Abstract
Periodic activity of the anaphase-promoting complex (APC) ubiquitin ligase determines progression through multiple cell cycle transitions by targeting cell cycle regulators for destruction. At the G1/S transition, phosphorylation-dependent dissociation of the Cdh1-activating subunit inhibits the APC, allowing stabilization of proteins required for subsequent cell cycle progression. Cyclin-dependent kinases (CDKs) that initiate and maintain Cdh1 phosphorylation have been identified. However, the issue of which cyclin-CDK complexes are involved has been a matter of debate, and the mechanism of how cyclin-CDKs interact with APC subunits remains unresolved. Here we substantiate the evidence that mammalian cyclin A-Cdk2 prevents unscheduled APC reactivation during S phase by demonstrating its periodic interaction with Cdh1 at the level of endogenous proteins. Moreover, we identified a conserved cyclin-binding motif within the Cdh1 WD-40 domain and show that its disruption abolished the Cdh1–cyclin A-Cdk2 interaction, eliminated Cdh1-associated histone H1 kinase activity, and impaired Cdh1 phosphorylation by cyclin A-Cdk2 in vitro and in vivo. Overexpression of cyclin binding-deficient Cdh1 stabilized the APC-Cdh1 interaction and induced prolonged cell cycle arrest at the G1/S transition. Conversely, cyclin binding-deficient Cdh1 lost its capability to support APC-dependent proteolysis of cyclin A but not that of other APC substrates such as cyclin B and securin Pds1. Collectively, these data provide a mechanistic explanation for the mutual functional interplay between cyclin A-Cdk2 and APC-Cdh1 and the first evidence that Cdh1 may activate the APC by binding specific substrates.
Proteins are marked for rapid destruction in the 26S proteasome by covalent attachment of polyubiquitin chains, a reaction catalyzed by an enzymatic cascade culminating on E3 ubiquitin ligases (10, 18). The anaphase-promoting complex (APC) ubiquitin ligase had been originally isolated as a component required for ubiquitination and degradation of B-type cyclins and later was identified as an essential factor promoting separation of the replicated chromosomes to daughter cells during anaphase (20, 22, 56). More-recent data from diverse experimental models provide compelling evidence for another important role for APC-regulated proteolysis besides its crucial functions in mitosis, namely, its involvement in imposing and maintaining the physiological length of the G1 phase (19, 27, 51, 55, 59). As such, APC represents a crucial cellular activity operating at multiple cell cycle transitions, which ensures error-free distribution of the genetic material between successive generations of eukaryotic cells.
To achieve its essential goals, APC activity during the cell cycle is highly periodic and subjected to a tight control by a combination of regulatory events such as phosphorylation, functional sequestration by kinetochore-associated checkpoint proteins, and recruitment of activating subunits to the APC core (39, 43, 66). The last regulatory mode has recently attracted much attention thanks to the isolation of Cdc20 (Slp1; Fizzy) and Cdh1 (Srw1/Ste9; Fizzy-related), two related but functionally distinct APC-activating subunits (14, 23, 37, 51, 54, 59, 64). Essential mitotic functions, such as initiation of sister chromatid separation and timing of exit from mitosis, are executed by the APC coupled to Cdc20 (11, 12, 53). The molecular mechanism which restricts formation of active APC-Cdc20 to mitosis reflects the requirement for phosphorylation of the APC structural subunits by mitotic kinases, in order to recruit Cdc20 and form an active ubiquitin ligase (24, 52). Although Cdc20 is quantitatively degraded at the end of mitosis, APC remains highly active throughout most of the G1 phase (7, 14, 47, 61). This is enabled by its assembly with another activating subunit, Cdh1, which unlike Cdc20 binds and activates both mitotic and interphase APC (14, 21, 23, 24, 67). APC activity is cancelled only at the G1/S transition due to Cdh1 phosphorylation and its abrupt dissociation from the APC core (5, 21, 23, 24, 31, 65, 67).
Inactivation of APC in early S phase represents an important switch in coordinating the subsequent cell cycle progression. Stabilization of proteins sensitive to APC-mediated proteolysis such as Cdc6 and Dbf4 contributes to the control of the formation of licensed origins of DNA replication and their subsequent firing, respectively (9, 15, 42, 45, 60). At the same time, it has been suggested that stabilization of geminin, another APC substrate, restricts initiation of the origins of DNA replication to only once within the same cell division cycle (38). Moreover, absence of APC activity during S and G2 phases allows accumulation of Polo-like kinase, B-type cyclins, securins, CENP-F/mitosin and Kid microtubule motors, and aurora-like kinases, proteins vital for productive cell division as well as establishment of a functional mitotic spindle checkpoint (3, 16, 39, 40, 55, 66). Finally, a period of inactive APC in interphase allows accumulation of Cdc20, a key prerequisite for APC reactivation in the subsequent mitosis (46, 47, 55). For all these reasons, mechanisms which inhibit APC at the G1/S transition and guard against its unscheduled reassociation with Cdh1 during S and G2 phases appear to be critically important to coordinate DNA replication with cell division. Indeed, forced reactivation of APC-Cdh1 in post-G1 mammalian cells and the resulting lack of APC periodicity induce endoreplication and nuclear abnormalities and ultimately result in cell death (55).
Evidence from yeast (Saccharomyces cerervisiae and Schizosaccharomyces pombe), Drosophila melanogaster, and vertebrate models indicates that cyclin-dependent kinase (CDK)-dependent phosphorylation of Cdh1 is important for regulation of APC-Cdh1 assembly and its periodic activity during the cell cycle (7, 21, 23, 24, 29, 31, 66). Thus, the binding of Cdh1 to the APC core upon exit from mitosis requires inhibition of mitotic CDKs and, at least in budding yeast, activation of the Cdc14 phosphatase (21, 58). Recent data obtained in the mammalian cell-free system suggest that in higher eukaryotes CDK-mediated regulation of APC-dependent proteolysis also operates in concert with a phosphatase activity specifically present in G1 cells (4). Cdh1 becomes phosphorylated on CDK consensus S/T-P sites during S phase, and this phosphorylation tightly correlates with a period of inactive APC (5, 21, 23, 24, 31, 65, 67). Importantly, replacement of the CDK phospho-acceptor residues of Cdh1 by alanines, but not by potentially phospho-mimicking aspartic acid residues, supports high APC activity and enables productive APC-Cdh1 assembly even in the presence of high CDK activity (24, 31). Finally, recent data suggest that CDK-mediated phosphorylation of Cdh1 accelerates its protein turnover, a mechanism that would further lower the risk of unscheduled reactivation of the APC (5, 65). Despite all this progress, several important issues, such as which cyclin-CDK complexes are involved in APC-Cdh1 inactivation and what the mechanistic requirements for Cdh1 recognition by cyclin-CDK complexes are remain unresolved. Thus, while there is strong genetic evidence from Drosophila for cyclin E being the major Cdh1 kinase (54), data from mammalian cells repeatedly indicated little or no effect of cyclin E-Cdk2 activity on the modulation of APC-Cdh1 assembly or the kinetics of stabilization of the APC substrates (7, 31). Instead, ectopically expressed human Cdh1 physically interacted with cyclin A but not cyclin E, suggesting that during S phase Cdh1 might be negatively regulated primarily by cyclin A and its associated CDKs (31). However, whether cyclin A-Cdk2 forms stable complexes also with endogenous Cdh1 and which component(s) of the cyclin A holoenzyme mediates such an interaction remain unclear. Moreover, whether the reported Cdh1-cyclin A interaction serves solely to regulate Cdh1 by phosphorylation or whether it also mediates substrate recognition of the APC-Cdh1 ubiquitin ligase has not been clarified. Stimulated by these open questions, we studied the mechanistic requirements for physical and functional interaction between Cdh1 and cyclin-CDK complexes. Our new data obtained by analysis of endogenous proteins in untransformed fibroblasts strengthen the evidence for mammalian cyclin A-Cdk2 playing an important role in preventing APC-Cdh1 reactivation in post-G1 cells. Moreover, we provide evidence for a cyclin A binding motif within the WD repeat domain of Cdh1 and demonstrate its requirement for both dissociation of Cdh1 from the APC core and for ubiquitination of cyclin A by the APC-Cdh1 holoenzyme.
MATERIALS AND METHODS
Plasmids, mutagenesis, and gene transfer.
Human Cdh1 cDNA (GenBank accession no. AF080397) and the phosphorylation-deficient Cdh1 4xA mutant (31) were tagged on the amino terminus with a myc epitope and subcloned either into the pX expression plasmid or into the pBI tetracycline-responsive plasmid (Clontech). Truncated versions of the human Cdh1 were produced by a PCR-based strategy; intragenic deletion of the RVL amino acids within the cyclin-binding domain (Cdh1 ΔRVL) and/or their replacement with alanines (Cdh1 RVL-AAA) were generated using the Quick Change method (Stratagene) according to the manufacturer's instructions. The pCMV-CD20 expression plasmid was described elsewhere (35). Calcium phosphate transfection and electroporation were performed as reported previously (33, 35).
Cell culture.
R12, a derivative of Rat-1 diploid fibroblasts, U-2-OS, a human osteosarcoma cell line, U-2-OS-TA, containing the tetracycline-responsive transcriptional activator, and U-2-OS/Cdh1, conditionally expressing myc-tagged wild-type Cdh1 in a tetracycline-dependent manner were characterized in detail elsewhere (55). Cells capable of conditional expression of the Cdh1 RVL-AAA mutant were generated by transient transfection of the U-2-OS-TA cells with the pBI plasmid encoding this Cdh1 mutant while culturing in the presence of tetracycline. Induction of myc-Cdh1, either the wild type or its cyclin binding-deficient derivative, by removal of tetracycline was performed according to procedures previously published (36). Synchronization of cells in metaphase was achieved by incubating the cells in the presence of nocodazole (40 ng/ml). Productively transfected cells expressing the CD20 surface were isolated using the anti-CD20-coated Dynabeads and a DYNAL magnetic particle concentrator according to the protocol of the manufacturer (Dynal A.S.). Proteasome inhibitor N-acetyl-Leu-Leu-norleucinal (LLnL; 25 μM) was in some cases added to the culture medium for the times specified in figure legends.
Immunochemical techniques.
Rabbit polyclonal antibodies used in this study were SC-751 (Santa Cruz Biotechnology) to human cyclin A and SC-163 (Santa Cruz Biotechnology) to human Cdk2. Rabbit sera for Cdc27 and Cdh1 (Sat-105) and Pds1 were described (17, 23, 55). Mouse monoclonal antibodies were as follows: SC-245 (Santa Cruz Biotechnology) to cyclin B1, CD20 and CD20FITC (Becton Dickinson) to the CD20 cell surface marker, 9E10 to the myc epitope (gift from G. Evan), DCS-141 to Mcm7 (55), MO-1 to Cdk7 (57), and SC-9972 (Santa Cruz Biotechnology) and C40920 (Transduction Laboratories) to Cdc27. DCS-266 against the full-length human Cdh1 was raised and characterized in our laboratory (Fig. 1A) (Sørensen et al., unpublished data) according to the established hybridoma technology (34). Immunoprecipitation, immunoblotting, and immunocytochemical techniques including detection of bromodeoxyuridine (BrdU) incorporation into newly synthesized DNA were described earlier (32, 35). Immunodepletion of cyclin A and Cdk2 from the cell lysates was achieved by three consecutive rounds of immunoprecipitation with Sepharose beads coated with the respective antibodies. The amount of protein in the resulting lysates was adjusted to that of the control sample depleted with a nonimmune antibody. In vitro kinase assays were performed essentially as described previously (32) with the exception that histone H1 (2 μg per reaction) or purified glutathione S-transferase (GST)-Cdh1 fusion proteins (6 μg per reaction) were used as substrates.
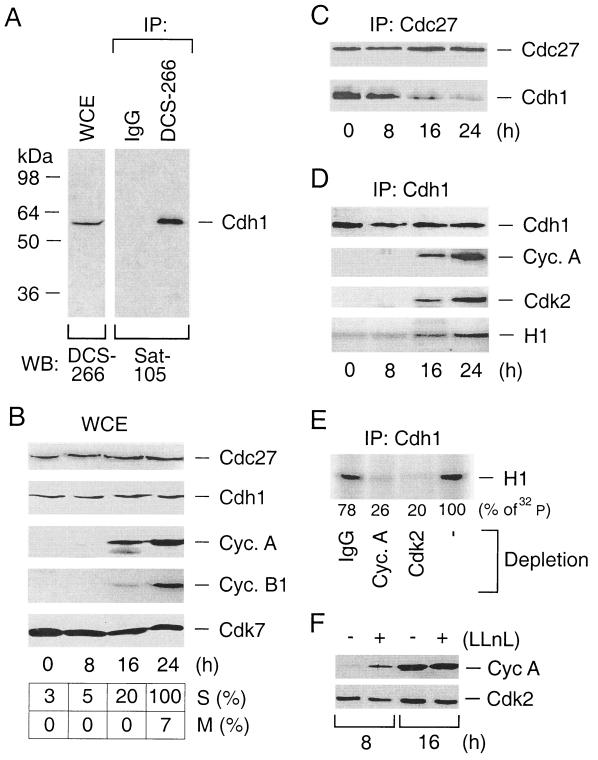
FIG. 1.
Endogenous Cdh1 redistributes from the APC to active cyclin A-Cdk2 complexes during the cell cycle. (A) Characterization of the DCS-266 mouse monoclonal antibody to Cdh1. Whole-cell extracts (WCE) prepared from R12 cells were either directly immunoblotted with DCS-266 (left) or immunoprecipitated (IP) with DCS-266 (right). DCS-266 immunoprecipitates were subjected to Western blotting analysis by Sat-105, an affinity-purified rabbit polyclonal antibody to Cdh1. IgG, immunoglobulin G. (B) R12 cells were starved for 48 h by incubating them in a mitogen-free medium and then induced to reenter the cell cycle by addition of FCS. At the indicated time points, the kinetics of cell cycle progression were measured by scoring BrdU-positive cells as an indication of productive entry into S phase (S) and by counting cells with condensed chromosomes as an indication of entry into mitosis (M). Expression profiles of Cdc27, Cdh1, cyclin A, cyclin B1, and Cdk7 (loading control) were assessed by Western blotting analysis of the WCE. (C) R12 cells were synchronized as for panel B. The APC was immunoprecipitated by an antibody to its Cdc27 structural subunit and analyzed for the presence of the Cdh1-activating subunit by Western blotting with Cdh1-specific antibody DCS-266. (D) R12 cells were synchronized as for panel B. Cdh1 complexes immunopurified by the DCS-266 antibody were analyzed for the presence of cyclin A and Cdk2 by Western blotting and for the associated kinase activity by an in vitro kinase assay using histone H1 as a substrate. (E) R12 cells were synchronized in early S phase by mitogen depletion for 48 h and subsequent restimulation by addition of FCS for 16 h. Cell extracts were immunodepleted with antibodies to cyclin A, Cdk2, or control IgG as indicated, and Cdh1 immunoprecipitates were subjected to an in vitro kinase reaction as for panel D. (F) R12 cells were starved as for panel B and stimulated for either 8 or 16 h by FCS to allow progression into G1 and early S phases, respectively. For the last 2 h, at each time point, the culture medium was supplied with LLnL. The cell extracts were analyzed for the abundance of cyclin A and Cdk2 (here serves as a loading control) by Western blotting.
Flow cytometry.
DNA distribution of the productively transfected cells was analyzed essentially as described previously (35). Briefly, cells were harvested with phosphate-buffered saline (PBS) containing EDTA (0.1%), immunostained with CD20FITC antibody, and fixed in 70% ice-cold methanol for 20 min. Subsequently, DNA was labeled by incubating the cells for 30 min at 37°C in a propidium iodide (PI) buffer (10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 50 μg of PI/ml, 10 μg of RNase A/ml). The stained cells were acquired and sorted by the FACSCalibur flow cytometer (Becton Dickinson), and the DNA content was analyzed using CellQuest software.
Expression and purification of the GST fusion proteins.
For expression in bacteria, wild-type Cdh1 and Cdh1 ΔRVL cDNAs were cloned in frame with the gene for GST using the GST-2TK plasmid (Pharmacia Upjohn). The fusion proteins were expressed in Escherichia coli, bound to glutathione-Sepharose, and eluted with reduced glutathione according to standard procedures (Pharmacia Upjohn). The purity of the eluted proteins was evaluated by the Coomassie staining of sodium dodecyl sulfate (SDS)-polyacrylamide gels.
Metabolic labeling and two-dimensional phosphopeptide mapping.
Exponentially growing U-2-OS cells were transfected with expression plasmids containing myc-tagged wild-type or cyclin binding-deficient versions of Cdh1 as specified in the figure legends and subsequently labeled for 3 h in phosphate-free medium supplemented with 20 mM HEPES, pH 7.2–10% dialyzed phosphate-free fetal calf serum (FCS)–2 mCi of [32P]orthophosphate (PBS43; Amersham)/ml. Ectopically expressed Cdh1 was immunoprecipitated with the antimyc 9E10 antibody followed by SDS-gel electrophoresis and protein transfer to a nitrocellulose membrane. The bands corresponding to Cdh1 were cut out and processed for tryptic digestion and two-dimensional phosphopeptide mapping as described by Boyle at al. (6).
In vitro ubiquitination assay.
A cyclin B fragment (amino acids 13 to 110) from sea urchins and full-length human cyclin A were radiolabeled as described previously (23). To obtain highly pure Cdh1-activated APC, the inactive APC core from interphase Xenopus laevis egg extracts was purified. Under such conditions, APC is not phosphorylated and thus not bound to Cdc20. Because Xenopus eggs do not contain any endogenous Cdh1, such APCs could be specifically activated by human Cdh1 prepared by coupled transcription-translation reactions in rabbit reticulocyte lysate (Promega). The in vitro ubiquitination reaction was performed essentially as described previously (23, 24). Samples were analyzed by SDS–5 to 15% polyacrylamide gel electrophoresis (PAGE) and phosphorimaging.
RESULTS
Endogenous Cdh1 periodically interacts with active cyclin A-Cdk2 in rat diploid fibroblasts.
We have previously demonstrated that human Cdh1 efficiently binds cyclin A when overexpressed in a human osteosarcoma cell line and proposed that this interaction represents an important part of the cellular mechanism(s) preventing APC-Cdh1 reassembly during S phase (31). To validate this prediction in an experimental system unbiased by potential side effects associated with protein overexpression, we studied whether Cdh1-cyclin A complexes could also be detected on the level of endogenous proteins and whether such an interaction is modulated during the cell cycle. We chose to perform this analysis with untransformed rat diploid fibroblasts (R12) synchronized by mitogen depletion, which allowed us to exclude the possibility that the previously observed Cdh1-cyclin A interaction was either specific for human tumor cells or influenced by drug-mediated synchronization protocols used in earlier studies. To minimize adverse consequences of antibody cross-reactions, we also generated a series of mouse monoclonal antibodies to Cdh1. One of these new reagents, DCS-266, recognized only one band when total cell lysates from human, mouse, and rat cells were analyzed by immunoblotting (Fig. 1A) (Sørensen et al., unpublished data), corresponding precisely to the predicted size of the Cdh1 protein (56 kDa). A single band of the same size was also detected when DCS-266 immunoprecipitates were probed by Sat-105, an independent and extensively characterized rabbit polyclonal antibody to Cdh1 (23). Together with the ability of DCS-266 to detect in vitro-translated and/or recombinant Cdh1 proteins (Sørensen et al., unpublished data), these data confirm that DCS-266 recognizes authentic Cdh1.
Upon stimulation by FCS, R12 cells reentered the cell cycle, as measured by increasing incorporation of BrdU into newly synthesized DNA and the gradual appearance of mitotic cells at later time points (Fig. 1B). Consistent with synchronous cell cycle progression, the cyclin A protein sharply increased at the G1/S transition, and cyclin B began to accumulate later during S phase (Fig. 1B). Both Cdc27, a structural component of the APC, and Cdh1 proteins were easily detectable already in starved R12 cells, and their levels did not change during the subsequent cell cycle progression, consistent with our earlier reports on cell cycle regulation of Cdc27 (24, 31) but different from the partial reduction of Cdh1 level during S phase that we previously observed. This discrepancy may be attributable to different synchronization methods, as the drug-based synchronization protocols used in all previous studies inevitably induce cell cycle checkpoints, thereby possibly affecting the protein stability of the APC-activating subunits. Importantly, in rat diploid cells synchronized by mitogen depletion and unbiased by any drug treatment, the ability of Cdh1 to interact with other proteins underwent dramatic changes. Thus, as the cells traversed from G1 to S phase, Cdh1 was progressively lost from Cdc27 immunocomplexes, indicating its dissociation from the APC (Fig. 1C). Cdh1 detachment from the APC coincided with Cdh1 appearance within the cyclin A-Cdk2 complexes and increased Cdh1-associated histone H1 kinase activity (Fig. 1D). To test directly whether the latter effect was indeed generated due to Cdh1 interaction with cyclin A-Cdk2, we immunodepleted cyclin A or Cdk2 from the cell lysates and reexamined the Cdh1-associated H1 kinase activity. Indeed, quantitative depletion of either cyclin A or Cdk2 removed the bulk of the kinase activity associated with Cdh1 and reduced the phosphate incorporation into the H1 substrate to very similar extents (Fig. 1E). Apart from cyclin A, we were unable to detect cyclins (D1, E, or B1) within the endogenous Cdh1 complexes immunopurified from different cell types (31; Sørensen et al., unpublished data). Likewise, affinity chromatography experiments revealed that in vitro-translated Cdh1 could be retained on columns with immobilized cyclin A but not cyclin E (Sørensen et al., unpublished data). Collectively, these data indicate that, in diploid mammalian cells, endogenous Cdh1 protein binds active cyclin A-Cdk2 and that the appearance of this association during unperturbed cell cycle progression coincides with dissociation of Cdh1 from the APC. Such a redistribution of Cdh1 may reflect an important switch between G1 phase, when a low rate of cyclin A synthesis allows its productive degradation by the APC, and S phase, when an abundant cyclin A inhibits APC reactivation, an issue explored in detail by subsequent experiments. The fact that a basal synthesis of cyclin A in early G1 phase is indeed actively counterbalanced by an ongoing proteolysis is documented by our observation that inhibition of the proteasome substantially increased the cyclin A protein level in early G1 cells but had no effect once the cells progressed beyond the G1/S transition (Fig. 1F).
Cyclin A interaction domain resides in the C-terminal part of Cdh1.
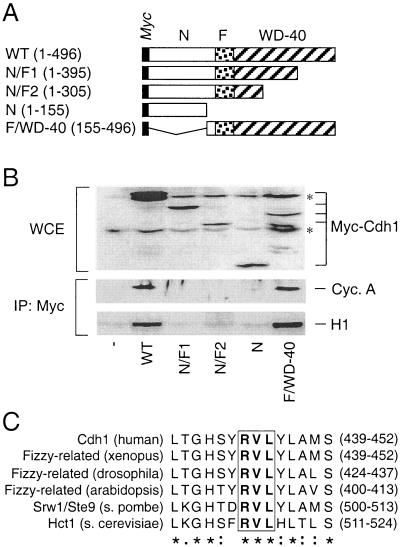
To gain a deeper insight into the requirements for the Cdh1-cyclin A interaction, we produced a series of truncation mutants in which the major Cdh1 structural domains were systematically disrupted (Fig. 2A). As illustrated, the WD-40 repeat-containing domain (amino acids 225 to 480) is localized in the C-terminal part of the molecule and was proposed to be involved in mediating the interaction of the APC-activating subunits with their regulatory proteins and/or APC substrates (66). The intramolecular Fizzy domain (amino acids 174 to 223) is highly conserved in both Cdh1 and Cdc20 and could be involved in docking these activating subunits to the APC core. Finally, the less-conserved N-terminal part of Cdh1 (amino acids 1 to 173) contains the majority of the conserved CDK phosphorylation sites and as such likely represents a Cdh1 regulatory domain. While all the truncated forms of Cdh1 were expressed upon transient transfection in human U-2-OS cells, only those containing the intact C terminus were able to coimmunoprecipitate with endogenous cyclin A and a significant histone H1 kinase activity (Fig. 2B). We concluded that both the N-terminal and the Fizzy domains are dispensable for Cdh1 association with active cyclin A-Cdk2 and that this potentially important interaction site localizes to the C-terminal part of the protein. Inspection of the C-terminal WD-rich domain revealed a putative cyclin-binding motif containing a core RxL amino acid sequence (x stands for any amino acid [V for Cdh1]) previously identified in other cell cycle regulators as an essential element to mediate their phosphorylation by cyclin-CDK complexes (1, 2, 8, 49, 68). The amino acid sequence including and surrounding the identified “RVL” motif is highly evolutionarily conserved in all known Cdh1 orthologs (Fig. 2C).
FIG. 2.
Cdh1 interaction with cyclin A is mediated by the C-terminal WD-40 repeat domain containing a conserved cyclin-binding motif. (A) Schematic representation of the myc-tagged Cdh1 truncation mutants. WT, wild type; N, N terminus; F, Fizzy domain; WD-40, the C-terminal domain containing multiple WD-40 repeats (the numbers in parentheses indicate the ranges of amino acids). (B) U-2-OS cells were transiently transfected with expression plasmids coding for the indicated myc-tagged deletion mutants. Control cells (−) were transfected with the empty pXmyc plasmid. Expression of the deletion mutants was verified by Western blotting the whole-cell extracts (WCE) prepared from the transfected cells (asterisks, nonspecific bands). Thirty-six hours after transfection, the cell lysates were subjected to immunoprecipitation (IP) with an antimyc antibody, and the purified immunocomplexes were either analyzed by Western blotting to detect associated cyclin A or subjected to an in vitro kinase assay with histone H1 as a substrate. (C) Identification of the cyclin-binding domain within the Cdh1 WD-40 repeat domain in diverse organisms. The degree of amino acid conservancy within the selected region is indicated; the core RVL sequence is boxed and in boldface. Identical (asterisks), conserved (colons), and semiconserved (dots) amino acids are indicated.
Disruption of the cyclin-binding motif prevents recognition and phosphorylation of Cdh1 by cyclin A-Cdk2.
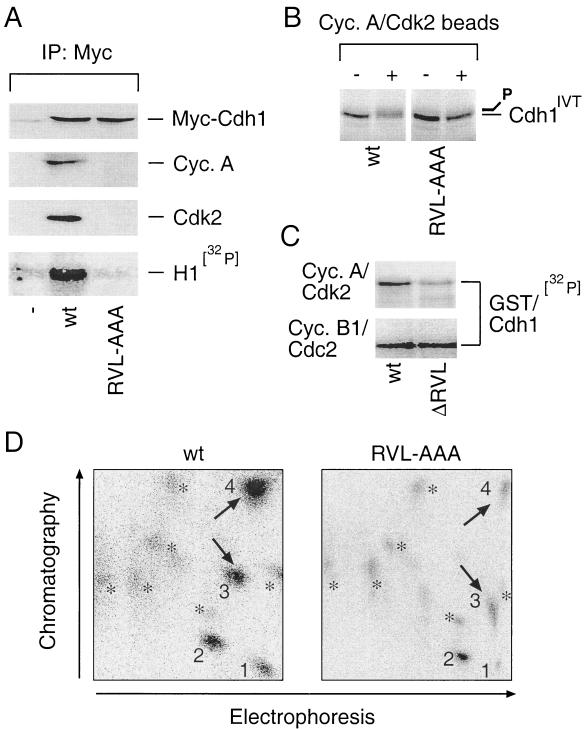
When transiently expressed in U-2-OS cells, a Cdh1 mutant where the RVL amino acids were converted to alanines (RVL-AAA) completely lost the ability to interact with endogenous cyclin A and Cdk2 (Fig. 3A). Likewise, the RVL-deficient Cdh1 was largely impaired in association with histone H1 kinase activity despite a protein level comparable to that of the wild-type Cdh1 generated by the same expression plasmid (Fig. 3A). Immunopurified cyclin A-Cdk2 phosphorylated in vitro-translated wild-type Cdh1, causing its shift on the SDS-PAGE gel, but was much less efficient in modification of the RVL-deficient Cdh1 mutant assayed under identical conditions (Fig. 3B). Likewise, the ability of cyclin A-Cdk2 to phosphorylate the bacterially purified cyclin-binding-deficient GST-Cdh1 fusion protein (ΔRVL) was reproducibly three- to fivefold lower than that of the wild-type GST-Cdh1 (Fig. 3C). However, the two forms of GST-Cdh1 were equally well phosphorylated by immunopurified cyclin B1-Cdc2, consistent with our conclusions that the Cdh1 cyclin-binding motif interacts specifically with cyclin A-Cdk2.
FIG. 3.
Integrity of the cyclin-binding domain is required for Cdh1 recognition and phosphorylation by cyclin A-Cdk2. (A) U-2-OS cells were transfected with the pXmyc expression plasmids containing either no insert (−) or the wild-type (wt) and the cyclin binding-deficient (RVL-AAA) forms of Cdh1. After 36 h cells were lysed and the myc immunocomplexes were analyzed for the presence of myc-Cdh1, cyclin A, and Cdk2 with the indicated antibodies and for associated histone H1-kinase activity as described for Fig. 2B. IP, immunoprecipitation. (B) Wild-type or RVL-AAA forms of Cdh1 translated in vitro were used as substrates in an in vitro kinase reaction mixture supplemented by Sepharose beads with active cyclin A-Cdk2 complexes immunopurified from U-2-OS cell extract (+). Beads coated with nonimmune rabbit immunoglobulin were preincubated in lysates from U-2-OS cells and added into the control reactions as indicated (−). Productive phosphorylation of wild-type (but not RVL-AAA) Cdh1 is manifested as a smear and retarded mobility after the kinase reaction was resolved by SDS-PAGE. (C) An in vitro kinase reaction with immunoprecipitated cyclin A-Cdk2 and cyclin B1-Cdc2 was performed essentially as for panel C except that the bacterially purified GST-tagged wild-type and ΔRVL forms of Cdh1 were used as substrates and the reaction mixture was supplemented with [γ-32P]ATP (ΔRVL indicates that the RVL amino acids representing the core of the Cdh1 cyclin-binding domain were deleted). (D) Tryptic phosphopeptide maps of 32P-labeled wild-type (wt) and cyclin binding-deficient (RVL-AAA) myc-tagged Cdh1 proteins transiently expressed in U-2-OS cells and immunoprecipitated with 9E10 antibody. 1 to 4, prominent, strongly labeled phosphopeptides; asterisks, weakly labeled spots; arrows, phosphopeptides most sensitive to cyclin-binding motif disruption.
To assess the importance of the cyclin-binding motif for Cdh1 phosphorylation in vivo, we labeled transiently transfected U-2-OS cells with [32P]orthophosphate and analyzed the pattern of phosphorylated peptides obtained by tryptic digestion of immunopurified wild-type and RVL-deficient Cdh1 proteins, respectively. Two-dimensional mapping of wild-type Cdh1 resolved four major phosphopeptides and several additional weakly labeled spots (Fig. 3D). Analysis of the RVL-AAA mutant revealed that, while phosphopeptide 2 as well as the majority of the weakly labeled spots remained unchanged, phosphate incorporation into peptides 1, 3, and 4 was reduced compared to that for the wild-type protein (Fig. 3D). Thus, disruption of the cyclin-binding motif impaired Cdh1 phosphorylation to an extent which could not be compensated for by other cellular kinases. Persisting phosphorylation of peptide 2 and all the weakly labeled spots found in the maps of the mutant protein could be mediated by cyclin B-Cdc2, consistent with our observation demonstrating that mutation of the Cdh1 cyclin-binding domain does not impair its phosphorylation by this mitosis-specific kinase complex (Fig. 3C). Additional experiments revealed that peptides 3 and 4 could be efficiently phosphorylated by purified cyclin A-Cdk2 in vitro (Sørensen et al., unpublished data), consistent with the interpretation that these phosphopeptides, both sensitive to the disruption of the cyclin-binding motif (Fig. 3D), contain authentic CDK recognition sites. These findings, taken together, indicate that the cyclin-binding motif within the WD-40 region of Cdh1 appeared necessary to mediate its productive interaction with and phosphorylation by cyclin A-Cdk2 both in vitro and in vivo.
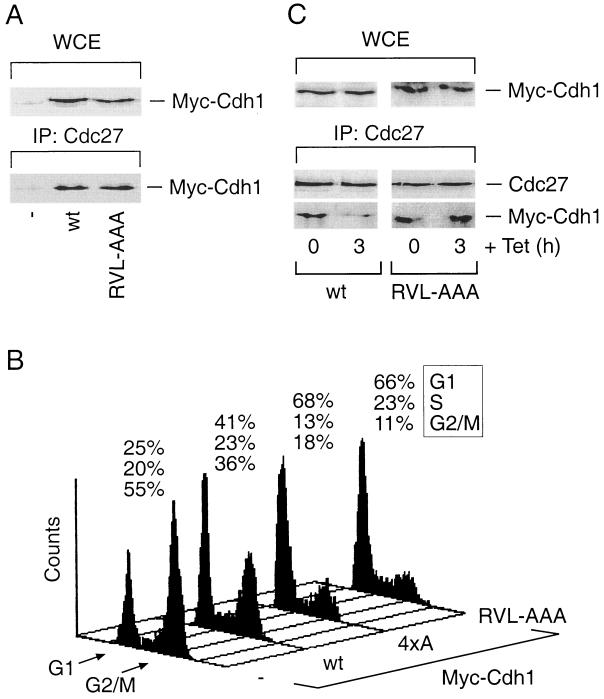
Uncoupling of Cdh1 from binding to cyclin A-Cdk2 stabilizes its interaction with APC and induces prolonged delay at the G1/S transition.
Next we asked whether disruption of the Cdh1-cyclin A interaction would influence the transition between G1 and S phases, when Cdh1 normally dissociates from the APC and enters cyclin A-Cdk2 complexes (Fig. 1). First, we tested whether the integrity of the cyclin-binding motif has any influence on APC-Cdh1 assembly in order to verify that any potential effect of the cyclin binding-deficient Cdh1 on cell cycle progression could be, in principle, explained by modulating the function of the active APC ubiquitin ligase. Unlike its profound effect on the cyclin A interaction, disruption of the cyclin-binding motif did not interfere with the capability of Cdh1 to assemble with the endogenous APC and to be readily recruited into Cdc27 immunocomplexes indistinguishable from those with the wild-type protein (Fig. 4A). To measure the impact of the cyclin-binding-deficient Cdh1 on the kinetics of G1/S transition, we designed an assay based on our previous finding that conditional elevation of wild-type Cdh1 delayed S-phase entry in mammalian cells (55), an observation consistent with the role of APC in modulating initiation of DNA replication (see the introduction). We therefore transiently transfected U-2-OS cells with expression plasmids encoding either the wild-type protein or selected Cdh1 mutants and supplemented the culture medium with nocodazole in order to inhibit cell division. Such treatment allowed us to assess the impact of the transiently expressed Cdh1 proteins on the kinetics of G1/S transition within one cell cycle. While more than 50% of the control-transfected cells effectively exited G1 phase and accumulated in mitosis, expression of wild-type Cdh1 significantly, albeit not completely, delayed the G1/S transition, reducing the percentage of cells reaching mitosis to about 35% in several independent experiments (Fig. 4B) (Sørensen et al., unpublished data). Expression of the 4xA mutant, where the four conserved CDK consensus serine/threonine residues within the Cdh1 N terminus were changed to alanines (31), further reduced the accumulation of cells in M phase (Fig. 4B), consistent with our recent report showing that an independently constructed phosphorylation-deficient Cdh1 allele gained the ability to arrest human cells in G1 (24). Importantly, cells expressing the RVL-AAA version of Cdh1 responded very similarly to those expressing the 4xA mutant in that both were impaired in progression through the cell cycle due to a robust arrest at the G1/S transition (Fig. 4B). A Cdh1 allele where the 4xA and RVL-AAA mutations were combined did not further increase the G1-arresting potential (Sørensen et al., unpublished data). Thus, the functional impact of interfering with Cdh1's ability to bind cyclin A-Cdk2 was virtually indistinguishable from that obtained by mutating its major phosphorylation sites.
FIG. 4.
Disruption of the cyclin-binding motif of Cdh1 delays its dissociation from the APC and induces prolonged G1/S block. (A) U-2-OS cells were transfected as for Fig. 3B. After 36 h, cell lysates were prepared and analyzed either for the total levels of both wild-type (wt) and RVL-AAA Cdh1 proteins in whole-cell extracts (WCE) or for their presence within the Cdc27 immunocomplexes by Western blotting using the antimyc antibody. IP, immunoprecipitation. (B) U-2-OS cells were transiently transfected with the pXmyc expression plasmid containing either no insert (−) or the wild-type, cyclin binding-deficient (RVL-AAA) and phosphorylation-deficient (4xA) versions of Cdh1. In each case, the expression plasmid for the CD20 surface marker was also included. After 36 h, the culture medium was supplemented with nocodazole (40 ng/ml) for another 12 h in order to trap cells in mitosis. Productively transfected cells were sorted and analyzed for their DNA content by flow cytometry. Positions of the peaks corresponding to G1 and G2/M cells are indicated, as well as the percentages of cells in distinct cell cycle phases at the end of the assay period. (C) U-2-OS cells containing tetracycline (Tet)-regulated myc-tagged versions of the wild-type and the cyclin binding-deficient (RVL-AAA) forms of Cdh1 were induced to express the transgenes for 12 h by culturing in a Tet-free medium. When indicated, transcription of the transgenes was inhibited by readdition of Tet for another 3 h. The cell extracts were subsequently analyzed for total levels of the ectopically expressed Cdh1 proteins (WCE) and for their presence in the Cdc27 immunocomplexes by Western blotting with the antimyc antibody.
Next we tested whether the quantitative difference between the wild-type and the RVL-AAA Cdh1 alleles in modulating exit from G1 phase could be explained by their differential abilities to undergo normal redistribution at the G1/S transition. To this end we employed the U-2-OS-derived cell lines, allowing conditional expression of the wild-type Cdh1 and the RVL-AAA mutant, respectively, in a tetracycline-dependent manner (see Materials and Methods). This system allowed us to limit the time of exposure of cells to the excess of Cdh1 proteins and thus minimize saturation of cellular factors that regulate APC-Cdh1 assembly. Derepression of the Cdh1 transgenes for 12 h was sufficient to induce formation of easily detectable complexes of endogenous APC with the wild-type and the cyclin binding-deficient Cdh1 proteins, respectively (Fig. 4C). Three hours after readdition of tetracycline into the culture media, which abruptly cancelled de novo synthesis of the transgenes, a substantial amount (60 to 70%) of wild-type Cdh1 dissociated from the APC, while the RVL-AAA mutant remained assembled and readily detectable within the Cdc27 immunocomplexes (Fig. 4C). The residual amount of APC-associated wild-type Cdh1, seen also at later time points after switching off transgene expression, could possibly be explained as a consequence of the prolonged G1 arrest in a fraction of cells expressing large amounts of the ectopic Cdh1 protein. Importantly, although the extension of the time course did reveal that the RVL-AAA protein was somewhat more stable than the wild-type Cdh1 (Sørensen et al., unpublished data), during the first 3 h after tetracycline addition the total levels of the Cdh1 forms remained essentially identical (Fig. 4C). Taken together, these data indicate that the wild-type Cdh1 could be effectively processed by the cellular enzymes and eventually released from the APC, explaining its significant but only transient ability to delay exit from G1. However, disruption of the cyclin-binding motif and the resulting inability of Cdh1 to bind cyclin A-Cdk2 appeared sufficient to stabilize the APC-Cdh1 interaction, consistent with the prolonged cell cycle delay at the G1/S transition.
Disruption of the cyclin-binding motif impairs APC-Cdh1-mediated ubiquitination and destruction of cyclin A but not other APC substrates.
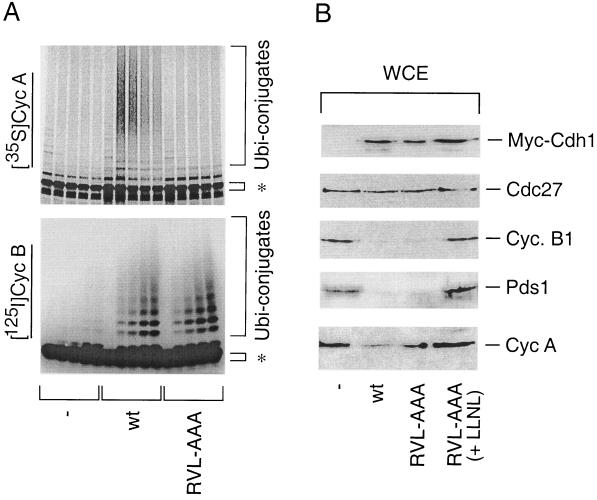
Having established the importance of the cyclin-binding motif for APC-Cdh1 dissociation at the G1/S transition, we asked whether the integrity of this Cdh1 cyclin-binding motif also determines recognition and productive ubiquitination of cyclins by APC-Cdh1, a key function executed by the APC during G1 phase (39, 66). Previous experiments already showed that cyclin binding-deficient Cdh1 could productively assemble with the APC (Fig. 4A and C). However, in a reconstituted in vitro ubiquitination assay, the cyclin binding-deficient RVL-AAA mutant completely lost its capability to promote ubiquitination of cyclin A, while its capability to modify cyclin B was indistinguishable from that of the wild-type Cdh1 protein (Fig. 5A). Consistent with the in vitro ubiquitination experiments, transient overexpression of wild-type Cdh1 triggered destruction of several established APC substrates such as cyclin B1 and securin Pds1, and to a somewhat lesser but still significant extent cyclin A (Fig. 5B). Under the same experimental conditions, the capability of the cyclin binding-deficient Cdh1 to degrade cyclin A was reproducibly reduced (Fig. 5B). This could not be a consequence of the general inability of the RVL-AAA mutant to activate the APC in vivo because its potential to induce quantitative and proteasome-dependent degradation of cyclin B1 and Pds1 was indistinguishable from that of wild-type Cdh1 (Fig. 5B). In essence, these data suggest that disruption of the cyclin-binding motif located within the WD repeat part of Cdh1 selectively undermined its ability to activate the APC toward cyclin A, while it did not impair ubiquitination and subsequent degradation of several other APC substrates, exemplified here by cyclin B and Pds1.
FIG. 5.
Integrity of the cyclin-binding motif of Cdh1 determines its ability to selectively activate productive ubiquitination and destruction of cyclin A. (A) Xenopus APC was activated with in vitro-translated wild-type or RVL-AAA versions of Cdh1. Radioactively labeled cyclin A and cyclin B were added as substrates to the ubiquitination reaction mixture as indicated. In the control reaction (−), APC was activated by wild-type Cdh1 but the reaction proceeded without addition of E2 enzymes. Samples from the ubiquitination reaction were taken at 0, 5, 10, 20, and 30 min. Asterisks, positions of unmodified radiolabeled substrates. (B) U-2-OS cells were transiently transfected with the pXmyc expression plasmid containing either no insert (−), wild-type Cdh1 (wt), or cyclin binding-deficient Cdh1 (RVL-AAA) together with the expression plasmid for a CD20 cell surface marker. After 36 h, transfected cells were sorted with anti-CD20-coated magnetic beads and analyzed for the levels of ectopically expressed Cdh1 proteins (antimyc antibody) and the indicated endogenous proteins by Western blotting. WCE, whole-cell extracts.
DISCUSSION
Our finding of a periodic interaction of endogenous Cdh1 with active cyclin A-Cdk2, which coincides with APC-Cdh1 disassembly during unperturbed cell cycle progression, provides an important and so far missing piece of evidence for Cdh1 being specifically recognized and regulated by this essential S- and G2-specific kinase complex. Identification of physiological CDK substrates and elucidating how distinct cyclin-CDK complexes recognize their targets have long been a central issue in cell cycle research. Cyclins are not only required for activation of CDKs per se but also play an essential role in mediating physical contact with the target proteins and thus determine the substrate specificity of a given cyclin-CDK complex (48). The RxL sequence has been identified as a core element of one essential motif that mediates interactions of cyclin-CDK complexes with important cell cycle regulators such as p27 and p21 CDK inhibitors (2, 8, 49), p107, p130, and pRb transcriptional repressors (1, 28, 62, 67), E2F-1 transcription factor (2), p53 tumor suppressor (30), and Cdc6 origin-binding protein (44). It has been postulated that the RxL-mediated recruitment of cyclin-CDKs can have a dual function. First, it can increase the local concentration of a substrate relative to the CDK catalytic site (50). Second, the cyclin-binding sequence provides a recognition signal allowing cyclin-CDK complexes to be themselves regulated by recruiting CDK inhibitors such as p21 and p27 (2, 8). The latter interaction can lead either to inhibition of Cdk2 bound to cyclin E and A or activation of Cdk4 and Cdk6 by promoting their assembly with the D-type cyclins. Which of these regulatory modes applies to the cyclin A–Cdk2-Cdh1 interaction reported in this study?
We report that the integrity of the cyclin-binding motif is required for timely dissociation of Cdh1 from the APC and suggest that Cdh1 belongs to the category of proteins, such as the E2F-1/DP-1 transcription factor, where the cyclin-binding motif evolved to facilitate their own specific phosphorylation required for coordinated progression through important cell cycle transitions (13, 25, 26, 63). Recruitment of Cdh1 into cyclin A-Cdk2 complexes appears to abolish its capability to bind and activate the APC and thus ensures that the APC-sensitive proteins required for cell cycle progression beyond the G1/S transition accumulate to a critical mass. Our observation that disruption of the RVL sequence impaired Cdh1 phosphorylation both in vitro and in vivo and that ectopic expression of the cyclin-binding- and/or CDK phosphorylation-deficient versions of Cdh1 had very similar impacts on cell cycle progression further supports the model that the integrity of the Cdh1 cyclin-binding motif has a vital role in phosphorylation-dependent regulation of the APC periodicity during the cell cycle.
In addition to the role of the cyclin binding motif in the timely inactivation of APC, we could demonstrate that the same interaction motif is also necessary for the APC-dependent ubiquitination of cyclin A in vitro and contributes to productive cyclin A degradation in vivo. To our knowledge, this is the first evidence that Cdh1 may activate the APC holoenzyme by binding specific substrates. The fact that the cyclin binding-deficient Cdh1 mutant retained its capability to bind the APC and promote destruction of well-established APC substrates such as cyclin B1 and Pds1 strongly argues that cyclin-binding motif-mediated substrate recognition by APC-Cdh1 is specific for cyclin A. Either other APC substrates must be recognized by a distinct region of Cdh1 or Cdh1 is not involved in their recognition at all but activates the APC through a different, yet-unknown mechanism. Although impaired in promoting cyclin A degradation, the cyclin binding-deficient Cdh1 still induced a potent G1 arrest. This observation supports the emerging view that, in addition to cyclin A, other cellular factors required for DNA replication are also regulated by APC-dependent proteolysis. Indeed, the recent report of APC-dependent destruction of Cdc6 provides the first evidence for the APC-sensitive origin-binding factor in mammalian cells (45). Lack of cyclin A ubiquitination and the reduced rate of its destruction in cells exposed to prolonged (3 to 4 days) exposure to the cyclin binding-deficient Cdh1 could, in principle, explain also the fact that these cells did not undergo substantial rereplication of the genomic DNA (Sørensen et al., unpublished data). The latter phenomenon was previously found to be a consequence of a nonperiodic expression of wild-type Cdh1, a situation where unscheduled cyclin A degradation could have impaired cellular mechanisms which normally prevent reinitiation of DNA replication within the same cell cycle (reference 55 and references therein). However, as the APC appears to regulate turnover of a larger spectrum of proteins involved in replication control (9, 15, 42, 45, 60), the differences between wild-type and cyclin binding-deficient Cdh1 in long-term impact on the cell cycle could be more complex. At this stage we cannot exclude the possibility that the propensity to endoreplicate could also reflect the difference in strength and duration of the G1 arrest elicited by the two Cdh1 forms.
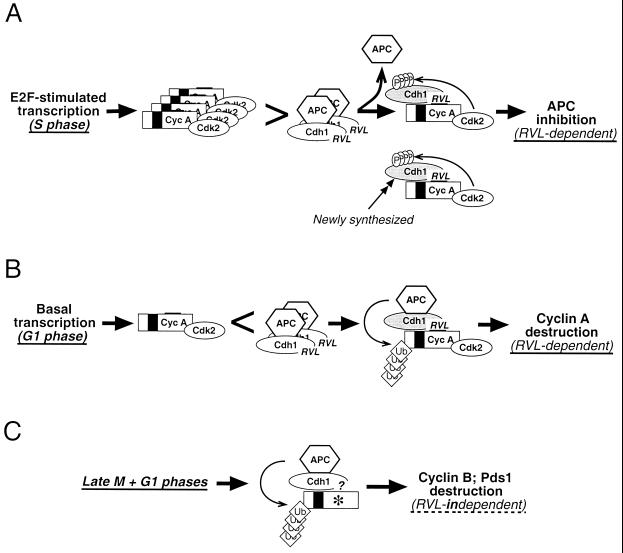
The facts that the APC could be a major source of cyclin A instability throughout most of the G1 phase on one hand and that, during S and G2 phases, cyclin A-Cdk2 prevents unscheduled reassembly with the Cdh1-activating subunit on the other hand raises an important conceptual question. How could the cyclin A protein accumulate to sufficiently high levels to compete with the APC for binding to Cdh1? In other words, what determines the switch between the upstream and downstream directions for cyclin A-Cdk2 and APC-Cdh1 enzymes at the G1/S transition? Based on the results presented in this study and our previous findings demonstrating the involvement of E2F-mediated cyclin A transcription in regulating APC-Cdh1 assembly (31) and the potential of deregulated Cdh1 expression to uncouple S-phase progression from cell division (55), we propose the following model (Fig. 6). The molecular basis for the switch between the APC and cyclin A functional interaction could be based on the burst of E2F-dependent expression of cyclin A at the G1/S transition, which overpowers the APC ubiquitin ligase potential as the rapidly emerging cyclin A-Cdk2 complexes shift the balance from being degraded by the APC toward Cdh1 phosphorylation and APC-Cdh1 disassembly. Once in molar excess (Fig. 6A), cyclin A-Cdk2 complexes may efficiently guard against precocious APC reactivation by the existing and/or newly synthesized Cdh1 until early stages of mitosis, when cyclin A becomes degraded presumably by the other form of the APC, specifically activated by the Cdc20 subunit. A conceptually similar scenario involving a positive-feedback loop between the fission yeast Cdc13/Cdc2 kinase and the APC/Srw1 ubiquitin ligase has been recently proposed as a mechanistic explanation of a point of no return at the G1/S transition when the fission yeast becomes committed to further cell cycle progression (65). During G1 phase, in contrast, basal transcription or the cyclin A mRNA persisting from the previous cycles or both support only limited synthesis of cyclin A, insufficient to reach the threshold of activity required for initiation of Cdh1 phosphorylation and its dissociation from the APC (Fig. 6B). Such conditions would support cyclin A ubiquitination and destruction, preventing its precocious accumulation and, consequently, unscheduled S-phase entry. In either case, the mutual interplay between APC-Cdh1 and cyclin A-Cdk2 would require the cyclin binding-dependent interaction between cyclin A and Cdh1 subunits. In contrast, APC-Cdh1-mediated destruction of other APC substrates such as cyclin B and Pds1 relies on a yet-unidentified motif within Cdh1 or perhaps other APC subunits (Fig. 6C). In conclusion, although our results support the central role of cyclin A in regulation of APC-Cdh1 assembly, a complete understanding of this complex and important process requires further experiments, which will have to involve highly accurate assays such as single-cell live imaging to precisely dissect the temporal relationship between accumulation of cyclins, phosphorylation of Cdh1, and stabilization of APC targets at the G1/S transition.
FIG. 6.
Schematic model for the functional interplay between APC-Cdh1 and cyclin A-Cdk2 during distinct stages of the cell cycle. (A) Derepression of E2F-dependent transcription at the G1/S transition generates an excess of cyclin A-Cdk2 over APC-Cdh1 and favors phosphorylation-dependent dissociation of the Cdh1-activating subunit from the APC and its redistribution into cyclin A-Cdk2 complexes. During subsequent S and G2 phases, the abundant and stabilized cyclin A-Cdk2 complexes guard against unscheduled APC reactivation by binding and phosphorylating the existing as well as the newly synthesized Cdh1. (B) During G1, basal transcription of the cyclin A gene and limited synthesis of the cyclin A protein do not allow the threshold needed to initiate Cdh1 phosphorylation to be reached. Such conditions result in an excess of APC-Cdh1 and create conditions permissive for ubiquitination of cyclin A and its subsequent destruction in the proteasome. The interaction of cyclin A and Cdh1 in panels A and B is dependent on the cyclin-binding motif within the Cdh1 C terminus. (C) In contrast, ubiquitination and destruction of other APC targets such as cyclin B and securin Pds1 during late M and G1 phases are independent of the RVL-containing cyclin-binding motif.
Finally, the striking conservancy of the WD-40 region containing the identified cyclin-binding motif in virtually all known Cdh1 orthologs suggests that Cdh1 phosphorylation and regulation of its assembly with the APC require a tight physical interaction with cyclins in other species as well. We noticed that a similar RVL-containing domain is also present at the C terminus of Cdc20, the mitotic activator of the APC. Indeed, it has been recently reported that cyclin A and Cdc20 interact in a yeast two-hybrid screen or when both proteins are overexpressed in mammalian cells and that this association appears to be mediated by the C-terminal Cdc20 WD-40 region (41). Although evidence for the in vivo association of the endogenous Cdc20 and cyclin A proteins is currently missing, these data are intriguing and suggest that, in addition to regulating APC-Cdh1 assembly, cyclin A can also influence the activity of APC-Cdc20 during early stages of mitosis. In addition to the APC-Cdc20 interaction, the recruitment of cyclin A-Cdk2 and/or cyclin A-Cdk1 complexes may control other regulatory steps required for precise and timely regulation of the mitotic APC such as phosphorylation of the APC structural subunits and/or interaction of APC-Cdc20 with the kinetochore checkpoint apparatus. Experiments along these lines are in progress in our laboratory.
ACKNOWLEDGMENTS
We are grateful to G. Evan, C. Gieffers, M. van Lohuizen, and S. Reed for donating diverse reagents, K. Hansen for invaluable advice with phosphopeptide mapping, and C. Lindeneg and I. Bull Olsen for excellent technical assistance.
This work was supported by grants from the Danish Cancer Society, the Human Frontier Science Program (RG-299/97), the Danish Medical Research Council (9600821), the Austrian Industrial Research Promoting Fund (FFF 3/12801), and Boehringer Ingelheim.
REFERENCES
- 1.Adams P D, Li X, Sellers W R, Baker K B, Leng X, Harper J W, Taya Y, Kaelin W G., Jr Retinoblastoma protein contains a C-terminal motif that targets it for phosphorylation by cyclin-cdk complexes. Mol Cell Biol. 1999;19:1068–1080. doi: 10.1128/mcb.19.2.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W G., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda A R, Vernos I. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell. 2000;102:425–435. doi: 10.1016/s0092-8674(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 4.Bastians H, Topper L M, Gorbsky G L, Ruderman J V. Cell cycle-regulated proteolysis of mitotic target proteins. Mol Biol Cell. 1999;10:3927–3941. doi: 10.1091/mbc.10.11.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco A M, Sanchez-Diaz A, de Prada J M, Moreno S. APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J. 2000;19:3945–3955. doi: 10.1093/emboj/19.15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 7.Brandeis M, Hunt T. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 1996;15:5280–5289. [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Saha P, Kornbluth S, Dynlacht B D, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng L, Collyer T, Hardy C F. Cell cycle regulation of DNA replication initiator factor Dbf4p. Mol Cell Biol. 1999;19:4270–4278. doi: 10.1128/mcb.19.6.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 11.Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93:1067–1076. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Fix O, Peters J M, Kirschner M W, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 13.Dynlacht B D, Flores O, Lees J A, Harlow E. Differential regulation of E2F transactivation by cyclin-CDK2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 14.Fang G, Yu H, Kirschner M W. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira F M, Santocanale C, Drury L S, Diffley J F. Dbf4p, an essential S phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol Cell Biol. 2000;20:242–248. doi: 10.1128/mcb.20.1.242-248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funabiki H, Murray A W. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 17.Gieffers C, Peters B H, Kramer E R, Dotti C G, Peters J M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA. 1999;96:11317–11322. doi: 10.1073/pnas.96.20.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochstrasser M. Protein degradation or regulation: Ub the judge. Cell. 1996;84:813–815. doi: 10.1016/s0092-8674(00)81058-2. [DOI] [PubMed] [Google Scholar]
- 19.Irniger S, Nasmyth K. The anaphase-promoting complex is required in G1 arrested yeast cells to inhibit B-type cyclin accumulation and to prevent uncontrolled entry into S-phase. J Cell Sci. 1997;110:1523–1531. doi: 10.1242/jcs.110.13.1523. [DOI] [PubMed] [Google Scholar]
- 20.Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81:269–278. doi: 10.1016/0092-8674(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 21.Jaspersen S L, Charles J F, Morgan D O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol. 1999;9:227–236. doi: 10.1016/s0960-9822(99)80111-0. [DOI] [PubMed] [Google Scholar]
- 22.King R W, Peters J M, Tugendreich S, Rolfe M, Hieter P, Kirschner M W. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- 23.Kramer E R, Gieffers C, Holzl G, Hengstschlager M, Peters J M. Activation of the human anaphase-promoting complex by proteins of the CDC20/Fizzy family. Curr Biol. 1998;8:1207–1210. doi: 10.1016/s0960-9822(07)00510-6. [DOI] [PubMed] [Google Scholar]
- 24.Kramer E R, Scheuringer N, Podtelejnikov A V, Mann M, Peters J M. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell. 2000;11:1555–1569. doi: 10.1091/mbc.11.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krek W, Ewen M E, Shirodkar S, Arany Z, Kaelin W G, Jr, Livingston D M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994;78:161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 26.Krek W, Xu G, Livingston D M. Cyclin A-kinase regulation of E2F-1 DNA binding function underlies suppression of an S phase checkpoint. Cell. 1995;83:1149–1158. doi: 10.1016/0092-8674(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 27.Kumada K, Su S, Yanagida M, Toda T. Fission yeast TPR-family protein nuc2 is required for G1-arrest upon nitrogen starvation and is an inhibitor of septum formation. J Cell Sci. 1995;108:895–905. doi: 10.1242/jcs.108.3.895. [DOI] [PubMed] [Google Scholar]
- 28.Lacy S, Whyte P. Identification of a p130 domain mediating interactions with cyclin A/cdk 2 and cyclin E/cdk 2 complexes. Oncogene. 1997;14:2395–2406. doi: 10.1038/sj.onc.1201085. [DOI] [PubMed] [Google Scholar]
- 29.Listovsky T, Zor A, Laronne A, Brandeis M. Cdk1 is essential for mammalian cyclosome/APC regulation. Exp Cell Res. 2000;255:184–191. doi: 10.1006/excr.1999.4788. [DOI] [PubMed] [Google Scholar]
- 30.Luciani G M, Hutchins J R, Zheleva D, Hupp T R. The C-terminal regulatory domain of p53 contains a functional docking site for cyclin A. J Mol Biol. 2000;300:503–518. doi: 10.1006/jmbi.2000.3830. [DOI] [PubMed] [Google Scholar]
- 31.Lukas C, Sørensen C S, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters J M, Bartek J, Lukas J. Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature. 1999;401:815–818. doi: 10.1038/44611. [DOI] [PubMed] [Google Scholar]
- 32.Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukas J, Muller H, Bartkova J, Spitkovsky D, Kjerulff A A, Jansen-Durr P, Strauss M, Bartek J. DNA tumor virus oncoproteins and retinoblastoma gene mutations share the ability to relieve the cell's requirement for cyclin D1 function in G1. J Cell Biol. 1994;125:625–638. doi: 10.1083/jcb.125.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumour cell lines. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- 35.Lukas J, Parry D, Aagaard L, Mann D J, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- 36.Lukas J, Sørensen C S, Lukas C, Santoni-Rugiu E, Bartek J. p16INK4a, but not constitutively active pRb, can impose a sustained G1 arrest: molecular mechanisms and implications for oncogenesis. Oncogene. 1999;18:3930–3935. doi: 10.1038/sj.onc.1202777. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto T. A fission yeast homolog of CDC20/p55CDC/Fizzy is required for recovery from DNA damage and genetically interacts with p34cdc2. Mol Cell Biol. 1997;17:742–750. doi: 10.1128/mcb.17.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGarry T J, Kirschner M W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 39.Morgan D O. Regulation of the APC and the exit from mitosis. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 40.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 41.Ohtoshi A, Maeda T, Higashi H, Ashizawa S, Hatakeyama M. Human p55(CDC)/Cdc20 associates with cyclin A and is phosphorylated by the cyclin A-Cdk2 complex. Biochem Biophys Res Commun. 2000;268:530–534. doi: 10.1006/bbrc.2000.2167. [DOI] [PubMed] [Google Scholar]
- 42.Oshiro G, Owens J C, Shellman Y, Sclafani R A, Li J J. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol Cell Biol. 1999;19:4888–4896. doi: 10.1128/mcb.19.7.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters J M. SCF and APC: the Yin and Yang of cell cycle regulated proteolysis. Curr Opin Cell Biol. 1998;10:759–768. doi: 10.1016/s0955-0674(98)80119-1. [DOI] [PubMed] [Google Scholar]
- 44.Petersen B O, Lukas J, Sørensen C S, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen B O, Wagener C, Marinoni F, Kramer E R, Melixetian M, Denchi E L, Gieffers C, Matteucci C, Peters J M, Helin K. Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev. 2000;14:2330–2343. doi: 10.1101/gad.832500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfleger C M, Kirschner M W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 47.Prinz S, Hwang E S, Visintin R, Amon A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr Biol. 1998;8:750–760. doi: 10.1016/s0960-9822(98)70298-2. [DOI] [PubMed] [Google Scholar]
- 48.Roberts J M. Evolving ideas about cyclins. Cell. 1999;98:129–132. doi: 10.1016/s0092-8674(00)81007-7. [DOI] [PubMed] [Google Scholar]
- 49.Russo A A, Jeffrey P D, Patten A K, Massague J, Pavletich N P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 50.Schulman B A, Lindstrom D L, Harlow E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwab M, Lutum A S, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- 52.Shteinberg M, Protopopov Y, Listovsky T, Brandeis M, Hershko A. Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem Biophys Res Commun. 1999;260:193–198. doi: 10.1006/bbrc.1999.0884. [DOI] [PubMed] [Google Scholar]
- 53.Sigrist S, Jacobs H, Stratmann R, Lehner C F. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigrist S J, Lehner C F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- 55.Sørensen C S, Lukas C, Kramer E R, Peters J M, Bartek J, Lukas J. Nonperiodic activity of the human anaphase-promoting complex–cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol Cell Biol. 2000;20:7613–7623. doi: 10.1128/mcb.20.20.7613-7623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sudakin V, Ganoth D, Dahan A, Heller H, Hershko J, Luca F C, Ruderman J V, Hershko A. The cyclosome, a large complex containing cyclin-selective ubiquitin ligase activity, targets cyclins for destruction at the end of mitosis. Mol Biol Cell. 1995;6:185–197. doi: 10.1091/mbc.6.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tassan J P, Schultz S J, Bartek J, Nigg E A. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase) J Cell Biol. 1994;127:467–478. doi: 10.1083/jcb.127.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visintin R, Craig K, Hwang E S, Prinz S, Tyers M, Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 59.Visintin R, Prinz S, Amon A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 60.Weinreich M, Stillman B. Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 1999;18:5334–5346. doi: 10.1093/emboj/18.19.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinstein J. Cell cycle-regulated expression, phosphorylation, and degradation of p55Cdc. A mammalian homolog of CDC20/Fizzy/slp1. J Biol Chem. 1997;272:28501–28511. doi: 10.1074/jbc.272.45.28501. [DOI] [PubMed] [Google Scholar]
- 62.Woo S M, Sanchez I, Dynlacht B D. p130 and p107 use a conserved domain to inhibit cellular cyclin-dependent kinase activity. Mol Cell Biol. 1997;17:3566–3579. doi: 10.1128/mcb.17.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu M, Sheppard K A, Peng C Y, Yee A S, Piwnica-Worms H. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol. 1994;14:8420–8431. doi: 10.1128/mcb.14.12.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi S, Murakami H, Okayama H. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol Biol Cell. 1997;8:2475–2486. doi: 10.1091/mbc.8.12.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaguchi S, Okayama H, Nurse P. Fission yeast Fizzy-related protein srw1p is a G(1)-specific promoter of mitotic cyclin B degradation. EMBO J. 2000;19:3968–3977. doi: 10.1093/emboj/19.15.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 67.Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282:1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]
- 68.Zhu L, Harlow E, Dynlacht B D. p107 uses a p21CIP1-related domain to bind cyclin-CDK2 and regulate interactions with E2F. Genes Dev. 1995;9:1740–1752. doi: 10.1101/gad.9.14.1740. [DOI] [PubMed] [Google Scholar]