This study shows that optical biometers based on different technologies provide repeatable measurements for different ocular parameters and, when compared, statistically significant differences between them were found when measuring most parameters.
Purpose:
To evaluate repeatability and agreement between various biometric parameters using 6 biometers based on different optical technologies.
Setting:
University of Valencia, Spain.
Design:
Prospective, comparative case series.
Methods:
150 eyes were measured using the Aladdin, AL-Scan, Argos, IOLMaster700, Lenstar LS900, and OA-2000 biometers. Keratometry (K1 and K2), J0 and J45, central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness (LT), axial length (AL), white to white (WTW), and pupil size (PS) were measured 5 times with each device. Intrasubject SD, coefficient of variability (CoV), coefficient of repeatability, intraclass correlation coefficient, and Bland -Altman graphs were analyzed.
Results:
CoV values were <0.30% for K1, K2, and AL and up to 1.61% for CCT, ACD, LT, and WTW. PS values were higher (from 4.2% to 7.68%). There was statistically significant differences between biometers for all parameters evaluated (P < .001), and these differences varied as a function of the parameter analyzed. The limit of agreement (LoA) width of some comparisons for K1 and the majority for K2 were >0.50 diopter. A similar pattern was found for J0/J45. For CCT, many comparisons showed LoA width values of >25 μm. The LoA width for ACD ranged from 0.366 mm to 0.175 mm and for LT was about 0.2 mm. AL showed a highest LoA width of 0.225 mm. The LoA width for WTW was, in most cases, about ≥0.50 mm. The LoA width for PS ranged from 1.578 mm to 3.541 mm.
Conclusions:
The 6 biometers provided repeatable measurements for the different parameters analyzed. The LoA obtained for each comparison should be analyzed carefully to consider the interchangeability of these devices.
One of the most important advances in ophthalmology and vision sciences is the measurement of ocular dimensions using noninvasive technologies. The quantitative information provided by optical devices has proven to be crucial and essential for clinical diagnosis and surgery. For example, ocular biometry is necessary for cataract and refractive surgery but also extremely useful for assessing the progression of myopia.
Since the introduction of the first-generation optical biometers using partial coherence interferometry (PCI), many publications have used this technology to properly assess various parameters for cataract surgery, including axial length (AL) or keratometry (K). Optical biometry overcomes important drawbacks compared with ultrasound biometry by increasing the accuracy of the measurements and reducing their variability.1 The advances made over the last 2 decades using different optical technologies have been enormous, converting optical biometry into the gold standard for measuring ocular biometric parameters. Research into this area has led to the development and application of various optical technologies that have improved ocular imaging and helped clinicians. Current optical biometry may be based on PCI, optical low coherence reflectometry (OLCR), optical low coherence interferometry (OLCI), and, most recently, swept-source optical coherence tomography (SS-OCT).2 It has been reported that SS-OCT has several advantages over the other technologies, such as deeper light penetration and long-range OCT imaging of posterior segment structures.3
The various optical biometers available must provide measurements of parameters needed for intraocular lens (IOL) power calculations for cataract or refractive surgery, as well as for assessing the progression of myopia. Agreement studies of the different biometers are aimed at finding whether they can be used interchangeably in clinical practice. Although there are a number of comparison studies between devices that have been conducted to investigate their repeatability and agreement using different technologies, these have principally compared just 2 devices, although in exceptional cases, they have involved 3 or 4. The purpose of this research was to assess 6 optical biometers, based on PCI, OLCR, OLCI, and SS-OCT optical technologies, to analyze their repeatability and, in particular, their agreement with regard to ocular dimensions.
METHODS
This prospective study was approved by the Ethics Committee of the University of Valencia (Valencia, Spain) and was conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients prior to their enrollment in this study.
Patients and Procedure
All the patients underwent a complete ophthalmic examination. The inclusion criteria were that the participants should be healthy and between 18 and 70 years of age. The exclusion criteria were ocular trauma, severe corneal or vitreous opacities, previous corneal surgery, retinal disease, systemic disease, glaucoma, or nystagmus. K1 (flat), K2 (steep), central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness (LT), AL, white to white (WTW), and pupil size (PS) parameters were measured 5 times consecutively, in a random order, using the 6 optical biometers, and by the same examiner, in a single session. Corneal astigmatism was converted into a vector representation: J0 and J45. Only the right eye from each patient was used for the data analysis. All the devices were calibrated prior to each measurement session.
Devices
Six optical biometers based on PCI, OLCI, OLCR, and SS-OCT were analyzed in this study: Aladdin (Topcon Corp.), AL-Scan (Nidek Co., Ltd.), Argos (Alcon Laboratories, Inc.), IOLMaster 700 (Carl Zeiss Meditec AG), Lenstar LS 900 (Haag Streit AG), and OA-2000 (Tomey Corp.).
Aladdin
The Aladdin biometer (v. 1.07) is based on OLCI, with an 820 mm superluminescent diode, for measuring AL, ACD, CCT, and LT. The K readings are calculated using 24-ring Placido disk-ring reflection at 1024 reference points in approximately 3.0 mm optical zones (from 2.8 to 3.2 mm). The WTW distance is calculated from the corneal topography. PS is determined via infrared and a white LED.
AL-Scan
This optical biometer is based on PCI and uses an 830 nm superluminescent diode to measure the AL. It uses a 970 nm LED for assessing K and PS, and a 525 nm LED for determining WTW. WTW and PS are ascertained by fitting the best circle with the lowest error square to the detected edge. K1 and K2 are obtained from an analysis of the images of 2 mires of spots over 2 areas (2.4 mm and 3.3 mm in diameter). For the purpose of this study, K values of 2.4 mm were recorded. The Scheimpflug principle is used for CCT and ACD measured with an LED of 470 nm. No LT values are obtained with this biometer.
Argos
This SS-OCT device provides a 2-dimensional OCT using 3000 A-scans/s and a wavelength of 1060 nm (20 nm bandwidth). K, using a 1.3375 corneal index of refraction, is measured from the OCT image in conjunction with a 2.2 mm diameter ring made up of 16 LEDs. The corneal diameter (CD) is measured using the OCT image; and the 1.5 version, with Alcon image guidance, also uses this reference image to compute the WTW value. CCT, ACD, LT, and AL are measured using the OCT, considering different refractive indices (cornea: 1.376; aqueous and vitreous humors: 1.336; lens: 1.410).
IOLMaster 700
This instrument is based on SS-OCT technology with a scanning rate of 2000 scans/s (a wavelength of 1055 nm, from 1035 to 1095 nm) that provides a 2-dimensional OCT scan (44 mm scan depth and 22 μm resolution in tissue). The scan informs about the CCT, ACD, LT, and AL values. A 950 nm light source is used to obtain telecentric K in 3 zones (1.5, 2.5, and 3.2 mm on an average cornea of 7.9 mm radius, using a refractive index of 1.3375). It measures PS and the horizontal WTW distance with an LED source of 800 nm.
Lenstar LS 900
The Lenstar LS 900 is based on OLCR and uses an 820 nm superluminescent diode to measure the CCT, ACD, LT, and AL. The K readings are calculated by analyzing the anterior corneal curvature at 32 reference points oriented in 2 circles in, approximately, the 2.30 mm and 1.65 mm optical zones. The WTW measurement is based on high-resolution color photography of the eye.
OA-2000
The OA-2000 is based on SS-OCT technology with a scanning rate of 1250 scans/s and a wavelength of 1060 nm to measure AL, ACD, CCT, and LT. The Placido disc, considering 9 rings (each having 256 points), is projected onto the cornea (5.5 mm) to obtain K values at different diameters (2.0, 2.5, and 3 mm, using a refractive index of 1.3375). For the purposes of this study, the 2.5 mm diameter was selected for K readings. PD and WTW were obtained from video analysis.
Statistical Analysis
Mean ± SD and range values were determined for all parameters. The data were entered into a Microsoft Excel spreadsheet (Microsoft Corp.) and statistically analyzed using SPSS software (22.0 vs, IBM Corp.). Repeatability and agreement were assessed based on standards adopted by the British Standards Institute and the International Organization for Standardization.4 Repeatability was assessed calculating within-subject SD (Sw), coefficient of repeatability (CoR), coefficient of variability (CoV), and the intraclass correlation coefficient (ICC). The CoR was expressed as a result from SD of the difference between measurements (). Thus, CoR was calculated as and can be approximated as 2.77 Sw. The CoV was calculated as the ratio between Sw and the mean value: CoV = . The ICC was evaluated as the absolute agreement through the 2-way mixed effect model, considering the examiner effect as fixed and the subject effects as random. The normality distribution was checked by the Shapiro-Wilk test. Statistically significant differences between the measurements taken using the 6 optical biometers were evaluated with repeated-measures analysis of variance. The Tukey test was used for post hoc analysis to compare the data between groups. The P value was considered statistically significant for <0.05. In addition, the agreement between the biometers was assessed using a Bland-Altman analysis. The mean difference, the CI of the mean difference at 95%, and 95% limits of agreement (LoA, calculated as the mean difference ±1.96 SD) were also ascertained.
The required sample size (n) was determined considering both repeatability and agreement. For repeatability, n was calculated considering the number of repeated measurements (m): = 0.1.5 Forty-eight eyes were required considering a 0.10% confidence in the estimate and 5-repeated measurements. For agreement, the following formula was used, where s is the SD of the differences: 1.96 = desired CI of LoA. We considered the desired CI for the LoA in our study to be 0.015 mm for the AL (primary outcome). With this value and the s value obtained in a subset of 50 eyes, the minimum n value required was 128 eyes. Then, taking into account both n values, we considered that this should be at least 128 eyes, with our target being 150 eyes.
RESULTS
In this study, a total of 150 healthy eyes from 150 patients (104 women) were evaluated. The mean age of the patients was 34.08 ± 16.52 years (ranging from 19 to 67 years). All eyes were measured using the 6 optical biometers, and no problems were encountered during the process. The mean spherical equivalent of the eyes analyzed was –1.38 ± 2.68 diopters (D) (mean ± SD), ranging from +5.75 to –8.38 D. Table 1 shows the mean values, SD, and ranges for the different parameters obtained using the 6 devices. Note that LT was not obtained from the AL-Scan.
Table 1.
Mean ± SD Values (Range) for the 6 Devices.
| Parameter | Aladdin | AL-Scan | Argos | IOLMaster 700 | Lenstar LS 900 | OA-2000 | P value |
| K1 (D) | 43.25 ± 1.48 38.61, 47.28 |
43.22 ± 1.49 38.50, 47.33 |
43.22 ± 1.48 38.52, 47.18 |
43.19 ± 1.48 38.51, 47.25 |
43.17 ± 1.48 38.45, 47.14 |
43.20 ± 1.49 38.57, 47.26 |
<.001* |
| K2 (D) | 44.18 ± 1.59 38.93, 49.71 |
44.21 ± 1.59 38.93, 49.81 |
44.26 ± 1.59 39.30, 49.51 |
44.14 ± 1.60 38.88, 49.43 |
44.14 ± 1.61 38.67, 49.48 |
44.16 ± 1.60 39.05, 49.63 |
<.001* |
| J0 (D) | 0.36 ± 0.34 −0.39, 1.41 |
0.37 ± 0.37 −0.43, 1.23 |
0.40 ± 0.36 −0.34, 1.26 |
0.35 ± 0.35 −0.46, 1.40 |
0.36 ± 0.36 −0.41, 1.23 |
0.37 ± 0.36 −0.45, 1.33 |
<.001* |
| J45 (D) | −0.01 ± 0.23 −0.93, 0.61 |
−0.01 ± 0.24 −0.90, 0.63 |
−0.08 ± 0.24 −0.94, 0.66 |
−0.01 ± 0.23 −0.82, 0.71 |
−0.02 ± 0.25 −0.93, 0.71 |
−0.02 ± 0.23 −0.79, 0.65 |
<.001* |
| CCT (μm) | 540.33 ± 33.65 425.80, 634.80 |
547.10 ± 32.52 448.40, 626.40 |
535.92 ± 33.46 428.20, 620.40 |
537.62 ± 35.48 432.60, 640.20 |
542.51 ± 34.10 442.20, 633.80 |
529.24 ± 32.87 429.20, 624.20 |
<.001* |
| ACD (mm) | 3.56 ± 0.34 2.33, 4.25 |
3.57 ± 0.34 2.33, 4.18 |
3.58 ± 0.34 2.32, 4.30 |
3.52 ± 0.35 2.29, 4.23 |
3.52 ± 0.34 2.30, 4.21 |
3.56 ± 0.34 2.34, 4.21 |
<.001* |
| LT (mm) | 3.88 ± 0.47 3.07, 5.01 |
NA | 4.00 ± 0.44 3.19, 5.04 |
3.89 ± 0.47 3.06, 4.92 |
3.91 ± 0.47 3.10, 5.02 |
3.89 ± 0.49 3.15, 5.07 |
<.001* |
| AL (mm) | 24.08 ± 1.37 20.46, 27.79 |
24.03 ± 1.37 20.42, 27.77 |
24.10 ± 1.34 20.43, 27.72 |
24.08 ± 1.38 20.43, 27.82 |
24.09 ± 1.38 20.43, 27.83 |
24.08 ± 1.38 20.45, 27.79 |
<.001* |
| WTW (mm) | 11.85 ± 0.33 10.95, 12.66 |
12.12 ± 0.38 11.12, 13.10 |
12.14 ± 0.39 11.10, 13.14 |
12.19 ± 0.34 11.32, 13.12 |
12.27 ± 0.38 11.34, 13.15 |
12.16 ± 0.36 11.26, 13.08 |
<.001* |
| PS (mm) | 3.63 ± 0.54 2.74, 5.08 |
5.04 ± 1.01 2.70, 7.74 |
4.00 ± 0.80 2.47, 6.63 |
4.18 ± 0.95 2.46, 6.82 |
3.80 ± 0.56 2.63, 5.64 |
4.58 ± 0.87 2.60, 6.96 |
<.001* |
ACD = anterior chamber depth; AL = axial length; CCT = central corneal thickness; J0 = Jackson cross-cylinder at 0 and 90 degrees; J45 = Jackson cross-cylinder at 45 degrees and 135 degrees; K = keratometry; LT = lens thickness; NA = not available; PS = pupil size; WTW = white to white
Significant differences <0.05
Repeatability of Devices
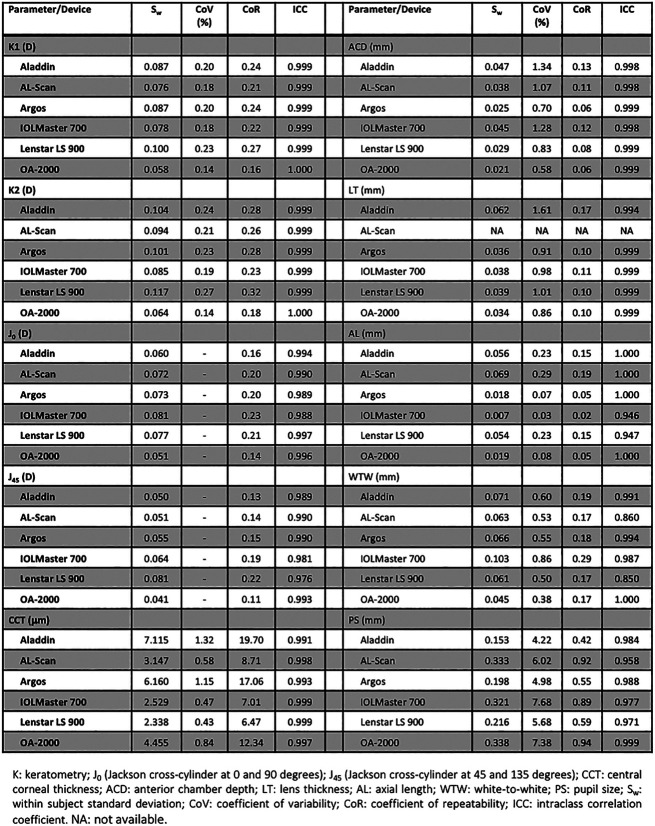
The repeatability outcomes of the 6 optical biometers for the different parameters analyzed are shown in Figure 1. The ICC for all ocular parameters was higher than 0.94 except for WTW measured using the AL-Scan and Lenstar LS 900, where it was 0.860 and 0.850, respectively. For K1 and K2, all CoV and CoR values were <0.24% and 0.32%, respectively. Smaller CoV and CoR values were found for the AL measurements. Specifically, the CoV values varied from 0.03% to 0.08% from the IOLMaster 700, Argos, and OA-2000 and up to 0.23% to 0.29% with the Aladdin, Lenstar LS 900, and AL-Scan. The CoR values were also distributed between these 2 groups of biometers, from 0.02 to 0.05 for the IOLMaster 700, Argos, and OA-2000 to 0.15 to 0.19 for the Aladdin, Lenstar LS 900, and AL-Scan. Higher CoV values were found for CCT, ACD, LT, and WTW, ranging from 0.38% to 1.61%. The PS measurement presented the highest CoV values, ranging from 4.2% with the Aladdin to 7.68% with the IOLMaster 700. Taking into account all the ocular parameters, the AL measurement provided the highest repeatability, measured with both CoV and CoR values, with PS being the parameter with the lowest repeatability.
Figure 1.
Repeatability of the 6 devices for the different parameters analyzed.
Agreement Between Devices
The outcomes for the agreement between the 6 optical biometers are shown in Supplemental Table 1 (http://links.lww.com/JRS/A377) for K1/K2 and astigmatism (J0 and J45), Supplemental Table 2 (http://links.lww.com/JRS/A378) for CCT, ACD, LT, and AL, and, finally, Supplemental Table 3 (http://links.lww.com/JRS/A379) for WTW and PS. For all pairwise comparisons between biometers, these tables show the mean difference ±SD, 95% CI, 95% LoA, and LoA width for the different parameters measured. Repeated-measures analysis of variance analysis revealed statistically significant differences between the 6 biometers for all the parameters evaluated (Table 1, P < .001), and the P values from the post hoc Tukey test are shown in these tables: Supplemental Table 1 (http://links.lww.com/JRS/A377) for K1/K2 and astigmatism; Supplemental Table 2 (http://links.lww.com/JRS/A378) for CCT, ACD, LT, and AL; and, finally, Supplemental Table 3 (http://links.lww.com/JRS/A379) for WTW and PS. Supplemental Figures 1–10 (http://links.lww.com/JRS/A367, http://links.lww.com/JRS/A368, http://links.lww.com/JRS/A369, http://links.lww.com/JRS/A370, http://links.lww.com/JRS/A371, http://links.lww.com/JRS/A372, http://links.lww.com/JRS/A373, http://links.lww.com/JRS/A374, http://links.lww.com/JRS/A375, and http://links.lww.com/JRS/A376) show the different Bland-Altman plots for K1 (1), K2 (2), J0 (3), J45 (4), CCT (5), ACD (6), LT (7), AL (8), WTW (9), and PS (10).
From Supplemental Figure 1 (http://links.lww.com/JRS/A367) and, in detail, Supplemental Table 1 (http://links.lww.com/JRS/A377), it can be seen that the mean difference for K1 ranged from −0.028 D (Lenstar LS 900 vs OA-2000) to 0.082 D (Aladdin vs Lenstar LS 900), whereas the comparison between the AL-Scan and IOLMaster 700 gave the highest LoA width (0.541 D). For K2 (Supplemental Figure 2, http://links.lww.com/JRS/A368, and Supplemental Table 1, http://links.lww.com/JRS/A377), these values varied from −0.075 D (Aladdin vs Argos) to 0.122 D (Argos vs IOLMaster 700), with the highest LoA width being 0.703 D (Argos vs AL-Scan). The values for J0 and J45 (Supplemental Figures 3, http://links.lww.com/JRS/A369, and 4, http://links.lww.com/JRS/A370, and Supplemental Table 1, http://links.lww.com/JRS/A377) ranged from −0.067 D (J0 Argos vs AL-Scan) to 0.067 D (J45 Aladdin vs Argos), with the highest LoA width of 0.457 D (Argos vs OA-2000) for J0,and 0.349 D (Argos vs IOLMaster 700) for J45. For CCT (Supplemental Figure 5, http://links.lww.com/JRS/A371, and Supplemental Table 2, http://links.lww.com/JRS/A378), the mean difference ranged from −11.173 μm (Argos vs AL-Scan) to 17.861 μm (AL-Scan vs OA-2000) with the highest LoA width being 37.600 μm (Aladdin vs Argos). The mean differences for ACD (Supplemental Figure 6, http://links.lww.com/JRS/A372, and Supplemental Table 2, http://links.lww.com/JRS/A378) ranged from −0.031 mm (IOLMaster 700 vs OA-2000) to 0.063 mm (Argos vs IOLMaster 700), with the highest LoA width being 0.366 mm (Aladdin vs Argos). The mean differences for LT (Supplemental Figure 7, http://links.lww.com/JRS/A373, and Supplemental Table 2, http://links.lww.com/JRS/A378) ranged from −0.120 mm (Aladdin vs Argos) to 0.114 mm (Argos vs IOLMaster 700) with the highest LoA width being 0.434 mm (Argos vs OA-2000). The mean differences for AL (Supplemental Figure 8, http://links.lww.com/JRS/A374, and Supplemental Table 2, http://links.lww.com/JRS/A379) ranged from −0.038 mm (AL-Scan vs Lenstar LS 900) to 0.036 mm (Argos vs AL-Scan) with the highest LoA width being 0.225 mm (Argos vs Lenstar LS 900). For WTW (Supplemental Figure 9, http://links.lww.com/JRS/A375, and Supplemental Table 3, http://links.lww.com/JRS/A379), the mean differences varied from −0.413 mm (Aladdin vs Lenstar LS 900) to 0.111 mm (Lenstar LS 900 vs OA-2000) with the highest LoA width being 0.620 mm (AL-Scan vs IOLMaster 700). Finally, the mean differences for PS (Supplemental Figure 10, http://links.lww.com/JRS/A376, and Supplemental Table 3, http://links.lww.com/JRS/A379) ranged from −1.519 mm (Argos vs AL-Scan) to 1.239 mm (AL-Scan vs OA-2000) with the highest LoA width being 3.541 mm (Argos vs AL-Scan).
DISCUSSION
In this study, we assessed the repeatability and agreement of the measurements obtained with the 6 optical biometers.
Our results revealed that the 6 devices showed high levels of repeatability. The ICC values were >0.94 except for WTW measured with the AL-Scan (0.86) and Lenstar LS 900 (0.85). As a general trend among the 6 biometers, we have to point out that AL is the parameter with the highest predictability (better for SS-OCTs) followed by K1, K2, J0, J45, CCT, and WTW. In fact, PS, ACD, and LT showed lower predictability values compared with the others. These 3 parameters may be directly affected by light and accommodation. We consider that the 6 biometers provide repeatable measurements for the different parameters analyzed. In general, our results agree with previously published studies, shown in the following section, that used the 6 biometers.
Aladdin
Mandal et al. assessed 75 cataract eyes and 22 healthy eyes.6 They evaluated interobserver variation by comparing the 2 groups (reproducibility) and concluded that it produces repeatable biometry measurements (95% CI of 0.05 mm for AL, 95% CI of 0.06 mm for ACD, and 95% CI of 0.17 D for K). Huang et al., who evaluated intraoperator repeatability in 52 healthy eyes and 46 with cataracts, concluded that it gave excellent repeatability.7 AL was the most repeatable parameter, with a CoV of about 0.10% in both groups, and ACD, K, and WTW measurements were highly repeatable in healthy eyes with a CoV of <0.89% and a ICC of >0.94. Notwithstanding, eyes with cataract showed a high repeatability only for the AL, ACD, and K measurements, whereas WTW had a CoR value of 0.80 mm and an ICC of 0.795. Sabatino et al. studied 109 patients and found values of about 3% CoV for AL, mean K, and CD and larger values for ACD (about 10%).8 McAlinden et al. assessed 102 right eyes and found Sw (and CoR, in brackets) values of 0.03 (0.08) mm, 0.03 (0.08) mm, 0.10 (0.29) D, 0.13 (0.35) D, 0.07 (0.20) D, 0.06 (0.16) D, and 0.08 (0.23) mm for AL, ACD, K1, K2, J0, J45, and WTW, respectively.9 Ruiz-Belda et al. evaluated 53 healthy eyes and recorded a mean Sw of 0.26 and 0.24 D for K1 and K2, respectively; recently, Fukumitsu et al., for 69 cataract eyes, reported Sw values of 0.03 to 0.05 mm and 0.03 to 0.09 for AL and ACD, respectively.10,11
AL-Scan
Huang et al. analyzed intraoperator repeatability for 68 cataract eyes measured by 2 operators.12 They found CoV values for the first operator of 0.35%, 0.64%, 0.27%, 0.34%, 0.08%, 1.70%, and 5.46% for CCT, ACD, K1, K2, AL, WTW, and PD, respectively, and concluded that it provides highly repeatable measurements of CCT, ACD, AL, and K. Srivannaboon et al. measured 137 eyes showing good repeatability for K, AL, ACD, and WTW (ICC >0.94); Kola et al. supported their conclusions, obtaining ICC values of >0.937 for K1, K2, WTW, CCT, ACD, and AL and 0.87 to 0.90 for PS.13,14 Yağcı et al. evaluated 62 healthy eyes and reported CoV values of 0.06%, 0.08%, 0.03%, 0.03%, and 0.03% for CCT, ACD, WTW, K1, and K2, respectively.15 Yağcı et al. also reported on 30 healthy eyes, with 1 of 2 observers, CoR values of 3.540 μm, 0.039 mm, 0.175 mm, 0.232 D, 0.303 D, and 0.088 mm for CCT, ACD, WTW, K1, K2, and AL, respectively.16 Güler et al. analyzed 65 cataract eyes and reported CoV values of 0.11%, 0.570%, 0.368%, 0.524%, 0.250%, and 0.284% for AL, aqueous depth (AQD), CCT, CD, K1, and K2, respectively, in eyes with an AL between 22 and 26 mm.17 These values were 0.18%, 0.576%, 0.372%, 0.501%, 0.304%, and 0.317%, respectively, for eyes with an of AL >26 mm. They concluded that this biometer gave excellent repeatability, with all eyes and parameters giving an ICC of >0.97. Mansoori et al. and Duman et al. reported an ICC of 0.999 for CCT in 127 healthy eyes and of >0.99 for CCT and ACD, but lower values, between 0.884 and 0.988, for WTW in 43 cataract eyes.18,19 Yu et al. assessed this biometer in 58 children, reporting CoR values of 0.09 mm, 5.1 μm, 0.04 mm, 0.28 D, 0.24 D, 0.39 mm, and 0.22 mm for AL, CCT, ACD, Kf, Ks, CD, and PD, respectively.20 CoV values were low, <0.35%, with an ICC of all parameters of >0.85. Specifically for CCT, Gokcinar et al. recorded an ICC of 0.992 (95% CI from 0.989 to 0.994) in 150 eyes; Doğan and Ertan an ICC of 0.91 (95% CI from 0.85 to 0.95) in 64 healthy eyes; and Can et al. an ICC of 0.980 in 80 healthy eyes.21–23 Chan et al. found CoR values of 0.155 mm, 0.268 D, 0.177 D, 8.813 μm, 0.042 mm, and 0.346 mm for AL, J0, J45, CCT, ACD, and WTW, respectively, in 52 cataract eyes.24
Argos
Shammas et al. reported Sw values of 0.01 μm, 0.11 mm, 0.01 mm, 0.02 mm, 0.01 mm, and 0.05 mm for CCT, WTW, AC, LT, AL, and PS, respectively.25 Nemeth and Modis analyzed 40 cataract eyes with CoV values of 0.34%, 0.32%, 1.59%, 0.95%, 1.35%, 0.79%, 0.09%, and 2.16% for K1, K2, CCT, WTW, ACD, LT, AL, and PD, respectively, and all parameters gave an ICC of >0.92.26 Huang et al. reported values of 0.02 mm for Sw, 0.10% for CoV, 0.06 mm for CoR, and an ICC of 0.999.27
IOLMaster 700
Several studies have reported the repeatability of the IOLMaster 700.24,27–42 In general, the ICC for K values was ≥0.97, with CoV and CoR values of about 0.22% and 0.25 D, respectively. For CCT, the Sw values were similar between studies (about 2 to 3 μm), with a small CoV (<1%) and a maximum CoR of about 10 μm. The ICC ranged from 0.87 to 0.99.24,28,31,34,36,38–41 WTW values were similar among studies, with an Sw of about 0.10 mm, CoR values between 0.2 and 0.3 mm, CoV <1% (when reported), and an ICC value that ranged from 0.87 to 0.99.24,34,41 For ACD, these studies showed an Sw of ≤0.05 mm, and all ICC values were ≥0.99. Similarly, the ICC values for LT were high (≥0.97), with Sw ranging from 0.01 to 0.07 mm, the highest CoV value being 1.35%.30,37 For AL, studies reported Sw values ≤0.01 mm, except for Shajari et al. who found a higher value (0.11 mm).42 The CoV was similar (from 0.02% to 0.05%), with high ICC values (1.00 in almost all the studies).
Lenstar LS 900
Buckhurst et al. evaluated 112 patients with cataract showing an intrasession repeatability of 0.079 mm, 0.077 mm, 0.14 D, 0.14 D, 0.003 μm, 0.051 mm, 0.089 mm, and 0.016 mm for PS, WTW, K1, K2, CCT, ACD, LT, and AL, respectively.43 Bjeloš Rončević et al. analyzed 32 cataract eyes, reporting a CoV of 0.004%, 0.018%, 0.019%, 0.000%, 0.003%, 0.003%, 0.027%, and 0.037% for CCT, ACD, LT, AL, K1, K2, WTW, and PS, respectively.44 Shammas and Hoffer evaluated 37 cataract eyes in 1 session, reporting CoV values of 0.001%, 0.006%, 0.004%, 0.003%, 0.002%, 0.003%, and 0.003% for AL, CCT, AQD, ACD, LT, CD, K1, and K2, respectively.45 They concluded that the precision of the measurements was extremely high. Chen et al. studied 40 eyes and reported Sw values of 3.10 μm, 0.02 mm, 0.04 mm, and 0.17 D for CCT, ACD, WTW, and mean K, respectively.46 Zhao et al. found higher values of Sw for 56 myopic eyes (0.018 mm, 0.052 mm, 0.181 D, 0.301 D, 0.274 mm, and 14.244 μm for AL, ACD, K1, K2, WTW, and CCT, respectively); Schulle and Berntsen published a value of 0.02 mm for AL, for 29 eyes.47,48 Shen et al., for a group of 33 myopic eyes, reported Sw values of 0.016 mm, 0.009 mm, 0.014 mm, 1.982 μm, and 0.061 mm for AL, ACD, LT, CCT, and WTW, respectively.49 CoV values were small and varied from 0.3% to 0.5%, except for AL, which was 0.06%. Tai et al. assessed CCT in 184 healthy eyes reporting a CoV of 1.51%; Ventura et al. analyzed K values in 32 eyes with Sw, CoV, and ICC values of 0.08 D, 0.14%, and 0.998, respectively.50,51 Huang et al. also specifically analyzed CCT repeatability in 55 healthy eyes with Sw, CoR, CoV, and ICC values of 2.4 μm, 6.7 μm, 0.5%, and 0.992, respectively (first operator).52 Shajari et al. analyzed ACD and WTW in 40 eyes, reporting CoR values of 0.1 mm for both parameters.53 Güler et al. reported CoV values of 0.06%, 0.702%, 0.403%, 0.628%, 0.312%, and 0.356% for AL, AQD, CCT, CD, K1, and K2, respectively, in eyes with an AL of between 22 and 26 mm and 0.12%, 0.641%, 0.385%, 0.579%, 0.310%, and 0.336%, respectively, for eyes with an AL of >26 mm.17 All the parameters for both groups gave an ICC >0.97. For 100 cataract eyes, Kurian et al. showed CoV values of 0.21%, 0.21%, 1.99%, 0.35%, and 1.62% for AL, mean K, ACD, CCT, and LT, respectively.30 McAlinden et al. reported in 102 patients Sw (and CoR, in brackets) values of 0.02 (0.05) mm, 0.02 (0.06) mm, 0.11 (0.29) D, 0.13 (0.36) D, 0.08 (0.22) D, 0.08 (0.23) D, and 0.07 (0.19) mm for AL, ACD, K1, K2, J0, J45, and WTW, respectively.9 Ferreira and Ribeiro reported in 30 eyes an ICC of >0.94 for K1, K2, J0, and J45.54 Ruiz-Mesa et al. analyzed Sw, CoR, CoV, and ICC in 40 normal eyes and reported 0.10 mm, 0.27 mm, 0.17%, and 0.998 for mean K; 5.58 μm, 14.44 μm, 0.58%, and 0.993 for CCT; 0.04 mm, 0.11 mm, 0.50%, and 0.997 for ACD; and 0.13 mm, 0.36 mm, 0.16%, and 0.998 for AL.55 These authors also analyzed 40 cataract eyes that gave similar values.
OA-2000
The OA-2000 was also evaluated in different studies.12,56–58 The Sw for K ranged from 0.05 to 0.15 D, with CoV values of around 0.20% and ICCs of ≥0.99.56,57 Good outcomes were also reported for CCT with Sw being approximately 4 μm, CoV <1%, and ICCs ≥0.98. All authors found similar WTW values (CoV <1% and CoR ≤0.55 mm), but those reported by Hua et al. were slightly higher.56 ACD values were similar for the 4 studies with Sw ranging from 0.02 to 0.03 mm.12,57–59 For LT, the Sw varied from 0.03 to 0.09 mm, with a CoV value from 0.6% to 2%, and ICCs ranged from 0.94 to 0.99.56,57,59 For AL, the ICC value was excellent (1); Sw values were 0.01 to 0.03 mm, and CoV was 0.03% to 0.10%.12,56,59 Hua et al. reported PS CoV and CoR values of 5.29% and 1.00 mm, respectively.56
Our results revealed that there were statistically significant differences between the 6 biometers (Table 1, P < .001). The post hoc Tukey analysis showed that statistical significance depended on which pairwise comparisons between biometers were analyzed and which parameter was being examined (see Supplemental Tables 1–3, http://links.lww.com/JRS/A377, http://links.lww.com/JRS/A378, and http://links.lww.com/JRS/A379).
K results showed that the minimum mean differences were obtained for the comparison between the Argos vs AL-Scan and IOLMaster 700 vs Lenstar LS 900 for K1 and K2, respectively (P > .8). The maximum mean difference for K1 was found for the comparison between the Aladdin vs Lenstar LS 900 (0.082 D) and for K2 between the Argos vs IOLMaster 700 (0.122 D), where both were statistically significant (P < .001). Hua et al. suggested that a difference of 1.0 D in the K value would cause a difference of about 1.40 D in IOL power calculation.56 If we plan to use these k values to calculate IOL power in the context of cataract surgery, and we consider 0.122 D as the maximum mean difference, it would lead to a difference of about 0.17 D in the IOL power. The small differences in agreement reported for all the K measurement comparisons led to nonsignificant changes in IOL power calculation because of the 0.50 D step in IOL manufacturing. However, it is important to note that the LoA width varied as a function of the comparison and some comparisons for K1, and almost all for K2, were >0.50 D (Supplemental Table 1, http://links.lww.com/JRS/A377, Supplemental Figures 1, http://links.lww.com/JRS/A367, and 2, http://links.lww.com/JRS/A368), broad enough to produce a significant change in IOL power. Similarly, both astigmatism vector components, J0 and J45, showed a comparable pattern but with an LoA width <0.50 D for all pairwise comparisons (Supplemental Table 1, http://links.lww.com/JRS/A377, Supplemental Figures 3, http://links.lww.com/JRS/A369, and 4, http://links.lww.com/JRS/A370).
In relation to CCT (Supplemental Figure 5, http://links.lww.com/JRS/A371, and Supplemental Table 2, http://links.lww.com/JRS/A378), the mean difference ranged from −11.173 μm (Argos vs AL-Scan) to 17.861 μm (AL-Scan vs OA-2000) with the highest LoA width being 37.600 μm (Aladdin vs Argos) and the lowest 11.980 μm (Lenstar LS 900 vs OA-2000). We should consider that CCT differences may affect the intraocular pressure measurement values because it has been estimated that there is about 1 mm Hg of correction for every 25-μm deviation from a mean CCT of 550 μm.59 Quite a few comparisons gave LoA width values of >25 μm, mainly involving the Aladdin biometer. In relation to IOL powering, this variable has little influence on classical IOL formulas, but it should be considered in those that may include it (i.e., Olsen). The mean ACD differences (Supplemental Figure 6, http://links.lww.com/JRS/A372, and Supplemental Table 2, http://links.lww.com/JRS/A378) were very small and all <±0.1 mm. It is interesting to consider that a difference in the ACD of 0.1 mm would lead to a change in the refraction in the eyes with a mean AL and corneal curvature.60 LoA width values ranged from 0.366 mm (Aladdin vs Argos) to 0.175 mm (IOLMaster vs OA-2000). Olsen indicated that a 1 mm deviation in the ACD could lead to a refractive error of 1.5 D in IOL power.61 We should therefore consider that the differences reported between biometers would not affect the calculation of the IOL power. We believe that a clinical criterion is necessary to judge whether the differences found should be considered negligible and hence used interchangeably (ie, for phakic IOL implantation, glaucoma screening, or postoperative ACD estimation).39 Mean differences for all pairwise comparison of LT lie within ±0.1 mm (Supplemental Figure 7, http://links.lww.com/JRS/A373, and Supplemental Table 2, http://links.lww.com/JRS/A378). For IOL calculation formulas that use this variable (ie, Olsen, Kane, and Holladay 2), it should be assessed as a possible source of error when comparing devices. Specifically, an LT increase of 0.2 mm would change the IOL power by 0.2 D; considering our mean differences, this may not have a clinically significant impact on the calculation of the IOL power when using the Holladay 2 or Olsen formulas.25,62 Broad ranges of LoA (≥0.5 mm) might have an important impact on IOL power calculation when using next-generation formulas. This was not our case, and we consider that the 5 devices can be used interchangeably for LT measurements.
For AL, we found small values, within about ±0.03 mm, for all pairwise comparisons (Supplemental Figure 8, http://links.lww.com/JRS/A374, and Supplemental Table 2, http://links.lww.com/JRS/A378). If we consider that a 0.1 mm AL error would yield a refraction error of about 0.27 D, the differences found between devices would not affect the IOL power calculation for a cataract surgery procedure.61 We therefore believe that they can be used interchangeably. Notwithstanding, if we consider the LoA width, some comparisons reach values of around 0.2 mm, which approach the limit to be considered clinically negligible. This should be kept in mind for both cataract IOL power calculation and studies on myopia progression. The mean differences reported for WTW measurements were larger than those reported for the other parameters (Supplemental Figure 9, http://links.lww.com/JRS/A375, and Supplemental Table 3, http://links.lww.com/JRS/A379). For example, the highest mean difference was −0.413 mm when comparing the Aladdin vs Lenstar LS 900. The LoA range (width) was, in most cases, around 0.50 mm or higher, which may be clinically meaningful. For IOL power, when the Holladay 2 and Barrett formulas use WTW as a variable, differences can be found; this is also true for phakic IOL sizing because surgeons choose the size to the nearest 0.50 mm. WTW measurements between some devices can be considered interchangeable to some extent, but not others. Different technologies used to ascertain this parameter (corneal topography, photography, and LED) may be responsible for this range of variation because the value depends on the image quality and the algorithms used for limbus edge detection.63 Finally, the mean differences for PS (Supplemental Figure 10, http://links.lww.com/JRS/A376, and Supplemental Table 3, http://links.lww.com/JRS/A379) showed the highest values and ranged about ±1.5 mm, which is clinically quite significant. Specifically, the LoA width ranged from 1.578 mm to 3.541 mm. This is an important difference between biometers, and they should not be considered interchangeable with regard to this parameter.
As a general consideration, we want to point out that for K, CCT, WTW, and PS, there is random variability in the agreement between devices using Placido rings, spots, OCT technology, and color photography. However, it seems that SS-OCT biometers showed the best agreement for axial distances, that is, ACD, LT, and AL, leading us to consider that biometers based on this technology are likely to become the gold standard for ocular biometry. Note that lower CoV and CoR values for AL were found for these biometers compared with the others.
Below, we present studies that analyze the agreement between the various biometers and discuss those found in our study. Note that no comparison studies were found for the Aladdin vs AL-Scan, Argos vs AL-Scan, or AL-Scan vs OA-2000.
Aladdin vs Argos
Nemeth and Modis analyzed 40 cataract and 46 pseudophakic eyes and concluded that the agreement was poor for all parameters, except AL and ACD for cataract eyes and AL for pseudophakic eyes.26 Our results agree because we found clinically significant differences between the 2 devices for all parameters except for ACD, LT, and AL.
Aladdin vs IOLMaster 700
Calvo-Sanz et al. reported good agreement in 55 cataract eyes for AL, K, LT, ACD, and CCT (ICC >0.93).64 We found good agreement for AL, LT, and ACD but slightly significant differences for K (LoA width of about 0.40 D) and CCT (LoA width of about 28 μm).
Aladdin vs Lenstar LS 900
McAlinden et al. compared both devices in 102 eyes and concluded that they may be used interchangeably because of the good agreement for all parameters except WTW.9 Yeu assessed 101 eyes and reported a high level of agreement for all biometric parameters, although there was a statistically significant difference in LT and WTW.65 Differences between the corneal topography and photography methods may be the source of this nonagreement. Ortiz et al. evaluated only AL, mean K, and ACD in a large sample of eyes (231) and concluded that there were no clinically significant differences.66 Our results support that but the LoA width for K does approach a clinically significant level.
Aladdin vs OA-2000
McAlinden et al. compared the AL agreement for 377 eyes, concluding that can be used interchangeably because of the small mean differences (about 0.01 mm) and narrow LoA.67 Savini et al. compared LT measurements for 88 eyes and found a mean difference of −0.06 ± 0.09 mm.68 Our results were similar: mean value of −0.091 ± 0.093 mm (Supplemental Table 1, http://links.lww.com/JRS/A377).
Argos vs IOLMaster 700
Sabatino et al., Tamaoki et al., and Yang et al. reported statistically significant differences for K values (about 0.10 D).39,69,70 This difference may be considered clinically nonsignificant, but the LoA width reported (about 0.80 D) should be considered. Our results reported lower values for K1 and K2, of about 0.50 D and 0.60 D, respectively. The mean differences in CCT varied as a function of the study, with the LoA width ranging from 6 to 25 μm and about 50 μm in some cases.39,70,71 In this study, the mean difference was about 2 μm with an LoA width of about 30 μm, which should be kept in mind when diagnosing glaucoma or for corneal refractive surgery candidates. The mean differences for WTW were also clinically relevant, being about 0.30 mm, with large LoA values (>2 mm).39,70 Our results were lower (mean difference of around −0.05 mm and LoA width of 0.60 mm) but clinically significant. With the exception of Yang et al., the other authors reported statistically significant differences when measuring ACD (mean difference of around −0.1 mm).39,69–71 Our results revealed small mean differences and a narrow LoA width (<0.35 mm) that was not clinically significant. In relation to LT, Omoto et al. did not find statistically significant differences between devices but the others did, with a large LoA width (0.50 to 1.50 mm).19,39,69–71 We found significant differences but with a small mean (0.114 mm) and LoA width (<0.4 mm), which were not clinically significant. For AL, the outcome comparison varied between the studies, being statistically significant for some but not for others (mean differences ranged from 0 to 0.07 mm).27,39,69–71 Tamaoki et al. and Omoto et al. reported a wide LoA width (about 0.30 mm), which could affect IOL power calculation.69,71 We found no statistically significant differences between devices as our LoA width was smaller (about 0.2 mm). Using different refractive indices for each segmental length (Argos) may play a role in this difference when calculating AL.
Argos vs Lenstar LS 900
Shammas et al. assessed 107 eyes and reported that there was good agreement for both AL and ACD measurements, and the mean differences were 0.01 ± 0.06 mm (Argos) and 0.08 ± 0.15 mm (Lenstar LS 900) (1.00 and 0.80).25 In addition, they found good correlation (0.93) for the CCT measurement between biometers and a nonobservable mean difference. A mean difference of −0.22 ± 0.20 mm was found for LT, with a correlation of 0.80. In general, the Lenstar LS 900 tends to measure a smaller pupil (−0.29 mm), and the WTW showed strong differences (mean −0.34 mm) and a poor correlation (0.41). Our results agree with small mean differences for all parameters evaluated (see Supplemental Table 2, http://links.lww.com/JRS/A378).
Argos vs OA-2000
Tamaoki et al. reported that the mean K, ACD, and LT values obtained using the Argos device were significantly higher than those measured with the OA-2000 (P < .0001), although they were all considered to be clinically negligible.69 In addition, there were statistically significant differences in AL (mean difference of 0.05 mm and LoA range of about 0.30 mm). However, Huang et al. reported excellent AL agreement with a mean difference of 0.00 ± 0.04 mm (95% LoA, −0.09 to 0.08 mm, P = 1.000) for 166 eyes.27 Our results revealed no statistically significant differences for AL (mean difference of 0.01 mm, P = .513), for which reason we consider both devices interchangeable.
AL-Scan vs IOLMaster 700
For a sample of 52 cataract eyes, Chan et al. found systematic differences in mean K, CCT, ACD, and WTW.24 The mean difference was <0.20 D (at 2.4 mm), 14.92 μm, −0.017 mm, and 0.283 mm for mean K, CCT, ACD, and WTW, respectively. These differences led to a statistically significant but clinically insignificant difference in IOL power prediction. We also obtained statistically significant differences, but in our case, these were lower.
AL-Scan vs Lenstar LS 900
Güler et al. analyzed 65 cataract eyes and reported significant differences for AQD, CD, K1, and K2, but not for AL or CCT.17 They concluded that there was good agreement for all parameters. Our results revealed statistically significant differences for all the parameters, but we consider the devices interchangeable for CCT, ACD, and AL.
IOLMaster 700 vs Lenstar LS 900
Kunert et al. reported good agreement for LT and CCT for 120 cataract eyes.29 Kurian et al. found significant differences for AL, ACD, CCT, and LT, but not for mean K.30 However, as we have presented in our results, the range for the 95% LoA was >0.50 D, which should be kept in mind. Hoffer et al. analyzed 183 eyes and found excellent agreement for AL, no significant differences for mean K (0.02 D), a significant but clinically insignificant difference for ACD (0.03 mm), and no statistical differences for LT and CCT.72 For 80 eyes, Arriola-Villalobos et al. reported excellent correlation, except for WTW and PD, concluding that there were no clinically relevant differences.73 We broadly agree with the differences reported for WTW and PS. Passi et al., for 63 eyes, also supported this good agreement, with a high ICC for AL, ACD, LT, K1, and K2 (>0.95).74 El Chehab et al., for 129 eyes, found significant differences only for the ACD and WTW measurements, with the agreement for AL being excellent (ICC = 1).75 Bullimore et al. compared CCT and LT and found small mean differences for normal (0.03 mm and 5.5 μm) and cataract eyes (0.08 mm and 4.5 μm), respectively; our results were similar.37 Cheng et al. also reported a high level of agreement for AL, mean K, ACD, KT, and CCT (ICC >0.86) for 164 cataract eyes.76
IOLMaster 700 vs OA-2000
Huang et al., Tamaoki et al., and Liao et al. showed that there was excellent agreement for AL.27,69,77 Tamaoki et al. found significant small K values with the IOLMaster 700, whereas Liao et al. reported comparable values, and the differences may be considered insignificant.69,77 Our results revealed no significant differences for either K1 or K2 (P > .05). Both studies also reported significant differences for CCT (LoA width of about 25 μm).69 The LoA width for WTW reported by Liao et al. was also high (about 1.30 mm).69 In contrast, our results revealed no significant differences with a small LoA range (about 0.50 mm). Tamaoki et al. reported significant differences for ACD values, but Liao et al. did not, finding a mean difference of 0.00 mm.32,33 Our results showed significant differences (mean difference of −0.03 mm). Significant differences in LT were found by Tamaoki et al. and Liao et al. (LoA width of 0.17 mm), similar to that found by us (about 0.27 mm).69,77
Lenstar LS 900 vs OA-2000
Goebels et al. assessed 138 cataract eyes and reported significant mean differences of −0.03 mm and 0.08 mm for AL and ACD, respectively.78 For 99 healthy eyes, Gao et al. reported good agreement for AL, AQD, ACD, and LT, with a narrow 95% LoA (−0.05 to 0.07 mm, −0.09 to 0.10 mm, −0.10 to 0.09 mm, and −0.06 to 0.22 mm, respectively).79 The CCT, K1, K2, J0, J45, and WTW parameters were in good agreement, with some of them having a statistically but not clinically significant difference. Our results revealed no differences only in the case of K1 and K2, but, like these authors, we also consider that they are not clinically significant, with the exception of WTW, which should not be considered interchangeable (LoA width of about 0.5 mm). Using the results from 140 eyes, Reiblat et al. also supported the high level of agreement for AL, ACD, and mean K. Wang et al. specifically compared AL measurements in eyes with the presence of a dense vitreous hemorrhage and reported excellent agreement with a mean difference of 0.04 mm and an LoA from −0.04 to 0.12 mm.80,81 Recently, Vasavada et al. measured 124 eyes with dense cataracts and showed that the OA-2000 gave a clinically higher AL than the Lenstar LS 900 (though not statistically significant), whereas the Lenstar LS 900 gave a higher CCT and lower LT than the OA-2000.82 The agreement for K and ACD was good.
In conclusion, our results reveal that the 6 biometers provide repeatable measurements for the different parameters analyzed. Comparing the devices, we found statistically significant differences between them when measuring most ocular parameters. However, depending on the particular parameter and its use, they may still be interchangeable because the clinical impact may be negligible. Our data could be considered to have underlined the clinical significance of using different biometers and how analyzing them is essential. In most cases, the outcomes should be judged in a clinical context to determine their interchangeability. It seems that SS-OCT biometers showed the best agreement for axial distances, that is, ACD, LT, and AL, leading us to consider that biometers based on this technology are likely to become the gold standard for ocular biometry. Further studies with a larger sample of both healthy eyes and those with cataracts are required to support these findings.
WHAT WAS KNOWN
Optical biometry has converted into the gold standard for measuring ocular biometric parameters.
Optical biometry may be based on partial coherence interferometry, optical low coherence reflectometry, optical low-coherence interferometry, and swept-source optical coherence tomography.
Comparison studies analyzing the agreement between biometers have been published, mainly comparing 2 or, in exceptional cases, 3 or 4 devices.
WHAT THIS PAPER ADDS
To our knowledge, this is the first study comparing 6 biometers, based on the different optical technologies available in the market.
The outcomes found by the 6 biometers showed statistically significant differences between most ocular parameters.
Depending on the specific parameter and its use, the biometers may still be interchangeable because the clinical impact may be negligible.
Acknowledgments
The author thanks Teresa Ferrer-Blasco for critical comments and laboratory data analysis and Francisco Pastor-Pascual, Ramón Ruiz-Mesa, and Pedro Tañá-Rivero for helpful discussion.
Footnotes
Supported by an investigator-initiated study grant from Alcon Laboratories, Inc. (IIT#56714317).
Presented in part at the 25th ESCRS Winter Meeting, Virtual, February 2021.
Disclosures: R. Montés-Micó declares research and consulting contracts with Alcon Laboratories Inc., Essilor, Glaukos, and Staar Surgical through the University of Valencia.

First author:
Robert Montés-Micó, PhD
University of Valencia, Valencia, Spain
REFERENCES
- 1.Montés-Micó R, Carones F, Buttacchio A, Ferrer-Blasco T, Madrid-Costa D. Comparison of immersion ultrasound, partial coherence interferometry, and low coherence reflectometry for ocular biometry in cataract patients. J Refract Surg 2011;27:665–671 [DOI] [PubMed] [Google Scholar]
- 2.Montés-Micó R, Pastor-Pascual F, Ruiz-Mesa R, Tañá-Rivero P. Ocular biometry with swept-source optical coherence tomography. J Cataract Refract Surg [Epub ahead of print December 10, 2020.] [DOI] [PubMed]
- 3.Grulkowski I, Liu JJ, Zhang JY, Potsaid B, Jayaraman V, Cable AE, Duker JS, Fujimoto JG. Reproducibility of a long-range swept-source optical coherence tomography ocular biometry system and comparison with clinical biometers. Ophthalmology 2013;120:2184–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlinden C, Khadka J, Pesudovs K. Statistical methods for conducting agreement (comparison of clinical tests) and precision (repeatability or reproducibility) studies in optometry and ophthalmology. Ophthalmic Physiol Opt 2011;31:330–338 [DOI] [PubMed] [Google Scholar]
- 5.McAlinden C, Khadka J, Pesudovs K. Precision (repeatability and reproducibility) studies and sample-size calculation. J Cataract Refract Surg 2015;41:2598–2604 [DOI] [PubMed] [Google Scholar]
- 6.Mandal P, Berrow EJ, Naroo SA, Wolffsohn JS, Uthoff D, Holland D, Shah S. Validity and repeatability of the Aladdin ocular biometer. Br J Ophthalmol 2014;98:256–258 [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Savini G, Wu F, Yu X, Yang J, Yu A, Yu Y, Wang Q. Repeatability and reproducibility of ocular biometry using a new noncontact optical low-coherence interferometer. J Cataract Refract Surg 2015;41:2233–2241 [DOI] [PubMed] [Google Scholar]
- 8.Sabatino F, Findl O, Maurino V. Comparative analysis of optical biometers. J Cataract Refract Surg 2016;42:685–693 [DOI] [PubMed] [Google Scholar]
- 9.McAlinden C, Gao R, Yu A, Wang X, Yang J, Yu Y, Chen H, Wang Q, Huang J. Repeatability and agreement of ocular biometry measurements: Aladdin versus Lenstar. Br J Ophthalmol 2017;101:1223–1229 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Belda C, Rodrigo F, Piñero DP. Validation of keratometric measurements obtained with an intraoperative image-guided system: intra-session repeatability and interchangeability with an optical biometer. Clin Exp Optom 2018;101:200–205 [DOI] [PubMed] [Google Scholar]
- 11.Fukumitsu H, Camps VJ, Piñero DP. Intrasession repeatability of biometric measurements obtained with a low-coherence interferometry system in pseudophakic eyes. Curr Eye Res 2020;45:221–226 [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Savini G, Li J, Lu W, Wu F, Wang J, Li Y, Feng Y, Wang Q. Evaluation of a new optical biometry device for measurements of ocular components and its comparison with IOLMaster. Br J Ophthalmol 2014;98:1277–1281 [DOI] [PubMed] [Google Scholar]
- 13.Srivannaboon S, Chirapapaisan C, Chonpimai P, Koodkaew S. Comparison of ocular biometry and intraocular lens power using a new biometer and a standard biometer. J Cataract Refract Surg 2014;40:709–715 [DOI] [PubMed] [Google Scholar]
- 14.Kola M, Duran H, Turk A, Mollamehmetoglu S, Kalkisim A, Erdol H. Evaluation of the repeatability and the reproducibility of AL-scan measurements obtained by residents. J Ophthalmol 2014;2014:739652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yağcı R, Kulak AE, Güler E, Tenlik A, Gürağaç FB, Hepşen İF. Comparison of anterior segment measurements with a dual Scheimpflug Placido corneal topographer and a new partial coherence interferometer in keratoconic eyes. Cornea 2015;34:1012–1018 [DOI] [PubMed] [Google Scholar]
- 16.Yağcı R, Güler E, Kulak AE, Erdoğan BD, Balcı M, Hepşen İF. Repeatability and reproducibility of a new optical biometer in normal and keratoconic eyes. J Cataract Refract Surg 2015;41:171–177 [DOI] [PubMed] [Google Scholar]
- 17.Güler E, Kulak AE, Totan Y, Yuvarlak A, Hepşen İF. Comparison of a new optical biometry with an optical low-coherence reflectometry for ocular biometry. Cont Lens Anterior Eye 2016;39:336–341 [DOI] [PubMed] [Google Scholar]
- 18.Mansoori T, Balakrishna N. Repeatability and agreement of central corneal thickness measurement with non-contact methods: a comparative study. Int Ophthalmol 2018;38:959–966 [DOI] [PubMed] [Google Scholar]
- 19.Duman R, Çetinkaya E, Duman R, Dogan M, Sabaner MC. Comparison of anterior segment measurements using Sirius Topographer and Nidek Axial Length-Scan with assessing repeatability in patients with cataracts. Indian J Ophthalmol 2018;66:402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Chen H, Savini G, Zheng Q, Song B, Tu R, Huang J, Wang Q. Precision of a new ocular biometer in children and comparison with IOLMaster. Sci Rep 2018;8:1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gokcinar NB, Yumusak E, Ornek N, Yorubulut S, Onaran Z. Agreement and repeatability of central corneal thickness measurements by four different optical devices and an ultrasound pachymeter. Int Ophthalmol 2019;39:1589–1598 [DOI] [PubMed] [Google Scholar]
- 22.Doğan M, Ertan E. Comparison of central corneal thickness measurements with standard ultrasonic pachymetry and optical devices. Clin Exp Optom 2019;102:126–130 [DOI] [PubMed] [Google Scholar]
- 23.Can E, Eser-Ozturk H, Duran M, Cetinkaya T, Arıturk N. Comparison of central corneal thickness measurements using different imaging devices and ultrasound pachymetry. Indian J Ophthalmol 2019;67:496–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan TCY, Wan KH, Tang FY, Wang YM, Yu M, Cheung C. Repeatability and agreement of a swept-source optical coherence tomography-based biometer IOLMaster 700 versus a Scheimpflug imaging-based biometer AL-scan in cataract patients. Eye Contact Lens 2020;46:35–45 [DOI] [PubMed] [Google Scholar]
- 25.Shammas HJ, Ortiz S, Shammas MC, Kim SH, Chong C. Biometry measurements using a new large-coherence-length swept-source optical coherence tomographer. J Cataract Refract Surg 2016;42:50–61 [DOI] [PubMed] [Google Scholar]
- 26.Nemeth G, Modis L, Jr. Ocular measurements of a swept-source biometer: repeatability data and comparison with an optical low-coherence interferometry biometer. J Cataract Refract Surg 2019;45:789–797 [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Chen H, Li Y, Chen Z, Gao R, Yu J, Zhao Y, Lu W, McAlinden C, Wang Q. Comprehensive comparison of axial length measurement with three Swept-Source OCT-based biometers and partial coherence interferometry. J Refract Surg 2019;35:115–120 [DOI] [PubMed] [Google Scholar]
- 28.Srivannaboon S, Chirapapaisan C, Chonpimai P, Loket S. Clinical comparison of a new swept-source optical coherence tomography-based optical biometer and a time-domain optical coherence tomography-based optical biometer. J Cataract Refract Surg 2015;41:2224–2232 [DOI] [PubMed] [Google Scholar]
- 29.Kunert KS, Peter M, Blum M, Haigis W, Sekundo W, Schütze J, Büehren T. Repeatability and agreement in optical biometry of a new swept-source optical coherence tomography-based biometer versus partial coherence interferometry and optical low-coherence reflectometry. J Cataract Refract Surg 2016;42:76–83 [DOI] [PubMed] [Google Scholar]
- 30.Kurian M, Negalur N, Das S, Puttaiah NK, Haria D, J TS, Thakkar MM. Biometry with a new swept-source optical coherence tomography biometer: repeatability and agreement with an optical low-coherence reflectometry device. J Cataract Refract Surg 2016;42:577–581 [DOI] [PubMed] [Google Scholar]
- 31.Kiraly L, Stange J, Kunert KS, Sel S. Repeatability and agreement of central corneal thickness and keratometry measurements between four different devices. J Ophthalmol 2017;2017:6181405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shajari M, Cremonese C, Petermann K, Singh P, Müller M, Kohnen T. Comparison of axial length, corneal curvature, and anterior chamber depth measurements of 2 recently introduced devices to a known biometer. Am J Ophthalmol 2017;178:58–64 [DOI] [PubMed] [Google Scholar]
- 33.Sel S, Stange J, Kaiser D, Kiraly L. Repeatability and agreement of Scheimpflug-based and swept-source optical biometry measurements. Cont Lens Anterior Eye 2017;40:318–322 [DOI] [PubMed] [Google Scholar]
- 34.Jung S, Chin HS, Kim NR, Lee KW, Jung JW. Comparison of repeatability and agreement between swept-source optical biometry and dual-Scheimpflug topography. J Ophthalmol 2017;2017:1516395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrer-Blasco T, Domínguez-Vicent A, Esteve-Taboada JJ, Aloy MA, Adsuara JE, Montés-Micó R. Evaluation of the repeatability of a swept-source ocular biometer for measuring ocular biometric parameters. Graefes Arch Clin Exp Ophthalmol 2017;255:343–349 [DOI] [PubMed] [Google Scholar]
- 36.References 36–82 are listed in the Supplemental References File http://links.lww.com/JRS/A418