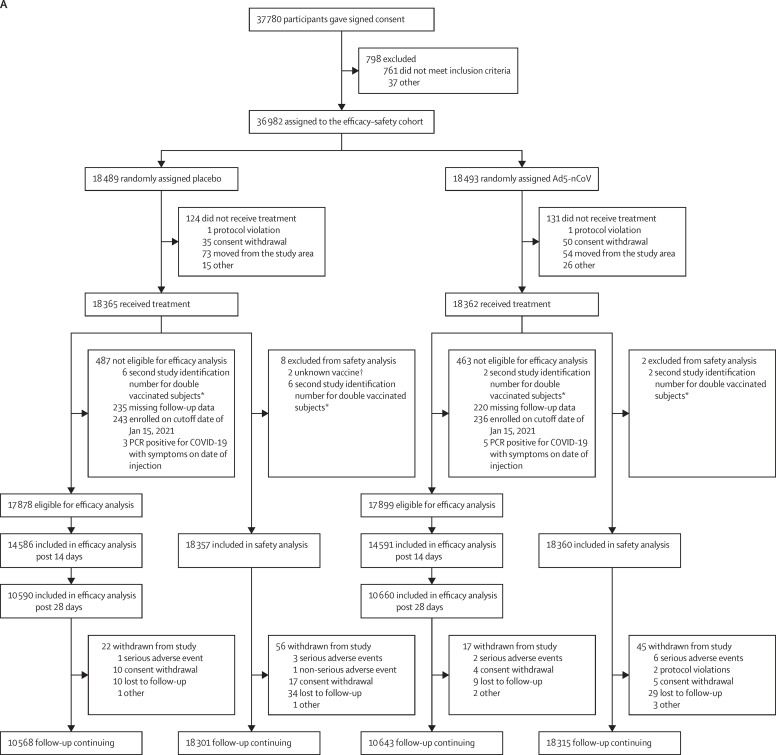
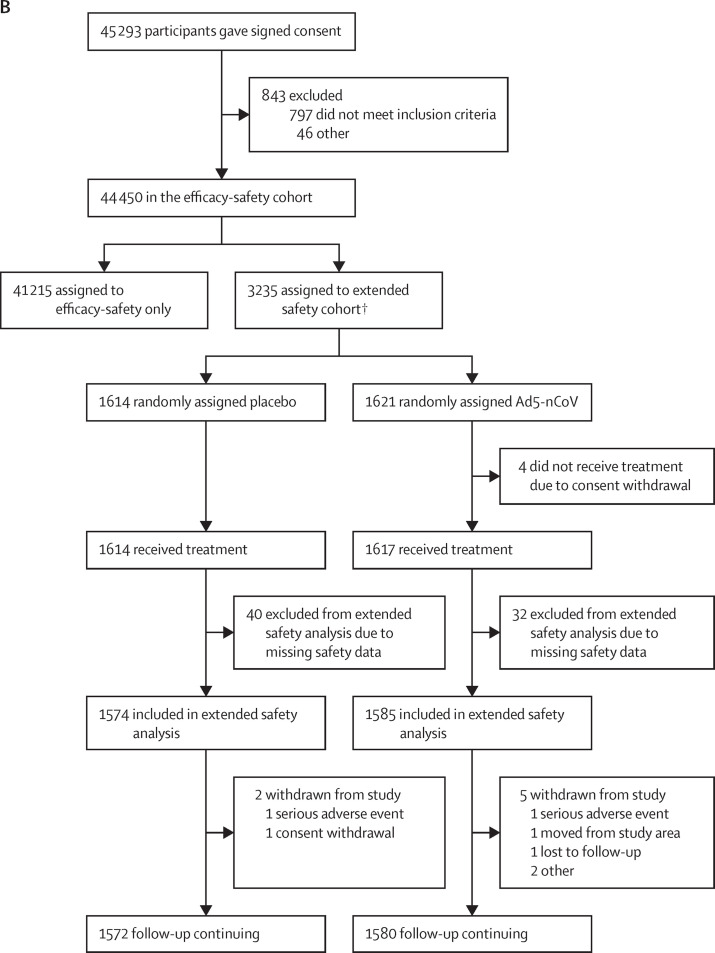
Figure 1.
Clinical trial profile
Disposition of clinical trial participants for the efficacy analysis and safety analysis at the cutoff date of the efficacy analysis (Jan 15, 2021; A), and the extended safety and immunogenicity analyses at the cut off date for the extended safety and immunogenicity analyses (March 15, 2021; B). A subset (n=538) of participants in the extended safety and immunogenicity analyses were in the immunogenicity subcohort, allocated randomly to placebo (n=267) or Ad5-nCoV (n=271). All were included in the immunogenicity analysis. The extended immunogenicity results will be presented at a later date. *Second study identification number for double vaccinated participants are individuals who, unknown to study staff, enrolled in the study twice and were randomly assigned twice and received two study injections. In the intention-to-treat analysis, they were analysed according to the first randomisation allocation. †The unknown vaccine arose because two participants were randomised with the same dispensing code, and it is unclear which vaccine they received.