On Dec 20, 2020, the Israeli Ministry of Health launched a national COVID-19 vaccination campaign aiming to rapidly vaccinate all adults using the Pfizer–BioNtech BNT162b2 mRNA vaccine given in two doses 21 days apart, and by June, 2021, following US Food and Drug Administration (FDA) approval, it was expanded to children and adolescents aged 12–16 years. By July 1, 2021, 5 184 169 Israelis (56·2% of the population) were fully vaccinated.
Although patients with cancer were excluded from the pivotal BNT162b2 vaccine trials, a series of studies examined the safety and efficacy of the vaccine in patients with solid tumours; all showed consistent data,1, 2, 3, 4 indicating the vaccine was safe and effective. At a median of 76 days after the second vaccine dose, 287 (88%) of 326 vaccine recipients who had cancer had protective antibody concentrations above the minimal threshold considered as seropositive, compared with 159 (97%) of 164 healthy individuals.3 However, absolute antibody concentrations were significantly lower in patients with cancer (931 AU/mL), compared with healthy individuals (2817 AU/mL; p=0·003).3
By July, 2021, Israel witnessed a resurgence of COVID-19 infections and severe illness, attributed to emergence of the delta (B.1.617.2) variant, but also to waning immunity of the vaccine.5 Thus, on July 30, 2021, the Israeli Ministry of Health approved administration of a third (booster) dose of the BNT162b2 vaccine. This strategy proved to be highly effective6 and helped pave the way for a US FDA emergency authorisation of a booster dose for individuals aged 65 years and older or those aged 18–64 years at high risk of severe COVID-19.7
Between Aug 1, 2021, and Sept 29, 2021, we prospectively assessed the safety and immunogenicity of the booster dose, 1 month after its administration, in a cohort of 72 actively treated patients with cancer, compared with a matched group of 144 healthy individuals. During this period, adult patients with solid tumours, with no known past SARS-CoV-2 infection, receiving active treatment (defined as any intravenous anticancer medication, administered up to 3 weeks before the administration of the booster to up to 3 weeks afterwards) at the day care centre of the Oncology Division of Tel-Aviv Sourasky Medical Center (TASMC) were invited to participate in the study. The control group consisted of fully vaccinated health-care workers at TASMC with no personal history of cancer, active immune suppressive medications, or a documented past infection with SARS-CoV-2, who participated in a parallel study conducted simultaneously.8
Each patient with cancer who had received the third vaccine dose was matched by sex and year of birth to a healthy individual from among the TASMC cohort (for antibody response comparisons, patients were matched with two control participants). Seven patients were not matched by year of birth and instead were matched according to closest age (up to 8 years).
Immunogenicity analysis was done using the SARS-CoV-2 IgG II Quant kit (Abbott, Sligo, Ireland) for patients with cancer9 and the ADVIA Centaur kit (Siemens Healthineers, Erlangen, Germany) for the control participants.8 Since each company has a different set of standard values for its respective antibody tests, WHO set a reference conversion method allowing comparison between the methods.10 We used this conversion method to compare between the tests used for the patients with cancer and the healthy control participants.8, 9, 10 The study was approved by the TASMC institutional review board. Following written informed consent, blood was withdrawn for immunogenicity analysis shortly before administration of the booster. Participants were contacted roughly 1 month after the booster, interviewed about side-effects, and asked to undergo repeat testing for immunogenicity analysis.
The median age of the patients was 62 years (IQR 48–71); 65% of both groups were women (appendix p 1).
Although the median time from second dose to third dose and time from second dose to first antibody testing were statistically significant longer in the healthy individuals compared with the patients with cancer (median time between dose two to three: 217 days (IQR 174–246) in healthy individuals vs 210 days (158–256) in patients with cancer [p<0·0001]; median time between dose two to first antibody test: 210 days (154–221) in healthy individuals vs 203 days (43–258) in patients with cancer [p<0·0001]), an absolute difference of 7 days was not deemed to have any clinical significance.
The median time from third dose to second antigenicity testing was 33 days (IQR 21–44) in the patients with cancer compared with 27 days (23–29) in the control group (p=0·0056). 53 (75%) of 72 patients had metastatic disease and 45 (63%) received a chemotherapy-based regimens (appendix p 1).
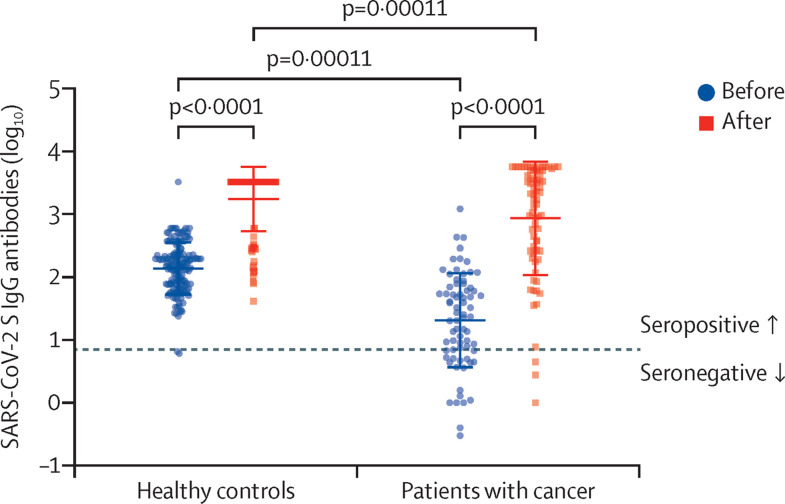
Before booster administration, 20 (28%) patients with cancer were found to be seronegative, compared with only two (1%) of the healthy individuals (p<0·0001; figure ; appendix p 2). After administration of the booster, three patients with cancer and none of the healthy individuals remained seronegative (appendix p 2). A significant increase in anti-SARS-CoV-2S IgG absolute antibodies concentrations in both groups was also noted (p<0·0001 for the comparison of pre-booster and post-booster concentrations within each group). Yet, higher pre-booster and post-booster antibody concentrations were noted in healthy individuals than in patients with cancer (p=0·00011; figure; appendix p 2). Multivariate analysis, including study group (patient or control) and time (before or after third dose) adjusted for the matching factors (age and gender) indicated that the only statistically significant variable associated with increased titre levels was administration of the booster (p<0·0001; appendix p 2).
Figure.
SARS-CoV-2 S IgG antibody values in serum samples of actively treated patients with cancer (n=72) and healthy controls (n=144) before (blue circles dots) and after (red squares) the third dose of BNT162b2 vaccine
Boxes represent median SARS-Cov-2 S IgG antibody concentrations and whiskers represent upper and lower quartiles. Each dot represents one participant. The y-axis (log10 scale) represents SARS-Cov-2 S IgG antibody WHO BAU/ML values transformed to log10 scale. Dashed line represents cutoff level for seropositivity. Significant differences were assessed by related-samples sign test for the comparison within a group and independent-samples Mann-Whitney U and Kolmogorov-Smirnov tests. After a third dose patients with cancer have lower plasma concentrations of SARS-Cov-2 S IgG antibodies compared with healthy individuals (p=0·00011).
Adverse effects were collected as previously described.4 The vaccine was well tolerated among all participants. The most common side-effects in patients with cancer were local, including local pain at the injection site (35 [49%] of 72 patients) and local swelling (four [6%]; data not shown), whereas the most common systemic side-effects were fatigue (20 [28%]), fever (nine [13%]), headache (six [8%]), muscle pain (five [7%]), and chills (five [7%]). Although patients with cancer reported statistically significant less headache (p=0·0046), muscle pain (p<0·0001), and chills (p=0·026) than healthy controls, there were no statistically significant differences in the frequency of other side-effects (appendix p 3). No severe side-effects, either life-threatening or requiring hospitalisation or any other intervention, were reported.
Because most patients with cancer in Israel were not known to have past SARS-CoV-2 infection, and because past infection might mask the response to vaccination, these patients who were known to have previous SARS-CoV-2 infection were excluded. Although this was a single-centre study, the TASMC is a large tertiary centre, treating more than 4500 new patients with solid tumours per year and serves as a national referral centre, with only 35% of the patients residing our immediate referral area. Thus, the study population is representative of the general population of Israeli patients with cancer on active treatment.
Our presented data suggest a high rate of waning immunogenicity in patients with cancer approximately 6 months after the administration of the second dose of BNT162b2, and support the use of a booster dose in this vulnerable population of actively treated patients with cancer. The modest side-effect profile further supports this recommendation. Although the study cohort was relatively small, we believe that the explicit data from this population, combined with the robustness of the national data,5 support the recommendation for a third dose booster for actively treated patients with cancer.
YA declares receiving research grants from Pfizer, outside the scope of this work. AS is partially supported by the Israeli Council for Higher Education via the Weizmann Data Science Research Center, and by a research grant from the Estate of Tully and Michele Plesser. IW reports speaker fees and fees for consultancy from Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Beyond Air, AstraZeneca, and Novartis; contracts or research funding from Roche, Novatis, Beyond Air, and Merck Sharp & Dohme; participation on a data safety monitoring board or advisory board of Merck Sharp & Dohme and AstraZeneca; participation in committee of Israeli Cancer Association; and stock options in Breath of life. All other authors declare no competing interests. COVI3 study investigators are listed at the appendix (p 4).
Supplementary Material
References
- 1.Waldhorn I, Holland R, Goshen-Lago T, et al. Six-month efficacy and toxicity profile of BNT162b2 vaccine in cancer patients with solid tumors. Cancer Discov. 2021;11:2430–2435. doi: 10.1158/2159-8290.CD-21-1072. [DOI] [PubMed] [Google Scholar]
- 2.Eliakim-Raz N, Massarweh A, Stemmer A, Stemmer SM. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol. 2021;7:1716–1718. doi: 10.1001/jamaoncol.2021.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligumsky H, Safadi E, Etan T, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst. 2021 doi: 10.1093/jnci/djab174. published online Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration FDA authorizes booster dose of Pfizer-BioNTech COVID-19 vaccine for certain populations. 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations
- 8.Saiag E, Goldshmidt H, Sprecher E, Ben-Ami R, Bomze D. Immunogenicity of a BNT162b2 vaccine booster in health-care workers. Lancet Microbe. 2021;2:e650. doi: 10.1016/S2666-5247(21)00272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tene Y, Levytskyi K, Adler A, et al. An outbreak of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infections among hospital personnel with high mRNA vaccine uptake. Infect Control Hosp Epidemiol. 2021 doi: 10.1017/ice.2021.412. published online Sept 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siemens Healthcare Diagnostics Inc Understanding SARS-CoV-2 IgG immunity thresholds and the process of standardization. 2021. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/b2406e708bf287c5/506564e9207f/Understanding-SARS-CoV-2-IgG-Immunity-Thresholds-and-the-Process-of-Standardization.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.