Abstract
Water extracts from sea lavender (Limonium algarvense Erben) plants cultivated in greenhouse conditions and irrigated with freshwater and saline aquaculture effluents were evaluated for metabolomics by liquid chromatography-tandem high-resolution mass spectrometry (LC-HRMS/MS), and functional properties by in vitro and ex vivo methods. In vitro antioxidant methods included radical scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ferric-reducing antioxidant power (FRAP), and copper and iron chelating assets. Flowers’ extracts had the highest compounds’ diversity (flavonoids and its derivatives) and strongest in vitro antioxidant activity. These extracts were further tested for ex vivo antioxidant properties by oxidative haemolysis inhibition (OxHLIA), lipid peroxidation inhibition by thiobarbituric acid reactive substances (TBARS) formation, and anti-melanogenic, anti-tyrosinase, anti-inflammation, and cytotoxicity. Extract from plants irrigated with 300 mM NaCl was the most active towards TBARS (IC50 = 81 µg/mL) and tyrosinase (IC50 = 873 µg/mL). In OxHLIA, the activity was similar for fresh- and saltwater-irrigated plants (300 mM NaCl; IC50 = 136 and 140 µg/mL, respectively). Samples had no anti-inflammatory and anti-melanogenic abilities and were not toxic. Our results suggest that sea lavender cultivated under saline conditions could provide a flavonoid-rich water extract with antioxidant and anti-tyrosinase properties with potential use as a food preservative or as a functional ingredient in herbal supplements.
Keywords: halophytes, herbal products, IMTA systems, saline agriculture, salinization, salt tolerant plants, sustainability
1. Introduction
The use of herbal medicines and supplements is widespread among patients with chronic health ailments [1], and as a result, the commercial interest in natural health supplements for improving biological functions and wellness is raising the market value of herbal supplements. The current market size value is USD 6.3 billion, and with a compound annual growth rate (CAGR) of 6.2% it is estimated to reach USD 8.5 billion by 2025. However, the European market is expected to record a CAGR of 8.2% [2,3]. Moreover, several studies show that the excessive consumption of synthetic food additives is related to several health problems, including attention deficit disorders, gut diseases, obesity, immune system problems, and allergies [4,5,6,7,8]. Consequently, an increasing number of consumers is doubting the use of artificial food additives, as they are increasingly being considered unhealthy [9], urging the need to find more efficient and safer alternatives and boosting a growing demand in the food industry for natural ingredients to create “clean label” products [10]. Different organisms have been explored as sources of bioactive ingredients to be used as herbal supplements and/or food additives, such as mushrooms, glycophytes, and algae [11,12]. However, despite the recognized biotechnological potential of salt tolerant plants, that is, halophytes [13], such plants remain mostly unexplored [14].
Halophytes have evolved to live in abiotic stressful environments (e.g., hypersalinity, high UV radiation, and fluctuating extreme temperatures) that enhance the production of free radicals and the occurrence of oxidative stress in plant cell tissues by developing diverse anatomical (e.g., alt glands, succulence) and biochemical (e.g., synthesis of specific solutes, antioxidant enzymes, and secondary metabolites, selective accumulation, or exclusion of specific ions) adaptations [15]. Specifically, secondary antioxidant metabolites which are produced, such as phenolic acids, flavonoids, sterols, and vitamins, are not only crucial for plant survival, but also exhibit beneficial properties for humans, such as antioxidant, anti-inflammatory, and antitumor properties, and are therefore of high interest for the food and pharmaceutical industries [16]. Halophytes are thus considered an important reservoir of compounds with biotechnological applications, and several species are currently being produced and commercially exploited as food, food supplements, and cosmetic ingredients, including quinoa (Chenopodium quinoa Willd.), sea asparagus (Salicornia spp.), sea fennel (Chritmum maritimum L.), and golden samphire (Inula chritmoides L.) [17,18,19]. Moreover, due to the high demand for more efficient and safer alternatives, the search for new sources of natural additives to replace the synthetic ones is rising in the food industry. For example, antioxidants and anti-browning agents are valuable for inhibiting detrimental changes of foods which are sensitive to oxidation. Due to halophytes’ chemical and biological richness, there is a growing interest in identifying halophyte species with potential uses in this sector that is thirsty for innovation [20].
Besides their commercial potential, halophytes have the advantage of being able to be cultivated in saline conditions, which is of particular importance in the context of the increasing scarcity of freshwater for agriculture. In fact, the United Nations (UN) have established several farming alternatives for sustainable crop production, which include the use of brackish and saltwater for irrigation. Salinity negatively affects the productivity of most of the conventional glycophytic crops, and therefore, halophytes are the most promising model for biosaline agriculture systems [21,22]. One of such systems is integrated multi-trophic aquaculture (IMTA), where aquaculture wastes are used as both irrigation and fertilization for halophytes [23,24]. IMTA systems contribute to the reduction of the environmental impacts of aquaculture while adding further income by producing crops with commercial food and/or medicinal uses, and were already optimized for the cultivation of different halophytic species, such as sea asparagus (Salicornia sp.) and sea purslane (Halimiones portulacoides (L.) Aellen) [23,24].
The halophyte Limonium algarvense Erben (sea lavender) is rich in several bioactive secondary metabolites, especially flavonoids and its derivatives, and displays important bioactivities that are highly relevant for the improvement of human health [25,26,27]. Moreover, this species can be cultivated under greenhouse conditions and irrigated with aquaculture wastewater up to 300 mM NaCl [28]. This work aimed to further explore sea lavender cultivated under irrigation with aquaculture wastewater as a source of innovative and sustainable natural products to be used as food preservatives and/or as a functional ingredient for herbal supplements. For that purpose, sea lavender water extracts were profiled for chemical and functional properties, including in vitro (antioxidant activity, tyrosinase inhibition, anti-melanogenic and anti-inflammatory properties, cytotoxicity) and ex vivo (antioxidant) methods.
2. Materials and Methods
2.1. Chemicals
Sigma-Aldrich (Lisbon, Portugal) provided the 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), butylated hydroxytoluene (BHT), and the mouse melanoma B16 5A4 cells. Further chemicals and solvents were supplied by VWR International (Leuven, Belgium). Methanol, acetonitrile, water LC-MS optima grade, and formic acid LC-MS grade were supplied by Fisher Scientific (Hampton, VA, USA).
2.2. Plant Material
Cultivated plants were derived from wild-collected seeds (June 2018) of sea lavender from “Ria de Alvor” (Algarve, Portugal; coordinates: 37°07′34.8″ N 8°35′54.9″ W) and grown under greenhouse conditions irrigated with freshwater (approx. 0 mM NaCl) and aquaculture wastewaters at two salinity concentrations, namely, 300 and 600 mM NaCl [28]. After 14 weeks of cultivation, plants were separated into flowers, peduncles, and leaves, dried for 3 days at 40 °C, and powdered and stored at −20 °C until needed.
2.3. Preparation of the Extracts
Dried biomass was extracted with distilled water by an ultrasound-assisted procedure (1:40, w/v) for 30 min (ultrasonic bath USC-TH (VWR, Leuven, Belgium), capacity of 5.4 L, frequency of 45 kHz, supply of 230 V, a tub heater of 400 W, temperature control made by a LED display). Extracts were filtered (Whatman n° 4), evaporated under reduced pressure and temperature in a rotary evaporator, weighted, dissolved at a concentration of 10 mg/mL in distilled water, and stored at −20 °C.
2.4. Liquid Chromatography-Tandem High-Resolution Mass Spectrometry (LC-HRMS/MS) Analysis
The extracts were analyzed by Liquid Chromatography (UHPLC Elute) interfaced with a QqTOF Impact II mass spectrometer equipped with an ESI source, operating at a negative mode (Bruker Daltonics, Bremen, Germany). Chromatographic separation was carried out on a C18 reversed-phase Halo column 100 Å (150 mm × 2.1 mm, 2.7 μm particle size; Advanced Materials Technology, Wilmington, DE, USA), using a gradient elution of 0.1% formic acid in water (phase A) and acetonitrile (phase B). For details on LC-HRMS/MS settings, see Rodrigues et al. [28].
2.5. In Vitro Antioxidant Activity
2.5.1. Radical Scavenging Activity (RSA) on DPPH• and ABTS+•
Samples and positive control (BHT) were tested for RSA against the DPPH and ABTS radicals at concentrations ranging from 10 to 1000 µg/mL, as described previously [25]. Results were expressed as inhibition percentage in relation to the negative control (distilled water), and as half-maximal inhibitory concentration (IC50 values, µg/mL), when possible.
2.5.2. Ferric Reducing Antioxidant Power (FRAP)
Samples’ ability to reduce Fe3+ was assessed by the method described by Rodrigues et al. [25]. Absorbance was measured at 700 nm (Biochrom EZ Read 400, Santa Clara, CA, USA), and increased absorbance of the reaction means higher reducing power. Results were expressed as a percentage relative to the standard (BHT, 1 mg/mL), and as IC50 values (µg/mL), when possible.
2.5.3. Metal Chelating Activity on Iron (ICA) and Copper (CCA)
ICA and CCA were tested on samples and the standard (EDTA) at different concentrations (10–1000 µg/mL), as described earlier [25]. Differences in absorbance were assessed on a microplate reader (Biochrom EZ Read 400). Results were expressed as an inhibition percentage compared to the negative control (distilled water), and as IC50 values (µg/mL), whenever possible.
2.6. Ex Vivo Antioxidant Activity
2.6.1. Oxidative Haemolysis Inhibition Assay (OxHLIA)
A sheep erythrocyte solution (2.8 %, v/v; 200 µL) prepared in phosphate-buffered saline (PBS, pH 7.4) was mixed with 400 µL of either: sample (0.0625–2 mg/mL), PBS (control), distilled water (baseline), or trolox (7.81–250 µg/mL). After pre-incubation at 37 °C for 10 min with shaking, 200 μL of AAPH (160 mM) were added and the optical density was measured kinetically at 690 nm (microplate reader Bio-Tek Instruments, ELX800, Santa Clara, CA, USA) until complete haemolysis [29]. Results were expressed as IC50 values (µg/mL) for a Δt of 60 min.
2.6.2. Inhibition of Lipid Peroxidation by the Thiobarbituric Acid Reactive Substances (TBARS) Assay
A porcine brain cell solution (1:2, w/v; 100 µL) was incubated with 200 µL of sample (0.0625–2 mg/mL) or trolox (3.125–100 µg/mL) plus 100 µL of FeSO4 (10 µM) and 100 µL of ascorbic acid (0.1 mM) at 37 °C for 1 h. Then, 500 µL of trichloroacetic acid (28 % w/v) and 380 µL of thiobarbituric acid (TBA; 2 % w/v) were added and the mixture was heated at 80 °C for 20 min. After centrifugation, the color intensity of the malondialdehyde (MDA)-TBA complexes formed in the system was measured at 532 nm [30]. Results were expressed as IC50 values (µg/mL).
2.7. In Vitro Tyrosinase Inhibition
Samples were tested at concentrations between 10 and 1000 μg/mL for tyrosinase inhibitory activity, as previously described [31]. Results were expressed as an inhibition percentage relative to a control containing ultrapure water, and when possible as IC50 values (μg/mL).
2.8. Cell Culture
The RAW 264.7 cells were maintained in RPMI 1640 culture media, whereas HEK 293, HepG2, and B16 4A5 cell lines were cultured in DMEM media. All media were supplemented with 10 % heat-inactivated fetal bovine serum, glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 mg/mL). All cell lines were kept in an incubator at 37 °C, with 5 % CO2 and under a humid atmosphere.
2.8.1. In Vitro Anti-Inflammatory Activity
RAW 264.7 macrophages were seeded with a cell density of 5 × 105 cells/mL in a 96-well microplate (300 µL/well) and left to adhere for 24 h. Afterwards, cells were treated with different concentrations of the samples (15 μL) at several concentrations (6.25–100 µg/mL) and incubated for 1 h. Then, 30 μL of liposaccharide (LPS; 1 mL/mL) was added to each well and incubated for an additional 24 h. Dexamethasone was used as a positive control. Quantification of nitric oxide was performed using a Griess reagent system kit (nitrophenamide, ethylenediamine, and nitrite solutions) using a sodium nitrite calibration curve. The samples’ absorbance was measured at 540 nm on a microplate reader (ELX800 Biotek, Santa Clara, CA, USA) and results were expressed as a percentage of inhibition of nitric oxide production relative to the negative control (ultrapure water), and, if possible, as half-maximal inhibitory concentration (IC50).
2.8.2. In Vitro Anti-Melanogenic Properties
The cellular melanin content was evaluated using B16 4A5 melanoma cells, as detailed by Rodrigues et al. [32]. Cells were plated at 3.5 × 104 into 12-well plates and allowed to adhere overnight. Then, extracts and positive control (arbutin) were applied at a concentration of 100 µg/mL (allowed cell viability higher than 80 %) for 72 h. After treatment, absorbance of the samples was measured (Biochrom EZ Read 400) and the melanin content was calculated using a standard curve of synthetic melanin (0–25 μg/mL).
2.8.3. In Vitro Cytotoxicity
Cells were plated at a concentration of 1 × 104 cells/well (RAW 264.7), 5 × 103 cells/well (HEK 293 and HepG2), and 2 × 103 cells/well (B16 4A5) in 96-well tissue plates and left to adhere overnight. Afterwards, extracts (100 µL) were applied at a concentration of 100 µg/mL for 72 h. An MTT colorimetric assay was used for cellular viability determination (Biochrom EZ Read 400), as previously described [33], and results were expressed in terms of cellular viability (%) in relation to a control containing the respective medium.
2.9. Statistical Analyses
Results were expressed as mean ± standard error of the mean (SEM), and experiments were conducted at least in triplicate. Significant differences were assessed by analysis of variance (ANOVA) followed by the Tukey HSD test (p < 0.05). All statistical analyses were performed using the XLSTAT statistical package for Microsoft Excel (version 2013, Microsoft Corporation). The IC50 values were calculated by the sigmoidal fitting of data using the GraphPad Prism v. 5.0 software.
3. Results and Discussion
All sea lavender plants, from all the irrigation conditions, survived until the end of the experiment. However, plants irrigated with 600 mM NaCl were not able to produce flower stems and flowers [28]. Thus, data on the in vitro antioxidant activity and the metabolomic profile of these plant organs cultivated under this condition were not possible to obtain.
Extraction is the first stage for obtaining natural products from raw ingredients. Solvent extraction is the most frequently used method, but common organic solvents, such as methanol, ethanol, and dichloromethane, have many limitations regarding their ecological and toxicity roles for food, pharmaceutical, or cosmetic applications. Thus, more eco-friendly and less harmful solvents and methodologies are preferred. The use of water as an alternative extraction solvent has a significant advantage over organic solvents, such as ethanol, previously used in the extraction of natural compounds from sea lavender. Regardless of extracting different compounds than organic solvents, water extraction may generate higher yields of bioactive molecules, besides having a reduced environmental impact and no hazard, and require simple extraction equipment [34]. Moreover, ultrasound-assisted extraction (UAE) has been increasingly used for extracting bioactive compounds from natural products for food and pharmaceuticals, since it is considered a more sustainable technique with higher levels of efficiency (lower solvent, time, and energy consumption). In this context, ultrasound-assisted water extraction was used to obtain a hydrophilic extract from sea lavender biomass as a more sustainable, efficient, and safe extraction procedure.
3.1. Metabolomic Profile
The metabolomic profile of the water extracts of sea lavender was established by LC-ESI-HRMS/MS, and the results are summarized in Table 1. The proposed compounds were identified based on their accurate m/z values released as deprotonated molecules [M-H]-, considering the accuracy and precision of measurement parameters such as error (ppm) and mSigma. The molecular formula was validated by extracting ionic chromatograms from the raw data, and accurate masses, isotopic patterns, and fragmentation paths were evaluated, supporting the respective chemical structures. A total of 81 compounds were tentatively identified in the water extracts of the sea lavender organs and, generally, the flowers showed higher chemical diversity, followed by leaves and peduncles (52, 47, and 28 compounds, respectively). Besides, several compounds were only identified in a specific plant organ or irrigation condition.
Table 1.
Liquid chromatography-tandem high-resolution mass spectrometry (LC-ESI-HRMS/MS) identification of the main metabolites present in water extracts of sea lavender (L. algarvense) organs (flowers, peduncles and leaves) irrigated with freshwater (FWt) and two dilutions of aquaculture wastewater, corresponding to 300 and 600 mM NaCl. For distinguishing amongst very low, low, medium, and high abundance, the symbols +, ++, +++, and ++++ were used, respectively.
| Id |
Rt
(min) |
Proposed Structure |
[M-H]−
[m/z (∆ ppm)] |
MS/MS
[(m/z) (∆ ppm) (Attribution) (%)] |
Proposed Compound | Flowers | Peduncles | Leaves | ||||
| FWt | 300 mM | FWt | 300 mM | FWt | 300 mM | 600 mM | ||||||
| 1 | 2.9 | - | 272.9591 | 158.9782 | n.i. | - | - | - | - | ++ | +++ | ++++ |
| 2 | 3.1 | C12H22O11 | 341.1094 (−1.3) | 179.0558 (+1.8) [C6H11O6]− (100) 119.0333 (+14.2) [C4H7O]− (70) |
Sucrose or isomers | - | - | - | - | ++ | +++ | ++++ |
| 3 | 3.3 | C6H7O6SO3 | 254.9820 (−1.4) | 175.0245 (+1.8) [C6H7O6]− (100) 115.0022 (+12) [C4H3O4]− (80) |
Ascorbic acid sulphate | - | - | - | - | ++++ | +++ | +++ |
| 4 | 3.4 | C6H6O7 | 189.0032 (+4.3) | 189.0034 (4.5) [C6H5O7]− (100) 127.0046 (−7.2) [C5H3O4]− (50) |
Oxalosuccinic acid | ++++ | +++ | ++++ | +++ | + | ++ | ++ |
| 5 | 3.6 | C11H17NO8 | 290.0885 (−1.3) | 170.0445 (8.5) [C7H8NO4]− (10) 128.0361 (−6.4) [C5H6NO3]− (100) |
2-Deoxy-2,3-dehydro N-acetylneuraminic acid |
- | ++++ | - | - | - | - | - |
| 6 | 3.7 | C6H8O7 | 191.0187 (+5.4) | 111.0088 (−9.9) [C5H3O4]− (100) | Citric acid | ++ | ++ | - | - | +++ | +++ | +++ |
| 7 | 3.8 | C13H10O8 | 293.0339 (−2.2) | 169.0133 (−5.6) [C7H5O5]− (100) 137.0220 (−12.6) [C7H5O3]− (7−0) 125.0261 (−10.2) [C6H5O3]− (40) |
Pyrogallol gallate | + | + | - | - | - | - | - |
| 8 | 3.9 | C13H16O8SO3 | 379.0348 (−2.1) | 299.0765 (+2.4) [C13H15O8]− (10) 241.0023 (+0.4) [C6H9O8S]− (100) |
Salicylic acid glucosyl sulphate | - | - | - | - | - | ++ | + |
| 9 | 3.9 | C7H6O5 | 169.0135(−5.0) | 125.0241 (−6.4) [C6H5O3]− (100) | Gallic acid | ++ | ++ | - | - | - | - | - |
| 10 | 4.0 | C13H16O10SO3 | 411.0235 (+0.9) | 331.0671 (−5.4) [C13H15O10]− (10) 241.0023 (+0.5) [C6H9O8S]− (100) 169.0134 (−4.9) [C7H5O5]− (20) |
Glucogallin sulphate | ++ | ++ | ++++ | +++ | +++ | ++++ | ++++ |
| 11 | 4.8 | C14H18O10SO3 | 425.0398 (−0.6) | 345.0828 (−0.3) [C14H17O10]− (7) 241.0023 (+0.5) [C6H9O8S]− (100) 183.0299(+0.1) [C8H7O5]− (50) |
Glucosyl methyl gallate sulphate | - | - | - | - | - | + | ++ |
| 12 | 5.0 | C15H20O10(SO3)2 | 519.01914 (−0.6) | 439.0552 (−0.3) [C15H19O13S]− (30) 241.0025 (−0.4) [C6H9O8S]− (100) |
Glucosyringic acid disulphate | ++ | + | - | - | ++++ | ++++ | +++ |
| 13 | 5.0 | C15H20O10SO3 | 439.0554 (−0.4) | 359.1010 (−8.1) [C15H19O10]− (10) 241.0023 (−0.5) [C6H9O8S]− (100) 197.0447 (−4.3) [C9H9O5] (10) |
Glucosyringic acid sulphate | ++ | ++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 14 | 5.4 | C15H18O8(SO3)2 | 485.0062 (−0.6) | 405.0498 (+0.2) [C15H17O15S]− (90) 325.0928 (+0.2) [C15H17O8]− (10) |
Glucosyl coumaric acid disulphate | - | - | - | - | ++ | ++ | +++ |
| 15 | 5.4 | C15H20O10SO3 | 439.0558 (−0.6) | 359.1010 (−8.1) [C15H19O10]− (20) 241.0026 (−0.8) [C6H9O8S]− (100) |
Glucosyringic acid sulphate isomer | +++ | +++ | - | - | - | - | - |
| 16 | 5.8 | C13H16O16SO3 | 379.0348 (−2.1) | 299.0768 (+1.4) [C13H15O8]− (15) 241.0023 (−0.5) [C6H9O8S]− (100) |
Salicylic acid glucoside | - | - | - | - | + | - | + |
| 17 | 5.9 | C18H24O13 | 447.1144 (−1.0) | 429.1041 (−0.6) [C18H21O12]− (60) 339.0722 (−0.1) [C15H15O9]− (30) 301.0568 (−1.1) [C12H13O9]− (20) |
Aralidioside | ++ | ++ | - | - | - | - | - |
| 18 | 5.9 | C15H18O8SO3 | 405.0496 (+0.1) | 325.0926 (+0.8) [C15H17O8]− (10) 241.0025 (−0.4) [C6H9O8S]− (100) 163.0396 (+2.2) [C9H7O3]− (10) |
Glucosyl coumaric acid sulphate | ++ | +++ | - | - | +++ | ++++ | ++++ |
| 19 | 6.2 | C15H18O9SO3 | 421.0451 (−1.1) | 341.0660 (−1.3) [C15H17O9]− (7) 241.0018 (+0.5) [C6H9O8S]− (100) 179.0344 (+3.4) [C9H7O4]− (40) |
Glucosyl-caffeic acid sulphate | + | + | - | - | - | - | - |
| 20 | 6.4 | C20H20O14 | 483.0779 (+0.3) | 313.0671 (−0.1) [C13H15O10]− (20) 313.0564 (+0.3) [C13H13O9]− (60) 271.0461 (−0.5) [C11H11O8]− (100)169.0129 (+8.1) [C7H5O5]− (40) |
Digalloyl glucose | + | ++ | ++ | ++ | - | - | - |
| 21 | 6.5 | C37H30O18 | 761.1356 (0.4) | 609.1249 (+0.1) [C30H25O14]− (20) 423.0721 (+0.0) [C22H15O9]− (100) 305.0667 (+0.0) [C15H13O7]− (70)169.0135 (−3.5) [C7H5O5]− (7) |
Theasinensin B | - | +++ | - | - | - | - | - |
| 22 | 6.5 | C15H18O8SO3 | 405.0496 (−1.6) | 241.0025 (−0.4) [C6H9O8S]− (100) 163.0395 (+3.6) [C9H7O3]− (30) |
Glucosyl coumaric acid sulphate isomer | ++ | - | ++ | +++ | - | - | - |
| 23 | 6.8 | C15H18O8(SO3)2 | 485.0061 (+0.7) | 405.0497 (+0.1) [C15H17O11S]− (40) 325.0928 (+0.2) [C15H17O8]− (5) 241.0024 (−0.1) [C6H9O8S]− (100) |
Glucosyl coumaric acid disulphate isomer | +++ | ++++ | - | - | +++ | +++ | ++++ |
| 24 | 6.8 | C15H18O8SO3 | 405.0498 (−0.3) | 325.0930 (−0.5) (C15H17O8)− (10) 241.0023 (−1.8) [C6H9O8S]− (100) 163.0397 (2.5) [C9H7O3]− (10) |
Glucosyl coumaric acid sulphate isomer | +++ | ++ | +++ | +++ | - | ++++ | ++++ |
| 25 | 7.0 | C37H28O18 | 759.1207 (−0.6) | 423.0724 (−0.6) [C22H15O9]− (100) 301.0354 (−0.2) [C15H9O7]− (80) |
ProdelphinidinA2 3′-gallate | ++++ | +++ | - | - | - | - | - |
| 26 | 7.0 | C20H22O12 | 453.1040 (−0.3) | 313.0567 (+0.7) [C13H13O9]− (100) 169.0131 (+6.5) [C7H5O5]− (40) |
Galloyl glucose derivative | - | - | +++ | +++ | - | - | - |
| 27 | 7.4 | C11H14O4SO3 | 289.0393 (−1.8) | 209.0826 (−3.1) [C11H13O4]− (60) 149.0599 (5.9) [C9H9O2]− (100) |
Sinapyl alcohol sulphate | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 28 | 7.5 | C15H16O10SO3 | 435.0240 (−0.2) | 355.0671 (−0.1) [C15H15O10]− (20) 197.0444 (+6.0) [C9H9O5]− (100) |
Caffeic acid-3-glucuronide sulphate | ++ | ++ | - | - | ++++ | +++ | ++++ |
| 29 | 7.7 | C17H30O10 | 393.1770 (−0.9) | 271.0610 (+0.8) [C15H11O5]− (20) 205.0709 (+4.3) [C8H13O6]− (100) 119.0337 (+7.8) [C4H7O4]− (80) |
Cis-3-hexenyl-b-primeveroside | ++++ | ++++ | - | - | - | - | - |
| 30 | 7.8 | C28H24O17 | 631.0944 (−0.6) | 479.0830 (+0.2) [C21H19O13]− (90) 316.019 (−1.8) [Y0−H]−∙ [C15H8O8]−∙ (100) |
Myricetin-3-O-galloyl-hexoside | +++ | +++ | +++ | +++ | ++ | +++ | ++++ |
| 31 | 8.0 | C18H24O12 | 431.1192 (−0.2) | 285.0625 (−3.1) [C12H13O8]− (10) 225.0409 (−1.8) [C10H9O8]− (100) |
Licoagroside B | + | +++ | - | - | - | - | - |
| 32 | 8.0 | C27H30O17 | 625.1418 (−1.2) | 316.0233 (−2.8) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-rutinoside | - | - | ++ | ++ | ++ | +++ | +++ |
| 33 | 8.2 | C21H20O13 | 479.0835 (+0.1) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) 271.0253 (−1.9) (C14H7O6)− (20) |
Myricetin-3-O-glucoside | +++ | +++ | - | - | ++ | +++ | ++++ |
| 34 | 8.5 | C22H22O12 | 477.1036 (+0.4) | 433.1145 (−1.1) (C21H21O10)− (20) 313.0567 (−0.7) (C13H13O9)− (100) 169.0141 (+1.0) [C7H5O5]− (40) |
Galloylhexoside derivative | ++ | ++ | ++ | +++ | - | - | - |
| 35 | 8.6 | C28H24O16 | 615.0985 (+0.1) | 463.0882 (+0.1) [C21H19O12]− (100) 301.0345 (+3.0) [Y0]− [C15H9O7]− (80) 300.0279 (−1.0) [Y0−H]−∙ [C15H8O7]−∙ (90) |
Quercetin-3-O-galloyl-hexoside | - | - | - | + | + | + | + |
| 36 | 8.7 | C18H20O10 | 371.0981 (+0.6) | 249.0615 (+0.2) [C9H13O8]− (100) | Hydroxyferuloylglucose | - | - | - | - | - | +++ | +++ |
| 37 | 8.7 | C27H30O16 | 609.1456 (+0.8) | 463.0882 (−0.1) [C22H19O12]− (400) 300.0275 (−0.8) [Y0−H]−∙ [C15H8O7]−∙ (100) |
Quercetin-3-O-rutinoside | - | - | - | - | ++ | ++ | +++ |
| 38 | 8.8 | C19H26O12 | 445.1350 (+0.2) | 285.0623 (−2.6) [C12H13O8]− (7) 225.0429 (−1.9) [C10H9O8]− (100) |
Methyl licoagroside B | +++ | +++ | - | - | - | - | - |
| 39 | 8.8 | C14H14O9 SO3 | 405.0145 (−1.2) | 325.0564 (0.3) [C14H13O9]− (20) 209.0454 (+0.5) [C10H9O5]− (50) 167.0344 (+3.2) [C8H7O4]− (100) |
Galloylshikimic acid sulphate | - | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 40 | 9.0 | C21H20O12 | 463.0872 (+2.2) | 316.020 (−1.8) [Y0−H]−∙ [C15H8O8]−∙ (100) 217.0250 (−0.8) [C14H7O6]− (20) |
Myricetin-3-O-rhamnoside | - | ++++ | ++ | ++ | ++ | +++ | ++++ |
| 41 | 9.0 | C15H16O10SO3 | 435.0251 (−2.9) | 355.0677 (−1.7) [C15H15O10]− (10) 197.0455 (+5.5) [C9H9O5]− (100) |
Caffeic acid glucuronic sulphate | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 42 | 9.3 | C21H20O13 | 479.0835 (+0.1) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-glucoside | + | + | - | - | - | - | - |
| 43 | 9.3 | C20H24O8SO3 | 471.0967 (−0.5) | 275.0228 +(0.9) [C10H11O4SO3]− (100) 195.0651 (+6.1) [C10H11O4]− (50) |
Acetosyringone sulphate | - | - | - | - | ++ | ++ | ++ |
| 44 | 9.3 | C28H24O15 | 599.1036 (+0.7) | 316.022 (−0.8) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-pentoside-gallate | - | - | - | - | - | - | + |
| 45 | 9.3 | C30H28O17 | 659.1244 (+1.5) | 316.019 (−1.7) [Y0−H]−∙ [C15H8O8]−∙ (100) 479.0811 (+4.2) [C21H19O13]− (20) |
Myricetin-3-O-(3-caffeic acid-glucoside) | - | - | - | - | + | ++ | +++ |
| 46 | 9.4 | C23H22O14 | 521.0931 (−1.1) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin 3-O-(6-acetylgalactoside) | - | - | + | ++ | +++ | ||
| 47 | 9.4 | C29H26O16 | 629.1143 (+0.9) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | 2’-C-methyl-myricetin-3-O-rhamnoside-gallate | - | - | - | - | - | + | ++ |
| 48 | 9.4 | C22H26O8SO3 | 599.1042 (+0.8) | 447.0932 (+0.2) [C21H19O11]− (30) 313.0563 (+0.5) [C13H13O9]− (100) 169.0141 (+1.0) [C7H5O5]− (40) |
Di-galloyl-hexose malic acid | - | - | + | + | - | - | - |
| 49 | 9.4 | C28H24O15 | 599.1045 (−0.4) | 447.0943 (−0.8) [C21H19O11]− (50) 285.0404 (0.1) [Y0−H]−∙ [C15H9O6]− (100) |
Kaempferol-galloyl-hexoside | - | ++ | - | - | - | - | - |
| 50 | 9.5 | C9H10O5 | 197.0445 (+5.5) | 124.0166 (−0.4) [C6H4O3]− (100) | Syringic acid | +++ | - | - | - | - | - | - |
| 51 | 9.5 | C28H24O15 | 599.1037 (+0.9) | 447.0931 (+0.3) [C21H19O11]− (30) 316.022 (−0.8) [Y0−H]−∙ [C15H8O8]−∙ (100) |
Myricetin-3-O-pentoside-gallate isomer | - | - | - | - | ++ | ++ | ++ |
| 52 | 9.6 | C29H26O16 | 629.1143 (+0.9) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | 2′-C-methyl-myricetin-3-O-rhamnoside-gallate isomer | - | - | - | - | + | ++ | - |
| 53 | 9.8 | C13H14N2O3 | 203.0818 (+4.0) | 142.0648 (+9.2) [C10H8N]− (40) | Tryptophan | - | - | ++++ | ++ | - | - | - |
| 54 | 9.8 | C13H14N2O3 | 245.0932 (+0.8) | 203.0818 (+4.0) [C11H11N2O2]− (100) 142.0648 (+9.2) [C10H8N]− (40) |
N-acetyl-tryptophan | ++++ | + | - | - | ++++ | +++ | +++ |
| 55 | 9.8 | C14H14N2O5 | 289.0830 (−0.1) | 203.0818 (+4.0) [C11H11N2O2]− (100) | N-manoyl-tryptophan | +++ | +++ | +++ | - | +++ | ++ | ++ |
| 56 | 9.8 | C24H22O5 | 549.0891 (−1.0) | 316.025 (−2.2) [Y0−H]−∙ [C15H8O8]−∙ (100) 217.0250 (−0.8) [C14H7O6]− (20) |
Myricetin-3-O-acetyl--malonyl-deoxyhexose | - | - | - | + | - | - | - |
| 57 | 10.0 | C27H36O12 | 551.2134 (+0.0) | 357.1346 (−0.7) [C20H12O6]− (100) | Pinoresinol derivative | - | - | + | - | - | - | - |
| 58 | 10.1 | C21H22O10 | 433.1136 (+1.0) | 271.0610 (−0.1) [Y0]− [C15H11O5]− (100) | Naringenin-7-O-glucoside | ++ | ++ | - | - | - | - | - |
| 59 | 10.1 | C21H18O11 | 445.0775 (+0.3) | 269.0456 (−0.1) [Y0]− [C15H9O5]− (100) | Apigenin-7-O-glucuronide | ++++ | +++ | - | - | - | - | - |
| 60 | 10.2 | C23H22O13 | 505.0995 (−1.2) | 316.0230 (−0.9)[Y0−H]−∙ [C15H8O8]−, (100) 217.0253 (−1.9) [C14H7O6]− (15) |
Myricetin-3-O-acetyl-deoxyhexose | - | - | - | - | +++ | +++ | ++++ |
| 61 | 10.5 | C28H26O14 | 585.1247 (+0.4) | 439.0880 (+0.5) [C19H19O13]− (100) 271.0608 (+1.2) [Y0]− [C15H11O5]− (10) |
Prunin-6′’-O-gallate | +++ | +++ | - | - | |||
| 62 | 10.5 | C18H18O7SO3 | 425.0545 (+0.3) | 345.0980 (+0.0) [C18H17O7]− (40) 315.0872 (+0.5) [C17H15O6]− (60) 300.0638 (+0.6) [C16H12O6]−∙ (100) |
3′,4′,5′-Trimethoxyflavanone sulphate | - | - | - | +++ | +++ | +++ | |
| 63 | 10.6 | C22H26O8SO3 | 497.1125 (−0.3) | 417.1556 (−0.3) [C22H25O8]− (100) 181.0493 (+7.4) [C9H9O4]− (90) |
Syringaresinol sulphate | ++ | +++ | ++ | ++ | +++ | +++ | ++++ |
| 64 | 10.7 | C17H24O7SO3 | 467.1019 (+1.1) | 387.1448 (+0.4) [C17H23O7]− (100) 372.1212 (+0.5) [C16H20O7]−∙ (90) 357.0978 (+0.4) [C19H17O7]− (40) 181.0494 (+7.0) [C9H9O4]− (50) 151.0309 (+6.8) [C8H7O3]− (40) |
Medioresinol sulphate | - | - | - | - | ++ | +++ | +++ |
| 65 | 10.7 | C23H22O12 | 489.1042 (−0.8) | 300.0280 (−1.6) [Y0−H]−∙ [C15H8O7]−∙ (100) 271.0153 (−1.9) [C14H7O6]− (30) |
Quercetin-3-O-acetyl-rhamnoside | + | ++ | - | - | - | - | - |
| 66 | 11.0 | C29H26O14 | 597.1245 (−0.5) | 413.0887 (−2.2) [C21H17O9]− (10) 301.03607 (−1.9) [C15H9O7]− (20) 269.0456 (−0.3) [Y0]− [C15H9O5]−∙ (100) |
Apigenin derivative | + | ++ | - | - | - | - | - |
| 67 | 11.2 | C15H12O6 | 287.0563 (+0.6) | 151.0031 (+3.9) [1,3A−] [C7H3O4]− (20) 135.0435 (−0.8) [1,3B−] [C8H7O2]− (100) |
2-Hydroxynaringenin | ++ | ++ | - | - | - | - | - |
| 68 | 11.3 | C18H18O7SO3 | 425.0553 (+0.9) | - | 3′,4′,5′-Trimethoxyflavanone sulphate isomer | - | - | - | - | ++ | ++ | ++ |
| 69 | 11.5 | C22H26O8SO3 | 497.1135 (−2.3) | 417.1164 (−2.1) [C22H25O8]− (50) 402.1326 (−1.5) [C21H22O8]− (90) 387.1093 (−2.0) [C20H19O8]− (60) 181.0500 (+3.4) [C9H9O4]− (100) |
Syringaresinol sulphate isomer | - | - | - | - | + | ++ | ++ |
| 70 | 11.5 | C20H22O6SO3 | 437.0904 (+1.0) | 357.1339 (+1.4) [C20H12O6]− (100) 342.1104 (+1.3) [C19H18O6]− (85) 151.0391 (+6.3) [C8H7O3] (70) |
Pinoresinol sulphate | ++++ | +++ | +++ | +++ | ++++ | ++++ | ++++ |
| 71 | 12.1 | C20H22O6SO3 | 437.0916 (+1.8) | 357.1339 (+1.4) [C20H12O6]− (100) | Pinoresinol sulphate isomer | ++ | + | - | - | +++ | +++ | +++ |
| 72 | 12.2 | C15H20O6 | 285.0403 (+0.6) | 199.0392 (+4.2) [C12H7O3]− (50) 175.0390 (+6.1) [C10H7O3]− (60) 151.0029 (+4.9) [1,3A−] [C7H3O4]− (40) 133.0279 (−8.3) [1,3B−] [C8H5O2]− (100) |
Luteolin | ++ | ++ | - | - | - | - | - |
| 73 | 12.2 | C25H24O14 | 547.1102 (−1.6) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-diacetylrhamnoside | + | + | - | + | +++ | ++ | ++++ |
| 74 | 12.3 | C15H12O6 | 287.0565 (−1.2) | 269.0458 (−1.0) [C15H9O5]− (30) 259.0614 (+0.6) [C14H11O5]− (30) 177.0546 (+6.6) [C10H9O3]− (100) 151.0031(+3.8) [1,3A−] [C7H3O4]− (40) |
Dihydrokaempferol | ++ | +++ | - | - | - | - | - |
| 75 | 13.3 | C18H22O5 | 327.2175 (−0.6) | - | Trihydroxy-10,15-octadecadienoic acid | +++ | +++ | +++ | +++ | ++ | ++ | ++ |
| 76 | 13.5 | C15H10O5 | 269.0457 (−0.6) | 227.0347 (−1.1) [C13H7O4]− (60) 151.0030 (+2.5) [1,3A−] [C7H3O4]− (70) 117.0324 (+9.1) [1,3B−] [C8H7O]− (100) |
Apigenin | +++ | + | - | - | - | - | - |
| 77 | 13.7 | C15H12O5 | 271.0615 (−1.3) | 187.0393 (+4.3) [C11H7O3]− (40) 151.0030 (+3.4) [1,3A−] [C7H3O4]− (50) 119.0490 (+9.9) [1,3B−] [C8H7O]− (100) |
Naringenin | +++ | ++++ | - | - | - | - | - |
| 78 | 14.1 | C18H34O5 | 329.2332 (−0.5) | - | Trihydroxy-10-octadecenoic acid | +++ | ++++ | ++ | ++ | ++ | ++ | ++ |
| 79 | 14.3 | C13H24O3SO3 | 307.1220(−0.2) | - | Oxo-tridecanoic acid sulphate | + | - | ++++ | ++ | ++ | +++ | +++ |
| 80 | 14.9 | C18H12O4 | 287.2227 (+0.4) | - | 10, 16-Dihydroxyhexadecanoic acid | + | - | - | - | +++ | ++ | ++ |
-: not detected.
Twenty tree compounds, corresponding to 28% of the identified compounds, were only detected in flowers, namely, 2,3-dehydro-2-deoxy-N-acetylneuraminic acid (5), gallic acid (9), an isomer of glucosyringic acid sulphate isomer (15), aralidioside (17), glucosyl-caffeic acid sulphate (19), theasinensin B (21), prodelphinidin A2 3′-gallate (25), cis-3-hexenyl-b-primeveroside (29), licoagroside B (31), methyl licoagroside B (38), myricitin-3-O-glucoside (42), kaempferol-galloyl-hexoside (49), syringic acid (50), naringenin-7-O-glucoside (58) apigenin-7-O-glucuroide (59), prunin-6″-O-gallate (61), quercetin-3-O-acetyl-rhamnoside (65), apigenin derivative (66) 2-hydroxynaringenin (69), luteolin (72), dihydrokaempferol (74), apigenin (76), and naringenin (77). In turn, 21 compounds (26% from total compounds) were only identified in leaves, namely, a non-identified compound (1), sucrose (2), ascorbic acid sulphate (3), a salicylic acid glucosyl sulphate (8), glucosyl methyl gallate sulphate (11), glucosyl coumaric acid disulphate (14), salicylic acid glucoside (16), hydroxyferuloylglucose (36), quercetin-3-O-rutinoside (37), acetosyringone sulphate (43), two isomers of myricetin-3-O-pentoside-gallate (44, 51), myricetin-3-O-(caffeic acid-glucoside) (45), myricetin-3-O-(6-acetylgalactoside) (46), two isomers of 2′-C-methyl-myricetin-3-O-rhamnoside-gallate (47, 52), myricetin-3-O-acetyl-deoxyhexose (60), two isomers of 3′,4′,5′-trimethoxyflavanone sulphate (62, 68), medioresinol sulphate (64), and an isomer of syringaresinol sulphate (69). Five compounds (6% from total) were only present in peduncles, namely, one galloyl glucose derivative (26), di-galloyl hexose malic acid (48), tryptophan (53), myricetin-3-O-acetyl-malonyl-deoxyhexose (56), and one pinoresinol derivative (57).
Additionally, the irrigation salinity influenced the relative abundance of some compounds. For instance, increasing salinity (including all tested salinities) led to a general increase in relative abundance of compounds 1, 2, 18, 20, 22, and 23. Compounds 10, glucosyl coumaryl acid sulphates (14 and 24), hydroxyferuloylglucose (36), and several myricetin glycoside derivatives (32, 40, 45, 46, 47, 60, and 73), two syringaresinol sulphates (63 and 69), and one medioresinol sulphate (64) only decreased in the leaves’ extracts. However, digalloyl glucose (20) and one galloylhexoside derivative (34) only decreased in flowers and peduncles, respectively. For some other compounds, as the irrigation salinity increased (among all tested salinities), their relative abundance decreased, namely compounds 3, 8, 12, 22, 24, syringic acid (50), tryptophan (53), and one pinoresinol sulphate isomer (71). Oxalosuccinic acid (4) decreased in both flowers and peduncles, apigenin (76) declined only in flowers, whereas for the pinoresinol derivative (57) only in peduncles.
Plants contain a wide variety of secondary metabolites, namely polyphenolic compounds, as, for example, flavonoids, tannins, and phenolic acids that are the most common plant-derived natural products, and that are widely present in the sea lavender aqueous extracts [35]. Moreover, enzymatic modifications of these known molecules result in the generation of many types of derivatives, such as prenylated, acetylated, methylated, sulphated, glucuronated, and glycosylated compounds [36]. Sulphated phenolics were usually found in plants from saline areas, which indicates a strong correlation between environments rich in salts and the biosynthesis of sulphated compounds. Despite their functional role in plants is still not being evident, these structural modifications can be considered ecological adaptations with important functions in co-pigmentation, plant growth regulation, molecular recognition, detoxification, and signalling pathways [36,37]. Moreover, sulphation, methylation, and glycosylation contribute to the improvement of the solubility, stability, and biological activities of these molecules, as, for example, negatively charged sulphated derivatives that have higher water solubility, important for interactions with biological targets [36,38,39]. Galloylation of phenolic compounds also affects their properties, which is important for their protective antioxidant mechanisms; for example, galloyl groups may increase the capacity to donate electrons, chelate iron, regenerate tocopherol, and for lipophilicity [40]. Thus, galloylation of polyphenols changes their biological properties, with more galloyl moieties in the structure resulting in increased biological activity when compared to the parent molecules [41]. Therefore, the high representativity of glycosylated, sulphated, and galloylated phenolic compounds in extracts of produced sea lavender plants may be related to stress resistance mechanisms, namely, to high UV radiation and temperature which they are subjected to in the greenhouse, as well as irrigation salinity. For example, salt stress promotes the accumulation of glucose derivatives for mitigating stress conditions, including osmoprotection, carbon storage, and scavenging free radicals, which may ultimately also affect the biological properties of the produced plants [42].
Twenty-eight of those identified have already been reported in infusions made from flowers of this species, including gallic (9) and syringic (46) acids, and apigenin (72). Furthermore, these and 24 more compounds (2, 26, 27, 30–34, 40, 46–47, 51, 54, 60, 66–67, 70, 72, 74–75, and 78–79) have also been described in ethanol extracts of sea lavender grown under saline cultivation [25,26,27,28]. Several other compounds were detected in L. algarvense for the first time; however, they were previously described in other Limonium species, as, for example, citric acid (6) detected in methanol leaf extract from L. globuliferum and L. quesadense [43,44] and quercetin-galloyl-hexoside (35) identified in L. delicatulum and L. quesadense [44]. Myricetin-3-O-glucoside (33) was previously detected in L. caspium and L. aureum [45,46], while tryptophan (53) was reported in L. doufourii and L. albuferae aerial parts grown under salt stress [47].
3.2. In Vitro Antioxidant Activity
In a preliminary approach to appraise the antioxidant properties of ultrasound-assisted water extracts, all samples were subjected to a preliminary evaluation by five complementary in vitro methods, including radical and redox metal assays, and the results are summarized in Table 2. Flowers’ extracts from freshwater-irrigated sea lavenders had the highest capacity to scavenge the ABTS radical, to chelate copper, and to reduce iron (IC50 values of 397, 642, and 129 μg/mL, respectively). However, when plants were irrigated with saline aquaculture wastewaters, the peduncles from plants watered with 300 mM NaCl had the best capacity to scavenge DPPH (IC50 = 383 μg/mL), whereas flowers exhibited the best activity in the ABTS, CCA, and FRAP assays (IC50 = 617, 720, and 191 μg/mL). None of the samples had significant ICA at the maximum concentration tested (1 mg/mL).
Table 2.
In vitro antioxidant activities of water extracts of different sea lavender (L. algarvense) organs (flowers, peduncles, and leaves) from greenhouse-produced plants irrigated with different salinities (freshwater, and 300 and 600 mM NaCl). Results are expressed as IC50 values (µg/mL).
| Irrigation Salinity/Treatment | Plant Organ | DPPH | ABTS | CCA | FRAP |
|---|---|---|---|---|---|
| Freshwater | Flower | 604 ± 4 d | 397 ± 2 b | 642 ± 21 b | 129 ± 6 a |
| Peduncles | 532 ± 17 c | 800 ± 13 e | 922 ± 31 d | 251 ± 22 c | |
| Leaves | 549 ± 7 c | 793 ± 11 e | 953 ± 27 d | 339 ± 14 d | |
| 300 mM NaCl | Flower | 692 ± 11 f | 617 ± 14 c | 720 ± 8 c | 191 ± 17 b |
| Peduncles | 383 ± 7 b | 745 ± 9 d | - | 228 ± 10 c | |
| Leaves | - | - | - | 351 ± 11 d | |
| 600 mM NaCl | Leaves | - | - | - | 251 ± 7 c |
| Positive control * | 111 ± 9 a | 142 ± 11 a | 171 ± 9 a | na |
-: activity lower than 50% at the higher concentration tested (1 mg/mL). na: not applicable. * Positive controls: BHT (RSA of DPPH and ABTS) and EDTA (CCA). Values represent the mean ± standard error of the mean (SEM) of at least three repetitions performed in triplicate (n = 9). In the same column, values marked with different letters are significantly different at p < 0.05 (Tukey HSD test).
As discussed in Section 3.1, the sea lavender water extracts are rich in phenolic compounds, mainly flavonoids, tannins, and phenolic acids, as well as their sulphated, glycosylated, and galloylated derivatives (Table 1). It is well-documented that phenols are great antioxidants that may act in several ways: (1) hydrogen-donating antioxidants react with reactive oxygen and nitrogen species, stopping the production of radicals by generating a molecule that is more chemically stable than the initial radical; (2) related to their metal chelating properties that participate in free radicals generation; or (3) interaction with proteins, due to their ability to inhibit enzymes implicated in free radicals formation, such as cytochrome P450, lipoxygenases, cyclooxygenase, and xanthine oxidase [35,48]. Therefore, natural products rich in these compounds, such as the water extract studied in this work, have the potential to be used as antioxidant supplements, to inactivate free radicals, and decrease the potential occurrence of cellular damage that leads to disease development [49], or as antioxidant food additives, to slow or stop the breakdown of fats and oils, thus contributing to food preservation [50].
The greater antioxidant capacity found in the flowers’ extracts may be related to the higher diversity of flavonoids and its derivatives detected in this plant organ (Table 1), since this group of molecules is well-known for this capacity; however, studies that support the activity of the most identified compounds alone are limited. Furthermore, the antioxidant activity of sea lavender water extracts generally decreased with increasing irrigation salinity. The presence of salt ions might affect plant growth and secondary metabolism, which influences the quantitative and qualitative variation of antioxidant molecules and its biological properties [20]; therefore, the decrease in the relative abundance of some of the detected compounds (stated in Section 3.1) with the increase in irrigation salinity is likely to be reflected in the reduced antioxidant activity observed. Still, these molecules are essential to reduce the levels of oxidative radicals induced by harsh environmental conditions [20]. Additionally, while the synthesis of some compounds (e.g., phenolics) can be impaired, other biosynthetic pathways can be activated by higher UV-radiation or salinity, such as pigments (e.g., chlorophylls, carotenoids) or proline [51].
Despite the few studies reporting on the impact of salinity on halophytes’ biological properties, some species display the same pattern as sea lavender. For example, acetone extracts from stems and leaves of Polygonum maritimum L. (sea knotgrass) showed reduced antioxidant capacity (in vitro radical-scavenging activity and copper chelation) with increasing irrigation salinity (from approx. 0 to 600 mM NaCl) when grown under greenhouse conditions [27]. The DPPH radical-scavenging activity of methanol extracts from Sesuvium portulacastrum (L.) (shoreline purslane or sea purslane) grown in outdoor containers was also influenced by salinity, while stems had reduced antioxidant capacity, and the leaves and roots displayed increased activity with augmented salt concentration (0 to 200 mM NaCl) [51]. Likewise, methanol extracts from greenhouse-cultivated Cakile maritima L. (sea rocket), obtained from seeds collected in Tabarka (Tunisia), exhibited reduced antioxidant activity when plants were irrigated with water containing higher NaCl concentrations (0–400 mM), but the same species obtained from seeds collected in Jerba (Tunisia) presented an opposite trend [52]. These data indicate that the antioxidant capacity of cultivated plants may be triggered by diverse factors, such as the local collection, the plant’s developmental stage, and plant organ, even for the same species, suggesting that many parameters may be involved and that adaptations may not be species-specific.
As the flowers’ extracts had the highest antioxidant capacity overall, they were therefore selected from fresh- and saltwater irrigated (300 mM NaCl) plants to be further appraised for their potential use as an ingredient source for herbal supplements and food additives. For that, extracts were analyzed for their ex vivo antioxidant, anti-melanogenic, and anti-inflammatory properties and toxicity, and for in vitro tyrosinase inhibition.
3.3. Ex Vivo Antioxidant Activity
Most methods for evaluating antioxidant activity are performed in vitro and based on radicals with reduced or nil biological relevance. Such in vitro methods are useful for screening purposes, but the obtained results should be confirmed in assays with biological targets that resemble those found in vivo, such as ex vivo methods, where cells are taken from an in vivo model and are used in vitro tests by using radicals and substrate targets with higher biological relevance compared to conventional assays.
In this work, the selected extracts were tested by two ex vivo antioxidant assays, which allowed to evaluate their ability to inhibit lipid peroxidation (by the TBARS formation) and oxidative haemolysis (OxHLIA), as shown in Table 3. The flower extract from plants irrigated with the aquaculture wastewater containing 300 mM NaCl was the most active (IC50 = 81 µg/mL) in TBARS compared to that from freshwater-irrigated plants (IC50 = 127 µg/mL). In the OxHLIA assay, no significant difference was found amongst the two irrigation systems (freshwater: IC50 = 136; 300 mM NaCl: IC50 = 140 µg/mL). To our best knowledge, this is the first report on the use of ex vivo assays to determine the antioxidant activity of sea lavender.
Table 3.
Functional properties of water extracts from greenhouse-cultivated sea lavender (L. algarvense) irrigated with freshwater and aquaculture wastewaters containing 300 mM of NaCl: ex vivo antioxidant (OxHLIA and TBARS), in vitro anti-melanogenic/anti-browning (tyrosinase inhibition), cellular anti-inflammatory (NO reduction), and inhibition of melanin synthesis on mouse melanoma (B16 4A5) cells. Results are expressed as IC50 values (µg/mL).
| Biological Activity | Method | Freshwater | 300 mM | Positive Control * |
|---|---|---|---|---|
| Antioxidant | TBARS | 127 ± 45 c | 81 ± 28 b | 9.1 ± 0.3 a |
| OxHLIA (Δt = 60 min) | 136 ± 4 b | 140 ± 4 b | 21 ± 1 a | |
| Anti-inflammatory | NO reduction | - | - | 16 ± 1 |
| Anti-melanogenic/anti-browning | Tyrosinase inhibition | - | 873 ± 59 b | 137 ± 6 a |
| Anti-melanogenic | Inhibition of melanin synthesis by B16 4A5 cells | - | - | 16 ± 1 |
-: activity lower than 50% at the maximum concentration tested (NO reduction: 400 µg/mL; tyrosinase inhibition: 1000 µg/mL; inhibition of melanin synthesis on B16 cells: 100 µg/mL); *: positive controls: Trolox (antioxidant), dexamethasone (anti-inflammatory), arbutin (anti-melanogenic/anti-browning) and ellipticine (cytotoxicity). TBARS: inhibition of lipid peroxidation using thiobarbituric acid reactive substances; OxHLIA: oxidative haemolysis inhibition assay; NO: nitric oxide. Values represent the mean ± standard error of the mean (SEM) of at least six repetitions (n = 6). In each line, values followed by different letters are significantly different at p < 0.05 (Tukey HSD test).
Lipid peroxidation is associated with several degenerative disorders due to the high susceptibility of lipids to oxidation. Moreover, the integrity of cell membranes is kept by lipids; thus, extensive lipid peroxidation alters their composition, structure, and function, promoting further damage of DNA and proteins that ultimately causes cellular death. Reducing cellular lipid peroxidation may be crucial to prevent the occurrence of degenerative and chronic disorders linked to oxidative stress. Furthermore, lipid peroxidation is also a major cause of deterioration in foods rich in fat, especially those containing polyunsaturated fats (PUFAs). BHT, BHA, and α-tocopherol are common lipid-soluble antioxidants used by food industries to prevent oxidation. However, they are volatile and decompose at high temperatures, and are also associated with several health concerns, such as skin allergic reactions, carcinogenic effects, and hormonal dysregulation [53,54,55]. In addition, they are toxic to aquatic organisms and have the potential to bioaccumulate [56]. Thus, the use of halophytes as antioxidants in foods is a promising alternative to the use of those of synthetic origin, mainly due to the increasing request of consumers for natural food additives [57,58]. Thus, TBARS and OxHLIA assays are good ex vivo models for evaluating inhibition of lipid peroxidation by the presence of antioxidants [59,60]. As discussed in former sections, sea lavender flower extracts’ richness in flavonoids and its derivatives may be related to the high antioxidant activity of these samples. This makes these extracts great candidates to be used as ingredients in herbal supplementation products with the aim of improving general health and well-being, and/or as food additives for preventing lipid oxidation of lipid-rich foods.
3.4. Tyrosinase Inhibition
Tyrosinase is a multi-copper enzyme with a key role in melanin biosynthesis and enzymatic browning. Excessive melanin production and accumulation occur in several types of skin diseases, such as melasma, periorbital hyperpigmentation, and lentigines, and is also linked with an increased risk of skin cancer and with neurodegenerative ailments, including Parkinson’s disease [61,62,63,64]. The demand for new tyrosinase inhibitors from natural sources is on the rise due to the problems presented by some tyrosinase inhibitors currently in use, such as hydroquinone, which is potentially mutagenic to mammalian cells and linked to several adverse reactions (e.g., contact dermatitis, transient erythema) and arbutin, which is chemically unstable [65,66]. Enzymatic browning is a major problem of fresh-cut fruits, which results from oxidation reactions with several enzymes and leads to modifications in the appearance of the nutritional value of foodstuffs [5,6,7]. Sulfiting agents are the most frequently used anti-browning products, but have adverse health effects [8,9]. Thus, safer anti-browning additives are much needed, and several natural products were already identified, including polyphenol-rich extracts [10,67]. In this work, selected extracts were tested for tyrosinase inhibition, and results are depicted on Table 3. The flower extracts from plants irrigated with 300 mM NaCl allowed for the lowest IC50 value (873 µg/mL), while those from freshwater-irrigated plants were not active up to the concentration of 1000 µg/mL. However, other Limonium species have showed lower IC50 values, namely, L. delicatulum methanol extracts from roots (9.87 µg/mL) and leaves (24.77 µg/mL) [68], as well as hexane and ethyl acetate fractions from L. effusum methanol extracts (148–295 µg/mL) [69].
A high number of natural tyrosinase inhibitors are phenolics, including flavonoids and their derivatives. Some synthetic inhibitors are based on natural flavonoid skeletons providing an effective scaffold for the development of novel tyrosinase inhibitors. For example, flavonoids containing a keto group (e.g., kaempferol and quercetin) are described to have potent tyrosinase inhibition due to their capacity to chelate cooper in the enzyme active site [70]. In this sense, the higher tyrosinase inhibition found in flower extracts irrigated with 300 mM NaCl may be related with the high abundance of flavonoid-containing keto groups in this extract, such as dihydrokaempferol, naringenin, apigenin, and some of their derivatives. Additionally, other studies indicate that the number and location of the hydroxyl group on the flavonoids affect their inhibitory capacity towards tyrosinase. For example, the number of hydroxyl groups on the B ring of flavonoids or catechins improves their tyrosinase inhibition, which may also be correlated with their enhanced antioxidant activity [71]. Thus, the occurrence of phenolics with multiple hydroxyl groups, such as theasinensin B, licoagroside B, myricetin-3-O-rhamnoside, galloylshikimic acid sulphate, kaempferol-galloyl-hexoside, quercetin-3-O-acetyl-rhamnoside, and dihydrokaempferol, found in higher abundance in the flower extract from plants irrigated with 300 mM NaCl, can be related with the higher tyrosinase inhibition observed. Furthermore, since copper is a cofactor of the tyrosinase enzyme, the presence of copper-chelating compounds can lead to enzyme inactivation. Thus, the inhibitory activity of this extract may also be associated with its high antioxidant and copper chelating capacity. Overall, our results suggest that the flower extracts from sea lavender irrigated with saline aquaculture wastewaters contain molecules with tyrosinase inhibitory properties, most probably phenolic acids and flavonoids, and therefore with interest in the cosmetic, pharmaceutical and food industries.
3.5. In Vitro Anti-Inflammatory Properties
A wide range of mental and physical health disorders involve inflammation, such as chronic inflammatory states (e.g., ischemic heart disease, stroke, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease, autoimmune and neurodegenerative conditions) and have been recognized to comprise 50% of all deaths worldwide [72]. In this work, RAW 264.7 macrophages were stimulated with LPS to produce nitric oxide (NO) to simulate chronic inflammation [73], a common method for assessing the anti-inflammatory potential of botanicals [33]. However, none of the tested samples showed the ability to reduce the LPS-induced NO production when tested up to 400 µg/mL (data not shown). However, previous studies have reported the capacity of aqueous extracts of L. algarvense flowers collected from the wild to reduce the NO production in RAW 264.7 cells (IC50 = 46–48 µg/mL) [26]. The probable reason for this discrepancy could be the biomass origin and extraction methodology, since the reported activity was detected in plants collected from the wild extracted by infusion (100 °C for 5 min) instead of plants produced in a greenhouse extracted by ultrasounds for 30 min. This variation of chemical and biological properties is often noticed by other authors, and usually attributed to different samples’ origin, divergent extraction methodologies, and/or interspecific variability [74].
3.6. Toxicological Evaluation
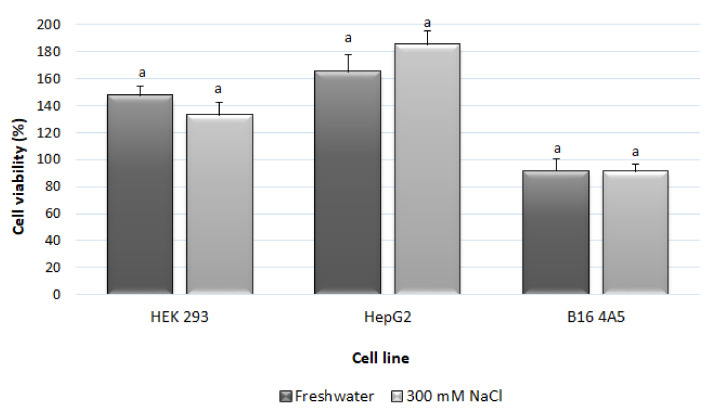
Although natural products are generally considered safer that its synthetic counterparts, it is well-known that “natural” does not necessary mean “safe” [75]. Thus, ensuring the toxicological safety of herbal ingredients is of major concern. Therefore, the cytotoxicity of the selected extracts was determined for three mammalian cell models, namely, a human hepatocellular carcinoma (HepG2) (if it was considered as a bioactive compound for oral dosage forms) and mouse melanoma (B16 4A5) (for a potential use as skin dosage forms), and one non-tumor cell line (human embryonic kidney, HEK 293). Incubating cells with the sea lavender flower extracts at a concentration of 100 µg/mL for 72 h resulted in cellular viabilities above 91% (Figure 1).
Figure 1.
Cytotoxicity of cultivated sea lavender flower extracts irrigated with freshwater and 300 mM NaCl towards non-tumoral human embryonic kidney (HEK 293), human hepatocellular carcinoma (HepG2) and mouse melanoma (B16 4A5) cell lines. Results are expressed as cellular viability (%) at a concentration of 100 µg/mL after 72 h. Values represent the mean ± standard error of the mean (SEM) of at least six repetitions (n = 6). For each cell line, values followed by the same letter (a) are significantly similar at p < 0.05 (Tukey HSD test).
The extracts triggered an increase in cell viability of HEK 293 and HepG2 cells (133–147% and 165–185%, respectively) (Figure 1). Former work on infusions made from this species also reported nil toxicity in mammalian cells (HepG2, mouse stromal bone marrow [S17] and mouse microglia [N9]), and against the brine shrimp Artemia salina [26]. Therefore, both extracts of sea lavender flowers (freshwater and 300 mM NaCl) can be considered non-toxic, suggesting that they can be used as safe ingredients for use in herbal health supplements and/or food additives.
4. Conclusions
This work reported on the effect of the irrigation salinity on the chemical and functional properties of water extracts obtained from greenhouse-produced sea lavenders. The irrigation salinity and plant organ affected the in vitro antioxidant capacity and chemical composition of the extracts, which were mainly composed of flavonoids and its derivatives (e.g., sulphated, methylated, and glycosylated), but both properties were preserved under fresh- and saltwater irrigation (up to 300 mM NaCl). The flower extract had the highest chemical diversity and in vitro antioxidant properties and exhibited high ex vivo antioxidant capacity in OxHLIA and TBARS assays, inhibition of tyrosinase, and was not toxic. Our results suggest that flowers from sea lavender cultivated in greenhouse conditions and irrigated with aquaculture wastewater with a concentration up to 300 mM NaCl could provide a flavonoid-rich water extract with potential uses in herbal health supplements and/or food additives, for its antioxidant and tyrosinase inhibitory properties.
Acknowledgments
The authors acknowledge the Faculty of Pharmacy and Centre for Neurosciences and Cell Biology (University of Coimbra, Portugal) that kindly offered the murine RAW 264.7 macrophages, and the Functional Biochemistry and Proteomics, and the Marine Molecular Bioengineering groups (Centre of Marine Sciences, Portugal) that provided the human embryonic kidney (HEK) 293, and the human hepatocellular carcinoma (HepG2) cell lines.
Author Contributions
Conceptualization, M.J.R., L.B., M.C.O., C.G.P. and L.C.; Data curation, M.J.R., V.C.-L., I.M. and J.P.; Formal analysis, M.J.R.; Funding acquisition, L.B., M.C.O. and L.C.; Investigation, M.J.R., V.C.-L., I.M., J.P., L.B., M.C.O. and C.G.P.; Methodology, M.J.R., V.C.-L., L.B. and C.G.P.; Project administration, L.C.; Resources, R.M.V.A. and L.C.; Supervision, C.R., F.S., P.P.-F. and L.C.; Validation, C.R. and L.C.; Writing—original draft, M.J.R., J.P. and M.C.O.; Writing—review & editing, M.J.R., L.B., R.M.V.A., C.R., F.S., P.P.-F., C.G.P. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
Work supported by the Foundation for Science and Technology (FCT) and the Portuguese National Budget through projects UIDB/04326/2020, UID/DTP/04138/2020 and PTDC/BAA-AGR/1391/2020 (Greenvalue: Exploring salt tolerant plants as sources of innovative food additives). It also received funding through Fundo Azul (XtremeAquaCrops project: FA-05-2017-028), and by RNEM (Portuguese Mass Spectrometry Network) (LISBOA-01-0145-FEDER-022125-IST). CIMO was financially supported by FCT through national funds FCT/MCTES (UIDB/00690/2020). Viana Castañeda-Loaiza acknowledge FCT for the PhD grant 2020.04541.BD. Luísa Custódio and José Pinela were supported by FCT Scientific Employment Stimulus (CEECIND/00425/2017 and CEECIND/01011/2018, respectively) and Lillian Barros through the institutional scientific employment program-contract.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset is available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Charen E., Harbord N. Toxicity of Herbs, Vitamins, and Supplements. Adv. Chronic Kidney Dis. 2020;27:67–71. doi: 10.1053/j.ackd.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Grand View Research Herbal Supplements Market Size, Share & Trends Analysis Report by Product (Turmeric, Echinacea), by Formulation (Capsules, Powder and Granules), by Consumer (Pregnant Women, Adults), and Segment Forecasts, 2018–2025. [(accessed on 30 October 2021)]. Available online: https://www.grandviewresearch.com/industry-analysis/herbal-supplements-market.
- 3.Persistance Market Research Botanical Supplements Market. Global Market Study on Botanical Supplements: Drugs Application Segment to Hold Maximum Value Share During 2017–2025. [(accessed on 30 October 2021)]. Available online: https://www.persistencemarketresearch.com/market-research/botanical-supplements-market.asp.
- 4.Anand S.P., Sati N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. IJPSR. 2013;4:2496. [Google Scholar]
- 5.Arnold E., Lofthouse N., Hurt E. Artificial Food Colors and Attention-Deficit/Hyperactivity Symptoms: Conclusions to dye for. Neurotherapeutics. 2012;9:599. doi: 10.1007/s13311-012-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tükoğlu S. Evaluation of genotoxic effects of five flavour enhancers (glutamates) on the root meristem cells of Allium cepa. Toxicol. Ind. Health. 2015;31:792. doi: 10.1177/0748233713475509. [DOI] [PubMed] [Google Scholar]
- 7.Minioti K.S., Sakellariou C.F., Thomaidis N.T. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta. 2007;583:103. doi: 10.1016/j.aca.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Kanarek R.B. Artificial food dyes and attention deficit hyperactivity disorder. Nutr. Rev. 2011;69:385–391. doi: 10.1111/j.1753-4887.2011.00385.x. [DOI] [PubMed] [Google Scholar]
- 9.Bearth A., Cousin M.-E., Siegrist M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Pref. 2014;38:14–23. doi: 10.1016/j.foodqual.2014.05.008. [DOI] [Google Scholar]
- 10.Huang H.-W. Current status and future trends of high-pressure processing in food industry. Food Control. 2017;72:1–8. doi: 10.1016/j.foodcont.2016.07.019. [DOI] [Google Scholar]
- 11.Scaglioni P.S., Badiale-Furlong E. Can Microalgae Act as Source of Preservatives in Food Chain? J. Food. Sci. Eng. 2017;7:283–296. [Google Scholar]
- 12.Che C.-T., Zhang H. Plant Natural Products for Human Health. Int. J. Mol. Sci. 2019;20:830. doi: 10.3390/ijms20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes M., Sanches-Silva A., Castilho M., Cavaleiro C., Ramos F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021:1–24. doi: 10.1080/10408398.2021.1959295. [DOI] [PubMed] [Google Scholar]
- 14.Guillerme J.-B., Couteau C., Coiffard L. Applications for Marine Resources in Cosmetics. Cosmetics. 2017;4:35. doi: 10.3390/cosmetics4030035. [DOI] [Google Scholar]
- 15.Parida A.K., Veerabathini S.K., Kumari A., Agarwal P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016;7:351. doi: 10.3389/fpls.2016.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano R., Saii Z., Fredsgaard M., Hulkko L.S.S., Poulsen T.B.G., Thomsen M.E., Henneberg N., Zucolotto S.M., Arendt-Nielsen L., Papenbrock J., et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules. 2021;26:3140. doi: 10.3390/molecules26113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barreira L., Resek E., Rodrigues M.J., Rocha M.I., Pereira H., Bandarra N., Silva M.M., Varela J., Custódio L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Anal. 2017;59:35–42. doi: 10.1016/j.jfca.2017.02.003. [DOI] [Google Scholar]
- 18.Pereira C.G., Barreira L., Neng N.R., Nogueira J.M.F., Marques C., Santos T.F., Varela J., Custódio L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017;107:581–589. doi: 10.1016/j.fct.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Castañeda-Loaiza V., Oliveira M., Santos T., Schüler L., Lima A.R., Gama F., Salazar M., Neng N.R., Nogueira J.M.F., Varela J., et al. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020;333:27536. doi: 10.1016/j.foodchem.2020.127536. [DOI] [PubMed] [Google Scholar]
- 20.Ksouri R., Ksouri W.M., Jallali I., Debez A., Magné C., Hiroko I., Abdelly C. Medicinal halophytes: Potent source of health promoting biomolecules with medical nutraceutical and food applications. Crit. Rev. Biotechnol. 2012;32:289–326. doi: 10.3109/07388551.2011.630647. [DOI] [PubMed] [Google Scholar]
- 21.Ladeiro B. Saline agriculture in the 21st Century: Using salt contaminated resources to cope food requirements. J. Bot. 2012;2012:310705. doi: 10.1155/2012/310705. [DOI] [Google Scholar]
- 22.Holguin Peña R.J., Medina-Hernández D., Ghasemi M., Rueda Puente E.O. Salt tolerant plants as a valuable resource for sustainable food production in arid and saline coastal zones. Acta Biol. Colomb. 2021;26:116–126. doi: 10.15446/abc.v26n1.82412. [DOI] [Google Scholar]
- 23.Custódio M., Villasante S., Cremades J., Calado R., Lillebø A.I. Unravelling the potential of halophytes for marine integrated multi-trophic aquaculture (IMTA)—A perspective on performance, opportunities and challenges. Aquac. Environ. Interact. 2017;9:445–460. doi: 10.3354/aei00244. [DOI] [Google Scholar]
- 24.Jerónimo D., Lillebø A.I., Maciel E., Domingues M.R.M., Cremades J., Calado R. Unravelling the fatty acid profiles of different polychaete species cultured under integrated multi-trophic aquaculture (IMTA) Sci. Rep. 2021;11:10812. doi: 10.1038/s41598-021-90185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues M.J., Soszynski A., Martins A., Rauter A.P., Neng N.R., Nogueira J.M.F., Varela J., Barreira L., Custódio L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015;77:315–322. doi: 10.1016/j.indcrop.2015.08.061. [DOI] [Google Scholar]
- 26.Rodrigues M.J., Neves V., Martins A., Rauter A.P., Neng N.R., Nogueira J.M.F., Varela J., Barreira L., Custódio L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis) Food Chem. 2016;200:322–329. doi: 10.1016/j.foodchem.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues M.J., Oliveira M., Neves V., Ovelheiro A., Pereira C., Neng N.R., Nogueira J.M.F., Varela J., Barreira L., Custódio L. Coupling Sea lavender (Limonium algarvense Erben) and green tea (Camellia sinensis (L.) Kuntze) to produce an innovative herbal beverage with enhanced enzymatic inhibitory properties. S. Afr. J. Bot. 2019;120:87–94. doi: 10.1016/j.sajb.2017.12.003. [DOI] [Google Scholar]
- 28.Rodrigues M.J., Monteiro I., Castañeda-Loaiza V., Placines C., Oliveira M.C., Caperta A.D., Pousão-Ferreira P., Pereira C., Custódio L. Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the salinity irrigation. Ind. Crop. Prod. 2020;143:111930. doi: 10.1016/j.indcrop.2019.111930. [DOI] [Google Scholar]
- 29.Silva de Sá I., Peron A.P., Leimann F.V., Bressan G.N., Krum B.N., Fachinetto R., Pinela J., Calhelha R.C., Barreiro M.F., Ferreira I.C.F.R., et al. In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions. Food Chem. Toxicol. 2019;125:29–37. doi: 10.1016/j.fct.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Corrêa R.C.G., de Souza A.H.P., Calhelha R.C., Barros L., Glamoclija J., Sokovic M., Peralta R.M., Bracht A., Ferreira I.C.F.R. Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 2015;6:2155–2164. doi: 10.1039/C5FO00465A. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues M.J., Pereira C., Oliveira M., Neng N.R., Nogueira J.M.F., Zengin G., Mahomoodally M.F., Custódio L. Sea rose (Armeria pungens (Link) Hoffmanns. & Link) as a potential source of innovative industrial products for anti-ageing applications. Ind. Crop. Prod. 2018;121:250–257. [Google Scholar]
- 32.Rodrigues M.J., Matkowski A., Slusarczyk S., Magné C., Poleze T., Pereira C., Custódio L. Sea knotgrass (Polygonum maritimum L.) as a potential source of innovative industrial products for skincare applications. Ind. Crop. Prod. 2019;128:391–398. doi: 10.1016/j.indcrop.2018.11.038. [DOI] [Google Scholar]
- 33.Rodrigues M.J., Gangadhar K.N., Vizetto-Duarte C., Wubshet S.G., Nyberg N.T., Barreira L., Varela J., Custódio L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs. 2014;12:2228–2244. doi: 10.3390/md12042228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petigny L., Ozel M.Z., Périno S., Wajsman J., Chemat F. Water as green solvent for extraction of natural products. In: Chemat F., Strube J., editors. Green Extraction of Natural Products. Wiley-VCH Verlag GmbH & Co.; Weinheim, Germany: 2015. pp. 237–264. [Google Scholar]
- 35.Pereira D.M., Valentão P., Pereira J.A., Andrade P.B. Phenolics: From Chemistry to Biology. Molecules. 2009;14:2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- 36.Teles Y.C.F., Souza M.S.R., Souza M.D.F.V.D. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules. 2018;23:480. doi: 10.3390/molecules23020480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barron D., Varin L., Ibrahim R.K., Harborne J.B., Williams C.A. Sulphated Flavonoids—An update. Phytochem. 1988;27:2375–2395. doi: 10.1016/0031-9422(88)87003-1. [DOI] [Google Scholar]
- 38.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correia-da-Silva M., Sousa E., Pinto M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014;34:223–279. doi: 10.1002/med.21282. [DOI] [PubMed] [Google Scholar]
- 40.Iglesias J., Pazos M., Lois S., Medina I. Contribution of galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J. Agric. Food Chem. 2010;58:7423–7431. doi: 10.1021/jf100832z. [DOI] [PubMed] [Google Scholar]
- 41.Karas D., Ulrichová J., Valentová K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017;105:223–240. doi: 10.1016/j.fct.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Gupta B., Huang B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014;2014:701596. doi: 10.1155/2014/701596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avaz S. Master’s Thesis. Afyon Kocatepe University; Afyonkarahisar, Turkey: 2010. Afyonkarahisar’da Doğal Olarak Yetişen Limonium Mill. Türlerinin Antimikrobiyal Aktiviteleri. [Google Scholar]
- 44.Ruiz-Riaguas A., Zengin G., Sinan K.I., Salazar-Mendías C., Llorent-Martínez E.J. Phenolic profile, antioxidant activity, and enzyme inhibitory properties of Limonium delicatulum (Girard) Kuntze and Limonium quesadense Erben. J. Chem. 2020;2020:1016208. doi: 10.1155/2020/1016208. [DOI] [Google Scholar]
- 45.Geng D., Chi X., Dong Q., Hu F. Antioxidants screening in Limonium aureum by optimized on-line HPLC–DPPH assay. Ind. Crop. Prod. 2015;67:492–497. doi: 10.1016/j.indcrop.2015.01.063. [DOI] [Google Scholar]
- 46.Gadetskaya A.V., Tarawneh A.H., Zhusupova G.E., Gemejiyeva N.G., Cantrell C.L., Cutler S.J., Ross S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia. 2015;104:80–85. doi: 10.1016/j.fitote.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Orenga S., Ferrer-Gallego P.P., Laguna E., López-Gresa M.P., Donat-Torres M.P., Verdeguer M., Vicente O., Boscaiu M. Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites. 2019;9:294. doi: 10.3390/metabo9120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miguel-Chávez R.S. Phenolic antioxidant capacity: A review of the state of the art. In: Soto-Hernandez M., Palma-Tenango M., Garcia-Mateos M.R., editors. Phenolic Compounds—Biological Activity. IntechOpen; London, UK: 2017. [Google Scholar]
- 49.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutiérrez-del-Río I., López-Ibáñez S., Magadán-Corpas P., Fernández-Calleja L., Pérez-Valero Á., Tuñón-Granda M., Miguélez E.M., Villar C.J., Lombó F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants. 2021;10:1264. doi: 10.3390/antiox10081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slama I., M’Rabet R., Ksouri R., Talbi O., Debez A., Abdelly C. Water deficit stress applied only or combined with salinity affects physiological parameters and antioxidant capacity in Sesuvium portulacastrum. Flora-Morphol. Distrib. Funct. Ecol. Plants. 2015;213:69–76. doi: 10.1016/j.flora.2015.04.004. [DOI] [Google Scholar]
- 52.Ksouri R., Megdiche W., Debez A., Falleh H., Grignon C., Abdelly C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007;45:244–249. doi: 10.1016/j.plaphy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 53.U.S. National Library of Medicine . Haz-Map: Occupational Exposure to Hazardous Agents. National Library of Medicine; Bethesda, US: 2010. [(accessed on 11 December 2021)]. Available online: http://hazmap.nlm.nih.gov. [Google Scholar]
- 54.International Agency for Research on Cancer. WHO; Paris, France: 1986. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 55.Study on Enhancing the Endocrine Disrupter Priority List with a Focus on Low Production Volume Chemicals, Revised Report to DG Environment. DHI Water and Environment; Hersholm, Denmark: 2007. [(accessed on 11 December 2021)]. Available online: http://ec.europa.eu/environment/endocrine/documents/final_report_2007.pdf. [Google Scholar]
- 56.UNEP and OECD, 2,6-di-tert-butyl-p-cresol (BHT) Screening Information Data Set: Initial Assessment Report. [(accessed on 11 December 2021)]. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=6d30349e-ef9f-496c-a2af-6d497d4f1cca.
- 57.Permal R., Chang W.L., Chen T., Seale B., Hamid N., Kam R. Optimising the spray drying of avocado wastewater and use of the powder as a food preservative for preventing lipid peroxidation. Foods. 2020;9:1187. doi: 10.3390/foods9091187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martínez-Tomé M., Jiménez A.M., Ruggieri S., Frega N., Strabbioli R., Murcia M.A. Antioxidant properties of mediterranean spices compared with common food additives. J. Food Prot. 2001;64:1412–1419. doi: 10.4315/0362-028X-64.9.1412. [DOI] [PubMed] [Google Scholar]
- 59.Takebayashi J., Chen J., Tai A. A Method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. In: Armstrong D., editor. Advanced Protocols in Oxidative Stress II. Humana Press; London, UK: 2009. pp. 287–296. [DOI] [PubMed] [Google Scholar]
- 60.Takebayashi J., Iwahashi N., Ishimi Y., Tai A. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA) Food Chem. 2012;134:606–610. doi: 10.1016/j.foodchem.2012.02.086. [DOI] [Google Scholar]
- 61.Hakozaki T., Swanson C.L., Bissett D.L. Hyperpigmentaion in aging skin. In: Farage M., Miller K., Maibach H., editors. Textbook of Aging Skin. Springer; Berlin/Heidelberg, Germany: 2015. pp. 495–501. [Google Scholar]
- 62.Cestari T.F., Dantas L.P., Boza J.C. Acquired hyperpigmentations. An. Bras. Dermatol. 2014;89:11–25. doi: 10.1590/abd1806-4841.20142353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nouveau S., Agrawal D., Kohli M., Bernerd F., Misra N., Nayak C.S. Skin hyperpigmentation in Indian population: Insights and best practice. Indian J. Dermatol. 2016;61:487–495. doi: 10.4103/0019-5154.190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorga S., Sarangal R. Pigmentary disorders: An insight. Pigment Int. 2014;1:5–7. [Google Scholar]
- 65.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zolghadri S., Bahrami A., Hassan Khan M.T., Munoz-Munoz J., Garcia-Molina F., Garcia-Canovas F., Saboury A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019;34:279–309. doi: 10.1080/14756366.2018.1545767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kusumawati I.G.A.W., Putra I.M.W.A., Yogeswara I.B.A. In vitro ACE inhibitory activity and bioactive-compounds of aqueous extract of Citrus amblycarpa. Trad. Med. J. 2021;26:118–122. [Google Scholar]
- 68.Bakhouche I., Toufik A., Tahar B., Lynda G., Aysen S., Yuva B. Phenolic contents and in vitro antioxidant, anti-tyrosinase, and antiinflammatory effects of leaves and roots extracts of the halophyte Limonium delicatulum. S. Afr. J. Bot. 2021;139:42–49. doi: 10.T/j.sajb.2021.01.030. [DOI] [Google Scholar]
- 69.Baysal I., Ekizoglu M., Ertas A., Temiz B., Agalar H.G., Yabanoglu-Ciftci S., Temel H., Ucar G., Turkmenoglu F.P. Identification of Phenolic Compounds by LC-MS/MS and Evaluation of Bioactive Properties of Two Edible Halophytes: Limonium effusum and L. sinuatum. Molecules. 2021;26:4040. doi: 10.3390/molecules26134040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Obaida R.J., Mughal E.U., Naeemb N., Sadiqc A., Alsantalid R.I., Jassase R.S., Moussa Z., Ahmed S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021;11:22159–22198. doi: 10.1039/D1RA03196A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuo A.R., Dong H.H., Yu Y.Y., Shu Q.L., Zheng L.X., Yu X.Y., Cao S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018;13:51. doi: 10.1186/s13020-018-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zamora R., Vodovotz Y., Billiar T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000;6:347–373. doi: 10.1007/BF03401781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaston T.E., Mendrick D.L., Paine M.F., Roe A.L., Yeung C.K. Natural” is not synonymous with “Safe”: Toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 2020;113:104642. doi: 10.1016/j.yrtph.2020.104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available upon request from the corresponding author.