trial for early COVID-19. March 10, 2021. https://investor.lilly.com/news-releases/news-release-details/lillys-bamlanivimab-and-etesevimab-together-reduced
- 8.Regeneron Phase 3 trial shows REGEN-COV™ (casirivimab with imdevimab) antibody cocktail reduced hospitalization or death by 70% in non-hospitalized COVID-19 patients. March 23, 2021. https://investor.regeneron.com/news-releases/news-release-details/phase-3-trial-shows-regen-covtm-casirivimab-imdevimab-antibody
- 9.GlobalNewswire Vir Biotechnology and GSK announce VIR-7831 reduces hospitalization and risk of death in early treatment of adults with COVID-19. March 10, 2021. https://www.globenewswire.com/news-release/2021/03/11/2190921/0/en/Vir-Biotechnology-and-GSK-Announce-VIR-7831-Reduces-Hospitalization-and-Risk-of-Death-in-Early-Treatment-of-Adults-with-COVID-19.html
- 10.Brii Biosciences Brii Biosciences announces positive data from the phase 3 ACTIV-2 trial evaluating combination BRII-196 and BRII-198 in non-hospitalized COVID-19 patients. Aug 25, 2021. https://www.briibio.com/news-detial.php?id=328
- 11.Murray DD, Babiker AG, Baker JV, et al. Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: therapeutics for inpatients with COVID-19 (TICO/ACTIV-3) Clinical Trials. 2021 doi: 10.1177/17407745211049829. published online Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACTIV-3/TICO LY-CoV555 Study Group A neutralizing monoclonal antibody for hospitalized patients with COVID-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuccori M, Ferraro S, Convertino I, et al. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline. MAbs. 2020;12 doi: 10.1080/19420862.2020.1854149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto D, Park Y-J, Beltramello M, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 15.Cathcart AL, Havenar-Daughton C, Lempp FA, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2021 doi: 10.1101/2021.03.09.434607. published online Sept 30. (preprint). [DOI] [Google Scholar]
- 16.Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 17.Datta-Mannan A. Mechanisms influencing the pharmacokinetics and disposition of monoclonal antibodies and peptides. Drug Metab Dispos. 2019;47:1100–1110. doi: 10.1124/dmd.119.086488. [DOI] [PubMed] [Google Scholar]
- 18.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Self WH, Semler MW, Leither LM, et al. Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Liu W, Yu X, Wu M, Reichert JM, Ho M. COVID-19 antibody therapeutics tracker: a global online database of antibody therapeutics for the prevention and treatment of COVID-19. Antib Ther. 2020;3:205–212. doi: 10.1093/abt/tbaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 22.Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RECOVERY Collaborative Group. Horby PW, Mafham M, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.06.15.21258542. published online June 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration Fact sheet for healthcare providers emergency use authorization (EUA) of sotrovimab. 2021. https://www.fda.gov/media/149534/download
- 25.US Food and Drug Administration Fact sheet for health care providers emergency use authorization (EUA) of bamlanivimab and etesevimab. 2021. https://www.fda.gov/media/145802/download#:~:text=The%20U.S.%20Food%20and%20Drug,patients%20(12%20years%20of%20age
- 26.US Food and Drug Administration Fact sheet for health care providers emergency use authorization (EUA) of REGEN-COV (casirivimab and imdevimab) 2021. https://www.fda.gov/media/145611/download
- 27.ACTIV-3/TICO Bamlanivimab Study Group. Lundgren JD, Grund B, et al. Clinical and virological response to a neutralizing monoclonal antibody for hospitalized patients with COVID-19. medRxiv. 2021 doi: 10.1101/2021.07.19.21260559. published online July 22. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuler CF, 4th, Gherasim C, O'Shea K, et al. Accurate point-of-care serology tests for COVID-19. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. 2021. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 30.Arvin AM, Fink K, Schmid MA, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584:353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 31.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from TICO trials will be made available 1 year after publication of final results from the platform. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data. Proposals for data use should be submitted using the Research Proposal Form on the INSIGHT website, www.insight-trials.org.